-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Genome-Wide, Fine-Scale Map of Natural Pigmentation Variation in
Various approaches can be applied to uncover the genetic basis of natural phenotypic variation, each with their specific strengths and limitations. Here, we use a replicated genome-wide association approach (Pool-GWAS) to fine-scale map genomic regions contributing to natural variation in female abdominal pigmentation in Drosophila melanogaster, a trait that is highly variable in natural populations and highly heritable in the laboratory. We examined abdominal pigmentation phenotypes in approximately 8000 female European D. melanogaster, isolating 1000 individuals with extreme phenotypes. We then used whole-genome Illumina sequencing to identify single nucleotide polymorphisms (SNPs) segregating in our sample, and tested these for associations with pigmentation by contrasting allele frequencies between replicate pools of light and dark individuals. We identify two small regions near the pigmentation genes tan and bric-à-brac 1, both corresponding to known cis-regulatory regions, which contain SNPs showing significant associations with pigmentation variation. While the Pool-GWAS approach suffers some limitations, its cost advantage facilitates replication and it can be applied to any non-model system with an available reference genome.
Published in the journal: . PLoS Genet 9(6): e32767. doi:10.1371/journal.pgen.1003534
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003534Summary
Various approaches can be applied to uncover the genetic basis of natural phenotypic variation, each with their specific strengths and limitations. Here, we use a replicated genome-wide association approach (Pool-GWAS) to fine-scale map genomic regions contributing to natural variation in female abdominal pigmentation in Drosophila melanogaster, a trait that is highly variable in natural populations and highly heritable in the laboratory. We examined abdominal pigmentation phenotypes in approximately 8000 female European D. melanogaster, isolating 1000 individuals with extreme phenotypes. We then used whole-genome Illumina sequencing to identify single nucleotide polymorphisms (SNPs) segregating in our sample, and tested these for associations with pigmentation by contrasting allele frequencies between replicate pools of light and dark individuals. We identify two small regions near the pigmentation genes tan and bric-à-brac 1, both corresponding to known cis-regulatory regions, which contain SNPs showing significant associations with pigmentation variation. While the Pool-GWAS approach suffers some limitations, its cost advantage facilitates replication and it can be applied to any non-model system with an available reference genome.
Introduction
Phenotypic variation is abundant in natural populations, but usually its genetic basis is unknown. That is, even when the biochemical pathways that underlie a trait are well-understood, the identities of the alleles or genes which contribute to variation in natural populations are often not known. This situation, however, is likely to soon change. Statistical genetic approaches originally developed for mapping complex genetic diseases have proved to be successful at mapping phenotypic differences segregating in natural populations, as long as the alleles underlying them are not rare and have reasonably large effects. As a result, these approaches are particularly successful at uncovering the genetic basis of traits that differ due to local adaptation (e.g., [1], [2]) or artificial selection (e.g., [3]–[5]).
Here, we examine the genetic basis of phenotypic variation in Drosophila cuticle pigmentation. In genetics, pigmentation has been a classically studied trait — indeed, many of the first markers identified in Drosophila melanogaster had pigmentation phenotypes [6] — and has a well-understood genetic basis of moderate complexity, consisting of neither a single Mendelian factor nor hundreds of genes [7]–[10]. For evolutionary genetics, pigmentation has a number of desirable properties. In D. melanogaster, pigmentation is highly variable in natural populations due to both genetic polymorphism and phenotypic plasticity [11], [12], can differ between closely-related species [10], [13]–[15], and often does differ between sexes in the Drosophila genus [16], [17]. In D. melanogaster, in particular, pigmentation shows patterns of spatial variation that suggest that local selection pressures might vary [18]–[20]. The identification of genes responsible for differences in pigmentation can thus shed light on evolution, plasticity and sexual dimorphism in Drosophila.
Currently, there are at least 9 genes known to be directly involved in pigment synthesis pathways in Drosophila [8], [21], and a number of other genes that can indirectly affect pigmentation patterns through spatial signaling or sex-specific regulation (e.g., bab [16] and Abd-B [22]). But it is not clear to what extent phenotypic variation in natural populations is due to variation at these loci, or to variation at uncharacterized pigmentation genes. Previously, variation in D. melanogaster has been studied via either studies of individual genes [18], [19], [23], [24], or by crossing strains with different phenotypes [11], [25]. Although these studies have identified interesting genes and regulatory regions, there has been, to date, no study simultaneously examining the contribution of all genes to pigmentation variation in a large sample of flies, as in a genome-wide association study (GWAS).
To accomplish this, we use a modified GWAS approach, Pool-GWAS, in which individuals with extreme phenotypes are pooled by phenotype, and the pools genotyped and analyzed for differences in allele frequencies (reviewed in [26]). Here, we use pooled next generation sequencing to estimate allele frequency differences between phenotypic classes [27], [28]. As in other GWAS studies, Pool-GWAS uses past recombination events in a large sample of individuals to map phenotypic variation to associated variants genome-wide, and can thus be used to survey much of the natural genetic diversity of an organism. Indeed, the short range over which linkage disequilibrium (LD) decays in D. melanogaster—significant LD declines over just 200 bp in most regions of the genome [29]–[31]—makes this species particularly well-suited for identifying the effect of small regions such as short cis-regulatory elements controlling pigmentation genes.
Results/Discussion
To obtain material for this study, we collected more than 30,000 wild D. melanogaster flies from each of two locations, Bolzano (Italy) and Vienna (Austria). We divided these flies into five independent replicates (two from Vienna and three from Bolzano), cultured a single generation of offspring in a common laboratory environment, and examined the abdominal pigmentation pattern of approximately 8000 of the female offspring (∼1500 females per replicate). We then selected from each replicate 100 of the darkest and lightest flies, as measured by the pigmentation area of the A7 tergite (Figure 1), for whole genome Illumina sequencing. After performing various quality filtering steps, we tested ∼3.3 million SNPs for allele frequency differences between dark and light flies using the Cochran-Mantel-Haenszel (CMH) test, a test designed for meta-analysis of contingency tables summarizing data from different samples. We found 17 SNPs that were significantly associated with female abdominal pigmentation [at a False Discovery Rate (FDR) cutoff of 5%; Text S1]. As expected, these SNPs lie in or near known pigmentation genes, specifically tan and bab1 (Figure 2). Most of the other highly ranked but non-significant SNPs also lie in or near pigmentation genes (79% of the 100 most highly ranked SNPs are within 20 kb from the boundaries of tan, bab1, or ebony; Text S2; Table S1). Interestingly, none of these high ranking SNPs lie in the coding sequence of those three genes, except at tan, where there is a single cluster of five synonymous mutations within 60 bp of one another. Instead, the significantly associated SNPs appear to cluster around previously characterized cis-regulatory regions of tan and bab1. For example, in the tan region, which harbors all but one of the 17 significant SNPs, a cluster of associated SNPs is located between the upstream genes CG15370 and Gr8a. This location corresponds precisely to the MSE cis-regulatory element, which harbors variants responsible for pigmentation differences between the closely-related species D. santomea and D. yakuba ([15]; Figure 2B, left). Within the limits of correct orthology assignment, none of the putative causal alleles that lighten the cuticle in D. santomea correspond to the significant SNPs in this study. In fact, no correspondence would be expected, as D. santomea and its close relatives appear to already have the inferred light allele seen here.
Fig. 1. Overview of the experimental design. 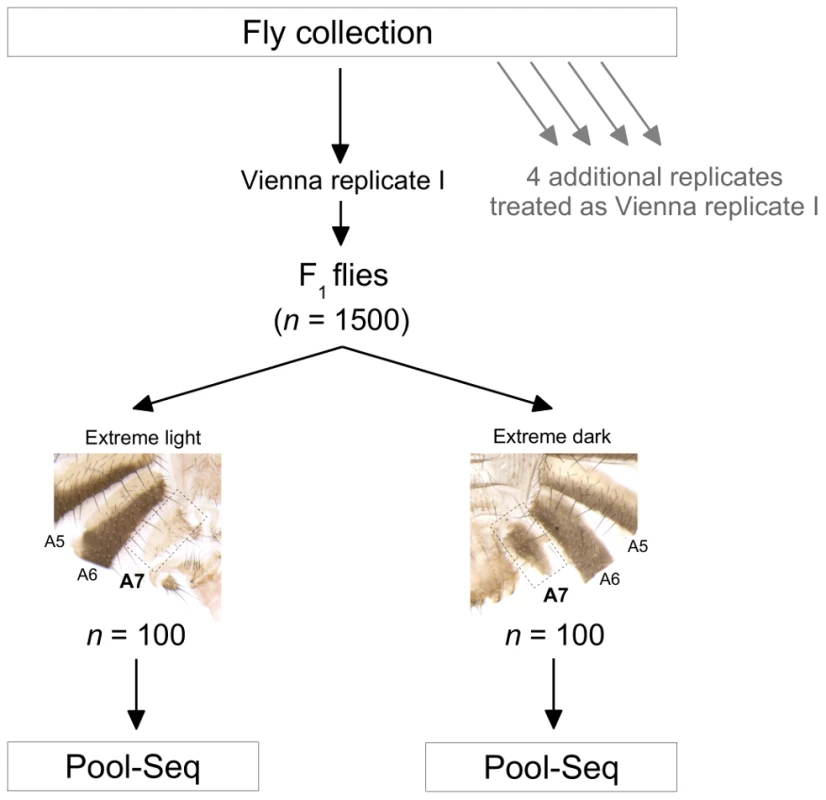
Wild D. melanogaster flies were collected from Vienna, Austria, and Bolzano, Italy, brought into a controlled environment in the laboratory, and treated as shown in the figure. The same procedure was used for all five replicates, with each replicate resulting in 1,500 females for phenotyping, and with 100 light and dark flies from each replicate sequenced. Fig. 2. Genome-wide association study of female abdominal pigmentation. 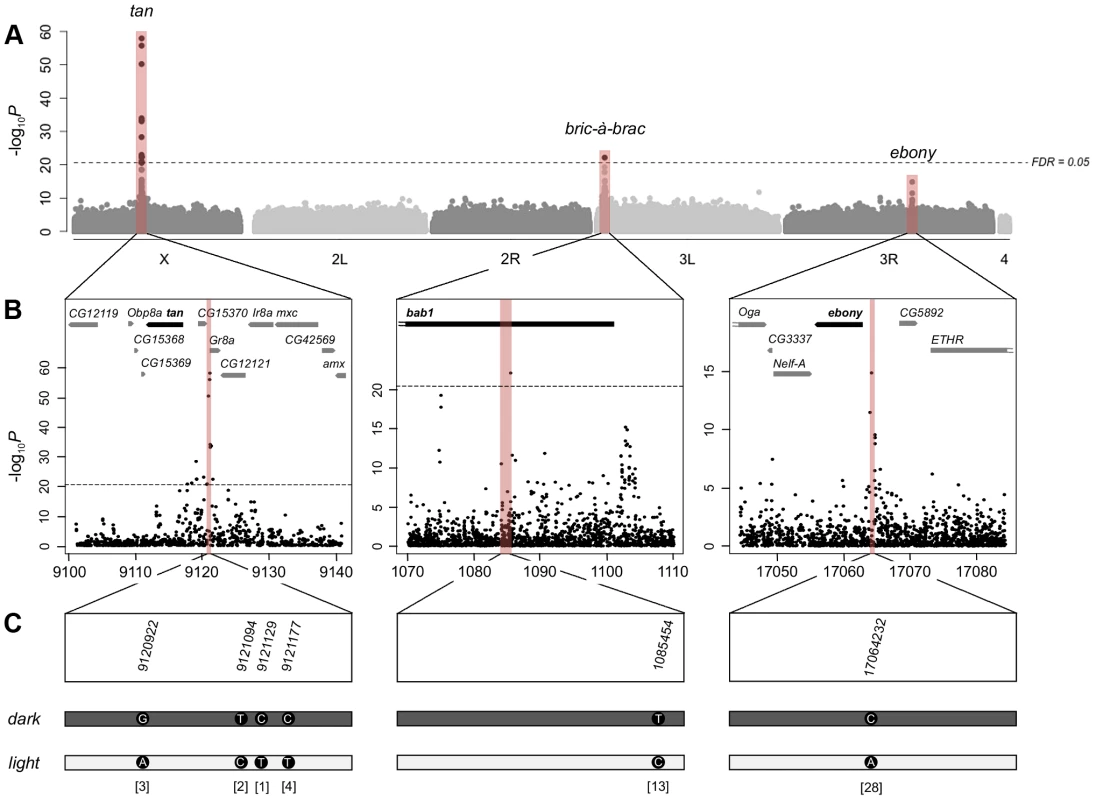
A) Manhattan plot for abdominal pigmentation in the full data set (including all five replicates). The–log10p-values are plotted against the position on each chromosome. The horizontal dashed line indicates the genome-wide significance threshold at an FDR of 0.05. The red bars indicate candidate genes previously shown to affect pigmentation. B) Detailed view of the tan (left), bab1 (middle) and ebony (right) regions. The positions on the x-axis are indicated in kb. The red bars indicate regulatory regions previously shown to affect pigmentation. C) Detailed view of top ranked polymorphisms located within or close to the three pigmentation genes. For every gene, the most significant SNPs fall in regulatory regions. Similarly, the most significant SNPs in the bab region also correspond to previously described regulatory regions. The bab locus consists of two paralogous genes, bab1 and bab2, both affecting abdominal pigmentation, with the segment scored here (A7) affected primarily by bab1 activity [17], [32]. The significant SNP in bab1 maps to the “dimorphic” regulatory element in the first intron of this gene, which upregulates bab1 in females, and represses it in males ([17]; Figure 2B, middle). The remaining highly ranked, but non-significant bab SNPs fall into regions that are plausibly also regulatory—a second region within the first intron of bab1 (downstream of the “dimorphic” element), and a third region near the promoter region of bab1—though they have not been validated with functional genetic studies. Finally, the highest ranked SNP outside of the tan and bab regions lies in the male repressor module of ebony, which affects pigmentation in males ([23]; Figure 2B, right), though this SNP was also non-significantly associated with the phenotype, likely due to the conservative criteria used to determine the FDR cutoff (see Text S1).
Thus far, we have considered all replicates from the two populations jointly. To gain insight into the strength and robustness of these associations, we also invested the consistency of the significant SNPs across populations. We therefore analyzed the Viennese and Bolzano samples separately. The tan region, which was strongly associated with pigmentation in the joint analysis, remained significantly associated in the separate analyses. All 3 of the SNPs in the Viennese sample that fell below the 5% FDR cutoff, and all 7 in the Bolzano sample, were in the tan region (with the three most significant SNPs being identical in the two analyses; Figures S1, S2, S3 and Tables S1, S2, S3). While no significant associations were found with SNPs lying in the bab region in the separate analyses, several bab SNPs remain highly ranked. We further investigated our power to detect significant associations using only one replicate, i.e., one light and one dark sample from either Vienna or Bolzano (analysis done with 5 paired replicates, not shown). Here, the decline in power is even more apparent; in these analyses, the number of significant associations ranged from 0 to 3 (mean = 0.8 across the 5 replicates at an FDR level of 0.05). This contrast clearly shows the utility of replication, as analyses of single replicates often did not identify even those SNPs with strong effects after accounting for multiple testing.
In general, then, our results are remarkably consistent across populations. This is perhaps not surprising, as European Drosophila populations are quite similar genetically [33], and as the CMH test favors associations that are consistent across populations. We were interested, however, in whether or not there were associations specific to either the Vienna or Bolzano populations. Of the four SNPs that were below the 5% FDR cutoff in the Bolzano, but not in the Viennese analysis, only one showed non-overlapping 95% confidence limits for the odds-ratios in the two populations, suggesting that these SNPs were not significant in the Viennese sample due only to a lack of statistical power (note that none of these SNPs were excluded for not meeting the filtering criteria in the Viennese population; data not shown). We further investigated the non-significant associations at tan, bab, or ebony regions for evidence of population-specificity, as population specificity may explain why some SNPs were highly ranked, but non-significant, in the combined analysis. There were some indications of other population-specific associations in the bab and ebony regions; e.g., SNPs in the bab1 promoter region discussed above tend to be highly ranked in only one of the populations (Text S3 and Figure S3). One explanation for such differences might be that there are unscored causative variants linked to different SNPs in each population, with two prime suspects for these unscored causal variants being segregating indels and transposable elements. We examined the data for evidence of differences in frequencies of transposable element insertions and indels in the tan, bab and ebony regions. In particular, we looked for indels or TE insertions with larger differences in frequencies than the surrounding SNPs. While some indels in these regions showed suggestive associations with pigmentation, none had frequency differences between light and dark pools that were as large as that of the least significant SNP (which showed an average difference in frequency between light and dark pools of ∼31%; Table S4). In addition, we found no evidence for strong differences in frequency of any transposable element insertions in these regions (using the method in [34]; Table S5).
Thus, we find that female abdominal pigmentation maps to regulatory regions of well-known pigmentation loci. This variation is also associated with sequence variants at ebony. Interestingly, regulatory variation in tan and ebony are also implicated in pigmentation differences between two North American Drosophila species (not closely related to D. melanogaster; [10]), and tan may also play a role in the pigmentation differences found between D. yakuba and the island species D. santomea [15], [35] (but see [36]). It is tempting to argue that there is some property of tan that predisposes it to respond to selection, such as might be the case for the Melanocortin Receptor 1 gene in vertebrates (reviewed in [37]), though there are other cases where tan does not appear to be the main factor affecting pigmentation variation (e.g., [38], [39]). In the case of D. melanogaster pigmentation variation, there is some evidence that pigmentation may be under balancing selection [19], [40]–[42], which can elevate causal alleles to intermediate frequencies [43]. Further, balancing selection may have acted on this phenotype long-term, as there appears to be adaptive variation in pigmentation in African D. melanogaster [19]; in this case, recombination may have had adequate time to break down linkage disequilibrium between the functional variants and nearby SNPs. As a result, the identification of functional variation mapping to very small regions within the Drosophila genome may have been facilitated beyond the genome-wide low linkage disequilibrium in D. melanogaster [29]–[31].
As proof of concept, this study demonstrates the power of a Pool-GWAS approach for investigating the genetic basis of natural variation. Comparison to previous studies analyzing the same or similar phenotypes highlights some of the advantages of Pool-GWAS. Our results are consistent with those of [19], who mapped pigmentation variation in a different (though correlated [44]) abdominal segment to the X and 3rd chromosomes in African populations, and were able to associate it to sequence variants at ebony. Genome-wide, however, their resolution was limited to the chromosome level. Previous quantitative trait locus (QTL) mapping of female abdominal pigmentation identified only the bab region as the major contributor to variation in the trait [11], [25], possibly because the small number of parental strains used in these studies had similar tan alleles. Pool-GWAS, however, unlike QTL mapping, surveys a large sample of natural variation, making it less likely that alleles contributing to the trait are excluded from the analysis. A follow-up association study did use a large sample of strains, but focused only on bab, and failed to identify individual SNPs strongly associated with pigmentation variation [24]. This result might be due to background variation at the tan locus confounding attempts to detect associations at bab. Pool-GWAS avoids these pitfalls by simultaneously surveying variation in many strains and genome-wide, revealing that both tan and bab influence pigmentation.
Pool-GWAS is appealing to biologists working on non-model organisms for various reasons. First, it is low cost relative to many other GWAS approaches—sequencing pools of individuals is far less expensive than individual sequencing [27], and unlike many standard GWAS analyses, it requires no development of SNP chips or resources other than a reference genome. And, as whole-genome sequencing is used, significant associations do not have to rely on high levels of long-range linkage disequilibrium between causal variants and genotyped SNPs; as a result, strong associations can be found with relatively small sample sizes [45]. Finally, while Drosophila pigmentation is a well-understood genetic trait, and the associated SNPs map to pigmentation genes, in principle, associated SNPs could have been detected anywhere in the genome, allowing for discovery of loci that affect a trait, but which have not been previously described.
Approaches similar to this one have been attempted in the past. Pooling of samples, of course, has long been a cost saving measure, and has been used in bulk segregant analysis (e.g., [46], [47]), and in standard GWAS (reviewed in [26]), coupled with whole genome sequencing [28], and in a quantitative trait mapping GWAS experiment [48]. Finally, several recent “evolve and resequence” experiments have used pooled sequencing to measure shifts in allele frequencies in populations subjected to multiple generations of natural or artificial selection, in an attempt to discover loci affected by the selection regime (e.g., [49]–[53]). Except for the larger number of generations, these experiments are similar to the process used here. However, in spite of the similarities between this and these previous approaches, there are some important differences. Unlike some of these previous approaches, we use replication as part of the experimental design, which increases the power to detect effects. In contrast to experimental designs requiring crosses or selection sustained over generation, no crosses or laboratory breeding of animals are required (although, for this study, we do rear one generation in a controlled environment). Eliminating this requirement not only reduces labor and costs, but also allows the method to be applied to organisms that cannot be easily bred in the laboratory. In practice, too, the need to propagate from parental populations or strains usually limits the amount of natural variation that can be surveyed, whereas starting with a very large natural sample allows the starting material to capture both large numbers of segregating alleles and as many historical recombination events as possible, rather than including only those that persist during an artificial or natural selection experiment.
Despite offering quite a few advantages, Pool-GWAS also suffers from some limitations. The cost-effectiveness of pooling samples comes at the expense of obtaining reliable estimates of the effects of individual alleles, though it seems that the alleles can be robustly ranked by the strength of their effects (as shown by simulation; Text S2, Figure S4; Table S6 for the ranks of SNPs in tan and bab1). Like other GWAS methods, Pool-GWAS is expected to have little power to analyze traits with very complex genetic bases, and the power to detect the contribution of rare alleles is limited. Consistent with this, the SNPs with the strongest signals in this study were at high frequencies in the control sample compared to unassociated SNPs [comparison of 100 random SNPs with 17 SNPs with FDR<0.05 : Wilcoxin rank sum test, p = 8.1e-05 (Vienna) and p = 0.000117 (Bolzano), Figure S10]. To investigate the effectiveness of Pool-GWAS under a range of biological conditions, we performed simulations using a coalescent sample of 8000 haploid individuals, each with a genome roughly equivalent to the euchromatic portion of the D. melanogaster genome, and with similar levels of variability and recombination rates (Text S4). These simulations show that, as expected, the power of Pool-GWAS is higher when the minor allele frequencies of causal SNPs are higher, and when there are few causal sites affecting the trait (Figures S5, S6, S7, S8, S9). Pool-GWAS is, therefore, unlikely to be particularly successful for mapping disease loci, which may usually be at low frequencies; rather, we anticipate Pool-GWAS will be best implemented as a means of uncovering the genetic basis of ecologically interesting traits in non-model organisms in a cost-effective manner.
Methods
Sample Collection
D. melanogaster were collected from vineyard waste heaps, with approximately 30,000 flies collected in Vienna, Austria in October 2010, and a similar number in Bolzano, Italy in September 2011. After collection, flies were randomly divided into two experimental replicates for Vienna or three replicates for Bolzano, put into fresh culture bottles at a density of ∼200 flies per bottle, and allowed to lay eggs at 25°C for 2–4 hours. Bottles were kept at 25°C until the emergence of F1 adults, after which the newly emerged flies were kept at 18°C for several days to allow adult pigmentation to fully develop [12].
Pigmentation Scoring
We scored approximately 3,500 adult F1 females from Vienna and almost 5,000 adult F1 females from Bolzano for abdominal pigmentation, measured via the extent of dark pigmentation on the last tergite (abdominal segment 7, A7) in lateral view. A7 pigmentation reflects overall posterior pigmentation [35], which is more variable than anterior pigmentation in females [12], [54]. To score pigmentation, all flies were initially divided into five pigmentation classes, ranging from 0 (no pigmentation) to 4 (completely pigmented). We then isolated 100 of the lightest class 0 flies and 100 of the darkest class 4 flies from each replicate for sequencing (i.e., a total of 400 and 600 flies from Vienna and Bolzano, respectively). In addition, three sets of control flies (not selected for pigmentation), consisting of between 100–160 flies each, were sequenced for each population.
DNA Extraction and Sequencing
Genomic DNA was extracted from each pool of female flies by chloroform extraction and ethanol precipitation, and used for paired-end libraries preparation with standard kits and protocols, to obtain fragments of ∼350 bp after gel-purification (or ∼450 bp for all samples from replicate I of the Viennese sample). Libraries were amplified using Phusion DNA polymerase (New England Biolabs, Ipswich, MA) using a 65°C annealing temperature and 10 cycles of amplification, and then purified and quantified using the Qubit HS Assay Kit (Invitrogen, Carlsbad, CA, USA). Libraries were sequenced using the 2×101 bp or 2×151 bp paired-end protocol on either a Genome Analyzer IIx or a HiSeq2000 (see Text S1 for details).
Mapping of Reads
Following [55], we trimmed raw reads to remove low quality bases and mapped them to the D. melanogaster reference genome (v5.18), Wolbachia pipientis wMel strain (NC_002978.6) and phiX174 (NC_001422.1) using BWA [v0.5.8c [56]] with the following parameters: seeding of the reads disabled (−l 200), 1% missing alignments assuming an error rate of 2% (−n 0.01), maximum number of two gap openings (−o 2) and a maximum gap extension of 12 bases (−e 12, −d 12). The mapped reads were filtered for a mapping quality of 20 and for proper pairs with samtools (v0.1.9 [57]).
Due to the overlap in ranges of D. melanogaster and D. simulans and the difficulty of distinguishing females of these species, we suspected some D. simulans contamination among the sequenced flies, which may yield false positive associations as the species can differ in pigmentation [54]. A screen of the wild caught males using diagnostic male genitalia differences [58] yielded an estimate of D. simulans contamination of around 1% in both collections. We thus filtered the mapped reads for D. simulans contamination, by simultaneously remapping the above reads to five D. melanogaster genomes (including the reference genome v.5.18 cited above and those of the MW6, MW28, RAL360, RAL732 strains from the Drosophila Population Genomics Project (http://www.dpgp.org/) and five D. simulans genomes (unpublished data), using GSNAP version 2011-12-28 [59], [60]. Reads were identified as D. melanogaster if they mapped at least as well to D. melanogaster as to D. simulans, as measured by mapping quality. The effectiveness of this filtering procedure was assessed by inspecting the filtered D. melanogaster specific reads for a target set of D. simulans specific variants (Text S1 and Figure S11A and S11B); we considered the filtering procedure to be effective when the median frequency of the target set of D. simulans alleles was reduced to zero. The filtered reads were then converted to mpileup format using samtools (without quality score adjustment, using option -B), with one replicate-phenotype combination per column.
All further analyses were performed using scripts contained in PoPoolation2 revision 98 [61]. We prepared the mpileup file for each population by converting them to synchronized files, requiring a base quality of at least 20, and by masking indels and repetitive regions (including five flanking nucleotides on both sides of an indel), separately for the two populations. Repetitive regions (e.g. transposable elements, microsatellites) were identified using RepeatMasker v. 3.2.8 (www.repeatmasker.org) with crossmatch version 0.990329 (http://www.phrap.org/phredphrapconsed.html) as the search engine; simple repeats were not masked (Repeatmasker option -nolow). After undergoing all filtering procedures, an average of 112-fold coverage per replicate was obtained (see Text S1 for individual coverage estimates for each of the 16 separate data sets).
Association Mapping
We tested SNPs showing an association with pigmentation using the Cochran-Mantel-Haenszel (CMH) test, a meta-analysis method for repeated measures of independence, as implemented in PoPoolation2 revision 176 [61]. Essentially, a 2×2 contingency table is created for each replicate, with phenotype (light vs. dark) and the two major allele variants at each SNP as the independent nominal variables, and the counts of each allele in each phenotypic category as dependent variables. The CMH test tests independence of the nominal variables across replicates. We analyzed only SNPs that met the following requirements: (i) the SNP must contain two alleles that occur at least five times each across all samples, (ii) the site must have a coverage of at least 10 in each sample, and (iii) to avoid including differences between paralogs and other artifacts due to copy number variation, the site must have a coverage lower than a maximum cutoff set independently for each sample. This maximum cutoff excludes sites with coverage in the upper 2% tail of coverage for all sites. To correct for multiple tests, we used a FDR control (see Text S1 and Figure S12). Indels and transposable element insertions in the regions surrounding the tan, bab, and ebony genes were identified and tested for associations as described in Text S1.
Feature Analysis of the Best Ranked SNPs
We used SnpEff v2.0.3 [62] and the D. melanogaster annotation v5.40 to assign candidate SNPs to genomic features. SNPs not further than 200 bases from the 5′or 3′UTR of a gene were considered upstream or downstream, SNPs further than were considered intergenic. Overlapping or alternative spliced genes were treated separately.
Accession Numbers
The FASTQ files are available from the European Sequence Read Archive (Accession no. ERP001827; http://www.ebi.ac.uk/ena/data/view/ERP001827).
Supporting Information
Zdroje
1. YiX, LiangY, Huerta-SanchezE, JinX, CuoZX, et al. (2010) Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329 : 75–78.
2. HancockAM, BrachiB, FaureN, HortonMW, JarymowyczLB, et al. (2011) Adaptation to climate across the Arabidopsis thaliana genome. Science 334 : 83–86.
3. HuangX, WeiX, SangT, ZhaoQ, FengQ, et al. (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet 42 : 961–967.
4. KarlssonEK, BaranowskaI, WadeCM, Salmon HillbertzNHC, ZodyMC, et al. (2007) Efficient mapping of mendelian traits in dogs through genome-wide association. Nat Genet 39 : 1321–1328.
5. Makvandi-NejadS, HoffmanGE, AllenJJ, ChuE, GuE, et al. (2012) Four loci explain 83% of size variation in the horse. PLoS One 7: e39929.
6. Morgan TH, Sturtevant AH, Muller HJ, Bridges CB (1915) The mechanism of Mendelian heredity. New York: Henry Holt and Company.
7. TrueJR (2003) Insect melanism: the molecules matter. Trends in Ecology & Evolution 18 : 640–647.
8. WittkoppPJ, CarrollSB, KoppA (2003) Evolution in black and white: genetic control of pigment patterns in Drosophila. Trends Genet 19 : 495–504.
9. SimpsonP (2007) The stars and stripes of animal bodies: evolution of regulatory elements mediating pigment and bristle patterns in Drosophila. Trends Genet 23 : 350–358.
10. WittkoppPJ, BeldadeP (2009) Development and evolution of insect pigmentation: genetic mechanisms and the potential consequences of pleiotropy. Semin Cell Dev Biol 20 : 65–71.
11. RobertsonA, BriscoeDA, LouwJH (1977) Variation in abdomen pigmentation in Drosophila melanogaster females. Genetica 47 : 73–76.
12. DavidJR, CapyP, GauthierJP (1990) Abdominal pigmentation and growth temperature in Drosophila melanogaster: Similarities and differences in the norms of reaction of successive segments. J Evol Biol 3 : 429–445.
13. LachaiseD, HarryM, SolignacM, LemeunierF, BenassiV, et al. (2000) Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from Sao Tome. Proc Biol Sci 267 : 1487–1495.
14. GibertJM, PeronnetF, SchlöttererC (2007) Phenotypic plasticity in Drosophila pigmentation caused by temperature sensitivity of a chromatin regulator network. PLoS Genet 3: e30.
15. JeongS, RebeizM, AndolfattoP, WernerT, TrueJ, et al. (2008) The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell 132 : 783–793.
16. KoppA, DuncanI, GodtD, CarrollSB (2000) Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 408 : 553–559.
17. WilliamsTM, SelegueJE, WernerT, GompelN, KoppA, et al. (2008) The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134 : 610–623.
18. Telonis-ScottM, HoffmannAA, SgroCM (2011) The molecular genetics of clinal variation: a case study of ebony and thoracic trident pigmentation in Drosophila melanogaster from eastern Australia. Mol Ecol 20 : 2100–2110.
19. PoolJE, AquadroCF (2007) The genetic basis of adaptive pigmentation variation in Drosophila melanogaster. Mol Ecol 16 : 2844–2851.
20. ParkashR, RajpurohitS, RamniwasS (2008) Changes in body melanisation and desiccation resistance in highland vs. lowland populations of D. melanogaster. J Insect Physiol 54 : 1050–1056.
21. TrueJR, YehSD, HovemannBT, KemmeT, MeinertzhagenIA, et al. (2005) Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genet 1: e63.
22. JeongS, RokasA, CarrollSB (2006) Regulation of body pigmentation by the Abdominal-B Hox protein and its gain and loss in Drosophila evolution. Cell 125 : 1387–1399.
23. RebeizM, PoolJE, KassnerVA, AquadroCF, CarrollSB (2009) Stepwise modification of a modular enhancer underlies adaptation in a Drosophila population. Science 326 : 1663–1667.
24. BickelRD, KoppA, NuzhdinSV (2011) Composite effects of polymorphisms near multiple regulatory elements create a major-effect QTL. PLoS Genet 7: e1001275.
25. KoppA, GrazeRM, XuS, CarrollSB, NuzhdinSV (2003) Quantitative trait loci responsible for variation in sexually dimorphic traits in Drosophila melanogaster. Genetics 163 : 771–787.
26. ShamP, BaderJS, CraigI, O'DonovanM, OwenM (2002) DNA pooling: A tool for large-scale association studies. Nat Rev Genetics 3 : 862–871.
27. FutschikA, SchlöttererC (2010) The next generation of molecular markers from massively parallel sequencing of pooled DNA samples. Genetics 186 : 207–218.
28. KimSY, LiYR, GuoYR, LiRQ, HolmkvistJ, et al. (2010) Design of association studies with pooled or un-pooled next-generation sequencing data. Genetic Epidemiology 34 : 479–491.
29. MiyashitaN, LangleyCH (1988) Molecular and phenotypic variation of the white locus region in Drosophila melanogaster. Genetics 120 : 199–212.
30. MackayTF, RichardsS, StoneEA, BarbadillaA, AyrolesJF, et al. (2012) The Drosophila melanogaster Genetic Reference Panel. Nature 482 : 173–178.
31. LangleyCH, StevensK, CardenoC, LeeYC, SchriderDR, et al. (2012) Genomic variation in natural populations of Drosophila melanogaster. Genetics 192 : 533–98.
32. CoudercJL, GodtD, ZollmanS, ChenJ, LiM, et al. (2002) The bric à brac locus consists of two paralogous genes encoding BTB/POZ domain proteins and acts as a homeotic and morphogenetic regulator of imaginal development in Drosophila. Development 129 : 2419–2433.
33. Orozco-terWengelP, CoranderJ, SchlöttererC (2011) Genealogical lineage sorting leads to significant, but incorrect Bayesian multilocus inference of population structure. Mol Ecol 20 : 1108–1121.
34. KoflerR, BetancourtAJ, SchlöttererC (2012) Sequencing of pooled DNA samples (Pool-Seq) uncovers complex dynamics of transposable element insertions in Drosophila melanogaster. PLoS Genet 8: e1002487.
35. CarboneMA, LlopartA, DeAngelisM, CoyneJA, MackayTFC (2005) Quantitative trait loci affecting the difference in pigmentation between Drosophila yakuba and D. santomea. Genetics 171 : 211–225.
36. MatuteDR, ButlerIA, CoyneJA (2009) Little effect of the tan locus on pigmentation in female hybrids between Drosophila santomea and D. melanogaster. Cell 139 : 1180–1188.
37. GompelN, Prud'hommeB (2009) The causes of repeated genetic evolution. Devel Biol 332 : 36–47.
38. BrissonJA, TempletonAR, DuncanI (2004) Population genetics of the developmental gene optomotor-blind (omb) in Drosophila polymorpha: Evidence for a role in abdominal pigmentation variation. Genetics 168 : 1999–2010.
39. NgCS, HamiltonAM, FrankA, BarminaO, KoppA (2008) Genetic basis of sex-specific color pattern variation in Drosophila malerkotliana. Genetics 180 : 421–429.
40. DavidJR, CapyP, PayantV, TsakasS (1985) Thoracic trident pigmentation in Drosophila melanogaster: differentiation of geographical populations. Genetics Sel Evol 17 : 211–223.
41. GibertP, MoreteauB, MoreteauJC, ParkashR, DavidJR (1998) Light body pigmentation in Indian Drosophila melanogaster: a likely adaptation to a hot and arid climate. J of Genetics 77 : 13–20.
42. MunjalAK, KaranD, GibertP, MoreteauB, ParkashR, et al. (1997) Thoracic trident pigmentation in Drosophila melanogaster: Latitudinal and altitudinal clines in Indian populations. Genetics Selection Evolution 29 : 601–610.
43. Hudson RR (1991) Gene genealogies and the coalescent process. In: Futuyma D, Antonivics J, editors. Oxford surveys in evolutionary biology. Oxford: Oxford University Press. pp. 1–44.
44. GibertP, MoreteauB, DavidJR (2000) Developmental constraints on an adaptive plasticity: reaction norms of pigmentation in adult segments of Drosophila melanogaster. Evol & Devel 2 : 249–260.
45. PritchardJK, PrzeworskiM (2001) Linkage disequilibrium in humans: models and data. Am J Hum Genet 69 : 1–14.
46. MichelmoreRW, ParanI, KesseliRV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci U S A 88 : 9828–9832.
47. EhrenreichIM, TorabiN, JiaY, KentJ, MartisS, et al. (2010) Dissection of genetically complex traits with extremely large pools of yeast segregants. Nature 464 : 1039–1042.
48. HuangW, RichardsS, CarboneMA, ZhuD, AnholtRR, et al. (2012) Epistasis dominates the genetic architecture of Drosophila quantitative traits. Proc Natl Acad Sci U S A 109 : 15553–15559.
49. BurkeMK, DunhamJP, ShahrestaniP, ThorntonKR, RoseMR, et al. (2010) Genome-wide analysis of a long-term evolution experiment with Drosophila. Nature 467 : 587–590.
50. ZhouD, UdpaN, GerstenM, ViskDW, BashirA, et al. (2011) Experimental selection of hypoxia-tolerant Drosophila melanogaster. Proc Natl Acad Sci U S A 108 : 2349–2354.
51. Orozco-terWengelP, KapunM, NolteV, KoflerR, FlattT, et al. (2012) Adaptation of Drosophila to a novel laboratory environment reveals temporally heterogeneous trajectories of selected alleles. Mol Ecol 21 : 4931–4941.
52. TurnerTL, StewartAD, FieldsAT, RiceWR, TaroneAM (2011) Population-based resequencing of experimentally evolved populations reveals the genetic basis of body size variation in Drosophila melanogaster. PLoS Genet 7: e1001336.
53. TurnerTL, MillerPM (2012) Investigating natural variation in Drosophila courtship song by the evolve and resequence approach. Genetics 191 : 633–642.
54. GibertP, MoreteauB, ScheinerSM, DavidJR (1998) Phenotypic plasticity of body pigmentation in Drosophila: correlated variations between segments. Genetics Sel Evol 30 : 181–194.
55. KoflerR, Orozco-terWengelP, De MaioN, PandeyRV, NolteV, et al. (2011) PoPoolation: a toolbox for population genetic analysis of next generation sequencing data from pooled individuals. PLoS One 6: e15925.
56. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
57. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence alignment/map format and SAMtools. Bioinformatics 25 : 2078–2079.
58. SturtevantAH (1919) A new species closely resembling Drosphila melanogaster.. Psyche 26 : 153–155.
59. WuTD, NacuS (2010) Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 26 : 873–881.
60. WuTD, WatanabeCK (2005) GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 21 : 1859–1875.
61. KoflerR, PandeyRV, SchlöttererC (2011) PoPoolation2: identifying differentiation between populations using sequencing of pooled DNA samples (Pool-Seq). Bioinformatics 27 : 3435–3436.
62. CingolaniP, PlattsA, Wang leL, CoonM, NguyenT, et al. (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6 : 80–92.
Štítky
Genetika Reprodukční medicína
Článek PARP-1 Regulates Metastatic Melanoma through Modulation of Vimentin-induced Malignant TransformationČlánek The Genome of : Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene ClusterČlánek Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish PathogenČlánek USF1 and hSET1A Mediated Epigenetic Modifications Regulate Lineage Differentiation and TranscriptionČlánek Comprehensive High-Resolution Analysis of the Role of an Arabidopsis Gene Family in RNA EditingČlánek Extensive Intra-Kingdom Horizontal Gene Transfer Converging on a Fungal Fructose Transporter Gene
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- BMS1 Is Mutated in Aplasia Cutis Congenita
- High Trans-ethnic Replicability of GWAS Results Implies Common Causal Variants
- How Cool Is That: An Interview with Caroline Dean
- Genetic Architecture of Vitamin B and Folate Levels Uncovered Applying Deeply Sequenced Large Datasets
- Juvenile Hormone and Insulin Regulate Trehalose Homeostasis in the Red Flour Beetle,
- Meiosis-Specific Stable Binding of Augmin to Acentrosomal Spindle Poles Promotes Biased Microtubule Assembly in Oocytes
- Environmental Dependence of Genetic Constraint
- H3.3-H4 Tetramer Splitting Events Feature Cell-Type Specific Enhancers
- Network Topologies and Convergent Aetiologies Arising from Deletions and Duplications Observed in Individuals with Autism
- Effectively Identifying eQTLs from Multiple Tissues by Combining Mixed Model and Meta-analytic Approaches
- Altered Splicing of the BIN1 Muscle-Specific Exon in Humans and Dogs with Highly Progressive Centronuclear Myopathy
- The NADPH Metabolic Network Regulates Human Cardiomyopathy and Reductive Stress in
- Negative Regulation of Notch Signaling by Xylose
- A Genome-Wide, Fine-Scale Map of Natural Pigmentation Variation in
- Transcriptome-Wide Mapping of 5-methylcytidine RNA Modifications in Bacteria, Archaea, and Yeast Reveals mC within Archaeal mRNAs
- Multiplexin Promotes Heart but Not Aorta Morphogenesis by Polarized Enhancement of Slit/Robo Activity at the Heart Lumen
- Latent Effects of Hsp90 Mutants Revealed at Reduced Expression Levels
- Impact of Natural Genetic Variation on Gene Expression Dynamics
- DeepSAGE Reveals Genetic Variants Associated with Alternative Polyadenylation and Expression of Coding and Non-coding Transcripts
- The Identification of -acting Factors That Regulate the Expression of via the Osteoarthritis Susceptibility SNP rs143383
- Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs
- The RNA Export Factor, Nxt1, Is Required for Tissue Specific Transcriptional Regulation
- Inferring Demographic History from a Spectrum of Shared Haplotype Lengths
- Histone Acetyl Transferase 1 Is Essential for Mammalian Development, Genome Stability, and the Processing of Newly Synthesized Histones H3 and H4
- PARP-1 Regulates Metastatic Melanoma through Modulation of Vimentin-induced Malignant Transformation
- DNA Methylation Restricts Lineage-specific Functions of Transcription Factor Gata4 during Embryonic Stem Cell Differentiation
- The Genome of : Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene Cluster
- Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
- Deregulation of the Protocadherin Gene Alters Muscle Shapes: Implications for the Pathogenesis of Facioscapulohumeral Dystrophy
- Evidence for Two Different Regulatory Mechanisms Linking Replication and Segregation of Chromosome II
- USF1 and hSET1A Mediated Epigenetic Modifications Regulate Lineage Differentiation and Transcription
- Methylation of Histone H3 on Lysine 79 Associates with a Group of Replication Origins and Helps Limit DNA Replication Once per Cell Cycle
- A Six Months Exercise Intervention Influences the Genome-wide DNA Methylation Pattern in Human Adipose Tissue
- The Gene Desert Mammary Carcinoma Susceptibility Locus Regulates Modifying Mammary Epithelial Cell Differentiation and Proliferation
- Hooked and Cooked: A Fish Killer Genome Exposed
- Distinct Neuroblastoma-associated Alterations of Impair Sympathetic Neuronal Differentiation in Zebrafish Models
- Mutations in Cause Autosomal Recessive Congenital Ichthyosis in Humans
- Integrated Transcriptomic and Epigenomic Analysis of Primary Human Lung Epithelial Cell Differentiation
- RSR-2, the Ortholog of Human Spliceosomal Component SRm300/SRRM2, Regulates Development by Influencing the Transcriptional Machinery
- Comparative Polygenic Analysis of Maximal Ethanol Accumulation Capacity and Tolerance to High Ethanol Levels of Cell Proliferation in Yeast
- SPO11-Independent DNA Repair Foci and Their Role in Meiotic Silencing
- Budding Yeast ATM/ATR Control Meiotic Double-Strand Break (DSB) Levels by Down-Regulating Rec114, an Essential Component of the DSB-machinery
- Comprehensive High-Resolution Analysis of the Role of an Arabidopsis Gene Family in RNA Editing
- Functional Analysis of Neuronal MicroRNAs in Dauer Formation by Combinational Genetics and Neuronal miRISC Immunoprecipitation
- DNA Ligase IV Supports Imprecise End Joining Independently of Its Catalytic Activity
- Extensive Intra-Kingdom Horizontal Gene Transfer Converging on a Fungal Fructose Transporter Gene
- Heritable Change Caused by Transient Transcription Errors
- From Many, One: Genetic Control of Prolificacy during Maize Domestication
- Neuronal Target Identification Requires AHA-1-Mediated Fine-Tuning of Wnt Signaling in
- Loss of Catalytically Inactive Lipid Phosphatase Myotubularin-related Protein 12 Impairs Myotubularin Stability and Promotes Centronuclear Myopathy in Zebrafish
- H-NS Can Facilitate Specific DNA-binding by RNA Polymerase in AT-rich Gene Regulatory Regions
- Prophage Dynamics and Contributions to Pathogenic Traits
- Global DNA Hypermethylation in Down Syndrome Placenta
- Fragile DNA Motifs Trigger Mutagenesis at Distant Chromosomal Loci in
- Disturbed Local Auxin Homeostasis Enhances Cellular Anisotropy and Reveals Alternative Wiring of Auxin-ethylene Crosstalk in Seminal Roots
- Causes and Consequences of Chromatin Variation between Inbred Mice
- Genome-scale Analysis of FNR Reveals Complex Features of Transcription Factor Binding
- Distinct and Atypical Intrinsic and Extrinsic Cell Death Pathways between Photoreceptor Cell Types upon Specific Ablation of in Cone Photoreceptors
- Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- BMS1 Is Mutated in Aplasia Cutis Congenita
- Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits
- Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
- Distinct Neuroblastoma-associated Alterations of Impair Sympathetic Neuronal Differentiation in Zebrafish Models
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



