-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
How Industry Uses the ICMJE Guidelines to Manipulate Authorship—And How They Should Be Revised
article has not abstract
Published in the journal: . PLoS Med 8(8): e32767. doi:10.1371/journal.pmed.1001072
Category: Perspective
doi: https://doi.org/10.1371/journal.pmed.1001072Summary
article has not abstract
Summary Points
-
Academic authorship boosts the credibility of industry publications and masks their commercial function.
-
Alongside traditional “guest authorship” and ghostwriting, industry may simply exaggerate the contribution of named academic authors and downplay that of commercial writers, who are excluded from authorship but listed as contributors in the small print.
-
Rather than obstructing industry, the current International Committee of Medical Journal Editors (ICMJE) authorship guidelines provide a ready tool for misattributing authorship. Industry also relies on selective interpretations of key authorship concepts.
-
The ICMJE guidelines should be fundamentally revised and the concept of origination given comparable importance to authorship and contributorship.
-
Companies and writers who work on industry publications should be listed as byline authors.
Introduction
Scientists and clinicians need to know the authorship, author interests, and origination of the articles they read to judge them appropriately. Since 1985, the International Committee of Medical Journal Editors (ICMJE) has provided evolving guidance on how authorship should be managed in the complex setting of modern biomedical science [1],[2], to the benefit of the published literature. Issues such as accountability, fraud, conflicts of interest, trial registration, and access to data have been considered by this voluntary, self-funded, closed-membership group of select general medical journal editors (http://www.icmje.org/) [3]–[5]. However, certain industry practices, including publications planning, ghostwriting, and guest authorship, have yet to be adequately addressed. On the basis of industry publications and documents, textual analysis, and direct working experience in the “medical communications” sector, I show here how pharma has succeeded not merely in outmaneuvering the ICMJE guidelines, but is able to use them as the basis for inappropriate attributions of authorship.
Commercial Origination
Industry trials and publications have scientific but also commercial functions. In its dealings with academia, industry takes the misguided view that it should exaggerate the former and conceal the latter. Effective publications planning based on this premise enables industry to exert substantial control over the literature on its products and configure understandings of medicine such that their use seems reasonable [6]–[10]. Industry has realized that origination is a key determinant of how publications are perceived, and one that current guidelines do not adequately address. Accordingly, while collaboration between industry and academia can have benefits, academic authors groomed as “key opinion leaders” (KOLs) [6],[11] may be used not only to endorse publications, but also to convey the impression the publications were originated by academics. “Medical communications” agencies bear joint responsibility for these practices, and for the systematic masking of corporate origination within the medical literature. Industry claims its activities are ethical, but this is disingenuous and rests on two subtle strategies: first, the use of weak definitions or convenient understandings of concepts such as accountability, responsibility, authority, intellectual contribution, contributorship, guest authorship, and ghostwriting; and second, the exploitation of flaws in current guidelines, particularly those of the ICMJE.
The Authorship-Contributorship Distinction Exploited
The important distinction between authorship and contributorship proves especially helpful to industry. Although contributorship was proposed as a replacement for authorship [12],[13], contributorship listings have acquired unintended parallels with advertising small print, and accordingly are used by industry to reduce its own visibility—despite, ironically, being used as the basis of claims to transparency. Through the exploitation of contributorship, crude ghostwriting and guest authorship are being replaced by more subtle exaggeration or understatement of authorial contributions. This practice is difficult to trace, since it involves subjective judgments, and the parties involved—companies, writers, and KOLs —all have incentives to allow their true levels of contribution to be aggrandized or downplayed. These practices gain succor from weak definitions of ghostwriting and ghost authorship, which the World Association of Medical Editors (WAME) and Council of Science Editors (CSE) deem not to have occurred if a writer is “mentioned in the manuscript” (WAME) or receives an “appropriate” place “in the author byline or Acknowledgments” (CSE) [14],[15]. Industry and medical writers' organizations are thus able publicly to condemn ghostwriting using comparable framings [16]–[18], while the misattribution of authorship remains widespread.
From industry's perspective, the most useful feature of the current ICMJE guidelines is the formula used to distinguish between authors and contributors (Figure 1). To qualify as an author, an individual must (1) contribute substantially to either conception and design, or acquisition of data, or analysis and interpretation of data; and (2) draft the article or revise it critically for important intellectual content; and (3) be responsible for final approval of the manuscript [2]. This “triple-lock” formula has become a de facto license for misrepresentation. Provided academics make some contribution to design or data analysis, some revisions to a manuscript, and approve it, they are required to be named as authors. By contrast, industry may conduct most of the design, data collection and analysis, and all the writing, but if sign-off is ceded to the academic, it is disqualified from authorship. Unsurprisingly, the practice of ceding final sign-off to academic “authors” is widespread in commercially driven publications.
Fig. 1. The ICMJE “triple lock” formula is a tool for industry. 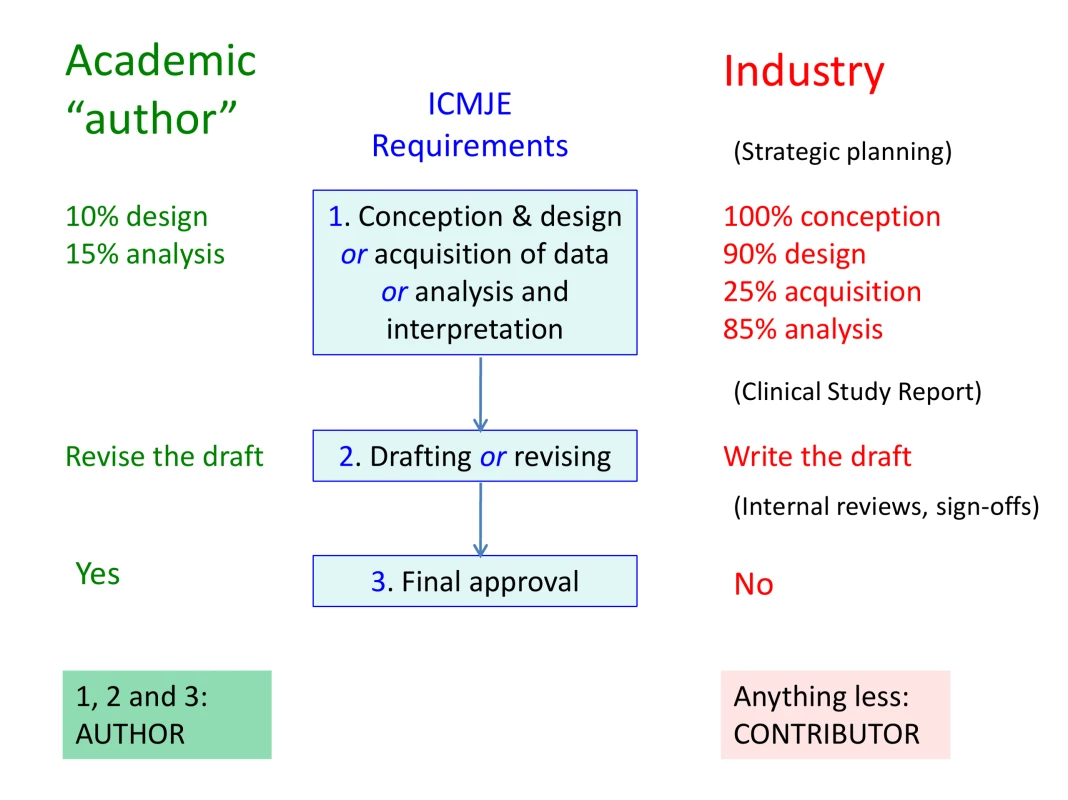
If final sign-off is ceded to the academic “author”, industry is disqualified from authorship even if it is responsible for most of the study and manuscript development. Manuscript writers are also disbarred even if they have sign-off, unless they were involved in other aspects of the study. These features give industry the opportunity to conceal its originating role behind the names of academic collaborators. Industry also carries out further originating activities not catered for in the ICMJE formula (shown in brackets). The percentage contributions shown here are for illustrative purposes only, and in reality vary widely. Further Hazards of the “Triple-Lock”
The “triple-lock” formula also helps downplay the importance of planning and writing texts. Only in clause 2 is “drafting” acknowledged as a component of authorship, but since this clause can be satisfied by revision, it enables the planner and writer to be excluded. In reality, drafting constitutes a substantial intellectual contribution to the form and content of manuscripts. It is for this reason that industry seeks to control it, while evading the visibility of byline authorship. The “triple lock” provides ideal support for these linked objectives.
Industry finds further justification for self-concealment behind KOL “authorship” in the ICMJE requirement that authors should “take responsibility for appropriate portions of the content” of publications [2]. This statement and the “triple lock” are both attempts to implement the connecting concepts of accountability, responsibility, and guarantorship [5],[12],[19]. One difficulty with the current ICMJE wording on “responsibility” is that it emphasizes “content” alone, rather than all aspects of the publication. Furthermore, “content” can be narrowly interpreted by industry to omit the framings, nuanced constructions, and selectivity of data that are crucial to drug marketing [6],[9]. Moreover, “responsibility” and “accountability” are readily conflated by industry with notions of authority and expertise, such that it becomes legitimate for KOLs to assume responsibility for texts they neither originated nor wrote, while the contributions of the true originators are downplayed on the grounds that they lack the “authority” to stand for them. Writers and companies with the ability to generate academic texts should not be permitted to step back from responsibility on such disingenuous grounds; indeed, the true “authority” behind industry publications belongs to corporations, not their academic collaborators. Ultimately, industry's KOL-focused construction of “authority” is designed once again to downplay its own role—but also to appeal to the vanity of KOLs. Sadly, it is a construction that finds a ready reception within the culture of contemporary medicine.
Further aspects of current authorship practices provide support for industry. Notably, the ICMJE guidelines place great emphasis on the contributions of named individuals. This approach reflects traditional authorship customs, but assists unethical practices in two respects. Firstly, it helps entities, and in particular companies, remain concealed, particularly if their authorial role involves many individuals, each of whom is only a minor contributor to the finished publication. Secondly, it gives insufficient exposure to the process of origination by which publications are conceived and come into being—for commercially driven articles that exist to promote specific products, this information is vital to the reader.
Moreover, another deficiency in the current ICMJE guidelines concerns their policy on author access to data [5]. There is no requirement that any authors have permanent access to the study data or the right to re-analyze the data as they choose. Rather, authors need only have had access to the trial data at the time the study was conducted and the publication prepared. This is a weak position for the guidelines to adopt, whereas pharma, by contrast, asserts company ownership of the data in the trials “authored” by its KOLs [20].
In sum, the current ICMJE guidelines provide pharmaceutical and medical communications companies with the opportunity to sequester their contributions in the small print of publications, despite bearing responsibility for conception, design, and analysis of many studies, retaining control of databases, and frequently writing manuscripts, scheduling publications, and selecting journals. KOLs whose contributions may be modest and who lack full permanent access to the data may be the only individuals who qualify for authorship under ICMJE criteria. The current guidelines are therefore not an obstacle but a vehicle through which origination and authorship can be misrepresented to readers in the services of marketing, while enabling the companies involved to claim their conduct is compliant and ethical.
Recommended Changes to Authorship Principles
For 26 years the ICMJE authorship guidelines have evolved by periodic adaptation, and in their current form have achieved broad support, notably from industry and commercial writers [20]–[23]. Clearly, however, a fundamental review is required. Indeed, such is the importance of the guidelines that the process by which they are devised and updated should itself be reviewed. In the meantime, guidelines developed by organizations involved with industry publications [21],[22] should not be recommended by journals to prospective authors. Furthermore, the commercially implanted phrase “industry-sponsored” trials (and publications), which is itself designed to downplay industry's role, should be replaced in the language of journals and medicine by the more truthful phrase “industry trials.”
With respect to the authorship issues discussed in this article, several philosophical clarifications are necessary. Firstly, while the categories of authorship, contributorship, and guarantorship remain important, comparable emphasis should be placed on the concept of origination, which differs from these categories in that it refers to a process rather than individual people. Some journals and indeed industry guidelines have made steps in this direction [24],[25], but these are insufficient. Crucial aspects of origination should be given immediate visibility: for instance, as depicted in Figure 2, companies and medical writers should be included among the named authors whenever appropriate, and both companies and specific drugs supported by the publication should be listed immediately below the author byline. This would enable readers immediately to recognize publications' commercial, as well as scientific, functions (Figure 2).
Fig. 2. Examples of origination bylines and of entities as authors in journal articles. 
Secondly, it should be explicitly acknowledged that planning, drafting, and writing generally constitutes a significant intellectual contribution to a publication, and in most cases should require the individual and/or entity responsible to be listed as a byline author. This position has previously been advocated by Gøtzsche [23] and is implicit in statements by WAME and the policies of some journals [25]–[27]. Receiving input or “direction” from KOLs should not disbar writers from authorship. It should be emphasized that whenever writers are omitted from byline authorship by underplaying their true contribution, this constitutes ghostwriting, including when writers are listed as contributors.
Thirdly, greater provision should be made for authorship by entities, and in particular companies. Whenever an entity carries out activities that in the case of an individual would justify authorship, it should be listed as a byline author.
Fourthly, it should be clarified that responsibility and accountability pertain to all aspects of manuscripts, and all individuals and entities involved in developing them must be accountable. Responsibility for “content” should not be ceded to academic authors alone if others helped plan, write, or revise the manuscript. Academics may remain the only authors with the expertise to guarantee some aspects of content, but other key originators must also take visible responsibility (and credit) as byline authors.
In keeping with these principles, several specific measures should be considered:
-
With respect to the ICMJE guidelines, the “triple-lock” formula for distinguishing authors from contributors should be discarded. A model in which a variety of contributions require an individual or entity to be listed as an author should replace it.
-
For submissions in which companies or similar entities played any role in finance, planning, or development, a separate schedule (or supplement to the competing interests statement) should be completed to identify salient features of origination, including who planned and wrote the piece, which specific products the publication supported, and how it was financed. To ensure completeness, a company lawyer should be required to sign off. A further option would be to treat the agreement to publish as a legal contract, with accurate completion of the schedule a contractual condition.
-
Companies should be encouraged to provide full, permanent data access to academic authors. In all cases in which a company retained control or ownership of a trial database, the company itself should be required to be listed as one of the first three (and therefore cited) byline authors.
Finally, journal publishers and groups such as the ICMJE, CSE, and WAME should seek to establish links with bodies responsible for other aspects of medical-scientific discourse, such as continuing medical education (CME) organizations, professional societies, Web sites, and congresses, with a view to establishing an integrated, international standard of transparency in science [6]. Such a standard should require truthful, prominent display of salient origination and authorship. Academics would be able to place greater trust in articles, presentations, and CME courses bearing the standard's logo, and exercise appropriate caution with those which did not.
The ICMJE guidelines will always be a work in progress, but the adjustments proposed here have the potential to end the self-concealment and authorial misrepresentations that mar industry's contributions to the literature. Furthermore, they have the potential to help industry achieve the enhanced respect its beneficial contributions to medicine deserve. Industry publications will always have a commercial valence alongside their scientific and medical content: this should henceforth be truthfully displayed, and no longer downplayed or concealed.
Zdroje
1. International Committee of Medical Journal Editors 1985 Guidelines on authorship. BMJ 291 722
2. International Committee of Medical Journal Editors 2011 Uniform requirements for manuscripts submitted to biomedical journals: writing and editing for biomedical publications. Available: http://www.icmje.org/. Accessed 6 July 2011
3. DavidoffFDeAngelisCDDrazenJMNichollsMGHoeyJ 2001 Sponsorship, authorship and accountability. CMAJ 165 786 788
4. De AngelisCDDrazenJMFrizelleFAHaugCHoeyJ 2005 Is this clinical trial fully registered? A statement from the International Committee of Medical Journal Editors. Lancet 365 1827 1829
5. DrazenJMde LeeuwPWLaineCMulrowCDeAngelisCD 2010 Toward more uniform conflict disclosures--the updated ICMJE conflict of interest reporting form. N Engl J Med 363 188 189
6. MathesonAD 2008 Corporate science and the husbandry of scientific and medical knowledge by the pharmaceutical industry. BioSocieties 3 355 382
7. Fugh-BermanAJ 2010 The haunting of medical journals: how ghostwriting sold “HRT”. PLoS Med 7 9 e1000335 doi:10.1371/journal.pmed.1000335
8. The PLoS Medicine Editors 2009 Ghostwriting: the dirty little secret of medical publishing that just got bigger. PLoS Med 6 9 e1000156 doi:10.1371/journal.pmed.1000156
9. SismondoSDoucetM 2010 Publication ethics and the ghost management of medical publication. Bioethics 24 273 283
10. EgilmanDDruarN 2011 Corporate versus public interests: community responsibility to defend scientific integrity. International Journal of Occupational and Environmental Health 17 181 185
11. Moynihan R 2008 Key opinion leaders: independent experts or drug representatives in disguise? BMJ 336 1402 1403
12. RennieDYankVEmanuelL 1997 When authorship fails. A proposal to make contributors accountable. JAMA 278 7 579 585
13. SmithR 1997 Authorship is dying: long live contributorship. BMJ 315 696
14. World Association of Medical Editors 2005 Ghost writing initiated by commercial companies. Available: http://www.wame.org/resources/policies#ghost. Accessed 6 July 2011
15. Council of Science 2009 White paper on promoting integrity in scientific journal publications. Available: http://www.councilscienceeditors.org/i4a/pages/index.cfm?pageid=3355. Accessed 6 July 2011
16. International Society for Medical Publication Professionals Issues and Actions Committee 2010 Professional medical writing. Available: http://www.ismpp.org/initiatives/Files/ISMPP_Ghost_Writing_vs_Professional_Medical_Writing.pdf. Accessed 6 July 2011
17. European Medical Writers Association Ghostwriting positioning statement. Available: http://www.emwa.org/Home/Ghostwriting-Positioning-Statement.html. Accessed 6 July 2011
18. American Medical Writers Association 2009 AMWA ethics FAQs. Available: http://www.amwa.org/default.asp?Mode=DirectoryDisplay&DirectoryUseAbsoluteOnSearch=True&id=466. Accessed 6 July 2011
19. IlakovacVFisterKMarusicMMarusicA 2007 Reliability of disclosure forms of authors' contributions. CMAJ 176 41 46
20. Pharmaceu Reasearch and Manufacturers of America 2009 Principles on conduct of clinical trials. Communication of clinical trial results. Washington (D.C.)Pharmaceutical Research and Manufacturers of America Available: http://www.phrma.org/sites/default/files/105/042009_clinical_trial_principles_final.pdf. Accessed 6 July 2011
21. GrafCBattistiWPBridgesDBruce-WinklerVConatyJM 2009 Research methods & Reporting. Good publication practice for communicating company sponsored medical research: the GPP2 guidelines. BMJ 339 b4330 doi:10.1136/bmj.b4330
22. JacobsAWagerE 2005 European Medical Writers Association (EMWA) guidelines on the role of medical writers in developing peer-reviewed publications. Curr Med Res Opin 21 2 317 322
23. GøtzschePCKassirerJPWoolleyKLWagerEJacobsA 2009 What should be done to tackle ghostwriting in the medical literature? PLoS Med 6 2 e1000023 doi:10.1371/journal.pmed.1000023
24. British Medical Journal 2011 Article submission. Provenance of articles. Available : http://resources.bmj.com/bmj/authors/authors/article-submission/submitting-an-article-to-the-bmj#provenance. Accessed 6 July 2011
25. World Association of Medical Editors 2007 Authorship. Available: http://www.wame.org/resources/policies#authorship. Accessed 6 July 2011
26. Neurology 2010 Information for authors. Available: http://www.neurology.org/misc/auth2.xhtml. Accessed 6 July 2011
27. Proceedings of the National Academy of Sciences 2011 Information for authors. Journal policies. Authorship. Available: http://www.pnas.org/site/misc/iforc.shtml#ii. Accessed 6 July 2011
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 8- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Are HIV Epidemics among Men Who Have Sex with Men Emerging in the Middle East and North Africa?: A Systematic Review and Data Synthesis
- Commercial Serological Tests for the Diagnosis of Active Pulmonary and Extrapulmonary Tuberculosis: An Updated Systematic Review and Meta-Analysis
- How Industry Uses the ICMJE Guidelines to Manipulate Authorship—And How They Should Be Revised
- Ghostwriting Revisited: New Perspectives but Few Solutions in Sight
- Legal Remedies for Medical Ghostwriting: Imposing Fraud Liability on Guest Authors of Ghostwritten Articles
- Can Mobile Phone Data Improve Emergency Response to Natural Disasters?
- Government Inaction on Ratings and Government Subsidies to the US Film Industry Help Promote Youth Smoking
- Building the Field of Health Policy and Systems Research: Framing the Questions
- Building the Field of Health Policy and Systems Research: Social Science Matters
- Building the Field of Health Policy and Systems Research: An Agenda for Action
- Corporate Social Responsibility and Access to Policy Élites: An Analysis of Tobacco Industry Documents
- Improved Response to Disasters and Outbreaks by Tracking Population Movements with Mobile Phone Network Data: A Post-Earthquake Geospatial Study in Haiti
- Changes in Drug Utilization during a Gap in Insurance Coverage: An Examination of the Medicare Part D Coverage Gap
- Serological Testing Versus Other Strategies for Diagnosis of Active Tuberculosis in India: A Cost-Effectiveness Analysis
- Neonatal Mortality Levels for 193 Countries in 2009 with Trends since 1990: A Systematic Analysis of Progress, Projections, and Priorities
- Reporting Guidelines for Survey Research: An Analysis of Published Guidance and Reporting Practices
- Four Arguments against the Adult-Rating of Movies with Smoking Scenes
- Being the Ghost in the Machine: A Medical Ghostwriter's Personal View
- Population Density, Water Supply, and the Risk of Dengue Fever in Vietnam: Cohort Study and Spatial Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Commercial Serological Tests for the Diagnosis of Active Pulmonary and Extrapulmonary Tuberculosis: An Updated Systematic Review and Meta-Analysis
- Are HIV Epidemics among Men Who Have Sex with Men Emerging in the Middle East and North Africa?: A Systematic Review and Data Synthesis
- Government Inaction on Ratings and Government Subsidies to the US Film Industry Help Promote Youth Smoking
- Serological Testing Versus Other Strategies for Diagnosis of Active Tuberculosis in India: A Cost-Effectiveness Analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



