-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Measuring hsCRP—An Important Part of a Comprehensive Risk Profile or a Clinically Redundant Practice?
article has not abstract
Published in the journal: . PLoS Med 7(2): e32767. doi:10.1371/journal.pmed.1000196
Category: Essay
doi: https://doi.org/10.1371/journal.pmed.1000196Summary
article has not abstract
With the publication of the JUPITER trial [1] there is now considerable interest in measuring high-sensitivity C-reactive protein (hsCRP). While treatment with rosuvastatin decreased the chance of clinically important events in the JUPITER trial, does this necessarily justify treatment based on hsCRP measurements? Here, we discuss issues surrounding hsCRP measurements in patients.
What Is the Suggested Role for hsCRP Measurements?
The median values for hsCRP are 2.5 mg/l (American women) and 1.5 mg/l (American men) [2]. Typical recommendations are to measure hsCRP in “intermediate risk” patients to help classify them into either a higher or lower risk category [3],[4]. Intermediate risk is usually, and arbitrarily, described as either a 10%–20% [3],[4] or 5%–20% [5],[6] 10-year risk of developing coronary heart disease (CHD). It has been suggested that hsCRP levels<1, 1–3, or >3 mg/l represent lower, moderate, and higher relative risk of future heart disease, respectively.
How Accurate Is hsCRP Measurement?
Between-subject standard deviation for hsCRP measurement is 1.7 mg/l. Within-subject standard deviation is 1.2 mg/l [7]. Clearly, within-subject standard deviation means that a patient with a reported hsCRP of 2 mg/l (moderate) when re-measured could readily be placed in a low (<1 mg/l) or high (>3 mg/l) range [7]. Some authors have therefore suggested hsCRP needs to increase or decrease by 120% to 175% before a “real” change can be considered to have occurred [8]. It has been estimated that “in order to reduce the intra-individual variation sufficiently, each subject is likely to require blood samples collected on at least 10 occasions” [9].
Does Measuring hsCRP Add Value to Already Established Risk Factors When It Comes to Assessing CHD or Cardiovascular Disease Risk?
Several studies have confirmed that using hsCRP in addition to established risk factors (age, gender, blood pressure, cholesterol, smoking, and diabetes) does not improve the estimation of risk of cardiovascular disease (CVD) to a clinically important degree. Folsom et al. found that elevated hsCRP was associated with an increased risk of CHD (hazard rate ratio 1.19, p<0.001) but that it was similar to, or less important than, many other markers including D-dimer (1.36), interleukin-6 (1.28), and lipoprotein-associated phospholipase A2 (1.17) [10]. Wang et al. used C statistics to assess CVD predictive models and found age, sex, and traditional risk factors provided 0.76 value compared to the 0.77 value when ten different biomarkers (including hsCRP) were added [11]. Shah et al. also assessed the additive value of hsCRP and found that “hsCRP does not perform better than the Framingham risk equation for discrimination. The improvement in risk stratification or reclassification …is small and inconsistent” [12].
How Does Measuring hsCRP Affect Absolute Risk Estimates of CVD in Individuals?
The Reynolds Risk Score [13] is an online calculator that incorporates hsCRP measurement with other risk factor information (age, sex, systolic blood pressure [SBP], smoking history, total cholesterol, high-density lipoprotein [HDL] cholesterol, and family history) to compute the risk (%) of heart attack, stroke, or other major heart disease in the next 10 years. We used two patients, one male and one female with a “moderate” hsCRP level (2 mg/l), and adjusted other factors so that they would have an overall absolute risk estimate of 15%—in the middle of the “intermediate” risk category. We then changed hsCRP to 0.5, 5, or 10 mg/l to see what would happen to their calculated risk (Table 1).
Tab. 1. Estimated 10 year risk of a heart attack, stroke, or other major heart disease based on the risk calculator at reynoldsriskscore.com. 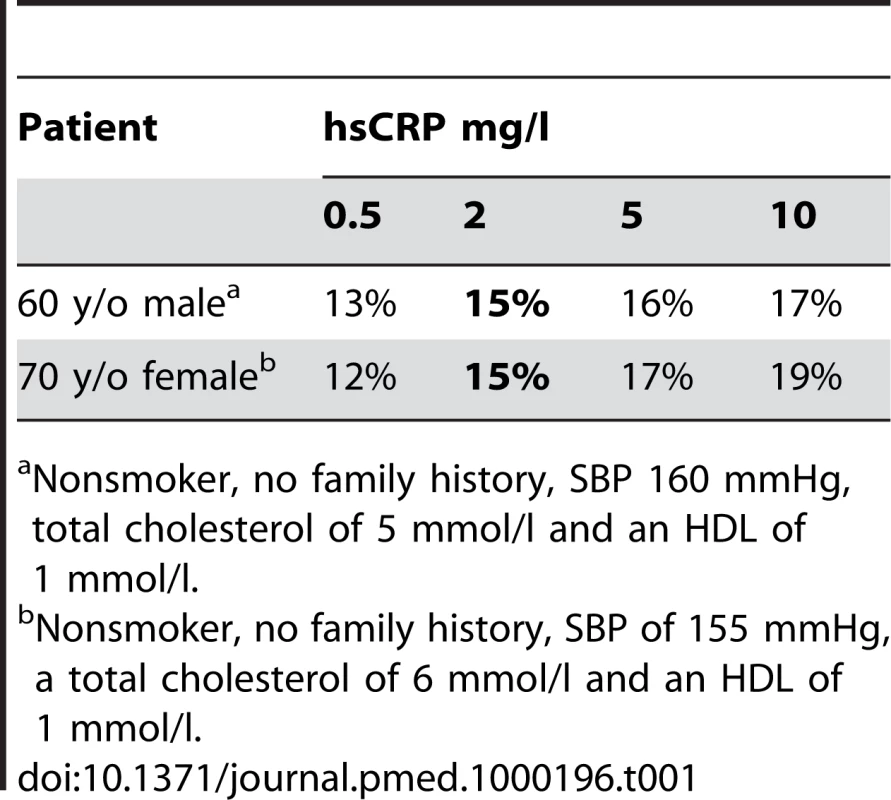
Nonsmoker, no family history, SBP 160 mmHg, total cholesterol of 5 mmol/l and an HDL of 1 mmol/l. Absolute risk estimates changed by just ±2% (male) and ±3%–4% (female). It is important to appreciate that the magnitude of these risk estimate changes are equal to or less than the confidence interval (CI) on the original risk estimate. Anderson et al. noted for the Framingham dataset that the 95% CIs for 10-year predictions of CHD of less than 10%, 10%–20%, and 30%–40% were ±1.5%–2%, ±3%, and ±15%, respectively [14]. Reynolds et al. recently calculated the CI of the estimates at 10%, 15%, 20%, and 30% thresholds to be ±4%, 5%, 6%, and 7%, respectively [15]. It seems obvious that refining such predictions by ±2%–4% by incorporating hsCRP measurements is of little clinical relevance.
Nonetheless, if we assume the changes in risk estimate using hsCRP are exact and the risk estimates are actually changed by the percentage suggested; are these changes in risk estimate clinically relevant? First, in these scenarios, one can see that none of the revised risk estimates actually end up recategorizing our particular patients into a different risk category. Second, would knowing one's risk was 17% or 13%, instead of 15% lead to a difference in the decision to consider taking a drug?
Let's assume statins produce a 25% relative risk reduction in CVD. If a patient has an absolute baseline risk of 17%, their risk, if they took a statin, would decline to 12.75%, an absolute difference of 4.25%. If their baseline risk was 15%, a statin would lower their risk to 11.25%, an absolute difference of 3.75%. In other words, in this patient, if the absolute risks were indeed different the difference in the estimate of absolute benefit would be 0.5% (4.25% minus 3.75%); a difference unlikely to change the decision to use or not use a statin.
Don't Studies Show hsCRP Measurements Reclassify Patients into Different Risk Categories?
Ridker et al. have shown that incorporating hsCRP “reclassifies” people into different risk categories [6],[16]. For instance, 14% of women and 12% of men were recategorized from intermediate to low risk. However, others have disputed these findings and found that only 5.6% of patients would end up being reclassified [17]. What is likely happening in these “reclassification” papers is a number of patients with risks just above or below these arbitrary thresholds (say an estimated 18%–19% risk) will be bumped up or down to a different risk category because their estimate has now changed by 2%–3%, but these absolute changes, as discussed above, have little clinical relevance [13].
Does Lowering hsCRP with Drugs Result in Improved Cardiovascular Outcomes?
In the JUPITER trial (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin), rosuvastatin was shown to reduce the chance of developing a clinically important cardiovascular event. Table 2 outlines the impact of other drugs on hsCRP and cardiovascular events. Although each drug has various pharmacological effects the impact of lowering hsCRP with medication on cardiovascular events is consistently inconsistent.
Tab. 2. Examples of drugs that lower hsCRP and the impact these drugs have had on clinical outcomes. 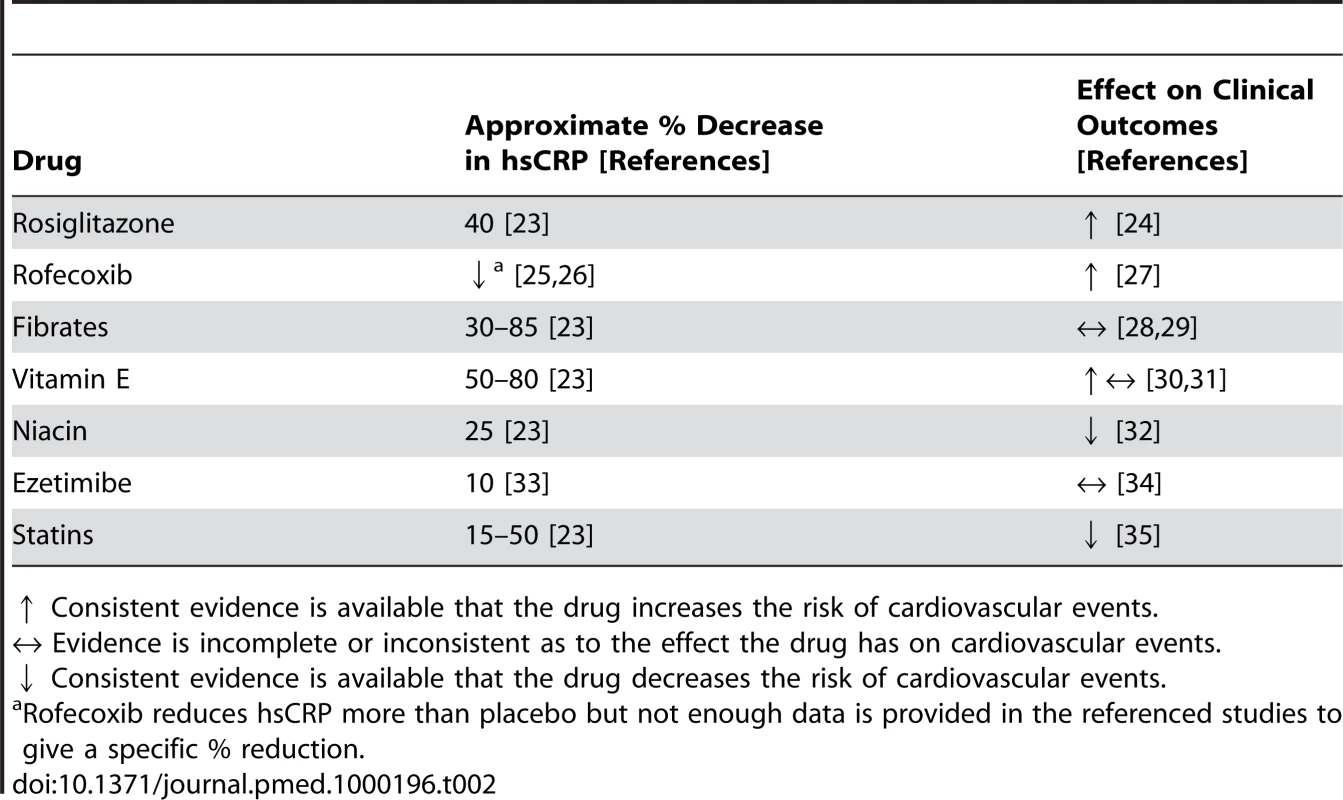
↑ Consistent evidence is available that the drug increases the risk of cardiovascular events. What Were the Outcomes of JUPITER?
The JUPITER investigators screened almost 90,000 people to enroll 17,802 who had an hsCRP≥2 mg/l and an LDL<3.4 mmol/l (130 mg/dl). Participants (mean age 66, median LDL of 2.8 mmol/l, average hsCRP of 4.2 mg/l) were randomized to rosuvastatin 20 mg daily or placebo. Based on the baseline characteristics of these participants, using the Reynolds risk score, the average participant in this trial would have had a 10-year risk of approximately 10%–15% [13]. The trial was stopped early, after a median follow-up of 1.9 years revealed that the combined endpoint of myocardial infarction, stroke, or death from cardiovascular causes occurred in 0.9% of participants taking rosuvastatin compared to 1.8% of patients taking placebo.
Ideally, the effects of hsCRP on risk assessment and treatment decisions would be evaluated using a randomized trial with two groups: one in which a risk assessment included hsCRP and one that did not. Then participants in each group would receive a drug known to improve cardiac outcomes at a predefined risk level. To date no trial, including JUPITER, has been designed to answer the question “Does the use of hsCRP in clinical practice result in reduced CVD outcomes and improved health?” A meta-analysis had already demonstrated that primary prevention with statins lowers the risk of CVD [18]. JUPITER might be considered unique in that the study participants had a reduced low-density lipoprotein (LDL) but ASCOT-LLA (Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm) has already demonstrated that primary prevention patients at risk for CVD benefit from a statin despite a lower than “normal” LDL, albeit not as low an LDL as was evaluated in JUPITER (ASCOT-LLA mean LDL 3.4 mmol/l, JUPITER median LDL 2.8 mmol/l) [19]. It is important to remember that JUPITER was an evaluation of a fixed dose of rosuvastatin and thus getting subjects to “targets” is purely extrapolation, as none of the statin studies done to date, including the JUPITER trial, actually attempted to get subjects to a targeted cholesterol or hsCRP level [20]. The reductions in outcomes found in JUPITER were greater than those seen in other studies of statins in primary prevention. For example, a meta-analysis of primary prevention statin trials [18] found a 29.2% reduction in relative risk for major coronary events compared to 54% for the same outcome in JUPITER [1]. It is tempting to think the enrollment of patients with hsCRP≥2 mg/l contributed to the increased benefit seen in JUPITER. However, JUPITER was stopped early for benefit, and there is some suggestion that trials stopped early for benefit yield exaggerated treatment benefits [21]. Additionally, it is important to keep in mind the 54% reduction relative risk of major coronary events is actually a 0.2% per year absolute reduction (number need to treat of 500) [1].
Is There Evidence That Baseline hsCRP Levels Predict Outcomes from Statin Therapy?
In a post-hoc analysis, Ridker et al. reviewed participants in the AFCAPS/TexCAPS study (Air Force/Texas Coronary Atherosclerosis Prevention Study). They stratified participants according to their baseline LDLs or hsCRPs (A, any hsCRP; H, LDL or hsCRP higher than the median; L, LDL or hsCRP lower than the median) to determine if different baseline levels of hsCRP or LDL could predict variations in the benefit of a statin. The main results are outlined in Table 3.
Tab. 3. AFCAPS/TexCAPS acute coronary disease results - Relative risks (and 95% CIs) associated with lovastatin therapy, according to baseline lipid and C-reactive protein levels. 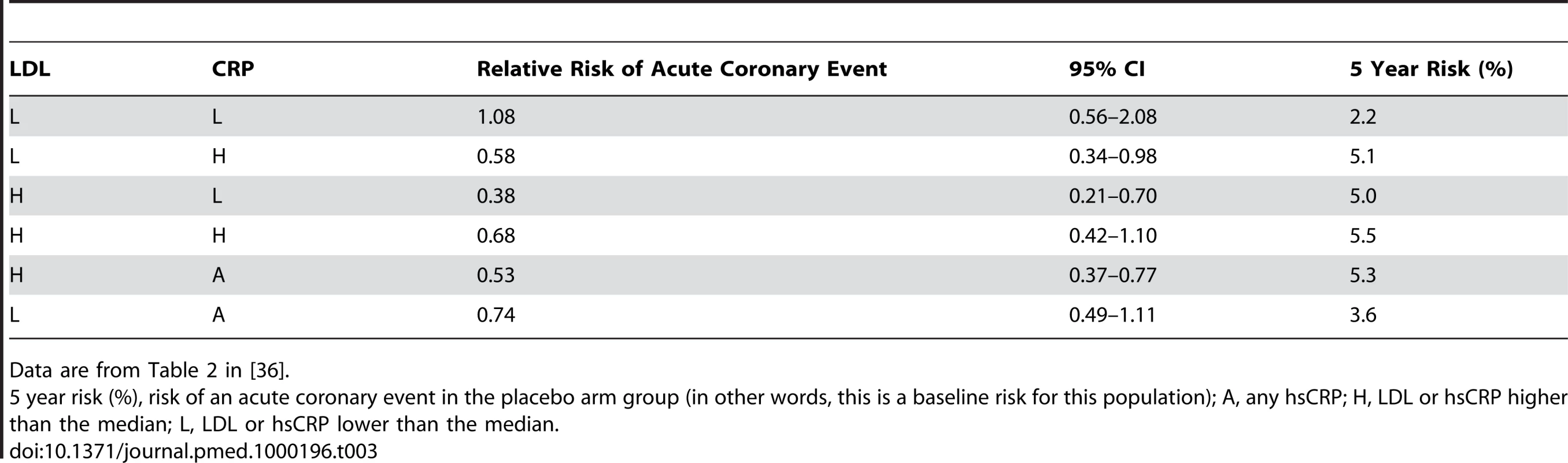
Data are from Table 2 in [36]. Key issues to consider about the AFCAPS/TexCAPS data:
-
Only the L/L and the L/A groupings showed a difference in baseline 5-year risk.
-
In three of the categories (L/H, H/L, and H/A), lovastatin produced a reduction in events that was superior to placebo; however, owing to the overlapping CIs, no subset had a statistically different magnitude of outcome from any other subset.
-
Post-hoc analysis of subgroups defined after randomization are subject to a high risk of bias.
Isn't There Evidence That Reducing Levels of hsCRP Predicts Outcomes?
The AFCAPS/TexCAPS retrospective evaluation was about what happened to subjects based on baseline LDL/hsCRP. Interestingly, no studies have actually looked prospectively at the question of getting patients to a target hsCRP or cholesterol. In the JUPITER study, a fixed-dose rosuvasatin trial, investigators did look at achieved (in contrast to AFCAPS/TexCAPS) hsCRP and LDL measurements to see if there was an association between achieved levels and outcome [22]. The categories they chose (above or below an LDL of 1.8, an hsCRP of 2 or 1) were prespecified. The key findings are outlined in Table 4.
Tab. 4. JUPITER results for cardiovascular events - Hazard ratios for incident cardiovascular events in JUPITER according to achieved concentrations of LDL cholesterol and hsCRP after initiation of rosuvastatin. 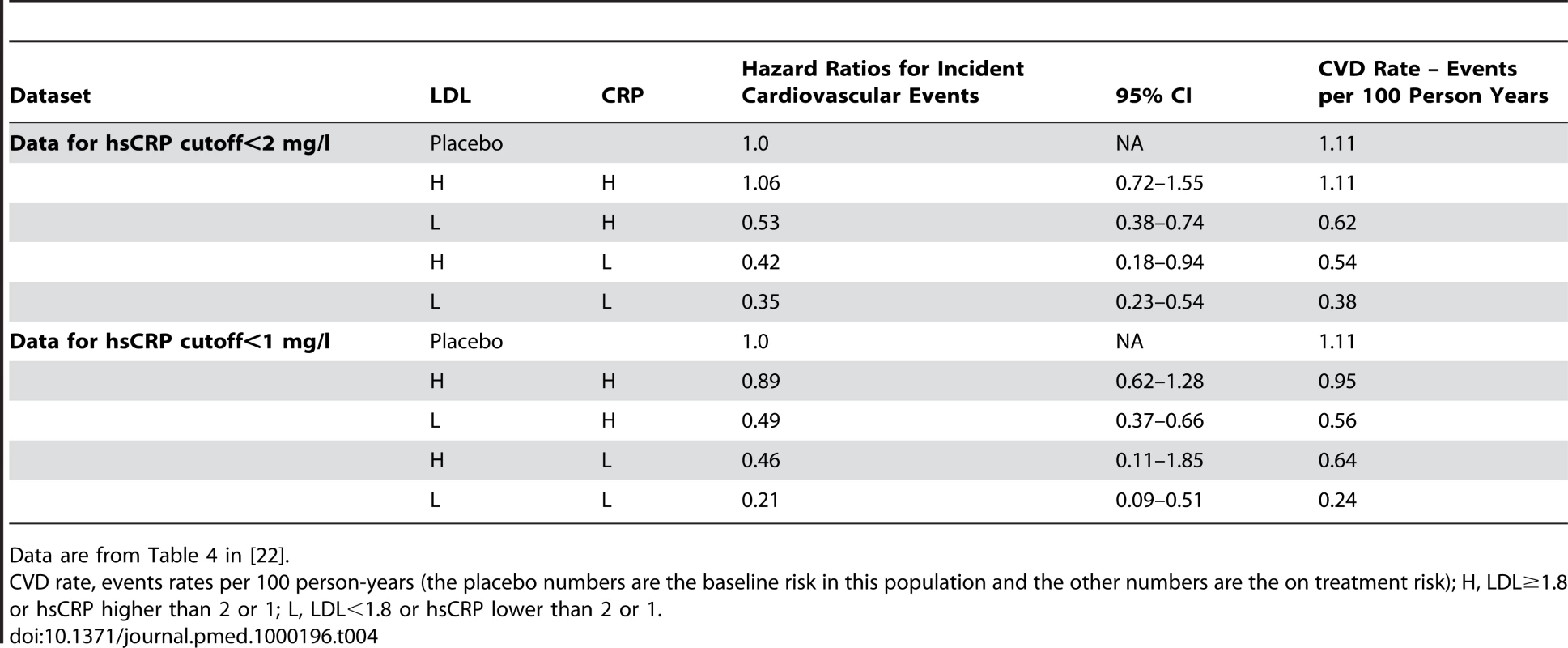
Data are from Table 4 in [22]. Key issues to consider about the JUPITER data:
-
While some information about baseline characteristics was provided, none was provided for any of the subsets in Table 4 other than the low LDL/low hsCRP.
-
No information is provided regarding how much (relatively) LDL and hsCRP went down in each of the LDL/CRP subsets.
-
Participants who took 20 mg of rosuvastatin and did not achieve an LDL<1.8 or hsCRP<1 (H/H) experienced no clinical benefit. While we are not necessarily endorsing the approach, if one were to follow this evidence, patients who do not attain an LDL<1.8 and a hsCRP of less than 1 or 2 while on 20 mg of rosuvastatin should stop taking the drug as they will not derive a clinical benefit.
-
There is little difference in the results among participants who achieved hsCRP of <1 mg/dl or <2 mg/d, which suggests that as long as you get the hsCRP below 2 mg/dl any further reduction yields no additional benefit.
-
Participants who achieved both an LDL<1.8 and an hsCRP<2 (L/L), had a lower point estimate of benefit than participants who only achieved one of these breakpoints. The authors state that overall there was a p-value for trend across LDL cholesterol and/or hsCRP strata. However, the CIs for a number of the groups clearly overlap to a degree that does not allow one to draw specific conclusions about the differences in benefit between specific subsets.
-
It is unknown if lower LDL/hsCRP levels were attained due to differences in adherence to rosuvastatin, a factor that might help explain why the group that did not achieve specific LDL and hsCRP levels did not appear to benefit from statin therapy.
Conclusion
The substantial intra-subject variation in hsCRP measurements makes it virtually impossible to assess the impact a therapy has on hsCRP in an individual patient. Even if the intra-subject variation is ignored, when hsCRP is used in addition to other established risk factors, the size of the absolute changes in risk estimates, even at the extremes of hsCRP levels, are unlikely to change an individual's decision to seek therapy. Finally, the evidence that cardiovascular events are reduced when a patient takes a drug that lowers hsCRP is inconsistent at best. Armed with this information, we hope clinicians can determine for themselves whether measuring hsCRP is an important part of a comprehensive risk profile or a clinically redundant practice.
Zdroje
1. RidkerPM
DanielsonE
FonsecaFA
GenestJ
GottoAMJr
2008 Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359 2195 2207
2. WoloshinS
SchwartzLM
2005 Distribution of C-reactive protein values in the United States. N Engl J Med 352 1611 1613
3. MitkaM
2003 Panel endorses limited role for CRP tests. JAMA 289 973 974
4. GrundySM
HansenB
SmithSC,Jr.
CleemanJI
KahnRA
2004 Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation 109 551 556
5. BardRL
RubenfireM
EagleK
ClarkeNS
BrookRD
2005 Utility of C-reactive protein measurement in risk stratification during primary cardiovascular disease prevention. Am J Cardiol 95 1378 1379
6. RidkerPM
PaynterNP
RifaiN
GazianoJM
CookNR
2008 C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation 118 2243 2251
7. OckeneIS
MatthewsCE
RifaiN
RidkerPM
ReedG
2001 Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem 47 444 450
8. MacyEM
HayesTE
TracyRP
1997 Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem 43 52 58
9. CampbellB
BadrickT
FlatmanR
KanowskiD
2002 Limited clinical utility of high-sensitivity plasma C-reactive protein assays. Ann Clin Biochem 39 85 88
10. FolsomAR
ChamblessLE
BallantyneCM
CoreshJ
HeissG
2006 An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: the atherosclerosis risk in communities study. Arch Intern Med 166 1368 1373
11. WangTJ
GonaP
LarsonMG
ToflerGH
LevyD
2006 Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 355 2631 2639
12. ShahT
CasasJP
CooperJA
TzoulakiI
SofatR
2009 Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol 38 217 231
13. 2009 Reynold's Risk Score. Available: http://www.reynoldsriskscore.org/. Accessed 30 March 2009
14. AndersonKM
OdellPM
WilsonPW
KannelWB
1991 Cardiovascular disease risk profiles. Am Heart J 121 293 298
15. ReynoldsTM
TwomeyP
WierzbickiAS
2002 Accuracy of cardiovascular risk estimation for primary prevention in patients without diabetes. J Cardiovasc Risk 9 183 190
16. CookNR
BuringJE
RidkerPM
2006 The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med 145 21 29
17. WilsonPWF
PencinaM
JacquesP
SelhubJ
D'AgostinoRSr
2008 C-Reactive Protein and Reclassification of Cardiovascular Risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes 1 92 97
18. BaigentC
KeechA
KearneyPM
BlackwellL
BuckG
2005 Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366 1267 1278
19. SeverPS
DahlofB
PoulterNR
WedelH
BeeversG
2003 Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361 1149 1158
20. 2009 Theheart.org. Available: http://www.theheart.org/editorial-program/926043.do. Accessed 30 March 2009
21. MontoriVM
DevereauxPJ
AdhikariNK
BurnsKE
EggertCH
2005 Randomized trials stopped early for benefit: a systematic review. JAMA 294 2203 2209
22. RidkerPM
DanielsonE
FonsecaFA
GenestJ
GottoAMJr
2009 Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 373 1175 1182
23. PrasadK
2006 C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev 24 33 50
24. SinghS
LokeYK
FurbergCD
2007 Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 298 1189 1195
25. MonakierD
MatesM
KlutsteinMW
BalkinJA
RudenskyB
2004 Rofecoxib, a COX-2 inhibitor, lowers C-reactive protein and interleukin-6 levels in patients with acute coronary syndromes. Chest 125 1610 1615
26. BogatyP
BrophyJM
NoelM
BoyerL
SimardS
2004 Impact of prolonged cyclooxygenase-2 inhibition on inflammatory markers and endothelial function in patients with ischemic heart disease and raised C-reactive protein: a randomized placebo-controlled study. Circulation 110 934 939
27. McCormackJP
RangnoR
2002 Digging for data from the COX-2 trials. CMAJ 166 1649 1650
28. KeechA
SimesRJ
BarterP
BestJ
ScottR
2005 Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366 1849 1861
29. 2000 Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation 102 21 27
30. VivekananthanDP
PennMS
SappSK
HsuA
TopolEJ
2003 Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 361 2017 2023
31. BjelakovicG
NikolovaD
GluudLL
SimonettiRG
GluudC
2007 Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297 842 857
32. 1975 Clofibrate and niacin in coronary heart disease. JAMA 231 360 381
33. SagerPT
CapeceR
LipkaL
StronyJ
YangB
2005 Effects of ezetimibe coadministered with simvastatin on C-reactive protein in a large cohort of hypercholesterolemic patients. Atherosclerosis 179 361 367
34. KasteleinJJ
AkdimF
StroesES
ZwindermanAH
BotsML
2008 Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 358 1431 1443
35. KearneyPM
BlackwellL
CollinsR
KeechA
SimesJ
2008 Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 371 117 125
36. RidkerPM
RifaiN
ClearfieldM
DownsJR
WeisSE
2001 Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 344 1959 1965
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 2- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Effectiveness of Non-nucleoside Reverse-Transcriptase Inhibitor-Based Antiretroviral Therapy in Women Previously Exposed to a Single Intrapartum Dose of Nevirapine: A Multi-country, Prospective Cohort Study
- The Global Research Neglect of Unassisted Smoking Cessation: Causes and Consequences
- Adolescent HIV—Cause for Concern in Southern Africa
- Impact of Antiretroviral Therapy on Incidence of Pregnancy among HIV-Infected Women in Sub-Saharan Africa: A Cohort Study
- Automated Detection of Infectious Disease Outbreaks in Hospitals: A Retrospective Cohort Study
- Event Rates, Hospital Utilization, and Costs Associated with Major Complications of Diabetes: A Multicountry Comparative Analysis
- Pretreatment CD4 Cell Slope and Progression to AIDS or Death in HIV-Infected Patients Initiating Antiretroviral Therapy—The CASCADE Collaboration: A Collaboration of 23 Cohort Studies
- Can Broader Diffusion of Value-Based Insurance Design Increase Benefits from US Health Care without Increasing Costs? Evidence from a Computer Simulation Model
- Causes of Acute Hospitalization in Adolescence: Burden and Spectrum of HIV-Related Morbidity in a Country with an Early-Onset and Severe HIV Epidemic: A Prospective Survey
- Developing Global Maps of the Dominant Vectors of Human Malaria
- Guidance for Developers of Health Research Reporting Guidelines
- Packages of Care for Attention-Deficit Hyperactivity Disorder in Low- and Middle-Income Countries
- A New Policy on Tobacco Papers
- Ghostwriting at Elite Academic Medical Centers in the United States
- Measuring hsCRP—An Important Part of a Comprehensive Risk Profile or a Clinically Redundant Practice?
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Packages of Care for Attention-Deficit Hyperactivity Disorder in Low- and Middle-Income Countries
- Measuring hsCRP—An Important Part of a Comprehensive Risk Profile or a Clinically Redundant Practice?
- Developing Global Maps of the Dominant Vectors of Human Malaria
- Guidance for Developers of Health Research Reporting Guidelines
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



