-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Developing Global Maps of the Dominant Vectors of Human Malaria
article has not abstract
Published in the journal: . PLoS Med 7(2): e32767. doi:10.1371/journal.pmed.1000209
Category: Health in Action
doi: https://doi.org/10.1371/journal.pmed.1000209Summary
article has not abstract
Introduction
Despite advances in mapping the geographical distribution and intensity of malaria transmission [1],[2], the ability to provide strategic, evidence-based advice for malaria control programmes remains constrained by the lack of range maps of the dominant Anopheles vectors of human malaria. This is because appropriate vector control depends on knowing both the distribution and epidemiological significance of Anopheles vectors [3]. Substantial investments by major donors in the distribution of long-lasting insecticide-treated nets and indoor residual spraying campaigns [4] are, therefore, not always fully informed by the basic biology of local anophelines.
Recent attempts to delineate Anopheles distributions have been conducted in Africa [5]–[11], the Americas [12]–[16], Europe [17], Central and South East Asia [18]–[22], and at the global scale [23]–[26]. The mapping techniques used in these various studies range from those based on expert opinion and simple interpolations to those employing more sophisticated statistical methods. Consequently, these studies are difficult to compare and impossible to synthesize globally. In addition, whereas in some regions Anopheles species distributions and their contribution to human malaria transmission are well known, uncertainty arises when suites of vectors contribute to local transmission, when the margins of the species ranges are poorly defined, and/or when there is simply a lack of any, or reliably identified, distribution records. Furthermore, as many regions attempt to maintain their malaria-free status against imported malaria [27] and others consider their prospects of malaria elimination [28],[29], contemporary maps of anophelines that are competent vectors for malaria are important in assessing local receptivity to reintroduction [30].
To help address these needs, the Malaria Atlas Project (MAP, http://www.map.ox.ac.uk) [31] has extended its activities to collate anopheline occurrence data to map the contemporary geographic distributions of the dominant mosquito vectors of human malaria. The plans for, and progress of, this initiative are described here.
Defining the Dominant Anopheles Vectors of Human Malaria
There are 462 formally named Anopheles species, with a further 50 provisionally designated and awaiting description [32]–[34]. Of these, approximately 70 have been shown to be competent vectors of human malaria [35] and from this set, 52 candidate dominant vector species (DVS) were initially chosen for inclusion in the MAP vector distribution mapping project. These DVS are species (or species complexes) that transmit the majority of human malaria parasites in an area by virtue of their abundance, their propensity for feeding on humans, their mean adult longevity (only old individuals incubate the parasite long enough to transmit the disease), or any combination of these and other factors that increase overall vectorial capacity [36]. The DVS were the inclusive set of those species identified as “main” [37],[38], “dominant” [24], or “principal” [23],[25] in major reviews of Anopheles distribution and biology. The list was then further refined by anopheline experts from the Americas, Europe, Africa, Asia, and the Pacific, who co-author this article, to exclude 11 species that were not considered important vectors either because few recent data had implicated them in transmission or because they acted as vectors in only restricted geographical areas (Text S1). Following the convention of the major reviews in this area [23]–[25],[37],[38], the DVS of the Anopheles (Cellia) gambiae complex are listed separately. We hope also to map at species level three other complexes, where examination of the primary literature has indicated sufficient species-specific data (the An. (Nyssorhynchus) albitarsis, An. (Cellia) culicifacies, and An. (Cellia) dirus complexes). Further details are provided in the legend of the maps of each complex in Text S3 (for the An. (Nyssorhynchus) albitarsis complex) and Text S5 (for the An. (Cellia) culicifacies and An. (Cellia) dirus complexes).
Comprehensive Literature Searches
An exhaustive and systematic search of formal and informal literature was conducted, mirroring the approaches developed by the MAP in building a global database of malaria parasite prevalence [39]. Only information collected after 31 December 1984 was searched. This criterion ensured that the data collected were representative of the contemporary distribution of the DVS and that the DVS occurrence records included only data collected using modern taxonomic species concepts [32],[33]. Following the introduction of cytological and then molecular methods to mosquito systematics, the taxonomy of the Anopheles changed radically, making many earlier species determinations potentially unreliable [32],[33],[40]–[43]. This date restriction also served to focus finite literature retrieval and abstracting resources on newer references, that are easier to retrieve from libraries, have sites that are less problematic to geo-position, and have authors that can often still be contacted with queries.
Records of the presence or absence of a DVS at a particular site and on a particular date were entered into the database so that information collected at different times from a locality was documented. Because abundance data have not been reported using methods that can be readily standardized across entomological surveys, only presence and absence data were used to generate the maps. Although the geographic distribution of the DVS in malaria-endemic countries is the first concern, data from any location was recorded because, as previously noted, information on DVS distribution is of major importance in those areas seeking to maintain their malaria-free status. Moreover, when modelling the fundamental niche of a species [44] using climate-envelope approaches [45], the aim is to be inclusive geographically, in an attempt to fully represent the environmental limits encompassed by its range.
Once a relevant literature source was identified, information was extracted using a list of data fields specified by a detailed pro forma (Text S2). Precise geo-positioning was conducted using established methods [39], so that any uncertainty associated with the positioning could be estimated [46]–[49]. Our strategy has been to first target the formally published literature and to use this base to direct further searches for informal (“grey”) literature sources and unpublished information held by relevant individuals and organisations. The results of this exercise were a total of 41,518 records with 22,249 spatially unique observations for all 41 DVS. These records are shown in full in a series of maps in Text S3, Text S4, and Text S5 for the American, Europe Africa, and Middle East and Asia Pacific region species, respectively. Short legends are included with each map indicating areas for which occurrence records are not well documented in the formal literature by comparison with digitised expert opinion distributions for each species. Informal searches are to be focussed on these areas of poor coverage and, where not prohibited by taxonomic identification issues, the inclusion date will be relaxed to the 31 December 1974. Ultimately, all these data will be made available in the public domain in accordance with the open access data sharing principles of the MAP [31].
Collaborative Online Databases
Many initiatives are being developed to provide information on the geographical distribution of disease vectors, including the Anopheles (Table 1; for example surveys of the geographical distribution of different forms of insecticide resistance [50]–[52]). These initiatives will be a significant help in data acquisition. Duplication of search effort will be minimized by ensuring compatibility between different data abstraction ontologies (e.g., [53] and Text S2), so that where possible, data exchange can be automated. Where this cannot be achieved, data will be incorporated manually into the MAP archives with its provenance clearly recorded.
Tab. 1. Summary of the online resources for <i>Anopheles</i>. 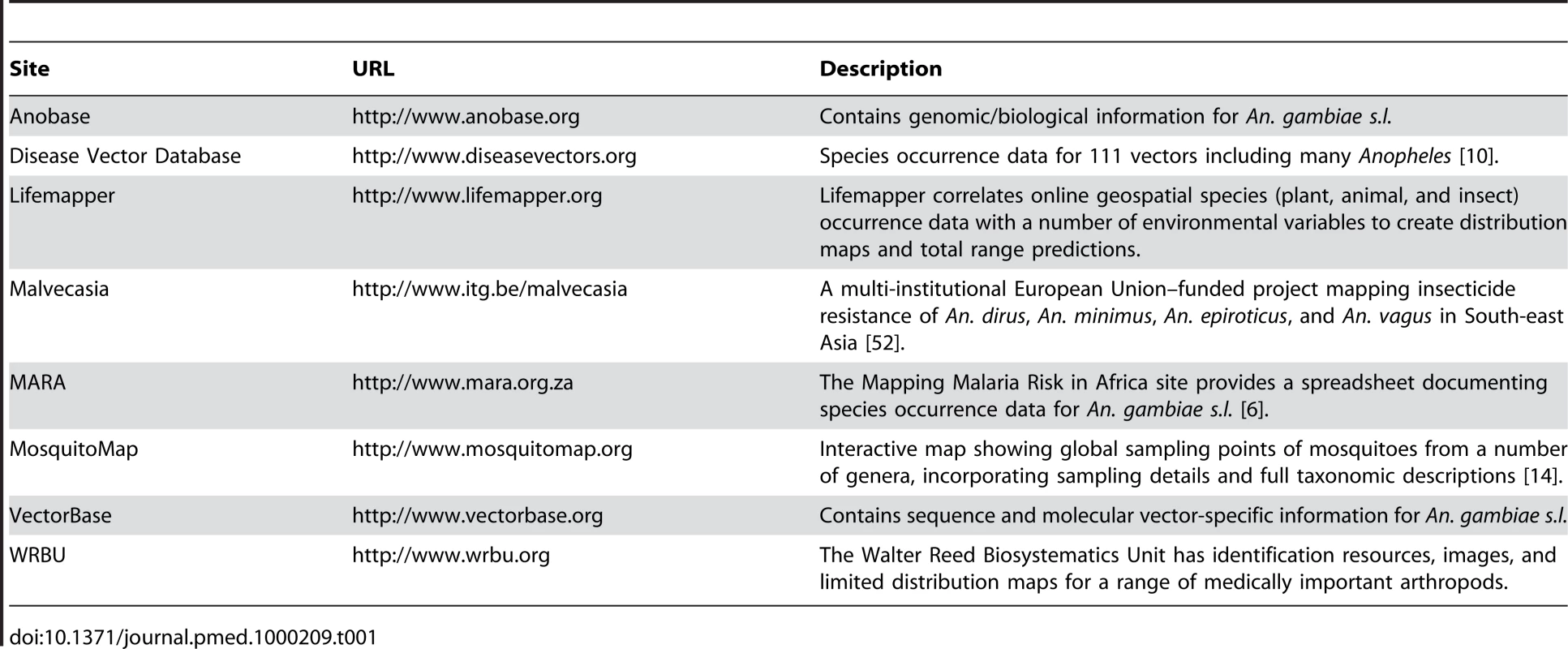
New Species Mapping Techniques
Recent years have seen the development of a number of new techniques to predict species ranges [54]–[59], of which the most promising include methods based on boosted regression trees [60],[61], generalised additive models [62], and maximum entropy approaches [63]. In addition, Bayesian statistical approaches [64]–[66], which have been widely used in mapping malaria prevalence [67]–[72], have recently begun to be applied to mapping the relative frequency of Anopheles species [73]. Bayesian models are able to integrate information from disparate sources and allow the comprehensive quantification of prediction uncertainty, something that is often overlooked in species mapping exercises [74].
An important input into the iterative mapping process is expert advice from entomologists and public health workers with extensive experience of DVS in the field. To facilitate this input, the DVS have been split into three biogeographical regions: the Americas (nine species); Africa, Europe, and the Middle East (13 species); and the Asia-Pacific region (19 species) (Text S1). These experts have helped refine the expert opinion distributions digitised from the literature for the 41 DVS. These are presented alongside the species occurrence summaries in Text S3, Text S4, and Text S5.
New Earth Observing Satellite Data
The statistical techniques we shall employ in future mapping efforts will model species occurrence as a function of environmental variables. We can then predict species distributions as a function of environmental conditions that can be obtained from Earth-observing satellite imagery [75]. During model formulation and validation we shall use coarse spatial resolution (∼8×8 km) multitemporal remotely sensed imagery [76] to reduce computational demand. Once the particular mapping technique is chosen, we will move to more contemporary Moderate Resolution Imaging Spectroradiometer (MODIS) satellite imagery, available globally at ∼1×1 km spatial resolution [77], to improve the spatial resolution of the predictions. Adapting temporal Fourier analyses techniques, which ordinate seasonal environmental data [78],[79], to cope with the irregular compositing periods of MODIS data, has been completed and the data has already been made available in the public domain [77].
New Bionomics Review
The usefulness of the species range maps when available online [80], can be improved by combining them with summaries of the species-specific life history characteristics or “bionomics” of the DVS. Anopheline vector bionomics are critical in defining the appropriate (and inappropriate) modes of control at the national and local level [81]–[83]. For example, indoor residual spraying of houses for the control of a vector that is predominantly an outdoor resting species and prefers biting animals (e.g., An. (Cellia) arabiensis) is unlikely to be an optimal control strategy [84]. Conversely, if the vector feeds predominantly indoors and at night (e.g., An. (Cellia) gambiae), insecticide-treated nets are likely to be a very appropriate intervention [85],[86]. Information on characteristics of specific larval habitats and range will also be informative. Public health and education measures aimed at larval reduction may be feasible across large parts of the Middle East and Asia [87], where An. (Cellia) stephensi is the major DVS. This species readily breeds in urban areas, often using human-made water containers as its preferred larval habitat. Conversely, environmental management techniques such as installing tidal gates or constructing drainage systems are likely to be more effective as a permanent means of reducing or eliminating suitable coastal habitats of members of the An. (Cellia) sundaicus complex across substantial areas of South East Asia [88].
A systematic review of life-history characteristics pertinent to control is also timely as previous summaries become out of date [3],[89]–[97]. For example, as the taxonomy of the genus is better understood, it is evident that previous accounts which do not separate the different members of species complexes may omit or confuse critical biological information relevant for pest management. Examples of this occur in the An. sundaicus [98] and An. (Cellia) minimus complexes [99]. In addition, it would be desirable to incorporate the latest information on the phylogeny of the Anopheles [33], so that modern comparative methods [100] can be used to infer species characteristics from evolutionary relationships when no observations are available. This assembled information will be particularly useful for extending models of malaria transmission beyond An. gambiae, the species that has been the subject of most [101]–[103], but not all [104], attention. This will become increasingly important as operational and research communities alike continue to model the impact of vector control on malaria transmission [30].
Since abundance cannot be modelled with these opportunistic data assemblies, the bionomics review will also facilitate a ranking of the importance in malaria transmission of the different DVS in each region. This ranking will enable multiple species maps to be overlaid to obtain a more accurate picture of the overall epidemiological significance of the local DVS community and thus provide a better understanding of the complexity of transmission in an area. It is clear that subregional ecological diversity, coupled with the behavioural plasticity of many DVS, will require that any maps, and associated bionomics information provided, be interpreted and acted on cautiously with local expert knowledge.
Conclusions
The completed DVS databases and predictive maps will be made available online once generated, alongside the wider portfolio of MAP products, including spatial limits and endemicity maps for the human malaria parasites [1],[2]. This juxtaposition of information should represent an important cartographic resource for those engaged in malaria control and where feasible, its elimination. The success and long-term sustainability of this DVS mapping initiative depends critically on its continued support, development, and refinement in the malaria vector control and research communities. We hope that the information on the aims and objectives provided here, and the commitment to providing data in an open access venue, will help ensure that support.
Supporting Information
Zdroje
1. GuerraCA
GikandiPW
TatemAJ
NoorAM
SmithDL
2008 The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med 5 e38 doi:10.1371/journal.pmed.0050038
2. HaySI
GuerraCA
GethingPW
PatilAP
TatemAJ
2009 A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med 6 e48 doi:10.1371/journal.pmed.1000048
3. ZaharAR
1984 Vector bionomics in the epidemiology and control of malaria. Part I. The WHO African region and the southern WHO Eastern Mediterranean region. Section I: malaria vectors of the Afrotropical region - general information. Section II: an overview of malaria control problems and the recent malaria situation. (VBC/84.6-MAP/84.3). Geneva World Health Organization 109
4. Kelly-HopeL
RansonH
HemingwayJ
2008 Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis 8 387 389
5. CoetzeeM
2004 Distribution of the African malaria vectors of the Anopheles gambiae complex. Am J Trop Med Hyg 70 103 104
6. CoetzeeM
CraigM
le SueurD
2000 Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today 16 74 77
7. LevineRS
Townsend PetersonA
BenedictMQ
2004 Geographic and ecologic distributions of the Anopheles gambiae complex predicted using a genetic algorithm. Am J Trop Med Hyg 70 105 109
8. LindsaySW
ParsonL
ThomasCJ
1998 Mapping the ranges and relative abundance of the two principal African malaria vectors, Anopheles gambiae sensu stricto and An. arabiensis, using climate data. Proc R Soc Lond B Biol Sci 265 847 854
9. RogersDJ
RandolphSE
SnowRW
HaySI
2002 Satellite imagery in the study and forecast of malaria. Nature 415 710 715
10. MoffettA
ShackelfordN
SarkarS
2007 Malaria in Africa: vector species' niche models and relative risk maps. PLoS One 2 e824 doi:10.1371/journal.pone.0000824
11. MoffettA
StrutzS
GudaN
GonzalezC
FerroMC
2009 A global public database of disease vector and reservoir distributions. PLoS Negl Trop Dis 3 e378 doi:10.1371/journal.pntd.0000378
12. Rubio-PalisY
ZimmermanRH
1997 Ecoregional classification of malaria vectors in the neotropics. J Med Entomol 34 499 510
13. LevineRS
PetersonAT
BenedictMQ
2004 Distribution of members of Anopheles quadrimaculatus Say s.l. (Diptera: Culicidae) and implications for their roles in malaria transmission in the United States. J Med Entomol 41 607 613
14. FoleyDH
WeitzmanAL
MillerSE
FaranME
RuedaLM
2008 The value of georeferenced collection records for predicting patterns of mosquito species richness and endemism in the Neotropics. Ecol Entomol 33 12 23
15. OsbornFR
Rubio-PalisY
HerreraM
FigueraA
MorenoJE
2004 Caracterización ecoregional de los vectores de malaria en Venezuela. Boletín de Malariología Y Salud Ambiental 44 77 92
16. LoaizaJR
BerminghamE
ScottME
RoviraJR
ConnJE
2008 Species composition and distribution of adult Anopheles (Diptera: Culicidae) in Panama. J Med Entomol 45 841 851
17. KuhnKG
Campbell-LendrumDH
DaviesCR
2002 A continental risk map for malaria mosquito (Diptera: Culicidae) vectors in Europe. J Med Entomol 39 621 630
18. ManguinS
GarrosC
DusfourI
HarbachRE
CoosemansM
2008 Bionomics, taxonomy, and distribution of the major malaria vector taxa of Anopheles subgenus Cellia in Southeast Asia: an updated review. Infect Genet Evol 8 489 503
19. SweeneyAW
BeebeNW
CooperRD
BauerJT
PetersonAT
2006 Environmental factors associated with distribution and range limits of malaria vector Anopheles farauti in Australia. J Med Entomol 43 1068 1075
20. ObsomerV
DefournyP
CoosemansM
2007 The Anopheles dirus complex: spatial distribution and environmental drivers. Malar J 6 26
21. FoleyDH
RuedaLM
PetersonAT
WilkersonRC
2008 Potential distribution of two species in the medically important Anopheles minimus Complex (Diptera: Culicidae). J Med Entomol 45 852 860
22. GarrosC
Van NguyenC
TrungHD
Van BortelW
CoosemansM
2008 Distribution of Anopheles in Vietnam, with particular attention to malaria vectors of the Anopheles minimus complex. Malar J 7 11
23. WhiteGB
1989 Malaria. Geographical distribution of arthropod-borne diseases and their principal vectors WHO/VBC/89967. Geneva World Health Organization, Division of Vector Biology and Control 7 22
24. KiszewskiA
MellingerA
SpielmanA
MalaneyP
SachsSE
2004 A global index representing the stability of malaria transmission. Am J Trop Med Hyg 70 486 498
25. MouchetJ
CarnevaleP
CoosemansM
JulvezJ
ManguinS
2004 Biodiversité du paludisme dans le monde. Montrouge, France John Libbey Eurotext 428
26. ManguinS
CarnevaleP
MouchetJ
CoosemansM
JulvezJ
2008 Biodiversity of malaria in the world. Montrouge, France John Libbey Eurotext 464
27. TatemAJ
RogersDJ
HaySI
2006 Estimating the malaria risk of African mosquito movement by air travel. Malar J 5 57
28. FeachemR
SabotO
2008 A new global malaria eradication strategy. Lancet 10 1633 1635
29. WernsdorferW
HaySI
ShanksGD
2009 Learning from history. Shrinking the Malaria Map: a Prospectus on Malaria Elimination 95 107
30. HaySI
SmithDL
SnowRW
2008 Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis 8 369 378
31. HaySI
SnowRW
2006 The Malaria Atlas Project: developing global maps of malaria risk. PLoS Med 3 e473 doi:10.1371/journal.pmed.0030473
32. HarbachRE
1994 Review of the internal classification of the genus Anopheles (Diptera: Culicidae): the foundation for comparative systematics and phylogenetic research. Bull Entomol Res 84 331 342
33. HarbachRE
2004 The classification of genus Anopheles (Diptera: Culicidae): a working hypothesis of phylogenetic relationships. Bull Entomol Res 94 537 553
34. HarbachRE
(2009) Mosquito taxonomic inventory (http://mosquito-taxonomic-inventory.info). Accessed 29 September 2009
35. ServiceMW
TownsonH
2002 The Anopheles vector.
GillesHM
WarrellDA
Essential Malariology. Fourth edition ed London Arnold 59 84
36. TakkenW
LindsaySW
2003 Factors affecting the vectorial competence of Anopheles gambiae: a question of scale.
TakkenW
ScottTW
Ecological Aspects for Application of Genetically Modified Mosquitoes Dordrecht Kluwer Academic Publishers 75 90
37. ServiceMW
1993 The Anopheles vector.
GillesHM
WarrellDA
Bruce-Chwatt's Essential Malariology. Third edition ed London Edward Arnold 96 123
38. ServiceMW
1993 Appendix II. Characteristics of some major Anopheles vectors of human malaria.
GillesHM
WarrellDA
Bruce-Chwatt's Essential Malariology. Third edition ed London Edward Arnold 305 310
39. GuerraCA
HaySI
LucioparedesLS
GikandiPW
TatemAJ
2007 Assembling a global database of malaria parasite prevalence for the Malaria Atlas Project. Malar J 6 17
40. KnightKL
1978 Supplement to “A catalog of the mosquitoes of the world (Diptera: Culicidae)”. College Park, Maryland, U.S.A. Thomas Say Foundation, Entomological Society of America 107
41. KnightKL
StoneA
1977 A catalog of the mosquitoes of the world (Diptera: Culicidae). College Park, Maryland, U.S.A. Thomas Say Foundation, Entomological Society of America
42. WardRA
1984 Second supplement to “A catalog of the mosquitoes of the world (Diptera: Culicidae)”. Mosq Syst 16 227 270
43. WardRA
1992 Third supplement to “A catalog of the mosquitoes of the world (Diptera: Culicidae)”. Mosq Syst 24 177 230
44. SouthwoodTRE
1977 Habitat, templet for ecological strategies? Presidential address to British Ecological Society, 5 January 1977. J Anim Ecol 46 337 365
45. RogersDJ
2006 Models for vectors and vector-borne diseases. Adv Parasitol 62 1 35
46. ChapmanAD
WieczorekJ
2006 Guide to best practices for georeferencing. Copenhagen Global Biodiversity Information Facility
47. WieczorekJ
GuoQ
HijmansRJ
2004 The point-radius method for georeferencing locality descriptions and calculating associated uncertainty. Int J Geogr Inf Sci 18 745 767
48. GuralnickRP
WieczorekJ
BeamanR
HijmansRJ
2006 BioGeomancer: automated georeferencing to map the world's biodiversity data. PLoS Biol 4 e381 doi:10.1371/journal.pbio.0040381
49. GuoQ
LiuY
WieczorekJ
2008 Georeferencing locality descriptions and computing associated uncertainty using a probabilistic approach. Int J Geogr Inf Sci 22 1067 1090
50. ColemanM
SharpB
SeocharanI
HemingwayJ
2006 Developing an evidence-based decision support system for rational insecticide choice in the control of African malaria vectors. J Med Entomol 43 663 668
51. HemingwayJ
BeatyBJ
RowlandM
ScottTW
SharpBL
2006 The Innovative Vector Control Consortium: improved control of mosquito-borne diseases. Trends Parasitol 22 308 312
52. Van BortelW
TrungHD
Thuan leK
SochanthaT
SocheatD
2008 The insecticide resistance status of malaria vectors in the Mekong region. Malar J 7 102
53. KoumG
YekelA
NdifonB
SimardF
2004 Design and implementation of a mosquito database through an entomological ontology. Bioinformatics 20 2205 2211
54. ArgaezJA
ChristenJA
NakamuraM
SoberonJ
2005 Prediction of potential areas of species distributions based on presence-only data. Environ Ecol Stat 12 27 44
55. ElithJ
GrahamCH
AndersonRP
DudikM
FerrierS
2006 Novel methods improve prediction of species' distributions from occurrence data. Ecography 29 129 151
56. SeguradoP
AraujoMB
2004 An evaluation of methods for modelling species distributions. J Biogeogr 31 1555 1568
57. LeathwickJR
ElithJ
HastieT
2006 Comparative performance of generalized additive models and multivariate adaptive regression splines for statistical modelling of species distributions. Ecol Model 199 188 196
58. PottsJM
ElithJ
2006 Comparing species abundance models. Ecol Model 199 153 163
59. TanCO
OzesmiU
BekliogluM
PerE
KurtB
2006 Predictive models in ecology: comparison of performances and assessment of applicability. Ecol Informatics 1 195 211
60. FriedmanJ
HastieT
TibshiraniR
2000 Additive logistic regression: a statistical view of boosting. Ann Stat 28 337 374
61. SextonJ
LaakeP
2007 Boosted regression trees with errors in variables. Biometrics 63 586 592
62. GuisanA
EdwardsTC
HastieT
2002 Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Model 157 89 100
63. PhillipsSJ
AndersonRP
SchapireRE
2006 Maximum entropy modeling of species geographic distributions. Ecol Model 190 231 259
64. GelfandAE
SchmidtAM
WuS
SilanderJA
LatimerA
2005 Modelling species diversity through species level hierarchical modelling. J Roy Stat Soc C-App 54 1 20
65. GelfandAE
SilanderJAJr
WuS
LatimerA
LewisPO
2006 Explaining species distribution patterns through hierarchical modeling. Bayesian Analysis 1 41 92
66. KeryM
RoyleJA
2008 Hierarchical Bayes estimation of species richness and occupancy in spatially replicated surveys. J Appl Ecol 45 589 598
67. DiggleP
MoyeedR
RowlingsonB
ThomsonM
2002 Childhood malaria in The Gambia: a case-study in model-based geostatistics. J Roy Stat Soc C-App 51 493 506
68. RattanasiriS
BohningD
RojanavipartP
AthipanyakomS
2004 A mixture model application in disease mapping of malaria. Southeast Asian J Trop Med Public Health 35 38 47
69. GemperliA
SogobaN
FondjoE
MabasoM
BagayokoM
2006 Mapping malaria transmission in West and Central Africa. Trop Med Int Health 11 1032 1046
70. GemperliA
VounatsouP
SogobaN
SmithT
2006 Malaria mapping using transmission models: application to survey data from Mali. Am J Epidemiol 163 289 297
71. GosoniuL
VounatsouP
SogobaN
SmithT
2006 Bayesian modelling of geostatistical malaria risk data. Geospat Health 1 127 139
72. NoorAM
ClementsACA
GethingPW
MoloneyG
BorleM
2008 Spatial prediction of Plasmodium falciparum prevalence in Somalia. Malar J 7 159
73. SogobaN
VounatsouP
BagayokoMM
DoumbiaS
DoloG
2007 The spatial distribution of Anopheles gambiae sensu stricto and An. arabiensis (Diptera: Culicidae) in Mali. Geospat Health 1 213 222
74. ElithJ
BurgmanMA
ReganHM
2002 Mapping epistemic uncertainties and vague concepts in predictions of species distribution. Ecol Model 157 313 329
75. TatemAJ
GoetzSJ
HaySI
2008 Fifty years of Earth-observation satellites. Am Sci 96 390 398
76. HaySI
TatemAJ
GrahamAJ
GoetzSJ
RogersDJ
2006 Global environmental data for mapping infectious disease distribution. Adv Parasitol 62 37 77
77. ScharlemannJPW
BenzD
HaySI
PurseBV
TatemAJ
2008 Global data for ecology and epidemiology: a novel algorithm for temporal Fourier processing MODIS data. PLoS One 3 e1408 doi:10.1371/journal.pone.0001408
78. RogersDJ
2000 Satellites, space, time and the African trypanosomiases. Adv Parasitol 47 129 171
79. RogersDJ
RobinsonTP
2004 Tsetse distribution.
MaudlinI
HolmesPH
MilesMA
The Trypanosomiases CAB International 139 179
80. Lozano-FuentesS
Elizondo-QuirogaD
Farfan-AleJA
Loroño-PinoMA
Garcia-RejonJ
2008 Use of Google Earth™ to strengthen public health capacity and facilitate management of vector-borne diseases in resource-poor environments. Bull World Health Organ 86 718 725
81. WalkerK
LynchM
2007 Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Med Vet Entomol 21 2 21
82. W.H.O 2006 Malaria vector control and personal protection: report of a WHO study group. WHO Technical Report Series, no 936 Geneva World Health Organization 72
83. W.H.O 2004 Global strategic framework for integrated vector management. Document WHO/CDS/CPE/PVC/2004.10 Geneva World Health Organization
84. ShililuJ
GhebremeskelT
SeuluF
MengistuS
FekaduH
2004 Seasonal abundance, vector behavior, and malaria parasite transmission in Eritrea. J Am Mosq Control Assoc 20 155 164
85. LengelerC
2004 Insecticide-treated bed nets and curtains for preventing malaria. The Cochrane Database of Systematic Reviews 2004, Issue 2. Art. No.:CD000363.pub2. DOI: 10.1002/14651858.CD000363.pub2
86. SnowRW
LindsaySW
HayesRJ
GreenwoodBM
1988 Permethrin-treated bed nets (mosquito nets) prevent malaria in Gambian children. Trans R Soc Trop Med Hyg 82 838 842
87. SharmaVP
1996 Re-emergence of malaria in India. Indian J Med Res 103 26 45
88. KonradsenF
van der HoekW
AmerasingheFP
MuteroC
BoeleeE
2004 Engineering and malaria control: learning from the past 100 years. Acta Trop 89 99 108
89. ZaharAR
1985 Vector bionomics in the epidemiology and control of malaria. Part I. The WHO African region and the southern WHO Eastern Mediterranean region. Section III: vector bionomics, malaria epidemiology and control by geographical areas (a) West Africa (VBC/85.1-MAP/85.1). Geneva World Health Organization 225
90. ZaharAR
1985 Vector bionomics in the epidemiology and control of malaria. Part I. The WHO African region and the southern WHO Eastern Mediterranean region. Section III: Vector bionomics, malaria epidemiology and control by geographical areas (b) equatorial Africa, (c) southern Africa (VBC/85.2-MAP/85.2). Geneva World Health Organization 136
91. ZaharAR
1985 Vector bionomics in the epidemiology and control of malaria. Part I. The WHO African region and the southern WHO Eastern Mediterranean region. Section III: Vector bionomics, malaria epidemiology and control by geographical areas (d) East Africa, (e) eastern outer islands, (f) southwestern Arabia (VBC/85.3-MAP/85.3). Geneva World Health Organization 244
92. ZaharAR
1988 Vector bionomics in the epidemiology and control of malaria. Part II. The WHO European region and the WHO Eastern Mediterranean region. Volume I: vector laboratory studies. (VBC/88.5-MAP/88.2). Geneva World Health Organization 228
93. ZaharAR
1990 Vector bionomics in the epidemiology and control of malaria. Part II. The WHO European region and the WHO Eastern Mediterranean region. Volume II: applied field studies. Section I: an overview of the malaria situation and current problems. Section II: vector distribution (VBC/90.1). Geneva World Health Organization
94. ZaharAR
1990 Vector bionomics in the epidemiology and control of malaria. Part II. The WHO European region and the WHO Eastern Mediterranean region. Volume II: applied field studies. Section III: vector bionomics, malaria epidemiology and control by geographical areas (a) the Mediterranean basin (VBC/90.2-MAL/90.2). Geneva World Health Organization 226
95. ZaharAR
1990 Vector bionomics in the epidemiology and control of malaria. Part II. The WHO European region and the WHO Eastern Mediterranean region. Volume II: applied field studies. Section III: vector bionomics, malaria epidemiology and control by geographical areas (b) Asia west of India (VBC/90.3-MAL/90.3). Geneva World Health Organization 352
96. ZaharAR
1994 Vector bionomics in the epidemiology and control of malaria. Part III. The WHO South East Asia Region and the WHO Western Pacific Region. (CDT/MAL/94.1). Geneva World Health Organization
97. ZaharAR
1996 Vector bionomics in the epidemiology and control of malaria. Part III. The WHO South East Asia Region and the WHO Western Pacific Region. (CDT/MAL/96.1). Geneva World Health Organization
98. DusfourI
HarbachRE
ManguinS
2004 Bionomics and systematics of the Oriental Anopheles sundaicus complex in relation to malaria transmission and vector control. Am J Trop Med Hyg 71 518 524
99. GarrosC
Van BortelW
TrungHD
CoosemansM
ManguinS
2006 Review of the Minimus Complex of Anopheles, main malaria vector in Southeast Asia: from taxonomic issues to vector control strategies. Trop Med Int Health 11 102 114
100. HarveyPH
PagelMD
1991 The comparative method in evolutionary biology;
HarveyPH
MayRM
Oxford Oxford University Press
101. SmithDL
McKenzieFE
2004 Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar J 3 13
102. KilleenGF
McKenzieFE
FoyBD
SchieffelinC
BillingsleyPF
2000 A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg 62 535 544
103. SmithDL
McKenzieFE
SnowRW
HaySI
2007 Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol 5 e42 doi:10.1371/journal.pbio.0050042
104. Le MenachA
TakalaS
McKenzieFE
PerisseA
HarrisA
2007 An elaborated feeding cycle model for reductions in vectorial capacity of night-biting mosquitoes by insecticide-treated nets. Malar J 6 10
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 2- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Effectiveness of Non-nucleoside Reverse-Transcriptase Inhibitor-Based Antiretroviral Therapy in Women Previously Exposed to a Single Intrapartum Dose of Nevirapine: A Multi-country, Prospective Cohort Study
- The Global Research Neglect of Unassisted Smoking Cessation: Causes and Consequences
- Adolescent HIV—Cause for Concern in Southern Africa
- Impact of Antiretroviral Therapy on Incidence of Pregnancy among HIV-Infected Women in Sub-Saharan Africa: A Cohort Study
- Automated Detection of Infectious Disease Outbreaks in Hospitals: A Retrospective Cohort Study
- Event Rates, Hospital Utilization, and Costs Associated with Major Complications of Diabetes: A Multicountry Comparative Analysis
- Pretreatment CD4 Cell Slope and Progression to AIDS or Death in HIV-Infected Patients Initiating Antiretroviral Therapy—The CASCADE Collaboration: A Collaboration of 23 Cohort Studies
- Can Broader Diffusion of Value-Based Insurance Design Increase Benefits from US Health Care without Increasing Costs? Evidence from a Computer Simulation Model
- Causes of Acute Hospitalization in Adolescence: Burden and Spectrum of HIV-Related Morbidity in a Country with an Early-Onset and Severe HIV Epidemic: A Prospective Survey
- Developing Global Maps of the Dominant Vectors of Human Malaria
- Guidance for Developers of Health Research Reporting Guidelines
- Packages of Care for Attention-Deficit Hyperactivity Disorder in Low- and Middle-Income Countries
- A New Policy on Tobacco Papers
- Ghostwriting at Elite Academic Medical Centers in the United States
- Measuring hsCRP—An Important Part of a Comprehensive Risk Profile or a Clinically Redundant Practice?
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Packages of Care for Attention-Deficit Hyperactivity Disorder in Low- and Middle-Income Countries
- Measuring hsCRP—An Important Part of a Comprehensive Risk Profile or a Clinically Redundant Practice?
- Developing Global Maps of the Dominant Vectors of Human Malaria
- Guidance for Developers of Health Research Reporting Guidelines
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



