-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaProton Pump Inhibitors and Hospitalization with Hypomagnesemia: A Population-Based Case-Control Study
Background:
Some evidence suggests that proton pump inhibitors (PPIs) are an under-appreciated risk factor for hypomagnesemia. Whether hospitalization with hypomagnesemia is associated with use of PPIs is unknown.Methods and Findings:
We conducted a population-based case-control study of multiple health care databases in Ontario, Canada, from April 2002 to March 2012. Patients who were enrolled as cases were Ontarians aged 66 years or older hospitalized with hypomagnesemia. For each individual enrolled as a case, we identified up to four individuals as controls matched on age, sex, kidney disease, and use of various diuretic classes. Exposure to PPIs was categorized according to the most proximate prescription prior to the index date as current (within 90 days), recent (within 91 to 180 days), or remote (within 181 to 365 days). We used conditional logistic regression to estimate the odds ratio for the association of outpatient PPI use and hospitalization with hypomagnesemia. To test the specificity of our findings we examined use of histamine H2 receptor antagonists, drugs with no causal link to hypomagnesemia. We studied 366 patients hospitalized with hypomagnesemia and 1,464 matched controls. Current PPI use was associated with a 43% increased risk of hypomagnesemia (adjusted odds ratio, 1.43; 95% CI 1.06–1.93). In a stratified analysis, the risk was particularly increased among patients receiving diuretics, (adjusted odds ratio, 1.73; 95% CI 1.11–2.70) and not significant among patients not receiving diuretics (adjusted odds ratio, 1.25; 95% CI 0.81–1.91). We estimate that one excess hospitalization with hypomagnesemia will occur among 76,591 outpatients treated with a PPI for 90 days. Hospitalization with hypomagnesemia was not associated with the use of histamine H2 receptor antagonists (adjusted odds ratio 1.06; 95% CI 0.54–2.06). Limitations of this study include a lack of access to serum magnesium levels, uncertainty regarding diagnostic coding of hypomagnesemia, and generalizability of our findings to younger patients.Conclusions:
PPIs are associated with a small increased risk of hospitalization with hypomagnesemia among patients also receiving diuretics. Physicians should be aware of this association, particularly for patients with hypomagnesemia.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(9): e32767. doi:10.1371/journal.pmed.1001736
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001736Summary
Background:
Some evidence suggests that proton pump inhibitors (PPIs) are an under-appreciated risk factor for hypomagnesemia. Whether hospitalization with hypomagnesemia is associated with use of PPIs is unknown.Methods and Findings:
We conducted a population-based case-control study of multiple health care databases in Ontario, Canada, from April 2002 to March 2012. Patients who were enrolled as cases were Ontarians aged 66 years or older hospitalized with hypomagnesemia. For each individual enrolled as a case, we identified up to four individuals as controls matched on age, sex, kidney disease, and use of various diuretic classes. Exposure to PPIs was categorized according to the most proximate prescription prior to the index date as current (within 90 days), recent (within 91 to 180 days), or remote (within 181 to 365 days). We used conditional logistic regression to estimate the odds ratio for the association of outpatient PPI use and hospitalization with hypomagnesemia. To test the specificity of our findings we examined use of histamine H2 receptor antagonists, drugs with no causal link to hypomagnesemia. We studied 366 patients hospitalized with hypomagnesemia and 1,464 matched controls. Current PPI use was associated with a 43% increased risk of hypomagnesemia (adjusted odds ratio, 1.43; 95% CI 1.06–1.93). In a stratified analysis, the risk was particularly increased among patients receiving diuretics, (adjusted odds ratio, 1.73; 95% CI 1.11–2.70) and not significant among patients not receiving diuretics (adjusted odds ratio, 1.25; 95% CI 0.81–1.91). We estimate that one excess hospitalization with hypomagnesemia will occur among 76,591 outpatients treated with a PPI for 90 days. Hospitalization with hypomagnesemia was not associated with the use of histamine H2 receptor antagonists (adjusted odds ratio 1.06; 95% CI 0.54–2.06). Limitations of this study include a lack of access to serum magnesium levels, uncertainty regarding diagnostic coding of hypomagnesemia, and generalizability of our findings to younger patients.Conclusions:
PPIs are associated with a small increased risk of hospitalization with hypomagnesemia among patients also receiving diuretics. Physicians should be aware of this association, particularly for patients with hypomagnesemia.
Please see later in the article for the Editors' SummaryIntroduction
Proton-pump inhibitors (PPIs) are among the most widely prescribed drugs in the world, with more than 147 million prescriptions dispensed in the United States in 2010 alone [1]. They are the mainstay of drug therapy for acid-related disorders, and have largely supplanted histamine H2 receptor antagonists owing to their superior efficacy [2]–[4].
Although widely regarded as safe, PPIs have been associated with a variety of adverse effects including Clostridium difficile-associated diarrhea [5]–[7], interstitial nephritis [8],[9], pneumonia [10], vitamin B12 deficiency [11], and osteoporosis and fractures [12]. More recently, long-term use of PPIs has been suggested as a potential cause of hypomagnesemia [13]. Magnesium is the second most abundant intracellular cation and its homeostasis is intricately regulated by intestinal absorption and renal excretion, although the estimated prevalence of hypomagnesemia in the general population ranges from 2.5% to 15% [14],[15]. The postulated mechanism of PPI-related hypomagnesemia involves inhibition of intestinal magnesium absorption via transient receptor potential melastin (TRPM) 6 and 7 cation channels [16]. Severe hypomagnesemia can be associated with malignant cardiac arrhythmias, tetany, generalized seizures, and other metabolic disturbances such as hypokalemia and hypocalcemia [17].
First described in 2006 [18], the evidence for PPI-induced hypomagnesemia has been mainly limited to case reports and small case series, with more than 30 cases published to date [18]–[30]. In 2011, the US Food and Drug Administration (FDA) issued a drug safety warning regarding the potential association of PPIs with hypomagnesemia, but observational studies have yielded conflicting findings [31]–[34]. The largest study to date evaluated patients in a single intensive care unit, and found that PPI use was associated with hypomagnesemia only among patients also receiving diuretics [31]. It is uncertain whether PPIs are a risk factor for hypomagnesemia in routine clinical practice.
Given the widespread use of PPIs, particularly in combination with other drugs that can promote hypomagnesemia, such an association may be difficult to appreciate clinically. We examined the association between outpatient PPI use and hospitalization with hypomagnesemia.
Methods
Ethics Statement
This study was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre. The Institute for Clinical Evaluative Sciences (ICES) is named as a prescribed entity under section 45 of the Personal Health Information Protection Act (Ontario Regulation 329/04, Section 18). Under this designation, ICES can receive and use anonymous health information without consent.
Setting
We conducted a population-based case-control study of all Ontario residents aged 66 years or older between April 1st, 2002 and March 31st, 2012. These individuals had universal access to physician services, hospital care, and prescription drug coverage.
Data Sources
We identified prescription records using the Ontario Drug Benefit Database, which contains comprehensive records of prescription drugs dispensed to Ontario residents aged 65 years or older. To avoid incomplete medication records, we excluded patients during their first year of eligibility for prescription drug coverage (age 65). We obtained hospitalization data from the Canadian Institute for Health Information Discharge Abstract Database, which contains detailed clinical information, including diagnoses, for all hospital admissions in Ontario. Emergency department records were obtained from the National Ambulatory Care Reporting System. We used the Ontario Health Insurance Plan database to identify claims for physician services, the Ontario Diabetes Database [35] to ascertain the presence of diabetes, and the Ontario Congestive Heart Failure Database [36] to identify individuals with congestive heart failure. We obtained basic demographic data and date of death from the Registered Persons Database, a registry of all Ontario residents eligible for health insurance. These databases were linked in an anonymous fashion using encrypted health card numbers, and are routinely used to study drug safety [37]–[39].
Study Patients
We defined case patients as those hospitalized with hypomagnesemia, defined using the International Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes E83.42 (hypomagnesemia) or E61.2 (magnesium deficiency). Only the first such hospitalization was considered for patients with multiple episodes. The date of hospital admission served as the index date for all analyses. For each individual enrolled as a case, we randomly selected four control patients not hospitalized with hypomagnesemia. Control patients were randomly assigned an index date within one calendar year of the corresponding case patient, and patients who were controls could later serve as cases. Four control patients were matched to each case patient according to age (within 3 years), sex, chronic kidney disease (CKD), or acute kidney injury (AKI) in the year preceding the index date, and receipt of thiazide, loop, or other diuretics in the 90 days preceding the index date, with each diuretic class considered separately. Each individual could only serve once as a control and unmatched cases were excluded. We also excluded patients with a diagnosis of hyperparathyroidism or inflammatory bowel disease in the year prior to index date because these disorders can influence magnesium balance, and we excluded individuals hospitalized for any reason in the month preceding the index date to avoid the potential confounding effects of recent hospitalization.
Assessment of PPI Exposure
We identified all outpatient prescriptions for omeprazole, lansoprazole, pantoprazole, rabeprazole, and esomeprazole. We classified PPI exposure according to the prescription closest to the index date as either current (within 90 days preceding the index date), recent (91 to 180 days prior to the index date), or remote (181 to 365 days prior to the index date). We used this approach because many patients take PPIs intermittently, particularly for symptoms of dyspepsia or gastroesophageal reflux. Moreover, any association should manifest in patients with ongoing PPI therapy, and would therefore be most likely in current users and least likely in remote users [13]. As well, in many published reports, magnesium levels have normalized shortly after discontinuation of the PPI [13].
To test the robustness and specificity of our findings, we also examined prescriptions for histamine H2 receptor antagonists, drugs with no plausible causal link to hypomagnesemia.
Statistical Analysis
We used conditional logistic regression to estimate the odds ratio and 95% confidence intervals for the association between hypomagnesemia and receipt of a PPI prescription. In all analyses, the reference group consisted of patients with no PPI prescription in the 365 days preceding the index date. A similar analysis was conducted for histamine H2 receptor antagonists. A subgroup analysis was performed to examine the risk of hypomagnesemia associated with PPI use amongst patients concomitantly prescribed diuretics.
We adjusted all models for confounders with standardized differences>0.10 including the number of drugs dispensed in the year preceding the index date (a validated measure of comorbidity) [40], systemic steroid use in the preceding year, any history of diabetes or heart failure in the 3 years prior to the index date, and the presence of systemic malignancy in the preceding year. We used SAS version 9.3 for all analyses (SAS Institute), and a two-tailed type 1 error rate of 0.05 as the threshold for statistical significance.
Results
During the ten-year study period, we identified 429 patients aged 66 years or older hospitalized with hypomagnesemia. We excluded 63 patients enrolled as cases (14.7%) who had been hospitalized in the prior month or had a diagnosis of inflammatory bowel disease in the previous year. The remaining 366 case patients were matched to 1,464 patients designated as controls. The characteristics of case and control patients are shown in Table 1. The median age was 78 (interquartile range [IQR] 71–83) years, and slightly more than half were women. As expected, compared with control patients, case patients received more medications and were more likely to have various comorbid conditions (Table 1).
Tab. 1. Characteristics of patients enrolled as cases and controls. 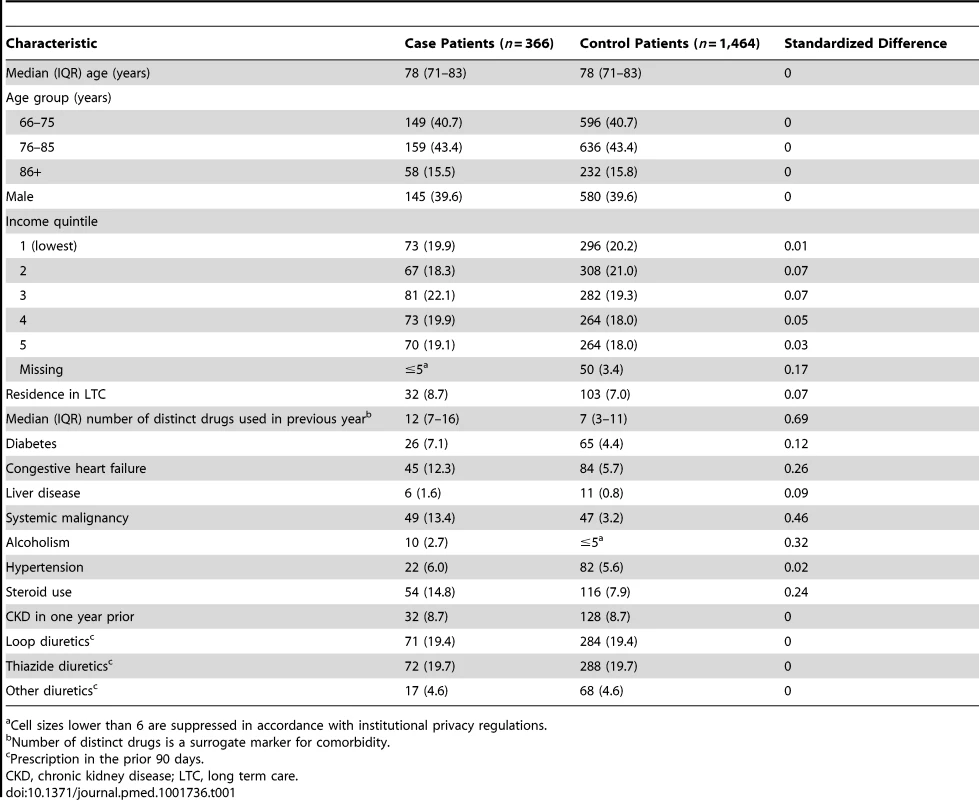
Cell sizes lower than 6 are suppressed in accordance with institutional privacy regulations. In the primary analysis, we found that patients hospitalized with hypomagnesemia were more likely than control patients to be current users of PPIs (adjusted odds ratio, 1.43; 95% CI 1.06–1.93). Neither recent nor remote PPI use was associated with a significantly increased risk of hypomagnesemia (Table 2). The median number of tablets dispensed to patients who were cases in the 5 years preceding the index date was 1,358 (IQR 540–1,829) versus 1,176 (IQR 540–1,831) among patients who were controls. As expected, we found no association between hospitalization with hypomagnesemia and current use of histamine H2 receptor antagonists (unadjusted odds ratio 2.12, 95% CI 1.17–3.86; adjusted odds ratio 1.06; 95% CI 0.54–2.06) (Table 3). In a subgroup analysis of diuretic users, there was a higher risk of hospitalization with hypomagnesemia in patients using PPIs who were also concomitantly prescribed diuretics (adjusted odds ratio, 1.73; 95% CI 1.11–2.70) (Table 4).
Tab. 2. Proton pump inhibitor use and hospitalization with hypomagnesemia. 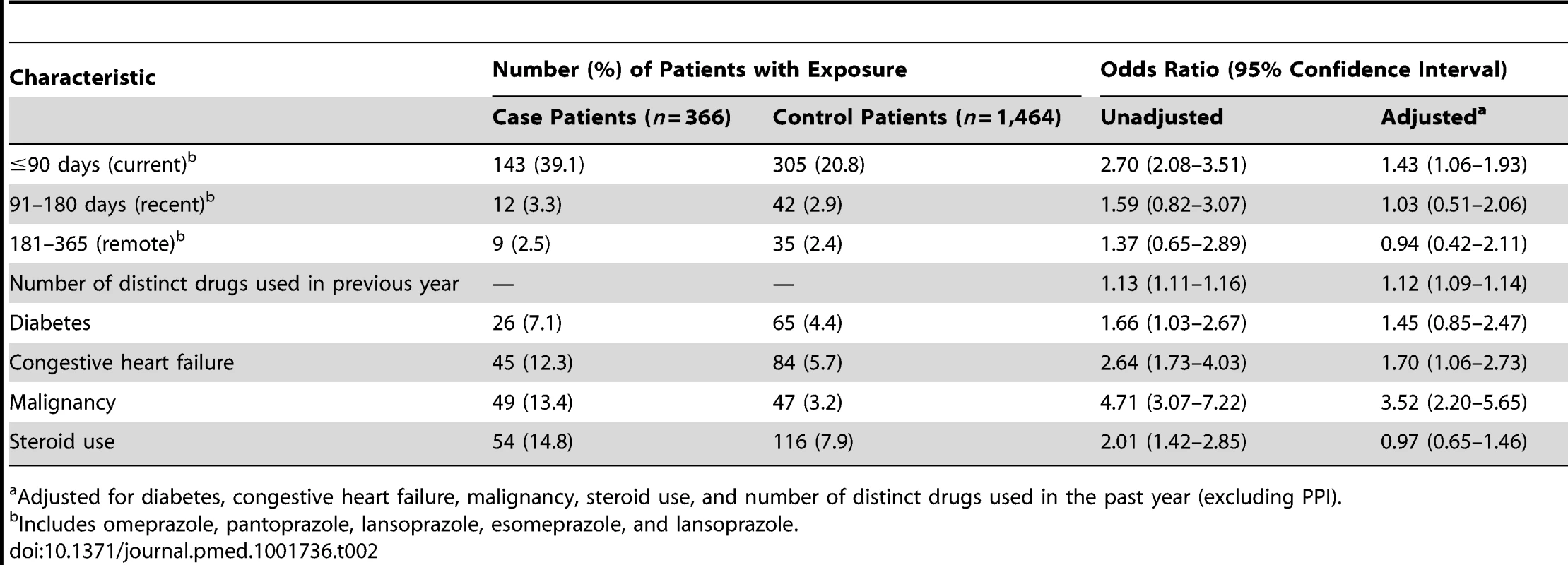
Adjusted for diabetes, congestive heart failure, malignancy, steroid use, and number of distinct drugs used in the past year (excluding PPI). Tab. 3. Histamine (H2) receptor blocker use and hospitalization with hypomagnesemia. 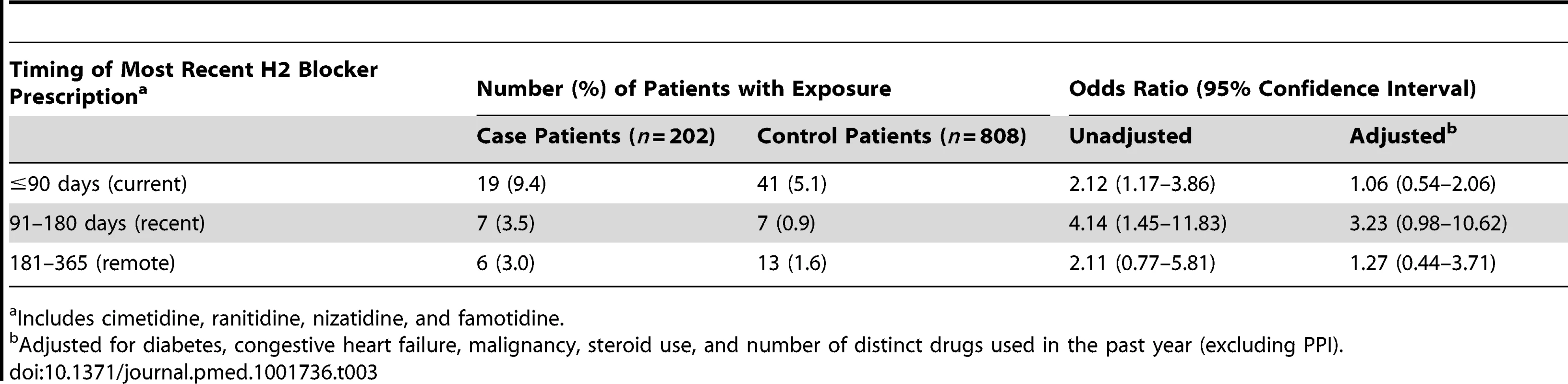
Includes cimetidine, ranitidine, nizatidine, and famotidine. Tab. 4. Proton pump inhibitor use and hospitalization with hypomagnesemia stratified by diuretic use. 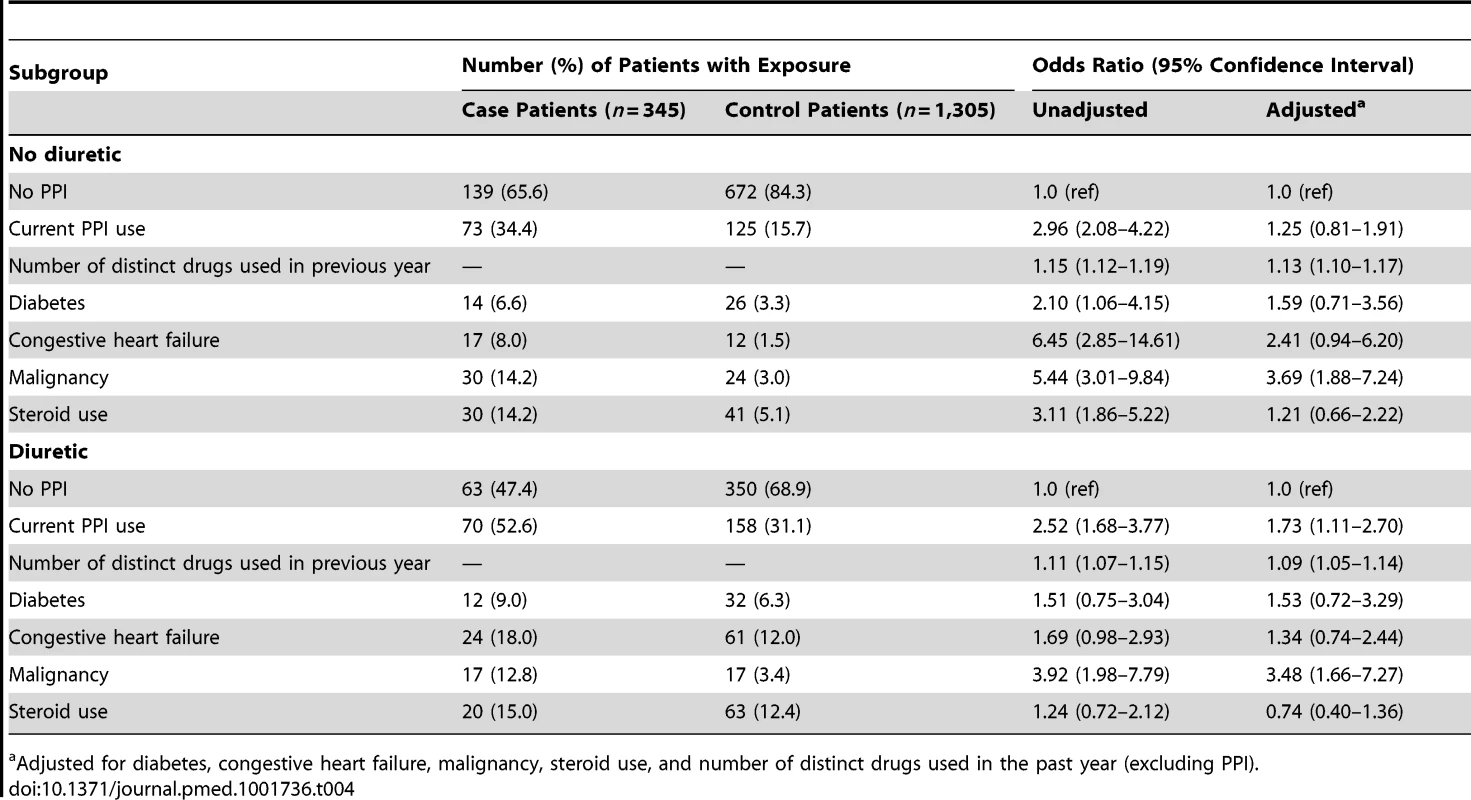
Adjusted for diabetes, congestive heart failure, malignancy, steroid use, and number of distinct drugs used in the past year (excluding PPI). We anticipated that hospitalization with hypomagnesemia would be uncommon among patients treated with a PPI. Among 1,042,765 patients who commenced treatment with a PPI during the study period, 15 were hospitalized with hypomagnesemia in the subsequent 90 days, representing 3.19 such admissions (95% CI 1.95–5.2) per 100,000 new PPI prescriptions. Among all eligible control patients (n = 1,040,875), fewer than six individuals were admitted with hypomagnesemia within 90 days, yielding a number needed to harm at 90 days of 76,591.
Discussion
We found that current PPI therapy was associated with a 43% increased relative risk of hospitalization with hypomagnesemia in a large population of older outpatients. In contrast, no such risk was evident with more remote use of PPIs or of histamine H2 receptor antagonists. Although mild hypomagnesemia may go unnoticed, severe cases can have significant neurologic and cardiac consequences. Our findings highlight an underappreciated albeit small risk of these commonly prescribed medications.
In an analysis stratified by diuretic use, concomitant users of PPIs and diuretics had an increased risk of hospitalization with hypomagnesemia, whereas those not taking diuretics did not have an increased risk. This finding accords with that of Danziger and colleagues, who studied the association between PPIs and hypomagnesemia in an intensive care unit setting [31]. While PPI-induced hypomagnesemia is rare and most PPI users are presumably unaffected, patients taking diuretics may be at particular risk.
Previous studies examining the association between PPIs and hypomagnesemia have found conflicting results. These studies [32]–[34] did not completely adjust for comorbidity and polypharmacy, important confounding factors contributing to hypomagnesemia, and two studies were limited to hospital inpatients [31],[32]. Another recent case-control study by Koulouridis and colleagues found no association between outpatient PPI use and hypomagnesemia at hospital admission [33]. Some reasons why this study's conclusion may have differed from ours include the potential for misclassification of PPI use, because physician and nursing medication administration records were the primary methods of ascertaining exposure, with specificity documented to be as low as 46.2%. In contrast, we examined population-based prescriptions records and accounted for timing of the prescription. Their definition (serum magnesium concentration <1.4 mEq/l) almost certainly included less severe cases of hypomagnesemia than in our study, which examined hospitalization with hypomagnesemia—a less sensitive but more clinically meaningful outcome. Finally, their study was limited to patients at a single center hospitalized for disorders of the upper gastrointestinal tract. The largest study to date evaluated hypomagnesemia in a single intensive care unit and found that PPIs were only associated with hypomagnesemia (serum magnesium concentration <1.6 mg/dl) in patients with concurrent use of diuretics [31]. While this study supports the notion that PPIs may be a risk factor for hypomagnesemia, the generalizability of these findings to patients in routine clinical practice is uncertain.
The mechanism of hypomagnesemia in the setting of PPI use is poorly understood but may reflect impaired gastrointestinal absorption of magnesium. TRPM 6 and 7 cation channels in the intestine are responsible for active transport of magnesium [16]. In contrast, most other drugs associated with hypomagnesemia cause renal magnesium wasting. In patients with suspected PPI-induced hypomagnesemia, renal magnesium handling is preserved [20],, although as found in our study and by others [31], this may be influenced by diuretic therapy.
We speculate that hypomagnesemia is an underappreciated consequence of PPI use, particularly given the high background use of PPIs in the general population. Whether some PPI recipients are genetically predisposed to this adverse effect is currently unknown. It has been postulated that individuals carrying heterozygous mutations of TRPM6/7 may be at especially high risk, as evidenced from observations that people with homozygous mutations of TRPM6 manifest severe hypomagnesemia [42]–[44]. The modest effect found in this study may in fact reflect a much larger problem with systemic magnesium balance, since only about 1% of total body magnesium is reflected in the blood [45].
The findings of this study are strengthened by the population-based nature of the data and our ability to account for virtually all PPI use, because the drugs are not sold over-the-counter in Canada. However, some limitations of our study merit emphasis. We had no access to serum magnesium levels, and the validity of hospital coding for hypomagnesemia using administrative databases is unknown. Second, our results derived from patients aged 66 years and older, and the generalizability to younger patients is unknown. Third, we cannot account for intermittent PPI use, although this would be expected to attenuate the association with hypomagnesemia. Finally, clinically significant cases of hypomagnesemia may have been missed in our analysis, particularly those presenting with arrhythmias or other metabolic abnormalities such as hypokalemia.
On the basis of the large number needed to harm (approximately 76,591), our study should not discourage clinicians from prescribing PPIs to appropriate patients. While routine screening of serum magnesium concentrations in patients on PPIs is likely unwarranted, clinicians should be aware of this association, particularly in patients on long-term PPI therapy and those with hypokalemia or associated cardiac or neurologic symptoms. In patients with hypomagnesemia in the setting of PPI therapy, we suggest reassessing the need for ongoing therapy and considering other treatment options.
In summary, we found an association between outpatient PPI therapy and hospitalization with hypomagnesemia among older patients. Our findings highlight what may be an underappreciated adverse effect of PPI therapy. Future research may help further characterize the significance of this effect, and the importance of cumulative dose and duration of PPI therapy. In the interim, we suggest that physicians recognize the potential causative role of PPIs in patients with hypomagnesemia, and reconsider PPI therapy in such patients.
Supporting Information
Zdroje
1. IMS Institute for Healthcare Informatics (2011) The Use of Medicine in the United States: Review of 2010. Available: http://www.imshealth.com/deployedfiles/imshealth/Global/Content/IMS%20Institute/Static%20File/IHII_UseOfMed_report.pdf. Accessed 10 April 2014.
2. RichardsonP, HawkeyCJ, StackWA (1998) Proton pump inhibitors. Pharmacology and rationale for use in gastrointestinal disorders. Drugs 56 : 307–335.
3. SalasM, WardA, CaroJ (2002) Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol 2 : 17.
4. PoynardT, LemaireM, AgostiniH (1995) Meta-analysis of randomized clinical trials comparing lansoprazole with ranitidine or famotidine in the treatment of acute duodenal ulcer. Eur J Gastroenterol Hepatol 7 : 661–665.
5. LeonardJ, MarshallJK, MoayyediP (2007) Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 102 : 2047–56; quiz 2057.
6. KwokCS, ArthurAK, AnibuezeCI, SinghS, CavallazziR, et al. (2012) Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol 107 : 1011–1019.
7. JanarthananS, DitahI, AdlerDG, EhrinpreisMN (2012) Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 107 : 1001–1010.
8. LeonardCE, FreemanCP, NewcombCW, ReesePP, HerlimM, et al. (2012) Proton pump inhibitors and traditional nonsteroidal anti-inflammatory drugs and the risk of acute interstitial nephritis and acute kidney injury. Pharmacoepidemiol Drug Saf 21 : 1155–1172.
9. BlankM-L, ParkinL, PaulC, HerbisonP (2014) A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int doi:10.1038/ki.2014.74
10. EomC-S, JeonCY, LimJ-W, ChoE-G, ParkSM, et al. (2011) Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ 183 : 310–319.
11. MarcuardSP, AlbernazL, KhazaniePG (1994) Omeprazole therapy causes malabsorption of cyanocobalamin (vitamin B12). Ann Intern Med 120 : 211–215.
12. YuEW, BauerSR, BainPA, BauerDC (2011) Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med 124 : 519–526.
13. FamularoG, GasbarroneL, MinisolaG (2013) Hypomagnesemia and proton-pump inhibitors. Expert Opin Drug Saf 1–8.
14. MaJ, FolsomAR, MelnickSL, EckfeldtJH, SharrettAR, et al. (1995) Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol 48 : 927–940.
15. SchimatschekHF, RempisR (2001) Prevalence of hypomagnesemia in an unselected German population of 16,000 individuals. Magnes Res 14 : 283–290.
16. SchlingmannKP, WaldeggerS, KonradM, ChubanovV, GudermannT (2007) TRPM6 and TRPM7–Gatekeepers of human magnesium metabolism. Biochim Biophys Acta 1772 : 813–821.
17. WeisingerJR, Bellorin-FontE (1998) Magnesium and phosphorus. Lancet 352 : 391–396.
18. EpsteinM, McGrathS, LawF (2006) Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med 355 : 1834–1836.
19. ArulananthamN, AndersonM (2011) A 63-year-old man with hypomagnesaemia and seizures. Clin Med (Northfield, Il) 11 : 591–593.
20. BroerenM, GeerdinkE (2009) Hypomagnesemia induced by several proton-pump inhibitors. Ann Intern 151 : 755–756.
21. Fernandez-FernandezF (2010) Intermittent use of pantoprazole and famotidine in severe hypomagnesaemia due to omeprazole. Neth J Med 68 : 329–330.
22. ShabajeeN, LambEJ, SturgessI, SumathipalaRW (2008) Omeprazole and refractory hypomagnesaemia. BMJ 337: a425.
23. CundyT, Dissanayakea (2008) Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clin Endocrinol (Oxf) 69 : 338–341.
24. FamularoG, MinisolaG, BraviMC, ColucciP, GasbarroneL (2012) Tetany, hypomagnesemia, and proton-pump inhibitors. Am J Med 125: e7–e8.
25. HoornEJ, van der HoekJ, de Man Ra, KuipersEJ, BolwerkC, et al. (2010) A case series of proton pump inhibitor-induced hypomagnesemia. Am J Kidney Dis 56 : 112–116.
26. MackayJD, BladonPT (2010) Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM 103 : 387–395.
27. HessMW, HoenderopJGJ, BindelsRJM, DrenthJPH (2012) Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 36 : 405–413.
28. FurlanettoTW, FaulhaberGAM (2011) Hypomagnesemia and proton pump inhibitors: below the tip of the iceberg. Arch Intern Med 171 : 1391–1392.
29. MatsuyamaJ, TsujiK, DoyamaH, KimF, TakedaY, et al. (2012) Hypomagnesemia Associated with a Proton Pump Inhibitor. Intern Med 51 : 2231–2234.
30. GandhiNY, SharifWK, ChadhaS, ShakherJ (2012) A patient on long-term proton pump inhibitors develops sudden seizures and encephalopathy: an unusual presentation of hypomagnesaemia. Case Rep Gastrointest Med 2012 : 632721.
31. DanzigerJ, WilliamJH, ScottDJ, LeeJ, LehmanL, et al. (2013) Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int 83 : 692–699.
32. GauJ, YangY (2012) Uses of proton pump inhibitors and hypomagnesemia. … drug Saf 553–559.
33. KoulouridisI, AlfayezM, TighiouartH, MadiasNE, KentDM, et al. (2013) Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis 62 : 730–737.
34. LukCP, ParsonsR, LeeYP, HughesJD (2013) Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother 47 : 773–780.
35. HuxJE, IvisF, FlintoftV, BicaA (2002) Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 25 : 512–516.
36. SchultzSE, RothwellDM, ChenZ, TuK (2013) Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can 33 : 160–166.
37. JuurlinkDN, MhscTG, KoDT, SzmitkoPE, AustinPC, et al. (2009) A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 180 : 713–718.
38. JuurlinkD, GomesT, LipsombeL, AustinPC, HuxJ, et al. (2009) Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ 339 : 1–6.
39. Park-WyllieLY, JuurlinkDN, KoppA, ShahBR, StukelTA, et al. (2006) Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med 354 : 1352–1361.
40. SchneeweissS, SeegerJD, MaclureM, WangPS, AvornJ, et al. (2001) Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 154 : 854–864.
41. RegolistiG, CabassiA, ParentiE, MaggioreU, FiaccadoriE (2010) Severe hypomagnesemia during long-term treatment with a proton pump inhibitor. Am J Kidney Dis 56 : 168–174.
42. WalderRY, LandauD, MeyerP, ShalevH, TsoliaM, et al. (2002) Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31 : 171–174.
43. ChubanovV, WaldeggerS, Mederos y SchnitzlerM, VitzthumH, SassenMC, et al. (2004) Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci U S A 101 : 2894–2899.
44. LainezS, SchlingmannKP, van der WijstJ, DworniczakB, van ZeelandF, et al. (2013) New TRPM6 missense mutations linked to hypomagnesemia with secondary hypocalcemia. Eur J Hum Genet 1–8.
45. ElinRJ (2010) Assessment of magnesium status for diagnosis and therapy. Magnes Res 23: S194–S1988.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Monitoring and Evaluating Progress towards Universal Health Coverage in Ghana
- Sorting Reality from What We Think We Know About Breast Cancer in Africa
- The PLOS “Monitoring Universal Health Coverage” Collection: Managing Expectations
- Monitoring and Evaluating Progress towards Universal Health Coverage in India
- Monitoring and Evaluating Progress towards Universal Health Coverage in Tanzania
- Monitoring and Evaluating Progress towards Universal Health Coverage in Brazil
- Monitoring and Evaluating Progress towards Universal Health Coverage in Thailand
- Monitoring and Evaluating Progress towards Universal Health Coverage in Estonia
- Monitoring and Evaluating Progress towards Universal Health Coverage in Chile
- Prioritizing Pregnant Women for Long-Lasting Insecticide Treated Nets through Antenatal Care Clinics
- Financial Risk Protection and Universal Health Coverage: Evidence and Measurement Challenges
- Convergence of Mortality Rates among Patients on Antiretroviral Therapy in South Africa and North America
- Malaria Prevention during Pregnancy—Is There a Next Step Forward?
- Beyond UHC: Monitoring Health and Social Protection Coverage in the Context of Tuberculosis Care and Prevention
- Intimate Partner Violence and Reproductive Coercion: Global Barriers to Women's Reproductive Control
- Genetic Predisposition to Increased Blood Cholesterol and Triglyceride Lipid Levels and Risk of Alzheimer Disease: A Mendelian Randomization Analysis
- Readmissions after Hospitalization for Heart Failure, Acute Myocardial Infarction, or Pneumonia among Young and Middle-Aged Adults: A Retrospective Observational Cohort Study
- Intermittent Preventive Treatment of Malaria in Pregnancy with Mefloquine in HIV-Infected Women Receiving Cotrimoxazole Prophylaxis: A Multicenter Randomized Placebo-Controlled Trial
- Equity-Oriented Monitoring in the Context of Universal Health Coverage
- The Clinical and Economic Impact of Point-of-Care CD4 Testing in Mozambique and Other Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effective Coverage: A Metric for Monitoring Universal Health Coverage
- Mortality in Patients with HIV-1 Infection Starting Antiretroviral Therapy in South Africa, Europe, or North America: A Collaborative Analysis of Prospective Studies
- Global Financing and Long-Term Technical Assistance for Multidrug-Resistant Tuberculosis: Scaling Up Access to Treatment
- Oral Cholera Vaccine Development and Use in Vietnam
- Monitoring and Evaluating Progress towards Universal Health Coverage in Bangladesh
- Monitoring and Evaluating Progress towards Universal Health Coverage in South Africa
- Monitoring and Evaluating Progress towards Universal Health Coverage in Ethiopia
- Preventing Acute Malnutrition in Young Children: Improving the Evidence for Current and Future Practice
- Monitoring and Evaluating Progress towards Universal Health Coverage in Singapore
- Monitoring and Evaluating Progress towards Universal Health Coverage in China
- Monitoring and Evaluating Progress towards Universal Health Coverage in Tunisia
- Receptor-Defined Subtypes of Breast Cancer in Indigenous Populations in Africa: A Systematic Review and Meta-Analysis
- WHO Essential Medicines Policies and Use in Developing and Transitional Countries: An Analysis of Reported Policy Implementation and Medicines Use Surveys
- Intermittent Preventive Treatment of Malaria in Pregnancy with Mefloquine in HIV-Negative Women: A Multicentre Randomized Controlled Trial
- Preventing Acute Malnutrition among Young Children in Crises: A Prospective Intervention Study in Niger
- Monitoring Progress towards Universal Health Coverage at Country and Global Levels
- Monitoring Intervention Coverage in the Context of Universal Health Coverage
- Regular Breakfast Consumption and Type 2 Diabetes Risk Markers in 9- to 10-Year-Old Children in the Child Heart and Health Study in England (CHASE): A Cross-Sectional Analysis
- Proton Pump Inhibitors and Hospitalization with Hypomagnesemia: A Population-Based Case-Control Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Proton Pump Inhibitors and Hospitalization with Hypomagnesemia: A Population-Based Case-Control Study
- Monitoring and Evaluating Progress towards Universal Health Coverage in Chile
- Malaria Prevention during Pregnancy—Is There a Next Step Forward?
- Financial Risk Protection and Universal Health Coverage: Evidence and Measurement Challenges
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



