-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Specific Missense Alleles of the Arabidopsis Jasmonic Acid Co-Receptor COI1 Regulate Innate Immune Receptor Accumulation and Function
Plants utilize proteins containing nucleotide binding site (NB) and leucine-rich repeat (LRR) domains as intracellular innate immune receptors to recognize pathogens and initiate defense responses. Since mis-activation of defense responses can lead to tissue damage and even developmental arrest, proper regulation of NB–LRR protein signaling is critical. RAR1, SGT1, and HSP90 act as regulatory chaperones of pre-activation NB–LRR steady-state proteins. We extended our analysis of mutants derived from a rar1 suppressor screen and present two allelic rar1 suppressor (rsp) mutations of Arabidopsis COI1. Like all other coi1 mutations, coi1rsp missense mutations impair Jasmonic Acid (JA) signaling resulting in JA–insensitivity. However, unlike previously identified coi1 alleles, both coi1rsp alleles lack a male sterile phenotype. The coi1rsp mutants express two sets of disease resistance phenotypes. The first, also observed in coi1-1 null allele, includes enhanced basal defense against the virulent bacterial pathogen Pto DC3000 and enhanced effector-triggered immunity (ETI) mediated by the NB–LRR RPM1 protein in both rar1 and wild-type backgrounds. These enhanced disease resistance phenotypes depend on the JA signaling function of COI1. Additionally, the coi1rsp mutants showed a unique inability to properly regulate RPM1 accumulation and HR, exhibited increased RPM1 levels in rar1, and weakened RPM1-mediated HR in RAR1. Importantly, there was no change in the steady-state levels or HR function of RPM1 in coi1-1. These results suggest that the coi1rsp proteins regulate NB–LRR protein accumulation independent of JA signaling. Based on the phenotypic similarities and genetic interactions among coi1rsp, sgt1b, and hsp90.2rsp mutants, our data suggest that COI1 affects NB–LRR accumulation via two NB–LRR co-chaperones, SGT1b and HSP90. Together, our data demonstrate a role for COI1 in disease resistance independent of JA signaling and provide a molecular link between the JA and NB–LRR signaling pathways.
Published in the journal: . PLoS Genet 8(10): e32767. doi:10.1371/journal.pgen.1003018
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003018Summary
Plants utilize proteins containing nucleotide binding site (NB) and leucine-rich repeat (LRR) domains as intracellular innate immune receptors to recognize pathogens and initiate defense responses. Since mis-activation of defense responses can lead to tissue damage and even developmental arrest, proper regulation of NB–LRR protein signaling is critical. RAR1, SGT1, and HSP90 act as regulatory chaperones of pre-activation NB–LRR steady-state proteins. We extended our analysis of mutants derived from a rar1 suppressor screen and present two allelic rar1 suppressor (rsp) mutations of Arabidopsis COI1. Like all other coi1 mutations, coi1rsp missense mutations impair Jasmonic Acid (JA) signaling resulting in JA–insensitivity. However, unlike previously identified coi1 alleles, both coi1rsp alleles lack a male sterile phenotype. The coi1rsp mutants express two sets of disease resistance phenotypes. The first, also observed in coi1-1 null allele, includes enhanced basal defense against the virulent bacterial pathogen Pto DC3000 and enhanced effector-triggered immunity (ETI) mediated by the NB–LRR RPM1 protein in both rar1 and wild-type backgrounds. These enhanced disease resistance phenotypes depend on the JA signaling function of COI1. Additionally, the coi1rsp mutants showed a unique inability to properly regulate RPM1 accumulation and HR, exhibited increased RPM1 levels in rar1, and weakened RPM1-mediated HR in RAR1. Importantly, there was no change in the steady-state levels or HR function of RPM1 in coi1-1. These results suggest that the coi1rsp proteins regulate NB–LRR protein accumulation independent of JA signaling. Based on the phenotypic similarities and genetic interactions among coi1rsp, sgt1b, and hsp90.2rsp mutants, our data suggest that COI1 affects NB–LRR accumulation via two NB–LRR co-chaperones, SGT1b and HSP90. Together, our data demonstrate a role for COI1 in disease resistance independent of JA signaling and provide a molecular link between the JA and NB–LRR signaling pathways.
Introduction
During their life cycle, plants have to fend off microbial pathogens including fungi, bacteria, viruses, and nematodes. To protect themselves, plants rely on the innate immune system of each plant cell to detect pathogen attack and subsequently activate disease resistance responses. The plant immune system relies on two inter-related branches. The first branch utilizes pattern recognition receptors (PRRs) to identify conserved pathogen associated molecular patterns (PAMPs). This recognition then initiates PAMP-triggered immunity (PTI) [1]–[3]. Although PTI can restrict further colonization in some cases, successful pathogens are still able to evade or suppress PTI with their effectors [4]. These proteins contribute to pathogen virulence by interfering with various plant defense-related cellular processes. However, effectors can also be recognized by the intracellular NB–LRR receptor proteins of the plant innate immune system [5]. Recognition of effectors results in effector-triggered immunity (ETI) and is the second branch of the plant immune system [1]–[3]. NB–LRR proteins contain a centrally located nucleotide binding site (NB) domain and a C-terminal leucine-rich repeat (LRR) domain. Mammalian NB–LRR containing (NLR) proteins mediate analogous processes in mammalian innate immunity [6].
NB–LRR-mediated ETI is typically associated with a form of programmed cell death at the infection site termed the hypersensitive response (HR) [1]–[3]. If not controlled, this strong response can lead to unnecessary tissue damage. Proper regulation of HR and therefore appropriate regulation of pre-activation, resting state NB–LRR proteins is critical [7]–[9]. Genetic analyses uncovered three genes, RAR1, SGT1 and HSP90, as key regulators of NB–LRR stability and activity [10]–[18]. RAR1, SGT1 and HSP90 proteins can interact independently with one another [13], [14], [16], and can cooperate as a molecular chaperone complex to regulate NB–LRR stability and function. HSP90 is usually thought to be the central subunit of the complex [19], [20]. RAR1 affects the conformational dynamics of HSP90, and modulates the “lid-open” conformation required for loading client NB–LRR proteins [21], [22]. However, the functional mechanism by which the RAR1-SGT1-HSP90 complex maintains NB–LRR levels remains poorly understood.
As highly conserved proteins, SGT1 and HSP90 also interact with each other in mammalian cells, and play essential roles in mammalian immune responses mediated by NLR proteins. By co-immunoprecipitation experiments, both SGT1 and HSP90 were found to associate with many NLR proteins including NOD1 (Nucleotide-binding Oligomerization Domain 1), NOD2 (Nucleotide-binding Oligomerization Domain 2), and NALP3 (NACHT, LRR and PYD domains-containing Protein 3) [23], [24]. In mammalian cells, treatment with geldanamycin (GDA), a chemical inhibitor of HSP90, impaired NOD2-induced NF-κB activity and NALP3-mediated inflammatory responses [24]. Knockdown of HSP90 by RNAi or GDA treatment also reduced the accumulation levels of NOD1 and NOD2 [23]. These results demonstrated that mammalian HSP90 is required for both NLR stability and function. In contrast, mammalian SGT1 is only required for NLR functions such as NOD1-mediated cytokine production, NOD1-mediated cell death, and NALP3-mediated inflammatory responses, but not for NLR stability [23], [24]. Plant SGT1, however, functions in both NB–LRR activity and stability [25]. Moreover, mammalian SGT1 knockdown reduced the association between HSP90 and the NALP3 LRR domain, indicating that mammalian SGT1 functions as a co-chaperone of mammalian HSP90 to regulate client NLR protein [24]. Unlike plant RAR1, CHP1 (CHORD-containing Protein 1), a homolog of RAR1 in mammals, is not involved in regulating NLR protein accumulation or function [24]. Taken together, the SGT1-HSP90 chaperone complex has functions for mammalian NLR protein stability and activity, analogous to its functions for plant NB–LRR biology [19], [20].
During infection, both host plants and pathogens regulate phytohormone signaling to enhance their defense and virulence respectively. Jasmonic Acid (JA) controls a well characterized example of phytohormone signaling required for both disease resistance and effector-induced susceptibility that is an outcome of the suppression of PTI [26], [27]. The JA receptor, COI1, is the key regulator of JA signaling [28]–[31]. Mutations in COI1 cause defects in JA responses and reproductive development [32], [33]. Of note, mutations in COI1 also affect, negatively or positively, disease resistance against various plant pathogens [29], [33]–[41].
COI1 encodes an F-box protein that is a component of the SCFCOI1 (Skp1/Cullin/F-boxCOI1) E3 ubiquitin ligase complex [31], [32], [42]. The function of COI1 is to specifically bind target proteins to promote ubiquitination and degradation by the 26S proteasome [31]. It is therefore assumed that COI1 regulates JA signaling and disease resistance via degradation of specific proteins. The connection between JA signaling and SCFCOI1-mediated protein degradation has been confirmed. The JASMONATE ZIM DOMAIN (JAZ) family proteins act as repressors of MYC2, a key transcriptional activator of JA responses, by directly interacting with MYC2. JA-Ile, a bioactive JA conjugate, induces the degradation of JAZ proteins by enhancing the protein interaction between JAZs and COI1, and thus de-represses JA-related transcription activation [28], [29], [31], [43]. The JAZ and MYC proteins also play a role in disease resistance. Overexpression of JAZ1Δ3A, a C-terminal deletion form of JAZ1, led to enhanced disease resistance against Pto DC3000 in Arabidopsis [29]. The triple mutant for transcription factor genes MYC2, MYC3, and MYC4, which are all repressed by JAZ proteins, was as resistant against Pto DC3000 as the coi1 mutant [44].
In this study, we extend our previously described suppressor screen for new mutants that recover impaired RPS5 function in rar1 [21]. We introduce two novel missense alleles of COI1 that suppress the disease resistance phenotypes associated with rar1 mutation. Surprisingly, these two coi1 rar1 suppressor (rsp) alleles are completely fertility, in contrast to the male sterility associated with all other coi1 mutant alleles [32], [43], [45]. Like sgt1b and the hsp90.2rsp alleles [21], these two coi1rsp alleles interact with rar1 to restore the disease resistance responses mediated by some NB–LRRs and the accumulation of at least RPM1. Moreover, we demonstrate that overexpression of SGT1b can partially inhibit the coi1rsp-enhanced accumulation of RPM1 and RPM1-mediated disease resistance in rar1. We also observe non-allelic non-complementation, a rare genetic interaction, between coi1rsp mutants and hsp90.2-7rsp mutant. These results support the hypothesis that coi1rsp proteins regulate NB–LRR levels via SGT1b and HSP90.
Results
Identification of new alleles of COI1 and of the rsp3 mutant
To identify new genes that act with RAR1 to regulate NB–LRR accumulation and activation, we performed a suppressor screen for new mutants which can suppress the disease susceptibility observed in rar1-21 (a stop mutation in Q52) [21]. Five rar1 suppressor (rsp) mutants were identified from approximately 200,000 M2 plants from 50 M2 pools that recover resistance responses to both Pto DC3000(avrPphB) and Pto DC3000(avrRpm1) [21]. Based on map-based cloning and subsequent allele sequencing, two of the five mutants were found to have mutations in COI1 (At2g39940). To follow accepted nomenclature conventions, we designated these two mutant alleles, coi1-21rsp and coi1-22rsp, respectively (Figure 1). Based on disease symptoms after inoculation of Pto DC3000(avrRpm1) on backcross F1 and F2 populations, both of the coi1rsp mutants were completely recessive (Table S1). This conclusion was also confirmed by growth assays of Pto DC3000(avrRpm1) in backcross F1 plants (Figure 2). The coi1-21rsp mutation is a G/A transition which leads to a G330E missense change in the COI1 protein. The coi1-22rsp mutation is a G/A transition resulting in a G434E missense change in the protein. Both mutations are within conserved LRR domains (Figure 1). Using the crystal structure of the Arabidopsis COI1 protein, we observed that neither coi1rsp mutation is localized in the interfaces of COI1 that make up the ASK1-binding region and the ligand-binding pocket [31].
Fig. 1. Mutations identified in COI1. 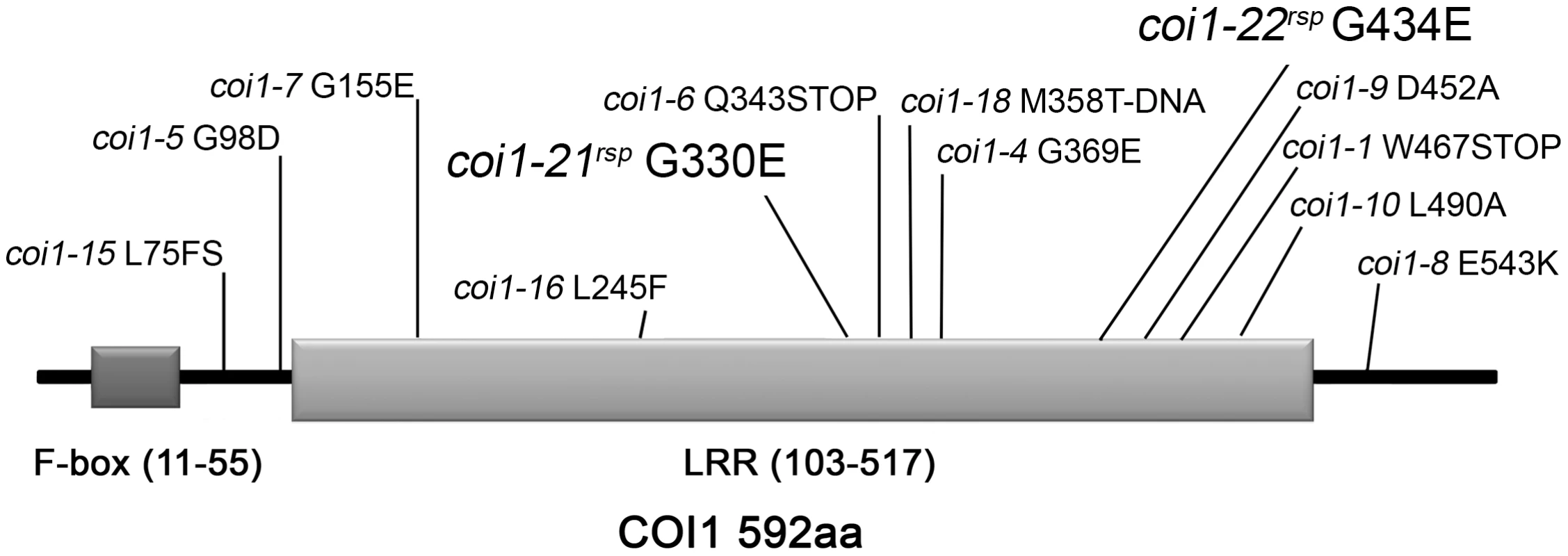
The F-box domain and the LRR domain are shown in dark and light gray, respectively. The allele designation and associated amino acid change is shown in relation to its linear position. New alleles introduced in this paper are shown with larger font. Fig. 2. COI1 and HSP90 interact genetically to regulate disease resistance. 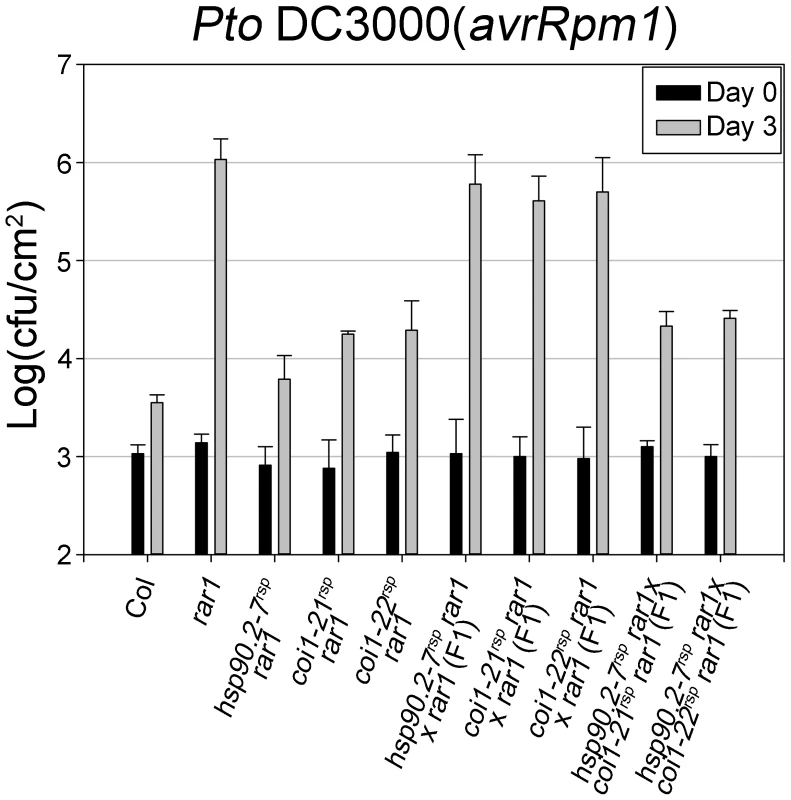
Bacteria Pto DC3000(avrRpm1) were hand-infiltrated into leaves of each indicated genotype and counted at day 0 and day 3. Error bars represent 2× SE. The result displayed is one of two independent analyses giving similar results. In addition, another rar1 suppressor (rsp) mutant called rsp3 was isolated from this screen. rsp3 suppressed all known rar1 phenotypes, and was localized in a 7 Mbp region on chromosome I (Figure S1). A single allele, dominant mutation was identified in rsp3; its detailed characterization is beyond the scope of this work.
COI1 and HSP90 interact genetically to regulate disease resistance
The disease resistance restoration phenotypes of hsp90.2-7rsp and either coi1rsp alleles in rar1 are fully recessive with respect to their respective wild type phenotypes ([21], Figure 2). We monitored in planta growth of Pto DC3000(avrRpm1) to measure RPM1-mediated disease resistance in F1 plants of hsp90.2-7rsp×coi1rsp crosses (Figure 2). The resulting F1 plants were as resistant to Pto DC3000(avrRpm1) as their parental coi1rsp plants. We also tested F1 plants of crosses between hsp90.2-7rsp and either coi1rsp allele for disease symptoms after inoculation of Pto DC3000(avrRpm1). The F1 plants displayed resistance against Pto DC3000(avrRpm1) (Table S2). In addition, we observed that a part of the F2 progenies from each F1 were susceptible to Pto DC3000(avrRpm1) (Table S2). These results clearly demonstrate non-allelic non-complementation between hsp90.2-7rsp and coi1rsp mutants, suggesting that the two proteins function in the same process, and likely do so in physical proximity [14], [46], [47].
coi1rsp alleles and coi1-16 partially suppress known rar1 phenotypes
hsp90.2rsp alleles isolated from our rar1 suppressor screen recover all known defective NB–LRR functions in a rar1 mutant background [21]. However, a previously published rar1 suppressor mutant, sgt1b, only affected a limited number of NB–LRR protein functions [25]. We therefore tested both coi1rsp alleles to determine whether they have any NB–LRR specificity in their suppression of rar1. The coi1rsp alleles partially suppress rar1 for RPS5 and RPM1 functions, and fully suppress rar1 for RPS2 function (Figure 2, Figure S2A and S2B, Figure 3A).
Fig. 3. coi1rsp mutants suppress rar1 phenotypes and are not null allele. 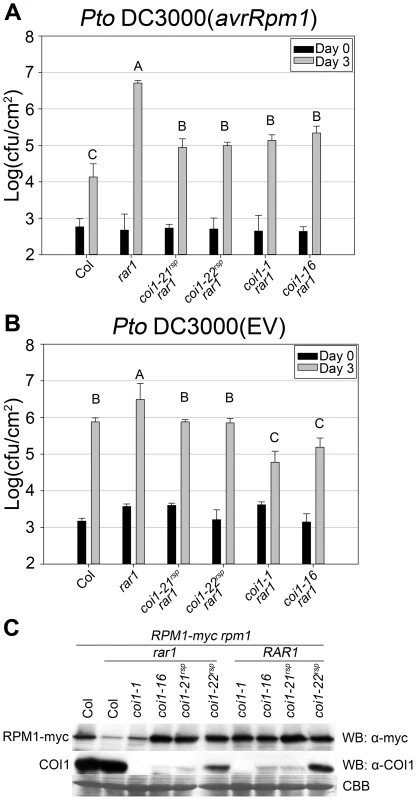
(A–B) Bacterial growth analysis of (A) Pto DC3000(avrRpm1) and (B) Pto DC3000(EV). Bacteria were hand-infiltrated into leaves of each indicated genotype and counted at day 0 and day 3. Error bars represent 2× SE. Pairwise comparisons for all means for bacterial growth on day 3 were performed with One-Way ANOVA test followed by Tukey-Kramer HSD at 95% confidence limits. (C) Western blot analysis of RPM1-myc and COI1 levels in the indicated genotypes. RuBisCo levels stained by Coomassie Brilliant Blue serve as loading controls. The pathogen growth assays were performed independently three times with similar results. The western blots were performed independently two times with similar results. Both RPM1-myc and COI1 blots used the same protein samples. rar1 exhibits enhanced disease susceptibility to the virulent bacterial strain Pto DC3000(EV) [18], [21], [25]. This phenotype might be due to a RAR1 function in basal defense, for example an additive effect of globally lowered accumulation of multiple NB–LRR proteins [1]–[3]. As measured by inhibition of bacterial growth, both coi1rsp alleles completely suppressed the enhanced disease susceptibility phenotype in rar1 (Figure 3B).
NB–LRR activation can trigger the hypersensitive response (HR) as well as disease resistance responses. RAR1 is required for HR mediated by many NB–LRR proteins. sgt1b is able to suppress the loss of RPS5-mediated disease resistance in a rar1 mutant, but not the loss of RPS5-mediated HR [25]. To test if NB–LRR-dependent HR is also recovered in coi1rsp rar1 double mutants, we measured ion leakage as a proxy for HR to quantify RPM1-mediated HR in plants. Notably, the coi1rsp alleles did not suppress rar1 for impaired RPM1-triggered HR (Figure S2C). However, the coi1rsp rar1 plants did recover RPM1-mediated disease resistance, measured via pathogen growth restriction (Figure 3A).
RPS5, RPM1 and RPS2 all belong to the CC-NB–LRR subclass. The functions of some TIR-NB–LRR proteins also require RAR1. The effect of coi1rsp on TIR-NB–LRR function was tested using the pathogenic oomycete Hyaloperonospora arabidopsidis (Hpa) isolate Emwa1 to trigger RAR1-dependent RPP4-mediated disease resistance [48]. Neither of the two coi1rsp rar1 double mutants inhibited the growth of Emwa1 (Figure S2D). This indicates that RPP4 function is not recovered in rar1 in the presence of either coi1rsp allele. Thus, the coi1rsp alleles possibly suppress rar1 only for CC-NB–LRR functions.
The accumulation of all tested NB–LRR proteins is reduced in rar1 plants, implying that the biochemical function of RAR1 is to maintain the stability of NB–LRR proteins [7], [18], [21], [25], [49]. We wondered whether coi1rsp alleles could suppress the decrease of NB–LRR protein accumulation in rar1. We introduced our transgenic, myc-tagged RPM1 [50] into the coi1rsp rar1 mutants by crossing and marker-assisted selection. The coi1rsp alleles suppressed the lowered RPM1-myc accumulation in rar1 (Figure 3C). Hence, the coi1rsp alleles suppress the biochemical phenotype of rar1.
The coi1rsp alleles are phenotypically different from two reference alleles, coi1-1 (a protein null (encoding W467STOP [32]; Figure 1) and coi1-16 (encoding L245F [45]; Figure 1), which are also completely or conditionally male sterile. We therefore tested whether either coi1-1 or coi1-16 could suppress rar1. Similar to the coi1rsp alleles, coi1-1 and coi1-16 enhanced disease resistance responses against both Pto DC3000(avrRpm1) and Pto DC3000(EV) in a rar1 background (Figure 3A, 3B). The increase in disease resistance against Pto DC3000(EV) was even higher than that caused by the coi1rsp alleles (Figure 3B). To our surprise, coi1-16 resulted in the recovery of RPM1-myc accumulation in rar1, but coi1-1 did not (Figure 3C). However, coi1-16 and coi1-1 express equivalent enhanced disease resistance in rar1. Thus, the “restoration” of disease resistance responses against Pto DC3000(avrRpm1) that we observed in coi1-1 rar1 is not due to restoration of NB–LRR protein levels, but rather to bypass suppression of rar1 disease susceptibility. This is likely caused by enhanced basal defense possibly related to the antagonistic relationship between JA - and SA-dependent signaling (Figure 3). The growth of Pto DC3000(avrRpm1) and Pto DC3000(EV) at 3 dpi was about the same in coi1-16 rar1 plants (Figure 3). Thus, the restored disease resistance in coi1-16 rar1 is likely due to enhanced basal defense, not RPM1 function, although there is a restoration of RPM1-myc accumulation in coi1-16 rar1.
Since the coi1-1 null allele cannot suppress rar1, we suggest that the coi1rsp alleles and coi1-16 are recessive gain-of-function alleles for the rar1 suppression phenotypes. They are also loss-of-function alleles for the JA response phenotypes as detailed below.
The coi1rsp mutations negatively regulate RPM1-dependent HR in otherwise wild-type plants
We introduced the coi1rsp alleles into an isogenic RAR1 background using marker-assisted breeding (see Methods). To further study the role of COI1 in regulating RPM1 function, we inoculated both coi1rsp alleles, coi1-1 and coi1-16 plants with Pto DC3000(avrRpm1) and measured bacterial growth (Figure 4B). The coi1rsp and coi1-16 mutants were as resistant as wild type. The coi1-1 mutant displayed slightly enhanced resistance compared with wild type. We also measured RPM1-mediated HR in these coi1 single mutants using the ion leakage assay (Figure 4C). Surprisingly, both coi1rsp alleles weakly suppressed RPM1-mediated HR. We crossed RPM1-myc into these coi1rsp, coi1-1 and coi1-16 single mutants and measured RPM1-myc protein levels (Figure 3C). We observed no obvious changes in RPM1-myc levels in any of the single coi1 mutant. We conclude from these data that coi1rsp mutations differentially regulate RPM1 function in rar1 or RAR1 backgrounds.
Fig. 4. coi1rsp alleles exhibit enhanced basal defense and additionally weakly suppress RPM1 HR function. 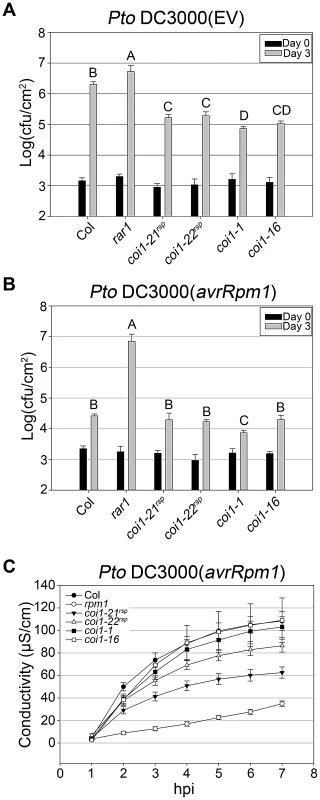
(A–B) Bacterial growth analysis of Pto DC3000(EV) (A) and Pto DC3000(avrRpm1) (B). Bacteria were hand-infiltrated into leaves of each indicated genotype and counted at day 0 and day 3. Error bars represent 2× SE. Pairwise comparisons for all means for bacterial growth on day 3 were performed with One-Way ANOVA test followed by Tukey-Kramer HSD at 95% confidence limits. (C) Conductivity measurements after inoculation with high concentration Pto DC3000(avrRpm1) (5×107 cfu/ml). Error bars represent 2× SE. The pathogen growth and HR assays were performed independently a minimum of three times with similar results. Increased RPM1 accumulation in coi1rsp and coi1-16 is post-transcriptionally regulated
Loss of COI1 leads to elevated levels of salicylic acid (SA) in plants [37], and elevated SA levels can induce the expression of some NB–LRR-encoding genes [51]–[53]. NB–LRR expression is not changed in rar1 (Figure S4, [49]). We measured RPM1 mRNA levels in the coi1rsp, coi1-1, and coi1-16 mutant plants in the context of wild-type RAR1 by RT-qPCR in order to determine whether the increased RPM1-myc protein levels noted in coi1rsp and coi1-16 were due to enhanced transcription. Wild type and rar1 plants were used as controls. We detected no enhancement of RPM1 mRNA levels among the tested coi1 mutants (Figure S4), indicating that the coi1rsp and coi1-16 alleles restore RPM1 protein levels by a post-transcriptional mechanism in rar1.
The coi1rsp alleles are JA–insensitive
COI1 has an essential role in JA signaling; all previously isolated COI1 mutations caused insensitivity to JA-mediated inhibition of seedling growth [32], [43], [45]. We compared JA-insensitivity phenotypes of the coi1rsp alleles to coi1-1 using a growth inhibition assay where plants were grown in the presence of MeJA, a functional JA derivative (Figure 5). Like coi1-1, the MeJA-treated coi1rsp seedlings grew on MeJA-containing media, while the growth of wild type seedlings was severely inhibited (Figure 5A). MeJA treated coi1rsp seedlings were clearly smaller than the untreated seedlings, suggesting that the coi1rsp alleles are not as insensitive to JA as coi1-1. We quantified these phenotypes with a root elongation assay (Figure 5B). The null allele coi1-1 displayed root growth inhibition of only about 14% in the presence of 50 µM MeJA. Compared with coi1-1, coi1-16 and both coi1rsp alleles displayed intermediate insensitivity to MeJA treatment. Their root growth was inhibited about 27%, 30% and 42% respectively, while the root growth inhibition was more than 60% in wild type seedlings. Thus, the coi1rsp alleles are JA-insensitive.
Fig. 5. coi1rsp alleles are insensitive to JA. 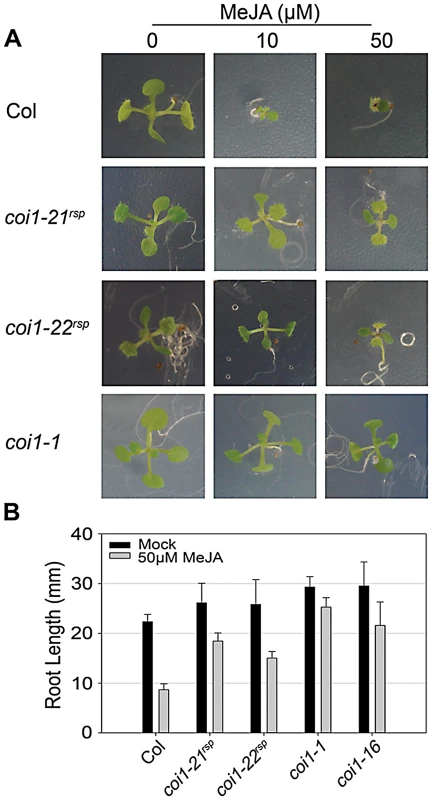
(A) Seedlings of the indicated genotypes were grown on MS medium (control) or medium containing 10 or 50 µM MeJA. (B) Inhibition of root elongation by 50 µM MeJA in at least twenty seedlings of indicated genotypes. This assay was performed independently three times with similar results. JA signaling is important in disease resistance responses. coi1 and other JA insensitive mutants exhibit enhanced resistance to the virulent bacterial strain Pto DC3000(EV) [29], [37], [38]. We measured the growth of Pto DC3000(EV) in our coi1rsp alleles, coi1-1, and coi1-16 (Figure 4A). The coi1rsp alleles also displayed enhanced resistance to Pto DC3000(EV), although the increase in the coi1rsp alleles was slightly lower than in the reference alleles coi1-1 and coi1-16.
coi1rsp alleles are not null alleles
The coi1rsp alleles are quantitatively different than the coi1-1 null allele with respect to JA responses (Figure 5B) and enhanced resistance to Pto DC3000(EV) (Figure 4A). We noted decreased COI1 protein accumulation levels in coi1-21rsp, coi1-22rsp and coi1-16 plants compared to wild type and rar1 plants (Figure 3C). As expected, no detectable amount of COI1 protein was observed in coi1-1. The residual accumulations of COI1 protein confirmed that the coi1rsp alleles and coi1-16 are not COI1 null alleles.
The sgt1b mutant is insensitive to JA responses
To determine whether other NB–LRR regulators function in regulating JA responses, we tested the JA response in the mutants of three NB–LRR co-chaperones, RAR1, SGT1b and HSP90.2 by the root elongation assay (Figure S3). All rar1 and hsp90.2 mutants were as sensitive to MeJA treatment as wild type, suggesting that neither RAR1 nor HSP90.2, plays a role in JA responses. As expected, the sgt1b mutant displayed an obvious insensitivity to MeJA [54]. We also noted MeJA insensitivity in the rar1 sgt1b double mutant (Figure S3). These results suggest that SGT1b is the only member of RAR1-SGT1-HSP90 NB–LRR co-chaperone complex required for JA signaling.
COI1 mutations do not affect the levels of RAR1, SGT1b, or HSP90 accumulation
coi1 mutations restored the disease resistance responses mediated by three NB–LRR proteins in rar1 (Figure 3A, Figure S2A and S2B) and thus possibly suppressed rar1 via effects upon NB–LRR regulators that control the accumulation, and hence the function, of multiple NB–LRR proteins. To examine this possibility, we determined the accumulation levels of three NB–LRR regulators, RAR1 (Figure S5A), SGT1b (Figure S5B), and HSP90 (Figure S5C), in the coi1rsp, coi1-1 and coi1-16 mutants in either RAR1 or rar1 backgrounds. These coi1 mutants did not exhibit any dramatic change of RAR1, SGT1b or HSP90 protein levels. Therefore, the coi1rsp and coi1-16 alleles do not suppress rar1 influencing by regulating the steady state levels of RAR1, SGT1b and/or HSP90.
SGT1b antagonizes coi1rsp-mediated RPM1 accumulation and RPM1-dependent disease resistance in rar1
The coi1rsp mutants displayed opposite phenotypes: increased NB–LRR accumulation and function in rar1 and decreased NB–LRR HR function in RAR1. A similar combination of phenotypes was previously observed in sgt1b as an rar1 suppressor [25]. The sgt1b mutation enhanced RPS5 accumulation and consequent restoration of RPS5-mediated disease resistance in rar1, but did not restore RPS5-triggered HR in RAR1 [25]. This similarity implies that coi1rsp mutants might regulate NB–LRR proteins by inhibiting the function of SGT1b and hence mimic sgt1b phenotypes.
Based on this hypothesis, we expected that a high dose of SGT1b would attenuate the rar1 suppression phenotypes of the coi1rsp mutants. To test this, we introduced a 35S:SGT1b-HA construct into coi1-21rsp rar1 plants containing RPM1-myc. Compared with parental coi1-21rsp rar1 plants, four independent T3 lines that expressed relatively high levels of SGT1b::HA exhibited both reduced RPM1-myc levels (Figure 6A) and RPM1-mediated disease resistance (Figure 6B). However, the RPM1 accumulation and RPM1-mediated disease resistance observed in these T3 plants were still much higher than rar1 plants (Figure 6A, 6B). These results demonstrated that modest over-expression of SGT1b can partially inhibit the rar1 suppression phenotypes of coi1rsp alleles. As a control, we measured the growth of Pto DC3000(EV) in the plants used in the Pto DC3000(avrRpm1) growth assay. No enhanced growth of Pto DC3000(EV) was observed in these T3 lines (Figure 6C), demonstrating that the reduction of RPM1-mediated disease resistance in 35S:SGT1b-HA transgenic plants are not due to a decrease in basal defense.
Fig. 6. SGT1b over-expression antagonizes coi1rsp-dependent RPM1 accumulation and RPM1-mediated disease resistance in rar1. 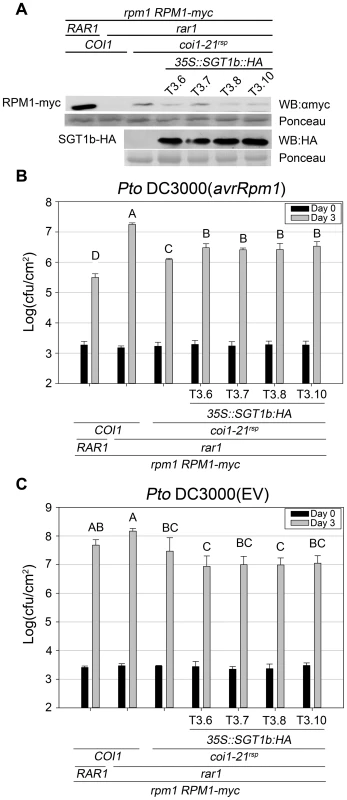
(A) Western blot analysis of RPM1-myc and SGT1b-HA protein levels in indicated genotypes. RuBisCo levels stained by Ponceau S serve as loading control. The result displayed is one of three independent blots giving similar results. (B–C) Bacterial growth analysis of Pto DC3000(avrRpm1) (B) and Pto DC3000(EV) (C). Bacteria were hand-infiltrated into leaves of each indicated genotype and counted at day 0 and day 3. Error bars represent 2× SE. Pair-wise comparisons for all means for bacterial growth on day 3 were performed with One-Way ANOVA test followed by Tukey-Kramer HSD at 95% confidence limits. The bacterial growth assays were performed independently three times (Pto DC3000(avrRpm1)) and twice (Pto DC3000(EV)) with similar results. In addition, we measured the HSP90 protein levels and RPM1-myc mRNA levels in the transgenic plants used in the western blot analysis. No obvious decrease of HSP90 protein level (Figure S6A) or RPM1-myc mRNA level was detected (Figure S6B), indicating that the reductions of RPM1-myc accumulation in 35S:SGT1b-HA transgenic plants are not due to the decrease of HSP90 accumulation or the silencing of RPM1-myc gene.
Discussion
We initially performed a suppressor screen for mutants that could recover the diminished NB–LRR RPS5-mediated disease resistance phenotype of rar1 [21]. These suppressors were isolated in the null rar1-21 background (Figure S5A), and thus likely represent mutations that either bypass or counteract rar1. We reported two novel HSP90 alleles derived from this screen that function to mimic the effects of RAR1 on the HSP90 lid open/close cycle required to stabilize NB–LRR clients [21]. Here, we detail the characterization of two coi1 alleles, coi1-21rsp and coi1-22rsp also identified in this screen (Figure 1), and we note that a third single allele locus defined by rsp3 has characteristics that suggest it might encode another new player in the regulation of NB–LRR accumulation (Figure S1). Because rsp3 is a single, dominant allele, its description beyond the mutant phenotype was not pursued as part of this study.
The F-box protein COI1 is a core component of the receptor complex for jasmonate (JA) [28], [29], [31]. In plants, mutations in COI1 impair all known JA responses and thus result in insensitivity to JA or functional JA derivatives [32], [33], [37], [43], [45]. As expected, both of the coi1rsp alleles were JA insensitive (Figure 5). However, the MeJA insensitivity in coi1rsp alleles is obviously weaker than in the null allele, coi1-1 (Figure 5B).
In addition to being insensitive to JA, all coi1 alleles identified previously are, at least partially, male-sterile [32], [33], [37], [43], [45]. To our surprise, the two coi1rsp alleles are completely fertile. Among the previously described alleles, only coi1-8 (encoding a missense change of E543K) exhibits partial fertility in regular growth conditions [43]. The other partially fertile allele, coi1-16 (encoding L245F), is fertile only at low temperature (16 degrees C) [45]. Similar to the coi1rsp alleles, coi1-8 exhibited drastically reduced but still detectable COI1 protein levels [43]. A pull-down assay demonstrated that the COI1-8E543K protein retains interaction with JAZ1, a substrate of COI1 in SCFCOI1-mediated protein degradation [43]. These results indicate that the weak MeJA insensitivity and intact fertility of the coi1rsp mutants are likely due to lower accumulation of functional COI1rsp proteins in these mutants. In other words, the coi1rsp mutations, G330E and G434E, cause relatively weaker impairments of the COI1 protein stability and activity than the other reported coi1 missense alleles.
COI1 functions in both basal defense and ETI
Mutations in COI1 affect, negatively or positively, disease resistance against various plant pathogens [29], [33]–[41]. It is widely accepted that the defense phenotypes of coi1 depend on signaling antagonism between SA and JA signaling pathways [55]. COI1 mutations disable JA-signaling and consequently enhance SA signaling and SA-induced defense responses by an as yet unknown mechanism.
In Arabidopsis, resistance against the virulent hemi-biotrophic pathogen Pto DC3000 is a measure of basal defense [56]. In our study, all four tested coi1 alleles, coi1-21rsp, coi1-22rsp, coi1-1, and coi1-16 displayed enhanced disease resistance against Pto DC3000(EV) in both rar1 and RAR1 backgrounds (Figure 3B, Figure 4A). These results correspond to previously published data [29], [37], [38], and confirm that COI1 represses basal defense, likely via JA-SA antagonism. Besides enhanced basal defense, the coi1 alleles also displayed enhanced ETI against Pto DC3000(avrRpm1) (Figure 3A, Figure 4B). Hence, COI1 also inhibits ETI. Since the enhancement of ETI was found in rar1 mutant plants, RAR1, which is necessary for NB–LRR-mediated ETI in this and many other cases, is not required by COI1 to repress ETI.
A plausible mechanism explaining COI1 effects on NB–LRR accumulation in rar1 and RAR1
Although all four coi1 alleles we analyzed restored resistance against Pto DC3000(avrRpm1) in rar1 (Figure 3A), we could classify them into three classes based on how they influence RPM1 accumulation and RPM1-mediated immune response (Figure 3C, Figure 4C). Class I, represented by the null allele coi1-1, does not alter RPM1 levels. Class II, represented by coi1-16, enhances RPM1 levels in rar1 and has no effect on RPM1-mediated HR in RAR1. Class III, represented by coi1-21rsp and coi1-22rsp, enhance RPM1 levels in rar1, but reduce RPM1-mediated HR in RAR1. Since the null coi1-1 does not exhibit any detectable effect on RPM1 accumulation, the enhancement of RPM1 levels in rar1 is a gain-of-function phenotype conferred by the COI1 mutant proteins accumulating in coi1-16 and the two coi1rsp alleles. However, these alleles are all recessive for JA response phenotypes. The coexistence of these distinct genetic characteristics demonstrates that coi1-16 and coi1rsp alleles are recessive gain-of-function alleles which have lost the JA signaling function of COI1, but gained new function, likely via interfering with the activity of other protein(s). RPM1 is associated with, and activated at, the plasma membrane; there is no current evidence suggesting that it shuttles into the nucleus [50], [57]. COI1 is expected to be localized in the nucleus, because it binds to the nucleus-localized JAZ proteins [58]. A biochemical mechanism to explain our genetic results would require a reconciliation of these findings. There may be sufficient coi1rsp protein at the plasma membrane to mediate the effects on RPM1 that we describe. Further, our inference that COI1 has a wild type function in mediating NB–LRR protein accumulation is consistent with suggestions that nucleo-cytoplasmic shuttling is required for the function of at least a subset of NB–LRR proteins [3].
Some publications suggest that the “target” protein with which recessive gain-of-function alleles interfere can share functional redundancy with it [14], [59]–[62]. We found that mutants of two NB–LRR co-chaperones, SGT1b and HSP90, have phenotypic similarities with coi1rsp alleles [14], [21], [25]. These include (Table S3): 1) enhanced NB–LRR accumulation in rar1: RPM1 in hsp90.2rsp rar1 [21], RPS5 in sgt1b rar1 [25], and RPM1 in coi1rsp rar1 (this work); 2) impaired NB–LRR-mediated HR in RAR1: RPM1-mediated HR in hsp90lra [14], RPS5-mediated HR in sgt1b [25], and RPM1-mediated HR in coi1rsp (this work). COI1 is an F-box protein which is a component of an SCF complex. Both SGT1b and HSP90 have been reported to associate and function with various SCF complexes in plants [13], [54], [63], [64]. These findings collectively imply that SGT1b and/or HSP90 are candidate target proteins of coi1rsp proteins in suppressing rar1.
Since the coi1rsp alleles did not affect steady state SGT1b levels (Figure S5B), coi1rsp alleles might inhibit SGT1b activity to suppress the rar1 phenotype of reduced NB–LRR accumulation. To test this hypothesis, we overexpressed SGT1b in a coi1-21rsp rar1 background. The rar1 suppression phenotypes of coi1-21rsp, restored RPM1-myc accumulation and RPM1-mediated disease resistance, were partially complemented by SGT1b overexpression (Figure 6A, 6B). This result supports our hypothesis, and suggests that SGT1 functions with COI1 to regulate NB–LRR accumulation. On the other hand, the incomplete complementation could mean that we need higher levels of SGT1b over-expression, or that coi1rsp proteins also down-regulate the activity of other targets, such as HSP90. Our speculation is supported by the non-allelic non-complementation observed between coi1rsp mutants and hsp90.2-7rsp mutant (Figure 2, Table S2). This specific genetic relationship suggests that COI1 and HSP90 physically interact with each other or belong to the same protein complex.
The RAR1-SGT1-HSP90 chaperone complex has been related to the SCF complex by two sorts of evidence: 1) SGT1b and HSP90 associate and function with various SCF complexes [13], [54], [63], [64]. RAR1 associates with the COP9 signalosome (CSN) which can inactivate the SCF complex [13], [64], [65]; 2) The SCFCPR1 complex negatively regulates the pre-activation steady state stability of two NB–LRR proteins, SNC1 and RPS2, via the F-box protein CPR1 [66]. The SCF component SKP1 is required for NB–LRR N protein-mediated resistance response against tobacco mosaic virus (TMV) [64]. This relationship suggests that RAR1-SGT1-HSP90 chaperone complexes function with an SCF-mediated protein degradation pathway to control the accumulation levels of NB–LRR protein and thus avoid inappropriate NB–LRR activation [19]. The phenotypes observed in our recessive gain-of-function coi1rsp mutants support this hypothesis. The coi1rsp mutants suppressed the rar1 mutant for reduced NB–LRR RPM1 accumulation, and showed non-allelic non-complementation with hsp90.2. Moreover, overexpression of SGT1b partially inhibited the phenotypes of the coi1rsp mutants. Similar to sgt1b and hsp90.2lra mutants, coi1rsp mutants caused impaired HR function when moved to a wild type background. The sum of these results is consistent the idea that the F-box protein COI1 functions with RAR1-SGT1-HSP90 chaperone complex and consequently affects NB–LRR protein accumulation and function.
Materials and Methods
Plant lines
We used coi1-1 [32] and coi1-16 [45] as reference alleles. For the pathology analyses and root elongation analyses, mutant lines used (all in Col-0 background) were rar1-21 [18], rpm1-1 [67], rps5-2 [68], rps2-101c [69], sgt1bedm1-1 [17], rar1-21 sgt1bedm1-1 [25], hsp90.2-2 [14], hsp90.2-5KO [14], hsp90.2-7 [21] and hsp90.2-8 [21]. Ecotype Ws was used as an rpp4 control [48]. We constructed coi1-1 rar1-21 and coi1-16 rar-21 double mutants by identifying F2s with PCR-based dCAP markers. The F2s with appropriate genotypes were selfed, and F3 individuals were further selected with PCR-based dCAP markers.
To make the 35S:SGT1b-HA construct, the coding sequence of SGT1b without its stop-codon was amplified by PCR, and then moved into pGWB14 vector [70]. The final destination vector, pGWB14/35S:SGT1b-HA was electropolated into the Agrobacterium strain GV3101 for transformation of appropriate genotypes. Transformed plants were selected on MS medium plate (PhytoTechnology Laboratories, KS, U.S.) containing Hygromycin B (SIGMA, St. Louis, MO, U.S.).
Pathogen strains, inoculation, growth quantification, and ion leakage assay
Pto DC3000 derivatives containing pVSP61(EV), avrRpm1, avrPphB, and avrRpt2 were maintained as described [71]. Plant inoculations and bacterial growth assays were performed as described (spray-inoculation [21]; dip-inoculation [72]; hand-inoculation [25]). The HR test and ion leakage assays were carried out as described [21].
Hyaloperonospora arabidopsidis (Hpa) isolate Emwa1 was used to inoculated ten-day-old cotyledons of plants as described [21]. Asexual sporangiophores were counted 7 days post-inoculation on at least 30 cotyledons for each genotype.
Identification and map-based cloning of mutations in COI1
The rar1 suppressor screen was previously described [21]. Standard genetic analyses and map-based cloning were performed as described [21]. We used 892 disease resistant F2 individuals to define a 60 Kb interval on the chromosome II containing COI1. By sequencing COI1 in the originally isolated rar1 suppressor mutant, a G/A transition at position 1849 (nucleotide positions relative to the translation start site of the published sequence of COI1; AT2G39940) was identified in coi1-21rsp. The other mutant, coi1-22rsp, also contains a G/A mutation at position 2161 in COI1. To obtain coi1-21rsp and coi1-22rsp single mutants, we backcrossed the coi1rsp alleles into an isogenic RAR1 background. PCR-based dCAP markers were designed for selecting these two coi1rsp mutations.
MeJA treatment
For growth inhibition assays, seedlings were grown on MS medium with different concentrations of Methyl Jasmonate (MeJA) (SIGMA) at 22°C under 16 h light/8 h dark photoperiod. 10-day-old seedlings were taken picture to show the inhibition effects.
For root elongation assays, seedlings were horizontally grown on MS medium at 22°C under 24 h light for 4 d. Then seedlings were transferred to new MS medium with or without 50 µM MeJA, and grown for additional 4 d. Root elongations during these four days were measured.
Western blots
For detection of RPM1-myc in the genotypes mentioned in this study, we introduced by crossing and segregation the mutants into plants expressing RPM1-myc from the native RPM1 promoter as described [14]. The protein extraction and western blot were performed as described [14]. For detection of SGT1b-HA in plants, the protein extraction and western blot were carried out based on the protocol that was previously used for RPS5-HA [25]. The anti-COI1 antiserum was kindly provided by Daoxin Xie (Tsinghua University, Beijing, China). The protein extraction and western blot were performed as described [42]. anti-SGT1 and anti-RAR1 polyclonal antibodies against the full length SGT1b and full length RAR1 with C-terminus GST tag were generated in rabbits (custom products of Cocalico Biologicals, Inc.). anti-HSP90-2 was the product of Agrisera company (Swedish). The detailed protocols for detection of SGT1a, SGT1b, RAR1, and HSP90 proteins are provided as Text S1.
RT–qPCR
Plant RNA was extracted with RNeasy Plant Mini Kit (Qiagen). To eliminate DNA contamination, RNA was purified by Turbo DNA Free Kit (Ambion) and RNeasy Mini Kit (Qiagen). 2 µg RNA was reverse transcribed with Random Decamers and RETROscript kit (Ambion).
RT-qPCR was performed in a total volume of 25 µl (12.5 µl SYBR Green PCR Master Mix (Applied Biosystems), 0.5 µl cDNA, 1 µl Primer 1 (10 µM), 1 µl Primer 2 (10 µM) and 10 µl H2O) with MJ White 96-well plate and a DNA Engine OPTICON 2 system (MJ Research). The reaction was run at 95°C for 5 min, followed by 40 cycles at 95°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec. Dissociation analysis was performed after each reaction to confirm the specificity. The relative expression of RPM1/RPM1-myc gene in different genotypes was calculated by ΔΔCt method (User Bulletin #2, Manual of Applied Biosystems). The primers were newly designed or obtained from previous publication [73], and are provided as Text S1.
Supporting Information
Zdroje
1. JonesJDG, DanglJL (2006) The plant immune system. Nature 444 : 323–329.
2. DoddsPN, RathjenJP (2010) Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet 11 : 539–548.
3. MaekawaT, KuferTA, Schulze-LefertP (2011) NLR functions in plant and animal immune systems: so far and yet so close. Nat Immunol 12 : 817–826.
4. MudgettMB (2005) New Insights to The Function of Phytopathogenic Bacterial Type III Effectors in Plants. Annual Review of Plant Biology 56 : 509–531.
5. NimchukZ, EulgemT, HoltBF3rd, DanglJL (2003) RECOGNITION AND RESPONSE IN THE PLANT IMMUNE SYSTEM. Annual Review of Genetics 37 : 579–609.
6. TingJPY, WillinghamSB, BergstralhDT (2008) NLRs at the intersection of cell death and immunity. Nat Rev Immunol 8 : 372–379.
7. BelkhadirY, NimchukZ, HubertDA, MackeyD, DanglJL (2004) Arabidopsis RIN4 Negatively Regulates Disease Resistance Mediated by RPS2 and RPM1 Downstream or Independent of the NDR1 Signal Modulator and Is Not Required for the Virulence Functions of Bacterial Type III Effectors AvrRpt2 or AvrRpm1. The Plant Cell 16 : 2822–2835.
8. MackeyD, HoltBF, WiigA, DanglJL (2002) RIN4 Interacts with Pseudomonas syringae Type III Effector Molecules and Is Required for RPM1-Mediated Resistance in Arabidopsis. Cell 108 : 743–754.
9. LiX, ClarkeJD, ZhangY, DongX (2001) Activation of an EDS1-Mediated R-Gene Pathway in the snc1 Mutant Leads to Constitutive, NPR1-Independent Pathogen Resistance. Molecular Plant-Microbe Interactions 14 : 1131–1139.
10. ShirasuK, LahayeT, TanM-W, ZhouF, AzevedoC, et al. (1999) A Novel Class of Eukaryotic Zinc-Binding Proteins Is Required for Disease Resistance Signaling in Barley and Development in C. elegans. Cell 99 : 355–366.
11. WarrenRF, MerrittPM, HolubE, InnesRW (1999) Identification of Three Putative Signal Transduction Genes Involved in R Gene-Specified Disease Resistance in Arabidopsis. Genetics 152 : 401–412.
12. AustinMJ, MuskettP, KahnK, FeysBJ, JonesJDG, et al. (2002) Regulatory Role of SGT1 in Early R Gene-Mediated Plant Defenses. Science 295 : 2077–2080.
13. AzevedoC, SadanandomA, KitagawaK, FreialdenhovenA, ShirasuK, et al. (2002) The RAR1 Interactor SGT1, an Essential Component of R Gene-Triggered Disease Resistance. Science 295 : 2073–2076.
14. HubertDA, TorneroP, BelkhadirY, KrishnaP, TakahashiA, et al. (2003) Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J 22 : 5679–5689.
15. MuskettPR, KahnK, AustinMJ, MoisanLJ, SadanandomA, et al. (2002) Arabidopsis RAR1 Exerts Rate-Limiting Control of R Gene–Mediated Defenses against Multiple Pathogens. The Plant Cell 14 : 979–992.
16. TakahashiA, CasaisC, IchimuraK, ShirasuK (2003) HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proceedings of the National Academy of Sciences 100 : 11777–11782.
17. TörM, GordonP, CuzickA, EulgemT, SinapidouE, et al. (2002) Arabidopsis SGT1b Is Required for Defense Signaling Conferred by Several Downy Mildew Resistance Genes. The Plant Cell 14 : 993–1003.
18. TorneroP, MerrittP, SadanandomA, ShirasuK, InnesRW, et al. (2002) RAR1 and NDR1 Contribute Quantitatively to Disease Resistance in Arabidopsis, and Their Relative Contributions Are Dependent on the R Gene Assayed. The Plant Cell 14 : 1005–1015.
19. ShirasuK (2009) The HSP90-SGT1 Chaperone Complex for NLR Immune Sensors. Annual Review of Plant Biology 60 : 139–164.
20. KadotaY, ShirasuK, GueroisR (2010) NLR sensors meet at the SGT1 HSP90 crossroad. Trends in biochemical sciences 35 : 199–207.
21. HubertDA, HeY, McNultyBC, TorneroP, DanglJL (2009) Specific Arabidopsis HSP90.2 alleles recapitulate RAR1 cochaperone function in plant NB–LRR disease resistance protein regulation. Proceedings of the National Academy of Sciences 106 : 9556–9563.
22. ZhangM, KadotaY, ProdromouC, ShirasuK, PearlLH (2010) Structural Basis for Assembly of Hsp90-Sgt1-CHORD Protein Complexes: Implications for Chaperoning of NLR Innate Immunity Receptors. Molecular Cell 39 : 269–281.
23. da Silva CorreiaJ, MirandaY, LeonardN, UlevitchR (2007) SGT1 is essential for Nod1 activation. Proceedings of the National Academy of Sciences 104 : 6764–6769.
24. MayorA, MartinonF, De SmedtT, PetrilliV, TschoppJ (2007) A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol 8 : 497–503.
25. HoltBF3rd, BelkhadirY, DanglJL (2005) Antagonistic control of disease resistance protein stability in the plant immune system. Science 309 : 929–932.
26. BrowseJ (2009) Jasmonate Passes Muster: A Receptor and Targets for the Defense Hormone. Annual Review of Plant Biology 60 : 183–205.
27. NomuraK, MelottoM, HeS-Y (2005) Suppression of host defense in compatible plant-Pseudomonas syringae interactions. Current Opinion in Plant Biology 8 : 361–368.
28. ChiniA, FonsecaS, FernandezG, AdieB, ChicoJM, et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448 : 666–671.
29. ThinesB, KatsirL, MelottoM, NiuY, MandaokarA, et al. (2007) JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 448 : 661–665.
30. KatsirL, SchilmillerAL, StaswickPE, HeSY, HoweGA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proceedings of the National Academy of Sciences 105 : 7100–7105.
31. SheardLB, TanX, MaoH, WithersJ, Ben-NissanG, et al. (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468 : 400–405.
32. XieD-X, FeysBF, JamesS, Nieto-RostroM, TurnerJG (1998) COI1: An Arabidopsis Gene Required for Jasmonate-Regulated Defense and Fertility. Science 280 : 1091–1094.
33. FeysBJF, BenedettiCE, PenfoldCN, TurnerJG (1994) Arabidopsis Mutants Selected for Resistance to the Phytotoxin Coronatine Are Male Sterile, Insensitive to Methyl Jasmonate, and Resistant to a Bacterial Pathogen. The Plant Cell 6 : 751–759.
34. ThommaBPHJ, EggermontK, PenninckxIAMA, Mauch-ManiB, VogelsangR, et al. (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proceedings of the National Academy of Sciences 95 : 15107–15111.
35. McDowellJM, CuzickA, CanC, BeynonJ, DanglJL, et al. (2000) Downy mildew (Peronospora parasitica) resistance genes in Arabidopsis vary in functional requirements for NDR1, EDS1, NPR1 and salicylic acid accumulation. The Plant Journal 22 : 523–529.
36. Norman-SetterbladC, VidalS, PalvaET (2000) Interacting Signal Pathways Control Defense Gene Expression in Arabidopsis in Response to Cell Wall-Degrading Enzymes from Erwinia carotovora. Molecular Plant-Microbe Interactions 13 : 430–438.
37. KloekAP, VerbskyML, SharmaSB, SchoelzJE, VogelJ, et al. (2001) Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. The Plant Journal 26 : 509–522.
38. MelottoM, MeceyC, NiuY, ChungHS, KatsirL, et al. (2008) A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine - and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. The Plant Journal 55 : 979–988.
39. ThatcherLF, MannersJM, KazanK (2009) Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis. The Plant Journal 58 : 927–939.
40. LorenzoO, ChicoJM, Sánchez-SerranoJJ, SolanoR (2004) JASMONATE-INSENSITIVE1 Encodes a MYC Transcription Factor Essential to Discriminate between Different Jasmonate-Regulated Defense Responses in Arabidopsis. The Plant Cell 16 : 1938–1950.
41. VijayanP, ShockeyJ, LévesqueCA, CookRJ, BrowseJ (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proceedings of the National Academy of Sciences 95 : 7209–7214.
42. XuL, LiuF, LechnerE, GenschikP, CrosbyWL, et al. (2002) The SCFCOI1 Ubiquitin-Ligase Complexes Are Required for Jasmonate Response in Arabidopsis. The Plant Cell 14 : 1919–1935.
43. YanJ, ZhangC, GuM, BaiZ, ZhangW, et al. (2009) The Arabidopsis CORONATINE INSENSITIVE1 Protein Is a Jasmonate Receptor. The Plant Cell 21 : 2220–2236.
44. Fernández-CalvoP, ChiniA, Fernández-BarberoG, ChicoJM, Gimenez-IbanezS, et al. (2011) The Arabidopsis bHLH Transcription Factors MYC3 and MYC4 Are Targets of JAZ Repressors and Act Additively with MYC2 in the Activation of Jasmonate Responses. The Plant Cell 23 : 701–715.
45. EllisC, TurnerJ (2002) A conditionally fertile coi1 allele indicates cross-talk between plant hormone signalling pathways in Arabidopsis thaliana seeds and young seedlings. Planta 215 : 549–556.
46. BelangerKD, KennaMA, WeiS, DavisLI (1994) Genetic and physical interactions between Srp1p and nuclear pore complex proteins Nup1p and Nup2p. J Cell Biol 126 : 619–630.
47. LarkinJC, WalkerJD, Bolognesi-WinfieldAC, GrayJC, WalkerAR (1999) Allele-Specific Interactions Between ttg and gl1 During Trichome Development in Arabidopsis thaliana. Genetics 151 : 1591–1604.
48. Van Der BiezenEA, FreddieCT, KahnK, ParkerJE, JonesJDG (2002) Arabidopsis RPP4 is a member of the RPP5 multigene family of TIR-NB–LRR genes and confers downy mildew resistance through multiple signalling components. The Plant Journal 29 : 439–451.
49. BieriS, MauchS, ShenQ-H, PeartJ, DevotoA, et al. (2004) RAR1 Positively Controls Steady State Levels of Barley MLA Resistance Proteins and Enables Sufficient MLA6 Accumulation for Effective Resistance. The Plant Cell 16 : 3480–3495.
50. BoyesDC, NamJ, DanglJL (1998) The Arabidopsis thaliana RPM1 disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response. Proceedings of the National Academy of Sciences 95 : 15849–15854.
51. KachrooA, LapchykL, FukushigeH, HildebrandD, KlessigD, et al. (2003) Plastidial Fatty Acid Signaling Modulates Salicylic Acid – and Jasmonic Acid–Mediated Defense Pathways in the Arabidopsis ssi2 Mutant. The Plant Cell 15 : 2952–2965.
52. ShiranoY, KachrooP, ShahJ, KlessigDF (2002) A Gain-of-Function Mutation in an Arabidopsis Toll Interleukin1 Receptor–Nucleotide Binding Site–Leucine-Rich Repeat Type R Gene Triggers Defense Responses and Results in Enhanced Disease Resistance. The Plant Cell 14 : 3149–3162.
53. YangS, HuaJ (2004) A Haplotype-Specific Resistance Gene Regulated by BONZAI1 Mediates Temperature-Dependent Growth Control in Arabidopsis. The Plant Cell 16 : 1060–1071.
54. GrayWM, MuskettPR, ChuangH-w, ParkerJE (2003) Arabidopsis SGT1b Is Required for SCFTIR1-Mediated Auxin Response. The Plant Cell 15 : 1310–1319.
55. KunkelBN, BrooksDM (2002) Cross talk between signaling pathways in pathogen defense. Current Opinion in Plant Biology 5 : 325–331.
56. ZipfelC, RobatzekS, NavarroL, OakeleyEJ, JonesJDG, et al. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428 : 764–767.
57. ChungE-H, da CunhaL, WuA-J, GaoZ, CherkisK, et al. (2011) Specific Threonine Phosphorylation of a Host Target by Two Unrelated Type III Effectors Activates a Host Innate Immune Receptor in Plants. Cell Host & Microbe 9 : 125–136.
58. PauwelsL, GoossensA (2011) The JAZ proteins: a crucial interface in the jasmonate signaling cascade. The Plant Cell 23 : 3089–3100.
59. LongJA, OhnoC, SmithZR, MeyerowitzEM (2006) TOPLESS Regulates Apical Embryonic Fate in Arabidopsis. Science 312 : 1520–1523.
60. DieavartA, DalalM, TaxFE, LaceyAD, HuttlyA, et al. (2003) CLAVATA1 Dominant-Negative Alleles Reveal Functional Overlap between Multiple Receptor Kinases That Regulate Meristem and Organ Development. The Plant Cell 15 : 1198–1211.
61. SijacicP, WangW, LiuZ (2011) Recessive Antimorphic Alleles Overcome Functionally Redundant Loci to Reveal TSO1 Function in Arabidopsis Flowers and Meristems. PLoS Genet 7: e1002352 doi:10.1371/journal.pgen.1002352
62. WürschumT, Groß-HardtR, LauxT (2006) APETALA2 Regulates the Stem Cell Niche in the Arabidopsis Shoot Meristem. The Plant Cell 18 : 295–307.
63. KimT-s, KimWY, FujiwaraS, KimJ, ChaJ-Y, et al. (2011) HSP90 functions in the circadian clock through stabilization of the client F-box protein ZEITLUPE. Proceedings of the National Academy of Sciences 108 : 16843–16848.
64. LiuY, SchiffM, SerinoG, DengXW, Dinesh-KumarSP (2002) Role of SCF ubiquitin-ligase and the COP9 signalosome in the N gene-mediated resistance response to Tobacco mosaic virus. The Plant Cell 14 : 1483–1496.
65. LyapinaS, CopeG, ShevchenkoA, SerinoG, TsugeT, et al. (2001) Promotion of NEDD8-CUL1 Conjugate Cleavage by COP9 Signalosome. Science 292 : 1382–1385.
66. ChengYT, LiY, HuangS, HuangY, DongX, et al. (2011) Stability of plant immune-receptor resistance proteins is controlled by SKP1-CULLIN1-F-box (SCF)-mediated protein degradation. Proceedings of the National Academy of Sciences 108 : 14694–14699.
67. GrantMR, GodiardL, StraubeE, AshfieldT, LewaldJ, et al. (1995) Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269 : 843–846.
68. WarrenRF, HenkA, MoweryP, HolubE, InnesRW (1998) A Mutation within the Leucine-Rich Repeat Domain of the Arabidopsis Disease Resistance Gene RPS5 Partially Suppresses Multiple Bacterial and Downy Mildew Resistance Genes. The Plant Cell 10 : 1439–1452.
69. MindrinosM, KatagiriF, YuG-L, AusubelFM (1994) The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell 78 : 1089–1099.
70. NakagawaT, KuroseT, HinoT, TanakaK, KawamukaiM, et al. (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. Journal of Bioscience and Bioengineering 104 : 34–41.
71. RitterC, DanglJL (1996) Interference between Two Specific Pathogen Recognition Events Mediated by Distinct Plant Disease Resistance Genes. The Plant Cell 8 : 251–257.
72. TorneroP, DanglJL (2001) A high-throughput method for quantifying growth of phytopathogenic bacteria in Arabidopsis thaliana. The Plant Journal 28 : 475–481.
73. CaoY, DaiY, CuiS, MaL (2008) Histone H2B Monoubiquitination in the Chromatin of FLOWERING LOCUS C Regulates Flowering Time in Arabidopsis. The Plant Cell 20 : 2586–2602.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 10- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- The Germline Genome Provides a Niche for Intragenic Parasitic DNA: Evolutionary Dynamics of Internal Eliminated Sequences
- Classical Genetics Meets Next-Generation Sequencing: Uncovering a Genome-Wide Recombination Map in
- Calpain-5 Mutations Cause Autoimmune Uveitis, Retinal Neovascularization, and Photoreceptor Degeneration
- Cofilin-1: A Modulator of Anxiety in Mice
- The Date of Interbreeding between Neandertals and Modern Humans
- Embryos of Robertsonian Translocation Carriers Exhibit a Mitotic Interchromosomal Effect That Enhances Genetic Instability during Early Development
- Viral Evasion of a Bacterial Suicide System by RNA–Based Molecular Mimicry Enables Infectious Altruism
- Phosphatase-Dead Myotubularin Ameliorates X-Linked Centronuclear Myopathy Phenotypes in Mice
- Full-Length Synaptonemal Complex Grows Continuously during Meiotic Prophase in Budding Yeast
- MOV10 RNA Helicase Is a Potent Inhibitor of Retrotransposition in Cells
- A Likelihood-Based Framework for Variant Calling and Mutation Detection in Families
- The Contribution of RNA Decay Quantitative Trait Loci to Inter-Individual Variation in Steady-State Gene Expression Levels
- New Partners in Regulation of Gene Expression: The Enhancer of Trithorax and Polycomb Corto Interacts with Methylated Ribosomal Protein L12 Its Chromodomain
- Mining the Unknown: A Systems Approach to Metabolite Identification Combining Genetic and Metabolic Information
- Mutations in (Hhat) Perturb Hedgehog Signaling, Resulting in Severe Acrania-Holoprosencephaly-Agnathia Craniofacial Defects
- The Many Landscapes of Recombination in
- Faster-X Evolution of Gene Expression in
- Loss of Slc4a1b Chloride/Bicarbonate Exchanger Function Protects Mechanosensory Hair Cells from Aminoglycoside Damage in the Zebrafish Mutant
- Regulation of ATG4B Stability by RNF5 Limits Basal Levels of Autophagy and Influences Susceptibility to Bacterial Infection
- and the BTB Adaptor Are Key Regulators of Sleep Homeostasis and a Dopamine Arousal Pathway in Drosophila
- Mutation and Fetal Ethanol Exposure Synergize to Produce Midline Signaling Defects and Holoprosencephaly Spectrum Disorders in Mice
- Specific Missense Alleles of the Arabidopsis Jasmonic Acid Co-Receptor COI1 Regulate Innate Immune Receptor Accumulation and Function
- Deep Genome-Wide Measurement of Meiotic Gene Conversion Using Tetrad Analysis in
- Mismatch Repair Balances Leading and Lagging Strand DNA Replication Fidelity
- Distinguishing between Selective Sweeps from Standing Variation and from a Mutation
- Cytokinesis-Based Constraints on Polarized Cell Growth in Fission Yeast
- Deposition of Histone Variant H2A.Z within Gene Bodies Regulates Responsive Genes
- Functional Antagonism between Sas3 and Gcn5 Acetyltransferases and ISWI Chromatin Remodelers
- The SET-Domain Protein SUVR5 Mediates H3K9me2 Deposition and Silencing at Stimulus Response Genes in a DNA Methylation–Independent Manner
- Morphogenesis and Cell Fate Determination within the Adaxial Cell Equivalence Group of the Zebrafish Myotome
- Muscle-Specific Splicing Factors ASD-2 and SUP-12 Cooperatively Switch Alternative Pre-mRNA Processing Patterns of the ADF/Cofilin Gene in
- Maize Is Required for Maintaining Silencing Associated with Paramutation at the and Loci
- Increasing Signal Specificity of the TOL Network of mt-2 by Rewiring the Connectivity of the Master Regulator XylR
- Use of Pleiotropy to Model Genetic Interactions in a Population
- RAB-Like 2 Has an Essential Role in Male Fertility, Sperm Intra-Flagellar Transport, and Tail Assembly
- Variants Affecting Exon Skipping Contribute to Complex Traits
- Topoisomerase II– and Condensin-Dependent Breakage of -Sensitive Fragile Sites Occurs Independently of Spindle Tension, Anaphase, or Cytokinesis
- Comparison of Family History and SNPs for Predicting Risk of Complex Disease
- Recovery of Arrested Replication Forks by Homologous Recombination Is Error-Prone
- A Mutation in the Gene Causes Alternative Splicing Defects and Deafness in the Bronx Waltzer Mouse
- Comparative Genomics Suggests an Independent Origin of Cytoplasmic Incompatibility in
- It Was Heaven: An Interview with Evelyn Witkin
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Mutation in the Gene Causes Alternative Splicing Defects and Deafness in the Bronx Waltzer Mouse
- Mutations in (Hhat) Perturb Hedgehog Signaling, Resulting in Severe Acrania-Holoprosencephaly-Agnathia Craniofacial Defects
- Classical Genetics Meets Next-Generation Sequencing: Uncovering a Genome-Wide Recombination Map in
- Regulation of ATG4B Stability by RNF5 Limits Basal Levels of Autophagy and Influences Susceptibility to Bacterial Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



