-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Receptor-Defined Subtypes of Breast Cancer in Indigenous Populations in Africa: A Systematic Review and Meta-Analysis
Background:
Breast cancer is the most common female cancer in Africa. Receptor-defined subtypes are a major determinant of treatment options and disease outcomes but there is considerable uncertainty regarding the frequency of poor prognosis estrogen receptor (ER) negative subtypes in Africa. We systematically reviewed publications reporting on the frequency of breast cancer receptor-defined subtypes in indigenous populations in Africa.Methods and Findings:
Medline, Embase, and Global Health were searched for studies published between 1st January 1980 and 15th April 2014. Reported proportions of ER positive (ER+), progesterone receptor positive (PR+), and human epidermal growth factor receptor-2 positive (HER2+) disease were extracted and 95% CI calculated. Random effects meta-analyses were used to pool estimates. Fifty-four studies from North Africa (n = 12,284 women with breast cancer) and 26 from sub-Saharan Africa (n = 4,737) were eligible. There was marked between-study heterogeneity in the ER+ estimates in both regions (I2>90%), with the majority reporting proportions between 0.40 and 0.80 in North Africa and between 0.20 and 0.70 in sub-Saharan Africa. Similarly, large between-study heterogeneity was observed for PR+ and HER2+ estimates (I2>80%, in all instances). Meta-regression analyses showed that the proportion of ER+ disease was 10% (4%–17%) lower for studies based on archived tumor blocks rather than prospectively collected specimens, and 9% (2%–17%) lower for those with ≥40% versus those with <40% grade 3 tumors. For prospectively collected samples, the pooled proportions for ER+ and triple negative tumors were 0.59 (0.56–0.62) and 0.21 (0.17–0.25), respectively, regardless of region. Limitations of the study include the lack of standardized procedures across the various studies; the low methodological quality of many studies in terms of the representativeness of their case series and the quality of the procedures for collection, fixation, and receptor testing; and the possibility that women with breast cancer may have contributed to more than one study.Conclusions:
The published data from the more appropriate prospectively measured specimens are consistent with the majority of breast cancers in Africa being ER+. As no single subtype dominates in the continent availability of receptor testing should be a priority, especially for young women with early stage disease where appropriate receptor-specific treatment modalities offer the greatest potential for reducing years of life lost.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(9): e32767. doi:10.1371/journal.pmed.1001720
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001720Summary
Background:
Breast cancer is the most common female cancer in Africa. Receptor-defined subtypes are a major determinant of treatment options and disease outcomes but there is considerable uncertainty regarding the frequency of poor prognosis estrogen receptor (ER) negative subtypes in Africa. We systematically reviewed publications reporting on the frequency of breast cancer receptor-defined subtypes in indigenous populations in Africa.Methods and Findings:
Medline, Embase, and Global Health were searched for studies published between 1st January 1980 and 15th April 2014. Reported proportions of ER positive (ER+), progesterone receptor positive (PR+), and human epidermal growth factor receptor-2 positive (HER2+) disease were extracted and 95% CI calculated. Random effects meta-analyses were used to pool estimates. Fifty-four studies from North Africa (n = 12,284 women with breast cancer) and 26 from sub-Saharan Africa (n = 4,737) were eligible. There was marked between-study heterogeneity in the ER+ estimates in both regions (I2>90%), with the majority reporting proportions between 0.40 and 0.80 in North Africa and between 0.20 and 0.70 in sub-Saharan Africa. Similarly, large between-study heterogeneity was observed for PR+ and HER2+ estimates (I2>80%, in all instances). Meta-regression analyses showed that the proportion of ER+ disease was 10% (4%–17%) lower for studies based on archived tumor blocks rather than prospectively collected specimens, and 9% (2%–17%) lower for those with ≥40% versus those with <40% grade 3 tumors. For prospectively collected samples, the pooled proportions for ER+ and triple negative tumors were 0.59 (0.56–0.62) and 0.21 (0.17–0.25), respectively, regardless of region. Limitations of the study include the lack of standardized procedures across the various studies; the low methodological quality of many studies in terms of the representativeness of their case series and the quality of the procedures for collection, fixation, and receptor testing; and the possibility that women with breast cancer may have contributed to more than one study.Conclusions:
The published data from the more appropriate prospectively measured specimens are consistent with the majority of breast cancers in Africa being ER+. As no single subtype dominates in the continent availability of receptor testing should be a priority, especially for young women with early stage disease where appropriate receptor-specific treatment modalities offer the greatest potential for reducing years of life lost.
Please see later in the article for the Editors' SummaryIntroduction
Breast cancer is the most common female malignancy in Africa, being the cancer with the first or second highest incidence and/or mortality in most African countries (Figure 1). Although breast cancer incidence rates are lower in Africa than in the rest of the world, mortality rates in certain African countries (e.g., Nigeria, Egypt, Ethiopia) are among the highest worldwide [1], reflecting the relatively poor survival from the disease in the continent. Different breast cancer subtypes are classified in the clinical setting by estrogen (ER), progesterone (PR), and human epidermal growth factor-2 (HER2) receptor status. These receptors are a fundamental characteristic of the epidemiology of this malignancy [2], as its aetiology and incidence trends are receptor-status specific, and they are also a major determinant of treatment options, disease outcomes, and survival [3].
Fig. 1. Breast cancer ranking among women for (a) incidence and (b) mortality, Africa, 2012 <em class="ref">[1]</em>. ![Breast cancer ranking among women for (a) incidence and (b) mortality, Africa, 2012 <em class="ref">[1]</em>.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/73f3a180b8be14119e05506810c7245d.png)
ER-positive (ER+) tumors typically have a better prognosis and are more receptive to hormonal treatment [4]. In white (i.e., European ancestry) women, ER+ tumors predominate, with 79% of breast tumors in US-born white women being ER+ (calculated amongst women with known ER-status) [5]. The proportion of ER+ tumors is lower among US-born black (i.e., of African ancestry) women (61% are ER+, all ages combined) [5],[6], but the extent to which this is also reflected in Africa is not well-established. Some studies [7],[8] have reported a markedly higher proportion of ER-negative (ER−) or basal-like breast cancers in indigenous populations in Africa, which may contribute to the poor survival from this malignancy, but others suggest that the relative frequency of the different subtypes in the continent may not differ substantially to that seen elsewhere [9],[10].
Knowledge of the relative frequency of breast cancer subtypes in Africa would be of relevance for several reasons. Firstly, if the distribution of receptor status is greatly different in Africa than elsewhere, the differing contribution of genetic and environmental risk factors to such a difference would need to be investigated, as is debated for ethnic differences in the US [11]. Secondly, where tumor receptor status is not routinely ascertained, the need for introducing it would be more urgent if one subtype does not greatly dominate and all subtypes are present. The latter scenario would call for the introduction of receptor testing to be prioritised, especially for patients who would have the prospect of good survival if given the appropriate treatment. Knowledge of the distribution of tumor receptor subtypes in Africa would also be of relevance globally as the continent would provide a better setting to study any subtypes that are rare elsewhere, but may be common there.
In the absence of large standardized multi-country studies of breast cancer subtypes in Africa, a rigorous systematic review of previously published studies will provide the timeliest answer to the debate on the receptor status distribution in Africa. Herein, we systematically review all studies that have reported receptor status of breast cancer in indigenous African populations and assess sources of between-study heterogeneity in prevalence estimates based on more than 17,000 women with breast cancer.
Methods
Search Methodology
The PRISMA guidelines (Text S1) were used to develop the study protocol (Text S2). We conducted a search of Medline, Embase, and Global Health [12] of studies published between 1st January 1980 and 15th April 2014. After an initial search using specific keywords, the search was broadened to “breast cancer” in “Africa” (with each country individually named; Text S3) in order to capture the studies where receptor status was not the focus of the paper but likely to be reported under patients' characteristics. No language restrictions were imposed. In addition, we searched African Journals Online (AJO) and the Breast Health Global Initiative – INCTR Breast Cancer Control Library [13].
The titles and abstracts were reviewed by one author (AE) twice independently. Abstracts were excluded if the studies did not focus on breast cancer (e.g., studies of “all cancers”) or did not include women with breast cancer (e.g., surveys of attitudes towards breast screening); if they exclusively focused on: males, African-American women, metastatic breast cancer, pregnant women, or specific treatment groups; or if the total number of women with breast cancer included was <50. The latter were predominantly clinical reports or unrepresentative small case series of women with breast cancer who had been selected because of their unusual clinical or pathological characteristics (e.g., high-risk familial cases, BRCA1/2 carriers, bilateral cases, gestational breast cancers), and were also more likely to have arisen from settings where there was less quality control in laboratory procedures for fixation and immunohistochemistry (IHC). Studies were also excluded if they focused exclusively on non-black populations (e.g., white or coloured women in South Africa). Reviews and conference proceedings were not included, but their references were cross-checked. A random sample of 80 titles/abstracts was also reviewed independently by another author (IdSS); this review revealed high between-reviewer reproducibility with no disagreements on which papers to select for full text review. The full text was retrieved for all potentially relevant papers and reviewed by the same author (AE) for reporting of receptor status. If there were multiple papers from the same study the paper with the most information on receptor status was selected for inclusion.
Data Extraction
The data extraction from each eligible paper was carried out independently by two reviewers (AE and IdSS or VM and IdSS) using a specifically developed and pre-tested computerised data extraction form (Text S2). Data were extracted on the number of women with breast cancer with available receptor status information, and the number of those with positive and negative tumors, as classified in the original article regardless of the criteria used to define positivity (Tables 1 and 2), for ER (ER+/ER−), PR (PR+/PR−), and HER2 (HER2+/HER2−) and, where available, for combined subtypes: luminal A (ER+ and/or PR+; HER2−), luminal B (ER+ and/or PR+; HER2+), HER2+-enriched (ER−; PR−; HER2+), and triple negative (ER−; PR−; HER2−). Information was also extracted on type of study, including study design (e.g., population-based, case series based on consecutive women diagnosed with breast cancer over a defined time period, or collection based on convenience [opportunistic] samples), source of the breast cancer patients (e.g., hospital/clinic or cancer registry), sample size and study period; tumor characteristics (e.g., histological type; tumor size, stage, and grade); collection and storage conditions of the tumor specimens (e.g., fresh-frozen, formalin-fixed paraffin-embedded [FFPE] blocks); receptor testing (e.g., timing, type of assay, positivity criteria); and on demographic and reproductive-related variables (e.g., ethnicity, age, and menopausal status at diagnosis) where available. Many studies had limited information on how women with breast cancer were selected, or on the time period from tumor specimen collection to receptor testing, and the details provided in their methods section were used to obtain as informed a description as was possible. We did not attempt to contact the authors because most of the missing information was from studies published in the early years, making it difficult to establish contact and unlikely that the missing information would still be available. A few studies included a small number of men with breast cancer; these were included in the review as the papers did not provide enough information to allow their exclusion. Disagreements were discussed by both reviewers and a consensus reached.
Tab. 1. Characteristics of the participating studies: North Africa (54 studies). 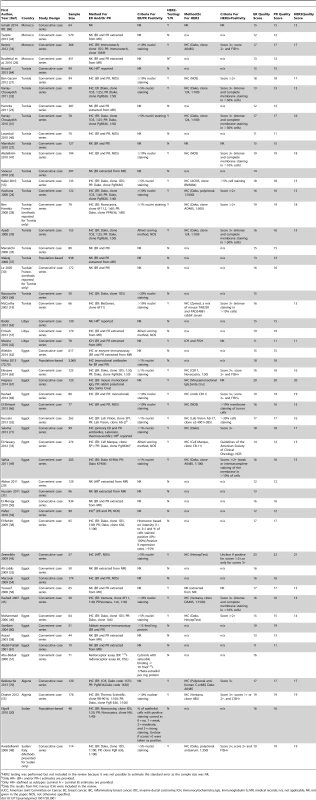
HER2 testing was performed but not included in the review because it was not possible to estimate the standard error as the sample size was NK. Tab. 2. Characteristics of the participating studies: Sub-Saharan Africa (26 studies). 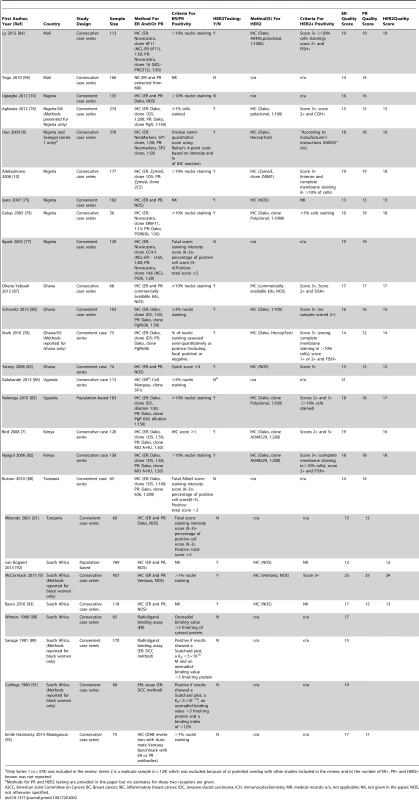
Only Series 1 (n = 378) was included in the review. Series 2 is a replicate sample (n = 129) which was excluded because of a) potential overlap with other studies included in the review; and b) the number of ER+, PR+ and HER2+ known was not reported. Study Quality
We adopted an approach similar to that used by the Cochrane Collaboration to develop a standardised quality assessment form for assessing the risk of bias in randomised studies [14]. We identified items within three quality domains to reflect the potential for selection bias, misclassification of receptor status, and availability of data on key correlates of receptor status. A list of items for each one of the three domains was developed. For each item, papers were allocated a score ranging from 0 (if it did not meet the criteria or if the information provided was unclear) to a maximum of 2 or 4, depending on the item, with more weight given to items in the selection bias and misclassification domains. Items in the selection bias domain included study design/case selection (score 0 if unclear; 2, if opportunistic case series; 4, if consecutive or population-based case series) and percentage of patients with known receptor status (score 0, if unclear; 2, if <70%; 4, if ≥70%). Items in the misclassification domain comprised timing of tumor specimen collection (score 0, if inferred that tumor samples were collected prior to the start of treatment but this is not clearly stated—studies stating that collection was done after treatment were excluded from the review; 2, if specified that collection was done prior to treatment onset); tumor tissue storage conditions (score 0, if unclear; 2, if FFPE; 4, if frozen); timing of receptor status testing (score 2, if retrospective based on archival samples; 4, if conducted at the time of diagnosis); assay method (score 0, if not given; 2, if method described); criteria used to ascertain receptor positivity (for ER and PR: score 0, if not given; 2, if criteria described; for HER2: score 0, if not given; 1, if criteria described but fluorescent in situ hybridization [FISH] [chromogenic in situ hybridization (CISH) or silver in situ hybridization (SISH)] not used; 2, if FISH [CISH or SISH] used). The domain on correlates of receptor status comprised availability of information on age and/or menopausal status, tumor grade, and tumor stage (all scored as 0 if missing, 1 if available). The overall quality of the study was expressed as the sum of its item-specific scores. The range of possible scores was from 0 (lowest) to 25 (highest); the higher the score the higher the methodological quality of the study and, hence, the lower the risk that its findings might have been affected by bias.
Two authors (AE and IdSS) reviewed the quality of individual studies and inconsistencies discussed to reach consensus. In the analysis, we opted for simply describing the distribution of scores for studies reporting on each specific receptor, rather than using an arbitrary cut-off to define high versus low quality studies, and for examining both the contribution of the overall quality score and of specific quality criteria to between-study heterogeneity in estimates.
Statistical Methods
As previous studies suggested differential ER+ proportions in women of African, rather than Arabic origin, results are presented separately for North Africa (i.e., Algeria, Egypt, Libya, Morocco, Sudan, Tunisia, and Western Sahara) and sub-Saharan Africa (i.e., all remaining African countries) according to their predominant population groups as defined by the United Nations [15]. For each receptor, the proportion of receptor-positive breast cancers (prop) was the statistic of interest, calculated as (number of receptor−positive tumors)/(n = number of tumors with known receptor status). Wilson score 95% CIs for this binomial prop were calculated and, on the basis of these, meta analyses were conducted in STATA version 12 (StataCorp), using the metaprop command to estimate pooled proportions using random effects models. Between-study heterogeneity was assessed using I2 (with its 95% CI estimated by the method of Higgins and Thomson [16]) and the p-value for heterogeneity (Cochrane's Q statistic). The I2 statistic represents the percentage of between-study variation due to heterogeneity rather than chance [17]. To examine potential sources of heterogeneity, study-specific estimates were stratified according to a priori defined geographical (i.e., two ad hoc sub-regions within North Africa—North-Eastern and North-Western—and three sub-regions in sub-Saharan Africa—Eastern, Southern, and Western—as defined by the United Nations [15]; see Results section), clinical factors (e.g., age, year, and menopausal status at diagnosis, tumor stage, and grade) and methodologically relevant variables (e.g., study design, timing of receptor testing, specimen storage conditions, study quality). Few studies provided information on reproductive-related variables except menopausal status; if data on the latter variable were not available, women aged >50 years were classified as post-menopausal. Meta-regression analyses were conducted to identify independent sources of between-study heterogeneity. These analyses necessitated an assumption of a single standard error that was estimated as √{prop(1−prop)/n}. Funnel plots and the Egger test [18] were performed to examine whether small study bias could have affected the results.
Results
Characteristics of Included Studies
The systematic search in Medline, Embase, and Global Health produced 2,032 abstracts, of which 243 were identified as potentially relevant and the full text reviewed (Figure 2). A further 13 studies were identified from African Journals Online or hand-searches of bibliographic references. Eighty studies reported on ER status (no studies reported on PR or HER2 status without also reporting on ER status) and were therefore included in the review, involving a total of 17,021 women with breast cancer. Tables 1 and 2 present the characteristics of each one of the 80 participating studies. Fifty-four studies from North Africa [19]–[73] and 26 from sub-Saharan Africa [7]–[10],[74]–[95] reported on ER status, with fewer also reporting on PR or HER2 status (Figure 3; Tables 1, 2, and 3). Eighty percent of the North African studies, corresponding to 81% of all women with breast cancer from this region, were conducted in Egypt or Tunisia; 50% of the sub-Saharan African studies, corresponding to 71% of women with breast cancer from the region, were from South Africa or Nigeria (the distribution by country is given in Table 3). Most studies had sample sizes <300 patients with known receptor status. Only four studies [9],[37],[50],[72],[73] had >900 women with breast cancer, with the largest one (n = 3,060) also being one of the few to be based on a population-based cancer registry (an Egyptian study [72],[73]). The most common method for assessing receptor status was monoclonal assays (i.e., the quantitative enzyme immunoassay and, more often, the semi-quantitative IHC approach), but ER status was ascertained by ligand binding assays (e.g., dextran-coated charcoal [DCC] method) in some earlier studies (Tables 1 and 2) [51],[89]–[91]. FISH, CISH, or SISH to ascertain the HER2 status of specimens with an equivocal IHC score of 2+ was only performed in a few studies (Tables 1 and 2) [34],[42],[48],[55],[65],[69],[76],[78],[82],[84],[87].
Fig. 2. Flow diagram detailing study identification, screening, and eligibility. 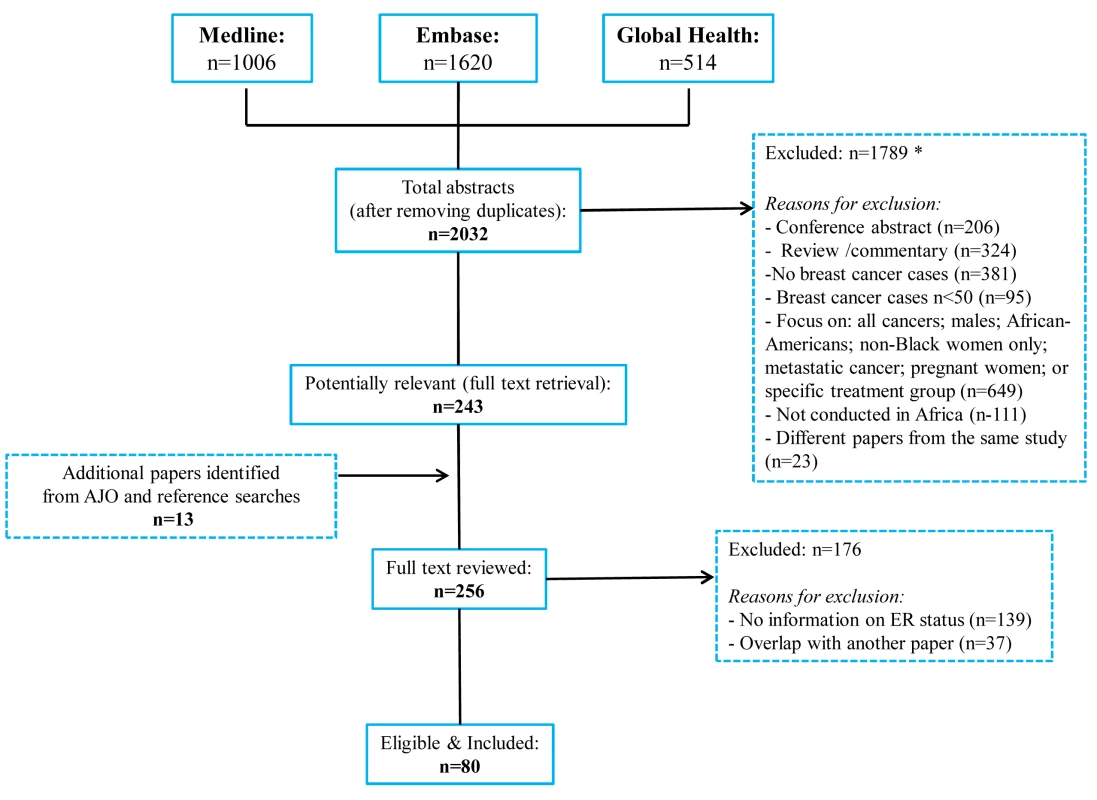
Many abstracts could fit into more than one exclusion category; these were allocated to the first eligible category in the order listed here. Fig. 3. Proportion of ER+, PR+, and HER2+ disease (ranked by increasing magnitude), North and sub-Saharan Africa. 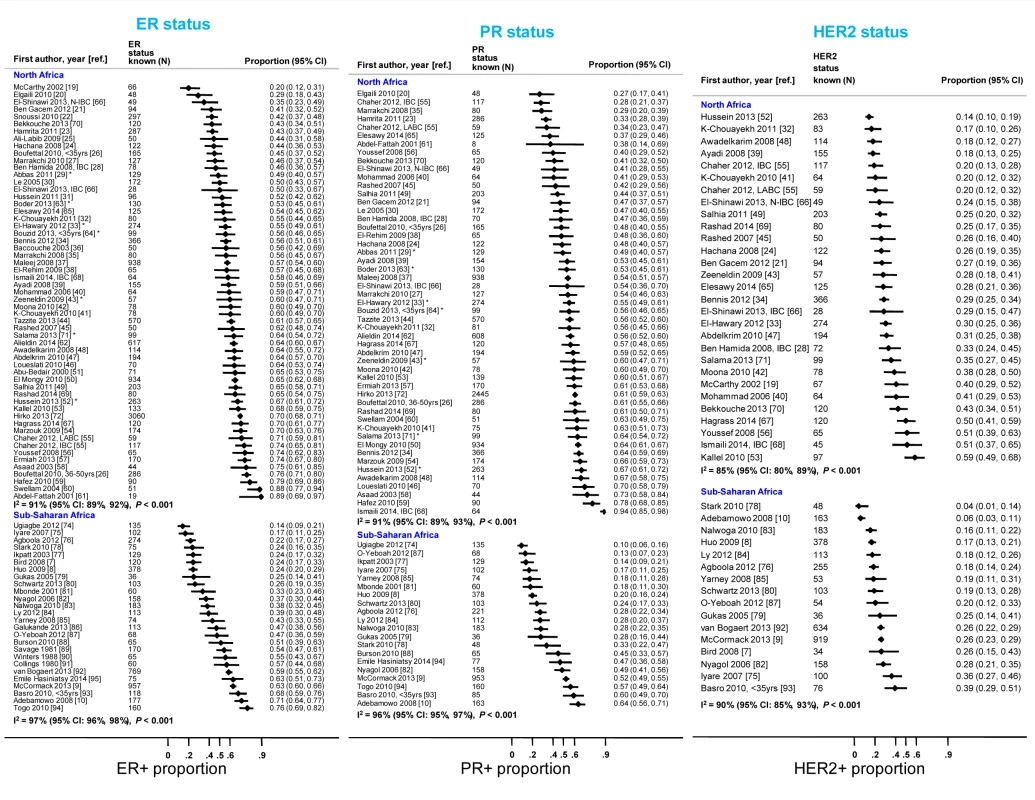
IBC, inflammatory breast cancer; LABC, non-IBC locally advanced breast cancer; N-IBC, non-inflammatory breast cancer. *These studies provided only a combined HR estimate for tumors that were either ER+ or PR+ [33] or ER+ and/or PR+ ([29]; [43]; [52]; [63]; [64]; [71]). These HR estimates were included in both the ER+ and PR+ plots. Tab. 3. Summary of the characteristics of the 80 participating studies. 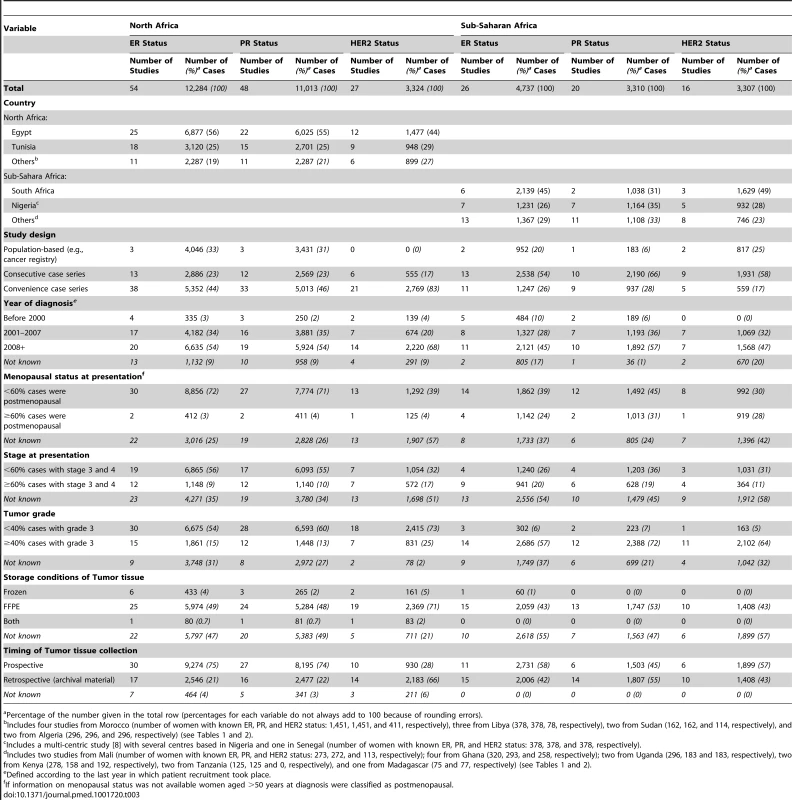
Percentage of the number given in the total row (percentages for each variable do not always add to 100 because of rounding errors). Figure 3 shows study-specific reported proportions of ER+, PR+, and HER2+ tumors, ranked according to their magnitude, for North and sub-Saharan Africa. There was marked between-study heterogeneity in the ER+ estimates in both regions (I2>90%), with the majority reporting proportions between 0.40 and 0.80 in North Africa and between 0.20 and 0.70 in sub-Saharan Africa. Similarly, large between-study heterogeneity was observed for PR+ and HER2+ estimates (I2>80%, in all instances). There were no clear differences in the reported proportions of HER2+ tumors according to whether they were classified with a IHC cut-off score of 2+/3+ or 3+ as HER2+, or whether they were, or were not, further tested with FISH, CISH, or SISH.
Between-Study Heterogeneity
Study design
Case series based on convenience samples predominated in North Africa whereas roughly half of the case series in sub-Saharan Africa were consecutive (Table 3). For North African studies, there were no consistent differences in the ER+ proportion by study design; for sub-Saharan African studies, the studies that yielded the highest ER+ estimates tended to be those based on population-based or consecutive series rather than those based on convenience samples but there was still wide between-study variability among the former (Figure 4). A similar pattern was observed for PR receptor status (Figure S1). There were no clear differences by study design for HER2 status in North or sub-Saharan Africa (Figure S2).
Fig. 4. Proportion of ER+ disease by study design, North and sub-Saharan Africa. 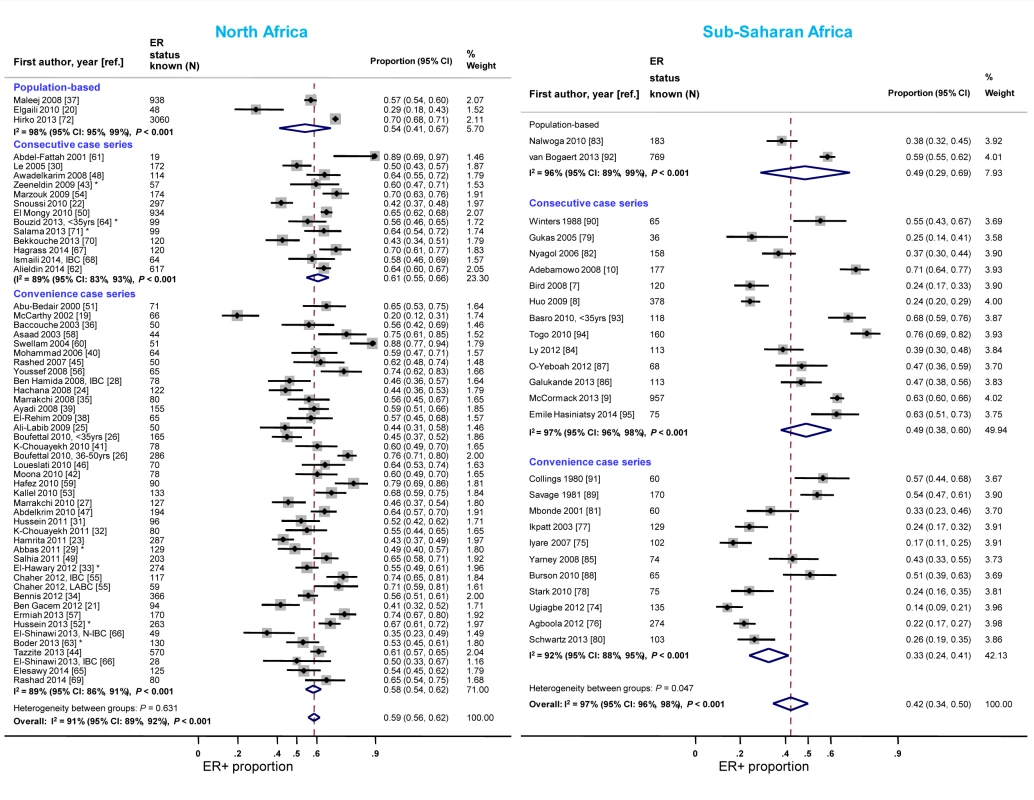
IBC, inflammatory breast cancer; LABC, non-IBC locally advanced breast cancer; N-IBC, non-inflammatory breast cancer. *These studies provided only a combined HR estimate for tumors that were either ER+ or PR+ [33] or ER+ and/or PR+ ([29]; [43]; [52]; [63]; [64]; [71]). Year of diagnosis
The majority of studies in both North and sub-Saharan Africa comprised women diagnosed with breast cancer after 2001 (Table 3). In each region, the study-specific ER+ proportion tended to increase over time. In North Africa, the rise was particularly noticeable when studies conducted before 2001 were compared to those completed after 2007 (Figure 5). An exception to this trend in sub-Saharan Africa was the generally higher ER+ proportion for studies conducted prior to 2001, driven by estimates from three South African studies [89]–[91], than for those conducted between 2001 and 2007. Similar increases over time in the proportion of PR+ disease were observed (Figure S3). In contrast, there was a slight decrease over time in the reported study-specific HER2+ proportion in North Africa; no sub-Saharan African study conducted prior to 2001 reported on HER2 status (Figure S4).
Fig. 5. Proportion of ER+ disease by year of diagnosis, North and sub-Saharan Africa. 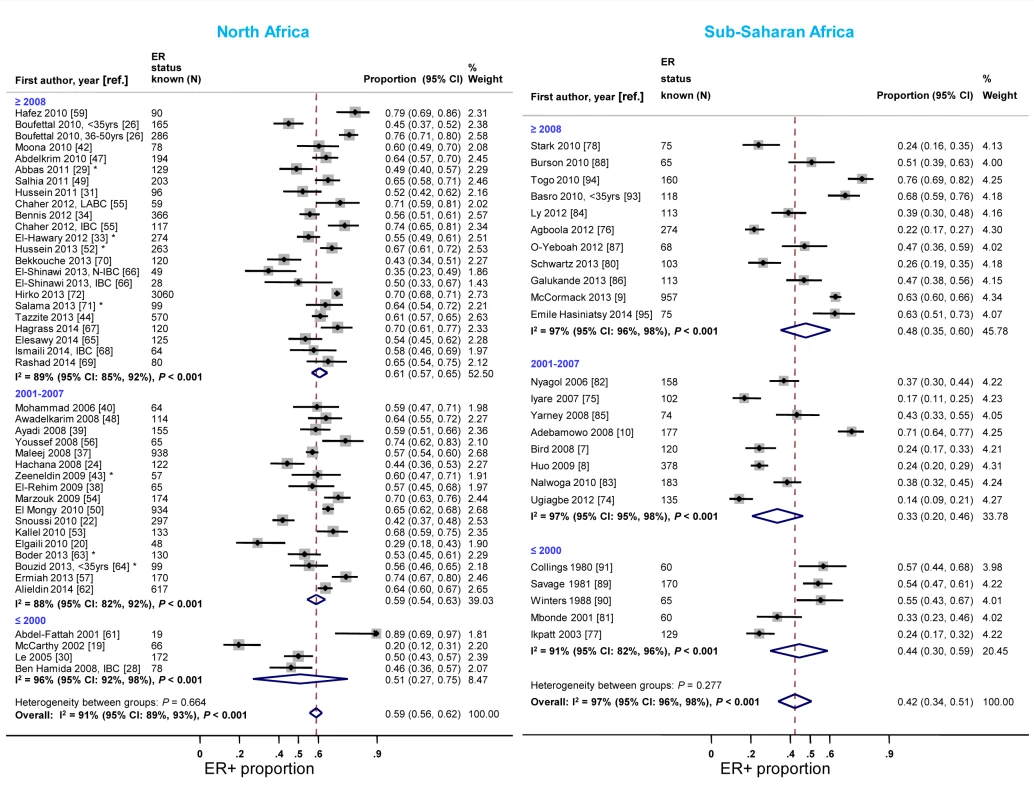
IBC: inflammatory breast cancer; LABC: non-IBC locally advanced breast cancer; N-IBC: non-inflammatory breast cancer. *These studies provided only a combined HR estimate for tumors that were either ER+ or PR+ [33] or ER+ and/or PR+ ([29]; [43]; [52]; [63]; [64]; [71]). Age and menopausal status at diagnosis
Study-specific proportions of ER+ disease tended to increase with increasing average (mean/median) age at breast cancer diagnosis in both North and sub-Saharan Africa (e.g., pooled ER+ prop [95% CI] for sub-Saharan studies with an average age at diagnosis of 31–46, 47–49.4, and 49.5+ years were 0.34 [0.24–0.44], 0.45 [0.28–0.62], and 0.49 [0.35–0.64]; I2>90%, p<0.01 for all). A similar age pattern was observed for the proportion of PR+ disease in both regions. No clear age trends were observed for HER2+ disease (e.g., pooled HER2+ prop [95% CI] for North African studies with an average age at diagnosis of 31–46, 47–49.4, and 49.5+ years were 0.31 [0.27–0.36], 0.32 [0.22–0.43], and 0.30 [0.24–0.36]; I2>70%, p≤0.01 for all except ages 31–46 for which I2 = 15%, p = 0.32). There were no clear differences in the frequency of ER+, PR+, and HER2+ disease by menopausal status, but few studies (two in North Africa; four in sub-Saharan Africa) were based on case series where ≥60% of the women were postmenopausal at breast cancer diagnosis (Table 3).
Tumor grade and stage
North African studies with ≥40% grade 3 tumors reported a lower proportion of ER+ disease relative to those with <40% of such tumors (Figure 6). A similar gradient was observed in sub-Saharan Africa; however, only three studies had <40% grade 3 tumors (Figure 6; Table 3), reflecting perhaps their late presentation. Twelve studies [7],[9],[10],[33],[34],[39],[41],[51],[65],[77],[81],[86] provided grade-specific ER+ estimates and they all consistently showed decreasing ER+ proportions with increasing grade (Figure S5). There were no notable differences in the frequency of PR+ and HER2+ tumors by grade in North Africa; the paucity of studies with <40% of grade 3 tumors in sub-Saharan Africa precluded the examination of this variable (Figures S6 and S7). There were no consistent differences in receptor status by tumor stage.
Fig. 6. Proportion of ER+ disease by tumor grade, North and sub-Saharan Africa. 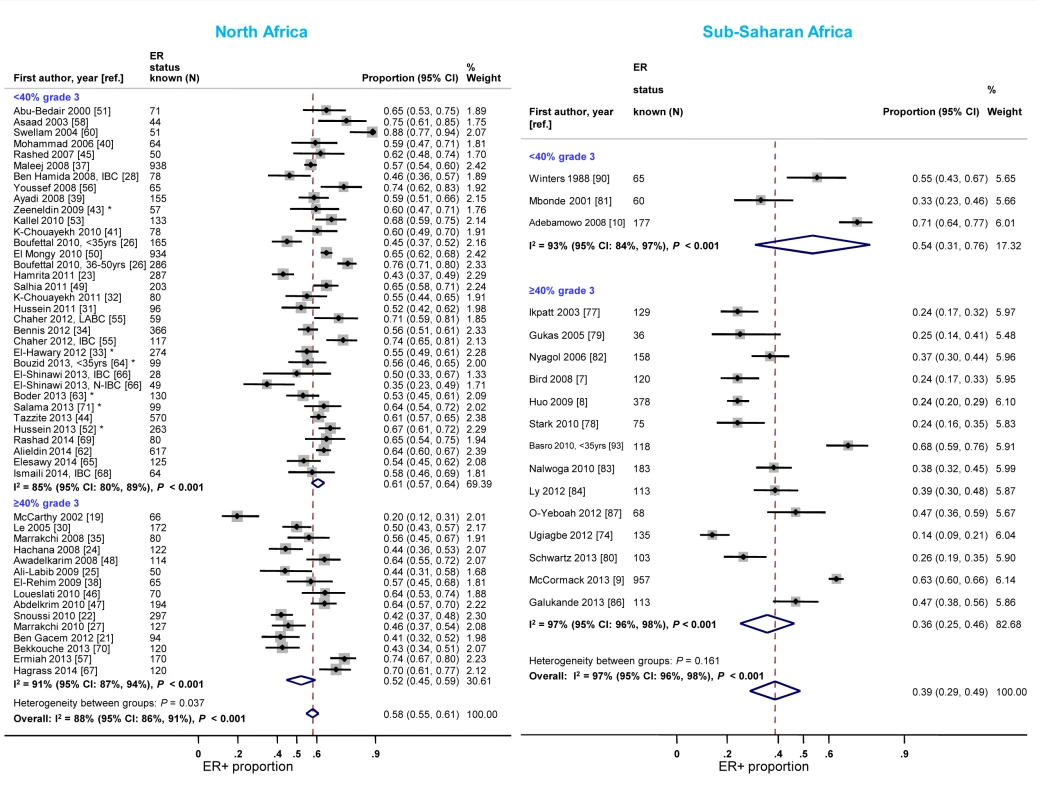
IBC, inflammatory breast cancer; LABC, non-IBC locally advanced breast cancer; N-IBC: non-inflammatory breast cancer. *These studies provided only a combined HR estimate for tumors that were either ER+ or PR+ [33] or ER+ and/or PR+ ([43]; [52]; [63]; [64]; [71]). Timing of receptor testing and specimen storage conditions
Reported proportions of ER+ and PR+ disease tended to be lower for studies where receptor status assays were conducted on retrospective (archival) tissue blocks than for those based on prospectively analysed specimens in sub-Saharan Africa, but not in North Africa (Figures 7 and S8). North African studies that used FFPE blocks tended to report lower ER+ (pooled prop = 0.57, 95% CI 0.52–0.62; I2 = 91%; p<0.01) and PR+ estimates (pooled prop = 0.51, 95% CI 0.46–0.55; I2 = 88%; p<0.01) than those based on frozen tissue samples (pooled ER+ prop = 0.64, 95% CI 0.52–0.76; I2 = 87%, p<0.01; pooled PR+ prop = 0.61; 95% CI 0.55–0.67; I2 = 0%; p = 0.88). Virtually all sub-Saharan African studies were based on FFPE tissue blocks (Table 3). No clear patterns in the frequency of HER2+ tumors by timing of receptor testing, or specimen storage conditions, were observed within each region (e.g., pooled prop [95% CI] for prospectively collected versus archival tissue: 0.36 [0.30–0.42] versus 0.28 [0.23–0.33] in North Africa; 0.22 [0.14–0.31] versus 0.20 [0.15–0.25] in sub-Saharan Africa [I2≥74% for all]; Figure S9).
Fig. 7. Proportion of ER+ disease by timing of receptor testing, North and sub-Saharan Africa. 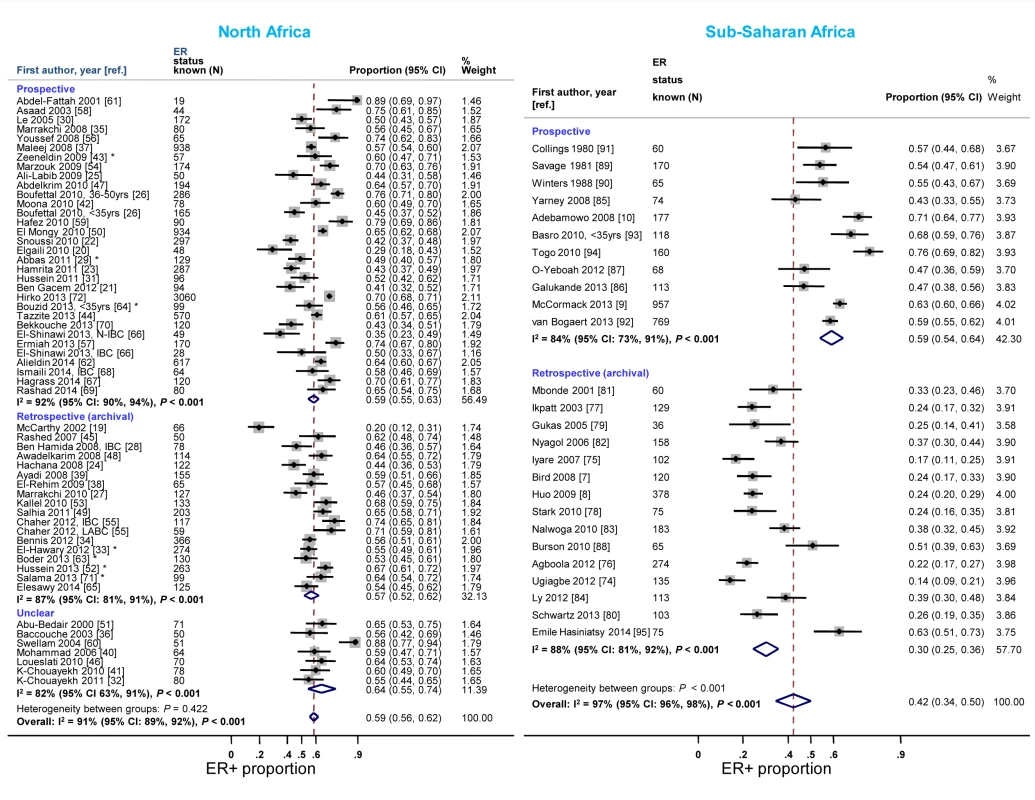
IBC, inflammatory breast cancer; LABC, non-IBC locally advanced breast cancer; N-IBC, non-inflammatory breast cancer. *These studies provided only a combined HR estimate for tumors that were either ER+ or PR+ [33] or ER+ and/or PR+ ([29]; [43]; [52]; [63]; [64]; [71]). Study quality
The median (inter-quartile range [IQR]) quality scores for studies reporting on ER, PR, and HER2 status for North Africa were 16 (14–17), 16 (15–18), and 15 (14–17), respectively (Table 1). The corresponding estimates for sub-Saharan Africa were 17 (15–19), 17 (15–19), and 16 (14–18) (Table 2). There were no clear differences in the frequency of ER+, PR+, and HER2+ disease by study quality scores, despite the differences observed for specific individual criteria (e.g., study tissue storage conditions, timing of receptor testing) described above.
Geographical sub-regions
Studies from North-Eastern Africa (i.e., Egypt, Sudan, and Libya) yielded higher ER+ proportions than those conducted in North-Western Africa (i.e., Morocco, Algeria, and Tunisia) (Figure 8). There was also a gradient within sub-Saharan Africa with the highest ER+ proportions being reported by studies from Southern Africa (i.e., South Africa) and the lowest by studies from Eastern Africa (i.e., Kenya, Uganda, Tanzania, and Madagascar) and Western Africa (i.e., Ghana, Mali, Nigeria, and Senegal) (Figure 8). Similar patterns by sub-region were observed for PR+ disease except that the gradient within North Africa was smaller (Figure S10). There was no variation in the frequency of HER2+ disease between the two North African sub-regions but, similarly to ER+ and PR+ disease, the proportion of HER2+ disease was highest for studies from Southern Africa and lowest for those from Western Africa (Figure S11).
Fig. 8. Proportion of ER+ disease by sub-regions within North and sub-Saharan Africa. 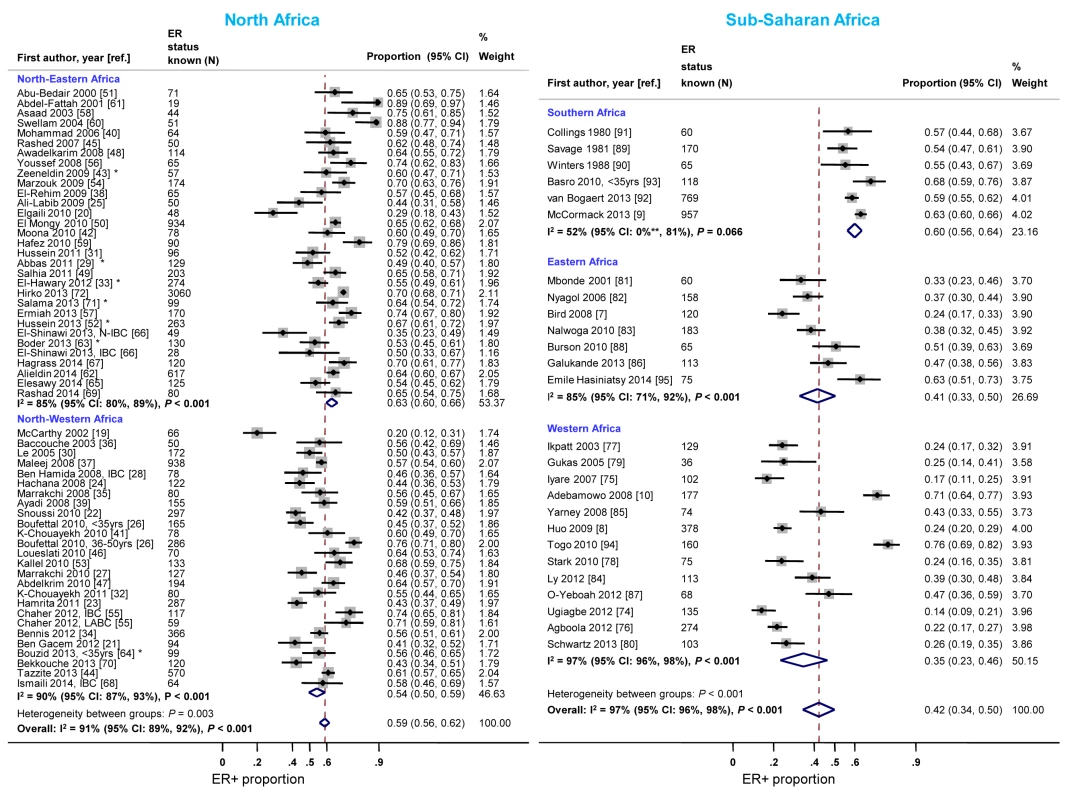
IBC, inflammatory breast cancer; LABC, non-IBC locally advanced breast cancer; N-IBC, non-inflammatory breast cancer. North-Western Africa: Morocco, Algeria, and Tunisia; North-Eastern Africa: Egypt, Sudan, and Libya; Eastern Africa: Kenya, Uganda, Tanzania, and Madagascar; Western Africa: Ghana, Mali, Nigeria, and Senegal; Sothern Africa: South Africa. *These studies provided only a combined HR estimate for tumors that were either ER+ or PR+ [33] or ER+ and/or PR+ ([29]; [43]; [52]; [63]; [64]; [71]). **Lower limit of 95% confidence interval for I2 statistic truncated at 0. Meta-regression analyses
Adjusted meta-regression analyses (Table 4) showed that the reported proportion of ER+ disease was 10% (95% CI 4%–17%) lower for studies based on archived tumor blocks versus those based on prospectively collected specimens, and 9% (2%–17%) lower for those with ≥40% versus those with <40% grade 3 tumors. The reported ER+ proportion was also higher for North African than sub-Saharan studies, but only among studies based on retrospective (archival) samples (p for interaction between region and time of receptor testing: <0.001). Similarly, further breakdown by sub-region showed that relative to North-Western Africa, the ER+ proportion was higher for North-Eastern (8.5%; 95% CI 1%–16%) and Southern Africa (5%; −8% to 18%), but lower for Western (−18%; −28% to −8%) and Eastern Africa (−11%; −24% to 1%). There was, however, an interaction with timing of receptor testing (p = 0.0001), with no differences in the ER+ proportion between sub-regions being observed among studies based on prospectively collected samples. There was a tendency for the proportion of ER+ disease to increase with increasing age and year at diagnosis. Similar patterns were observed for proportion of PR+ disease. The patterns for HER2+ were less clear but the reported proportions tended to be slightly higher for studies based on prospectively collected specimens, those conducted before 2001, and those from North Africa regardless of the timing of receptor testing (Table 4).
Tab. 4. Sources of between-study heterogeneity in the proportions of ER+, PR+, and HER2+ cases from meta-regression analyses. 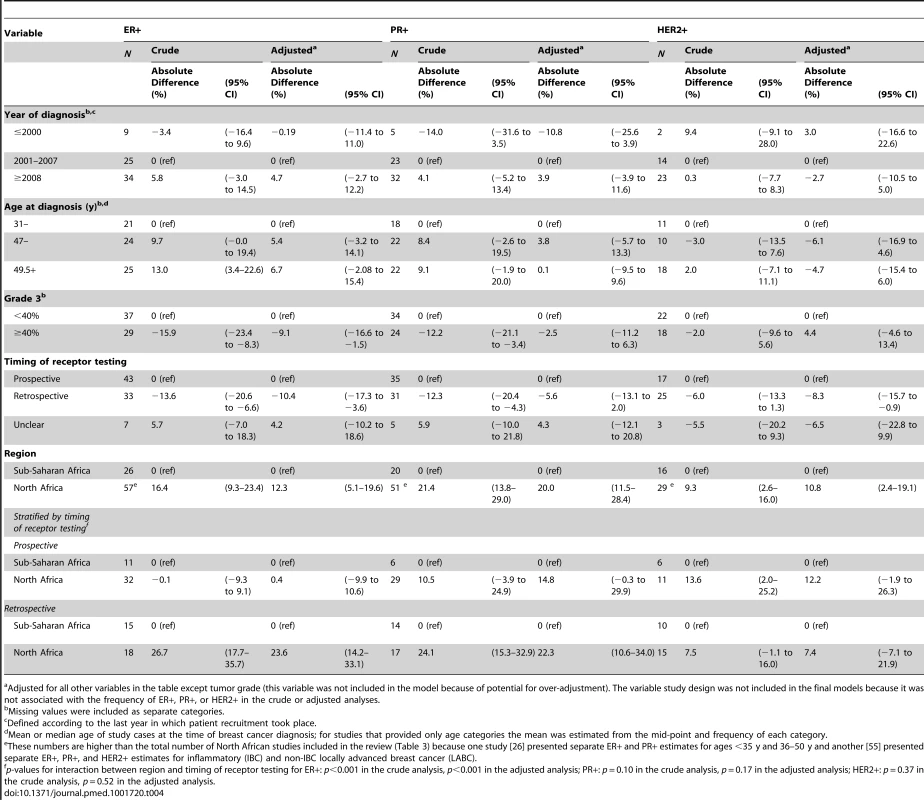
Adjusted for all other variables in the table except tumor grade (this variable was not included in the model because of potential for over-adjustment). The variable study design was not included in the final models because it was not associated with the frequency of ER+, PR+, or HER2+ in the crude or adjusted analyses. Combined ER/PR/HER2 Tumor Subtypes
Eighteen North African [21],[32]–[34],[39],[41],[42],[47]–[49],[52],[55],[56],[65],[69]–[71] and 12 sub-Saharan African studies [7]–[10],[75],[76],[78],[80],[82]–[84],[92] provided information on the frequency of one or more subtypes. Consistent with the findings reported above, the proportion of triple negative tumors was lower for studies based on prospectively collected samples and those with <40% grade 3 tumors (Figure 9). The opposite was true for luminal A and, to a lesser extent, luminal B tumors. In contrast, there was little variation in the frequency HER2+-enriched tumors according to these two variables. However, marked between-study heterogeneity was still present within each stratum (Figure 9).
Fig. 9. Frequency of tumor subtypes by year of diagnosis, grade, and timing of receptor testing. 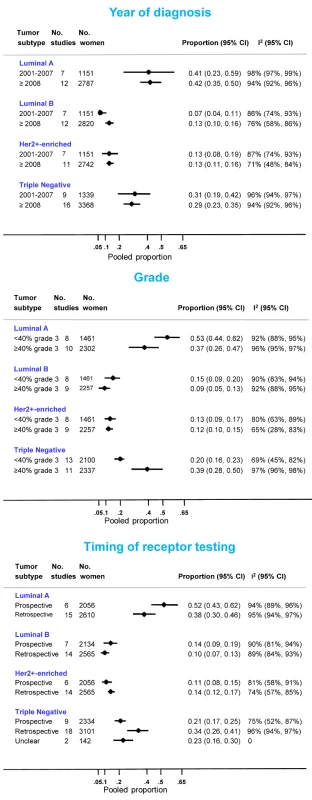
International and Ethnic Comparisons
Figure 10 presents the findings from studies that involved international or ethnic comparisons. The international comparisons highlighted the striking differences between indigenous African and Western white women with breast cancer, with the former showing a much younger age as well as larger tumor sizes and higher grade and stage, consistent with a more advanced disease at presentation. Despite these differences, Le and colleagues [30] reported similarly low proportions (∼0.50) of ER+ disease among both Tunisian and French women with breast cancer (the two series were selected to ensure they had broadly similar percentages of inflammatory breast cancers (T4d) Figure 10). In contrast, Ben Hamida and colleagues (Figure 10) [28] reported a higher proportion of ER+ disease among French (0.74) relative to Tunisian (0.46) patients; however, all Tunisian tumors, but none of the French ones, were inflammatory breast cancers. Stark and colleagues [78] reported large differences in the proportion of ER+ disease between Ghanaian (0.24), African-American (0.64), and white American (0.78) women; however, the differences were far less marked when the analysis was restricted to advanced stage disease (Figure 10). Awadelkrim and colleagues [48] reported a ER+ proportion of 0.64 among Sudanese women versus a proportion of 0.83 among Italian women, but the proportion of advanced tumors was much higher for the former (Figure 10). Three studies from South Africa [9],[89],[91], presented remarkably consistent between-ethnic differences despite covering a 30-year period, with all reporting smaller differences in the frequency of ER+ disease between black and white women than those described above (Figure 10), with this magnitude being broadly in line with the magnitude of the ethnic differences between black and white women in the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute, US (SEER) data (data downloaded from [6] using the same methods as in [9]) (Figure 10). The pooled proportions of ER+ disease yielded by this review for North (0.59) and sub-Saharan studies (0.59) on the basis of the possibly better quality prospectively collected samples, were broadly similar to the ER+ proportion for US black women in the SEER data (0.64). Notably, when the analysis was further restricted to studies in this review with <40% grade 3 tumors, a case mix more akin to that seen in the US series, the pooled ER+ proportions for North (0.59; 95% CI 0.54–0.64) and sub-Saharan studies (0.64; 0.49–0.90; based on two studies) were similar to the ER+ proportion seen among US black women (0.64) (Figure 10).
Fig. 10. International and ethnic comparisons in the proportion of ER+ disease. 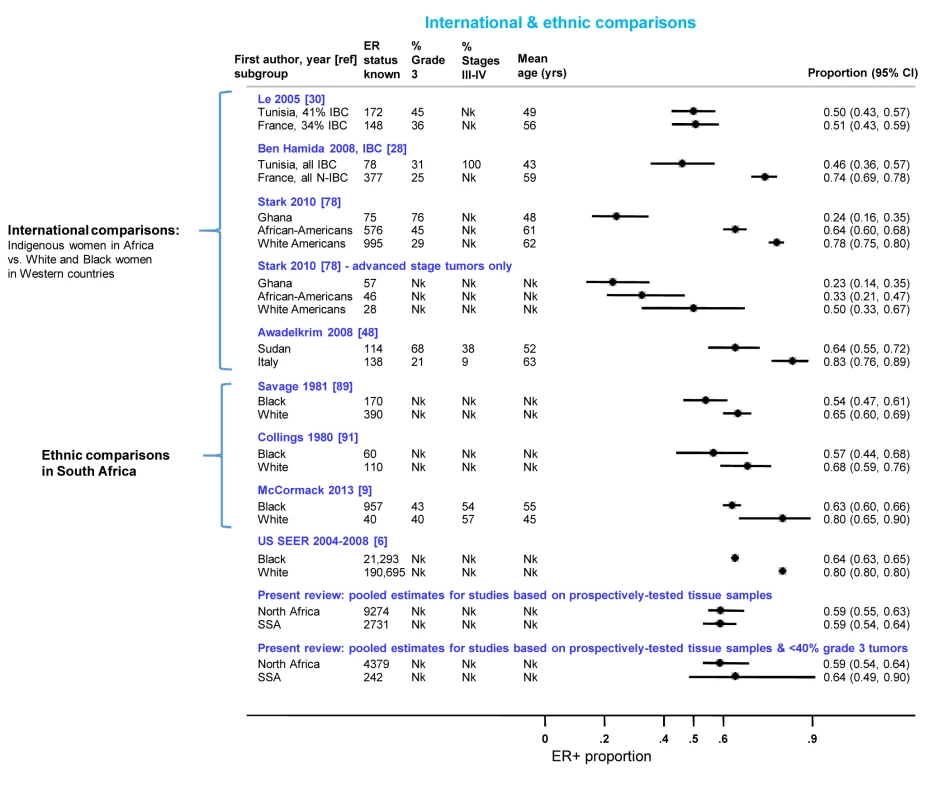
IBC, inflammatory breast cancer; N-IBC, non-inflammatory breast cancer; Nk, information not given in the original paper; SEER, Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute, US (data downloaded from [6] using the same methods as in [9]); SSA, sub-Saharan Africa. Small Study Bias
The funnel plots (Figure S12) and Egger's test for small study effects provide evidence of small study bias for North African studies only (p-values for studies reporting on ER, PR, and HER2 status: p = 0.004, 0.03, and 0.01, respectively).
Discussion
Main Findings
This systematic review aimed to characterize the distribution of receptor-defined subtypes of breast cancer in indigenous populations in Africa. It highlighted the extent to which data on these receptors, which are important prognostic markers of the disease, is scarce in the continent. Nevertheless, we identified 80 studies, comprising >17,000 women with breast cancer, with information on at least ER status, thus providing the largest synthesis so far to our knowledge of breast cancer subtypes in Africa. The review revealed large between-study heterogeneity in the reported frequency of ER+ tumors, ranging approximately from 1 in 4 to 3 in 4 tumors being ER+ within each region. This heterogeneity may have arisen as a result of regional and temporal differences in the prevalence of subtype-specific risk factors, differences in tumor characteristics (e.g., grade, stage) at presentation, or artefacts caused by unrepresentative case series and varying quality in the procedures used to collect, store, and analyse tumor specimens.
The review revealed a tendency for studies based on archival tissue and/or FFPE blocks to yield lower ER+ and PR+ frequency estimates, in line with archival samples being particularly susceptible to antigen degradation [96],[97]. Additionally, such archival samples tended to be from older studies where quality control on pre-analytical factors may have been suboptimal. More recent studies have demonstrated the vulnerability of hormone receptor (HR) testing to false negatives and the importance of pre-analytic factors, with errors introduced by delays, inadequate or prolonged fixation and variability in fixatives used, dehydration procedures, and quality of paraffin. The present review also found that the proportion of ER+ disease decreased with increasing tumor grade, reflecting perhaps the accelerated growth rate of ER − tumors, loss of estrogen expression in more advanced forms of the disease, and higher likelihood of false-negative results (due to difficulties in obtaining a biopsy of the original tumor). Although the observed increase in the frequency of ER+ disease over time may reflect improvements in methodology as well as the change in the tumor nuclei staining intensity score threshold for ER positivity from ≥10% to ≥1% (following the introduction of new guidelines in 2010 [98]), they may also represent a genuine rise in ER+ disease as African women became more westernised (as illustrated by declines in fertility [99] and rises in body mass index [100],[101] and, consequently, age at menarche in the continent).
A few studies in this review included international or ethnic comparisons in the distribution of ER status. None of the international studies appeared to have conducted centralized receptor status testing, with none reporting on cross-centre evaluation of comparability in measurements and quality control procedures, but each one of the three ethnic studies was conducted within a single institution and hence using the same procedures for all their participants. These comparisons consistently reported a lower frequency of ER+ tumours in indigenous women in Africa relative to Western white women, or in black relative to white women in South Africa, consistent with the well documented ethnic differences in the US. The existence of, and reasons for, the black-white differences in the US may shed light on the situation in Africa. Over age 35 years, a higher ER+ proportion among US white than black women with breast cancer is driven by the latter group's slightly higher absolute incidence rate of triple negative disease, in combination with their much lower incidence rate of better prognosis ER+/PR+ HER2 − tumors [102]. However, the magnitude of the black-white difference in the ER+ proportion has changed somewhat over time and the reasons driving these differences are much debated [11]. As risk factors are subtype-specific, ethnic differences in the prevalence of hormonal-related risk factors may contribute to ethnic differences in the incidence of the various breast cancer subtypes. Pre-menopausal obesity and higher parity may be associated with raised risk of tripe-negative disease, in contrast to their protective effects on ER+ disease [103],[104], and oral contraceptive use may increase more markedly the risk of triple negative disease than the risk of other subtypes [105]. Equally, or in addition, ethnic differences may derive from genetic susceptibility to triple negative or ER-negative breast cancer in some African populations [106],[107].
In the present study, relative to breast cancer in Western white women, the disease in indigenous women in Africa was characterized by a younger age, an advanced stage, and a higher grade at presentation (Figure 10). Both young age and more advanced forms of the disease at presentation are associated with lower prevalence of ER+ tumors. Thus, the observed lower frequency of ER+ tumors in indigenous African women may simply reflect a much younger demographic structure of the indigenous African populations rather than a more intrinsic aggressive biology of the disease, as incidence rates at young ages are lower than among Western white women [1], as well as a tendency for late presentation due to lack of breast cancer awareness and screening activities, the unavailability of appropriate healthcare facilities, and the influence of socio-cultural and logistic factors that could limit access to health-care. In fact, our finding that the proportion of ER+ disease reported by African studies based on prospectively collected samples with predominantly low grade tumors was virtually the same as among US black women (all ∼64%) argues against breast cancer being a much more biologically aggressive disease in Africa than in the West.
Two subtypes are known to be associated with particularly poor breast cancer outcomes: triple negative and HER2+-enriched tumors. Few studies provided information on these subtypes and even fewer were based on prospectively collected samples. Nevertheless, the estimates based on the latter for triple negatives (pooled prop = 0.21; Figure 9) were slightly above the range of frequencies usually seen in white populations (10%–16%) [2],[108], but similar to that seen in US black women (e.g., 26% in [109]). The prevalence of HER2+-enriched tumors (pooled prop = 0.11) (Figure 9) was slightly higher than that seen in white populations [2] or US black women [109] (6%–10% for both) but similar to that reported for Chinese women [108]. However, considerable misclassification of HER2 status may have occurred as few African studies used FISH (or CISH/SISH) to ascertain the true HER2 status of tumors with an equivocal IHC score of 2+.
It is noteworthy to highlight that although between-study differences in the proportion of ER+ disease reflect the ratios of the underlying receptor-specific incidence rates (assuming no bias is present), they cannot be used to infer anything about the differences in incidence rates. The proportion of ER+ disease represents the ratio of the number of women who developed ER+ disease in a given population over a certain time period (thus, reflecting the underlying incidence rate of ER+ disease) by the total number of women who develop any type of breast cancer in the same population during the same time period (reflecting the incidence rate of ER+ and ER − disease combined). Thus, differences in the proportion of ER+ disease among women with breast cancer could arise from two populations with (i) the exact same incidence rates of ER − disease, but different incidence rates of ER+ disease, or (ii) equal incidence rates of ER+ disease, but different rates of ER − disease, or (iii) any combinations of these two. Case-only studies are unable to disentangle these different alternatives. Consequently, the findings from this review cannot be used to infer differences in the underlying incidence rates of receptor-specific disease across populations, e.g., between North and sub-Saharan Africa.
Strengths and Limitations
Major strengths of this review are the very comprehensive and inclusive search strategy (with inclusion of African-specific journals, the use of broad search terms rather than specific keywords, and the decision not to impose any language restrictions), the large number of eligible studies (comprising >17,000 women with breast cancer), and the use of well-established methodologies to provide an unbiased synthesis of the published evidence. The study had several weaknesses too. Firstly, the systematic review includes data from all countries in North Africa except Western Sahara, but with a predominance of studies from Egypt and Tunisia (Table 3). The proportion of sub-Saharan countries represented in the review was much smaller—only nine (i.e., South Africa, Nigeria, Senegal, Mali, Ghana, Uganda, Kenya, Tanzania, and Madagascar) out of 49 countries, albeit together these countries represent 46% of the total African female population [1]. Furthermore, no receptor status testing is performed in many of the countries not represented in the review. Secondly, the representativeness of the case series was not only compromised by the poor design of many of the participating studies, particularly those based on convenience samples, but also by the limited access to appropriate diagnostic and treatment facilities experienced by most indigenous African women affected with breast cancer. For instance, in many countries, receptor status testing in public hospital attendees is only available to those who can afford it. Thirdly, it is also possible that women with breast cancer may have contributed to more than one study. When multiple papers from the same study were identified, only the one with the most information on receptor status was included in the review. However, it was often impossible to ascertain potential overlaps in study populations, particularly among studies conducted within the same institution. This was a particular issue for Egyptian and Tunisian studies published in the early years, most of which provided a poor description of how their study populations were recruited, but sensitivity analyses including only studies in each institution whose recruitment dates did not overlap yielded similar estimates to those reported here. Fourthly, there was no suggestion that small study bias affected the results for receptor status in sub-Saharan Africa, but for North African studies, the smaller studies tended to have lower-than-average ER+ and PR+ proportions and higher-than-average HER2+ proportions. If this small study bias is real, the true ER+ and PR+ proportions would be higher and the HER2+ lower than the pooled estimates reported here. Finally, real geographical or temporal differences in the frequency of breast cancer subtypes may have been obscured by the lack of standardisation in pre-analytical and analytical procedures across studies.
Implications
Large well-designed studies, incorporating standardised high-quality procedures for receptor testing, are required to accurately quantify the distribution of the various breast cancer subtypes across Africa. In the meantime, this systematic review provides the strongest evidence yet that the distribution of receptor-defined subtypes is not dramatically different to that found in Western populations given their younger age structure and late presentation. The availability of receptor testing should be a priority in Africa, especially for young women with early stage disease where the potential to improve survival and reduce years of life-lost is greatest. In the absence of such testing, it would be appropriate to presume that the majority of tumors are ER+.
The findings have important implications for both research needs and public health in Africa. In addition to the need for high-quality characterisation of receptor-status, etiologic studies on breast cancer in the continent need to be conducted separately by subtype, to gain a better insight into risk factors for each. For the rare subtypes, such as triple negatives, this will require collaborative efforts to provide sufficient numbers of cases. In terms of public health implications, despite relatively low incidence rates, African women have mortality rates from breast cancer that are as high as in high incidence countries [1]. If more aggressive breast tumors predominated, the potential to improve survival rates would be curtailed using current therapies. However, the present synthesis suggests that this is not the case, and that two-thirds of women with breast cancer have a less aggressive disease form for which targeted endocrine treatments have been shown to produce good survival rates. Tamoxifen [4], in particular, may provide an effective therapeutic option because of its low cost and ease of administration. Improving prognosis for such cancers will also hinge on the ability to diagnose and commence treatment at earlier stages of the disease, which is needed across many African countries as several hospitals have over 70% of breast cancer patients being diagnosed at stage III/IV. With a majority of ER+ tumors, this less-aggressive disease is also consistent with relatively long (6–18 months) symptomatic periods reported by women prior to diagnosis. This is a time-window during which efforts to encourage earlier presentation and faster referral through health systems to treatment centres can be focussed.
Supporting Information
Zdroje
1. International Agency for Research on Cancer (2012) GLOBOCAN 2012. Available: http://globocaniarcfr/. Accessed 17 April 2014.
2. YangXR, Chang-ClaudeJ, GoodeEL, CouchFJ, NevanlinnaH, et al. (2011) Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst 103 : 250–263.
3. BlowsFM, DriverKE, SchmidtMK, BroeksA, van LeeuwenFE, et al. (2010) Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 7: e1000279.
4. DaviesC, GodwinJ, GrayR, ClarkeM, CutterD, et al. (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378 : 771–784.
5. JemalA, FedewaSA (2012) Is the prevalence of ER-negative breast cancer in the US higher among Africa-born than US-born black women? Breast Cancer Res Treat 135 : 867–873.
6. Surveillance Epidemiology and End Results (SEER) Program (Available: www.seer.cancer.gov) SEER*Stat Database: Incidence, SEER 17 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases, Nov 2010 Sub (1973–2008 varying), Linked To County Attributes. 2010. Total U.S., 1969–2009 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch released April 2011 (updated 10/28/2011). Ref Type: Report
7. BirdPA, HillAG, HoussamiN (2008) Poor hormone receptor expression in East African breast cancer: evidence of a biologically different disease? Ann Surg Oncol 15 : 1983–1988.
8. HuoD, IkpattF, KhramtsovA, DangouJM, NandaR, et al. (2009) Population differences in breast cancer: Survey in indigenous african women reveals over-representation of triple-negative breast cancer. J Clin Oncology 27 : 4515–4521.
9. McCormackVA, JoffeM, van den BergE, BroezeN, dos Santos SilvaI, et al. (2013) Breast cancer receptor status and stage at diagnosis in over 1,200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast Cancer Res 15: R84.
10. AdebamowoCA, FamootoA, OgundiranTO, AniagwuT, NkwodimmahC, et al. (2008) Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat 110 : 183–188.
11. KriegerN, ChenJT, WatermanPD (2011) Temporal trends in the black/white breast cancer case ratio for estrogen receptor status: disparities are historically contingent, not innate. Cancer Causes Control 22 : 511–514.
12. Global Health (2014) Available: http://www.cabi.org/publishing-products/online-information-resources/global-health/.
13. LodgeM, CorbexM (2011) Establishing an evidence-base for breast cancer control in developing countries. Breast 20 Suppl 2S65–69.
14. The Cochrane Collaboration Cochrane Handbook for Systematic Reviews of Interventions 2009. Available: http://handbook.cochrane.org/
15. United Nations (2013) United Nations Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. Available: http://unstats.un.org/unsd/methods/m49/m49regin.htm. Accessed 10 December 2013.
16. HigginsJ, ThompsonS (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21 : 1539–1558.
17. HigginsJP, ThompsonSG, DeeksJJ, AltmanDG (2003) Measuring inconsistency in meta-analyses. BMJ 327 : 557–560.
18. EggerM, Davey SmithG, SchneiderM, MinderC (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315 : 629–634.
19. McCarthyNJ, YangX, LinnoilaIR, MerinoMJ, HewittSM, et al. (2002) Microvessel density, expression of estrogen receptor alpha, MIB-1, p53, and c-erbB-2 in inflammatory breast cancer. Clin Cancer Res 8 : 3857–3862.
20. ElgailiEM, AbuidrisDO, RahmanM, MichalekAM, MohammedSI (2010) Breast cancer burden in central Sudan. Int J Womens Health 2 : 77–82.
21. Ben GacemR, HachanaM, ZiadiS, Ben AbdelkarimS, HidarS, et al. (2012) Clinicopathologic significance of DNA methyltransferase 1, 3a, and 3b overexpression in Tunisian breast cancers. Hum Pathol 43 : 1731–1738.
22. SnoussiK, MahfoudhW, BouaouinaN, FekihM, KhairiH, et al. (2010) Combined effects of IL-8 and CXCR2 gene polymorphisms on breast cancer susceptibility and aggressiveness. BMC Cancer 10 : 283.
23. HamritaB, Ben NasrH, GabboujS, BouaouinaN, ChouchaneL, et al. (2011) Apolipoprotein A1 −75 G/A and +83 C/T polymorphisms: susceptibility and prognostic implications in breast cancer. Mol Biol Rep 38 : 1637–1643.
24. HachanaM, TrimecheM, ZiadiS, AmaraK, GaddasN, et al. (2008) Prevalence and characteristics of the MMTV-like associated breast carcinomas in Tunisia. Cancer Lett 271 : 222–230.
25. Ali-LabibR, El-MonemF (2009) Hypermethylation of the tumour suppressor RassF1A gene in malignant and benign breast tissues from Egyptian patients. The Egyptian Journal of Biochemistry & Molecular Biology 27 : 83–100.
26. BoufettalH, NounM, SamouhN (2010) [Breast cancer in young patient in Morocco]. Cancer Radiother 14 : 698–703.
27. MarrakchiR, KhadimallahI, OuerhaniS, GamoudiA, KhomsiF, et al. (2010) Expression of WISP3 and RhoC genes at mRNA and protein levels in inflammatory and noninflammatory breast Cancer in Tunisian patients. Cancer Invest 28 : 399–407.
28. Ben HamidaA, LabidiIS, MradK, Charafe-JauffretE, Ben ArabS, et al. (2008) Markers of subtypes in inflammatory breast cancer studied by immunohistochemistry: prominent expression of P-cadherin. BMC Cancer 8 : 28.
29. AbbasH, SalemAAS, SalemMAE, BinziadS, GamalB (2011) Breast cancer: radiotherapy at the South Egypt Cancer Institute. Gastric and Breast Cancer 10 : 180–186.
30. LeMG, ArriagadaR, ContessoG, CammounM, PfeifferF, et al. (2005) Dermal lymphatic emboli in inflammatory and noninflammatory breast cancer: A French-Tunisian joint study in 337 partients. Clin Breast Cancer 6 : 439–445.
31. HusseinYM, GharibAF, EtewaRL, El-ShalAS, Abdel-GhanyME, et al. (2011) The melanoma-associated antigen-A3, -A4 genes: relation to the risk and clinicopathological parameters in breast cancer patients. Mol Cell Biochem 351 : 261–268.
32. Karray-ChouayekhS, TrifaF, KhabirA, BoujelbeneN, Sellami-BoudawaraT, et al. (2011) Methylation status and overexpression of COX-2 in Tunisian patients with ductal invasive breast carcinoma. Tumour Biol 32 : 461–468.
33. El-HawaryAK, AbbasAS, ElsayedAA, ZalataKR (2012) Molecular subtypes of breast carcinoma in Egyptian women: clinicopathological features. Pathol Res Pract 208 : 382–386.
34. BennisS, AbbassF, AkasbiY, ZnatiK, JouteiKA, et al. (2012) Prevalence of molecular subtypes and prognosis of invasive breast cancer in north-east of Morocco: retrospective study. BMC Research Notes 5 : 436.
35. MarrakchiR, OuerhaniS, BenammarS, RouissiK, BouhahaR, et al. (2008) Detection of cytokeratin 19 mRNA and CYFRA 21-1 (cytokeratin 19 fragments) in blood of Tunisian women with breast cancer. Int J Biol Markers 23 : 238–243.
36. BaccoucheS, DaoudJ, FrikhaM, Mokdad-GargouriR, GargouriA, et al. (2003) Immunohistochemical Status of p53, MDM2, bcl2, bax, and ER in Invasive Ductal Breast Carcinoma in Tunisian Patients. Ann N Y Acad Sci 1010 : 752–763.
37. MaalejM, HentatiD, MessaiT, KochbatiL, El MayA, et al. (2008) Breast cancer in Tunisia in 2004: a comparative clinical and epidemiological study. Bull Cancer 95: E5–9.
38. El-RehimD, AliM (2009) Aberrant expression of beta-catenin in invasive ductal breast carcinomas. Journal of the Egyptian National Cancer Institute 21 : 185–195.
39. AyadiL, KhabirA, AmouriH, KarrayS, DammakA, et al. (2008) Correlation of HER-2 over-expression with clinico-pathological parameters in Tunisian breast carcinoma. World J Surg Oncol 6 : 112.
40. MohammadAM, AbdelHA, AbdelW, AhmedAM, WaelT, et al. (2006) Expression of cyclooxygenase-2 and 12-lipoxygenase in human breast cancer and their relationship with HER-2/neu and hormonal receptors: impact on prognosis and therapy. Indian J Cancer 43 : 163–168.
41. Karray-ChouayekhS, TrifaF, KhabirA, BoujelbaneN, Sellami-BoudawaraT, et al. (2010) Aberrant methylation of RASSF1A is associated with poor survival in Tunisian breast cancer patients. J Cancer Res Clin Oncol 136 : 203–210.
42. MoonaMS, AlarabiRAR, HussainA, AhmadM, MehdiI (2010) The study of ER (Oestrogen Receptor), PR (Progesterone Receptor) and HER-2/neu status in patients with breast cancer. Jamahiriya Medical Journal 10 : 141–143.
43. ZeeneldinAA, MohamedAM, AbdelHA, TahaFM, GodaIA, et al. (2009) Survival effects of cyclooxygenase-2 and 12-lipooxygenase in Egyptian women with operable breast cancer. Indian J Cancer 46 : 54–60.
44. TazziteA, JouhadiH, SaissK, BeniderA, NadifiS (2013) Relationship between family history of breast cancer and clinicopathological features in moroccan patients. Ethiop J Health Sci 23 : 150–157.
45. RashedMM, RagabNM, GalalMK (2007) The association of HER-2/neu over-expression in relation to p53 nuclear accumulation, hormonal recceptor status and common clinico-pathological prognostic parameters in a series of Egyptian women with invasive ductal carcinoma. European Journal of General Medicine 4 : 73–79.
46. LoueslatiBY, TroudiW, CherniL, RhomdhaneKB, Mota-VieiraL (2010) Germline HVR-II mitochondrial polymorphisms associated with breast cancer in Tunisian women. Genet Mol Res 9 : 1690–1700.
47. AbdelkrimSB, TrabelsiA, MissaouiN, BeizigN, BdiouiA, et al. (2010) Distribution of molecular breast cancer subtypes among Tunisian women and correlation with histopathological parameters: A study of 194 patients. Pathol Res Pract 206 : 772–775.
48. AwadelkarimKD, ArizziC, ElaminEOM, HamadHMA, BlasioPd, et al. (2008) Pathological, clinical and prognostic characteristics of breast cancer in Central Sudan versus Northern Italy: implications for breast cancer in Africa. Histopathology 52 : 445–456.
49. SalhiaB, TapiaC, IshakEA, GaberS, BerghuisB, et al. (2011) Molecular subtype analysis determines the association of advanced breast cancer in Egypt with favorable biology. BMC Womens Health 11.
50. El MongyM, El HossienyH, HaggagF, FathyR (2010) Clinico-pathological study and treatment results of 1009 operable breast cancer cases: Experience of NCI Cairo University, Egypt. Chinese-German Journal of Clinical Oncology 9 : 409–415.
51. Abu-BedairFA, El-GamalBA, IbrahimNA, El-AaserAA (2000) Hormonal profiles and estrogen receptors in Egyptian female breast cancer patients. Tumori 86 : 24–29.
52. HusseinO, MosbahM, FaroukO, FaragK, El-SaedA, et al. (2013) Hormone receptors and age distribution in breast cancer patients at a university hospital in northern Egypt. Breast Cancer: Basic and Clinical Research 7 : 51–57.
53. KallelI, KharratN, Al-fadhlyS, RebaiM, KhabirA, et al. (2010) HER2 polymorphisms and breast cancer in Tunisian women. Genet Test Mol Biomarkers 14 : 29–35.
54. MarzoukD, GaafaryM, DamatyS, SabbourS, MeckyF, et al. (2009) Breast cancer and hormonal intake among Egyptian females. Eur J Oncol 14 : 37–51.
55. ChaherN, Arias-PulidoH, TerkiN, QuallsC, BouzidK, et al. (2012) Molecular and epidemiological characteristics of inflammatory breast cancer in Algerian patients. Breast Cancer Res Tr 131 : 437–444.
56. YoussefN, HewediI, RabohN (2008) Immunohistochemical expression of survivin in breast carcinoma: relationship with clinicopathological parameters, proliferation and molecular classification. Journal of the Egyptian National Cancer Institute 20 : 348–357.
57. ErmiahE, BuhmeidaA, KhaledBR, AbdallaF, SalemN, et al. (2013) Prognostic value of bcl-2 expression among women with breast cancer in Libya. Tumour Biol 34 : 1569–1578.
58. AsaadNY, KandilMAEH, ShabanMI (2003) Prognostic significance of maspin expression in breast carcinoma. Cancer Mol Biol 10 : 1937–1951.
59. HafezN, TahounN (2010) Assessment of the reliability of immunocytochemical detection of estrogen and progesterone receptors status on the cytological aspiarates of breast carcinoma. Journal of the Egyptian National Cancer Institute 22 : 217–225.
60. SwellamM, IsmailM, EissaS, HamdyM, MokhtarN (2004) Emerging role of P53, Bcl-2 and telomerase activity in Egyptian breast cancer patients. IUBMB Life 56 : 483–490.
61. Abdel-FattahM, LotfyNS, BassiliA, AnwarM, MariE, et al. (2001) Current treatment modalities of breast-cancer patients in Alexandria, Egypt. Breast 10 : 523–529.
62. AlieldinNH, Abo-ElazmOM, BilalD, SalemSE, GoudaE, et al. (2014) Age at diagnosis in women with non-metastatic breast cancer: Is it related to prognosis? Journal of the Egyptian National Cancer Institute 26 : 23–30.
63. BoderJ, AbdallaF, ElfagiehM, BuhmeidaA, CollanY (2013) Proliferative activity in Libyan breast cancer with comparison to European and central African patients. BioMed Research International 2013.
64. BouzidN, LahmarR, TebraS, BouaouinaN (2013) Breast cancer in woman younger than 35 years in Tunisia: Retrospective study about 124 cases. [French] Cancer du sein chez la femme jeune de moins de 35 ans en Tunisie: etude retrospective a propos de 124 cas. Gynecologie Obstetrique Fertilite 41 : 356–360.
65. ElesawyBH, Abd El HafezA, ShawkyAEA, ArafaM (2014) Immunohistochemistry-based subtyping of breast carcinoma in Egyptian women: a clinicopathologic study on 125 patients. Ann Diagn Pathol 18 : 21–26.
66. El-ShinawiM, MohamedHT, El-GhonaimyEA, TantawyM, YounisA, et al. (2013) Human cytomegalovirus infection enhances NF - kappa B/p65 signaling in inflammatory breast cancer patients. PLoS ONE 8: e55755.
67. HagrassHA, PashaHF, ShaheenMA, Abdel BaryEH, KassemR (2014) Methylation status and protein expression of RASSF1A in breast cancer patients. Mol Biol Rep 41 : 57–65.
68. IsmailiN, ElyaakoubiH, BensoudaY, ErrihaniH (2014) Demographic, clinical, pathological, molecular, treatment characteristics and outcomes of nonmetastatic inflammatory breast cancer in Morocco: 2007 and 2008. Experimental Hematology and Oncology 3.
69. RashadYA, ElkhodaryTR, El-GayarAM, EissaLA (2014) Evaluation of serum levels of HER2, MMP-9, nitric oxide, and total antioxidant capacity in Egyptian breast cancer patients: correlation with clinico-pathological parameters. Scientia Pharmaceutica 82 : 129–145.
70. BekkoucheZ, GuedouarY, Ben AliF, El KebirFZ (2013) Characteristics of triple-negative breast carcinomas in west Algeria. [French] Caracteristiques des carcinomes mammaires triple-negatifs dans l'Ouest-algerien. Journal Africain du Cancer 5 : 155–161.
71. SalamaA, El-FendyH, TalaatS, BayomiB, AminA (2013) Prognostic value of immunohistochemical stratification of invasive duct carcinoma of the breast. Chinese-German Journal of Clinical Oncology 12: P265–P272.
72. HirkoKA, SolimanAS, HablasA, SeifeldinIA, RamadanM, et al. (2013) Trends in breast cancer incidence rates by age and stage at diagnosis in gharbiah, Egypt, over 10 years (1999–2008). Journal of Cancer Epidemiology 2013.
73. DeyS, SolimanAS, HablasA, SeifeldinIA, IsmailK, et al. (2010) Urban-rural differences in breast cancer incidence by hormone receptor status across 6 years in Egypt. Breast Cancer Res Treat 120 : 149–160.
74. UgiagbeEE, ObasekiDE, OluwasolaAO, Olu-EddoAN, AkhiwuWO (2012) Frequency of distribution of oestrogen and progesterone receptors positivities in breast cancer cases in Benin-City, Nigeria. Nigerian Postgraduate Medical Journal 19 : 19–24.
75. IyareF (2007) Immunohistochemical characteristics of breast cancers in South East Nigeria. Ebonyi Medical Journal 6 : 9–12.
76. AgboolaAJ, MusaAA, WanangwaN, Abdel-FatahT, NolanCC, et al. (2012) Molecular characteristics and prognostic features of breast cancer in Nigerian compared with UK women. Breast Cancer Res Treat 135 : 555–569.
77. IkpattOF, Ndoma-EgbaR (2003) Oestrogen and progesterone receptors in Nigerian breast cancer: relationship to tumour histopathology and survival of patients. Cent Afr J Med 49 : 122–126.
78. StarkA, KleerCG, MartinI, AwuahB, Nsiah-AsareA, et al. (2010) African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer 116 : 4926–4932.
79. GukasID, JenningsBA, MandongBM, IgunGO, GirlingAC, et al. (2005) Clinicopathological features and molecular markers of breast cancer in Jos, Nigeria. West Afr J Med 24 : 209–213.
80. SchwartzT, StarkA, PangJ, AwuahB, KleerCG, et al. (2013) Expression of aldehyde dehydrogenase 1 as a marker of mammary stem cells in benign and malignant breast lesions of Ghanaian women. Cancer 119 : 488–494.
81. MbondeMP, AmirH, AkslenLA, KitinyaJN (2001) Expression of oestrogen and progesterone receptors, Ki-67, p53 and BCL-2 proteins, cathepsin D, urokinase plasminogen activator and urokinase plasminogen activator-receptors in carcinomas of the female breast in an African population. East Afr Med J 78 : 360–365.
82. NyagolJ, Nyong'oA, ByakikaB, MuchiriL, CoccoM, et al. (2006) Routine assessment of hormonal receptor and her-2/neu status underscores the need for more therapeutic targets in Kenyan women with breast cancer. Anal Quant Cytol Histol 28 : 97–103.
83. NalwogaH, ArnesJB, WabingaH, AkslenLA (2010) Expression of aldehyde dehydrogenase 1 (ALDH1) is associated with basal-like markers and features of aggressive tumours in African breast cancer. Br J Cancer 102 : 369–375.
84. LyM, AntoineM, DembeleAK, LevyP, RodenasA, et al. (2012) High incidence of triple-negative tumors in sub-saharan Africa: a prospective study of breast cancer characteristics and risk factors in Malian women seen in a Bamako university hospital. Oncology 83 : 257–263.
85. YarneyJ, VanderpuyeV, Clegg LampteyJN (2008) Hormone receptor and HER-2 expression in breast cancers among Sub-Saharan African women. Breast J 14 : 510–511.
86. GalukandeM, WabingaH, MirembeF, KaramagiC, AseaA (2013) Difference in risk factors for breast cancer by ER status in an indigenous African population. ISRN Oncology 1.
87. Ohene-YeboahM, AdjeiE (2012) Breast cancer in Kumasi, Ghana. Ghana Medical Journal 46 : 8–13.
88. BursonAM, SolimanAS, NgomaTA, MwaiselageJ, OgweyoP, et al. (2010) Clinical and epidemiologic profile of breast cancer in Tanzania. Breast Dis 31 : 33–41.
89. SavageN, LevinJ, De MoorNG, LangeM (1981) Cytosolic oestrogen receptor content of breast cancer tissue in blacks and whites. S Afr Med J 59 : 623–624.
90. WintersZ, MannellA, EsserJD (1988) Breast cancer in black South Africans. S Afr J Surg 26 : 69–70.
91. CollingsJR, LevinJ, SavageN (1980) Racial differences in oestrogen receptor and peroxidase status of human breast cancer tissue. S Afr Med J 57 : 444–446.
92. Van BogaertLJ (2013) Breast cancer molecular subtypes as identified by immunohistochemistry in South African black women. Breast Journal 19 : 210–211.
93. BasroS, ApffelstaedtJP (2010) Breast cancer in young women in a limited-resource environment. World J Surg 34 : 1427–1433.
94. TogoA, KanteL, DembeleBT, TraoreA, DiakiteI, et al. (2010) Breast cancer in Bamako hospitals: epidemiologic and diagnostic aspects. Medecine d'Afrique Noire 57 : 249–253.
95. Emile HasiniatsyNR, VololonantenainaCR, RabarikotoHF, RazafimanjatoN, RanoharisonHD, et al. (2014) First results of hormone receptors' status in Malagasy women with invasive breast cancer. Pan African Medical Journal 17.
96. KhouryT, SaitS, HwangH, ChandrasekharR, WildingG, et al. (2009) Delay to formalin fixation effect on breast biomarkers. Mod Pathol 22 : 1457–1467.
97. WasielewskiR, HasselmannS, RuschoffJ, Fisseler-EckhoffA, KreipeH (2008) Proficiency testing of immunohistochemical biomarker assays in breast cancer. Virchows Arch 453 : 537–543.
98. HammondME, HayesDF, WolffAC, ManguPB, TeminS (2010) American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 6 : 195–197.
99. MoultrieTA, SayiTS, TimaeusIM (2012) Birth intervals, postponement, and fertility decline in Africa: a new type of transition? Popul Stud (Camb) 66 : 241–258.
100. PelletierD, RahnM (1998) Trends in body mass index in developing countries. Food Nutr Bull 19 : 223–238.
101. JamesP (2004) Obesity: the worldwide epidemic. Clin Dermatol 22 : 276–280.
102. ClarkeC, KeeganT, YangJ, PressD, KurianA, et al. (2012) Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst 104 : 1094–1101.
103. Vona-DavisL, RoseD, HazardH, Howard-McNattM, AdkinsF, et al. (2008) Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev 17 : 3319–3324.
104. PhippsA, ChlebowskiR, PrenticeR, McTiernanA, Wactawski-WendeJ, et al. (2011) Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst 103 : 470–477.
105. DolleJ, DalingJ, WhiteE, BrintonL, DoodyD, et al. (2009) Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev 18 : 1157–1166.
106. PalmerJ, Ruiz-NarvaezE, RotimiC, CupplesL, CozierY, et al. (2013) Genetic susceptibility loci for subtypes of breast cancer in an African American population. Cancer Epidemiol Biomarkers Prev 22 : 127–134.
107. Ruiz-NarvaezE, RosenbergL, RotimiC, CupplesL, BoggsD, et al. (2010) Genetic variants on chromosome 5p12 are associated with risk of breast cancer in African American women: the Black Women's Health Study. Breast Cancer Res Treat 123 : 525–530.
108. SuY, ZhengY, ZhengW, GuK, ChenZ, et al. (2011) Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study. BMC Cancer 11 : 292.
109. CareyLA, PerouCM, LivasyCA, DresslerLG, CowanD, et al. (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295 : 2492–2502.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Monitoring and Evaluating Progress towards Universal Health Coverage in Ghana
- Sorting Reality from What We Think We Know About Breast Cancer in Africa
- The PLOS “Monitoring Universal Health Coverage” Collection: Managing Expectations
- Monitoring and Evaluating Progress towards Universal Health Coverage in India
- Monitoring and Evaluating Progress towards Universal Health Coverage in Tanzania
- Monitoring and Evaluating Progress towards Universal Health Coverage in Brazil
- Monitoring and Evaluating Progress towards Universal Health Coverage in Thailand
- Monitoring and Evaluating Progress towards Universal Health Coverage in Estonia
- Monitoring and Evaluating Progress towards Universal Health Coverage in Chile
- Prioritizing Pregnant Women for Long-Lasting Insecticide Treated Nets through Antenatal Care Clinics
- Financial Risk Protection and Universal Health Coverage: Evidence and Measurement Challenges
- Convergence of Mortality Rates among Patients on Antiretroviral Therapy in South Africa and North America
- Malaria Prevention during Pregnancy—Is There a Next Step Forward?
- Beyond UHC: Monitoring Health and Social Protection Coverage in the Context of Tuberculosis Care and Prevention
- Intimate Partner Violence and Reproductive Coercion: Global Barriers to Women's Reproductive Control
- Genetic Predisposition to Increased Blood Cholesterol and Triglyceride Lipid Levels and Risk of Alzheimer Disease: A Mendelian Randomization Analysis
- Readmissions after Hospitalization for Heart Failure, Acute Myocardial Infarction, or Pneumonia among Young and Middle-Aged Adults: A Retrospective Observational Cohort Study
- Intermittent Preventive Treatment of Malaria in Pregnancy with Mefloquine in HIV-Infected Women Receiving Cotrimoxazole Prophylaxis: A Multicenter Randomized Placebo-Controlled Trial
- Equity-Oriented Monitoring in the Context of Universal Health Coverage
- The Clinical and Economic Impact of Point-of-Care CD4 Testing in Mozambique and Other Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effective Coverage: A Metric for Monitoring Universal Health Coverage
- Mortality in Patients with HIV-1 Infection Starting Antiretroviral Therapy in South Africa, Europe, or North America: A Collaborative Analysis of Prospective Studies
- Global Financing and Long-Term Technical Assistance for Multidrug-Resistant Tuberculosis: Scaling Up Access to Treatment
- Oral Cholera Vaccine Development and Use in Vietnam
- Monitoring and Evaluating Progress towards Universal Health Coverage in Bangladesh
- Monitoring and Evaluating Progress towards Universal Health Coverage in South Africa
- Monitoring and Evaluating Progress towards Universal Health Coverage in Ethiopia
- Preventing Acute Malnutrition in Young Children: Improving the Evidence for Current and Future Practice
- Monitoring and Evaluating Progress towards Universal Health Coverage in Singapore
- Monitoring and Evaluating Progress towards Universal Health Coverage in China
- Monitoring and Evaluating Progress towards Universal Health Coverage in Tunisia
- Receptor-Defined Subtypes of Breast Cancer in Indigenous Populations in Africa: A Systematic Review and Meta-Analysis
- WHO Essential Medicines Policies and Use in Developing and Transitional Countries: An Analysis of Reported Policy Implementation and Medicines Use Surveys
- Intermittent Preventive Treatment of Malaria in Pregnancy with Mefloquine in HIV-Negative Women: A Multicentre Randomized Controlled Trial
- Preventing Acute Malnutrition among Young Children in Crises: A Prospective Intervention Study in Niger
- Monitoring Progress towards Universal Health Coverage at Country and Global Levels
- Monitoring Intervention Coverage in the Context of Universal Health Coverage
- Regular Breakfast Consumption and Type 2 Diabetes Risk Markers in 9- to 10-Year-Old Children in the Child Heart and Health Study in England (CHASE): A Cross-Sectional Analysis
- Proton Pump Inhibitors and Hospitalization with Hypomagnesemia: A Population-Based Case-Control Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Proton Pump Inhibitors and Hospitalization with Hypomagnesemia: A Population-Based Case-Control Study
- Monitoring and Evaluating Progress towards Universal Health Coverage in Chile
- Malaria Prevention during Pregnancy—Is There a Next Step Forward?
- Financial Risk Protection and Universal Health Coverage: Evidence and Measurement Challenges
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



