-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Research Agenda to Underpin Malaria Eradication
The interruption of malaria transmission worldwide is one of the greatest challenges for international health and development communities. The current expert view suggests that, by aggressively scaling up control with currently available tools and strategies, much greater gains could be achieved against malaria, including elimination from a number of countries and regions; however, even with maximal effort we will fall short of global eradication. The Malaria Eradication Research Agenda (malERA) complements the current research agenda—primarily directed towards reducing morbidity and mortality—with one that aims to identify key knowledge gaps and define the strategies and tools that will result in reducing the basic reproduction rate to less than 1, with the ultimate aim of eradication of the parasite from the human population. Sustained commitment from local communities, civil society, policy leaders, and the scientific community, together with a massive effort to build a strong base of researchers from the endemic areas will be critical factors in the success of this new agenda.
Published in the journal: . PLoS Med 8(1): e32767. doi:10.1371/journal.pmed.1000406
Category: Review
doi: https://doi.org/10.1371/journal.pmed.1000406Summary
The interruption of malaria transmission worldwide is one of the greatest challenges for international health and development communities. The current expert view suggests that, by aggressively scaling up control with currently available tools and strategies, much greater gains could be achieved against malaria, including elimination from a number of countries and regions; however, even with maximal effort we will fall short of global eradication. The Malaria Eradication Research Agenda (malERA) complements the current research agenda—primarily directed towards reducing morbidity and mortality—with one that aims to identify key knowledge gaps and define the strategies and tools that will result in reducing the basic reproduction rate to less than 1, with the ultimate aim of eradication of the parasite from the human population. Sustained commitment from local communities, civil society, policy leaders, and the scientific community, together with a massive effort to build a strong base of researchers from the endemic areas will be critical factors in the success of this new agenda.
Summary Points
-
Malaria remains a major global public health problem, but a recent paradigm shift has moved the emphasis from control of malaria to the interruption of malaria transmission and ultimately malaria eradication
-
The Malaria Eradication Research Agenda (malERA) initiative was convened in 2008 to define the knowledge base, strategies, and tools required to eradicate malaria from the human population
-
A two-year consultative process has resulted in the preparation of a detailed research and development agenda for malaria eradication, which is reported in this Supplement
-
Implementation of this research agenda might enable the elimination of malaria, even in the most difficult areas
-
However, to achieve the aim of malaria eradication in a timely manner, commitment to implementing this agenda must begin immediately
Introduction
The unacceptable health burden of malaria, and its economic and social impacts on development, have made it a focal point of the international development agenda, and the world has embraced an ambitious plan for scaling up malaria control that progresses towards country-by-country and regional elimination and the ultimate goal of global eradication [1]. Over the past decade, resources and control efforts have intensified to a level not seen since the early days of the World Health Organization's Global Malaria Eradication Program (GMEP) in the late 1950s. Nonetheless, in 2009, with 3.28 billion people living in areas that have some risk of malaria transmission and about 1.2 billion people (one-fifth of the world's population) living in areas with a high risk of transmission (more than one reported case per 1,000 population per year), there were about 225 million cases of clinical malaria and 781,000 malaria-related deaths. Today, there is ongoing malaria transmission in 106 countries. Eighty-one of these countries are focusing on control, while 25 are in pre-elimination, elimination, and prevention of reintroduction phases; Morocco, the United Arab Emirates, and Turkmenistan have recently been certified as malaria free [2]–[4].
These statistics emphasize the direness of the current malaria burden but also benchmark the accomplishments and progress that have been achieved in malaria control. Following declarations at the Malaria Forum in October 2007 convened by the Bill & Melinda Gates Foundation, and subsequent support voiced by the World Health Organization (WHO), the Roll Back Malaria (RBM) Partnership, and many other organizations and institutions, the paradigm of malaria control and elimination has been extended to encompass an ultimate goal of malaria eradication [1],[2],[5]. The question is no longer whether international agencies and national health authorities should be mobilized to pursue the goal of malaria eradication, but rather when and how.
A key question, however, is whether elimination from all regions of the world (eradication) is feasible with the current tools and state of knowledge. For a number of reasons, we believe that the answer is “no.” First, malaria is not a single disease. The five Plasmodium species (falciparum, vivax, ovale, malariae, knowlesi) that cause human malaria are transmitted by more than 30 Anopheline mosquito species with diverse breeding and feeding habits, and result in different disease spectra in different population target groups and epidemiological settings. Second, current malaria control and elimination programs face remarkable heterogeneity of transmission dynamics of malaria in endemic areas, including differences in parasite, vector, human, social, and environmental factors. Third, operational limitations include underperforming health services, lack of political will, insufficient financial, social and human resources, and for some areas, inadequate tools to interrupt transmission given an exceedingly high force of transmission. Each country presents different combinations of these problems and their determinants. Thus, a widely held view suggests that with currently available tools, much greater gains could be achieved, including elimination from a number of countries and regions, but that even with maximal effort we will fall short of elimination in many areas and of global eradication [6]. For definitions of terms used regarding malaria eradication see Box 1.
Box 1. Clarifying the Goals and Definitions
-
Control: Reduction of disease incidence, prevalence, morbidity, or mortality to a locally acceptable level as a result of deliberate efforts; continued intervention measures are required to maintain the reduction.
-
Elimination: Reduction to zero of the incidence of locally transmitted malaria infection in a defined geographical area as a result of deliberate efforts; continued intervention measures are required to prevent reestablishment of transmission.
-
Eradication: Permanent reduction to zero of the global incidence of malaria as a result of deliberate efforts; intervention measures are no longer needed [1].
-
What species? Although the eradication of P. falciparum, the most serious form of malaria, would constitute an historic public health achievement, the coexistence of transmission of P. falciparum and P. vivax in many areas of the world together with the fact that they are the species responsible for the major burden of disease, make it necessary to aim for the eradication of both.
Mixed Success and Failure of Past Malaria Control and Elimination Efforts
A detailed discussion of all the factors involved in the partial success of the past eradication campaign is beyond the scope of this introduction, but three critical elements can be highlighted. First, there was insufficient recognition of the heterogeneity of malaria transmission and disease. Much of the optimism that inspired WHO GMEP in 1955 was based on the successful outcomes of earlier control programs that benefited from a combination of biological, parasitological, social, and environmental factors that favoured success (e.g., the rarity of DDT-resistant Anophelines and of chloroquine-resistant parasites). Second, the first WHO GMEP (1955-1969) was predicated on an assumption that the available knowledge and tools were sufficient to achieve worldwide eradication. A single strategy that would work everywhere—“one size fits all”—proved to be ill-founded because it underestimated the challenges of dealing with the extremely efficient vectors in Africa (An. gambiae) and with transmission by outdoor-feeding mosquitoes that were not susceptible to attack by indoor residual insecticide. It also did not allow for the lack of safe drugs for mass administration to remove all infectious parasites from symptomatic and asymptomatic carriers, particularly from people carrying P. vivax or P. ovale, species that establish latent liver infections that are responsible for relapses months or years following initial infection. Third, insufficient research in biomedical and social sciences and inadequate local application of research findings across a wide variety of settings are widely viewed to have contributed to demoralization and waning effort when tools proved ineffective or could not be adequately implemented. The neglect of malaria research during and after the campaign did long-term damage. These elements resulted in a lack of progress that in turn compromised continued financial support [7].
Current Malaria Control Efforts: The Goal of Eradication and Its Research and Development Implications
The past decade has witnessed renewed investment in malaria control and substantial increases in funding for malaria research. The Roll Back Malaria Global Malaria Action Plan (GMAP) and WHO have recently revised and updated the strategy and the steps for scaling up and sustaining malaria control (Figure 1). In addition, the Malaria Elimination Group (MEG), a group of scientists, public health decision makers, control program managers, and funders, has compiled a guide to policy makers for areas that embark or have embarked on elimination strategies [8].
Fig. 1. Epidemiological milestones [1],[23]. ![Epidemiological milestones <em class="ref">[<b>1</b>]</em>,<em class="ref">[23]</em>.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/7da8bf322903e9f14f61e5d43ac9ff37.png)
Image credit: Fusión Creativa. Reductions in disease incidence are being documented, even in some areas of sub-Saharan Africa that constitute the heartland of malaria transmission [2]. There are, however, significant threats to current progress that cannot be ignored, and unmet needs that will continue to be central to the global research agenda for improving malaria control and eventually achieving eradication. Notable examples are the emergence of artemisinin resistance and the consequent need for improved strategies to contain dissemination of resistant parasite strains coupled with accelerated research into potential new drugs for first-line treatment [9],[10]. Similarly, new insecticides are urgently needed to replace those threatened by increased mosquito resistance [11], and accelerated development of vaccines that can impact on malaria incidence, disease, and death remains a high priority [12].
Complementing the current research agenda—primarily directed towards improving malaria control and reducing morbidity and mortality—with research on developing tools, interventions, and strategies to interrupt transmission and ultimate eradication of the parasite from the human population constitutes a true paradigm shift.
The malERA Initiative
To catalyze this paradigm shift towards malaria elimination and eradication, it was necessary to design a process to bring together the best scientific minds in the malaria community. That process is the Malaria Eradication Research Agenda (malERA) initiative, which was established to complement GMAP and which aims to define the critical knowledge base, strategies, and tools required to reduce the basic reproduction rate (R0 or the number of secondary cases arising from a single case) to less than one.
Scientists involved in malaria research were challenged to develop a multidisciplinary, global research and development agenda that would be actionable by research and public health agencies and funders/sponsors and available for discussion and debate through publication in a readily accessible format. The process engaged more than 250 scientists in a series of 20 consultations around the world (Figure 2) and was managed by a three-tier governance structure (Figure 3). The rest of this article briefly introduces the work undertaken by the various malERA Consultative Groups and presented in the other articles in this Supplement.
Fig. 2. Consultative process towards a consolidated research and development agenda for malaria eradication. 
Image credit: Fusión Creativa. Fig. 3. The malERA governance bodies. 
Image credit: Fusión Creativa. Tools to Interrupt Malaria Transmission
To reduce the basic reproduction rate to less than 1, and hence to interrupt transmission, interventions are needed to reduce the reservoir of infection, the time that a person or a mosquito is infectious, and the rate at which infections are spread. This goal can be achieved by drugs or vaccines directed against the parasite or by new tools that attack the vector, with the support of improved diagnostics and surveillance.
Drugs: Single Encounter Radical Cure and Prophylaxis
In the recent past, drug development efforts were guided by the need for first-line drugs to treat P. falciparum infections with an increasing emphasis on drugs with a short half-life that potentially minimize the risk of development of resistance rather than on drugs with a long half-life that have benefits for dosing and post-treatment prophylaxis [13]. Treatment of infected individuals with a variety of drug regiments has been used successfully in combination with intensive vector control to eliminate malaria from areas with relatively strong health systems and stable populations. However, interruption of malaria transmission is likely to require a new set of drugs and formulations.
As described in more detail in the article by the malERA Consultative Group on Drugs [14], such drugs will need to be used both in stable transmission areas and in complex urban or remote rural areas, with poorly functioning health systems where concerted campaigns may be the only way of achieving high coverage or preventing reintroduction by migrants or travelers from endemic regions. For such campaigns to impact effectively on inaccessible populations, a single encounter between health providers and target populations is critical. Single Encounter Radical Cure and Prophylaxis (SERCaP) has a target product profile (TPP) that includes radical cure, defined as elimination of all parasites (including the long-lived hypnozoites of P. vivax or P. ovale in the liver), suitability for mass administration (including administration to healthy subjects and the consequent need of a very good safety profile), and prophylaxis for at least 1 month after treatment, to outlast the typical development period of Plasmodia parasites in Anopheline mosquitoes. A drug with this profile would perform in a similar way to a highly efficacious pre-erythrocytic (infection-preventing) vaccine.
A drug with this TPP may take a long time to develop, but the development of new drugs that meet some of these essential requirements could dramatically improve chances of eradication. For example, development of new safe and effective drugs that block the infectivity of the mature sexual forms of P. falciparum gametocytes and/or the dormant hepatic forms (hypnozoites) of P. vivax could have a profound impact on transmission rates and would be valuable tools in the efforts to contain and eliminate parasite strains resistant to first-line treatment drugs. Presently, only the 8-aminoquinolines are known to be effective against both P. vivax hypnozoites and P. falciparum stage-five gametocytes. Unfortunately this class of drugs has significant side-effects in some individuals, particularly hemolysis in those with G6PD deficiency, that compromise their widespread use in mass administration for elimination [14].
Vaccines that Interrupt Malaria Transmission
Vaccines currently in clinical development have the primary aim of reducing morbidity and mortality from P. falciparum in young children living in highly endemic countries. However, with the new goal of elimination and eradication, vaccines that will reduce and contribute to interruption of transmission also need to be developed. The broader concept of “vaccines that interrupt malaria transmission (VIMT)” is introduced by the malERA Consultative Group on Vaccines to replace the term “transmission blocking vaccines” (TBVs), which has been used widely to refer to vaccines that target only the sexual and mosquito stages of the parasite [15]. VIMT could include antivector vaccines that target mosquito molecules essential for parasite development, highly effective pre-erythrocytic or erythrocytic stage vaccines, and vaccines targeting parasite antigens of sexual and mosquito stages of the infection. The desired TPP identified by the Consultative Group for VIMT indicates that they should be effective against both P. falciparum and P. vivax, suitable for administration to all age groups, and should impact transmission. Other issues discussed by the group in their article include the need for validated functional assays that measure the reduction in infectivity at the individual level after vaccination that could be used as surrogate measures to predict reductions in transmission rates at the community level. Such surrogate measures will be critical components of a regulatory pathway leading to licensure. Standardized, specific and sensitive methods for assessment of transmission rates, particularly when intensity is low, will be critical in the assessment of vaccine efficacy in interrupting transmission following large-scale deployment of vaccination as an elimination tool [15],[16].
Vector Control
The overarching goal of vector control is to reduce the vectorial capacity of local vector populations below the critical threshold to prevent ongoing or epidemic transmission. Because it takes a relatively long time (days) after ingestion for Plasmodia to become infective to humans in its Anopheles vectors, the most effective vector control strategies currently in use rely on interventions like indoor residual insecticide spraying and insecticide treated bednets (ITNs) that reduce vector daily survival rates [17].
The malERA Consultative Group on Vector Control identifies three critical challenges in its article [18]. The most pressing challenge is the development of a coherent research agenda for discovering and developing a broader range of insecticides, with novel modes of action that can circumvent emerging resistance to existing insecticides, in particular, pyrethroid-based insecticides [11]. The second challenge is the development of interventions that affect vectors that do not rest or feed indoors and are therefore not susceptible to current tools. The final critical challenge is the development of novel approaches that permanently reduce the high vectorial capacities of the dominant malaria vectors in sub-Saharan Africa. Genetic control programs based on permanent reduction of the vectorial capacities of natural vector populations have received the most attention to date [19],[20], but the Consultative Group also considers the development of other novel approaches [18].
Diagnostics
Methods for measuring transmission are central to an elimination agenda. Current methods for measuring transmission that may be applied in endemic areas are time-consuming, expensive, and too insensitive for use in conditions of low and nonuniform infection [21],[22]. Some years after regional elimination, as immunity declines, infection is likely to be symptomatic and may become the best marker of resumed transmission. However, during the early elimination phase in regions previously experiencing high transmission, populations will retain clinical immunity and will not experience symptomatic disease with every infection [23]. Thus, the main challenge identified by the malERA Consultative Group on Diagnoses and Diagnostics and discussed in detail in their article and in the article on Cross-cutting Issues for Eradication [24],[25] is to find a robust, sensitive, and specific standardized method for assessment of transmission intensity in the intervening period when transmission continues at low and nonrandom levels. Improved serological tests have been suggested [26], but other minimally invasive biomarkers could be considered. This information will be essential for modeling potential effects of various interventions alone, or in combination, and for assessing efficacy of transmission–reducing vaccines and drugs. Other challenges for diagnostics discussed by the Consultative Group include the need for tools that can rapidly detect and monitor unexpectedly high transmission that leads to outbreaks and that can identify reintroduction of infections that may be asymptomatic [16],[24].
Beyond the Tools: Supporting Strategies and the Knowledge Base
Modeling and Harmonized Data Systems
Substantial advances have been made recently in computational approaches for modeling malaria epidemiology and in model-based approaches to economic evaluation [27]–[29]. As discussed by the malERA Consultative Group on Modeling [16], a significant research challenge for malaria eradication will be to integrate these new approaches into the planning of elimination, surveillance, monitoring, and evaluation, and to create appropriate interfaces for different user communities, including researchers, global and national policy makers, and local-level planners. Modeling can inform the definition of TPPs for new tools and intervention strategies and will be needed throughout a global eradication campaign to analyze the likely effects on malaria and of various elimination strategies and the costs of these strategies [30].
Importantly, a single unifying model will be insufficient to meet all these needs, so multiple modeling efforts need to be coordinated and made accessible to everyone. This harmonization and validation process will require close, iterative collaboration between software engineers, researchers, and malariologists to develop the necessary computer systems and connectivity (cyberinfrastructure). It will also necessitate the creation and maintenance of properly annotated and accessible malariometric databases that include all the research results needed to insert parameters into the models and the model outputs. How this can be achieved is considered in detail by the Consultative Group in their article [16].
Enabling Technologies and Platforms
The development of new tools for elimination is critically dependent on a vibrant and coherent agenda for basic sciences. We believe there are at least two potentially transformative developments that need to be pursued. First, continuous laboratory culture of P. vivax, P. ovale, and P. malariae needs to be developed to provide an essential platform for studying the biology of the liver stages and sexual forms of these parasites. These forms could be important targets of intervention strategies with drugs, vaccines, or other biological or chemical agents aimed at interrupting transmission. Second, systems analyses of transcription, proteome, and metabolome libraries, rapid screening of drug libraries, high-throughput approaches to antigen identification, and the functional definition of gene products are all feasible but not yet fully exploited, but would bring important new tools to the bench scientist and to field operations. These and other aspects of enabling technologies and platforms are considered in detail in the articles prepared by the malERA Consultative Groups on Basic Science and Enabling Technologies and on Cross-cutting Issues for Eradication [25],[31].
Health Systems Integration, Operational Research, and Effectiveness-Decay Analysis
The previous formal attempt at global eradication of malaria (1955–1969) depended largely on vertical operations that often bypassed health systems and their health services because it was assumed that eradication operations could be run most efficiently in this way. Many of the elimination efforts failed, because the health systems failed, leading to a pessimistic view that malaria can only be eliminated where economic progress, governance, and efficient health systems are in place to support maintenance of conditions necessary to block transmission [32],[33].
It is now clear that the long-term solution to malaria elimination and eradication will require a systems approach in which malaria-specific interventions and actions are integrated into existing health systems [34]. To achieve this, research is needed into health systems, their readiness to optimize novel programs, systems, tests, or other interventions, and their continuing performance [35]–[37]. During their deliberations, the malERA Consultative Group on Health Systems and Operational Research identified the need for a substantial research approach to establish and validate a tool kit that allows effectiveness-decay analysis of health system impediments to effective and equitable coverage of malaria interventions and that allows decisions to be made on the degree of possible integration of interventions into an existing health system [16],[38]. A further critical component of the research agenda identified by this Consultative Group is the development and validation of a decision-making framework to guide the move from control to elimination.
Finally, but equally importantly, the article by the malERA Consultative Group on Monitoring, Evaluation, and Surveillance considers the need to investigate the performance of surveillance, monitoring, and evaluation by new and old technologies [39],[40] and to evaluate optimal strategies for implementation of surveillance as an active responsive intervention to further reduce transmission [41].
Training
The last time the world community tried to eliminate malaria, so the joke goes, the only thing that was eliminated was malariologists. For a renewed malaria eradication campaign to have a chance to succeed, it will be essential to train the malariologists and scientists in the multiple disciplines needed for an eradication campaign that might last 50 years, especially in endemic countries. This need cannot be overemphasized. The malaria research community remains small and often dominated by the views and strategies of scientists who sit far away from the problems. A massive effort to train, empower, and sustain research capacity in the endemic countries will be a critical factor for the success of improved control efforts and for the ultimate elimination and eradication of malaria.
Concluding Remarks
The past 2 years have reinvigorated an old malaria paradigm in which reduction of transmission is the driving strategy for malaria interventions. The malaria community has now used the malERA process to propose a research and development agenda that will be essential for regional elimination and eventual global eradication of malaria. Not every tool or strategy considered by the malERA Consultative Groups (see Box 2) will be essential in every situation (see Figure 4), but the complexity and heterogeneity, and in some places, the sheer intensity of transmission, demand that we start without delay to prepare for the most difficult challenges. This focus on the end goal of eradication must not displace our determination and efforts to continue to scale up ongoing efforts for control and to include a research agenda for reducing morbidity in areas of continuing moderate or high transmission. Rather, it must encourage us to supplement our efforts with a structured agenda that can realize the ultimate goal of eradication envisaged by the Global Malaria Action Plan and the Roll Back Malaria Partnership. An important lesson we can learn from other disease elimination efforts is that complacency is dangerous. The parasite and the vector are always evolving, and the human environment is always changing. Thus, new research questions will continually arise during the course of elimination [42], and active malaria research, particularly on the development of new tools, must continue up to the point when eradication is finally achieved. We anticipate that the results of research efforts proposed by our Consultative Groups for each stage of progression, from scaling up for improved control to the elimination phases, will have great synergy in design and application.
Box 2. Key Examples of Critical Research Needed to Support Elimination and Eradication of Plasmodium falciparum and Plasmodium vivax
-
In vitro culture and study of hypnozoites (persistent liver stages) of P. vivax
-
Drugs to be used for mass drug administration to clear infections and provide prophylaxis to prevent new infections
-
Vaccines that target different stages of the parasite life cycle, or the mosquito, with the key goal of interrupting transmission
-
New vector control approaches for (i) outdoor biting/resting mosquitoes and (ii) achieving permanent reductions of vectorial capacity in areas where transmission is predominantly due to the highly efficient vector A. gambiae
-
New approaches for fast and accurate assessment of transmission at community level
-
When to press the elimination button? Tool kits to scientifically determine “health system readiness” for a switch to elimination efforts
-
New collaborative approaches to use of mathematical modeling to inform TPPs, and expected outcomes of mixes of intervention
-
Strengthened monitoring and evaluation tools and strategies for interrupting transmission that are linked and embedded in the health and social systems
Fig. 4. Key research and development issues and their position in relation to the different epidemiological phases towards eradication. 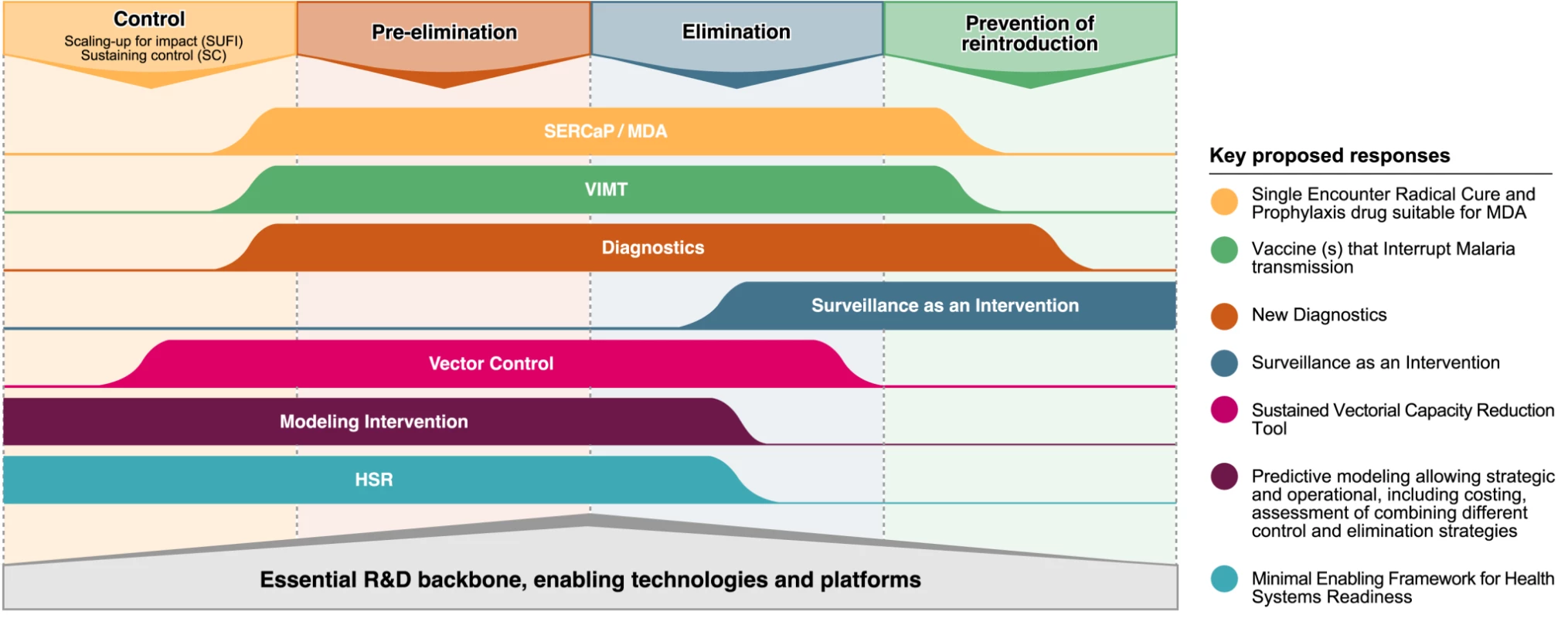
Image credit: Fusión Creativa. Past efforts at disease eradication, successful or otherwise, have highlighted the importance of sustained commitment from local communities, civil society, policy leaders, and the scientific community to implement research in the context of the desired integration of services, sector wide approaches, harmonisation of activities, and long-term funding commitment. Thus, research areas such as social science or research into direct and indirect economic benefits of malaria eradication also need to be strengthened. With these drivers in place, and the development of the new tools we describe briefly here and in the other articles in this Supplement, it may be possible to fulfil the dream that malaria eradication can be achieved within the lifetime of young scientists just embarking on their careers, even in the most difficult areas where current tools/strategies have proven to be insufficient. The time course may be long, but to have a chance of realizing that dream, the commitment to starting those research and development efforts must begin now.
Supporting Information
Zdroje
1. Roll Back Malaria Partnership 2008 The global malaria action plan for a malaria free world. Geneva RBM Available: http://www.rollbackmalaria.org/gmap/gmap.pdf.
2. World Health Organization 2010 World malaria report. Geneva World Health Organization Available: http://whqlibdoc.who.int/publications/2010/9789241564106_eng.pdf
3. World Health Organization 2010 Weekly epidemiological record 85, 229-236. Available: http://www.who.int/wer/2010/wer8524.pdf.
4. World Health Organization 2010 Weekly epidemiological record 85, 461-472. Available: http://www.who.int/wer/2010/wer8547/en/index.html
5. Bill & Melinda Gates Foundation Malaria Forum 2007 Day-2 transcript. 17-10-2007. Available: http://www.gatesfoundation.org/speeches-commentary/Pages/melinda-french-gates-2007-malaria-forum.aspx
6. MendisK
RietveldA
WarsameM
BosmanA
GreenwoodB
2009 From malaria control to eradication: The WHO perspective. Trop Med Int Health 14 802 809
7. NájeraJ
González-SilvaM
Alonso, PL 2010 Some lessons for the future from the Global Malaria Eradication Programme (1955-1969). PLoS Med 8 e1000412 doi:10.1371/journal.pmed.1000412
8. FeachemRGA
PhillipsAA
TargettGA
2009 Shrinking the malaria map: A prospectus on malaria elimination. San Francisco The Global Health Group, Global Health Sciences University of California, San Francisco
9. PloweCV
2009 The evolution of drug-resistant malaria. Trans R Soc Trop Med Hyg 103 S11 S14
10. DondorpAM
NostenF
YiP
DasD
PhyoAP
2009 Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361 455 467
11. RansonH
AbdallahH
BadoloA
GuelbeogoWM
Kerah-HinzoumbéC
2009 Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malar J 8 299
12. GreenwoodB
FidockDA
KyleDE
KappeSH
AlonsoPL
2008 Malaria: Progress, perils and prospects for eradication. J Clin Invest 118 1266
13. NyuntMM
PloweCV
2007 Pharmacologic advances in the global control and treatment of malaria: combination therapy and resistance. Clin Pharmacol Ther 82 601 605
14. The malERA Consultative Group on Drugs 2011 A research agenda for malaria eradication: Drugs. PLoS Med 8 e1000402 doi:10.1371/journal.pmed.1000402
15. The malERA Consultative Group on Vaccines 2011 A research agenda for malaria eradication: Vaccines. PLoS Med 8 e1000398 doi:10.1371/journal.pmed.1000398
16. The malERA Consultative Group on Modeling 2011 A research agenda for malaria eradication: Modeling. PLoS Med 8 e1000403 doi:10.1371/journal.pmed.1000403
17. EnayatiA
HemingwayJ
2010 Malaria management: Past, present, and future. Ann Rev Entomol 55 569 591
18. The malERA Consultative Group on Vector Control 2011 A research agenda for malaria eradication: Vector control. PLoS Med 8 e1000401 doi:10.1371/journal.pmed.1000401
19. TereniusO
MarinottiO
SieglaffD
JamesAA
2008 Molecular genetic manipulation of vector mosquitoes. Cell Host Microbe 13 417 423
20. SinkinsSP
GouldF
2006 Gene drive systems for insect disease vectors. Nat Rev Genet 7 427 35
21. BellD
WongsrichanalaiC
BarnwellJW
2006 Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol S7 S20
22. ParisDH
ImwongM
FaizAM
HasanM
YunusEB
2007 Loop-mediated isothermal PCR (LAMP) for the diagnosis of falciparum malaria. Am J Trop Med Hyg 77 972 976
23. World Health Organization 2007 Malaria elimination: a field manual for low and moderate endemic countries. Geneva World Health Organization
24. The malERA Consultative Group on Diagnoses and Diagnostics 2011 A research agenda for malaria eradication: Diagnosis and diagnostics. PLoS Med 8 e1000396 doi:10.1371/journal.pmed.1000396
25. The malERA Consultative Group on Integration Strategies 2011 A research agenda for malaria eradication: Cross-cutting issues for eradication. PLoS Med 8 e1000404 doi:10.1371/journal.pmed.1000404
26. BousemaT
YoussefRM
CookJ
CoxJ
AleganaVA
2010 Serologic markers for detecting malaria in areas of low endemicity, Somalia 2008. Emerging Infect Dis 16 392 399
27. GhaniAC
SutherlandCJ
RileyEM
DrakeleyCJ
GriffinetJT
2009 Loss of population levels of immunity to malaria as a result of exposure-reducing interventions: consequences for interpretation of disease trends. PLoS One 4 e4383 doi:10.1371/journal.pone.0004383
28. McKenzieFE
BossertWH
2005 An integrated model of Plasmodium falciparum dynamics. J Theor Biol 232 411 426
29. SmithT
KilleenGF
MaireN
RossA
MolineauxL
2006 Mathematical modeling of the impact of malaria vaccines on the clinical epidemiology and natural history of Plasmodium falciparum malaria: Overview. Am J Trop Med Hyg 75 1 10
30. SmithDL
SmithT
HaySI
2009 “Measuring malaria for elimination.
FeachemRGA
PhillipsAA
TargettGA
Shrinking the malaria map: A prospectus on malaria elimination San Francisco The Global Health Group, Global Health Sciences University of California, San Francisco 108 126
31. The malERA Consultative Group on Basic Science and Enabling Technologies 2011 A research agenda for malaria eradication: Basic science and enabling technologies. PLoS Med 8 e1000399 doi:10.1371/journal.pmed.1000399
32. AlilioMS
BygbjergIC
BremanJG
2004 Are multilateral malaria research and control programs the most successful? Lessons from the past 100 years in Africa. Am J Trop Med Hyg 71 268 278
33. NájeraJA
2001 Malaria control: Achievements, problems and strategies. Parassitologia 43 1 89
34. TannerM
de SavignyD
2008 Malaria eradication back on the table. Bull World Health Organ 86 82 83
35. de SavignyD
AdamT
2009 Health systems thinking for health systems strengthening. Geneva World Health Organization
36. World Health Organization 2007 Everybody's business: Strengthening health systems to improve health outcomes: WHO's framework for action. Geneva World Health Organization
37. World Health Organization 2008 World health report 2008 – Primary health care: More than ever. Geneva World Health Organization
38. The malERA Consultative Group on Health Systems and Operational Research 2011 A research agenda for malaria eradication: Health systems and operational research. PLoS Med 8 e1000397 doi:10.1371/journal.pmed.1000397
39. HaySI
SmithDL
SnowRW
2008 Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis 8 369 378
40. DrakeleyC
CookJ
2009 Potential contribution of sero-epidemiological analysis for monitoring malaria control and elimination: Historical and current perspectives. Adv Parasitol 69 299 352
41. The malERA Consultative Group on Monitoring, Evaluation, and Surveillance 2011 A research agenda for malaria eradication: Monitoring, evaluation, and surveillance. PLoS Med 8 e1000400 doi:10.1371/journal.pmed.1000400
42. BremanJG
QuadrosCA
DowdleWR
FoegeWH
HendersonDA
2011 The role of research in viral disease eradication and elimination programs: Lessons for malaria eradication. PLoS Med 8 e1000405 doi:10.1371/journal.pmed.1000405
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 1- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Magnosolv a jeho využití v neurologii
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- A Simple Novel Method for Determining Mortality Rates in HIV Treatment Programs Worldwide
- Setting Implementation Research Priorities to Reduce Preterm Births and Stillbirths at the Community Level
- A Research Agenda for Malaria Eradication: Monitoring, Evaluation, and Surveillance
- A Research Agenda for Malaria Eradication: Cross-Cutting Issues for Eradication
- A Research Agenda to Underpin Malaria Eradication
- Correcting Mortality for Loss to Follow-Up: A Nomogram Applied to Antiretroviral Treatment Programmes in Sub-Saharan Africa
- The Impact of eHealth on the Quality and Safety of Health Care: A Systematic Overview
- Setting Research Priorities to Reduce Almost One Million Deaths from Birth Asphyxia by 2015
- Predicting Live Birth, Preterm Delivery, and Low Birth Weight in Infants Born from In Vitro Fertilisation: A Prospective Study of 144,018 Treatment Cycles
- Some Lessons for the Future from the Global Malaria Eradication Programme (1955–1969)
- A Research Agenda for Malaria Eradication: Basic Science and Enabling Technologies
- A Research Agenda for Malaria Eradication: Vector Control
- The Role of Research in Viral Disease Eradication and Elimination Programs: Lessons for Malaria Eradication
- The Influence of Distance and Level of Care on Delivery Place in Rural Zambia: A Study of Linked National Data in a Geographic Information System
- Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies
- Development of a Standardized Screening Rule for Tuberculosis in People Living with HIV in Resource-Constrained Settings: Individual Participant Data Meta-analysis of Observational Studies
- WHO/PLoS Collection “No Health Without Research”: A Call for Papers
- Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study
- A Research Agenda for Malaria Eradication: Vaccines
- A Research Agenda for Malaria Eradication: Health Systems and Operational Research
- A Research Agenda for Malaria Eradication: Diagnoses and Diagnostics
- A Research Agenda for Malaria Eradication: Drugs
- A Research Agenda for Malaria Eradication: Modeling
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Impact of eHealth on the Quality and Safety of Health Care: A Systematic Overview
- A Research Agenda for Malaria Eradication: Cross-Cutting Issues for Eradication
- Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study
- Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



