-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Expanding Disease Definitions in Guidelines and Expert Panel Ties to Industry: A Cross-sectional Study of Common Conditions in the United States
Background:
Financial ties between health professionals and industry may unduly influence professional judgments and some researchers have suggested that widening disease definitions may be one driver of over-diagnosis, bringing potentially unnecessary labeling and harm. We aimed to identify guidelines in which disease definitions were changed, to assess whether any proposed changes would increase the numbers of individuals considered to have the disease, whether potential harms of expanding disease definitions were investigated, and the extent of members' industry ties.Methods and Findings:
We undertook a cross-sectional study of the most recent publication between 2000 and 2013 from national and international guideline panels making decisions about definitions or diagnostic criteria for common conditions in the United States. We assessed whether proposed changes widened or narrowed disease definitions, rationales offered, mention of potential harms of those changes, and the nature and extent of disclosed ties between members and pharmaceutical or device companies.
Of 16 publications on 14 common conditions, ten proposed changes widening and one narrowing definitions. For five, impact was unclear. Widening fell into three categories: creating “pre-disease”; lowering diagnostic thresholds; and proposing earlier or different diagnostic methods. Rationales included standardising diagnostic criteria and new evidence about risks for people previously considered to not have the disease. No publication included rigorous assessment of potential harms of proposed changes.
Among 14 panels with disclosures, the average proportion of members with industry ties was 75%. Twelve were chaired by people with ties. For members with ties, the median number of companies to which they had ties was seven. Companies with ties to the highest proportions of members were active in the relevant therapeutic area. Limitations arise from reliance on only disclosed ties, and exclusion of conditions too broad to enable analysis of single panel publications.Conclusions:
For the common conditions studied, a majority of panels proposed changes to disease definitions that increased the number of individuals considered to have the disease, none reported rigorous assessment of potential harms of that widening, and most had a majority of members disclosing financial ties to pharmaceutical companies.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(8): e32767. doi:10.1371/journal.pmed.1001500
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001500Summary
Background:
Financial ties between health professionals and industry may unduly influence professional judgments and some researchers have suggested that widening disease definitions may be one driver of over-diagnosis, bringing potentially unnecessary labeling and harm. We aimed to identify guidelines in which disease definitions were changed, to assess whether any proposed changes would increase the numbers of individuals considered to have the disease, whether potential harms of expanding disease definitions were investigated, and the extent of members' industry ties.Methods and Findings:
We undertook a cross-sectional study of the most recent publication between 2000 and 2013 from national and international guideline panels making decisions about definitions or diagnostic criteria for common conditions in the United States. We assessed whether proposed changes widened or narrowed disease definitions, rationales offered, mention of potential harms of those changes, and the nature and extent of disclosed ties between members and pharmaceutical or device companies.
Of 16 publications on 14 common conditions, ten proposed changes widening and one narrowing definitions. For five, impact was unclear. Widening fell into three categories: creating “pre-disease”; lowering diagnostic thresholds; and proposing earlier or different diagnostic methods. Rationales included standardising diagnostic criteria and new evidence about risks for people previously considered to not have the disease. No publication included rigorous assessment of potential harms of proposed changes.
Among 14 panels with disclosures, the average proportion of members with industry ties was 75%. Twelve were chaired by people with ties. For members with ties, the median number of companies to which they had ties was seven. Companies with ties to the highest proportions of members were active in the relevant therapeutic area. Limitations arise from reliance on only disclosed ties, and exclusion of conditions too broad to enable analysis of single panel publications.Conclusions:
For the common conditions studied, a majority of panels proposed changes to disease definitions that increased the number of individuals considered to have the disease, none reported rigorous assessment of potential harms of that widening, and most had a majority of members disclosing financial ties to pharmaceutical companies.
Please see later in the article for the Editors' SummaryIntroduction
Changes in technologies, treatments, medical knowledge, and cultural norms provide cause to review and change disease definitions and diagnostic thresholds, a task that is commonly undertaken by expert panels, consensus meetings, or influential workgroups who publish findings as statements, special reports, or as part of clinical practice guidelines. While such changes can be beneficial, there is an increasing recognition that widening of disease definitions may be one factor contributing to the problem of over-diagnosis, occurring across a range of conditions including pulmonary embolism, breast and prostate cancers [1],[2]. The concern expressed by some researchers is that for some people with milder symptoms, at lower risks, or in earlier stages of possible disease, the harms of a diagnostic label and treatment may outweigh benefits [3],[4].
At the same time there is accumulating evidence about pervasive financial ties between pharmaceutical companies and health professionals [5], including those writing guidelines [6] and disease definitions [7]. While noting the value of professional–industry collaborations, a 2009 Institute of Medicine (IOM) report found “widespread relationships with industry have created significant risks that individual and institutional financial interests may unduly influence professionals' judgments,” and that these “conflicts of interest” threaten the integrity of research, the objectivity of education, the quality of patient care, and public trust in medicine [5].
The 2009 report recommended professional societies and other organisations drafting clinical practice guidelines should “generally exclude as panel members individuals with conflicts of interest.” A subsequent 2011 IOM report on how to produce trustworthy guidelines included recommendations that “whenever possible,” guideline developers “should not have” conflicts of interest, that only a minority should have conflicts, and that chairs should be free of conflicts [8].
As both reports make clear, in addition to financial ties there are non-financial or intellectual conflicts such as academic advancement, and there should be no assumption that having a conflict is unethical, or “that any particular professional will necessarily let financial gain influence his or her judgment” [5].
A 2011 systematic review found many clinical guideline panels have failed to disclose financial ties, and those that did disclose had a “high percentage” of individuals with financial conflicts of interest [6]. Studies analysing ties of working groups for the Diagnostic and Statistical Manual of Mental Disorders (DSM), which set definitions and diagnostic criteria, have also found a majority of members with ties [7]. Kung and colleagues recently found two-thirds of individuals chairing guideline committees had conflicts of interest [9].
Few studies [7] have examined the financial ties of members of panels reviewing and changing definitions of common conditions, whether as part of practice guideline development or other processes. Our aim was to identify guideline panels in the US setting that have most recently made decisions about definitions or diagnostic thresholds for common conditions, and to report on any proposed changes and their industry ties.
Methods
List of Conditions
On the basis of the method previously used by Choudhry and colleagues [10], we derived a list of common conditions in the United States, drawing from a list of the ten most costly adult diseases [11], the top 20 therapeutic classes of drugs, and the top 25 individual drugs by expenditure [12]. Consistent with that method, drugs used to treat many non-specific conditions were excluded (e.g., pain killers). For situations in which a drug was approved for a number of conditions, we identified the most common condition for inclusion (e.g., etanercept ultimately mapped to rheumatoid arthritis, not psoriatic arthritis). If a condition in the top ten costly disease list was too broad or diffuse, or covered many specific conditions, it was excluded (e.g., back problems). A flowchart of the method appears in Figure 1.
Fig. 1. Flowchart identifying study conditions and panels reviewing definitions. 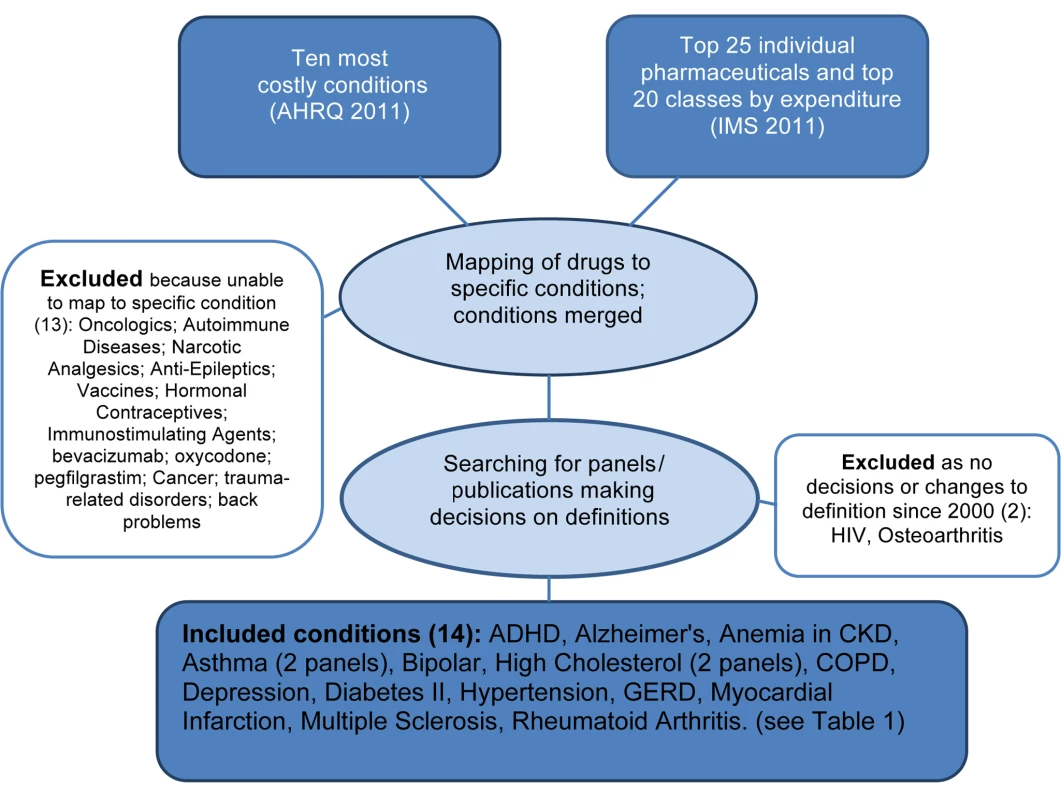
Note: bipolar/depression was one panel. List of Panels and Publications
We aimed to identify the most recent publication from panels making decisions about disease definition and diagnosis. A panel publication was eligible for inclusion if it was generated or supported by a widely recognised US-based organisation, published between 2000 and April 2013, and included deliberations and decisions on disease definitions and/or diagnostic criteria, classification, or assessment. If the panel made decisions, but proposed no changes, our search would continue for the most recent publication proposing changes, to include as well. If the focus of the panel publication was limited to specific sub-groups of patients, (e.g., adolescents), specific sub-categories of the condition (e.g., work-related asthma), it came from a single entity (e.g., a health maintenance organisation), or it included treatment recommendations but no review and deliberation on disease definition or diagnostic criteria, it was excluded.
During a pilot phase, using the searches for the most recent hypertension and asthma panel publications, an explicit search strategy using standardized keywords was iteratively developed in order to maximise sensitivity. We searched Medline (Ovid) using terms for each disease/condition and combined these terms with a standardized search strategy consisting of a string of MeSH and keyword terms to identify panels and publications (example in Table S1). Searches were run over 26–31 July 2012, updated 17–18 April 2013, and limited to English language from 2000.
To further improve sensitivity and try to ensure recent publications were not missed, two authors (RNM, GPEC) independently analysed the results of the standardised Medline searches for all conditions, and supplemented this with independent individual searches of the websites of the relevant National Institutes of Health and the National Guideline Clearing House. For two conditions, minor discrepancies in independent suggestions were resolved by discussion and, in one case (diabetes II), by consultation with a third author (PPG). Because of their global prominence and influence, if a panel was constituted under the umbrella of the National Institutes of Health (NIH), or the American Psychiatric Association's DSM, and met our inclusion criteria, these panels were identified for inclusion in our study. If there was a more recent panel publication that also met the study's inclusion criteria, in addition to the NIH or DSM panel, we included the more recent publication as well. This occurred twice (asthma and high cholesterol), resulting in two publications being identified for each condition.
Information on the Panels' Decisions
For each publication we extracted information on key proposed changes to definitions/diagnostic criteria, the rationale offered, and any mention of potential harms associated with the proposed changes (e.g., over-diagnosis, overtreatment, medicalizing normality, labelling asymptomatic people). All six authors then made an assessment of whether the panel's proposed key changes would tend to widen (e.g., earlier diagnosis, lower thresholds, adding symptoms, increasing numbers diagnosed) or narrow the disease definition, or whether it was unclear.
Information on Industry Ties
Using published disclosure sections from the panel publications, duplicate independent extraction of data was conducted (RNM and research assistant Peter Coxeter), with a third party resolving any disagreement (PGG). Ties were categorized as speaker/honorarium, consultant/adviser, grant/research, stock, employee, travel, or royalties. Panel members were those listed as authors or identified as the group with primary responsibility for generating the publications. In line with the IOM approach [5], an industry tie was defined as a tie to a pharmaceutical, diagnostic, device, or biotechnology company, but not a communications or medical education company. If there was any lack of clarity as to the nature of the company, or uncertainty if it met study criteria, a tie was not recorded. Once all industry ties were recorded for each panel, websites of companies with financial ties to the three highest proportions of panel members were searched to determine whether those companies were active in the specific therapeutic area. Where they appeared in disclosure sections, the disclosure of any ties to public agencies, non-government organizations, and publishers was also recorded.
Results
After analyzing source documents [11],[12], the following drug classes, individual drugs, and conditions were excluded when identifying study conditions, as they were too non-specific or too broad, and did not map to specific conditions enabling analysis: oncologics; autoimmune diseases; narcotic analgesics; anti-epileptics; vaccines; hormonal contraceptives; immunostimulating agents; bevacizumab; oxycodone; pegfilgrastim; cancer; trauma-related disorders; and back problems.
From an initial list of 16 included common conditions, for two—osteoarthritis, HIV—we could identify no panel that made decisions about definitions or diagnostic thresholds since 2000 in the US context specifically. For the remaining 14 conditions, we identified the most recent panels that deliberated and made decisions about disease definitions, all of which proposed changes. For asthma and high cholesterol we identified two panels each, one constituted under the government funded NIH [13],[14] and one by professional societies [15],[16], reflecting the two main types of panels identified in this study. A single panel, the DSM-V Mood Disorders working group, proposed changes to two different conditions, bipolar and depression, in two separate web-based publications [17]. A full list of the final 14 conditions, 15 panels and 16 publications, key changes and rationale, analysis of panel decisions, and disclosed ties appears in Table 1.
Tab. 1. Conditions and characteristics of the panels and publications included in the study. 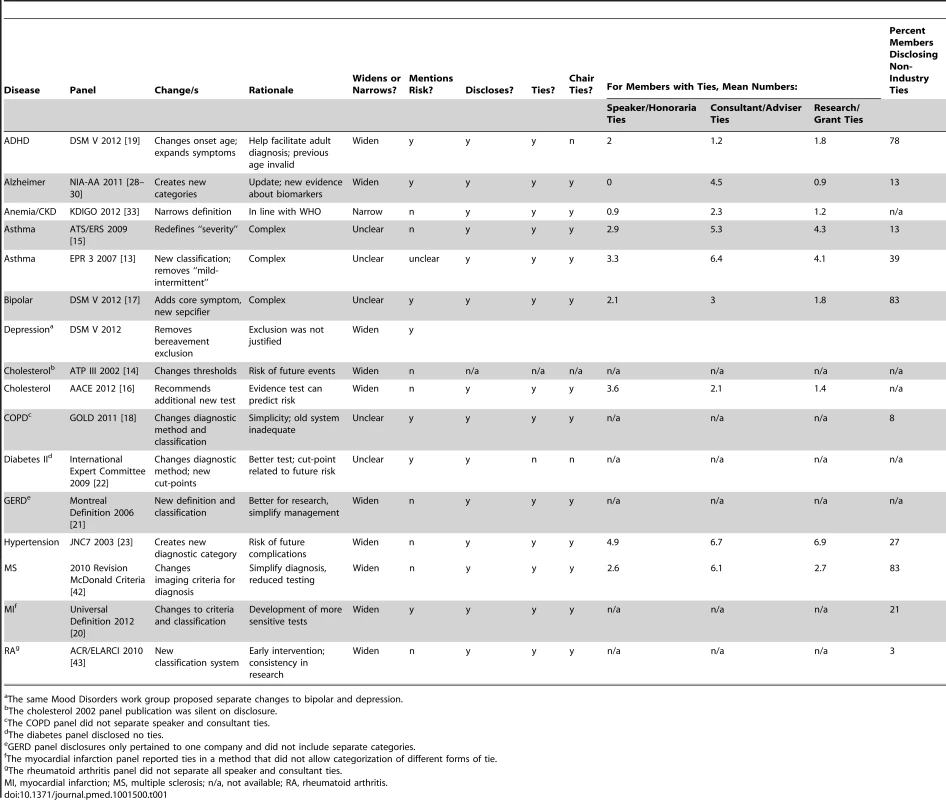
The same Mood Disorders work group proposed separate changes to bipolar and depression. Among 16 publications, all authors in our study agreed that proposals in ten publications would tend to widen definitions (Table 2) and for one, narrow the definition. For the remaining five publications the impact was unclear. Rationales for the benefits of widening definitions or expanding diagnostic categories included: evidence about the risk of future adverse events for people previously considered normal (pre-hypertension); simplification (gastroesophageal reflux disease [GERD]); standardisation for research (rheumatoid arthritis); and the emergence of new evidence about biomarkers, tests, or treatments (Alzheimer disease). Among 15 panels, six included mention of possible harms of proposed changes (Table 3), albeit briefly, with three of those including citations in that mention [17]–[19], two citing primary studies [18],[19], and one of those citing a review of primary studies as well [18]. One publication referred to the potential negative consequences for those who would be labelled by the expanded definition [20], and only one referred to overdiagnosis [21].
Tab. 2. Different ways to expand disease definitions. 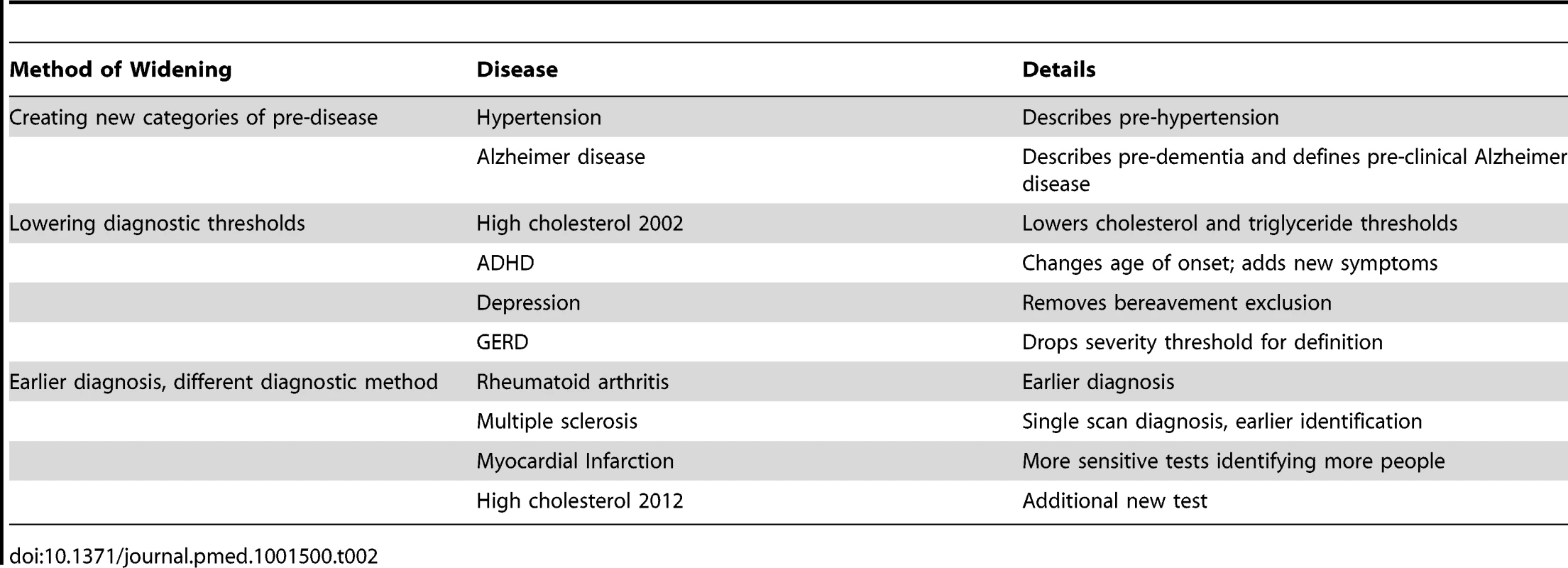
Tab. 3. Mention of possible harms of proposed changes to definitions. 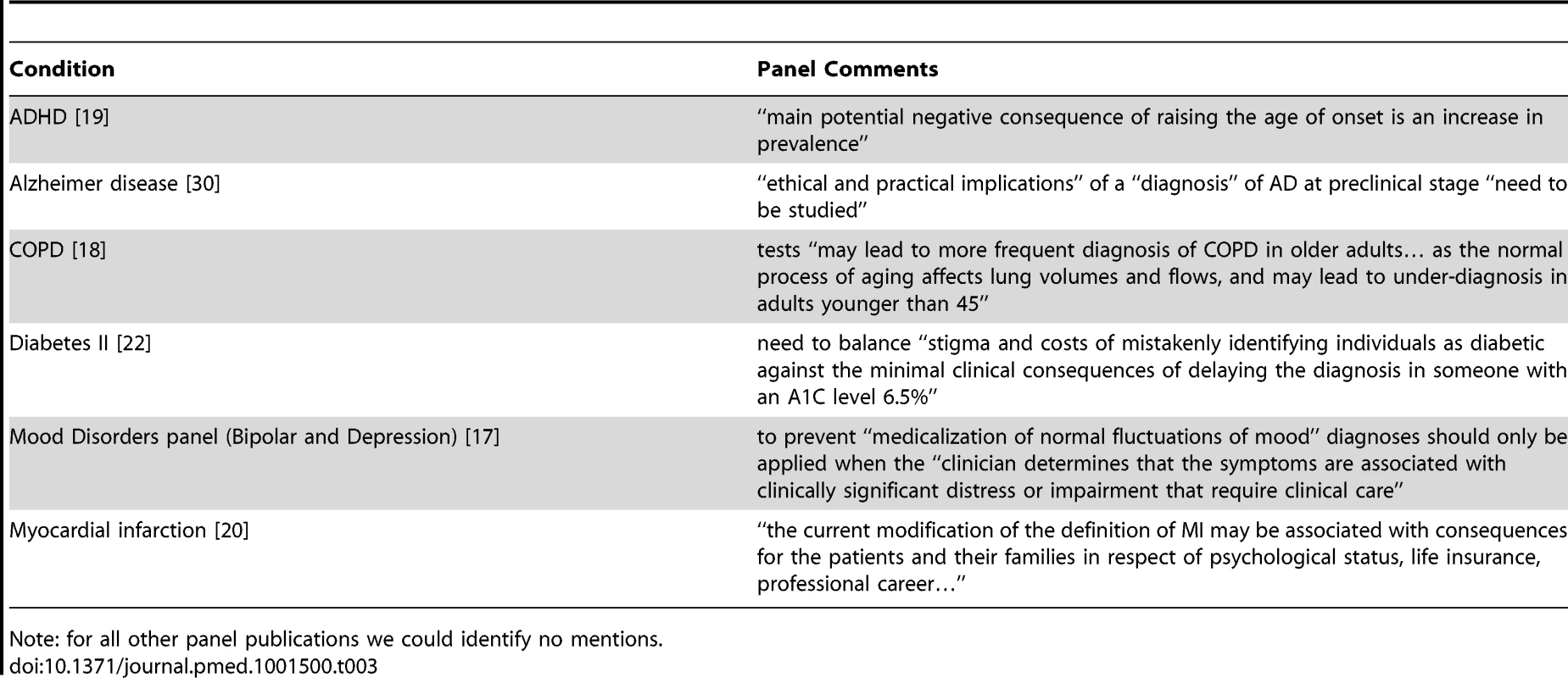
Note: for all other panel publications we could identify no mentions. The average number of panel members was 21 (range, five to 52). Among 15 panels, 12 included members disclosing financial ties to multiple companies, one panel disclosed ties to a single company only (GERD) [21], one stated that members had no relevant conflicts of interest (diabetes II) [22], and one had no disclosure section (high cholesterol 2002) [14], also the oldest panel. For a total of 2,081 individual ties across all categories recorded in the study, there were 55 discrepancies, 2.6%, arising from the independent extraction, mainly involving one or other extractor accidentally missing or adding a specific tie, or making errors by entering a specific tie into an adjacent column or row in a spreadsheet. All were resolved by discussion.
Among 14 panels with disclosure sections, the average proportion of members with industry ties was 75% (range 0%–100%) (Table 4). For members with ties, the median number of pharmaceutical or device companies to which they had declared ties to was seven (Table 4). For the nine panel publications disclosing multiple separate categories of tie, on average, members with industry ties were a consultant/adviser for four companies, received speaker fees/honoraria from two companies, and they or their institutions received research support from three. Twelve panels were chaired or publications led by authors with industry ties, most commonly to multiple companies. Among panels disclosing any ties to government agencies, non-government organisations, or publishers, on average around one-third of panel members disclosed these ties.
Tab. 4. Nature and extent of disclosed ties, by panel. 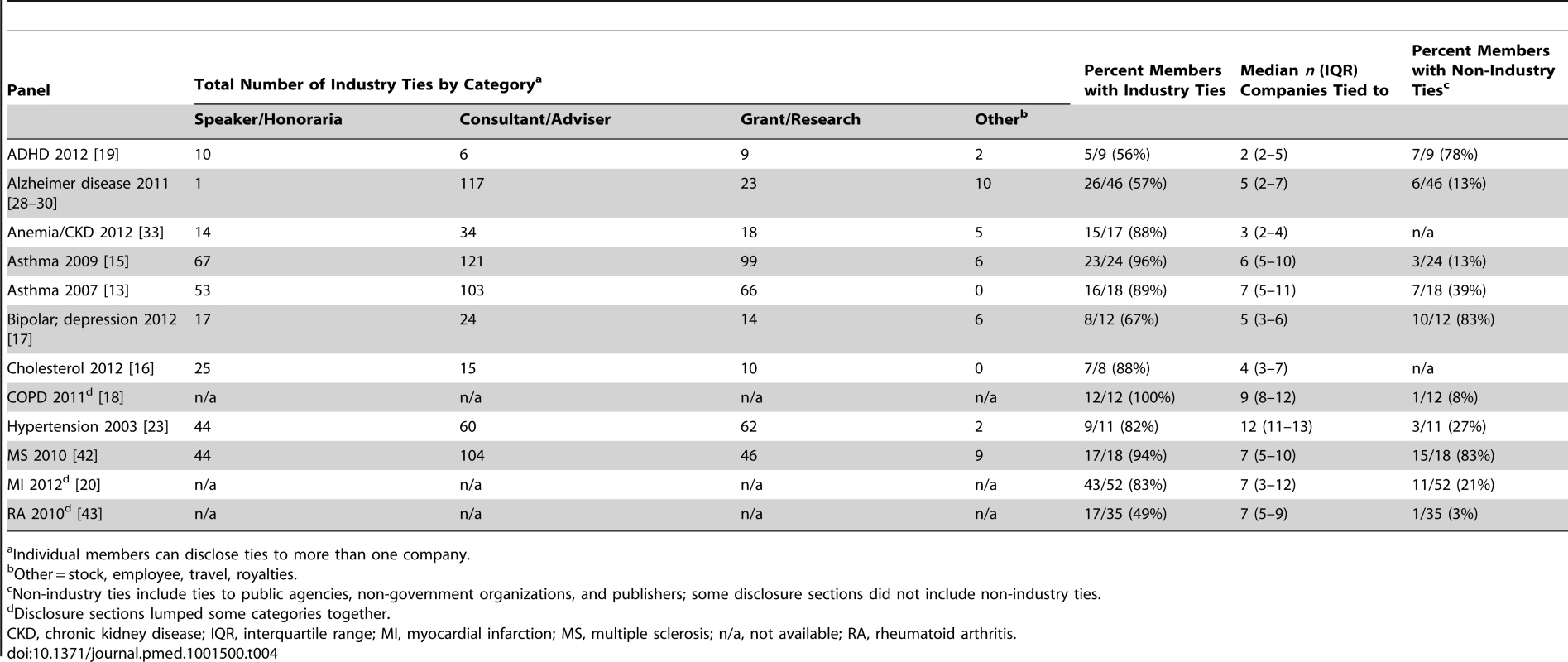
Individual members can disclose ties to more than one company. For the 12 panels for which ties were disclosed to more than one company, almost all companies with ties to the three highest proportions of panel members were also active in the market for that panel's condition, with at least one drug on the market or in the research pipeline (Table 5). For example, with the chronic obstructive pulmonary disease [COPD] publication, Astra Zeneca, Boehringer-Ingelheim, and GSK—all companies with drugs for the condition—each had financial ties to 11 of 12 members, including the chair [18]. With the DSM-V Mood Disorders work group, Pfizer and Lilly—with drugs for depression and bipolar—had ties to five of the 12 members [17]. Similarly, companies marketing hypertension drugs—Bristol-Myers Squibb, Merck, Novartis—each had financial ties to eight of the 11 members of the panel which created the new diagnostic category “pre-hypertension” [23].
Tab. 5. Companies with highest proportions of ties, and drugs in therapeutic area. 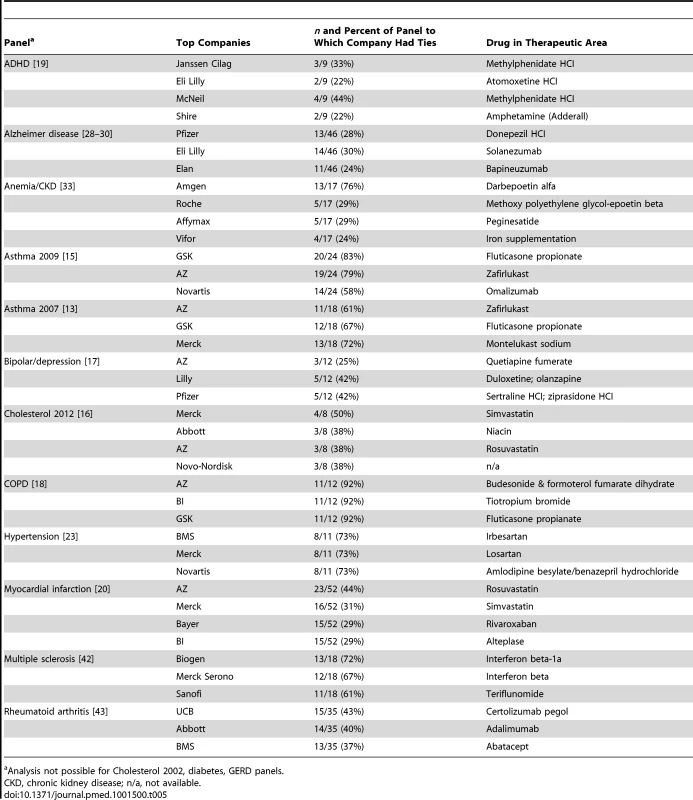
Analysis not possible for Cholesterol 2002, diabetes, GERD panels. To evaluate any potential impact of the IOM recommendations regarding industry ties, we compared the panel publications released in 2012—after both IOM reports [5],[8]—to those released earlier. We found similar proportions of members disclosing industry ties (76% was the average across 2012 panels; 74% was the average across pre-2012 panels); a small reduction in the median number of companies to which those members disclosed ties in the 2012 panels (four in 2012 panels; seven pre-2012 panels); and similar proportions of panel publications widening definitions (four of six of 2012 publications; six of ten of pre-2012 publications).
Discussion
In this cross-sectional analysis of panels making recent decisions on definitions of common conditions in the US context, we found most panels proposed widening definitions and most had a majority of members with multiple ties to pharmaceutical companies. Proposals to widen fell into three inter-related categories: creating new categories of “pre-disease”; lowering diagnostic thresholds; and proposing earlier diagnosis or different diagnostic methods (Table 2). In some cases a clear rationale was offered for these changes—as when the hypertension panel cited evidence from original studies and meta-analysis linking normal blood pressure with elevated risks as the reason to create “pre-hypertension” [23]. In other publications, including the 2007 panel proposing changes to the diagnosis of asthma [13], the rational was less clear, more complex and diffuse.
Notwithstanding the problem of under-diagnosis, a growing body of evidence suggests over-diagnosis may be occurring across a range of common conditions, including hypertension [24], asthma [25], attention deficit hyperactivity disorder (ADHD) [26], and COPD [27]. Yet less than half of the study publications mentioned potential harms of proposed changes to definitions, and none included a rigorous evidence-informed discussion of those risks or how they might be mitigated.
In a three-part publication in 2011 [28]–[30] proposing new categories of “pre-clinical” Alzheimer disease (for research only at this stage) and “predementia”—which would clearly expand the population labelled—there was one short reference to the need to study the “ethical and practical implications” of diagnosing people at a “preclinical” stage [30]. The panel proposing changes to assessment and classification of COPD briefly mentioned that diagnostic methods “may lead to more frequent diagnosis of COPD in older adults with mild COPD as the normal process of aging affects lung volumes and flows” [18], but did not explicitly refer to the risk of “over-diagnosis” as it had done in a previous version of its report [31]. Proposing changes to ADHD diagnostic criteria—in part to make the condition more amenable to being a “lifespan” disorder involving adults as well as children—the DSM-V panel mentioned potential increases in prevalence but suggested they would be “negligible” (Table 3) [32].
Among panels disclosing ties, almost all chairs had financial ties to industry, and an average of three-quarters of members had ties to a median of seven companies, commonly working as consultants, advisers and/or speakers, as well as receiving research support. Companies with financial relationships with the greatest proportion of panel members were marketing or developing drugs for the same conditions about which those members were making critical judgements. GSK for example, marketing top-selling products for asthma, had financial ties to 20 of the 24 members of the 2009 asthma panel, and all 20 were consultant/advisers and/or declared speaker/honoraria ties to GSK [15].
This study has several important limitations. First, the lack of a comparison group means it is impossible to draw any inference of association between frequency of industry ties and proposals to change disease definitions. The exclusion of common conditions too broad to enable a focussed analysis of single panel publications (e.g., back problems) means it may have missed potentially important examples of changing disease definitions and limits generalizability of findings. The focus on the United States—chosen explicitly because of its globally influential panels such as DSM-V workgroups—also limits generalizability. A fourth limitation is reliance solely on disclosed ties, likely leading to an underestimate of their extent. Finally, we note that while we tried to ensure an exhaustive and multi-layered search strategy, we are unaware of any established method for identifying panel publications that review or propose changes to disease definitions.
Notwithstanding these limitations, the study has strong clinical, research, and policy relevance. Its novel focus on panels reviewing and proposing changes to common disease definitions or diagnostic criteria will help deepen understanding of the nature of what's been described as the “modern epidemic” of over-diagnosis [2]. Moreover, the group of 16 publications includes influential articles affecting the definition of 14 common conditions and impacting directly on medical policy and practice around the world.
The study findings are consistent with and help augment the evidence-base about industry ties of influential medical professionals. The 2011 systematic review found 56%–87% of clinical guideline writers had conflicts of interest [6], similar to our finding of 75% across disease-defining panels. Kung and colleagues found 71% of guideline committee chairs had conflicts [9], again similar to our findings. While these proportions may reflect the level of ties among medical specialists more generally, they are in stark contrast to IOM 2009 and 2011 reports calling for panels to generally exclude people with conflicts of interest [5],[8]. As reported above, we found no change in the proportion of members disclosing ties in the 2012 publications, after release of both IOM reports.
At least two publications [20],[21] made reference to members believing industry ties did not influence their decision-making, and we make no suggestion to the contrary. Indeed our data do not support any inference industry ties are associated with widening definitions or failure to rigorously assess potential harms of that widening. With anemia in chronic kidney disease, a panel with a high proportion of ties raised thresholds, effectively narrowing the definition [33]. There will doubtless be other cases where diseases have been widened by panels of medical specialists without industry ties. Moreover, as Lurie and colleagues found in the context of drug regulation, the financial conflicts of expert advisory committees did not correlate significantly with their voting outcomes [34]. Medicalization and over-diagnosis are driven by many factors—technological, professional, commercial, legal, and cultural [3].
While inferences of association or causation between industry ties and expanding disease definitions cannot be drawn, our findings can be considered in the context of broader evidence about potentially distorting biases associated with widespread industry sponsorship and financial ties in medical research [35]–[37], education [38], and practice [5], and in relation to “key opinion leaders” who speak and consult for industry [39].
In 1999 Schwartz and Woloshin found changes to definitions of high blood pressure, high cholesterol, diabetes, and overweight would “dramatically inflate disease prevalence” and “ultimately label 75% of the adult U.S. population as diseased” [40]. They concluded the “extent to which new ‘patients’ would ultimately benefit from early detection and treatment of these conditions is unknown. Whether they would experience important physical or psychological harm is an open question.” To what extent newly created “patients” produced by widening disease definitions will experience important harms remains a largely unanswered question, over a decade later.
This study did not investigate the merits of the proposed changes to the conditions identified. However, findings that diagnostic thresholds are being lowered by panels dominated by those with financial ties to multiple companies that may benefit directly from those decisions raise questions about current processes of disease definition. While it may be more difficult to locate senior specialists without industry ties, two recent IOM reports have encouraged such a change [5],[8], and models already exist for panels free of such conflicts, including the NIH consensus development program [41].
Several unanswered questions arise from this study, which could benefit from further investigation. Researchers might fruitfully examine how definitions are changing over time, what dollar amounts are being received from industry by panel members and organisations that auspice them, and how panel proposals impact on potential markets of sponsors. Most importantly enhanced research and policy attention might be directed at designing new processes for reviewing disease definitions, free of financial conflicts of interest and informed by rigorous analysis of benefits and harms.
Supporting Information
Zdroje
1. Welch HG, Schwartz LM, Woloshin S (2011) Overdiagnosed: making people sick in the pursuit of health. Boston: Beacon.
2. HoffmanJ, CooperR (2012) Overdiagnosis of disease: a modern epidemic. Arch Intern Med 172 : 1123–1124.
3. MoynihanR, DoustJ, HenryD (2012) Preventing overdiagnosis: how to stop harming the healthy. BMJ 344: e3502.
4. BrodyH, LightD (2011) The inverse benefit law: how drug marketing undermines patient safety and public health. Am J Public Health 101 : 399–404.
5. Lo B, Field MJ (2009) Conflict of interest in medical research, education, and practice. Summary. Washington (D.C.): Institute of Medicine National Academies of Science.
6. NorrisSL, HolmerHK, OgdenLA, BurdaBU (2011) Conflict of interest in clinical practice guideline development: a systematic review. PloS One 6: e25153 doi:10.1371/journal.pone.0025153
7. CosgroveL, KrimskyS (2012) A comparison of DSM-IV and DSM-5 panel members' financial associations with industry: a pernicious problem persists. PLoS Med 9: e1001190 doi:10.1371/journal.pmed.1001190
8. IOM (Institute of Medicine) (2011) Clinical practice guidelines we can trust. Washington (D.C.): The National Academies Press.
9. KungJ, MillerRR, MackowiakPA (2012) Failure of clinical practice guidelines to meet institute of medicine standards: two more decades of little, if any, progress. Arch Intern Med 172 : 1628–1633.
10. ChoudryN, StelfoxHT, DetskyA (2002) Relationships between authors of Clinical Practice Guidelines and the Pharmaceutical Industry. JAMA 287 : 1–6.
11. Soni A (2011) Top 10 most costly conditions among men and women, 2008: estimates for the U.S. civilian noninstitutionalized adult population, age 18 and older. Statistical brief #331. Rockville (Maryland): Agency for Healthcare Research and Quality. Available: http://www.meps.ahrq.gov/mepsweb/data_files/publications/st331/stat331.pdf Accessed 13 February 2013.
12. IMS Institute (2011) Use of medicine in the United States Review of 2010. Available: http://www.imshealth.com/deployedfiles/imshealth/Global/Content/IMS%20Institute/Static%20File/IHII_UseOfMed_report.pdf Accessed 13 February 2013.
13. National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program (2007) Expert panel report 3: guidelines for the diagnosis and management of asthma. Full report. Washington (D.C.): US Department of Health and Human Services.
14. National Cholesterol Education Program (2002) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Bethesda (Maryland): National Institutes of Health. National Heart, Lung, and Blood Institute.
15. ReddelHK, TaylorDR, BatemanED, BouletL-P, BousheyHA, et al. (2009) An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Resp Crit Care 180 : 59–99.
16. JellingerPS, SmithDA, MehtaAE, GandaO, HandelsmanY, et al. (2012) American Association of Clinical Endocrinologists' guidelines for management of dyslipidemia and prevention of artherosclerosis. Endocr Pract 18 : 1–78.
17. The Mood Disorders Work group of the fifth edition of the DSM. Membership, proposed changes and disclosures. Available: http://www.dsm5.org/MeetUs/Pages/MoodDisorders.aspx Accessed 19 October 2012.
18. Global Initiative for Chronic Obstructive Lung Disease (2011) Global Strategy for the diagnosis management and prevention of chronic obstructive pulmonary disease. Updated 2011. Global Initiative for Chronic Obstructive Lung Disease, Inc.
19. ADHD and Disruptive Behavior Disorders Work Group of fifth edition of the DSM. Membership, proposed changes, and disclosures. Available: http://www.dsm5.org/MeetUs/Pages/ADHD.aspx. Accessed 19 October 2012.
20. ThygesenK, AlpertJS, JaffeAS, SimoonsML, ChaitmanBR, et al. (2012) Third universal definition of myocardial infarction. Circulation 126 : 2020–2035.
21. VakilN, van ZantenSV, KahrilasP, DentJ, JonesR (2006) The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 101 : 1900–1920.
22. The International Expert Committee (2009) International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care 32 : 1327–1334.
23. National High Blood Pressure Education Program (2004) The seventh report of the joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Complete report. Bethesda (Maryland): US Department of Health and Human Services, National Institutes of Health. National Heart, Lung, and Blood Institute.
24. HodgkinsonJ, MantJ, MartinU, GuoB, HobbsFDR, et al. (2011) Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ 342: d3621–d3621.
25. AaronSD, VandemheenKL, BouletL-P, McIvorRA, FitzgeraldJM, et al. (2008) Overdiagnosis of asthma in obese and nonobese adults. CMAJ 179 : 1121–1131.
26. MorrowRL, GarlandEJ, WrightJM, MaclureM, TaylorS, et al. (2012) Influence of relative age on diagnosis and treatment of attention-deficit/hyperactivity disorder in children. CMAJ 184 : 755–762.
27. HardieJA, BuistAS, VollmerWM, EllingsenI, BakkePS, et al. (2002) Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J 20 : 1117–1122.
28. McKhannGM, KnopmanDS, ChertkowH, HymanBT, JackCR, et al. (2011) The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7 : 263–269.
29. AlbertMS, DeKoskyST, DicksonD, DuboisB, FeldmanHH, et al. (2011) The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7 : 270–279.
30. SperlingRA, AisenPS, BeckettLA, BennettDA, CraftS, et al. (2011) Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7 : 280–292.
31. Global Initiative for Chronic Obstructive Lung Disease (2010) Global strategy for the diagnosis management and prevention of chronic obstructive pulmonary disease. Updated 2010. Global Initiative for Chronic Obstructive Lung Disease, Inc.
32. Rationale for Changes in ADHD in DSM-5 from the ADHD and Disruptive Behavior Disorders Workgroup Updated May 3, 2012. Available: http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=383# Accessed 19 October 2012.
33. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease (2012) Kidney International 2(4) August (2) (Supplements).
34. LurieP, AlmeidaC, StineN, StineA, WolfeS (2006) Financial conflict of interest disclosure and voting patterns at food and drug administration advisory committee meetings. JAMA 295 : 1921–1928.
35. SchottG, PachlH, LimbachU, Gundert-RemyU, LudwigW-D, LiebK (2010) The financing of drug trials by pharmaceutical companies and its consequences. Dtsch Arztebl Int 107 : 279–285.
36. LundhA, SismondoS, LexchinJ, BusuiocOA, BeroL (2012) Industry sponsorship and research outcome. Cochrane Database of Syst Rev 12: MR000033.
37. BekelmanJE, LiY, GrossCP (2003) Scope and impact of financial conflicts of interest in biomedical research. JAMA 289 : 454–465.
38. Fletcher S (2008) Continuing education in the health professions: improving healthcare through lifelong learning: chairman's summary of the conference. Josiah Macy Jr Foundation. Available: http://macyfoundation.org/docs/macy_pubs/Macy_ContEd_1_7_08.pdf Accessed 13 February 2013.
39. WangAT, McCoyCP, MuradMH, MontoriVM (2010) Association between industry affiliation and position on cardiovascular risk with rosiglitazone: cross sectional systematic review. BMJ 340: c1344–c1344.
40. SchwartzLM, WoloshinS (1999) Changing disease definitions: implications for disease prevalence. Analysis of the Third National Health and Nutrition Examination Survey, 1988–1994. Effective Clinical Practice 2 : 76–85.
41. About the consensus development program. Office of Disease Prevention, National Institutes of Health. Available: http://prevention.nih.gov/cdp/about.aspx Accessed 13 February 2013.
42. PolmanCH, ReingoldSC, BanwellB, ClanetM, CohenJA, et al. (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69 : 292–302.
43. AletahaD, NeogiT, SilmanAJ, FunovitsJ, FelsonDT, et al. (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62 : 2569–2581.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 8- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Better Reporting of Scientific Studies: Why It Matters
- Towards Universal Voluntary HIV Testing and Counselling: A Systematic Review and Meta-Analysis of Community-Based Approaches
- Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non-Inferiority Trial in Thailand
- Public Engagement in Health Priority Setting in Low- and Middle-Income Countries: Current Trends and Considerations for Policy
- Expanding Disease Definitions in Guidelines and Expert Panel Ties to Industry: A Cross-sectional Study of Common Conditions in the United States
- Pediatric AIDS in the Elimination Agenda
- Country Contextualization of the Mental Health Gap Action Programme Intervention Guide: A Case Study from Nigeria
- Reproductive and Maternal Health in the Post-2015 Era: Cervical Cancer Must Be a Priority
- Effect of Household-Based Drinking Water Chlorination on Diarrhoea among Children under Five in Orissa, India: A Double-Blind Randomised Placebo-Controlled Trial
- First Diagnosis and Management of Incontinence in Older People with and without Dementia in Primary Care: A Cohort Study Using The Health Improvement Network Primary Care Database
- Risk of Early-Onset Neonatal Infection with Maternal Infection or Colonization: A Global Systematic Review and Meta-Analysis
- Inclusion of Ethical Issues in Dementia Guidelines: A Thematic Text Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non-Inferiority Trial in Thailand
- Risk of Early-Onset Neonatal Infection with Maternal Infection or Colonization: A Global Systematic Review and Meta-Analysis
- Inclusion of Ethical Issues in Dementia Guidelines: A Thematic Text Analysis
- Country Contextualization of the Mental Health Gap Action Programme Intervention Guide: A Case Study from Nigeria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



