-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Packages of Care for Attention-Deficit Hyperactivity Disorder in Low- and Middle-Income Countries
article has not abstract
Published in the journal: . PLoS Med 7(2): e32767. doi:10.1371/journal.pmed.1000235
Category: Neglected Diseases
doi: https://doi.org/10.1371/journal.pmed.1000235Summary
article has not abstract
This is the last in a series of articles highlighting the delivery of “packages of care” for mental health disorders in low - and middle-income countries. Packages of care are combinations of treatments aimed at improving the recognition and management of conditions to achieve optimal outcomes.
Summary Points
-
Attention-deficit/hyperactivity disorder (AD/HD) is a multidimensional disorder that, although commonest in childhood and adolescence, can be diagnosed across the age span. Worldwide prevalence is about 5%.
-
An appropriate package of treatment for AD/HD in low - and middle-income countries (LMICs) should include screening of high-risk groups, psychoeducational interventions with caregivers, methylphenidate, and behavioral interventions.
-
Strategies to facilitate the delivery of effective interventions in LMICs should increase demand for services, access to AD/HD interventions, and the capacity of health care teams, as well as improve recognition of AD/HD, develop community-based and practice-based programs, and address the impact of AD/HD on other health and social outcomes.
-
Interventions to address AD/HD should be part of a more comprehensive package of services for mental disorders.
Introduction
Attention-deficit hyperactivity disorder (AD/HD) is a chronic, pervasive developmental disorder that, although usually diagnosed in childhood, spans the preschool to adult years. The most recent version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) defines the disorder using the core features of age-inappropriate hyperactivity, impulsivity, and inattention (Box 1) [1]. The 10th edition of the International Classification of Diseases (ICD-10) provides operational criteria for the similar, but more severe and narrowly defined hyperkinetic disorder (HKD) [2]. We have used the term AD/HD throughout this paper because most of the published literature relates to the broader concept of AD/HD rather than to HKD.
Box 1. DSM-IV-TR Criteria for Attention Deficit Hyperactivity Disorder and International Classification of Diseases 10 Criteria for Hyperkinetic Disorder
According to the DSM-IV-TR, to satisfy the criteria for AD/HD, a patient must manifest six or more symptoms of inattention or hyperactivity-impulsivity that have persisted for at least six months “to a degree that is maladaptive and inconsistent with developmental level.” Examples of symptoms of inattention include often having difficulty in sustaining attention in tasks or play activities, being easily distracted by extraneous stimuli, and often being forgetful in daily activities. Examples of symptoms of hyperactivity–impulsivity include often fidgeting with hands or feet or squirming in seat, often talking excessively, and often having difficulty awaiting turn. In addition, some of the symptoms causing impairment must have been present before the age of seven years, some impairment from the symptoms must be present in two or more settings, there “must be clear evidence of clinically significant impairment in social, academic, or occupational functioning,” and the symptoms should “not occur exclusively in the course of a Pervasive Developmental Disorder, Schizophrenia, or other Psychotic Disorder and are not better accounted for by another mental disorder” [1].
According to the ICD-10, to satisfy the criteria for hyperkinetic disorder, a patient must demonstrate “abnormality of attention, activity and impulsivity at home, for the age and developmental level of the child,” as evidenced at least three of a set of attention problems, plus at least three of a set of activity problems, plus at least three of a set of impulsivity problems. In addition, the patient must demonstrate “abnormality of attention at school or nursery (if applicable), for the age and developmental level of the child,” as evidenced by at least two of a set of attention problems and at least three of a set of activity problems. Also, there must be “directly observed abnormality of attention or activity,” which is “excessive for the child's age and developmental level.” Finally, the child must not meet criteria for selected other conditions, onset must be before the age of seven years, the duration must be at least six months, and the IQ must be above 50 [2].
A rapidly expanding body of literature from low - and middle-income countries (LMICs) largely refutes the notion that AD/HD is a “Western” concept, although cultural factors clearly influence illness perceptions and help-seeking behavior [3]–[7]. A systematic review and meta-regression analysis of 102 studies from all continents concluded that the worldwide pooled prevalence of AD/HD is 5.3%, and that the geographic variability between AD/HD prevalence estimates is best explained by the methodological characteristics of the studies [8].
A strong body of evidence from high-income countries (HICs) suggests that AD/HD is a neurobiological syndrome with complex genetic factors primarily implicated in its etiology. Although individual risk alleles identified by molecular genetic studies increase the risk of AD/HD only slightly, the mean estimate of total heritability is just under 80% [9]. A wide range of social determinants significantly influence the symptomotology of AD/HD in HICs. These include low socioeconomic status, low parental education, family conflict, parental mental disorder, severe early deprivation, and institutional upbringing [9]–[11]. The involvement of these multiple determinants in the symptomatology of AD/HD is consistent with the hypothesis that AD/HD is an etiologically heterogeneous, final common pathway disorder that is influenced by genes, environment, and gene–environment interactions. Other nongenetic causes of AD/HD identified in HICs are factors that affect early brain development, such as perinatal stress, low birth weight, prenatal smoking and alcohol use, obstetric complications, head injury, epilepsy, and HIV/AIDS [9]–[11]. The mediating and moderating influence of these variables on the development of AD/HD amongst children in LMICs is less well researched and does not always match with that seen in HICs [11].
The Evidence on the Treatment of AD/HD
Detection
Accurate detection and diagnosis of AD/HD is crucial for the effective management of individuals with the disorder [12]–[14]. Two structured approaches are available to detect and diagnose AD/HD: clinical diagnostic interviews and rating scales (Table 1). The only clinical diagnostic interview that we are aware has been assessed in LMICs is the Diagnostic Interview Schedule for Children (DISC-IV) [15], which has been validated for use in China [16] and for Xhosa-speaking South Africans [17]. In China, the test-retest reliability of the AD/HD component was excellent for the version of the interview given to parents (k = 0.81) and poor for the version given to adolescents (k = 0.25). Similarly, for the South African Xhosa version of the DISC-IV the parent version resulted in substantial reliability (k = 0.56) whereas the youth version resulted in poor reliability (k = 0.23). Although systematic reviews have investigated the numerous rating scales developed to diagnose AD/HD in HICs [12]–[14], only a few scales have been developed for use in LMICs. In Brazil, the test-retest reliability of a scale for use by teachers based on DSM-III-R diagnostic criteria ranged from 0.56 to 0.70 [18], and the diagnostic performance of the Child Behavior Checklist Attention problem scale resulted in moderate areas under the curve (AUC = 0.78) [19].
Tab. 1. The evidence in support of AD/HD treatment. 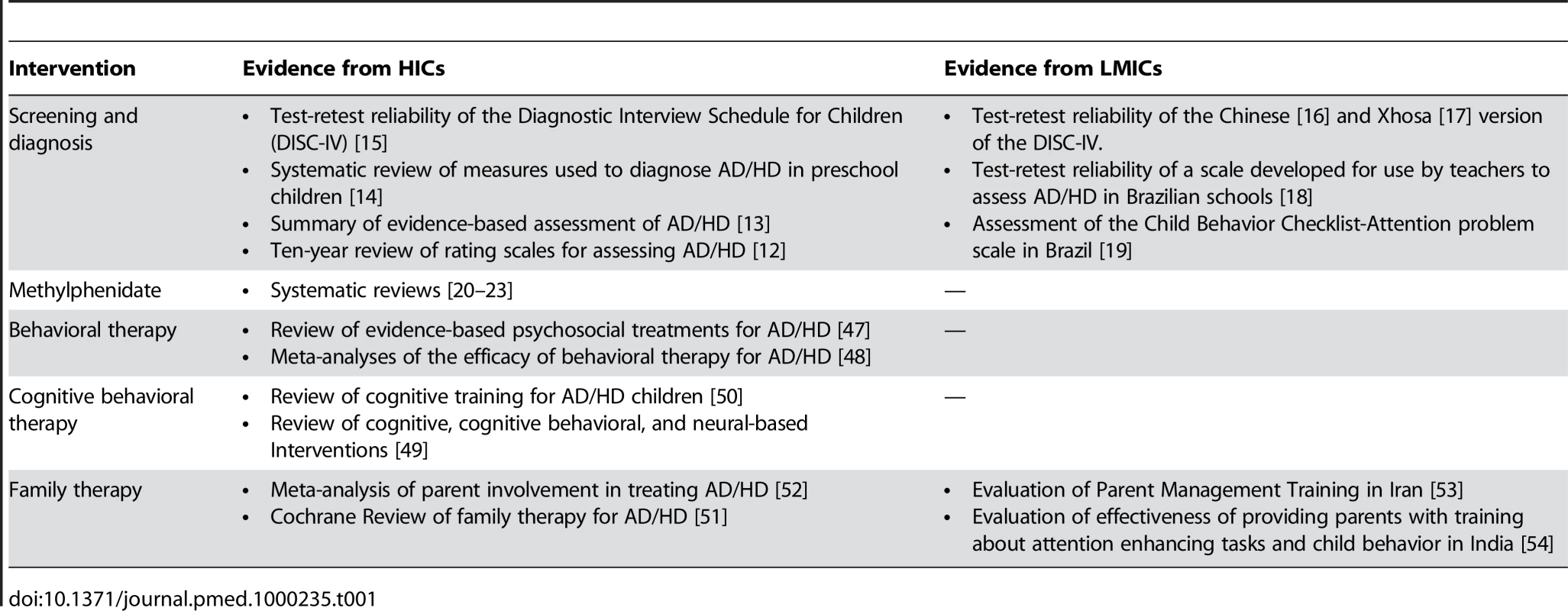
Medication
The efficacy of pharmacological interventions for the treatment of AD/HD in HICs is well established (Table 1) [20]–[23]. Although there are few studies on medication for AD/HD from LMICs, efficacy data from studies conducted in HICs are likely to be applicable to these settings. Pharmacologic agents used in the treatment of AD/HD include the psychostimulants (methylphenidate or amphetamine), atomoxetine, bupropion, tricyclic antidepressants (TCAs), and alpha-agonists [24],[25]. The psychostimulants, which have been used clinically for more than 50 years, are supported by the most robust efficacy and safety data and are available as affordable generic immediate and sustained release formulations [25]. Methylphenidate has been established as an effective short-term treatment for school-age children and adults with AD/HD, and there are some data, albeit sparse, that support its efficacy in preschoolers [26],[27]. The short-term efficacy of atomoxetine, a newer medication, is also supported by numerous large-scale clinical trials, although direct comparison studies with the psychostimulants have demonstrated greater treatment effect sizes for methylphenidate [28],[29]. Also, the psychostimulants are less expensive than atomoxetine and more likely to be available in LMICs. Longer-term open label studies in HICs for methyphenidate and atomoxetine support the efficacy and safety of both these medications [30],[31].
Before the introduction of atomoxetine, tricyclic antidepressants (TCAs) were a common alternative to the psychostimulants. However, few studies have compared the efficacy of these two drug classes and the association of TCAs with troublesome side effects, potential cardiotoxicity, and the risk of death in the event of overdose has taken TCAs out of favor [21],[24],[25]. The evidence base for other medications in the treatment of AD/HD is weak.
The most frequent troublesome side effects associated with the psychostimulant medications are reduced appetite, weight loss, and sleep difficulties [32],[33]. Compared to the pyschostimulants, atomoxetine has less-pronounced effects on appetite and sleep but is more likely to be associated with gastrointestinal upset [34]. Both the pyschostimulants and atomoxetine may be associated with slight increases in heart rate and blood pressure. Finally, the psychostimulants are controlled substances and concerns exist regarding the risk of pyschostimulant misuse and drug diversion. However, there is no clear evidence that pyschostimulant-treated AD/HD children abuse prescribed medication when they are appropriately diagnosed and carefully monitored [35].
Children treated with medication require careful clinical monitoring for side effects, clinical response, adherence, treatment acceptability, and dose adjustment. Treating clinicians must have a proficient understanding of the pharmacokinetic and pharmacodynamic properties of these medications, the potential for adverse events, and strategies for addressing emergent side effects. Medication monitoring should include: a baseline assessment followed by regular assessment of symptoms, function, side effects, adherence, and the emergence of comorbid disorders or other medical conditions; measurement of height, weight, blood pressure, and pulse; and provision of psychoeducation that addresses emerging questions or concerns from the patient or family.
There is limited empirical evidence (all from HICs) to inform the duration of treatment with pharmacological agents in AD/HD. Six - and eight-year follow-up data from the Multimodal Treatment Study of Children with AD/HD (MTA) failed to provide support for long-term medication treatment where care was provided in community settings without ongoing careful titration and monitoring [36]. Decisions about continuing or discontinuing medication should be individualized, with periodic discontinuations to assess need and benefit.
Finally, decisions regarding the use of pharmacological therapy should be informed by the level of impairment, symptom severity, availability of appropriately trained health personnel and support, accessibility to monitoring and follow-up services, acceptability to patient and the family, and cost. Pharmacologic treatment is not indicated if symptoms are mild, if there is minimal impairment or an unclear diagnosis, if there are inadequate services for close monitoring and follow-up, or if the use of medication is unacceptable to the patient or family.
Structured Psychotherapies
Two of the largest studies of psychotherapies for the treatment of AD/HD ever conducted are the New York-Montreal Study and the MTA study [37]–[41]. In the New York-Montreal Study, 103 children with AD/HD (ages 7–9), who did not have comorbid conduct or learning disorders and who had responded to methylphenidate were allocated randomly to three groups for two years: (1) methylphenidate treatment alone; (2) methylphenidate combined with multimodal psychosocial treatment that included parent training and counseling, academic assistance, psychotherapy, and social skills training; or (3) methylphenidate plus attention control treatment that excluded specific aspects of the psychosocial intervention. Individual assessments were conducted on outcome measures such as academic and emotional status [39] and symptomatic improvement [40]. This study found no significant difference between the combined-treatment groups and the medication-only group. In the MTA study, 579 children aged 7.0 to 9.9 years with AD/HD were randomly assigned to four treatment groups (medication, behavioral treatment, combined treatment, and community care) [38]. The authors concluded that after the 24-month follow-up, medication was superior over the other individual treatment options and that there was no significant difference between the combined treatment and medication-only [41]. However, further analysis of the data concluded that patients with AD/HD and either comorbid disorders [42]–[44] or psychosocial stressors [45],[46] did benefit from adjunctive psychosocial interventions (parent training and behavioral interventions).
Several reviews have investigated whether psychosocial interventions are effective for treating children and adolescents with AD/HD (Table 1). A systematic literature review [47] and a meta-analysis that investigated 174 AD/HD treatment studies concluded that behavioral treatment is highly effective [48]. Reviews of cognitive-behavioral [49],[50] and family therapy [51],[52] interventions have yielded promising but inconclusive findings.
We were not able to locate any randomized controlled trials addressing the efficacy of structured psychotherapies for the treatment of AD/HD in LMICs. However, an Iranian study [53] reported that Parent Management Training (eight sessions, once a week for 1.5 hours) improved the behavior of 15 children with AD/HD and the general health of their parents. Similarly, an Indian study investigated the effectiveness of a program providing five training sessions to ten parents of boys aged 6–10 years with AD/HD. The training, which addressed attention-enhancing tasks and child behavior principles and techniques, had significant effects on the behavior of the children, and parent-reported study habits, pro-social behavior, and academic performance [54].
Delivery of Effective Interventions
Interventions to Increase Demand for Services
Similarly to the other disorders discussed in this series, stigma and a lack of awareness about mental health problems are important contributors to a relatively low demand for services for AD/HD in LMICs. In the case of AD/HD, however, the salience of these factors is magnified because AD/HD is most common among children and adolescents, an age group in which stigma and lack of awareness are particularly evident [55]. Among populations where stigma is high and knowledge levels low, efforts should be directed in the first instance to increasing general awareness and recognition of the disorder (Table 2). Once this goal has been achieved, attention may need to be focused on minority groups, inattentive type AD/HD, girls with AD/HD, and adult AD/HD. While specialists in mental health, education, and pediatrics should take a leading role in reducing stigma and increasing awareness, the potential contribution of community-based advocacy groups should not be ignored. School is a particularly important setting for stigma reduction and information raising activities. While we were unable to locate any reports that evaluated awareness or recognition programs for AD/HD specifically, Hoven et al. [56] assessed children, parents, and teachers before and after an awareness program to determine changes in knowledge, attitudes, and understanding of mental health in nine countries including Azerbaijan, Brazil, China, Georgia, Russia, and Uganda. The authors reported a positive change in awareness in all the countries and an increased willingness to discuss emotional problems freely.
Tab. 2. Delivery treatments for AD/HD. 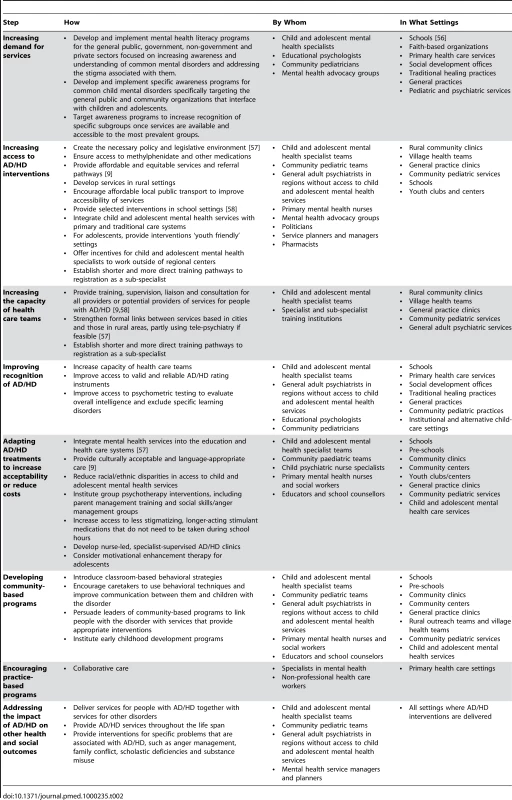
Interventions to Increase Access to Interventions
Important elements of a strategy for increasing access include advocacy for the development of new, or the revision of existing, policy and legislative frameworks that ensure the promotion of mental health and the development of services that are accessible, affordable, appropriate, and acceptable to the children, families, and communities they are meant to serve (Table 2). Other important elements include ensuring that evidence-based, cost-effective treatments are both accessible and consistently available and that appropriately trained, skilled, and experienced staff are available [57]. A crucial aspect of access to effective treatment for AD/HD is access to the psychostimulants and other pharmacological agents for which there is evidence of effectiveness. This may require that child and adolescent psychiatrists and pharmacists attend financial committees and other managerial structures to ensure that the appropriate psychopharmacological agents are available and affordable, that financing is sustainable, and that health and supply systems are reliable. Clearly, the inclusion of methylphenidate in national and transnational lists of essential drugs is crucial in facilitating such circumstances.
Interventions to Increase the Capacity of Health Care Teams
People with AD/HD may interface with health providers in many settings. Thus, a broad range of health care providers may be potential targets of capacity development activities. Depending on the context, providers may include child and adolescent mental health specialists, pediatricians, general psychiatrists, general medical service providers, and other nonphysician health providers, particularly those who work closely with children and adolescents. Opportunities for continual learning and support through mentorship, supervision, and continuing education should be built into capacity development initiatives. Training content should be tailored to the needs of target groups but should be based on an understanding of AD/HD as a chronic, pervasive disorder requiring a holistic, multisectoral approach to management and should be provided as part of a broader curriculum that addresses the comorbid mental health issues associated with AD/HD. Ensuring that capacity development initiatives are accessible and available to target groups can be a challenge in any context, and how training is delivered may have to be tailored to best meet the needs of the recipients. General psychiatrists in rural areas might benefit from telepsychiatry to improve their prescribing practices, for example. Similarly, educators might benefit from continuing education seminars that focus on the identification of children with AD/HD and on classroom interventions, and pediatric primary health care nurses might benefit from supervised clinical practice followed by direct access to a nursing or medical specialist consultation.
Interventions to Improve Recognition of AD/HD
Strategies to improve recognition of AD/HD should be implemented only once access to services has been ensured and health care providers have sufficient capacity to respond to an increased demand for interventions. There are three aspects to improving recognition: (1) awareness of the risk factors for AD/HD and the identification of persons at risk; (2) screening of persons at risk for ADHD; and (3) determination of a diagnosis of AD/HD. As mentioned above, there are many useful clinical instruments and rating scales for both screening and assessment of AD/HD. Depending on the context, informal health services, schools, faith-based organizations, and other community-based groups can be invaluable in the early recognition of risk factors for potential mental health problems including AD/HD. Where available, psychometric testing may also help to assess the extent to which low levels of intellectual functioning or deficits in academic skill contribute to the presentation. Clearly, such instruments, rating scales, and tests are most likely to produce optimum benefits when they are administered by people with the necessary skill, training, and facility in the language of the individuals who are being assessed.
Interventions to Adapt Treatments to Increase Acceptability or Reduce Costs
Both methylphenidate and amphetamine, the best-studied and most cost-effective medicines for AD/HD, are available in long-acting preparations that require once daily dosing. Such dosing is often preferable to multiple daily dosing, particularly for school-aged children and adolescents, and should be made available where possible. Psychosocial interventions may be delivered in either individual or group format and may be offered in various settings including schools. Individual-level interventions, while having advantages over group-level interventions, are not always feasible because of the shortages of appropriately trained staff. While group-level interventions are implemented in many settings in both LMICs and HICs, there is a dearth of evaluations of such interventions, a gap that begs filling.
Integration of child and adolescent mental health services into the education and/or primary health care system can also increase the acceptability and affordability of AD/HD treatments [58]. There are numerous models for integrating mental health services into community and primary care that require the development of continuous care pathways linking different levels of the health system. One example is the development of nurse-led but specialist-supervised clinics. The strategy adopted will be highly dependent on the resources and infrastructure available in a particular setting.
Interventions to Develop Community-Based Programs
The establishment of community-based initiatives in partnership with formal health services allows the development of a continuous network of care and support for children with AD/HD and their families. Such care and support is essential to achieving and maintaining optimal functioning and ameliorating the disability associated with AD/HD. Schools, where most children spend most of their time, can be the cornerstone of such programs. Educators can be helped to implement classroom-based behavioral interventions such as token economy systems in which children are rewarded immediately for desirable behavior with tokens that can be exchanged later for a reward, or response cost systems in which a “fine” is imposed for bad behavior. Other interventions that could be introduced include increasing teacher-to-child classroom ratios and breaking the school day into brief academic assignments interspersed with brief periods of physical exercise [58]. Community-based organizations can also be used to deliver parenting interventions and support for families and caregivers, to provide gatekeeper functions and public mental health education, and to assist with accessing medications for which there is evidence of effectiveness.
Interventions to Encourage Practice-Based Programs
Collaborative care programs that are specific to a particular disorder (as opposed to more general strategies such as the integration of mental health care into the primary health care system) have been successfully implemented for depression [59]. Furthermore, there is evidence that with appropriate diagnostic and pharmacological training, both general practitioners and pediatricians can apply this intervention effectively in HICs for AD/HD [60],[61]. However, we were not able to locate any studies in which practice-based programs to address AD/HD have been implemented and evaluated in LMICs.
Interventions to Address the Impact of AD/HD on Other Health and Social Outcomes
AD/HD is frequently comorbid with other mental health problems and is associated with a range of negative health and social outcomes. Thus, services for people with AD/HD should not be delivered in isolation from services for other disorders. Also, because most children with AD/HD will retain the diagnosis into adulthood or continue to be deleteriously affected by the symptoms associated with AD/HD, it is crucial that interventions are not terminated in adolescence, as frequently occurs. By continuing to receive intervention, the probability of long-term adverse consequences of the disorder is reduced. Finally, it is important to offer interventions for selected specific problems that are associated with AD/HD, such as anger management, family conflict, scholastic deficiencies, and substance misuse.
Packages of Care for AD/HD in LMICs
AD/HD is a common developmental disorder that affects individuals throughout their lives and across all cultural contexts, and is associated with considerable social, psychological, and economic adversity. Recognition of the disorder can be improved by the use of screening instruments, some of which have been shown to have adequate psychometric properties in LMICs. Although there are few studies from LMICs that address the effectiveness of methylphenidate, the consistent and strong effects that have emerged from HICs suggest that this drug will be effective universally. In addition, although the evidence for structured psychotherapies is mixed, there is sufficient justification to include behavioral interventions in a package of care for AD/HD in LMICs (Table 3). However, given that most people worldwide with AD/HD will not receive interventions from a mental health specialist such as a psychiatrist or psychologist, it is essential that efforts to deliver this package of care in LMICs are not dependent on such specialists. There are risks associated with such an approach. For example, the quality of the interventions may be less than would have been the case if they were delivered by specialists. However, such risks need to be weighed against the certainty that services will be less accessible if they are delivered by specialists. Finally, it is crucial that this package of care for AD/HD form part of a more comprehensive package of services in which other disorders are also addressed.
Zdroje
1. American Psychiatric Association (APA) 2004 Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition Text Revision (DSM-IV-TR). Washington DC APA
2. World Health Organization (WHO) 1992 The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva WHO
3. BuitelaarJK
BartonJ
DanckaertsM
GillbergC
HazellPL
2006 A comparison of North American versus non-North American AD/HD study populations. Europ Child Adolesc Psychiat 15 177 181
4. FaraoneS
SergeantJ
GillbergC
BidermanJ
2003 The worldwide prevalence of AD/HD: is it an American condition? World Psychiat 2 104 113
5. RohdeLA
SzobotC
PolanczykG
SchmitzM
MartinsS
Tramontina S. 2005 Attention-deficit/hyperactivity disorder in a diverse culture: Do research and clinical findings support the notion of a cultural construct for the disorder? Biol Psychiat 57 1436 1441
6. CaninoG
AlegríaM
2008 Psychiatric diagnosis - is it universal or relative to culture? J Child Psychol Psychiatry 49 237 50
7. FlisherAJ
HatherillS
DhansayY
Swartz L. 2007 ADHD, Culture and the DSM-V (abstract). J Child Adolesc Mental Health 19 173
8. PolanczykG
De LimaMS
HortaBL
BiedermanJ
RohdeLA
2007 The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiat 164 942 948
9. BiedermanJ
2005 Attention-deficit/hyperactivity disorder: A selective overview. Biol Psychiatry 57 1215 1220
10. BiedermanJ
FaraoneSV
2005 Attention deficit hyperactivity disorder. Lancet 366 237 248
11. PatelV
LundC
HatherillS
PlagersonS
CorrigallJ
(in press) Social Determinants of Mental Disorders.
BlasE
Sivasankara KurupA
Priority public health conditions: from learning to action on social determinants of health Geneva WHO
12. CollettB
OhanJ
MyersK
2009 Ten-Year Review of Rating Scales. V: Scales Assesing Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolsc Psychiat 42 1015 1037
13. PelhamW
FabianoG
MassettiG
2005 Evidence-Based Assessment of Attention Deficit Hyperactivity Disorder in Children and Adolescents. J Clin Child Adolesc Psychol 34 449 476
14. SmithK
CorkumP
2007 Systematic Review of Measures Used to Diagnose Attention-Deficit/Hyperactivity Disorder in Research on Preschool Children. Topics Early Child Spec Educ 27 164
15. ShafferD
FisherP
LucasCP
DulcanMK
Schwab-StoneME
2000 NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 39 28 38
16. LeungPW
LeeCC
TangCP
HungSF
KwongSL
2005 Test-retest reliability of the Chinese version of the Diagnostic Interview Schedule for Children-Version 4 (DISC-IV). J Child Psychol Psychiatry 46 1135 1138
17. FlisherAJ
LundC
SorsdahlK
RobertsonB
2009 Test-Retest Reliability of the Xhosa Version of the Diagnostic Interview Schedule for Children (abstract). J Child Adolesc Mental Health 21 88
18. BritoG
PintoR
LinsM
1995 A behavioral assessment scale for attention deficit disorder in Brazilian children based on DSM-IIIR criteria. J Abnorm Child Psychol 23 509 520
19. LampertTL
PolanczykG
TramontinaS
MardiniV
RohdeLA
2004 Diagnostic performance of the CBCL-Attention Problem Scale as a screening measure in a sample of Brazilian children with AD/HD. J Atten Disord 8 63 71
20. VitielloB
2001 Methylphenidate in the treatment of children with attention-deficit hyperactivity disorder. Can Med Assoc J 165 1505 1506
21. BanaschewskiT
CoghillD
SantoshP
ZuddasA
AshersonP
2006 Long – acting medications for the hyperkinetic disorders: a systematic review and European treatment guideline. Eur Child Adolesc Psychiatry 15 476 495
22. KarandeS
2005 Attention deficit hyperactivity disorder – a review for family physicians. Indian J Med Sci 59 546 555
23. KratochvilCJ
EggerH
GreenhillLL
McGoughJJ
2006 Pharmacological management of preschool ADHD. J Am Acad Child Adolesc Psychiatry 45 115 118
24. TaylorE
DöpfnerM
SergeantJ
AshersonP
BanaschewskiT
2004 A. European clinical guidelines for hyperkinetic disorder – first upgrade. Eur Child Adolesc Psychiatry 13 Suppl 1 17 130
25. American Academy of Child and Adolescent Psychiatry 2007 Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 46 894 921
26. GreenhillLL
KollinsS
AbikoffH
McCrackenJ
RiddleM
2006 Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry 45 1284 1293
27. WigalT
GreenhillLL
ChuangS
McGoughJ
VitielloB
2006 Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry 45 1294 1303
28. MichelsonD
AllenAJ
BusnerJ
CasatC
DunnD
2002 Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 159 1896 1901
29. MichelsonD
FariesD
WernickeJ
KelseyD
KendrickF
2001 Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 108 1 9
30. WilensT
McBurnettK
SteinM
LernerM
SpencerT
WolraichM
2005 ADHD treatment with once daily OROS methylphenidate treatment: final results from a long term open-label study. J Am Acad Child Adolesc Psychiatry 44 1015 1023
31. WilensT
GaoH
ThomasonC
GelowitzD
KratochvilC
NewcornJ
2004 Longer term treatment with atomoxetine in adolescents with ADHD. Scientific Proceedings of the American Psychiatric Association, No. 578, New York, May
32. MTA Cooperative Group 2004 National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics 113 762 769
33. VitielloB
SevereJB
GreenhillLL
ArnoldLE
AbikoffHB
2001 Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. J Am Acad Child Adolesc Psychiatry 40 188 196
34. GreenhillLL
NewcornJH
GaoH
FeldmanPD
2007 Effect of two different methods of initiating atomoxetine on the adverse event profile of atomoxetine. J Am Acad Child Adolesc Psychiatry 45 566 572
35. WilensTE
FaraoneSV
BiedermanJ
GunawardeneS
2003 Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics 111 179 185
36. MolinaBS
HinshawSP
SwansonJM
ArnoldLE
VitielloB
2009 The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry 48 484 500
37. KleinRG
AbikoffH
HechtmanL
WeissG
2004 Design and rationale of controlled study of long-term methylphenidate and multimodal psychosocial treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry 43 792 801
38. The MTA Cooperative Group 1999 A 14-month randomized clinical trial of treatment strategies for attention deficit/hyperactivity disorder (AD/HD). Arch Gen Psychiatry 56 1073 1086
39. HechtmanL
AbikoffH
KleinRG
WeissG
RespitzC
2004 Academic achievement and emotional status of children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Amer Acad Child Adolesc Psychiatry 43 812 819
40. AbikoffH
HechtmanL
KleinRG
WeissG
FleissK
2004 Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Amer Acad Child Adolesc Psychiatry 43 802 811
41. MTA Cooperative Group 2004 National Institute of Mental Health Multimodal Treatment Study of AD/HD Follow-up: 24-Month Outcomes of Treatment Strategies for Attention-Deficit/Hyperactivity Disorder. Pediatrics 113 754 761
42. ConnersCK
EpsteinJN
MarchJS
AngoldA
WellsKC
2001 Multimodal treatment of ADHD in the MTA: An alternative outcome analysis. J Am Acad Child Adolesc Psychiatry 40 159 167
43. MarchJS
SwansonJM
ArnoldLE
HozaB
ConnersCH
2000 Anxiety as a predictor and outcome variable in the multimodal treatment study of children with ADHD MTA. J Abnorm Child Psychol 28 527 541
44. JensenPS
HinshawSP
SwansonJM
GreenhillLL
ConnersCK
2001 Findings from the NIMH Multimodal Treatment Study of ADHD MTA: implications and applications for primary care providers. J Dev Behav Pediatr 22 60 73
45. ArnoldLE
ElliotM
SachsL
BirdH
KraemerHC
2003 Effects of ethnicity on treatment attendance, stimulant response/dose, and 14-month outcome in ADHD. J Consult Clin Psychol 71 713 727
46. MTA Cooperative Group 1999 Moderators and mediators of treatment response for children with attention deficit hyperactivity disorder: the MTA Study. Arch Gen Psychiatry 56 1088 1096
47. ChronisA
JonesH
RaggiVL
2006 Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. Clin Psychol Review 26 486 502
48. FabianoG
PelhamWE
ColesEK
GnagyEM
Chronis-TuscanoA
2009 A meta-analysis of behavioural treatments for attention-deficit/hyperactivity disorder. Clin Psychol Rev 29 129 140
49. ToplakME
ConnorsL
ShusterJ
KnezevicB
ParksS
2008 Review of cognitive, cognitive-behavioral, and neural-based interventions for Attention-Deficit/Hyperactivity Disorder (AD/HD). Clin Psychol Rev 28 801 823
50. AbikoffH
1985 Efficacy of Cognitive Training Interventions in Hyperactive Children: A Critical Review. Clin Psychol Rev 5 479 512
51. BjornstadGJ
MontgomeryP
2009 Family therapy for attention-deficit disorder or attention-deficit/hyperactivity disorders in children and adolescents. Cochrane Database of Systematic Reviews. Art.No CD005042 doi:10.1002/14651858.CD005042.pub2
52. CorcoranJ
DattaloP
2006 Parent Involvement in Treatment for AD/HD: A Meta-Analysis of the Published Studies. Res Soc Work Pract 16 561 570
53. GhanizadehA
ShahrivarFZ
2005 The Effect of Parent Management Training on Children with Attention Deficit Hyperactivity Disorder. J Child Adolesc Mental Health 17 31 34
54. SaugataB
AniruddhaD
1996 Parent training in children with attention deficit hyperactivity disorder: An integrated approach for greater effectiveness. Ind J Clin Psychol 23 184 191
55. WalkerJS
ColemanD
LeeJ
SquirePN
FriesenBJ
2008 Children's stigmatization of childhood depression and AD/HD: magnitude and demographic variation in a national sample. J Am Acad Child Adolesc Psychiatry 47 912 920
56. HovenCW
DoanT
MusaGJ
JaliashviliT
DuarteCS
2008 WPA Awarness Task Force. Worldwide child and adolescent mental health begins with awareness: a preliminary assessment in nine countries. Int Rev Psychiatry 20 261 270
57. Graeff-MartinsAS
FlamentMF
FayyadJ
TyanoS
JensenP
2008 Diffusion of efficacious interventions for children and adolescents with mental health problems. J Child Psychol Psychiatry 49 335 352
58. JitendraAK
DupaulGJ
SomekiF
TrescoKE
2008 Enhancing academic achievement for children with Attention-Deficit Hyperactivity Disorder: evidence from school-based intervention research. Dev Disabil Res Rev 14 325 330
59. PatelV
SimonG
ChowdharyN
KaayaS
ArayaR
2009 Packages of Care for Depression in Low - and Middle-Income Countries. PLoS Med 6 e1000159 doi:10.1371/journal.pmed.1000159
60. RushtonJL
FantKE
ClarkSJ
2004 Use of practice guidelines in the primary care of children with attention-deficit/hyperactivity disorder. Pediatrics 11 e23 28
61. EpsteinJN
RabinerD
JohnsonDE
FitzgeraldDP
ChrismanA
2007 Improving attention-deficit/hyperactivity disorder treatment outcomes through use of a collaborative consultation treatment service by community-based pediatricians: A cluster randomized trial. Arch Pediatr Adolesc Med 161 835 840
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 2- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Effectiveness of Non-nucleoside Reverse-Transcriptase Inhibitor-Based Antiretroviral Therapy in Women Previously Exposed to a Single Intrapartum Dose of Nevirapine: A Multi-country, Prospective Cohort Study
- The Global Research Neglect of Unassisted Smoking Cessation: Causes and Consequences
- Adolescent HIV—Cause for Concern in Southern Africa
- Impact of Antiretroviral Therapy on Incidence of Pregnancy among HIV-Infected Women in Sub-Saharan Africa: A Cohort Study
- Automated Detection of Infectious Disease Outbreaks in Hospitals: A Retrospective Cohort Study
- Event Rates, Hospital Utilization, and Costs Associated with Major Complications of Diabetes: A Multicountry Comparative Analysis
- Pretreatment CD4 Cell Slope and Progression to AIDS or Death in HIV-Infected Patients Initiating Antiretroviral Therapy—The CASCADE Collaboration: A Collaboration of 23 Cohort Studies
- Can Broader Diffusion of Value-Based Insurance Design Increase Benefits from US Health Care without Increasing Costs? Evidence from a Computer Simulation Model
- Causes of Acute Hospitalization in Adolescence: Burden and Spectrum of HIV-Related Morbidity in a Country with an Early-Onset and Severe HIV Epidemic: A Prospective Survey
- Developing Global Maps of the Dominant Vectors of Human Malaria
- Guidance for Developers of Health Research Reporting Guidelines
- Packages of Care for Attention-Deficit Hyperactivity Disorder in Low- and Middle-Income Countries
- A New Policy on Tobacco Papers
- Ghostwriting at Elite Academic Medical Centers in the United States
- Measuring hsCRP—An Important Part of a Comprehensive Risk Profile or a Clinically Redundant Practice?
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Packages of Care for Attention-Deficit Hyperactivity Disorder in Low- and Middle-Income Countries
- Measuring hsCRP—An Important Part of a Comprehensive Risk Profile or a Clinically Redundant Practice?
- Developing Global Maps of the Dominant Vectors of Human Malaria
- Guidance for Developers of Health Research Reporting Guidelines
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání