-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Patented Drug Extension Strategies on Healthcare Spending: A Cost-Evaluation Analysis
Background:
Drug manufacturers have developed “evergreening” strategies to compete with generic medication after patent termination. These include marketing of slightly modified follow-on drugs. We aimed to estimate the financial impact of these drugs on overall healthcare costs and also to examine the impact of listing these drugs in hospital restrictive drug formularies (RDFs) on the healthcare system as a whole (“spillover effect”).Methods and Findings:
We used hospital and community pharmacy invoice office data in the Swiss canton of Geneva to calculate utilisation of eight follow-on drugs in defined daily doses between 2000 and 2008. “Extra costs” were calculated for three different scenarios assuming replacement with the corresponding generic equivalent for prescriptions of (1) all brand (i.e., initially patented) drugs, (2) all follow-on drugs, or (3) brand and follow-on drugs. To examine the financial spillover effect we calculated a monthly follow-on drug market share in defined daily doses for medications prescribed by hospital physicians but dispensed in community pharmacies, in comparison to drugs prescribed by non-hospital physicians in the community.
Estimated “extra costs” over the study period were €15.9 (95% CI 15.5; 16.2) million for scenario 1, €14.4 (95% CI 14.1; 14.7) million for scenario 2, and €30.3 (95% CI 29.8; 30.8) million for scenario 3. The impact of strictly switching all patients using proton-pump inhibitors to esomeprazole at admission resulted in a spillover “extra cost” of €330,300 (95% CI 276,100; 383,800), whereas strictly switching to generic cetirizine resulted in savings of €7,700 (95% CI 4,100; 11,100). Overall we estimated that the RDF resulted in “extra costs” of €503,600 (95% CI 444,500; 563,100).Conclusions:
Evergreening strategies have been successful in maintaining market share in Geneva, offsetting competition by generics and cost containment policies. Hospitals may be contributing to increased overall healthcare costs by listing follow-on drugs in their RDF. Therefore, healthcare providers and policy makers should be aware of the impact of evergreening strategies.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(6): e32767. doi:10.1371/journal.pmed.1001460
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001460Summary
Background:
Drug manufacturers have developed “evergreening” strategies to compete with generic medication after patent termination. These include marketing of slightly modified follow-on drugs. We aimed to estimate the financial impact of these drugs on overall healthcare costs and also to examine the impact of listing these drugs in hospital restrictive drug formularies (RDFs) on the healthcare system as a whole (“spillover effect”).Methods and Findings:
We used hospital and community pharmacy invoice office data in the Swiss canton of Geneva to calculate utilisation of eight follow-on drugs in defined daily doses between 2000 and 2008. “Extra costs” were calculated for three different scenarios assuming replacement with the corresponding generic equivalent for prescriptions of (1) all brand (i.e., initially patented) drugs, (2) all follow-on drugs, or (3) brand and follow-on drugs. To examine the financial spillover effect we calculated a monthly follow-on drug market share in defined daily doses for medications prescribed by hospital physicians but dispensed in community pharmacies, in comparison to drugs prescribed by non-hospital physicians in the community.
Estimated “extra costs” over the study period were €15.9 (95% CI 15.5; 16.2) million for scenario 1, €14.4 (95% CI 14.1; 14.7) million for scenario 2, and €30.3 (95% CI 29.8; 30.8) million for scenario 3. The impact of strictly switching all patients using proton-pump inhibitors to esomeprazole at admission resulted in a spillover “extra cost” of €330,300 (95% CI 276,100; 383,800), whereas strictly switching to generic cetirizine resulted in savings of €7,700 (95% CI 4,100; 11,100). Overall we estimated that the RDF resulted in “extra costs” of €503,600 (95% CI 444,500; 563,100).Conclusions:
Evergreening strategies have been successful in maintaining market share in Geneva, offsetting competition by generics and cost containment policies. Hospitals may be contributing to increased overall healthcare costs by listing follow-on drugs in their RDF. Therefore, healthcare providers and policy makers should be aware of the impact of evergreening strategies.
Please see later in the article for the Editors' SummaryIntroduction
To balance the at times competing goals of increasing access to new drugs on the one hand, and rewarding drug innovation via patents on the other hand, drug manufacturers are granted exclusive manufacturing rights for periods of up to 20 y [1]. This can generate large revenues that often exceed initial investments, thus providing an incentive for pharmaceutical companies to develop new drugs [2]. However, profits have increasingly come under pressure because of stricter regulatory procedures for drug approval, implementation of price control policies, and increased competition by generic drugs [3],[4]. Pharmaceutical companies have responded by developing a number of tactics to extend market monopoly. These are known as “evergreening” strategies, or more euphemistically as “life cycle management”, with sometimes questionable benefit to society [5].
One common strategy is the patenting and marketing of a single enantiomer of an already approved drug [6]. When large-scale production of enantiopure compounds was first possible in the 1980s, it was expected that the use of these drugs would translate into direct health benefits for the patient, e.g., in the form of better tolerability [6]. However, there is currently no clear evidence of increased efficacy or tolerability of enantiopure compounds over racemic combinations [5],[7]. The marketing of enantiopure compounds with questionable advantages over the original drug is just one example of an evergreening strategy [8]. Other evergreening techniques include patenting combination formulations, structural analogues, active metabolic types, and slow-release forms [9]. The specific impact of these second-generation products, or “follow-on” drugs, on overall healthcare costs has not been well studied.
Hospitals usually adopt a payer perspective strategy, trying to minimise acquisition costs for their medications [11]. Usually, pharmaceutical companies offer high rebates to hospitals on their brand or follow-on drugs to assure that the hospitals will buy and use their drugs, speculating that hospital prescription patterns may influence prescription patterns in the community in their favour [12]. The objective of our study was to assess the overall costs associated with the prescription of follow-on drugs as a result of an “evergreening” strategy over a 9-y period in the Swiss canton of Geneva. In addition, we aimed to calculate the financial impact of the Geneva University Hospitals (HUG) restrictive drug formulary (RDF) on overall healthcare costs, the so called “spillover effect” [10].
Methods
Study Population and Settings
The Swiss canton of Geneva has a single public hospital system (HUG) providing primary and tertiary care to a total population of 464,000 inhabitants (2010), with 2,000 beds (2008) and approximately 50,000 admissions and 800,000 outpatient visits each year. Community physicians account for an additional 1.2 million outpatient consultations per year [12].
The Swiss healthcare system provides mandatory health insurance with universal access to healthcare for everyone [13]. To encourage utilisation of generic medications, Swiss regulations have allowed pharmacists to substitute brand drug prescriptions with generic equivalents since 2001. In 2006, a 20% patient co-payment was introduced instead of the usual 10% for brand drug prescriptions, when brand drugs did not lower their price.
Like many other hospitals, HUG has implemented a RDF trying to minimise acquisition costs for medications (which may be well below the official market price for some drugs) and to limit the number of medications available in the hospital. Drugs are selected based on their efficacy, safety, and costs. For the purpose of this study we differentiated three settings: (1) inpatient setting: all the prescriptions generated during a hospitalisation, (2) hospital spillover setting: medications prescribed by HUG physicians but dispensed by community pharmacies (e.g., at hospital discharge or in outpatient clinics), and (3) community setting: drug prescriptions dispensed by community pharmacies and not issued by HUG physicians. These settings have different rules. Drug prices are negotiated and prescriptions are restricted for the inpatient setting, while in the other settings, prices are fixed and prescriptions are unrestricted.
Follow-On Drugs Based on Evergreening Strategies
For each evergreening situation we differentiated three categories of drugs marketed at different prices: the initially patented drug, commonly called the brand drug (e.g., brand omeprazole); the generic version of the brand drug, marketed after patent expiration (e.g., generic omeprazole); and the follow-on drug, defined as the brand drug to which an evergreening strategy is applied (e.g., esomeprazole as an active isomer of omeprazole). The follow-on drug is usually marketed by the pharmaceutical company that owns the brand drug, and both drugs are marketed at the same time in most cases, effectively making them competitors.
We identified eight follow-on drugs available in the canton of Geneva between 1 January 2000 and 31 December 2008: three drugs for which an isomer had been marketed (levocetirizine as follow-on drug for cetirizine, escitalopram for citalopram, esomeprazole for omeprazole), one active metabolite (desloratadine for loratadine), two combination formulations of the originally patented drug (alendronic acid combined with colecalciferol for alendronic acid alone, simvastatin combined with ezetimibe for simvastatin alone), one slow-release formulation (zolpidem extended release), and one structural analogue (pregabalin for gabapentin).
To analyse the impact of evergreening strategies on overall healthcare spending, we calculated a monthly follow-on market share score—as an indicator of market competitiveness—as the percentage of follow-on drugs (in defined daily doses [DDDs]) of all prescriptions of follow-on, generic, and brand drugs in that category. Follow-on market share scores could therefore range from 0% (no use of the follow-on drug) to 100% (exclusive use of the follow-on drug) [14].
Data Sources
We combined three different administrative registries for our analyses: the HUG hospital registry for patient characteristics, the HUG hospital pharmacy database for drugs dispensed in the inpatient setting, and the OFAC database for drugs dispensed both in the hospital spillover and the community settings. OFAC is a Swiss pharmacist professional organization that serves as an administrative intermediary for 92% of affiliated pharmacies and health insurance companies and covers 80% of the insured population in the canton of Geneva. The pharmacies not affiliated with OFAC are comparable with regard to patient population and location, but patients obtaining prescriptions at a non-affiliated pharmacy send their bills directly to their health insurer.
Institutional Review Board Approval
The HUG Ethics Committee considered the study to be exempt from formal institutional review since it was based upon administrative data without direct patient involvement. All confidential health information was removed to create anonymous analytic datasets in conformity with Swiss data protection regulations.
Costs Calculation and RDF Spillover Effect
“Extra costs” associated with brand and follow-on drug prescriptions in the community were calculated using the World Health Organization's recommended metric, the 2008 DDD, defined as the assumed average maintenance dose per day for a drug used for its main indication in an adult [15]. To analyse the impact of evergreening strategies on healthcare spending, we combined the hospital spillover and community settings. Costs were analysed under three scenarios assuming a replacement with the corresponding generic, when available, of (1) all brand drug prescriptions, (2) all follow-on drug prescriptions, and (3) both follow-on and brand drug prescriptions. The “extra cost” was assessed as the difference between the total cost based on the observed data and the total cost estimated in the three scenarios. Costs were converted from Swiss francs to Euros at the established 2011 exchange rate of €1 = 1.20 CHF. Inflation was not taken into account.
RDF Spillover Effect
We defined the RDF spillover effect by comparing the follow-on market share for the three settings outlined above, i.e., inpatient, hospital spillover, and community. We hypothesised that hospital physicians accustomed to prescribing according to the RDF for their inpatients would be influenced when prescribing in the outpatient clinic, the emergency department, and at discharge, even if under these conditions prescriptions are unrestricted. We analysed the monthly dynamic RDF spillover effects for the only two evergreening strategies that were directly affected by a change in the HUG RDF during the study period. At admission, all patients using a proton-pump inhibitor were switched to the follow-on drug esomeprazole from 1 October 2002 onwards, and all patients on cetirizine and levocetirizine were switched to generic cetirizine from 1 December 2004 onwards.
RDF-Spillover-Associated Costs
To explore the spillover costs or benefits we hypothesised that if the hospital would not have implemented a RDF, the follow-on market share of the hospital spillover and community settings would be equivalent and thus represent the market-driven force. We defined the financial spillover-associated costs as the difference in market share between these two settings. We therefore applied the community follow-on market share to that of the hospital spillover setting and calculated the corresponding “extra costs”.
Statistical Analysis
Demographic variables were expressed as percentages or means with standard deviation. The global “extra costs” and those related to the spillover were assessed using a simulation-based approach. First, monthly community drug consumption was simulated (for each drug and reference) to reproduce the observed data and to introduce the variability of monthly drug consumption into the cost calculation. Monthly drug consumption values were generated from a normal distribution with mean and variance derived from the observed data. A one-way sensitivity analysis was conducted by varying the correlation between successive months from 0.0 to 0.5, and a correlation of 0.5 was selected in a conservative way (95% confidence interval was larger). The changes in consumption over time were captured by this simulation procedure. These simulated data corresponded to the base-case scenario. We checked graphically whether the generated monthly drug consumption values fitted to the effective medication use. Second, drug consumption was extrapolated under the three scenarios (generic replacement of brand drugs, follow-on drugs, or both) by applying new prescription rates to the simulated data. In the first scenario, the prescription rate of brand drugs was set to 0 if a generic was available, and the brand drug DDDs were transferred to generic equivalence. The model uncertainty related to the extrapolations was accounted for by introducing a random effect on the prescription rates of generic references: the DDDs transferred to generic drugs were split in the references with rates that varied by 30% (relatively) compared to the observed data. The “extra costs” were the cost differences between the base-case scenario and each of the three scenarios, and the simulations were run 10,000 times. The reported results were the mean extra costs and the 95% confidence interval (percentiles 0.025 and 0.975 of the set of 10,000 values of extra costs). We used a similar approach to derive extra costs from the spillover. Details are given in Text S1. Simulations were performed with R 2.15.1 software (R Foundation for Statistical Computing).
The spillover effect dynamic was analysed under robust time series analysis using autoregressive integrated moving average models according to the Box-Jenkins methodology, which allows the stochastic dependence of consecutive data to be modelled [16],[17]. We used dummy variables (0 before intervention, 1 after) to assess changes in level and slope after introduction of a RDF in the hospital and generics coming to market. Significance tests for parameter estimates at a p-value of <0.05 were used to eliminate the unnecessary terms. Among different models, we chose the most parsimonious one, i.e., the model with the fewest parameters. All final model residuals passed a “white noise” test (based on Ljung-Box statistics). R2 represents the overall fitting of a model. Statistical analysis was performed with Eviews 7 software (QMS).
Results
Study Population Characteristics
During the study period the number of patients receiving either a brand or follow-on product increased from 56,686 patients in 2001 to 131,193 patients in 2008. The most commonly prescribed follow-on medications were esomeprazole and escitalopram (55% and 32% of all patients prescribed follow-on drugs over the entire study period, respectively). Table 1 summarises study population characteristics and medications prescribed.
Tab. 1. Characteristics of patients and medication prescriptions in the hospital and community. 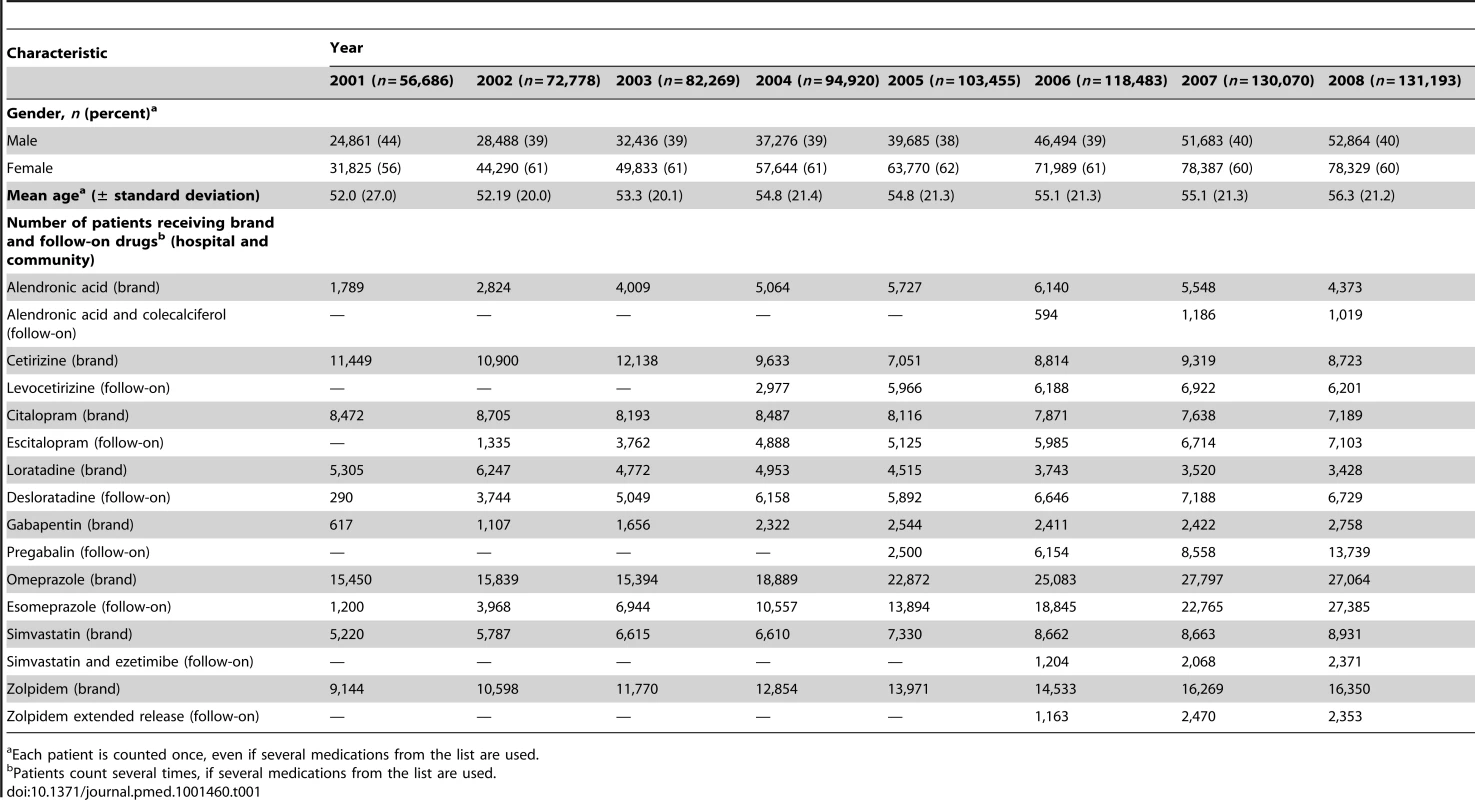
Each patient is counted once, even if several medications from the list are used. Costs and “Extra Costs” Associated with Brand and Follow-On Drug Prescriptions in the Community
Figure 1 demonstrates that between 2000 and 2008, the total cost for all studied drugs was €171.5 (95% CI 170.2; 172.9) million. By category of drug, the total cost was €103.2 (95% CI 102.0; 104.3) million for brand drugs, €41.1 (95% CI 40.6; 42.0) million for follow-on drugs, and €27.2 (95% CI 26.8; 27.6) million for generics. Based on the “extra costs” calculated from scenario 1 (generic replacement of brand drugs) and scenario 2 (generic replacement of follow-on drugs), the healthcare system could have saved, over the entire study period, €15.9 (95% CI 15.5; 16.2) million and €14.4 (95% CI 14.1; 14.7) million if brand and follow-on drug prescriptions, respectively, had been replaced. This amounts to €30.3 (95% CI 29.8; 30.8) million over the entire study period if both brand and follow-on drug prescriptions were replaced at their corresponding community generic selling price equivalents when available (scenario 3).
Fig. 1. Costs and “extra costs” of brand, follow-on, generic, and total prescriptions in millions of Euros. 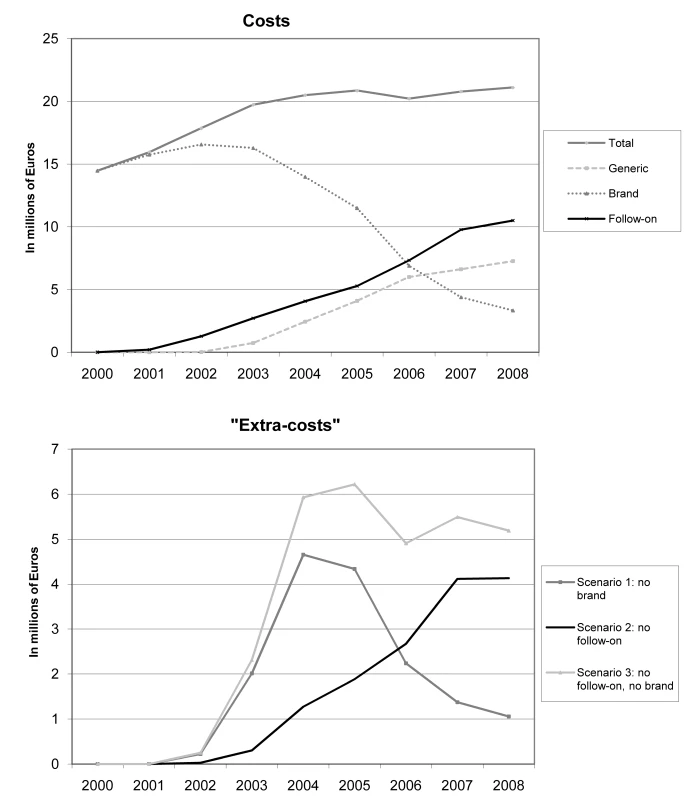
It is noteworthy that “extra costs” attributable to brand drug prescriptions increased sharply between 2002 until 2004. This is a consequence of the increasing availability of generic counterparts: citalopram from 1 October 2002, omeprazole from 1 July 2003, simvastatin from 1 May 2004, cetirizine from 1 September 2004, zolpidem from 1 August 2005, loratadine from 1 July 2006, and alendronic acid and gabapentin from 1 July 2007. After 2004, the potential savings of replacing brand-name drugs with generics gradually decreased, particularly from 2006 onwards, when generic substitution through an additional co-payment cost containment policy was incentivised in Switzerland (Figure 1). This decrease was fully offset by the progressive increase in costs due to the replacement of brand drugs by follow-on prescriptions in the community. Table 2 illustrates the impact of each follow-on drug on total “extra costs” over the study period by scenario. Esomeprazole (41.5%), escitalopram (31.7%), and the combination of simvastatin and ezetimibe (17.6%) were the major contributors to these “extra costs”.
Tab. 2. “Extra costs” in millions of Euros, 95% CIs, and percent of total prescriptions for three scenarios. 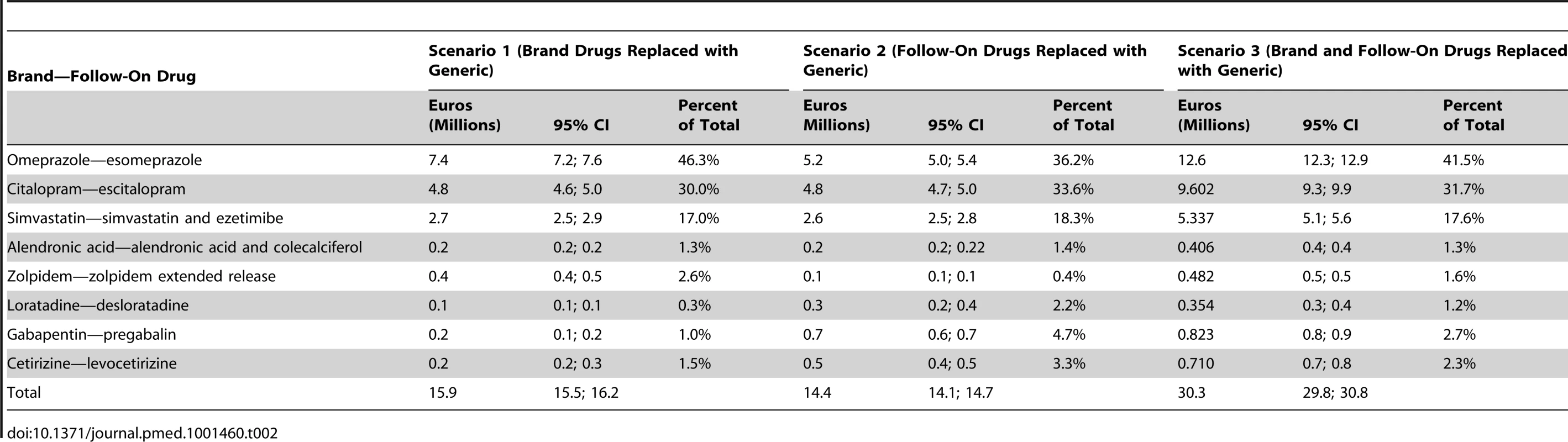
Spillover Effect and Associated “Extra Costs”
Esomeprazole spillover
The RDF spillover effect is illustrated for esomeprazole (Figure 2A) and generic cetirizine (Figure 2B) in Figure 2. From October 2002, when the HUG RDF switched to esomeprazole, until July 2003, when generic omeprazole was marketed, the esomeprazole hospital spillover market share moved from 5.2% (p<0.05) to 35.8% (p<0.05). During this same period, no statistically significant change in trend or level was observed in the community setting. From July 2003 onwards, we observed a statistically significant increase in trend in both hospital spillover and community settings, leading to 70.3% (p<0.05) and 41.0% (p<0.05) esomeprazole market share, respectively, in December 2008. We found a significant first-order correlation (p<0.05) for the hospital spillover setting, and R2 for both autoregressive integrated moving average models was 99%.
Fig. 2. Esomepraprole and levocetirizine market share. 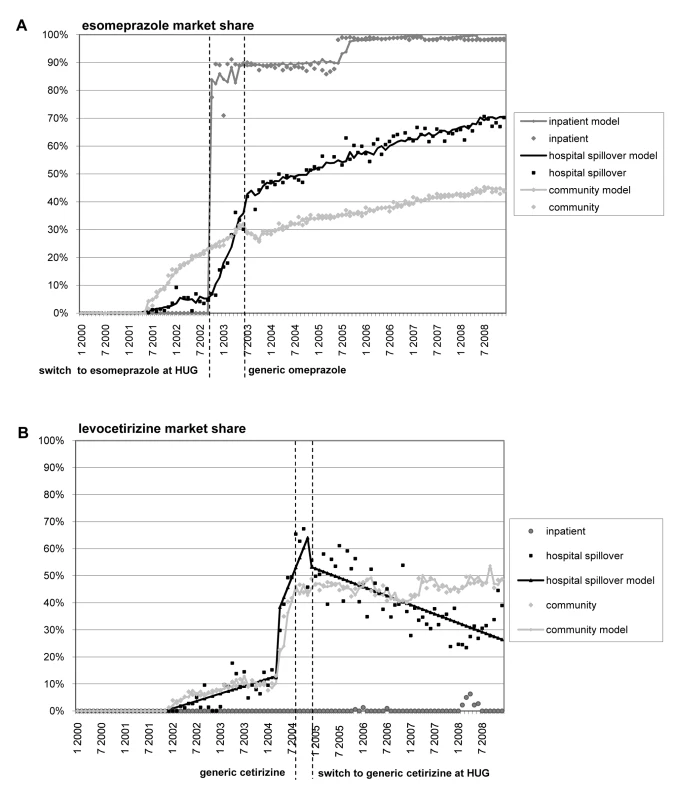
This figure shows changes in esomepraprole (A) and levocetirizine (B) market share before and after changes for these drugs in the HUG RDF and generics coming to market. Generic cetirizine spillover
Six months prior to the generic drug entering the market (September 2004), the pharmaceutical company producing brand cetirizine removed the drug from the reimbursement list, shifting the levocetirizine market share from 12.8% (p<0.05) to 56.7% (p<0.05) in the hospital spillover setting, and from 10.2% (p<0.05) to 43.2% (p<0.05) in the community setting. From December 2004 onwards, when the RDF switched from brand to generic cetirizine, we observed a statistically significant decrease in trend only in the hospital discharge setting, leading to a 26.4% (p<0.05) levocetirizine market share in December 2008, compared to 48.6% (p<0.05) in the community setting. There was no autocorrelation for the hospital spillover setting. R2 was 90% for the hospital spillover setting and 98% for the community setting.
Spillover-associated extra costs
Table 3 shows the time frame of the specific decisions taken by the hospital for its RDF and the associated “extra costs” of these decisions. It demonstrates that the diffusion of hospital prescription patterns into the community resulted in an “extra cost” of €503,600 (95% CI 444,500; 563,100) over the study period, mainly attributable to esomeprazole (€330,300 [95% CI 276,100; 383,800]) and escitalopram (€192,300 [95% CI 168,500; 215,900]). However, we demonstrated that spillover can also be beneficial for the healthcare system, e.g., if a generic drug is listed in the RDF (cetirizine −€7,700 [95% CI −11,100; −4,100]).
Tab. 3. Time frame of changes in the hospital drug formulary (RDF) and spillover-associated “extra costs” (95% CI) in thousands of Euros. 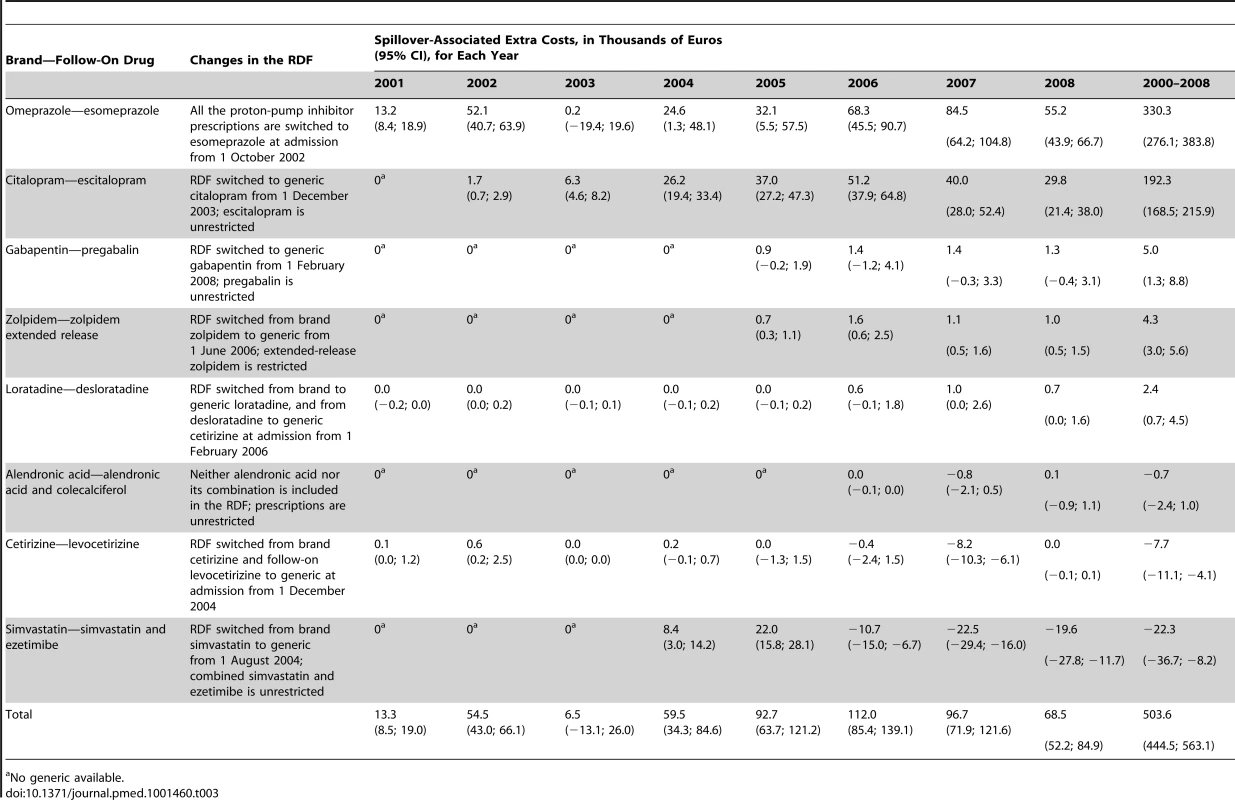
No generic available. Discussion
Our study demonstrates that the evergreening strategies of the pharmaceutical industry have been successful in the canton of Geneva with regard to several brand drugs facing intense price competition from generics after losing their patent protection. The generic competition and co-payment incentive implemented in Switzerland in 2006 most likely contributed to an increasing replacement of brand with generic drugs and also reduced prices for brand drugs [18]. However, we found that this effect was fully offset by the successful marketing of follow-on drugs.
We demonstrate that the total healthcare expenditure “volume” for the examined drugs was constant over the study period, despite the increasing availability of cheap generics; this is comparable to the “squeezing the balloon” phenomenon [19]. These results suggest that, absent other policies and strategies to offset evergreening marketing tactics, the potential of generic medicines as a key strategy to decrease drug costs is unlikely to be successful.
While the patent system's main purpose is to incentivise innovation, it is sometimes used (or abused) to stifle competition. A recently published study identified 108 patents for two antiretroviral drugs (ritonavir and lopinavir/ritonavir) that could delay competition with generics until at least 2028, well beyond the usual 20-y period [9]. Some of these patents were judged by Amin et al. [9] to shelter “innovations” of very limited value. A similar argument may be made for some of the follow-on drugs examined in this study. The results of several studies suggest that the clinical benefit of follow-on drugs over the original brand drugs (or their generics) is unclear or marginal at best [5],[6],[20],[21]. To determine the true benefits of follow-on drugs, well-conducted randomised controlled clinical trials comparing them with the corresponding brand-name or generic drugs at equivalent dosages would be necessary, but few of these trials exist, and for most follow-on drugs, it is unlikely that these trials will ever be conducted [21],[22]. In order to minimise the impact of follow-on drug prescriptions on healthcare costs in the United Kingdom, Hughes and colleagues have suggested that before “evergreened” drugs are marketed, they should first undergo a cost-efficacy comparison process with their generic or brand-name counterparts [5],[23]. In the absence of direct comparative data, however, this may prove difficult.
While cost containment policies seem vital in a time of steadily increasing healthcare costs in many Western countries, it should be kept in mind that excessive pressure on pharmaceutical companies may also lead to the unintended consequence of reduced investment in innovation. Therefore, Hitchings et al. recently recommended that policy makers not cut follow-on drug access, but alert prescribers and medical students about evergreening strategies [7]. It remains to be seen if such an approach can be successful, given that it would have to compete with the intense promotional activities by pharmaceutical representatives in many settings [24].
Our study also confirmed the “spillover effect” of the hospital RDF on prescriptions in the community, leading to an increase in healthcare expenditures as a whole. Other studies have found similar results. Feely et al. found that hospital-initiated prescriptions were responsible for an increase in the volume and subsequent cost of prescriptions in general practices in Ireland [25]. Another study in California demonstrated that physicians who had many patients receiving Medicaid (the program for low-income families and individuals) generated a significant increase in prescriptions of drugs on Medicaid's drug list in their non-Medicaid-affiliated patients [10].
To our knowledge, our study is the first to show the specific impact on costs of follow-on drugs integrated into a hospital RDF. We demonstrated that this can influence prescription patterns in the community and benefit drug manufacturers: gains generated by increased prescription of follow-on drugs in the community through the “spillover phenomenon” can greatly exceed the cost of rebates offered to hospitals. On the other hand, we also showed with the example of generic cetirizine that a RDF can contribute to reduced overall costs for the healthcare system. Furthermore, our study illustrates that the drug manufacturer's removing brand cetirizine from the reimbursement list prior to the generic drug coming onto the market accelerated the therapeutic switch from brand cetirizine to levocetirizine both in the hospital spillover and community settings.
Strengths and Limitations
This study has several strengths. First, we used a single data source to analyse the financial impact of follow-on and brand prescriptions in the canton of Geneva over a 9-y period. This database includes more than 73% of the total of insured patients, thus guaranteeing a uniform and large data collection system [26]. Second, we analysed a specific geographical area that includes not only one major public, university-affiliated hospital but also private clinics and physician practices. This made it possible to measure the interaction between hospital and community prescriptions and to show how the hospital contributed to increased healthcare costs by taking an exclusive payer perspective when selecting follow-on drugs into its RDF. Third, by measuring prescriptions over an 9-y time period we not only were able to measure the follow-on market share at a given time point, but—using time series analysis—could also demonstrate that a hospital RDF can have a significant impact on drug prescriptions.
Our study also has several limitations. First, we assumed that health outcomes for patients would be the same regardless of which type of drug was prescribed (brand, generic, or follow-on). Whether this assumption is correct in all cases still needs to be demonstrated [5],[27]. Second, the three scenarios analysed were based on the assumption that all brand and/or follow-on prescriptions would be switched to generics. This approach does not take into account the fact that some patients may prefer the galenic formulation of certain brand or follow-on drugs and may thus be reluctant to switch to the generic equivalent [28]. Third, we were also unable to measure the impact on adherence to treatment and health outcome of the substitution of patients' personal medications for RDF drugs at hospital admission [29]. Fourth, our study analysed only a single Swiss canton, limiting the generalisability of our findings. Finally, we did not examine complementary strategies developed by drug manufacturers to promote brand and follow-on drugs, such as physician education and visits of pharmaceutical representatives.
Conclusion
Drug manufacturers have developed various “evergreening” strategies that contribute to increased overall healthcare costs. The study provides further evidence that cost-saving policies encouraging generic medicine prescriptions, which can have substantial savings for healthcare expenditures, may be offset by increased costs from follow-on drugs. A hospital's attempts to minimise its own medication costs can, as an unintended consequence, lead to increased overall community healthcare expenditure through “spillover effects”.
Supporting Information
Zdroje
1. AgranatII, CanerH (1999) Intellectual property and chirality of drugs. Drug Discov Today 4 : 313–321.
2. MunosB (2009) Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov 8 : 959–968.
3. TimonenJ, KarttunenP, BengtstromM, AhonenR (2009) The impact of generic substitution on the turnover and gross margin of pharmaceutical companies a survey 1 year and 5 years after the introduction of generic substitution in Finland. Health Policy 92 : 116–123.
4. LexchinJ (2004) The effect of generic competition on the price of brand-name drugs. Health Policy 68 : 47–54.
5. HughesDA, FernerRE (2010) New drugs for old: disinvestment and NICE. BMJ 340: c572.
6. SomogyiA, BochnerF, FosterD (2004) Inside the isomers: the tale of chiral switches. Australian Prescriber 27 : 47–49.
7. HitchingsAW, BakerEH, KhongTK (2012) Making medicines evergreen. BMJ 345: e7941.
8. AgranatI, CanerH, CaldwellJ (2002) Putting chirality to work: the strategy of chiral switches. Nat Rev Drug Discov 1 : 753–768.
9. AminT, KesselheimAS (2012) Secondary patenting of branded pharmaceuticals: a case study of how patents on two HIV drugs could be extended for decades. Health Aff (Millwood) 31 : 2286–2294.
10. WangYR, PaulyMV (2005) Spillover effects of restrictive drug formularies: a case study of PacifiCare in California. Am J Manag Care 11 : 24–26.
11. TordoffJM, MurphyJE, NorrisPT, ReithDM (2006) Use of centrally developed pharmacoeconomic assessments for local formulary decisions. Am J Health Syst Pharm 63 : 1613–1618.
12. LjungbergC, LindbladAK, TullyMP (2007) Hospital doctors' views of factors influencing their prescribing. J Eval Clin Pract 13 : 765–771.
13. The Federal Authorities of the Swiss Confederation (1994) Loi fédérale du 18 mars 1994 sur l'assurance-maladie (LAMal). RS 832.10. Available: http://www.admin.ch/ch/f/rs/c832_10.html. Accessed 30 April 2013.
14. MuijrersPE, GrolRP, SijbrandijJ, JanknegtR, KnottnerusJA (2005) Differences in prescribing between GPs: impact of the cooperation with pharmacists and impact of visits from pharmaceutical industry representatives. Fam Pract 22 : 624–630.
15. WHO Collaborating Centre for Drug Statistics Methodology (2013) ATC/DDD index 2013 [database]. Available: http://www.whocc.no/atc_ddd_index/. Accessed 30 April 2013.
16. HelfensteinU (1996) Box-Jenkins modelling in medical research. Stat Methods Med Res 5 : 3–22.
17. MorganOW, GriffithsC, MajeedA (2007) Interrupted time-series analysis of regulations to reduce paracetamol (acetaminophen) poisoning. PLoS Med 4: e105 doi:10.1371/journal.pmed.0040105
18. DylstP, SimoensS (2011) Does the market share of generic medicines influence the price level?: a European analysis. Pharmacoeconomics 29 : 875–882.
19. PetersonLR (2005) Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistance. Clin Microbiol Infect 11(Suppl 5): 4–16.
20. SvenssonS, MansfieldPR (2004) Escitalopram: superior to citalopram or a chiral chimera? Psychother Psychosom 73 : 10–16.
21. LandefeldCS, SteinmanMA (2009) The Neurontin legacy—marketing through misinformation and manipulation. N Engl J Med 360 : 103–106.
22. VedulaSS, BeroL, SchererRW, DickersinK (2009) Outcome reporting in industry-sponsored trials of gabapentin for off-label use. N Engl J Med 361 : 1963–1971.
23. RichterJE, KahrilasPJ, JohansonJ, MatonP, BreiterJR, et al. (2001) Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol 96 : 656–665.
24. SondergaardJ, VachK, KragstrupJ, AndersenM (2009) Impact of pharmaceutical representative visits on GPs' drug preferences. Fam Pract 26 : 204–209.
25. FeelyJ, ChanR, McManusJ, O'SheaB (1999) The influence of hospital-based prescribers on prescribing in general practice. Pharmacoeconomics 16 : 175–181.
26. AchermannR, SuterK, KronenbergA, GygerP, MuhlemannK, et al. (2011) Antibiotic use in adult outpatients in Switzerland in relation to regions, seasonality and point of care tests. Clin Microbiol Infect 17 : 855–861.
27. GrantK, Al-AdhamiN, TordoffJ, LiveseyJ, BarbezatG, et al. (2006) Continuation of proton pump inhibitors from hospital to community. Pharm World Sci 28 : 189–193.
28. GreeneJA, KesselheimAS (2011) Why do the same drugs look different? Pills, trade dress, and public health. N Engl J Med 365 : 83–89.
29. WuEQ, YuAP, LauzonV, RamakrishnanK, MarynchenkoM, et al. (2011) Economic impact of therapeutic substitution of a brand selective serotonin reuptake inhibitor with an alternative generic selective serotonin reuptake inhibitor in patients with major depressive disorder. Ann Pharmacother 45 : 441–451.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 6- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Uncovering Treatment Burden as a Key Concept for Stroke Care: A Systematic Review of Qualitative Research
- Bigotry and Oppressive Laws in Africa Drive HIV in Men Who Have Sex with Men
- Household Air Pollution in Low- and Middle-Income Countries: Health Risks and Research Priorities
- The Health Effects of Motorization
- The Role of Adiposity in Cardiometabolic Traits: A Mendelian Randomization Analysis
- Patented Drug Extension Strategies on Healthcare Spending: A Cost-Evaluation Analysis
- The Effect of Intermittent Antenatal Iron Supplementation on Maternal and Infant Outcomes in Rural Viet Nam: A Cluster Randomised Trial
- Prevalence of Consensual Male–Male Sex and Sexual Violence, and Associations with HIV in South Africa: A Population-Based Cross-Sectional Study
- Associations between Active Travel to Work and Overweight, Hypertension, and Diabetes in India: A Cross-Sectional Study
- Addressing the Wicked Problem of Obesity through Planning and Policies
- Serum Iron Levels and the Risk of Parkinson Disease: A Mendelian Randomization Study
- Targeting Asymptomatic Malaria Infections: Active Surveillance in Control and Elimination
- Malignant Neglect: The Failure to Address the Need to Prevent Premature Non-communicable Disease Morbidity and Mortality
- Diet and Physical Activity for the Prevention of Noncommunicable Diseases in Low- and Middle-Income Countries: A Systematic Policy Review
- Modern Medicine Is Neglecting Road Traffic Crashes
- Integrating Health Care Delivery and Data Collection in Rural India Using a Rapidly Deployable eHealth Center
- Rising Health Care Costs and Life-Cycle Management in the Pharmaceutical Market
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Diet and Physical Activity for the Prevention of Noncommunicable Diseases in Low- and Middle-Income Countries: A Systematic Policy Review
- Addressing the Wicked Problem of Obesity through Planning and Policies
- Modern Medicine Is Neglecting Road Traffic Crashes
- Uncovering Treatment Burden as a Key Concept for Stroke Care: A Systematic Review of Qualitative Research
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



