-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non-Inferiority Trial in Thailand
Background:
Viral load (VL) is recommended for monitoring the response to highly active antiretroviral therapy (HAART) but is not routinely available in most low - and middle-income countries. The purpose of the study was to determine whether a CD4-based monitoring and switching strategy would provide a similar clinical outcome compared to the standard VL-based strategy in Thailand.Methods and Findings:
The Programs for HIV Prevention and Treatment (PHPT-3) non-inferiority randomized clinical trial compared a treatment switching strategy based on CD4-only (CD4) monitoring versus viral-load (VL). Consenting participants were antiretroviral-naïve HIV-infected adults (CD4 count 50–250/mm3) initiating non-nucleotide reverse transcriptase inhibitor (NNRTI)-based therapy. Randomization, stratified by site (21 public hospitals), was performed centrally after enrollment. Clinicians were unaware of the VL values of patients randomized to the CD4 arm. Participants switched to second-line combination with confirmed CD4 decline >30% from peak (within 200 cells from baseline) in the CD4 arm, or confirmed VL >400 copies/ml in the VL arm. Primary endpoint was clinical failure at 3 years, defined as death, new AIDS-defining event, or CD4 <50 cells/mm3. The 3-year Kaplan-Meier cumulative risks of clinical failure were compared for non-inferiority with a margin of 7.4%. In the intent to treat analysis, data were censored at the date of death or at last visit. The secondary endpoints were difference in future-drug-option (FDO) score, a measure of resistance profiles, virologic and immunologic responses, and the safety and tolerance of HAART. 716 participants were randomized, 356 to VL monitoring and 360 to CD4 monitoring. At 3 years, 319 participants (90%) in VL and 326 (91%) in CD4 were alive and on follow-up. The cumulative risk of clinical failure was 8.0% (95% CI 5.6–11.4) in VL versus 7.4% (5.1–10.7) in CD4, and the upper-limit of the one-sided 95% CI of the difference was 3.4%, meeting the pre-determined non-inferiority criterion. Probability of switch for study criteria was 5.2% (3.2–8.4) in VL versus 7.5% (5.0–11.1) in CD4 (p = 0.097). Median time from treatment initiation to switch was 11.7 months (7.7–19.4) in VL and 24.7 months (15.9–35.0) in CD4 (p = 0.001). The median duration of viremia >400 copies/ml at switch was 7.2 months (5.8–8.0) in VL versus 15.8 months (8.5–20.4) in CD4 (p = 0.002). FDO scores were not significantly different at time of switch. No adverse events related to the monitoring strategy were reported.Conclusions:
The 3-year rates of clinical failure and loss of treatment options did not differ between strategies although the longer-term consequences of CD4 monitoring would need to be investigated. These results provide reassurance to treatment programs currently based on CD4 monitoring as VL measurement becomes more affordable and feasible in resource-limited settings.Trial registration:
ClinicalTrials.gov NCT00162682
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(8): e32767. doi:10.1371/journal.pmed.1001494
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001494Summary
Background:
Viral load (VL) is recommended for monitoring the response to highly active antiretroviral therapy (HAART) but is not routinely available in most low - and middle-income countries. The purpose of the study was to determine whether a CD4-based monitoring and switching strategy would provide a similar clinical outcome compared to the standard VL-based strategy in Thailand.Methods and Findings:
The Programs for HIV Prevention and Treatment (PHPT-3) non-inferiority randomized clinical trial compared a treatment switching strategy based on CD4-only (CD4) monitoring versus viral-load (VL). Consenting participants were antiretroviral-naïve HIV-infected adults (CD4 count 50–250/mm3) initiating non-nucleotide reverse transcriptase inhibitor (NNRTI)-based therapy. Randomization, stratified by site (21 public hospitals), was performed centrally after enrollment. Clinicians were unaware of the VL values of patients randomized to the CD4 arm. Participants switched to second-line combination with confirmed CD4 decline >30% from peak (within 200 cells from baseline) in the CD4 arm, or confirmed VL >400 copies/ml in the VL arm. Primary endpoint was clinical failure at 3 years, defined as death, new AIDS-defining event, or CD4 <50 cells/mm3. The 3-year Kaplan-Meier cumulative risks of clinical failure were compared for non-inferiority with a margin of 7.4%. In the intent to treat analysis, data were censored at the date of death or at last visit. The secondary endpoints were difference in future-drug-option (FDO) score, a measure of resistance profiles, virologic and immunologic responses, and the safety and tolerance of HAART. 716 participants were randomized, 356 to VL monitoring and 360 to CD4 monitoring. At 3 years, 319 participants (90%) in VL and 326 (91%) in CD4 were alive and on follow-up. The cumulative risk of clinical failure was 8.0% (95% CI 5.6–11.4) in VL versus 7.4% (5.1–10.7) in CD4, and the upper-limit of the one-sided 95% CI of the difference was 3.4%, meeting the pre-determined non-inferiority criterion. Probability of switch for study criteria was 5.2% (3.2–8.4) in VL versus 7.5% (5.0–11.1) in CD4 (p = 0.097). Median time from treatment initiation to switch was 11.7 months (7.7–19.4) in VL and 24.7 months (15.9–35.0) in CD4 (p = 0.001). The median duration of viremia >400 copies/ml at switch was 7.2 months (5.8–8.0) in VL versus 15.8 months (8.5–20.4) in CD4 (p = 0.002). FDO scores were not significantly different at time of switch. No adverse events related to the monitoring strategy were reported.Conclusions:
The 3-year rates of clinical failure and loss of treatment options did not differ between strategies although the longer-term consequences of CD4 monitoring would need to be investigated. These results provide reassurance to treatment programs currently based on CD4 monitoring as VL measurement becomes more affordable and feasible in resource-limited settings.Trial registration:
ClinicalTrials.gov NCT00162682
Please see later in the article for the Editors' SummaryIntroduction
Since the mid 1990s, highly active antiretroviral therapy (HAART) has radically modified AIDS prognosis by suppressing viral replication and thus allowing immune restoration [1]–[3]. Maximal and durable viral suppression is expected to impede the development of drug resistance and to lead to the restoration of immunological function, improvement of quality of life, and reduction of HIV-related morbidity, mortality, and transmission. Monitoring of viral load (VL) is central to this therapeutic approach and to national guidelines in most resource-rich settings and was recently recommended as part of the WHO 2013 consolidated guidelines [4],[5].
However, in low - and middle-income countries, with limited resources and restricted access to more costly second and third-line drugs, the utility of this approach is debated [6]–[8]. Moreover, a VL-based monitoring strategy may lead to more frequent treatment changes, limiting future drug options. Three randomized trials compared clinical monitoring with clinical-plus-laboratory monitoring in adult patients in sub-Saharan Africa [9]–[11], but none of them directly compared CD4-monitoring versus CD4 plus VL monitoring. We therefore designed this study to determine whether, in therapy-naïve patients, monitoring by VL is optimal for therapeutic decision making, or whether a CD4-based strategy would lead to non-inferior clinical outcomes. The purpose of the study was to test the non-inferiority of a CD4-based monitoring and switching strategy compared to the standard VL-based monitoring and switching strategy among antiretroviral (ARV)-naive adults treated with non-nucleotide reverse transcriptase inhibitor (NNRTI)-containing regimens in Thailand.
Methods
Trial Design
This was a multicenter, randomized, non-inferiority trial conducted in 21 public hospitals throughout Thailand (ClinicalTrials.gov NCT00162682). The primary objective was to compare the 3-y clinical outcomes of HIV-infected adults initiating HAART, followed according to a monitoring-switching strategy either based on CD4 cell count (CD4), or on VL. The secondary objectives were (i) to compare future ARV treatment options, taking into account the profile of resistance mutations; (ii) to assess virologic and immunologic responses by arm; and (iii) to evaluate the safety and tolerance of HAART.
Participants
Confirmed HIV-infected patients, 18 y or older, were eligible if they could be followed at a study site and provided written informed consent. Inclusion criteria included confirmed CD4-cell count of 50–250 cells/mm3 and absence of prior ARV therapy (except zidovudine during pregnancy or nevirapine during labor). Exclusion criteria included pregnancy, opportunistic infection or medical condition interfering with study participation, hepatitis B or C co-infection, or any of the following: hemoglobin <8.0 g/dl, neutrophil count <1,000 cells/mm3, alanine transaminase (ALT), aspartate aminotransferase (AST), or total bilirubin >5.0× upper limit of normal (ULN), serum creatinine >1.0× ULN, platelet count <50,000/mm3, pancreatic amylase >2.0× ULN, or total amylase >2.0× ULN plus symptoms of pancreatitis.
Randomization and Switching Criteria
After treatment initiation, participants were randomly assigned in blocks of four, stratified by site and CD4 level (±100 cells/mm3), to one of two monitoring-switching strategies: (1) VL arm: switch if confirmed decreased <1 log at 3 mo or confirmed VL above 400 copies/ml thereafter; (2) CD4 arm: switch if confirmed CD4 count declined >30% from peak value (defined as the highest average of two consecutive CD4 counts) unless CD4 remained >200 cells above baseline. Using a pseudorandom number generator (Mersenne twister), a statistician produced the randomization lists and encrypted them in a database before the initiation of the study. Only the study statisticians were allowed to access the randomization lists to maintain blinding of other research staff. Randomization was performed centrally at the study coordination center in Chiang Mai, by a research assistant. The arm assigned to each patient was disclosed to the site physician after randomization. Even though blinding was not feasible, clinicians who were responsible for enrolling and following-up with study participants were unaware of the VL values of patients randomized to the CD4 arm.
Follow-up
Patients were seen 2 wk after ARV initiation for a medical examination and blood chemistry evaluation, to ensure adherence and detect early toxicities. Patients were then seen monthly for clinical evaluation, adherence assessment by pill count and self-report questionnaire, safety and tolerance evaluation, and drug refill. Cotrimoxazole and fluconazole prophylaxis was provided per WHO guidelines [12]. Hematology, ALT, CD4 count, and pregnancy tests and VL were performed at enrollment and then every 3 mo. Creatinine, bilirubin, AST, glucose, triglycerides, cholesterol, and amylase were measured every 6 mo. In case of intolerance to one drug, that drug was replaced. Serious adverse events (as defined by the International Conference on Harmonization, Good Clinical Practices [ICH GCP]) were reported to the Ministry of Public Health. Adverse event grading was based on the Division of AIDS, NIAID Table [13]. Patients were monitored according to protocol until the last enrollee had been on study for 3 y.
Laboratory Assessments
Plasma HIV RNA was measured using the Cobas Amplicor HIV-1 Monitor kit (version 1.5, Roche Molecular Systems). HIV genotypic resistance was performed retrospectively for all patients who met the per-protocol switching criteria and had detectable VL on the last sample available before switch using ViroSeq HIV-1 Genotyping system version 2.0 (Applied Biosystems). Both were performed at the Faculty of Associated Medical Sciences, Chiang-Mai University and quality assured by the Virology Quality Assurance Proficiency Program (VQA). CD4 counts were measured using a flow cytometer at each hospital laboratory with quality control from the Center of Excellence for Flow Cytometry, Mahidol University, Bangkok, Thailand and the United Kingdom National External Quality Assessment Service (UKNEQAS). Resistance mutations were identified using the 2009 International AIDS Society (US) tables. Each mutation was assigned a penalty score derived from the Stanford HIVdb Sequence Analysis Programs (version 6.0.8) and ARV drug resistance was inferred by adding the penalty scores of each mutation.
Antiretroviral Treatment
Upon enrollment, participants initiated a regimen containing nevirapine or efavirenz, plus lamivudine with stavudine or zidovudine. From April 2006, tenofovir plus emtricitabine (Truvada) became available and was widely used in combination with efavirenz. When switching criteria were reached, a protease inhibitor (PI)-based regimen, usually indinavir/ritonavir or lopinavir/ritonavir, depending on availability, was provided. Before treatment switches, causes for viral rebound or immunological deterioration were investigated, with attention to adherence, toxicities, and co-infections. Drug changes within class or between classes for reasons of toxicity were not considered “protocol switches” in the analysis.
Outcomes
The primary endpoint was clinical failure defined as confirmed CD4 <50 cells/mm3, first or new AIDS-defining event, or death. An independent committee reviewed and classified all AIDS-defining events. The main secondary endpoint was the number of drugs remaining available for treatment at the time of switch (future drug options, denoted FDO), calculated from resistance mutations [14]. Two FDO scores were calculated: FDO-1 based on the number of drug classes with one or more drugs to which the virus was susceptible (NC) with extra credit (0.3) for full susceptibility in NRTI or PI classes; and FDO-2 calculated as NC + the number of drugs to which the virus was susceptible (ND) divided by the total number (19) of drugs available +1, i.e., NC + (ND/20). Other secondary endpoints were the rate of switch to second-line regimens per protocol criteria, virologic response (percent of subjects below 50 copies/ml at 3 y), immunological status (CD4 cell count at 3 y), and serious adverse events.
Sample Size
On the basis of a literature review at the time the study was planned, the Kaplan-Meier cumulative 3-y risk of clinical failure on VL monitoring was expected to be 5% per year, or 14% over 3 y [15]–[18]. For the primary analysis, a noninferiority margin (delta) of 7.4%, corresponding to a hazard ratio of 1.6, was considered acceptable in view of the expected benefit of the CD4 monitoring strategy. Using a one-sided confidence interval (CI) approach, a sample size of 304 evaluable patients per arm ensured 80% power to rule out a difference greater than delta. Assuming two interim analyses and 15% unevaluable, 350 patients per arm were required.
Statistical Methods
The primary analysis compared the CD4 versus VL arm based on the Kaplan-Meier estimates of clinical/immunologic failure at 36 mo. All randomized patients were included in the final intent to treat analysis. Participants who died, withdrew from the study, or were lost to follow-up (defined as those who missed all visits for over 6 mo and no contact) were included and data were censored at date of death or at last visit. Participants who completed the study schedule were censored on April 1, 2010. Distributions were compared using the Fisher exact test and Wilcoxon rank-sum test. Additional analyses studied baseline factors associated with clinical failure using Log rank tests and Cox proportional hazards models after testing that the Cox proportional hazards model assumptions were met (covariate effects not changing over time, and flat slope in the regression of time versus residuals).
Study Monitoring
In addition to the Data and Safety Monitoring Board (DSMB), a Resistance Experts Committee was constituted to provide expertise in support of the DSMB regarding resistance mutations and their clinical implications. Outcomes, safety and resistance profiles by arm, were presented during the two interim analyses.
The study protocol was approved by the Ethical Committees of the Thai Ministry of Public Health, Chiang Mai University Faculty of Associated Medical Sciences, Harvard School of Public Health Institutional Review Board, and local hospitals when applicable.
Results
From May 2005 to April 2007, 716 participants were recruited and randomized: 356 to the VL arm and 360 to the CD4 arm (Figure 1). All participants were included in the analysis. Baseline characteristics are provided in Table 1. The two arms were balanced with respect to all baseline characteristics except sex (58% female in the VL arm and 66% in the CD4 arm, p = 0.03). Initial regimens were efavirenz-based in 65% and nevirapine-based in 35% of participants; 66% were in combination with tenofovir-emtricitabine and 34% with zidovudine or stavudine plus lamivudine.
Fig. 1. Patients' disposition. 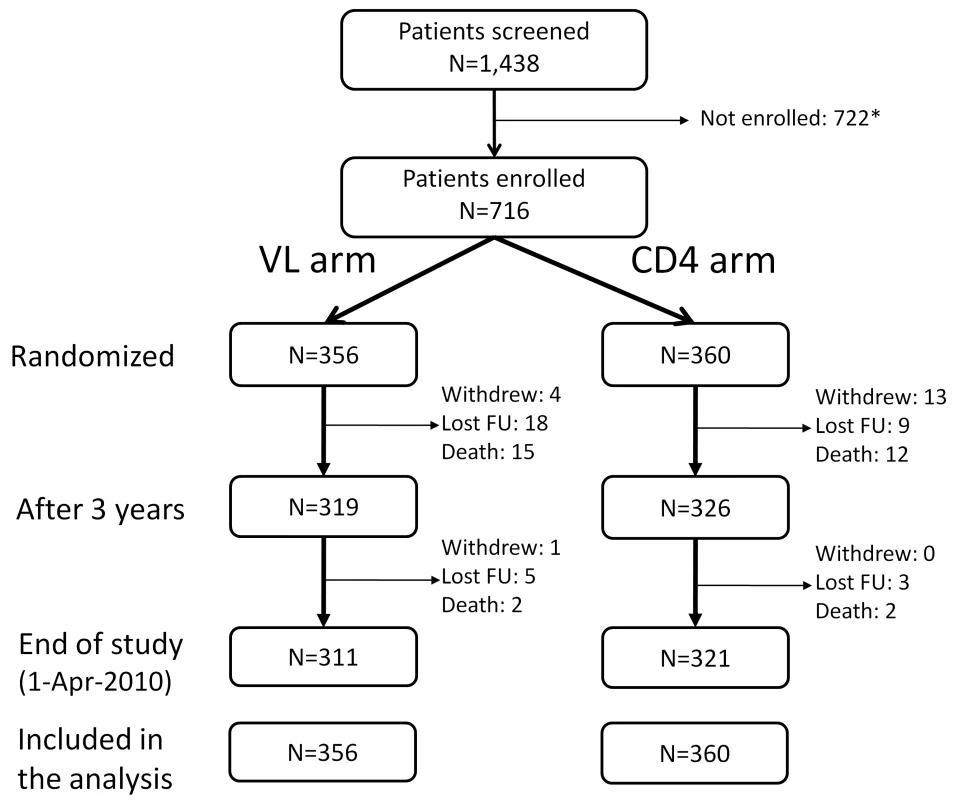
All patients randomized were included in the final analysis with patients who were lost to follow-up (FU), withdrew, or died considered as censored when last seen or at the time of death. *Reasons for not being enrolled: 158 subjects with CD4 <50 or >250 cells/mm3; 284 drop-out before enrollment (no information available for more than 2 mo); 101 with hepatitis B or C infection; 179 with other exclusion criteria such as pregnancy, opportunistic infections, or laboratory values outside the required ranges. 18 patients withdrew from the study: 11 because they moved to another region, five because they found the follow-up too frequent, and two because of side effects of the treatments. Tab. 1. Characteristics of the patients at baseline, according to randomization arm. 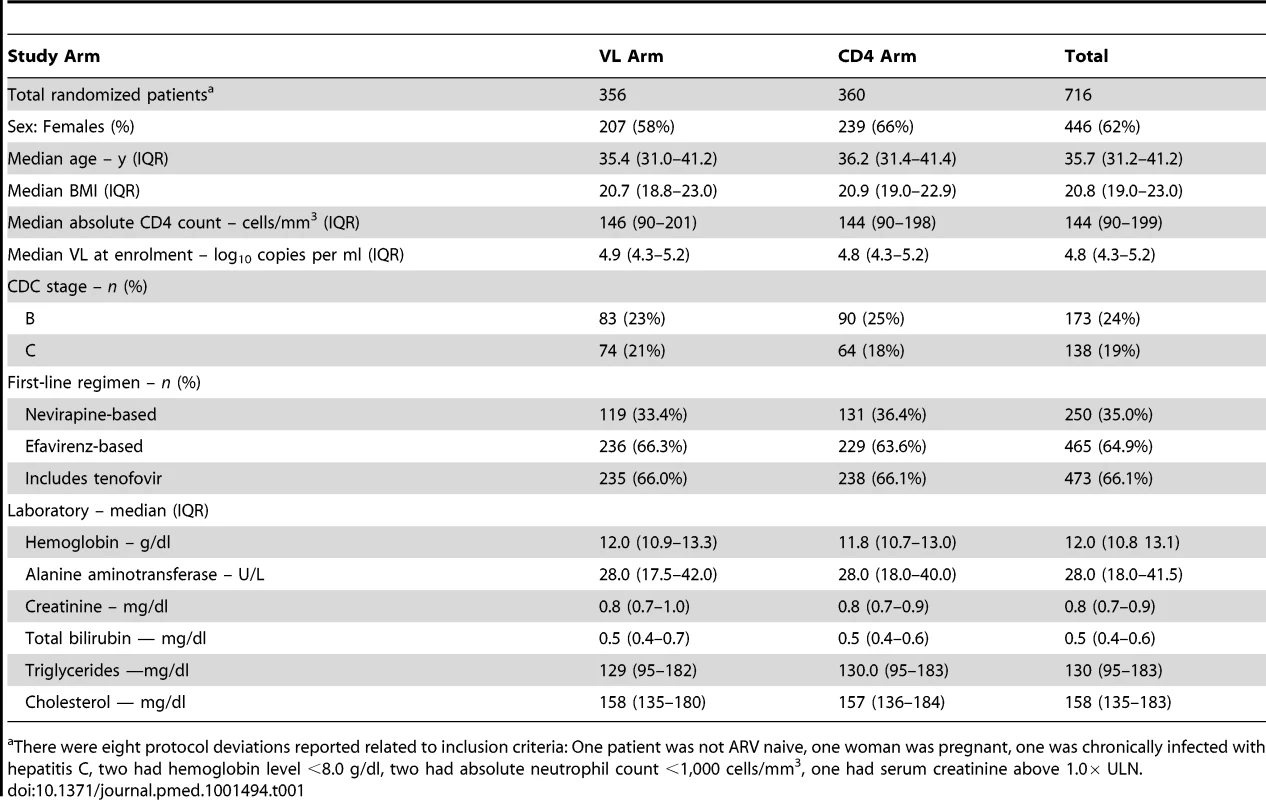
There were eight protocol deviations reported related to inclusion criteria: One patient was not ARV naive, one woman was pregnant, one was chronically infected with hepatitis C, two had hemoglobin level <8.0 g/dl, two had absolute neutrophil count <1,000 cells/mm3, one had serum creatinine above 1.0× ULN. The study was completed and unblinded on April 1, 2010, after the last-enrolled patient reached 3 y of follow-up. The DSMB recommended pursuit of the study as planned at the two interim analyses. At 3 y following enrollment, 27 patients had died, 27 were lost-to-follow up, and 18 had withdrawn (Figure 1), with 319 participants (90%) in VL and 326 (91%) in CD4 alive and on follow-up. There was no statistically significant difference between arms in terms of loss to follow-up or withdrawal (p = 0.63). At the end of study, after a median follow-up of 43.5 mo (interquartile range [IQR] 38.8–48.4), retention remained high (93.7%); the ARV regimens were PI-based in 14% of the participants (indinavir/ritonavir in 62%, lopinavir/ritonavir in 23%, saquinavir/ritonavir in 13%, and atanazavir/ritonavir in 2%), efavirenz-based in 64%, and nevirapine-based in 22% of participants; 76% were in combination with tenofovir-emtricitabine or -lamivudine.
Compliance to scheduled visits and adherence to therapy were both excellent with no difference between arms—4.2% of patients' visits (213 among a total of 5,020 patients' visits) with >15 d difference between scheduled and actual dates, and 7.3% (52/716) reporting >1 missed dose/week. Seventy-two patients experienced treatment interruption (64 patients one episode, six patients two, and two patients three). The reasons for the first interruptions were intolerance or toxicity in 48 cases (67%), self-interruption or missed visits in 22 cases (31%), and surgery in two cases. The median duration of the first interruption was 3.8 wk (IQR 1.3–8.6) with no difference between arms.
Primary Outcome: Clinical Failure
Overall, 58 patients (30 in the VL arm and 28 in the CD4 arm) reached the primary endpoint of clinical failure: three experienced a CD4 count decrease below 50 cells/mm3, 33 developed a new AIDS-defining event (including nine followed by death), and 22 died (Table 2). Nineteen of the 26 AIDS-defining events that occurred in the first 6 mo were classified as possibly related to immune reconstitution. The AIDS-defining events included 13 tuberculosis or mycobacterium infections (four fatal), seven cryptococcal diseases (one fatal), five sepsis (four fatal), four Pneumocystis jerovicii pneumonia, and four systemic Penicillium marneffei infections.
Tab. 2. Number of primary outcomes by arm. 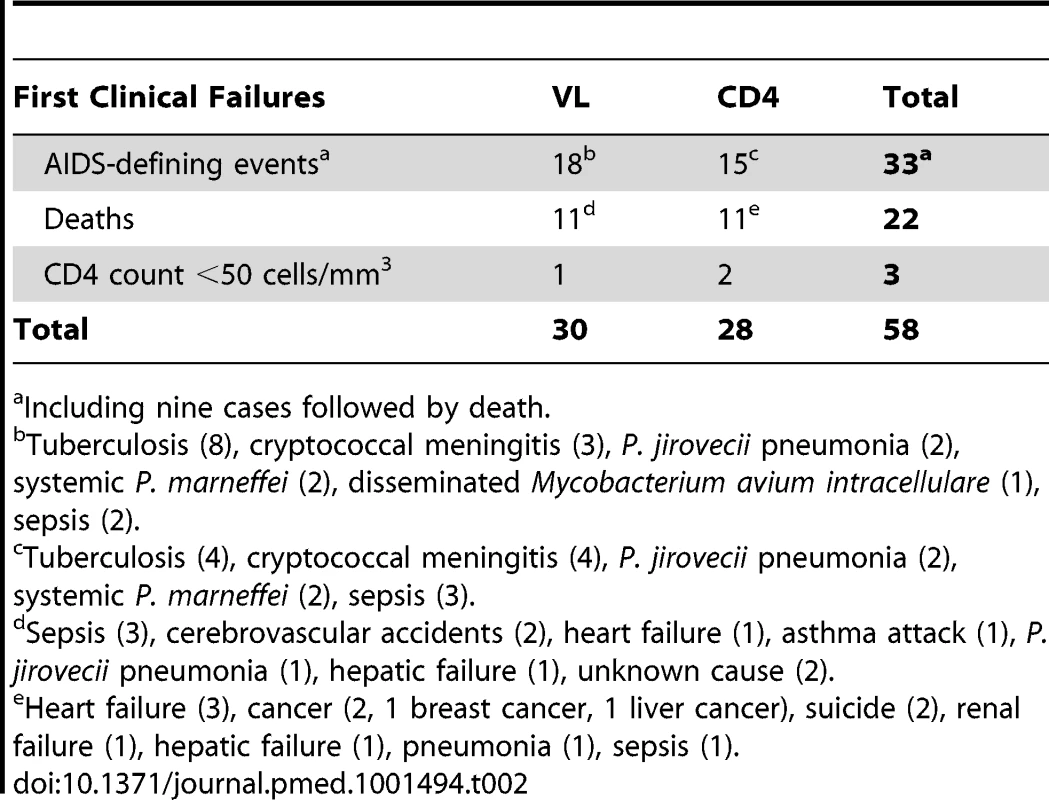
Including nine cases followed by death. The Kaplan-Meier risk of clinical failure at 3-y was not significantly different between the two arms: 8.0% (95% CI 5.6–11.4) in the VL versus 7.4% (5.1–10.7) in the CD4 arm (p = 0.74) (Figure 2; Table 3). The 95% CI of the difference, −0.6%, was −4.5% to 3.4%. The upper limit of this CI was below the pre-determined criterion for non-inferiority, 7.4%. The corresponding hazard ratio was 0.92, and its 95% CI was 0.55–1.53, also within the preset hazard ratio non-inferiority limit of 1.6. Nearly half of the failures occurred during the first 3 mo of therapy (15/30 and 11/28 in the VL and CD4 arms, respectively) before the tested monitoring strategies could have any clinical impact. At 3-y, the cumulative risk of death was 4.3% (2.6–7.1) versus 3.4% (2.0–6.0), respectively (p = 0.57).
Fig. 2. Kaplan Meier curve of occurrence of clinical failure. 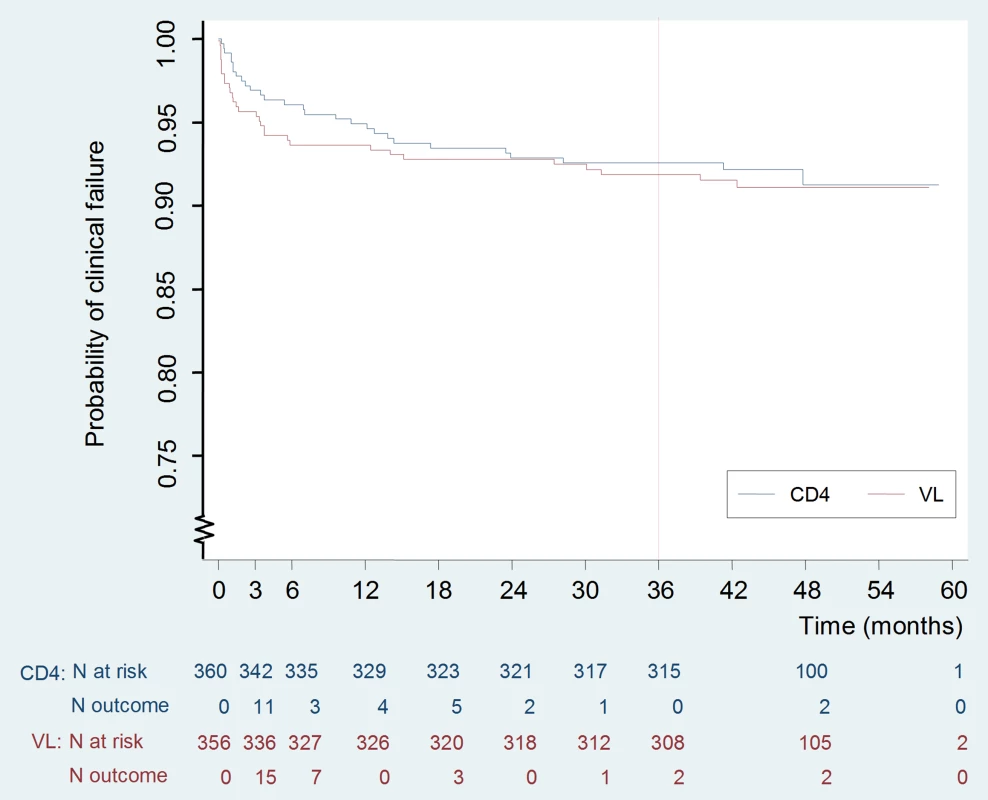
Tab. 3. Primary outcomes (clinical failure) at 3 y: death, new AIDS-defining events, or confirmed CD4 <50/mm3. 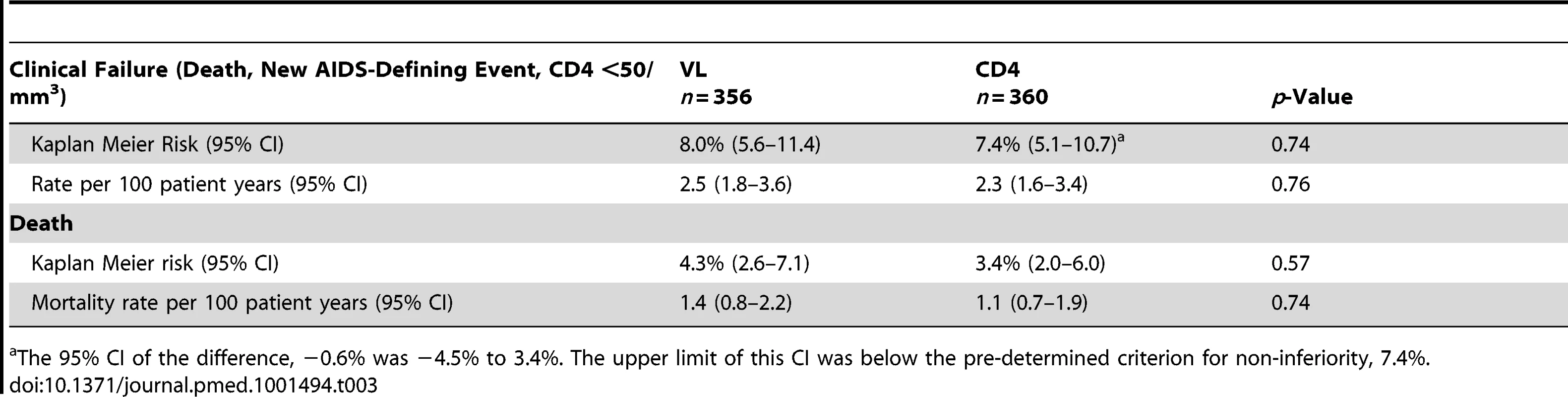
The 95% CI of the difference, −0.6% was −4.5% to 3.4%. The upper limit of this CI was below the pre-determined criterion for non-inferiority, 7.4%. Baseline factors associated with clinical failure by univariate analysis included US Centers for Disease Control and Prevention (CDC) stage B or C, anemia (hemoglobin ≤10 g/dl), CD4 count below median, i.e., 150 cells/mm3 (all p<0.001), VL above 5 log10 copies/ml (p = 0.001), and body mass index (BMI) below 18.5 (p = 0.002) (Table 4). Age, sex, initial ARV regimen, and switching strategy were not associated with the primary outcome. Upon multivariable analysis, factors independently associated with clinical failure were baseline anemia (p = 0.001), CDC stage B or C (p = 0.002), and low CD4 count (p = 0.04).
Tab. 4. Risk factors for clinical failure. 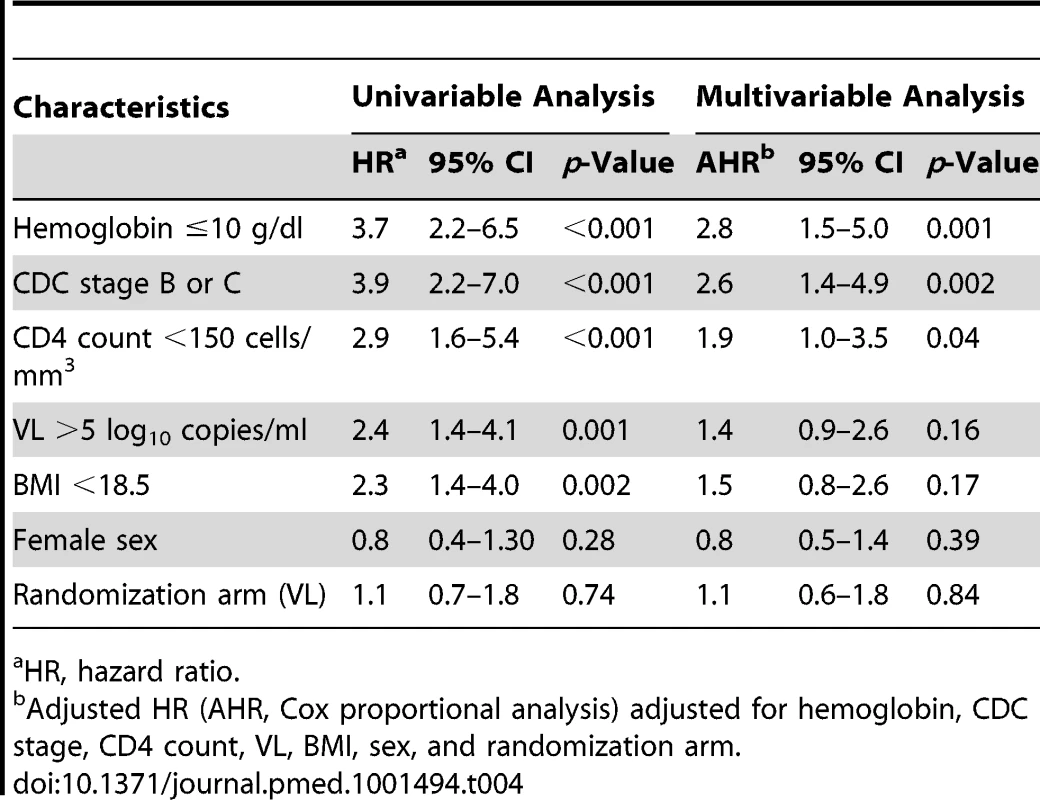
HR, hazard ratio. Secondary Outcomes
Switch for study criteria
Fifty of the 716 enrolled patients (14%) were switched to second-line regimens as per protocol criteria (19 in the VL arm and 31 in the CD4 arm). The 36-mo probabilities of switch for protocol were not significantly different, 5.2% (3.2–8.4) in VL versus 7.5% (5.0–11.1) in CD4, p = 0.10. However, the median time from enrollment to switch was significantly shorter in the VL arm (11.7 versus 24.7 mo, p = 0.001) (Table 5).
Tab. 5. Treatment switch to second-line, PI-based treatment. 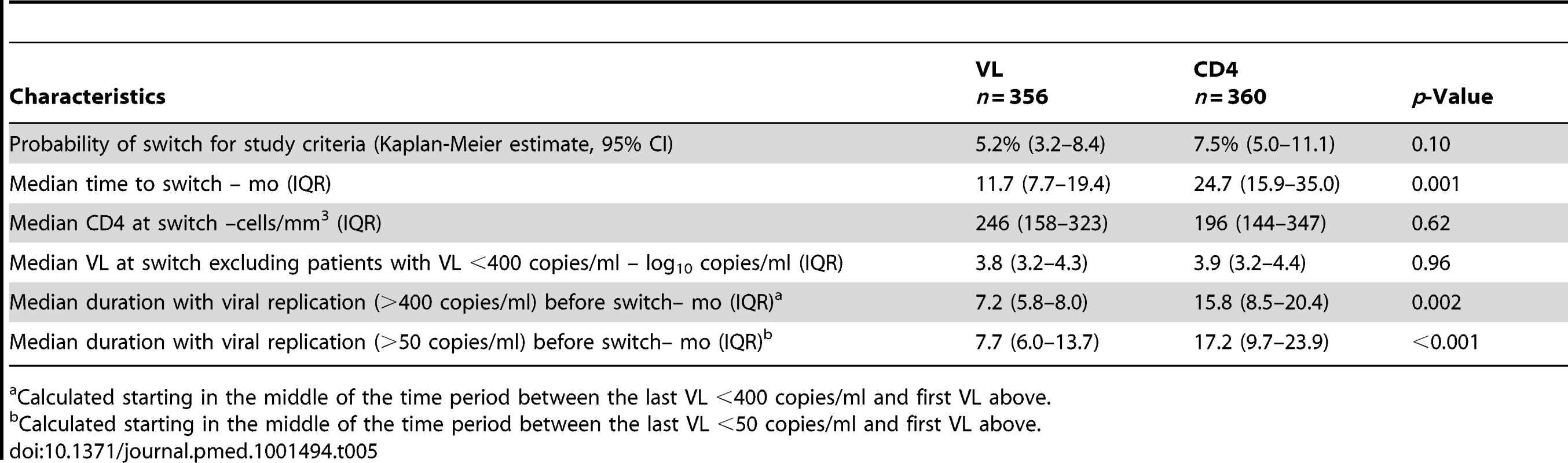
Calculated starting in the middle of the time period between the last VL <400 copies/ml and first VL above. Among the 31 patients who switched to a second-line regimen for protocol criteria in the CD4 arm, 15 had VL <400 copies/ml at time of switch. In six of these cases the CD4 count subsequently increased, in seven it remained stable, and in two it fell further. Of the 19 patients who switched for criteria in the VL arm, four would have switched earlier if the CD4 criteria had been used.
At the time of switch, median CD4 count and VL among those with VL >400 copies/ml were not different between arms (Table 5). However, patients had a significantly shorter median duration of viremia >400 copies/ml in the VL arm than in the CD4 arm. At the 50 copies/ml threshold, the difference was even greater.
Resistance and Future Drug Options
In the VL arm (Table 6), 18 of the 19 participants reaching switching criteria had interpretable genotyping results: 15 had at least one resistance mutation to NNRTI and 13 to NRTI, of whom two had at least three NRTI resistance mutations. In the CD4 arm (Table 7), all of the 16 participants reaching switching criteria with detectable VL had at least one resistance mutation to NNRTI and 13 to NRTI, of whom six had at least three NRTI resistance mutations. Thymidine analogue mutations (TAMs) were observed in two patients, but both had already one major NNRTI resistance mutation (Y181C) at baseline.
Tab. 6. Resistance mutations found at first ARV switch (VL arm). 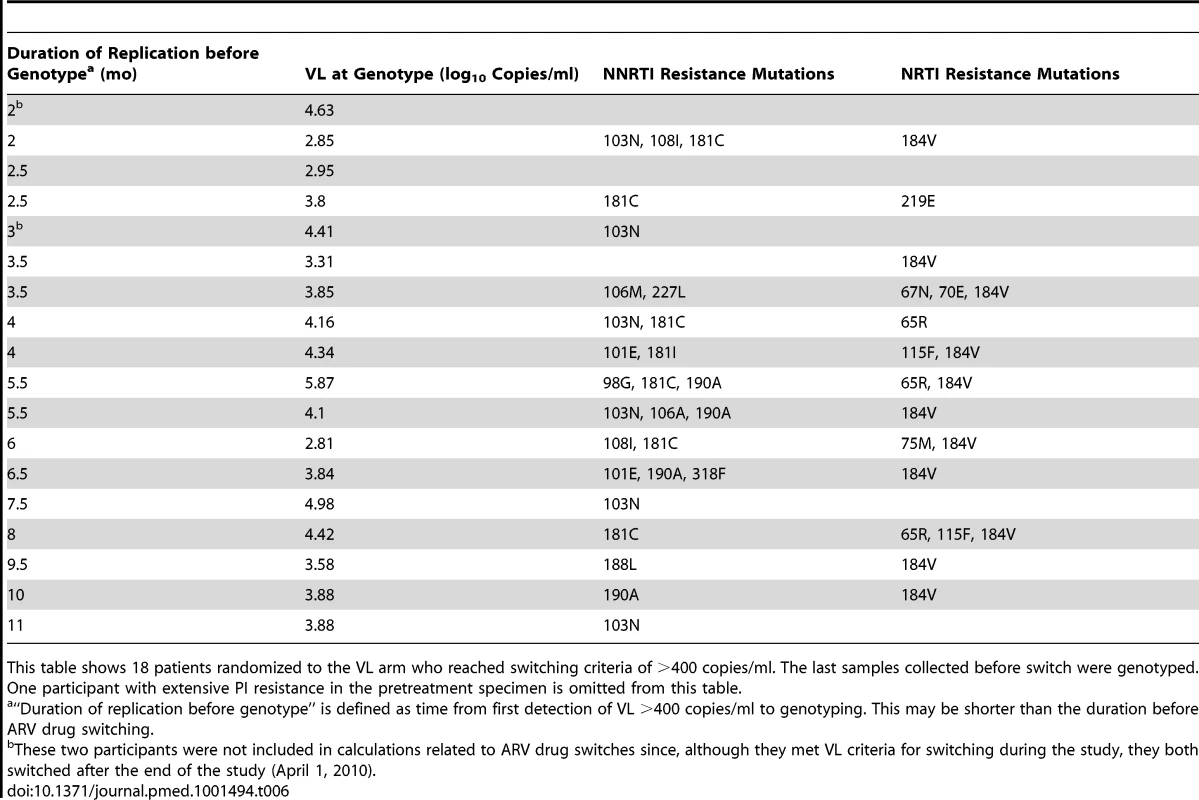
This table shows 18 patients randomized to the VL arm who reached switching criteria of >400 copies/ml. The last samples collected before switch were genotyped. One participant with extensive PI resistance in the pretreatment specimen is omitted from this table. Tab. 7. Resistance mutations found at first ARV switch (CD4 arm). 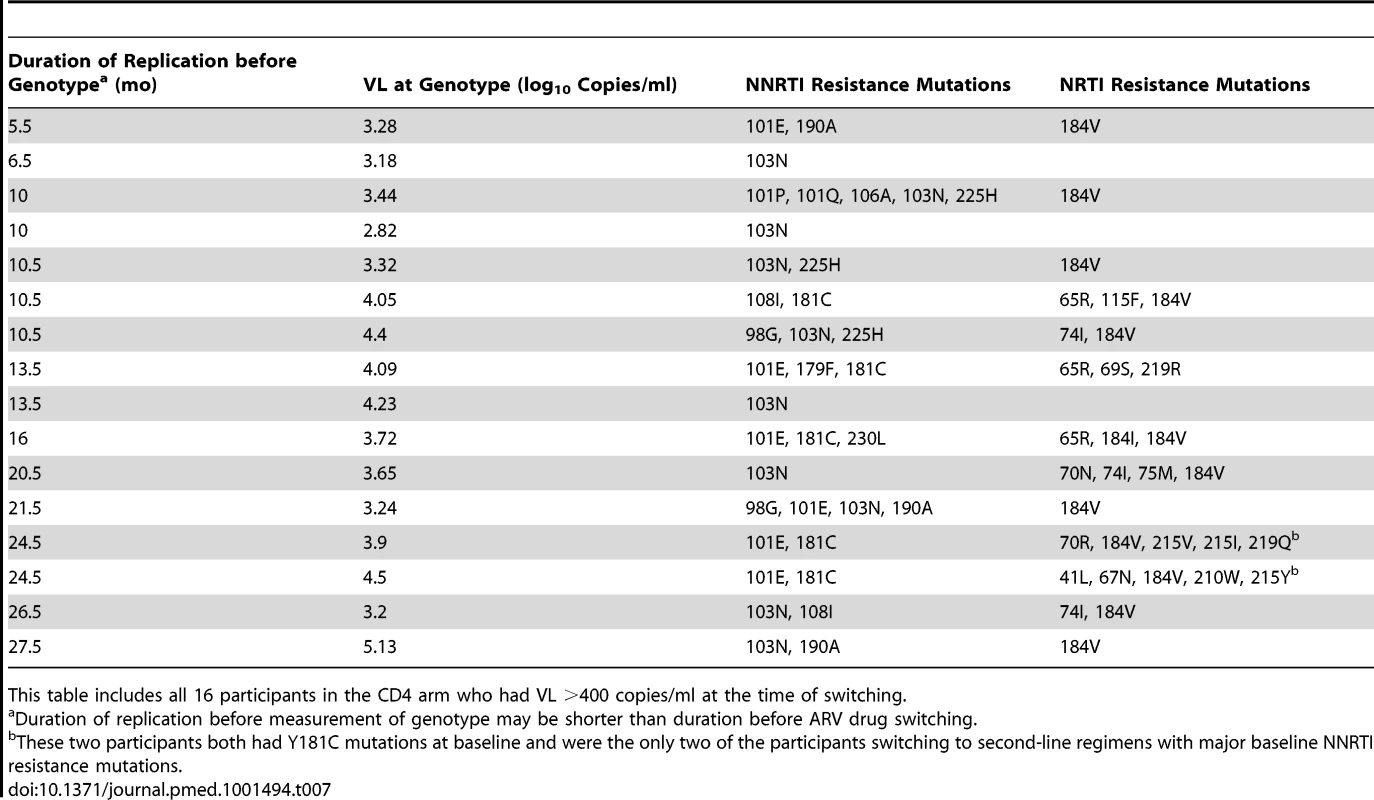
This table includes all 16 participants in the CD4 arm who had VL >400 copies/ml at the time of switching. FDO scores were comparable in the two study arms (Table 8). At the time of switching, FDO-1 and FDO-2 scores were slightly higher in the VL arm than in the CD4 arm. Scores at the end of the study were similar to those at the time of switch.
Tab. 8. Mean, 95% CI, median, and IQR of the Future Drug Options scores, by trial arm. 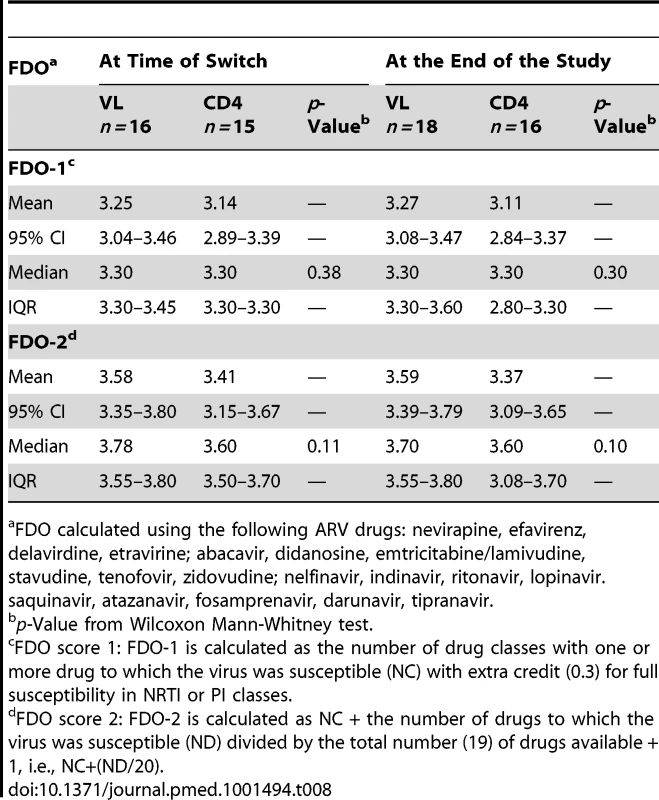
FDO calculated using the following ARV drugs: nevirapine, efavirenz, delavirdine, etravirine; abacavir, didanosine, emtricitabine/lamivudine, stavudine, tenofovir, zidovudine; nelfinavir, indinavir, ritonavir, lopinavir. saquinavir, atazanavir, fosamprenavir, darunavir, tipranavir. Virologic and Immunologic Responses at 3 y
At 3 y, the percentages of participants with VL <50 copies/ml were very high in both arms: 98.4% in the VL and 98.2% in CD4 arm. Similarly, the median CD4 cell counts at 3 y were high in both arms: 420 cells/mm3 (IQR 321–527) in VL and 426 (IQR 335–538) in the CD4 arm.
Toxicity and Safety
There were 335 serious adverse events reported during the study among 198 patients, with no difference between arms: 112 (33.4%) were possibly or probably related to HIV and 67 (20.0%) to ARV toxicity (Table 9). At 3 y, the probability of occurrence of a serious event was 31.8% in the VL-s arm versus 29.5% in the CD4-s arm (p = 0.76). A total of 151 grade 3 adverse events were observed in 92 participants (73 in 45 patients in the VL arm and 78 in 47 patients in the CD4 arm). One hundred and twenty seven grade 4 adverse events were reported, 56 in 52 patients in the VL arm and 72 in 57 patients in the CD4 arm. Fifty patients (19 in the VL and 31 in the CD4 arm) changed treatment from NNRTI-based to PI-based regimens because of toxicities, most of which were rashes (11 cases in the VL arm and ten in the CD4 arm) or hepatic enzyme abnormalities (12 cases in the VL arm and seven in the CD4 arm).
Tab. 9. Serious adverse events by trial arm. 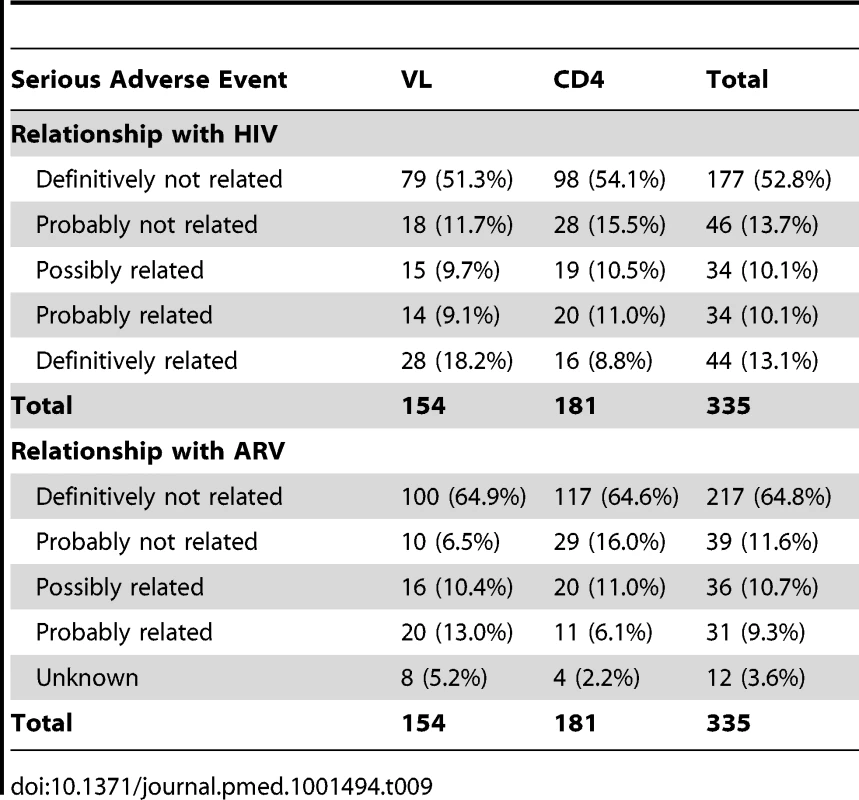
Discussion
In this randomized clinical trial of NNRTI-based HAART in an HIV-infected treatment-naïve population with 50 to 250 CD4/mm3, a CD4-based switching strategy was non-inferior in terms of clinical outcomes at 3 y of follow-up, compared to a reference VL-based switching strategy. Moreover, at study end there were no differences in terms of viral suppression and immune restoration between arms. Although the duration of detectable viral replication was longer in the CD4 arm, resistance testing showed similar profiles in the two arms.
Three other randomized monitoring trials conducted in sub-Saharan Africa have been published [9]–[11]. In all three the primary question was whether outcomes with clinical monitoring were as good as with clinical-plus-laboratory monitoring, using CD4 cells alone [11], or VL plus CD4 [9]. All three concluded that laboratory monitoring gave a better outcome. Only one study, the HBAC trial in Uganda, directly compared outcomes of CD4 - versus viral-load–based monitoring, as part of their secondary analysis [16]. Participants in that study had more advanced disease at entry than in our study, with higher risk of death and new AIDS defining events, and no difference in outcomes at 3 y of follow-up was also observed. A preliminary report from a cluster randomized trial in Zambia comparing the effect of routine VL testing to the standard of care in which VL is used sparingly to adjudicate discrepancies between CD4 and clinical assessments, showed that VL monitoring did not reduce mortality over the first 36 mo of ART but resulted in earlier regimen change [19]. One international data-base analysis found earlier failure or death in Zambia and Malawi where patients were monitored with CD4 only, in comparison to South Africa where VL measurements were available [20]. This result may also reflect differences in health care environment.
The health consequences of the longer duration of viral replication in the CD4 arm of the study are difficult to gauge. The exploratory AIDS Clinical Trials Group Study A5115 that compared switching at high versus low VL thresholds in a population with median CD4 concentration of 421 cells/mm3 also found no differences in total or activated CD4 cells or FDO scores at study end [21],[22]. The SMART treatment interruption study raised concerns, subsequently confirmed [23], that viral replication leads to more rapid immunologic deterioration and immune activation, increasing the risk of cardiovascular events, cancer, and hepatic dysfunction [24],[25]. No excess of these events was observed in the CD4 arm in our study, but the numbers were not large, ARV drugs were not discontinued with likely consequent partial control of viral replication, and CD4 cell numbers were preserved during viremia.
Although more switches were expected in the VL than in the CD4 arm, the opposite was observed. In the CD4 arm, 31 patients switched for study criteria but almost half of them (15) had VL <400 copies/ml at the time of switch. It is well known that a drop in CD4 cells does not necessarily correlate with virological failure [26]–[28], and while these switches might be viewed as unnecessary, they did not appear to do harm. On the other hand, they have economic implications since PI-based regimens are substantially more expensive. Another unexpected result was that there were as many changes to PI-based regimens for toxicity as for switching criteria. These treatment changes to more expensive regimens should also be considered at the programmatic level regardless of the monitoring strategy.
This study had several limitations. First, although all patients satisfied contemporary WHO criteria for starting ARV therapy, none had a CD4 cell count below 50 cells/mm3 by protocol design, and fewer than anticipated primary endpoints were reached, many of them in the first 3 mo of follow-up before the switching strategy could take effect. When we compared outcomes occurring only after 3 mo, similar results were found. Even though our results are consistent with the observations made in the HBAC study and indicate that, at least over 3–5 y, monitoring by CD4 or VL leads to essentially the same outcome, they must be interpreted with caution. The long term outcomes, including response to second-line treatment have not been thoroughly studied. While the FDO score did not differ between arms, six out of 16 patients in the CD4-monitoring arm developed ≥3 NRTI mutations, in contrast to two out of 18 participants in the VL-monitoring arm. From this finding, had the study continued longer, less optimal response to second-line treatment may be observed when monitoring with CD4 only. The fairly similar resistance patterns at failure may be related, at least in part, to the low barrier to mutation toward high-level resistance in NNRTIs and 3TC. The same NNRTI and 3TC resistance mutations were probably selected in both arms, with only thymidine analogue mutation (TAM) accumulation differing, perhaps because of longer duration of failure in the CD4 arm.
The generalizability of our findings to routine care settings must also be considered: participants in this trial were seen and counseled every month throughout the entire study. The overall rates of virologic failure and loss to follow-up were lower than those reported in other settings, most likely due to close follow up. It is also important to note that laboratory evaluations were performed every 3 mo rather than every 6 mo as recommended by WHO. It is not clear how less frequent monitoring would have affected the outcomes in this study.
Economic evaluations, mostly in sub-Saharan Africa, have generated conflicting results regarding the cost-effectiveness of VL and CD4 monitoring strategies [29]. The DART analysis concluded that no form of laboratory monitoring was cost-effective in Uganda and Zimbabwe [30], while the HBAC analysis considered CD4 monitoring “desirable clinically and economically” [31]. Both rejected VL monitoring as not cost-effective. However, published analyses do not take fully into account the wider benefit of VL monitoring in supporting adherence and thus preventing drug-resistance or in reducing HIV transmission [29]–[35]. A preliminary report by Keiser et al. indicated a substantial improvement in cost-effectiveness of VL when the effect on adherence and HIV transmission were considered [36]. Moreover, it is possible that newly developed, point of care VL tests would further reduce the cost and increase the feasibility of routine VL monitoring in many settings [37].
In summary, at 3 y, rates of clinical failure and loss of treatment options did not differ between the two monitoring strategies, although the longer-term consequences of CD4 monitoring are unknown. These findings confirm that access to life-saving ARV treatment should continue to be expanded even in settings without virological monitoring, and provide reassurance to treatment programs currently based on CD4 monitoring alone, as VL measurement becomes more affordable and feasible in resource-limited settings.
Supporting Information
Zdroje
1. EggerM, MayM, CheneG, PhillipsAN, LedergerberB, et al. (2002) Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 360 : 119–129.
2. ThompsonMA, AbergJA, CahnP, MontanerJS, RizzardiniG, et al. (2010) Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 304 : 321–333.
3. ART-CC (2008) Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 372 : 293–299.
4. Panel on Antiretroviral Guidelines for Adults and Adolescents (2011). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington (D.C.): Department of Health and Human Services. October 14, 2011. pp. 1–167.
5. World Health Organization (2013). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. June 2013. Geneva: WHO.
6. KoenigSP, KuritzkesDR, HirschMS, LeandreF, MukherjeeJS, et al. (2006) Monitoring HIV treatment in developing countries. BMJ 332 : 602–604.
7. KentDM, McGrathD, IoannidisJP, BennishML (2003) Suitable monitoring approaches to antiretroviral therapy in resource-poor settings: setting the research agenda. Clin Infect Dis 37: S13–24.
8. KumarasamyN, FlaniganTP, MahajanAP, CarpenterCC, MayerKH, et al. (2002) Monitoring HIV treatment in the developing world. Lancet Infect Dis 2 : 656–657.
9. LaurentC, KouanfackC, Laborde-BalenG, AghokengAF, MbouguaJB, et al. (2011) Monitoring of HIV viral loads, CD4 cell counts, and clinical assessments versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratall ANRS 12110/ESTHER): a randomised non-inferiority trial. Lancet Infect Dis 11 : 825–833.
10. MerminJ, EkwaruJP, WereW, DegermanR, BunnellR, et al. (2011) Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomised trial. BMJ 343: d6792.
11. MugyenyiP, WalkerAS, HakimJ, MunderiP, GibbDM, et al. (2010) Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet 375 : 123–131.
12. World Health Organization (2002) Scaling up antiretroviral therapy in resource-limited settings. Guidelines for a public health approach. Geneva: World Health Organization.
13. Division of AIDS table for grading the severity of adult and pediatric adverse events Version 1.0, December, 2004; clarification August 2009. Available: http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf. Accessed 8 February 2012.
14. JiangH, DeeksSG, KuritzkesDR, LallemantM, KatzensteinD, et al. (2003) Assessing resistance costs of antiretroviral therapies via measures of future drug options. J Infect Dis 188 : 1001–1008.
15. GrabarS, Le MoingV, GoujardC, LeportC, KazatchkineMD, et al. (2000) Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med 133 : 401–410.
16. HoggRS, YipB, ChanKJ, WoodE, CraibKJ, et al. (2001) Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 286 : 2568–2577.
17. JunghansC, LowN, ChanP, WitschiA, VernazzaP, et al. (1999) Uniform risk of clinical progression despite differences in utilization of highly active antiretroviral therapy: Swiss HIV Cohort Study. AIDS 13 : 2547–2554.
18. SterlingTR, ChaissonRE, MooreRD (2001) HIV-1 RNA, CD4 T-lymphocytes, and clinical response to highly active antiretroviral therapy. AIDS 15 : 2251–2257.
19. Saag M, Westfall A, Luhanga D, Mulenga P, Chi B, et al.. (2012) A cluster randomized trial of routine vs discretionary viral load monitoring among adults starting ART: Zambia. Abstract 87. 19th Conference on Retroviruses and Opportunistic Infections. Seattle (Washington), US.
20. KeiserO, ChiBH, GsponerT, BoulleA, OrrellC, et al. (2011) Outcomes of antiretroviral treatment in programmes with and without routine viral load monitoring in Southern Africa. AIDS 25 : 1761–1769.
21. RiddlerSA, JiangH, TenorioA, HuangH, KuritzkesDR, et al. (2007) A randomized study of antiviral medication switch at lower - versus higher-switch thresholds: AIDS Clinical Trials Group Study A5115. Antivir Ther 12 : 531–541.
22. TenorioAR, JiangH, ZhengY, BastowB, KuritzkesDR, et al. (2009) Delaying a treatment switch in antiretroviral-treated HIV type 1-infected patients with detectable drug-resistant viremia does not have a profound effect on immune parameters: AIDS Clinical Trials Group Study A5115. AIDS Res Hum Retroviruses 25 : 135–139.
23. ZhangS, van SighemA, KesselringA, GrasL, SmitC, et al. (2012) Episodes of HIV viremia and the risk of non-AIDS diseases in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr 60 : 265–272.
24. El-SadrWM, LundgrenJD, NeatonJD, GordinF, AbramsD, et al. (2006) CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 355 : 2283–2296.
25. KullerLH, TracyR, BellosoW, De WitS, DrummondF, et al. (2008) Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 5: e203 doi:10.1371/journal.pmed.0050203
26. KeiserO, MacPhailP, BoulleA, WoodR, SchechterM, et al. (2009) Accuracy of WHO CD4 cell count criteria for virological failure of antiretroviral therapy. Trop Med Int Health 14 : 1220–1225.
27. MeeP, FieldingKL, CharalambousS, ChurchyardGJ, GrantAD (2008) Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. AIDS 22 : 1971–1977.
28. RawizzaHE, ChaplinB, MeloniST, EisenG, RaoT, et al. (2011) Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis 53 : 1283–1290.
29. WalenskyRP, CiaranelloAL, ParkJE, FreedbergKA (2010) Cost-effectiveness of laboratory monitoring in sub-Saharan Africa: a review of the current literature. Clin Infect Dis 51 : 85–92.
30. Medina LaraA, KigoziJ, AmurwonJ, MuchabaiwaL, Nyanzi WakaholiB, et al. (2012) Cost effectiveness analysis of clinically driven versus routine laboratory monitoring of antiretroviral therapy in Uganda and Zimbabwe. PLoS ONE 7: e33672 doi:10.1371/journal.pone.0033672
31. KahnJG, MarseilleE, MooreD, BunnellR, WereW, et al. (2011) CD4 cell count and viral load monitoring in patients undergoing antiretroviral therapy in Uganda: cost effectiveness study. BMJ 343: d6884.
32. BraithwaiteRS, NuciforaKA, YiannoutsosCT, MusickB, KimaiyoS, et al. (2011) Alternative antiretroviral monitoring strategies for HIV-infected patients in east Africa: opportunities to save more lives? J Int AIDS Soc 14 : 38.
33. CohenMS, ChenYQ, McCauleyM, GambleT, HosseinipourMC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365 : 493–505.
34. KimmelAD, WeinsteinMC, AnglaretX, GoldieSJ, LosinaE, et al. (2010) Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness. J Acquir Immune Defic Syndr 54 : 258–268.
35. PhillipsAN, GilksC, LundgrenJD (2009) Cost-effectiveness of strategies for monitoring the response to antiretroviral therapy in resource-limited settings. Arch Intern Med 169 : 904; author reply 904–905.
36. Keiser O, Estill J, Egger M (2012) Viral load versus CD4 monitoring - impact on mortality, transmission and cost-effectiveness. MSF-UNITAID Co-Hosted Satellite Event, XIX International AIDS Conference. Washington (District of Columbia).
37. Murtagh M (2011) HIV/AIDS Diagnostic Landscape. UNITAID technical report. Geneva: UNITAID.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 8- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Better Reporting of Scientific Studies: Why It Matters
- Towards Universal Voluntary HIV Testing and Counselling: A Systematic Review and Meta-Analysis of Community-Based Approaches
- Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non-Inferiority Trial in Thailand
- Public Engagement in Health Priority Setting in Low- and Middle-Income Countries: Current Trends and Considerations for Policy
- Expanding Disease Definitions in Guidelines and Expert Panel Ties to Industry: A Cross-sectional Study of Common Conditions in the United States
- Pediatric AIDS in the Elimination Agenda
- Country Contextualization of the Mental Health Gap Action Programme Intervention Guide: A Case Study from Nigeria
- Reproductive and Maternal Health in the Post-2015 Era: Cervical Cancer Must Be a Priority
- Effect of Household-Based Drinking Water Chlorination on Diarrhoea among Children under Five in Orissa, India: A Double-Blind Randomised Placebo-Controlled Trial
- First Diagnosis and Management of Incontinence in Older People with and without Dementia in Primary Care: A Cohort Study Using The Health Improvement Network Primary Care Database
- Risk of Early-Onset Neonatal Infection with Maternal Infection or Colonization: A Global Systematic Review and Meta-Analysis
- Inclusion of Ethical Issues in Dementia Guidelines: A Thematic Text Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non-Inferiority Trial in Thailand
- Risk of Early-Onset Neonatal Infection with Maternal Infection or Colonization: A Global Systematic Review and Meta-Analysis
- Inclusion of Ethical Issues in Dementia Guidelines: A Thematic Text Analysis
- Country Contextualization of the Mental Health Gap Action Programme Intervention Guide: A Case Study from Nigeria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



