-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Reproductive and Maternal Health in the Post-2015 Era: Cervical Cancer Must Be a Priority
article has not abstract
Published in the journal: . PLoS Med 10(8): e32767. doi:10.1371/journal.pmed.1001499
Category: Essay
doi: https://doi.org/10.1371/journal.pmed.1001499Summary
article has not abstract
Summary Points
-
Over the last two decades, there has been unprecedented global action on tackling high maternal mortality rates in low - and middle-income countries (LMICs) and on expanding access in these countries to reproductive health interventions, including family planning.
-
The attention has paid enormous dividends: from 1990 to 2010, the annual number of maternal deaths worldwide fell from 546,000 to 287,000. While this positive trend is cause for celebration, we also believe that the global health community is failing women in a crucial way: it has neglected prevention, screening, and treatment for cervical cancer in LMICs.
-
Such neglect is difficult to understand. Unlike some other cancers, including selected reproductive cancers, cervical cancer is detectable, highly preventable, and curable if detected early.
-
There is an enormous international disparity in the incidence of, and survival from, cervical cancer, which are both also closely aligned with country income.
-
The incidence of cervical cancer is 52.8 per 100,000 women in sub-Saharan Africa compared to 6.8 per 100,000 women in Western countries. Women are more likely to die in low-resource settings due to lack of infrastructure for screening.
-
In this Essay, we propose four arguments for why cervical cancer screening and treatment should be included when it comes to operationalizing these two goals and thus to improving reproductive and maternal health outcomes. Each of the four arguments is illustrative of a larger framework that has equity and socioeconomic, gender, public health, and health services dimensions.
Over the last two decades, there has been unprecedented global action on tackling high maternal mortality rates in low - and middle-income countries (LMICs) and on expanding access in these countries to reproductive health interventions, including family planning [1]. Four major focusing events were particularly important in mobilizing awareness and funding. First, the International Conference on Population and Development, held in Cairo in 1994, provided the foundation for a comprehensive approach to women's health [2]. Second, in 2000, all 193 United Nations member states agreed to adopt Millennium Development Goal 5, which has both a maternal health and a reproductive health target. Target 5A is to reduce the maternal mortality ratio by three-quarters between 1990 and 2015, and Target 5B is to achieve “universal access to reproductive health” by 2015 [3]. Third, in 2010 the United Nations launched the Global Strategy for Women's and Children's Health, aimed at mobilizing US$40 billion to save the lives of 16 billion women and children over five years [4]. Finally, in July 2012, at the London Summit on Family Planning, donors pledged US$2.6 billion to scale up contraception [5], an important commitment given that about a third of all maternal deaths could be averted by scaling up comprehensive family planning services [4].
The attention has paid enormous dividends: from 1990 to 2010, the annual number of maternal deaths worldwide fell from 546,000 to 287,000 [6]. While this positive trend is cause for celebration, we also believe that the global health community is failing women in a crucial way: it has neglected prevention, screening, and treatment for cervical cancer in LMICs.
Such neglect is difficult to understand. Unlike some other cancers, including selected reproductive cancers, cervical cancer is detectable, highly preventable, and curable if detected early.
There is an enormous international disparity in the incidence of and rate of survival from cervical cancer, which are both also closely aligned with country income [7]. The incidence of cervical cancer is 52.8 per 100,000 women in sub-Saharan Africa, compared to 6.8 per 100,000 women in Western countries [8]. Women are more likely to die in low-resource settings because of lack of infrastructure for screening.
With the 2015 Millennium Development Goals deadline rapidly approaching, the international community is currently debating a “post-2015 development agenda,” which we believe should include taking global action on cervical cancer. The May 2013 report of the High-Level Panel of Eminent Persons on the Post-2015 Development Agenda recommends that there should be a single universal health goal (Goal 4) [9]. This goal would include reducing maternal mortality, ensuring “universal sexual and reproductive health and rights,” and reducing the burden of infectious diseases and “priority non-communicable diseases.” The panel also recommends a global goal on empowering girls and women and achieving gender equality (Goal 2).
In this Essay, we propose four arguments for why cervical cancer screening and treatment should be included when it comes to operationalizing these two goals and thus to improving reproductive and maternal health outcomes. Each of the four arguments is illustrative of a larger framework that has equity and socioeconomic, gender, public health, and health services dimensions (Figure 1). While we focus specifically on cervical cancer, we acknowledge that women in LMICs face a high burden of other cancers, such as breast and ovarian cancers.
Fig. 1. Cervical cancer and the post-2015 agenda: a multidimensional framework. 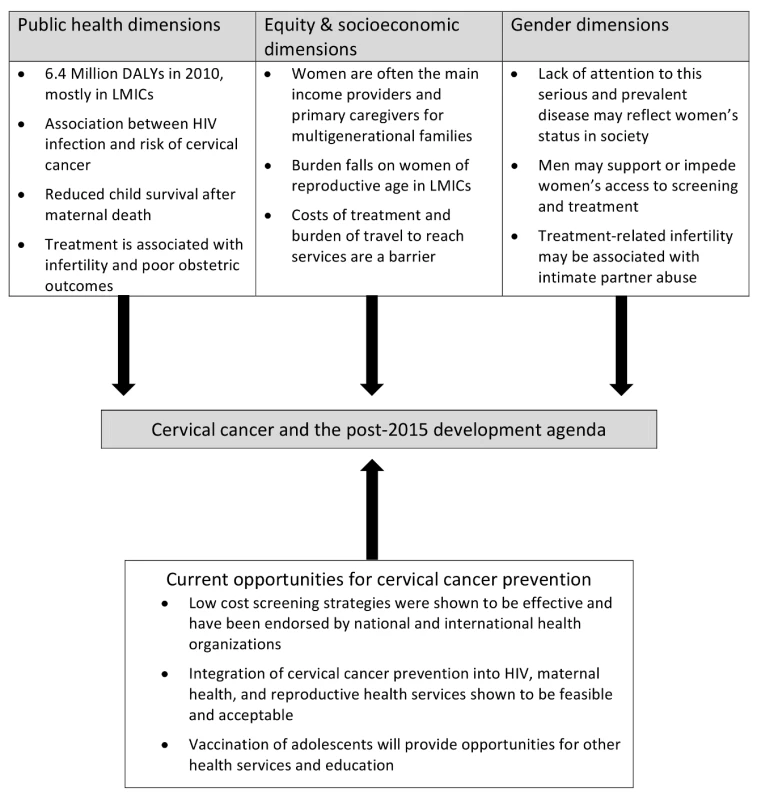
The Burden Falls on Women of Reproductive Age
Our first argument is that the enormous global burden of cervical cancer falls mostly on women of reproductive age in LMICs. Each year, over half a million women globally are diagnosed with cervical cancer, and just over a quarter of a million die of the disease [8]. About nine in ten of these deaths occur in LMICs, where the mortality rate is 85% [10]. In many LMICs, women of reproductive age are the main income providers and primary caregivers for children and elderly relatives, and so these deaths have profound economic and social consequences.
Early maternal deaths from cervical cancer have intergenerational consequences for children, since children who lose their mothers are at higher risk of developmental delays and other poor health outcomes [11]–[13]. Two recent studies conducted in Bangladesh showed the detrimental impact of maternal death on child survival. The first study found that about six in ten children born shortly before the mother's death died in the first 90 months after her death [14]. The second found that children whose mothers die are three times less likely to survive to their tenth birthday compared to those with living mothers [15]. Maternal deaths also place an increased burden on extended family members, who often end up caring for the children.
In addition to the mortality consequences, the morbidity consequences of cervical cancer can profoundly affect a woman's quality of life. Women living with late stage cervical cancer may experience irregular bleeding, back pain, pelvic pain, fatigue, leg swelling, loss of appetite, and weight loss [16]. Early death due to cervical cancer and years affected by disease-related disability contributed to the loss of 6.4 million disability-adjusted life years (DALYs) globally in 2010, mostly in LMICs [17].
This burden of death and disability falls particularly heavily on sub-Saharan Africa, a situation related not only to the lack of preventive and treatment services, but also to the high HIV prevalence rate. HIV-infected women are at higher risk of developing cervical precancer and invasive cancer than uninfected women [18],[19].
The vast majority of women with cervical cancer in LMICs present to care when they have advanced disease. These women need the kind of treatment or palliative care only available at a major medical center [20]. For women in rural settings, the nearest tertiary care center can be located hundreds of miles away. In addition to the hospital fees, the costs of travel and lodging mean that poor women may not be able to afford treatment [21].
Association with Reproductive Capacity
A second reason why cervical cancer should be central to Goal 4 of the post-2015 development agenda is the association between treatment for the disease and a woman's ability to conceive and deliver a healthy child. Treatments for cervical precancer and cancer are associated with increased risk of infertility and poor obstetric outcomes, including preterm delivery, low birth weight, and premature rupture of the membranes [22],[23]. Outpatient treatments can also lead to cervical stenosis in a small number of women, making pregnancy more difficult to achieve [24].
When available, the appropriate treatment for invasive cervical cancer includes a hysterectomy or radiation with chemotherapy [25], both of which render women infertile. Although fertility-sparing options have been explored in higher-income countries, they are limited to the small proportion of women with early invasive cancer, they require oncologic surgeons with specialized training and intensive follow-up, and they can result in poor obstetric outcomes [26],[27]. Such treatments are not widely available in most LMICs [27].
Infertility, a result of definitive treatment for cervical cancer, is associated with a high risk of familial and intimate partner abuse, depression, anxiety, and stigma [28]–[30]. The stigma of infertility may have an additional gendered dimension. In southern Ghana, for example, about two-thirds of women in one study reported feeling anxious and stigmatized by infertility, which included a fear of being expelled from the home by their husbands [31].
Cancer Prevention Can Be Integrated into HIV, Maternal Health, or Reproductive Health Services
There is growing evidence of the feasibility of integrating cervical cancer prevention into HIV, maternal health, or reproductive health services using low-cost screening strategies coupled with treatment for precancerous lesions [32]. Conventional screening methods, using Pap smears and biopsies, require infrastructure and clinical expertise and are hard to scale up in LMICs. But simpler, cheaper screening techniques, such as visual inspection with acetic acid (VIA) and human papillomavirus (HPV) DNA testing, hold great promise and are undergoing widespread evaluation [33].
The World Health Organization global action plan on noncommunicable diseases describes screening with VIA as a “best buy,” meaning that it is both highly cost-effective (i.e., it costs less than the per capita gross domestic product to avert one DALY) and feasible to implement in settings with constrained health systems [34]. There are promising results from large trials suggesting that VIA can reduce cervical cancer incidence by 25%–30% [35],[36]. Although screening with HPV DNA testing is more expensive than with VIA, a study by Goldie and colleagues in five LMICs found that HPV DNA screening is very cost-effective, and a single test at age 35 years reduces lifetime cancer risk by 25%–36% [37]. Integrating screening into primary care services for women should increase the likelihood that precancer is detected, as is seen in high-income countries, where effective screening averts progression to cervical cancer.
Integrating care for HIV, sexual health, reproductive health, and maternal health has been shown to improve uptake of services, reduce HIV-related stigma, and improve the quality of care received by women [38],[39]. A recent study in western Kenya showed that it was feasible to integrate cervical cancer screening into HIV outpatient clinics [40].
Furthermore, integrating cervical cancer prevention services into primary care facilities provides an opportunity to include and educate male partners, which may be particularly important in regions where men have control over health care decisions [41],[42].
HPV Vaccination Can Protect Girls from a Fatal Disease
Finally, almost all women with cervical cancer are infected with HPV. The World Health Organization has approved two HPV vaccines that could dramatically reduce cervical cancer deaths in LMICs if vaccination coverage can be scaled up [43]. A recent multinational trial showed that the vaccine can reduce precancerous lesions by up to 90% [44].
Ensuring that adolescent girls have the opportunity to receive a vaccine that protects them from death, infertility, and other morbidity related to cervical cancer, should be a key global health priority. Vaccination of adolescent girls also provides an opportunity to provide them with other reproductive health services and health education (including education on family planning and menstrual hygiene).
Next Steps
Including cervical cancer in the post-2015 agenda would give the disease the policy priority that it deserves, and could help to attract greater domestic and international donor funding. Lowering the burden of cervical cancer in LMICs will not happen by chance—it requires national and international leadership, attention, and resource mobilization to roll out primary disease prevention through HPV vaccination programs, and secondary prevention through low-cost screening strategies. Rwanda is a good example of how prioritizing cervical cancer at the national level, mobilizing key actors (public, private, and international), and working across multiple sectors (including education) are effective in rolling out HPV vaccination [43]. The country's national campaign to vaccinate school girls achieved over 90% coverage rates.
An important step forward is that primary prevention through scaled up HPV vaccination will be rolled out in eight LMICs in 2013 (Ghana, Kenya, Madagascar, Malawi, Niger, Sierra Leone, Tanzania, and Lao People's Democratic Republic) through the support of the GAVI Alliance [45]. Julio Frenk, former Minister of Health of Mexico, and current dean of the Harvard School of Public Health, has argued that the GAVI Alliance's decision to include HPV vaccine in its financing portfolio is “a visionary investment that will improve the health of girls and women, equity, and development.”
The GAVI Alliance announced on May 9, 2013, that the world's poorest countries will be able to obtain, with its support, HPV vaccine for as low as US$4.50 per dose through the United Nations Children's Fund Supply Division [45]. While this low price is clearly good news, modeling by Goldie and colleagues in 72 countries eligible for this GAVI Alliance–supported program suggested that the intervention would become very cost-effective only at a price of about US$2 per dose [46]. Eventually, we look forward to an era in which all girls and boys are offered the public health benefits of HPV vaccination.
The health of women and girls must continue to be prioritized in the post-2015 development agenda. Assuming that the maternal mortality rate continues to fall, we are likely to see a continuing rise in chronic diseases in women in LMICs, including cancers. Thus, the post-2015 agenda needs to include a concerted global plan to curb the “cancer crisis” of developing countries. For cervical cancer, we fortunately now have a wide range of feasible, affordable, and effective prevention options, which make dramatic global reductions in cervical cancer incidence a realistic goal in our lifetime.
Zdroje
1. ShiffmanJ (2007) Generating political priority for maternal mortality reduction in 5 developing countries. Am J Public Health 97 : 796–803.
2. El FekiS (2004) The birth of reproductive health: a difficult delivery. PLoS Med 1: e9 doi:10.1371/journal.pmed.0010009
3. Millennium Development Goals (2013) Goal 5: improve maternal health. Available: http://www.un.org/millenniumgoals/maternal.shtml. Accessed 17 June 2013.
4. United Nations Secretary-General Ban Ki-moon (2010) Global strategy for women's and children's health. Geneva: Partnership for Maternal, Newborn and Child Health. Available: http://www.who.int/pmnch/topics/maternal/201009_globalstrategy_wch/en/. Accessed 17 June 2013.
5. London Summit on Family Planning (2012) New financial commitments by donors and private sector at the London Summit on Family Planning (USD millions). Available: http://www.londonfamilyplanningsummit.co.uk/1530%20CommitmentSummary_Final_.pdf. Accessed 17 June 2013.
6. Maternal Mortality Estimation Inter-Agency Group (2012) Trends in maternal mortality 1990 to 2010: WHO, UNICEF, UNFPA, and the World Bank estimates. Geneva: World Health Organization.
7. FarmerP, FrenkJ, KnaulFM, ShulmanLN, AlleyneG, et al. (2012) Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet 376 : 1186–1193.
8. Globocan 2008 (2013) Fast stats: most frequent cancers—men, women, both sexes, summary statistics. Available: http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=900. Accessed 17 June 2013.
9. High-Level Panel of Eminent Persons on the Post-2015 Development Agenda (2013) A new global partnership: eradicate povery and transform economies through sustainable development. Available: http://www.post2015hlp.org/wp-content/uploads/2013/05/UN-Report.pdf. Accessed 7 July 2013.
10. United Nations Population Fund (2011) Comprehensive cervical cancer and control: programme guidelines for countries. Available: http://www.unfpa.org/webdav/site/global/shared/ENGLISH - Cervical Cancer Guidance.pdf. Accessed 17 June 2013.
11. World Health Organization (2009) Women and health: today's evidence tomorrow's agenda. Geneva: World Health Organization. Available: http://whqlibdoc.who.int/publications/2009/9789241563857_eng.pdf. Accessed 17 June 2013.
12. ClarkSJ, KahnK, HouleB, ArtecheA, CollinsonMA, et al. (2013) Young children's probability of dying before and after their mother's death: a rural South African population-based surveillance study. PLoS Med 17: e1001409 doi:10.1371/journal.pmed.1001409
13. KalibalaS, SchenkKD, WeissDC, ElsonL (2012) Examining dimensions of vulnerability among children in Uganda. Psychol Health Med 17 : 295–310.
14. Razzaque A, Akhtar H, DaVanzo J, Enamul Hoque M, Nurul A, et al.. (2012) Effect of maternal mortality on survival of under-five children: evidence from Matlab, Bangladesh. Available: http://paa2012.princeton.edu/papers/120545. Accessed 17 June 2013.
15. RonsmansC, ChowdhuryME, DasguptaSK, AhmedA, KoblinskyM (2010) Effect of parent's death on child survival in rural Bangladesh: a cohort study. Lancet 375 : 2024–2031.
16. JonesSC, JohnsonK (2012) Women's awareness of cancer symptoms: a review of the literature. Womens Health (Lond Engl) 8 : 579–591.
17. MurrayCJ, VosT, LozanoR, NaghaviM, FlaxmanAD, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 380 : 2197–2223.
18. AbrahamAG, StricklerHD, JingY, GangeSJ, SterlingTR (2012) Invasive cervical cancer risk among HIV-infected women: North American multi-cohort collaboration prospective study. J Acquir Immune Defic Syndr E-pub ahead of print. doi:10.1097/QAI.0b013e31828177d7
19. PhelpsRM, SmithDK, HeiligCM, GardnerLI, CarpenterCC, et al. (2001) Cancer incidence in women with or at risk for HIV. Int J Cancer 94 : 753–757.
20. World Health Organization (2013) WHO guidance note: comprehensive cervical cancer prevention and control: a healthier future for girls and women. Geneva: World Health Organization. Available: http://apps.who.int/iris/bitstream/10665/78128/3/9789241505147_eng.pdf. Accessed 17 June 2013.
21. McKinnonB, HarperS, MooreS (2011) Decomposing income-related inequality in cervical screening in 67 countries. Int J Public Health 56 : 139–152.
22. KyrgiouM, KoliopoulosG, Martin-HirschP, ArbynM, PrendivilleW, et al. (2006) Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 367 : 489–498.
23. World Health Organization (2012) Biomedical infertility care in poor resource countries: barriers, access and ethics. Geneva: World Health Organization. Available: http://www.who.int/reproductivehealth/publications/infertility/biomedical_infertility_care/en/index.html. Accessed 17 June 2013.
24. BaldaufJJ, DreyfusM, RitterJ, MeyerP, PhilippeE (1996) Risk of cervical stenosis after large loop excision or laser conization. Obstet Gynecol 88 : 933–938.
25. WethingtonSL, CibulaD, DuskaLR, GarrettL, KimCH, et al. (2012) An international series on abdominal radical trachelectomy: 101 patients and 28 pregnancies. Int J Gynecol Cancer 22 : 1251–1257.
26. TestaR, RamirezPT, FerreyraH, SaadiJ, FrancoG, et al. (2013) Abdominal radical trachelectomy: a safe and feasible option for fertility preservation in developing countries. J Low Genit Tract Dis E-pub ahead of print. doi:10.1097/LGT.0b013e31827cce89
27. DennyL, AnorluR (2012) Cervical cancer in Africa. Cancer Epidemiol Biomarkers Prev 21 : 1434–1438.
28. Widge A (2002) Sociocultural attitudes towards infertility and assisted reproduction in India. In: Vayena E, Rowe PJ, Griffin PD, editors. Current practices and controversies in assisted reproduction. Geneva: World Health Organization.
29. UpkongD, OrjiE (2006) Mental health of infertile women in Nigeria. Turk Psikiyatri Derg 17 : 259–265.
30. RouchouB (2013) Consequences of infertility in developing countries. Perspect Public Health 133 : 174–179.
31. DonkorES, SandallJ (2007) The impact of perceived stigma and mediating social factors on infertility-related stress among women seeking infertility treatment in Southern Ghana. Soc Sci Med 65 : 1683–1694.
32. LindegrenML, KennedyCE, Bain-BrickleyD, AzmanH, CreangaAA, et al. (2012) Integration of HIV/AIDS services with maternal, neonatal and child health, nutrition, and family planning services. Cochrane Database Syst Rev 9: CD010119.
33. GageJC, AjenifujaKO, WentzensenN, AdepitiAC, StolerM, et al. (2012) Effectiveness of a simple rapid human papillomavirus DNA test in rural Nigeria. Int J Cancer 131 : 2903–2909.
34. World Health Organization (2011) Scaling up action against NCDs: how much will it cost? Geneva: World Health Organization. Available: http://www.who.int/nmh/publications/cost_of_inaction/en/. Accessed 5 June 2013.
35. SankaranarayananR, NessaA, EsmyPO, DangouJM (2012) Visual inspection methods for cervical cancer prevention. Best Pract Res Clin Obstet Gynaecol 26 : 221–232.
36. SankaranarayananR, NeneBM, ShastriSS, JayantK, MuwongeR, et al. (2009) HPV screening for cervical cancer in rural India. N Engl J Med 360 : 1385–1394.
37. GoldieSJ, GaffikinL, Goldhaber-FiebertJD, Gordillo-TobarA, LevinC, et al. (2005) Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med 353 : 2158–2168.
38. International Planned Parenthood Federation, United Nations Population Fund, World Health Organization, Joint United Nations Programme on HIV/AIDS, Global Network of People Living with HIV, International Community of Women Living with HIV/AIDS, Young Positives (2009) Rapid assessment tool for sexual and reproductive health and HIV linkages: a generic guide. Available: http://data.unaids.org/pub/Manual/2009/2009_rapid_assesment_brochure_en.pdf. Accessed 15 June 2013.
39. KennedyCE, SpauldingAB, BrickleyDB, AlmersL, MirjahangirJ, et al. (2010) Linking sexual and reproductive health and HIV interventions: a systematic review. J Int AIDS Soc 13 : 26.
40. HuchkoMJ, BukusiEA, CohenCR (2011) Building capacity for cervical cancer screening in outpatient HIV clinics in the Nyanza province of Western Kenya. Int J Gynaecol Obstet 114 : 106–110.
41. WilliamsMS, AmoatengP (2012) Knowledge and beliefs about cervical cancer screening among men in Kumasi, Ghana. Ghana Med J 46 : 147–151.
42. GarrettJJ, BarringtonC (2013) ‘We do the impossible’: women overcoming barriers to cervical cancer screening in rural Honduras—a positive deviance analysis. Cult Health Sex 15 : 637–651.
43. BinagwahoA, WagnerCM, GateraM, KaremaC, NuttCT, et al. (2012) Achieving high coverage in Rwanda's national human papillomavirus vaccination programme. Bull World Health Organ 90 : 623–628.
44. LehtinenM, PaavonenJ, WheelerCM, JaisamrarnU, GarlandSM, et al. (2012) Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 13 : 89–99.
45. GAVI Alliance (2013) Human papillomavirus vaccine support. Available: http://www.gavialliance.org/support/nvs/human-papillomavirus-vaccine-support/. Accessed 17 June 2013.
46. GoldieSJ, O'SheaM, Gastineau CamposM (2008) Health and economic outcomes of HPV 16,18 vaccination in 72 GAVI-eligible countries. Vaccine 26 : 4080–4093.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 8- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Better Reporting of Scientific Studies: Why It Matters
- Towards Universal Voluntary HIV Testing and Counselling: A Systematic Review and Meta-Analysis of Community-Based Approaches
- Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non-Inferiority Trial in Thailand
- Public Engagement in Health Priority Setting in Low- and Middle-Income Countries: Current Trends and Considerations for Policy
- Expanding Disease Definitions in Guidelines and Expert Panel Ties to Industry: A Cross-sectional Study of Common Conditions in the United States
- Pediatric AIDS in the Elimination Agenda
- Country Contextualization of the Mental Health Gap Action Programme Intervention Guide: A Case Study from Nigeria
- Reproductive and Maternal Health in the Post-2015 Era: Cervical Cancer Must Be a Priority
- Effect of Household-Based Drinking Water Chlorination on Diarrhoea among Children under Five in Orissa, India: A Double-Blind Randomised Placebo-Controlled Trial
- First Diagnosis and Management of Incontinence in Older People with and without Dementia in Primary Care: A Cohort Study Using The Health Improvement Network Primary Care Database
- Risk of Early-Onset Neonatal Infection with Maternal Infection or Colonization: A Global Systematic Review and Meta-Analysis
- Inclusion of Ethical Issues in Dementia Guidelines: A Thematic Text Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non-Inferiority Trial in Thailand
- Risk of Early-Onset Neonatal Infection with Maternal Infection or Colonization: A Global Systematic Review and Meta-Analysis
- Inclusion of Ethical Issues in Dementia Guidelines: A Thematic Text Analysis
- Country Contextualization of the Mental Health Gap Action Programme Intervention Guide: A Case Study from Nigeria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



