-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mutator Suppression and Escape from Replication Error–Induced Extinction in Yeast
Cells rely on a network of conserved pathways to govern DNA replication fidelity. Loss of polymerase proofreading or mismatch repair elevates spontaneous mutation and facilitates cellular adaptation. However, double mutants are inviable, suggesting that extreme mutation rates exceed an error threshold. Here we combine alleles that affect DNA polymerase δ (Pol δ) proofreading and mismatch repair to define the maximal error rate in haploid yeast and to characterize genetic suppressors of mutator phenotypes. We show that populations tolerate mutation rates 1,000-fold above wild-type levels but collapse when the rate exceeds 10−3 inactivating mutations per gene per cell division. Variants that escape this error-induced extinction (eex) rapidly emerge from mutator clones. One-third of the escape mutants result from second-site changes in Pol δ that suppress the proofreading-deficient phenotype, while two-thirds are extragenic. The structural locations of the Pol δ changes suggest multiple antimutator mechanisms. Our studies reveal the transient nature of eukaryotic mutators and show that mutator phenotypes are readily suppressed by genetic adaptation. This has implications for the role of mutator phenotypes in cancer.
Published in the journal: . PLoS Genet 7(10): e32767. doi:10.1371/journal.pgen.1002282
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002282Summary
Cells rely on a network of conserved pathways to govern DNA replication fidelity. Loss of polymerase proofreading or mismatch repair elevates spontaneous mutation and facilitates cellular adaptation. However, double mutants are inviable, suggesting that extreme mutation rates exceed an error threshold. Here we combine alleles that affect DNA polymerase δ (Pol δ) proofreading and mismatch repair to define the maximal error rate in haploid yeast and to characterize genetic suppressors of mutator phenotypes. We show that populations tolerate mutation rates 1,000-fold above wild-type levels but collapse when the rate exceeds 10−3 inactivating mutations per gene per cell division. Variants that escape this error-induced extinction (eex) rapidly emerge from mutator clones. One-third of the escape mutants result from second-site changes in Pol δ that suppress the proofreading-deficient phenotype, while two-thirds are extragenic. The structural locations of the Pol δ changes suggest multiple antimutator mechanisms. Our studies reveal the transient nature of eukaryotic mutators and show that mutator phenotypes are readily suppressed by genetic adaptation. This has implications for the role of mutator phenotypes in cancer.
Introduction
Accurate DNA replication ensures the faithful transmission of genetic information between mother and daughter cells. To accomplish this important task, organisms have evolved a network of conserved pathways that govern DNA replication fidelity (reviewed in [1]). Polymerase proofreading and postreplication mismatch repair (MMR) are key determinants of fidelity, functioning to correct errors introduced by DNA polymerases during cell division (reviewed in [2]–[4]). Defects in these and other DNA repair pathways produce mutator phenotypes, which are characterized by increased rates of spontaneous mutation.
Mutator phenotypes arise spontaneously in nature and have mixed biological consequences (reviewed in [5]–[10]). In Escherichia coli and other bacteria, changing environmental conditions favor high mutation rates, which increase the likelihood of genetic adaptation [11]–[16]. However, after adaptation, mutator bacteria progressively lose fitness as they accumulate deleterious mutations in other genes [14], [17], and clones with lower mutation rates can evolve from mutator populations [14], [16], [18]–[20]. Thus, mutation rates in E. coli rise and fall as populations cycle through periods of adaptive and non-adaptive growth.
Mutators also impact eukaryotes. In mammals, mutator phenotypes fuel oncogenesis by providing the genetic diversity necessary for emergence of malignant clones [21], [22]. Many sporadic human tumors show signs of an elevated mutation rate [23], and inherited defects in polymerase proofreading [24]–[26] or MMR (reviewed in [27], [28]) confer mutator phenotypes and increase cancer risk. In the budding yeast Saccharomyces cerevisiae, loss of proofreading or MMR also elevates spontaneous mutation [29]–[34], and defective MMR can facilitate adaptation to changing environments [35], [36].
Similar to bacteria, eukaryotic mutator alleles are detrimental in the long-term. Deleterious mutations accumulate faster in mutator compared to non-mutator yeast strains [37], [38], and mutators eventually become extinct in a mutational meltdown process after serial passage through population bottlenecks [39]. This decline is accelerated in yeast with extreme mutation rates. Diploids that are homozygous defective for both proofreading and MMR grow slowly and have mutations rates that are elevated 10,000-times above wild-type levels [40]–[42]. Double-mutant spores germinate but arrest at various cell-cycle stages after 6–7 mitotic divisions [40], suggesting that the accumulation of DNA replication errors drives the extinction of haploid mutator strains.
Here, we experimentally define the threshold of error-induced extinction in haploid S. cerevisiae and show that cells readily escape extinction via genetic suppression. These escape mutants emerge rapidly and carry second-site mutations that suppress the mutator phenotype. Our findings show that mutators are intrinsically unstable and that spontaneous suppressors moderate high mutation rates in yeast.
Results
Abrupt Loss in Viability with Increased Mutation Rate
To obtain a range of mutator strains suitable for defining the maximal mutation rate in yeast, we conducted a mutagenesis screen of the POL3 gene, which encodes the catalytic subunit of DNA polymerase δ (Pol δ). We used a plasmid shuffling strategy [29], [43] to introduce mutated pol3 alleles into strains proficient (MSH6) or deficient (msh6Δ) for MMR (Figure S1). The screen focused on conserved residues in the proofreading exonuclease domain of Pol δ [44], [45] that, when mutated in msh6Δ cells, are expected to preferentially increase base-substitution and ±1 frameshift mutations [40], [46]–[49].
Our analysis identified 21 amino acid substitutions in the Pol δ proofreading domain that individually conferred a range of increased spontaneous mutation frequencies (Figure S2). These alleles had no observable effect on colony formation in MSH6 cells. However, four alleles (pol3-01, pol3-F406A, pol3-D407A and pol3-Y516F) did not yield visible colonies when shuffled into msh6Δ cells. This result is consistent with previous reports of synthetic lethality between the proofreading-deficient pol3-01 allele and MMR-defective alleles pms1Δ, msh2Δ, or msh6Δ [40], [41], [50].
To determine whether the loss of growth capacity correlated with mutator strength, we quantified the spontaneous mutation rates of a subset of pol3 alleles in the presence or absence of MSH6 (Figure 1A). Alleles that imparted a 2 - to 8-fold increase in the mutation rate of MSH6 cells (R459A, G400A, Y401A, D396A, Y410A, K491R and D463A) were compatible with survival when MSH6 was deleted. These pol3 msh6Δ double-mutants had mutation rates that were 15 - to 150-times greater than the corresponding pol3 MSH6 strains, consistent with synergy between pol3 mutators and msh6Δ. In contrast, pol3 alleles that increased the mutation rate 25 - to 50-fold in MSH6 cells (D407A, pol3-01, Y516F and F406A) conferred a loss of colony-forming capacity in msh6Δ cells (<1 colony/105 cells plated; Figure 1B). Thus, the transition to no colony formation occurred over a narrow range of increasing mutation rates. This abrupt loss of growth capacity implies the existence of a threshold for error-induced extinction.
Fig. 1. Evidence for a threshold of error-induced extinction. 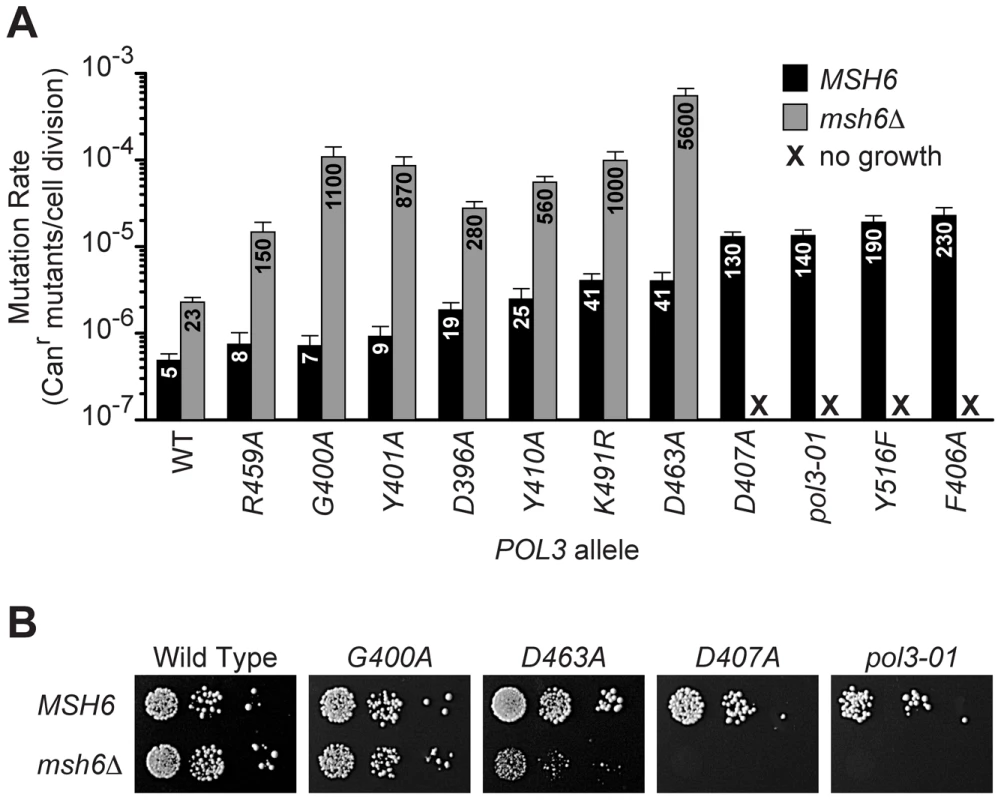
A) Entry into error-induced extinction. Mutated pol3 alleles were introduced into haploid MSH6 and msh6Δ yeast by plasmid shuffling (Figure S1), and mutation rates were measured by fluctuation assays and calculated using the maximum likelihood method. Each bar represents the spontaneous mutation rate, expressed as canavanine-resistant (Canr) mutants per cell division, conferred by a specific POL3 allele in MSH6 or msh6Δ cells. Mutation rate values (x 10−7) are indicated on each column. Error bars show 95% confidence intervals. WT, wild-type POL3; black, MSH6; gray, msh6Δ; X, no growth. B) Synthetic lethality of strong pol3 mutator alleles with msh6Δ. Serial dilutions of haploid yeast containing POL3–URA3 and pol3–LEU2 plasmids were plated on SC FOA medium to select for cells that spontaneously lost POL3–URA3. Similar numbers of cells (∼105, 104 and 103) were plated for each set of alleles in the MSH6 and msh6Δ strains. Failed growth of msh6Δ cells carrying pol3-D407A or pol3-01 indicates synthetic lethality (right two panels). pol3-F406A and pol3-Y516F also failed to support colony formation in msh6Δ cells (not shown). Note the small size of pol3-D463A msh6Δ colonies. Mutants That Escape Error-Induced Extinction
During our shuffling experiments, we observed occasional colonies that escaped pol3 msh6Δ synthetic lethality (Figure 2A). We speculated that second-site changes in Pol δ might rescue yeast from error-induced extinction by increasing DNA replication fidelity and thereby reducing the spontaneous mutation burden. To test this idea, we sequenced pol3 plasmids from error-induced extinction (eex) mutants that escaped synthetic lethality between msh6Δ and pol3-01, pol3-F406A or pol3-D407A. The plasmids retained the original pol3 mutator alleles, but also harbored additional second-site mutations in each pol3 sequence. Our initial experiment yielded three eex mutants encoding single amino-acid substitutions in Pol δ (E594G or W821C in pol3-01; T711P in pol3-D407A) and two mutants with multiple substitutions (K689E, S725L and I1076V in pol3-F406A; R470C and T655A in pol3-D407A). Another mutation (A894G) was found in a large-colony variant of pol3-D463A msh6Δ cells. When the second-site eex mutations were re-engineered into new plasmids together with their corresponding mutator alleles (pol3-01, pol3-F406A or pol3-D407A), they rescued colony-forming capacity in msh6Δ cells and decreased the mutation rate of MSH6 cells 10 - to 33-fold (Figure 2B). The eex mutations appeared to be functionally interchangeable; T711P (the pol3-D407A suppressor) also rescued pol3-01 msh6Δ lethality, and either T711P or E594G (a pol3-01 suppressor) restored normal growth to pol3-D463A msh6Δ cells. Considered together, these initial findings suggested that eex mutations within POL3 confer escape from error-induced extinction by exerting an antimutator phenotype.
Fig. 2. Escape from error-induced extinction. 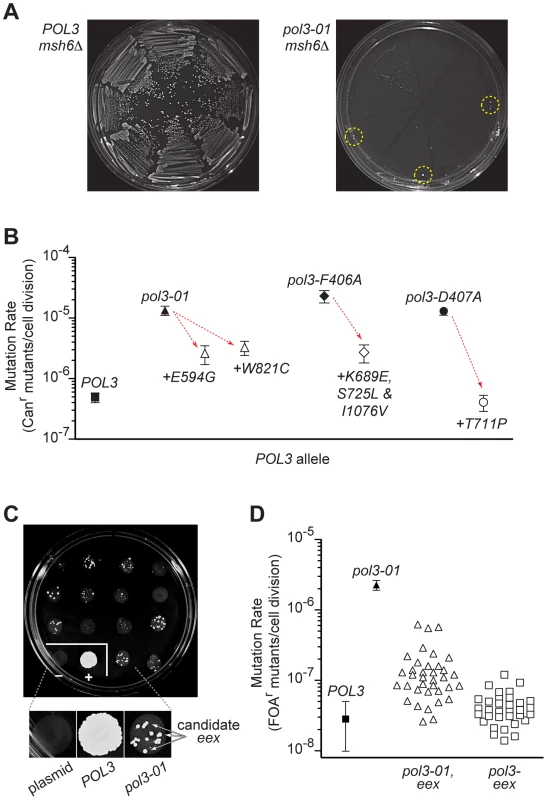
A) Emergence of colonies that escape pol3-01 msh6Δ synthetic lethality. Each segment of an FOA-containing SC plate (eight segments per plate) was streaked with an individual colony of POL3–LEU2 POL3–URA3 msh6Δ (left) or pol3-01–LEU2 POL3–URA3 msh6Δ (right) cells to select for loss of the POL3–URA3 plasmid (see Figure S1). Resultant POL3 msh6Δ cells formed abundant visible colonies (left), whereas pol3-01 msh6Δ cells did not (right). Colonies that escape pol3-01 msh6Δ synthetic lethality (eex mutants) arose at low frequency near the outer margins of the plate (circled) where cell densities were highest. Similar results were seen when pol3-F406A or pol3-D407A were shuffled into msh6Δ cells (not shown). B) Antimutator effects of eex alleles encoding second-site changes in Pol δ. Rates of spontaneous mutation to canavanine-resistance (Canr) conferred by pol3-01, pol3-F406A or pol-D407A alone (filled symbols) and combined with intragenic eex alleles (open symbols) were determined in MSH6 cells. Downward arrows illustrate the reduction in mutation rates (i.e., the antimutator effect) caused by the second-site, amino-acid substitutions indicated beneath each datum point. Error bars show 95% confidence intervals. C) Representative plate from large-scale screen for eex mutants. Approximately 106 cells from multiple independent pol3-01–LEU POL3–URA msh6Δ parent colonies were plated separately in ∼1-cm spots on FOA-containing SC medium. LEU-only and POL3–LEU plasmids were also shuffled into msh6Δ cells as controls. FOA-resistant colonies arose at varied frequencies from each parent clone. Insert, magnified view showing colonies that are candidate eex mutants. plasmid, LEU-only plasmid with no POL3 gene. D) Mutation rates of eex mutants. Rates of spontaneous mutation to FOA-resistance (FOAr) were measured in a MMR-proficient strain with a chromosomal URA3 reporter gene. Each datum point represents a different POL3 allele. Mutation rates were determined from multiple independent fluctuation analyses of each allele. Confidence intervals (95%) are shown for POL3 and pol3-01. Mutation rates and confidence intervals of individual eex alleles are in Table 1 and Table 2. To obtain a broader view of escape mechanisms, we performed a large-scale screen for mutants that suppress the synthetic lethality between pol3-01 and msh6Δ (Figure 2C). Mutants emerged from nearly every pol3-01 msh6Δ parent clone, and there was wide fluctuation in the number and size of mutant colonies, suggesting that escape variants arise randomly prior to selection on FOA. We isolated 113 independent eex mutants (Table S1). Seventy-four of these eex mutants carried pol3-01 plasmids that still conferred lethality in a fresh msh6Δ strain. We infer that these mutants harbor mutations in chromosomal genes that influence DNA replication fidelity. The remaining 39 eex mutants carried pol3-01 plasmids that did not cause synthetic lethality when isolated and independently re-shuffled into msh6Δ cells. DNA sequencing of these plasmids showed that, in addition to the pol3-01 allele, each plasmid contained a different secondary mutation in pol3. These secondary mutations encoded single amino-acid changes in Pol δ (Figure 3 and Figure 4) and rescued colony-forming capacity when engineered de novo into pol3-01 plasmids and shuffled into naïve msh6Δ cells. Consistent with our initial experiment, all of these intragenic eex mutations suppressed the pol3-01 mutator phenotype, as measured at two different genetic loci (Figure 2D and Table 1). The weakest eex alleles suppressed mutation rates three-fold, while the strongest suppressors lowered mutation rates to wild-type levels (Figure 2D). Thus, cells escape pol3-01 msh6Δ lethality by acquiring any one of a variety of second-site mutations that suppress the mutator effect of Pol δ proofreading deficiency.
Fig. 3. Amino-acid changes in Pol δ eex mutants. 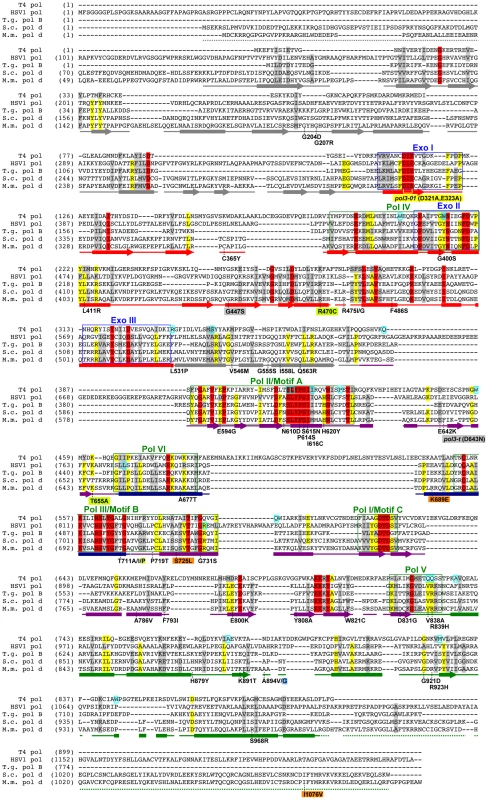
Aligned sequences of five B-family DNA polymerases: Saccharomyces cerevisiae Pol δ (S.c. pol d), Mus musculus Pol δ (M.m. pol d), Thermococcus gorgonarius (T.g. pol B), bacteriophage T4 (T4 pol), and herpes simplex virus 1 (HSV1 pol). Secondary structural elements of yeast Pol δ [74] are indicated below the alignment and color coded to depict their domain locations (see Figure 4A): rectangles, α-helices; arrows, β-strands; solid lines, loops; dotted lines, structure not solved. Conserved polymerase (Pol) and exonuclease (Exo) motifs are framed [44], [45], [120]. Amino-acid substitutions of interest in yeast Pol δ are placed underneath the alignment at the relevant positions, highlighted according to the following scheme: no highlight, pol3-01,eex mutations; green, pol3-D407A,eex mutations (R470C and T655A in one mutant, T711P in another); orange, pol3-F406A,eex mutations (three substitutions in the same mutant); blue, A894G mutation that rescued slow growth of pol3-D463A msh6Δ cells; yellow, pol3-01 (D321A,E323A); gray, pol3-t (D643N) and G447S (previously identified antimutator alleles; [121], [122]). Residues that increase polymerase fidelity when mutated in T4 or HSV1 are indicated by aqua boxes in the alignment [4], [84]-[89], [123]. Fig. 4. Locations of eex amino-acid substitutions in the S. cerevisiae Pol δ structure. 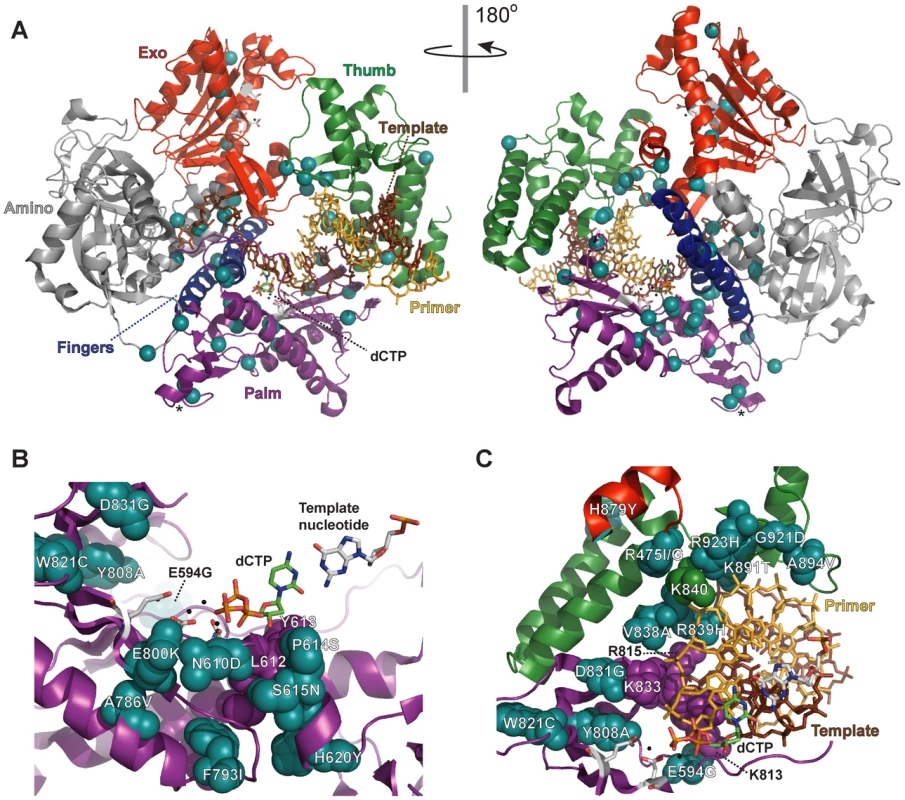
A) Overall distribution of eex amino-acid substitutions. The catalytic subunit of yeast Pol δ is shown as a ribbon diagram with color-coded structural domains: amino, gray; exonuclease (Exo), red; palm, purple; fingers, blue; thumb, green. Other important elements are indicated as follows: DNA template strand, brown sticks; DNA primer strand, yellow sticks; incoming dCTP, green CPK sticks; metal ions, small black spheres; active-site residues, gray CPK sticks extending out from the α-carbon backbone in the palm and exonuclease domains. Residues changed by eex mutations are shown as light blue spheres. The asterisks mark adjacent E642K and D643N eex substitutions located on the solvent-exposed surface of Pol δ. Structure from [74] (Protein Data Bank accession code 3IAY). B) Amino-acid substitutions near the polymerase active site. Palm domain eex residues are shown as space-filling spheres (light blue) and labeled to indicate the amino-acid substitutions. Important non-mutated residues proximal to the eex substitutions are also shown as space-filling spheres (purple). The fingers and thumb domains have been removed for clarity. C) Amino-acid substitutions in the DNA binding track. View looking down the DNA helical axis. The primer (yellow) and template (brown) are held by a series of interactions along the DNA minor groove. eex residues are light blue. Amino acids positioned by eex residues and minor-groove ‘sensing’ residues in the palm domain (K813, K814 and R815; [74]) are shown as space-filling spheres colored according to domain as in panel (A). The three unpaired 5′ nucleotides of the template have been removed for clarity. Tab. 1. Mutation Rates (×10−7) of pol3-01,eex Mutant Strains. 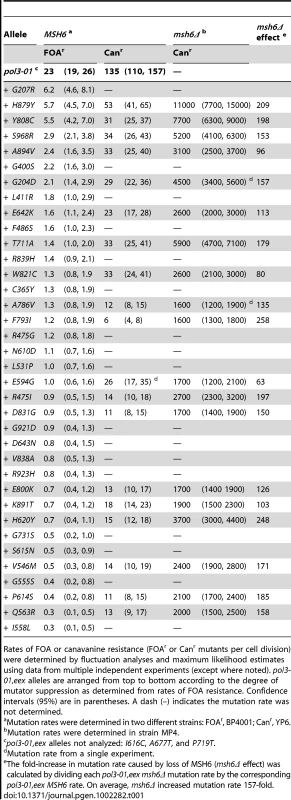
Rates of FOA or canavanine resistance (FOAr or Canr mutants per cell division) were determined by fluctuation analyses and maximum likelihood estimates using data from multiple independent experiments (except where noted). pol3-01,eex alleles are arranged from top to bottom according to the degree of mutator suppression as determined from rates of FOA resistance. Confidence intervals (95%) are in parentheses. A dash (–) indicates the mutation rate was not determined. Contributions of MMR and Proofreading to DNA Replication Fidelity In Vivo
The eex mutants provided an opportunity to assess the proportion of Pol δ errors that are repaired by proofreading and MMR in vivo. Taking advantage of the viability of pol3-01,eex msh6Δ cells, we first compared the mutation rates of isogenic strains that lack Pol δ proofreading and differ only in their MMR activity. The average increase in mutation rate in pol3-01,eex strains after deletion of MSH6 was 157-fold (Table 1), consistent with Msh6-dependent repair of greater than 99% of the errors generated by proofreading-deficient Pol δ. As expected from the mutation biases conferred by pol3-01 or msh6Δ alone [40], [46]–[48], spontaneous mutations in pol3-01,eex msh6Δ strains were almost exclusively base substitutions (Figure S3 and Table S2). Thus, our estimate primarily reflects the efficiency of base-base mismatch repair. This estimate is an average across multiple scoreable sites in CAN1; MMR efficiencies at individual sites may vary widely [51].
To similarly estimate the efficiency of Pol δ proofreading in vivo, we initially determined the influence of eex alleles on mutation rates in the presence of proofreading. Most MMR-proficient pol3-eex strains had no discernable mutator phenotype (Figure 2D, Table 2). However, in the absence of MSH6 many of the pol3-eex alleles produced slightly higher mutation rates than the POL3 msh6Δ control (Table 2). These alleles were excluded from our analysis, because their weak mutator phenotypes may result from altered partitioning or other defects that reduce proofreading efficiency [4], [52]. Eight pol3-eex msh6Δ strains exhibited mutation rates within two-fold of POL3 msh6Δ: G204D, H620Y, T711A, E594G, Y808C, W821C, H879Y, and S968R. The mutation rates of these pol3-eex msh6Δ strains were compared to rates of the corresponding pol3-01,eex msh6Δ cells. This strategy allowed us to examine isogenic strains that differ only in their Pol δ proofreading activity and lack the masking effects of Msh6-mediated MMR. Proofreading deficiency increased mutation rates an average of 163-fold, indicating that the Pol δ exonuclease corrects greater than 99% of polymerase errors across the CAN1 reporter gene. Assuming Pol δ proofreading and Msh6-dependent MMR act in series [40], we estimate their combined contribution to DNA replication fidelity in yeast at greater than 104. Proofreading and MMR contribute similarly to replication fidelity in bacteria [53].
Tab. 2. Mutation Rates (×10−7) of pol3-eex Mutant Strains. 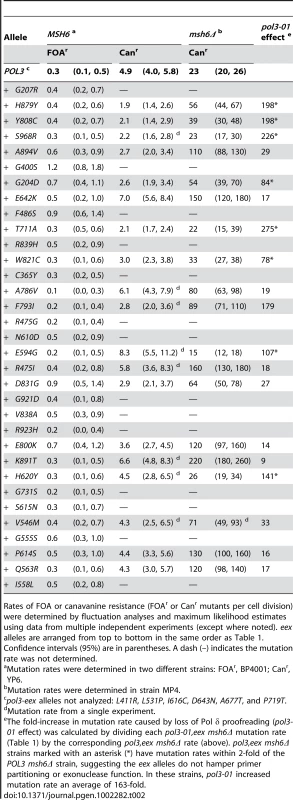
Rates of FOA or canavanine resistance (FOAr or Canr mutants per cell division) were determined by fluctuation analyses and maximum likelihood estimates using data from multiple independent experiments (except where noted). eex alleles are arranged from top to bottom in the same order as Table 1. Confidence intervals (95%) are in parentheses. A dash (–) indicates the mutation rate was not determined. Defining the Threshold of Error-Induced Extinction
The pol3-01,eex MMR-proficient strains formed colonies with similar size and efficiency as the POL3 control (Figure 5A, left). Thus, the corresponding eex mutant polymerases must suffice for the essential functions of Pol δ in replication [54]. However, in the absence of MSH6, pol3-01,eex alleles with the strongest mutator phenotypes impaired growth (Figure 5A, center). We used these mutants, together with synthetic-lethal alleles, to estimate the maximal mutation rate compatible with haploid yeast proliferation.
Fig. 5. Defining the threshold of error-induced extinction. 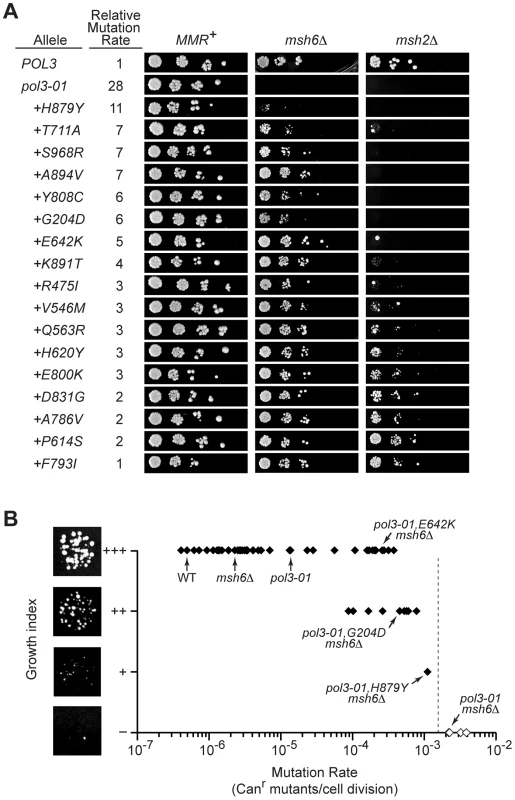
A) Plasmid shuffling experiments to reveal synthetic interactions between pol3-01,eex alleles and MMR mutations. Ten-fold serial dilutions of yeast containing POL3–URA3 and pol3–LEU2 plasmids were plated on FOA-containing SC medium to select for cells that spontaneously lost POL3–URA3. Similar numbers of colony forming units were plated for each set of alleles in the MMR+, msh6Δ and msh2Δ strains. Failed growth indicates synthetic lethality. Small colonies reflect slow-growth phenotypes. Relative mutation rates are the Canr mutation rates conferred by each pol3 allele relative to wild-type POL3 in MMR-proficient cells (see Table 1 and Table 2). Alleles are listed in decreasing order of mutator strengths. Some alleles with statistically similar mutation rates (as reflected by overlapping confidence intervals) have slightly different relative rates due to mathematical round-off. B) Relationship between growth capacity and CAN1 mutation rate for 62 haploid yeast strains. Colonies of pol3-01,E642K msh6Δ, pol3-01,G204D msh6Δ, pol3-01,H879Y msh6Δ, and pol3-01 msh6Δ cells are shown to illustrate wild-type (+++), moderately defective (++), severely defective (+), and failed (–) growth, respectively. The vertical dashed line indicates the estimated maximal mutation rate compatible with haploid yeast colony formation, which is our functional definition of the replication error threshold. Filled symbols, rates measured by fluctuation analyses. Open symbols, rates estimated as described in the text. Data in brackets with an asterisk (*) are pol3-01,T711A msh6Δ, pol3-01,S968R msh6Δ, pol3-01,G204D msh6Δ, and pol3-01,Y808C msh6Δ. The upper and lower limits of the maximal rate were determined as follows. First, we calculated the predicted mutation rates of msh6Δ strains harboring synthetically lethal pol3 alleles (pol3-01, pol3-F406A, pol3-D407A or pol3-Y516F) as the mutation rate of each pol3 MSH6 strain (Figure 1A) times 157 (the average increase in rate observed upon deletion of MSH6; see preceding section). These predicted rates ranged from 2×10−3 Canr mutants per cell division for pol3-01 msh6Δ and pol3-D407A msh6Δ to 4×10-3 for pol3-F406A msh6Δ (Figure 5B). Second, we determined the growth capacities of all mutator and suppressor strains in our collection using a semi-quantitative scale based on colony size. Wild-type colony-forming capacity (+++) was consistently observed at rates as high as 5×10−5 Canr mutants per cell division (Figure 5B). As the mutation rate exceeded 5×10-5, several strains exhibited a slow-growth phenotype (++), and a single strain (pol3-01,H879Y msh6Δ) showed a severe growth deficit (+) at a mutation rate of 1×10−3. These results demonstrate that the maximal mutation rate is reached when there are ∼10−3 inactivating mutations in CAN1 per cell division (Figure 5B). Rates exceeding this maximum result in a failure to form visible colonies (i.e., error-induced extinction).
If the observed decline in viability is due to an error threshold, additional mutation stress should exacerbate the growth defect. We introduced pol3-01,eex alleles into msh2Δ cells, which are defective in both Msh6 - and Msh3-mediated MMR and thus have Canr mutation rates that are 2 - to 3-fold higher than msh6Δ cells [3], [41], [47], [48]. Colonies were observed only in pol3-01 msh2Δ strains with the strongest mutator suppressor alleles (Figure 5A, right). Collectively, these data suggest that pol3-01 msh6Δ cells with weak mutator suppressors are on the edge of error-induced extinction and that eliminating MSH2 increases mutation rates beyond an extinction threshold. Although pol3-01 msh2Δ strains with strong mutator suppressors formed distinct colonies, these colonies were generally smaller and less uniform than the POL3 msh2Δ control. This variability in colony size suggests that viable pol3-01,eex msh2Δ cells quickly accumulate deleterious mutations that compromise replicative fitness. The observation that growth is impaired at mutation rates 10-times less than the 10−3 threshold (Figure 5B) suggests that accumulation of random mutations can impose a loss in fitness and shows that the growth capacity of haploid yeast declines even under conditions of non-lethal mutation burden.
Discussion
Mutators accelerate microbial adaptation and mammalian oncogenesis. However, the fitness cost of increased mutation imposes indirect selection pressure to reduce mutation rates. This counter-selection will occur after adaptation to a stable environment where conditions no longer favor the genetic potential of mutators. One possible mechanism to reduce mutation rates is the acquisition of compensatory alleles at modifier loci that suppress the mutator phenotype.
In this study, we took advantage of synergies between Pol δ proofreading and MMR to titrate yeast mutation rates up to lethal levels and study the fate of mutators under strong counter-selection. We found that msh6Δ cells carrying hypomorphic proofreading alleles abruptly lose viability over a narrow range of increasing mutation rates (Figure 1). Thus, cell survival requires both proofreading and MMR to limit potentially lethal mutations introduced by Pol δ. Mutant clones that escape this error-induced extinction arose spontaneously (Figure 2), frequently due to second-site changes in Pol δ (Figure 3 and Figure 4) that conferred antimutator phenotypes (Figure 2 and Table 1). Using our collection of mutator and antimutator strains, we found that the maximum mutation rate compatible with haploid yeast survival corresponds to ∼10-3 inactivating mutations in CAN1 per cell division (Figure 5). These studies provide evidence for an error threshold in yeast and demonstrate that genetic suppressors of error-prone replication spontaneously arise in eukaryotic mutator cells.
Below we consider error thresholds in relation to genetic complexity and mutational robustness, and we discuss potential mechanisms of mutator suppression.
Error Thresholds, Genetic Complexity, and Mutational Robustness
We observed loss of growth capacity when the CAN1 mutation rate exceeds ∼10−3 inactivating mutations per cell division (Figure 5). The yeast genome is comprised of ∼6000 genes (http://www.yeastgenome.org). Thus, a mutation rate of 10−3 corresponds to the random inactivation of ∼6 genes per cell per replication cycle (assuming CAN1 is typical). On average, one of these six mutations will involve a gene required for haploid cell viability [55], [56]. Thus, there is a high probability that cells above the maximal mutation rate will acquire a lethal mutation after a few cell divisions. The restoration of cell growth via antimutator alleles (Figure 2) supports this hypothesis. Stalled DNA synthesis at nascent 3′ mispairs [57] and S-phase checkpoint signalling [58] could also contribute to growth arrest in strong mutators. However, it is not evident how MMR defects would exacerbate 3′ mispair extension by Pol δ, and simultaneous loss of proofreading and MMR does not halt growth specifically in S-phase [40]. Rather, proofreading/MMR double mutants arrest with varied cell morphologies [40] that resemble those observed in a systematic promoter-repression screen of essential genes [59] (Figure S4). Considered together, the evidence suggests that random mutations in essential genes are a primary cause of error-induced extinction. Synthetic cooperative interactions of non-lethal alleles will also contribute as cells accrue multiple mutations [60], [61]. In a similar manner, bacteria exhibit a replication error threshold that correlates with the number of indispensable genes [62], suggesting that maximal mutation rates can be used to estimate the genetic complexity of vital pathways in other organisms.
Error thresholds are also evident in diploids. Although diploid genomes generally buffer cells against the deleterious effects of mutation accumulation [63], haploinsufficient alleles still pose a significant threat to fitness. In a comprehensive library of diploid yeast heterozygotes, up to 20% of the hemizygous mutant strains exhibit reduced fitness during growth competition [64]. Observations of mutation meltdown in MMR-deficient cells [39] and lethality conferred by a hyper-mutator Pol δ variant [65] argue that diploid yeast are subject to an error threshold. The combined loss of Pol δ proofreading and MMR is also synthetically lethal in mice [25]. Similar to the situation in yeast [40], mouse cells defective for both proofreading and MMR are initially viable but arrest after a limited number of mitotic divisions [25]. This cessation of growth presumably results from an accumulation of mutations in genes required for cell propagation and embryo development.
Although cells eventually succumb to error-induced extinction, they tolerate substantial increases in mutation rate before losing viability. This mutational robustness is apparent in yeast, E. coli and mouse cells (Figure 6). We show that haploid yeast tolerate more than a 1,000-fold increase in mutation rate before exhibiting overt loss of colony-forming capacity (Figure 5B), and a comparable increase in mutation rate is required to cause catastrophic errors in E. coli [62], suggesting that prokaryotes and haploid eukaryotes share similar degrees of robustness toward DNA polymerase errors. In comparison, diploid yeast and mouse cells retain replication capacity at mutation rates 10,000-times higher than wild-type levels (Figure 6; [25], [40], [66]). Thus, diploidy extends the threshold of error-induced cell death by five - to tenfold. These data suggest that cells can survive and persist during periods of high mutational loads. The maximal mutation rate will likely vary, depending on environmental conditions [67], [68], genetic redundancy [63], [69], [70], the plasticity of genetic interactions [71], [72], and the ability of cells to buffer deleterious changes in essential proteins [73].
Fig. 6. Mutational robustness of yeast, E. coli, and mice. 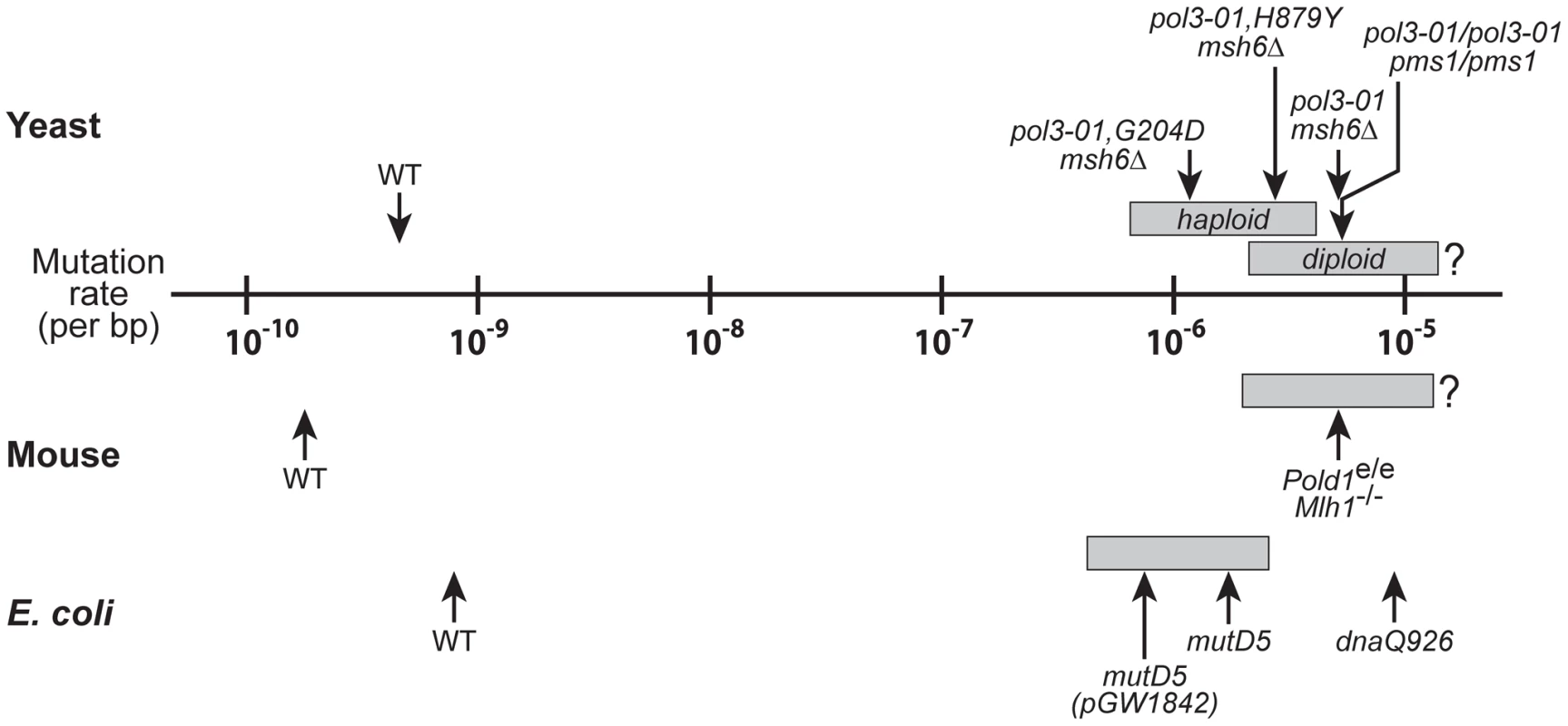
Comparison of spontaneous per-base-pair mutation rates of wild-type (WT) and strong mutator strains of yeast (haploid and diploid), E. coli and mouse cells. Gray boxes indicate the mutation rate intervals that coincide with the transition from wild-type growth (leftmost boundary) to failed growth (rightmost boundary). The data for haploid yeast mutators are from Figure 5B, with the left boundary corresponding to the mutation rate of pol3-01,E642K msh6Δ cells (6.5×10−7) and the right boundary corresponding to the lethal threshold (4.1×10−6). The mutation rates of pol3-01,G204D msh6Δ, pol3-01,H879Y msh6Δ, and pol3-01 msh6Δ haploid yeast are shown as examples of progressively stronger mutators with slow (++), very slow (+) and no-growth (–) phenotypes, respectively. The data for pol3-01/pol3-01 pms1/pms1 diploid yeast are from Morrison et al. [40]; pol3-01/pol3-01 pms1/pms1 cells divide very slowly with a growth phenotype presumably in the range of + to ++. The mouse Pold1e/e Mlh1Δ/Δ mutation rate is extrapolated from ouabain-resistance rates of cultured Pold1+/e Mlh1Δ/Δ fibroblasts as described in Materials and Methods; a growth phenotype between + and ++ is assumed [25]. E. coli mutation rates and growth phenotypes are from Fijalkowska et al. [62]; mutD5(pGW1842), mutD5 and dnaQ926 exhibit slow (++), very slow (+) and no-growth (–) phenotypes, respectively. The positions of the gray boxes for diploid yeast, mouse and E. coli are estimates based on the mutation rate and growth capacity relationships observed in haploid yeast (Figure 5B). The error thresholds (rightmost boundaries) for diploid yeast and mouse cells are not known. The yeast wild-type rate is the average of multiple independent determinations (data herein and [40], [108], [114], [124]). Wild-type mouse and E. coli mutation rates are from Drake et al. [9], [114]. Structural Implications of the eex Amino-Acid Substitutions
We observed that escape mutants readily emerge when moderate mutators are pushed above the error threshold (Figure 2). One-third of the escape mutants resulted from second-site changes in Pol δ that suppress the proofreading-deficient mutator phenotype. Recent structural studies of S. cerevisiae Pol δ [74] provide insight into potential mechanisms of mutator suppression by these intragenic eex alleles (Figure 4, Figures S5 and S6).
Many eex mutations alter amino acids around the polymerase active site that are predicted to influence dNTP binding or catalysis (Figure 4B). Effects may be mediated by direct interactions of mutated residues with the metal•dNTP substrate or via packing interactions that indirectly affect the substrate binding pocket. Other eex mutations map to a stretch of amino acids that bind the template near the active site and buttress the fingers domain, which contains residues that contour the template•dNTP base pair (Figure S5). Amino-acid changes affecting active-site geometry, positioning of the template nucleotide, or stability of the catalytic conformation may act as antimutators by increasing selectivity for correct dNTPs or by slowing the rate of catalysis so that mispaired template•primers have more time to dissociate from Pol δ. A model of dissociation and subsequent editing by an alternative enzyme [20], [75] may best explain eex mutations that change amino acids along the DNA binding track (Figure 4C). Similarly, eex mutations in the exonuclease domain may impart structural changes that promote Pol δ dissociation during failed proofreading attempts (Figure S6). Intriguingly, two eex amino-acid substitutions (E642K and D643N) are located on the solvent-exposed surface of Pol δ (asterisk in Figure 4A), suggesting that changes in protein-protein interactions influence mutagenesis. Proteins encoded by eex loci extragenic to POL3 (Table S1) are candidate interacting partners.
Several alternative enzymes may function to edit Pol δ errors in the eex mutants. One candidate is proofreading by Pol ε. Yeast with deficiencies in both Pol δ and Pol ε proofreading exhibit a synergistic increase in mutation rate, suggesting one or both polymerases may proofread for the other [46]. Other candidates include the 3′→5′ exonuclease activities of MRE11 [76] and Apn2 [77], or endonucleases such as Rad1/Rad10 or Mus81/Mms4 that cleave 3′ flap structures during replication fork restart [78]–[82]. An important consideration is that such alternative editing pathways may be redundant, with multiple activities masking the contributions of any one nuclease.
The locations of several eex substitutions in Pol δ resemble those of antimutators previously identified in bacteriophage T4 polymerase [4], [83]–[86] and in herpes simplex virus polymerase [87]–[89], two B-family DNA polymerases similar to Pol δ (Figure 3). Genetic screens have also identified E. coli DNA polymerase I and III antimutator variants, and similar to our findings, these E. coli antimutators result from diverse amino-acid substitutions throughout the polymerase structures [19], [20], [90]–[92]. Some amino-acid substitutions in T4 pol are thought to increase polymerase fidelity by promoting ‘hyper-editing’ of the primer terminus by the integral proofreading exonuclease (reviewed in [4]). However, the eex mutations we describe mediate their antimutator effects without the aid of an active exonuclease domain, similar to previously isolated E. coli antimutators [19], [20], [90].
Taken together, this structural analysis suggests two general antimutator mechanisms for Pol δ eex mutations: 1) increased dNTP discrimination, thereby making Pol δ more accurate, and 2) increased dissociation from mispaired primer-templates, thereby allowing other enzymes to proofread Pol δ errors. eex mutations could also decrease errors at Okazaki fragment junctions by suppressing the strand-displacement activity of proofreading-deficient Pol δ [52], [93]–[95].
Evidence for Other Pathways of Mutator Suppression
Our study took advantage of synthetically lethal interactions between Pol δ proofreading and MMR alleles to select for antimutators. Several lines of evidence indicate that mutator suppressors also arise under non-lethal conditions and are not restricted to the Pol δ proofreading – MMR pathway. Morrison and Sugino observed mutator suppression in a yeast clone defective for Pol ε proofreading and MMR [46], and an engineered second-site mutation in Pol ε suppresses the mutator effect of Pol ε proofreading deficiency [96]. In E. coli, suppressors of diverse mutator pathways (MMR, proofreading and DNA damage repair) emerge spontaneously in strains that are well below the error threshold [14], [18]–[20]. In our studies, large-colony variants of slow-growing mutators were frequently observed (see, for example, Figure 5A), and in the one variant we pursued, we found the A894G suppressor mutation. Collectively, these studies show that many defects in DNA replication fidelity can be genetically suppressed and suggest that both moderate and strong mutators are intrinsically unstable.
The facile emergence of mutator suppressors that we observed in yeast suggests that similar pathways of suppression exist in multicellular eukaryotes. This has implications for the role of mutator phenotypes in cancer [22], [97]. During neoplastic transformation, mutator alleles that promote the formation of tumor cells are likely to incur a fitness cost due to an increase in mutational load. To offset this cost, suppressor alleles that reduce the mutation rate may emerge during the later stages of oncogenesis after genetic barriers to immortalization and metastasis have been overcome. Although recent findings suggest that a mutator phenotype persists in at least some types of human tumors [23], our results raise the prospect that mutator phenotypes may be transient during tumor progression due to genetic suppression. An analysis of mutation rate dynamics in cancer is warranted.
Materials and Methods
Media and Growth Conditions
Yeast were grown at 30°C using YPD, synthetic complete (SC) media or SC drop-out media deficient in specific amino acids as needed to select for prototrophy [98]. Pre-formulated nutrient supplements for SC and SC lacking uracil and leucine were purchased from Bufferad. All other drop-out supplements were made as described [98]. URA3-deficient cells were selected on SC medium containing 1 mg/ml 5-fluroorotic-acid (FOA; Zymo Research) and an additional 50 mg/L uracil [43]. TRP1-deficient strains were selected on FAA selection media containing 0.5 mg/ml 5-fluroanthranillic acid (FAA) made as described [99]. Canavanine-resistant mutants were scored on SC plates lacking arginine that contained 60 µg/ml of canavanine. Reagents were obtained from Sigma-Aldrich or Fisher Scientific unless otherwise indicated.
Yeast Plasmids and Strains
Plasmids
pGL310 is the CEN4/ARS1/URA3 plasmid, YCp50 [100], modified to carry SUP11 and the wild-type POL3 gene under control of its native promoter [29], [101]. YCplac111POL3 and YCplac111pol3 derivatives are CEN4/ARS1/LEU2 plasmids derived from YCplac111 [102] and contain the entire wild-type POL3 or mutant pol3 genes (with native promoters) flanked by HindIII and EcoRI restriction sites. pRS414POL3 and pRS414pol3-01 are derivatives of the CEN6/ARS4/TRP1 plasmid, pRS414 [103], carrying the HindIII-EcoRI POL3 DNA fragments from YCplac111POL3 and YCplac111pol3-01, respectively. The construction of YCplac111 and pRS414 vectors and subsequent subcloning of eex mutants are described in detail in Text S1.
Strains
Yeast strains and their genotypes are listed in Table S3. Chromosomal gene disruptions were introduced using PCR products generated with primers, templates, and protocols detailed in Table S4 and Text S1. YGL27-3D (a kind gift from Michel Simon and Gerard Faye, Institut Curie) is a haploid strain that carries a lethal, partial deletion of chromosomal POL3 substituted by HIS3 and complemented by pGL310. Chromosomal MSH6 was replaced with TRP1 to create YGL27-3Dmsh6dis4. To limit gene conversion between the pol3 plasmids and residual POL3 sequences in the chromosome, the entire POL3 coding sequences in YGL27-3D and YGL27-3Dmsh6dis4 were replaced with kanMX [104], creating YP6 (previously called YGL27-pol3Δ [105]) and MP4, respectively.
The S288c derivative, BY4733 [103], was modified to create a set of isogenic strains in a standard genetic background. BY4733 was transformed with pGL310, and chromosomal POL3 was deleted and replaced with HIS3 to create P3H3a. MSH2 was deleted in P3H3a and replaced with TRP1 to create BP0109. MSH6 was deleted in P3H3a and replaced with kanMX [106] to create BP1506. P3H3a was modified as follows to allow mutation rate measurements at URA3. First, P3H3a was transformed with pRS414POL3 and plated on SC FOA to isolate a strain that lost pGL310. Then, to create BP4001, AGP1 on Chromosome III was replaced with URA3 oriented with the direction of transcription towards ARS306 [107].
Plasmid Shuffling
Plasmid shuffling with pGL310-containing strains was carried out essentially as described [29], [43] (Figure S1). Cells transformed with YCplac111pol3 plasmids, YCplac111POL3 (positive control), or YCplac111 (negative control) were plated on SC lacking uracil and leucine. Cells transformed with pRS414pol3-01, pRS414POL3 (positive control), or pRS414 (negative control) were plated on SC lacking uracil and tryptophan. After three days at 30°C, individual colonies were picked and resuspended in sterile H2O, and serial dilutions containing approximately 105, 104, 103, or 102 cells were plated onto SC or SC FOA to select for clones that spontaneously lost the URA3 plasmid pGL310. A similar approach was used for shuffling in strains carrying the TRP1 plasmid pRS414POL3; BP4001 transformants containing both pRS414POL3 and YCplac111-based plasmids were selected on SC lacking tryptophan and leucine and then plated onto SC FAA to select for clones that spontaneously lost the TRP1 plasmid.
eex Mutant Screen
For the systematic isolation of spontaneous pol3-01,eex mutant alleles (Figure 2C), a pol3-01 plasmid was transformed into pol3Δ msh6Δ + pGL310 yeast, and 1–5×106 cells from independent transformants were plated separately on SC FOA. FOA-resistant clones were isolated in the MP4 strain carrying YCplac111pol3-01 or in BP1506 carrying YCplac111pol3-01 or pRS414pol3-01. Bona fide eex mutants were distinguished from FOA-resistant clones that result from ura3 mutation or pol3-01→POL3 gene conversion by using a genotyping assay described in Text S1. pol3-01 plasmids from individual eex mutants were recovered and reshuffled into naïve pol3Δ msh6Δ + pGL310 cells to identify suppressors intragenic to pol3-01. Plasmids that conferred consistent survival upon reshuffling were purified, and the pol3 genes were sequenced (primer sequences available on request). Intragenic eex alleles thus identified were individually re-engineered into fresh YCplac111POL3 and YCplac111pol3-01 vectors and re-transformed into MP4 or BP1506 stock strains as a final confirmation of the ability of each allele to confer the eex phenotype. The re-engineered mutants were used to assess the effects of eex alleles on mutation rates and plating efficiencies.
Mutation Frequencies and Rates
For the scanning mutagenesis screen (Figure S2), sequence-verified YCplac111pol3 plasmids were shuffled into YP6 or MP4 cells immediately prior to each experiment. Twelve to thirteen independent FOA-resistant colonies of each genotype were streaked onto SC plates in ∼1-cm patches, grown two days at 30°C, and then replica-plated to canavanine plates to qualitatively assess mutant frequencies based on the number of canavanine-resistant colonies [47] (Figure S2A).
To measure mutation rates at the CAN1 locus, freshly streaked YP6 or MP4 strains were transformed with YCplac111POL3 or YCplac111pol3 plasmids, and multiple independent transformants were shuffled on SC FOA plates to obtain well-isolated single colonies. For each genotype, seven to eleven independent colonies, 1–2 mm in diameter, were excised as an agar plug, resuspended in 1 ml of dH2O, and sonicated briefly. To estimate the number of cell divisions (Nt) during colony formation, serial dilutions were plated on SC media, and the number of colony-forming units was counted after two days at 30°C. To determine the number of mutants for wild-type and weak mutator strains, all of the remaining cells were plated on canavanine plates; for stronger mutators, the cell suspension was diluted 1∶10 to 1∶200 in dH20 prior to plating. The numbers of canavanine-resistant colonies on each plate were scored after three to four days at 30°C.
To measure URA3 mutation rates, BP4001 was transformed with YCplac111POL3, YCplac111pol3-01, or their respective eex mutant derivatives. Four FAA-resistant colonies from independent transformants with each plasmid were inoculated into separate 100-µl SC overnight cultures. The following morning the cultures were diluted to 1000 cells/ml and, for each of the four isolates, 12 parallel 100-µl cultures (100 cells/culture) were set up in 96-well microtiter plates. The plates were sealed with adhesive PCR plate sealers (Abgene, AB-0558) to minimize evaporation [108]. After two days of growth at 30°C, the cells were re-suspended by vigorous vortexing, and nine of the replicate cultures were spot-plated in 200-µl volumes on SC FOA plates. To estimate the total number of cell divisions, the remaining three replica cultures from each isolate were combined, diluted, and plated on SC plates. Colony numbers were scored after 3–4 days. We confirmed that spot plating accurately determines the number of FOA-resistant colonies for the strongest mutator by dividing test cell suspensions in half and comparing colony counts in a 100-µl spot with 100 µl of the same suspension spread over an entire SC FOA plate.
Mutation rates were determined from the number of mutant colonies in each replica by calculating an estimate for m by maximum likelihood [109] using newtonLD in Salvador 2.1 [110] with Mathematica 6.0 (Wolfram Research) and dividing by the number of cell divisions inferred from colony forming units. Where values for Nt from independent experiments differed by less than 2-fold, the data sets were combined for the mutation rate calculations [109]. In some instances, Nt values from independent experiments differed by more than 2-fold. In most cases, the independently-derived mutation rates were similar and a single value was reported (noted in Table 1). Confidence intervals were calculated in Salvador 2.1 using LRIntervalLD, which relies on likelihood ratios [110].
From these mutation rates, the efficiency of Msh6-dependent MMR (em6), expressed as the percentage of errors corrected, was calculated using equation 1:(1)where Mr is the relative mutation rate of the strain indicated in the subscript. The efficiency of Pol δ proofreading (eδexo), expressed as a percentage of errors corrected, was calculated similarly from equation 2:(2)
CAN1 Mutation Spectra
For each strain, we isolated up to 48 canavanine-resistant mutants from 48 independent shuffling experiments. Cells were treated with Zymolyase (ICN Biomedicals; 50 u/ml in 10 mM Tris•HCl/0.1 mM EDTA, pH7.5 at 37°C for 30 min then 95°C for 10 min), and the can1 coding sequence was PCR-amplified in 50-µl reactions with Phusion polymerase (NEB) using primers can1F1N (5′-GGTTAAGATAAGTAGATAAGAGAATGATACG-3′) and can1S1 (5′-GCGTGGAAATGTGATCAAAGG-3′) with the following PCR conditions: 98°C, 1 min.; 35× (98°C, 10 sec.; 45°C, 30 sec.; 72°C, 90 sec.); 72°C, 1 min. The samples were treated with 5 units each of Antarctic phosphatase and Exo1 (New England Biolabs) to degrade excess primer and dNTPs, heated at 80°C for 20 min to inactivate the enzymes, and then sequenced with primers can1S1, can1S2 (5′-CCAAAGCGCCAAATGCAGCAG-3′), can1S3 (5′-TCCAATAACGGAATCCAACTG-3′) and can1S4 (5′-GGGCAATCATACCAATATGTC-3′). Mutation spectra were tabulated and compared using iMARS [111].
Per-Base-Pair Mutation Rates
Phenotypic mutation rates were converted to per-base-pair rates using the approach of Drake [112]–[114] according to equations 3 – 5:(3)
(4)
(5)C and C′ ´ (equations 3 and 4) are correction factors to adjust for undetected (phenotypically silent) base-substitution mutations in a reporter gene. BCT = the number of chain-terminating base substitutions (3 possible codons), B = the number of all base substitutions (64 possible codons), and I = the number of insertions+deletions (indels) in representative mutation spectra from M mutants sequenced. The mutation rate per base pair (μb) is calculated using equation 5 from the experimentally determined phenotypic mutation rate (μT) multiplied by the correction factor C′ ´ and divided by the number of base pairs in the mutation-reporter target sequence (T). The effective target size (τ) is estimated by T / C ´′.
In our collection of 484 Canr mutants from proofreading - and MMR-deficient yeast (Figure S3 and Table S2), there were 442 base substitutions (101 chain-terminating + 341 missense) and 42 indels (including infrequent complex mutations) in CAN1 (T = 1773). Thus, C = 4.87 and τ = 391 base pairs. These values from mutator yeast strains are similar to those previously determined by others scoring spontaneous mutation in wild-type yeast (C = 4.73, [113]; τ = 236, [108]). The per-base-pair rates for haploid yeast plotted in Figure 6 were calculated from our Canr μT values (Table 1 and Table 2) with C = 4.87, C′ ´ = 4.53 and T = 1773. For diploid pol3-01/pol3-01 pms1/pms1 yeast, we used the FOAr mutation rate (μT) of 3.5×10-4 reported by Morrison et. al. [40]; C = 8.18 (determined from the data of Lang and Murray [108]), C′ ´ = 6.79 and T = 804 base pairs for the URA3 target gene. Thus, τ is 118 base pairs, and the per-base-pair mutation rate of pol3-01/pol3-01 pms1/pms1 diploids at the URA3 locus is [(3.5×10−4) ×6.79 / 804] = 3.0×10−6. In Figure 6 we multiply this rate by 1.8 to adjust for the lower intrinsic mutation rate of URA3 compared to CAN1 (Table 1 and Table 2 and [108]).
For mouse cells, per-base-pair mutation rates were calculated from ouabain-resistance (Ouar) rates determined in our laboratory using spontaneously immortalized mouse embryo fibroblasts ([25] and unpublished data). The effective target size (τ) is estimated as follows. Base substitution mutations in any one of sixteen codons in the Na,K-ATPase α1 gene (Atp1a1) are known to confer genetically dominant resistance to µM concentrations of ouabain in human cells [115]. Mouse cells, however, are naturally resistant to µM concentrations of ouabain due to differences at 2 of these 16 codons (Q111R and N122D; [116], [117]. Our fluctuation assays were conducted with 2 mM ouabain [25], conditions expected to only detect mutations that confer exceptionally high ouabain resistance. We estimate the target size to be ∼5 base pairs per allele, corresponding to two Atp1a1 codons (D121 and T797) known to effect >50-fold ouabain-resistance when mutated [115], [118]. Mouse fibroblast cell lines are typically tetraploid [119]. Therefore τ = 5 base pairs per allele×4 alleles = 20 base pairs. Pold1+/e Mlh1Δ/Δ cells, which are heterozygous defective for Pol δ proofreading and nullizygous for MMR, exhibited a mutation rate of 65×10−7 Ouar mutants per cell division (95% confidence interval = 56–75×10−7). This phenotypic rate corresponds to a per-base-pair rate of 65×10−7 / 20 base pairs = 3.3×10−7. Mouse cells that are homozygous deficient for both Pol δ proofreading and MMR (Pold1e/e Mlh1Δ/Δ) are viable but divide slowly up to embryonic day E9.5 [25]. Based on the relative mutation rates of MMRΔ/Δ diploid yeast with +/ - or −/ − Pol δ proofreading alleles [40], we estimate the per-base-pair rate of Pold1e/e Mlh1Δ/Δ mouse cells to be 5×10−6.
Supporting Information
Zdroje
1. FriedbergECWalkerGCSiedeWWoodRDSchultzRA 2006 DNA Repair and Mutagenesis. Washington, D.C. ASM Press
2. McCullochSDKunkelTA 2008 The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res 18 148 161
3. IyerRRPluciennikABurdettVModrichPL 2006 DNA mismatch repair: functions and mechanisms. Chem Rev 106 302 323
4. Reha-KrantzLJ 2010 DNA polymerase proofreading: Multiple roles maintain genome stability. Biochim Biophys Acta 1804 1049 1063
5. de VisserJA 2002 The fate of microbial mutators. Microbiology 148 1247 1252
6. GiraudARadmanMMaticITaddeiF 2001 The rise and fall of mutator bacteria. Curr Opin Microbiol 4 582 585
7. SturtevantAH 1937 Essays on evolution. I. On the effects of selection on mutation rate. Q Rev Biol 12 464 467
8. DenamurEMaticI 2006 Evolution of mutation rates in bacteria. Mol Microbiol 60 820 827
9. DrakeJWCharlesworthBCharlesworthDCrowJF 1998 Rates of spontaneous mutation. Genetics 148 1667 1686
10. ElenaSFLenskiRE 2003 Evolution experiments with microorganisms: the dynamics and genetic bases of adaptation. Nat Rev Genet 4 457 469
11. ChaoLCoxEC 1983 Competition between high and low mutating strains of Escherichia coli. Evolution 37 125 134
12. SniegowskiPDGerrishPJLenskiRE 1997 Evolution of high mutation rates in experimental populations of E. coli. Nature 387 703 705
13. MaoEFLaneLLeeJMillerJH 1997 Proliferation of mutators in a cell population. J Bacteriol 179 417 422
14. GiraudAMaticITenaillonOClaraARadmanM 2001 Costs and benefits of high mutation rates: adaptive evolution of bacteria in the mouse gut. Science 291 2606 2608
15. NilssonAIKugelbergEBergOGAnderssonDI 2004 Experimental adaptation of Salmonella typhimurium to mice. Genetics 168 1119 1130
16. Notley-McRobbLSeetoSFerenciT 2002 Enrichment and elimination of mutY mutators in Escherichia coli populations. Genetics 162 1055 1062
17. FunchainPYeungAStewartJLLinRSlupskaMM 2000 The consequences of growth of a mutator strain of Escherichia coli as measured by loss of function among multiple gene targets and loss of fitness. Genetics 154 959 970
18. TröbnerWPiechockiR 1984 Selection against hypermutability in Escherichia coli during long term evolution. Mol Gen Genet 198 177 178
19. SchaaperRMCornacchioR 1992 An Escherichia coli dnaE mutation with suppressor activity toward mutator mutD5. J Bacteriol 174 1974 1982
20. FijalkowskaIJSchaaperRM 1995 Effects of Escherichia coli dnaE antimutator alleles in a proofreading-deficient mutD5 strain. J Bacteriol 177 5979 5986
21. LoebLASpringgateCFBattulaN 1974 Errors in DNA replication as a basis of malignant changes. Cancer Res 34 2311 2321
22. LoebLABielasJHBeckmanRA 2008 Cancers exhibit a mutator phenotype: clinical implications. Cancer Res 68 3551 3557
23. BielasJHLoebKRRubinBPTrueLDLoebLA 2006 Human cancers express a mutator phenotype. Proc Natl Acad Sci USA 103 18238 18242
24. GoldsbyRELawrenceNAHaysLEOlmstedEAChenX 2001 Defective DNA polymerase-δ proofreading causes cancer susceptibility in mice. Nat Med 7 638 639
25. AlbertsonTMOgawaMBugniJMHaysLEChenY 2009 DNA polymerase ε and δ proofreading suppress discrete mutator and cancer phenotypes in mice. Proc Natl Acad Sci USA 106 17101 17104
26. GoldsbyREHaysLEChenXOlmstedEASlaytonWB 2002 High incidence of epithelial cancers in mice deficient for DNA polymerase δ proofreading. Proc Natl Acad Sci USA 99 15560 15565
27. WeiKKucherlapatiREdelmannW 2002 Mouse models for human DNA mismatch-repair gene defects. Trends Mol Med 8 346 353
28. PeltomäkiP 2005 Lynch syndrome genes. Fam Cancer 4 227 232
29. SimonMGiotLFayeG 1991 The 3′ to 5′ exonuclease activity located in the DNA polymerase δ subunit of Saccharomyces cerevisiae is required for accurate replication. EMBO J 10 2165 2170
30. MorrisonABellJBKunkelTASuginoA 1991 Eukaryotic DNA polymerase amino acid sequence required for 3′ → 5′ exonuclease activity. Proc Natl Acad Sci USA 88 9473 9477
31. WilliamsonMSGameJCFogelS 1985 Meiotic gene conversion mutants in Saccharomyces cerevisiae. I. Isolation and characterization of pms1-1 and pms1-2. Genetics 110 609 646
32. ReenanRAKolodnerRD 1992 Characterization of insertion mutations in the Saccharomyces cerevisiae MSH1 and MSH2 genes: evidence for separate mitochondrial and nuclear functions. Genetics 132 975 985
33. ProllaTAChristieDMLiskayRM 1994 Dual requirement in yeast DNA mismatch repair for MLH1 and PMS1, two homologs of the bacterial mutL gene. Mol Cell Biol 14 407 415
34. StrandMProllaTALiskayRMPetesTD 1993 Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature 365 274 276
35. DesaiMMFisherDSMurrayAW 2007 The speed of evolution and maintenance of variation in asexual populations. Curr Biol 17 385 394
36. ThompsonDADesaiMMMurrayAW 2006 Ploidy controls the success of mutators and nature of mutations during budding yeast evolution. Curr Biol 16 1581 1590
37. WlochDMSzafraniecKBortsRHKoronaR 2001 Direct estimate of the mutation rate and the distribution of fitness effects in the yeast Saccharomyces cerevisiae. Genetics 159 441 452
38. ZeylCde VisserJAGM 2001 Estimates of the rate and distribution of fitness effects of spontaneous mutation in Saccharomyces cerevisiae. Genetics 157 53 61
39. ZeylCMizeskoMde VisserJAGM 2001 Mutational meltdown in laboratory yeast populations. Evolution 55 909 917
40. MorrisonAJohnsonALJohnstonLHSuginoA 1993 Pathway correcting DNA replication errors in Saccharomyces cerevisiae. EMBO J 12 1467 1473
41. TranHTGordeninDAResnickMA 1999 The 3′→5′ exonucleases of DNA polymerases δ and ε and the 5′→3′ exonuclease Exo1 have major roles in postreplication mutation avoidance in Saccharomyces cerevisiae. Mol Cell Biol 19 2000 2007
42. GreeneCNJinks-RobertsonS 2001 Spontaneous frameshift mutations in Saccharomyces cerevisiae: accumulation during DNA replication and removal by proofreading and mismatch repair activities. Genetics 159 65 75
43. BoekeJDLaCrouteFFinkGR 1984 A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet 197 345 346
44. BernadABlancoLLazaroJMMartinGSalasM 1989 A conserved 3′→5′ exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell 59 219 228
45. ShevelevIVHübscherU 2002 The 3′–5′ exonucleases. Nat Rev Mol Cell Biol 3 364 376
46. MorrisonASuginoA 1994 The 3′→5′ exonucleases of both DNA polymerases δ and ε participate in correcting errors of DNA replication in Saccharomyces cerevisiae. Mol Gen Genet 242 289 296
47. MarsischkyGTFilosiNKaneMFKolodnerR 1996 Redundancy of Saccharomyces cerevisiae MSH3 and MSH6 in MSH2-dependent mismatch repair. Genes Dev 10 407 420
48. JohnsonREKovvaliGKPrakashLPrakashS 1996 Requirement of the yeast MSH3 and MSH6 genes for MSH2-dependent genomic stability. J Biol Chem 271 7285 7288
49. AlaniE 1996 The Saccharomyces cerevisiae Msh2 and Msh6 proteins form a complex that specifically binds to duplex oligonucleotides containing mismatched DNA base pairs. Mol Cell Biol 16 5604 5615
50. SokolskyTAlaniE 2000 EXO1 and MSH6 are high-copy suppressors of conditional mutations in the MSH2 mismatch repair gene of Saccharomyces cerevisiae. Genetics 155 589 599
51. HawkJDStefanovicLBoyerJCPetesTDFarberRA 2005 Variation in efficiency of DNA mismatch repair at different sites in the yeast genome. Proc Natl Acad Sci USA 102 8639 8643
52. JinYHGargPStithCMAl-RefaiHSterlingJF 2005 The multiple biological roles of the 3′→5′ exonuclease of Saccharomyces cerevisiae DNA polymerase δ require switching between the polymerase and exonuclease domains. Mol Cell Biol 25 461 471
53. SchaaperRM 1993 Base selection, proofreading, and mismatch repair during DNA replication in Escherichia coli. J Biol Chem 268 23762 23765
54. PavlovYIShcherbakovaPVRogozinIB 2006 Roles of DNA polymerases in replication, repair, and recombination in eukaryotes. Int Rev Cytol 255 41 132
55. GiaeverGChuAMNiLConnellyCRilesL 2002 Functional profiling of the Saccharomyces cerevisiae genome. Nature 418 387 391
56. WinzelerEAShoemakerDDAstromoffALiangHAndersonK 1999 Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285 901 906
57. GoodmanMFCreightonSBloomLBPetruskaJ 1993 Biochemical basis of DNA replication fidelity. Crit Rev Biochem Mol Biol 28 83 126
58. DattaASchmeitsJLAminNSLauPJMyungK 2000 Checkpoint-dependent activation of mutagenic repair in Saccharomyces cerevisiae pol3-01 mutants. Mol Cell 6 593 603
59. YuLPena CastilloLMnaimnehSHughesTRBrownGW 2006 A survey of essential gene function in the yeast cell division cycle. Mol Biol Cell 17 4736 4747
60. OoiSLPanXPeyserBDYePMeluhPB 2006 Global synthetic-lethality analysis and yeast functional profiling. Trends Genet 22 56 63
61. BooneCBusseyHAndrewsBJ 2007 Exploring genetic interactions and networks with yeast. Nat Rev Genet 8 437 449
62. FijalkowskaIJSchaaperRM 1996 Mutants in the Exo I motif of Escherichia coli dnaQ: defective proofreading and inviability due to error catastrophe. Proc Natl Acad Sci USA 93 2856 2861
63. SliwaPKluzJKoronaR 2004 Mutational load and the transition between diploidy and haploidy in experimental populations of the yeast Saccharomyces cerevisiae. Genetica 121 285 293
64. DelneriDHoyleDCGkargkasKCrossEJMRashB 2008 Identification and characterization of high-flux-control genes of yeast through competition analyses in continuous cultures. Nat Genet 40 113 117
65. DaeeDLMertzTMShcherbakovaPV 2010 A cancer-associated DNA polymerase δ variant modeled in yeast causes a catastrophic increase in genomic instability. Proc Natl Acad Sci USA 107 157 162
66. TreutingPMAlbertsonTMPrestonBD 2010 Case series: acute tumor lysis syndrome in mutator mice with disseminated lymphoblastic lymphoma. Toxicol Pathol 38 476 485
67. SzafraniecKBortsRHKoronaR 2001 Environmental stress and mutational load in diploid strains of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA 98 1107 1112
68. HillenmeyerMEFungEWildenhainJPierceSEHoonS 2008 The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science 320 362 365
69. GuZSteinmetzLMGuXScharfeCDavisRW 2003 Role of duplicate genes in genetic robustness against null mutations. Nature 421 63 66
70. KafriRLevyMPilpelY 2006 The regulatory utilization of genetic redundancy through responsive backup circuits. Proc Natl Acad Sci USA 103 11653 11658
71. HarrisonRPappBPalCOliverSGDelneriD 2007 Plasticity of genetic interactions in metabolic networks of yeast. Proc Natl Acad Sci USA 104 2307 2312
72. WagnerA 2000 Robustness against mutations in genetic networks of yeast. Nat Genet 24 355 361
73. RutherfordSLLindquistS 1998 Hsp90 as a capacitor for morphological evolution. Nature 396 336 342
74. SwanMKJohnsonREPrakashLPrakashSAggarwalAK 2009 Structural basis of high-fidelity DNA synthesis by yeast DNA polymerase δ. Nat Struct Mol Biol 16 979 986
75. AlbertsonTMPrestonBD 2006 DNA replication fidelity: proofreading in trans. Curr Biol 16 R209 211
76. TrujilloKMSungP 2001 DNA structure-specific nuclease activities in the Saccharomyces cerevisiae Rad50·Mre11 complex. J Biol Chem 276 35458 35464
77. UnkIHaracskaLPrakashSPrakashL 2001 3′-Phosphodiesterase and 3′→5′ exonuclease activities of yeast Apn2 protein and requirement of these activities for repair of oxidative DNA damage. Mol Cell Biol 21 1656 1661
78. BardwellAJBardwellLTomkinsonAEFriedbergEC 1994 Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science 265 2082 2085
79. Bastin-ShanowerSAFrickeWMMullenJRBrillSJ 2003 The mechanism of Mus81-Mms4 cleavage site selection distinguishes it from the homologous endonuclease Rad1-Rad10. Mol Cell Biol 23 3487 3496
80. BoddyMNGaillardPHMcDonaldWHShanahanPYatesJR3rd 2001 Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell 107 537 548
81. ChenXBMelchionnaRDenisCMGaillardPHBlasinaA 2001 Human Mus81-associated endonuclease cleaves Holliday junctions in vitro. Mol Cell 8 1117 1127
82. KaliramanVMullenJRFrickeWMBastin-ShanowerSABrillSJ 2001 Functional overlap between Sgs1-Top3 and the Mms4-Mus81 endonuclease. Genes Dev 15 2730 2740
83. DrakeJWAllenEFForsbergSAPreparataRMGreeningEO 1969 Genetic control of mutation rates in bacteriophage T4. Nature 221 1128 1132
84. Reha-KrantzLJ 1988 Amino acid changes coded by bacteriophage T4 DNA polymerase mutator mutants. Relating structure to function. J Mol Biol 202 711 724
85. Reha-KrantzLJ 1995 Use of genetic analyses to probe structure, function, and dynamics of bacteriophage T4 DNA polymerase. Methods Enzymol 262 323 331
86. Reha-KrantzLJWongC 1996 Selection of bacteriophage T4 antimutator DNA polymerases: a link between proofreading and sensitivity to phosphonoacetic acid. Mutat Res 350 9 16
87. HwangYTZuccolaHJLuQHwangCB 2004 A point mutation within conserved region VI of herpes simplex virus type 1 DNA polymerase confers altered drug sensitivity and enhances replication fidelity. J Virol 78 650 657
88. HallJDCoenDMFisherBLWeisslitzMRandallS 1984 Generation of genetic diversity in herpes simplex virus: an antimutator phenotype maps to the DNA polymerase locus. Virology 132 26 37
89. GibbsJSChiouHCBastowKFChengYCCoenDM 1988 Identification of amino acids in herpes simplex virus DNA polymerase involved in substrate and drug recognition. Proc Natl Acad Sci USA 85 6672 6676
90. LohEChoeJLoebLA 2007 Highly tolerated amino acid substitutions increase the fidelity of Escherichia coli DNA polymerase I. J Biol Chem 282 12201 12209
91. FijalkowskaIJDunnRLSchaaperRM 1993 Mutants of Escherichia coli with increased fidelity of DNA replication. Genetics 134 1023 1030
92. SchaaperRM 1996 Suppressors of Escherichia coli mutT: antimutators for DNA replication errors. Mutat Res 350 17 23
93. JinYHObertRBurgersPMKunkelTAResnickMA 2001 The 3′→5′ exonuclease of DNA polymerase δ can substitute for the 5′ flap endonuclease Rad27/Fen1 in processing Okazaki fragments and preventing genome instability. Proc Natl Acad Sci USA 98 5122 5127
94. GargPStithCMSabouriNJohanssonEBurgersPM 2004 Idling by DNA polymerase δ maintains a ligatable nick during lagging-strand DNA replication. Genes Dev 18 2764 2773
95. StithCMSterlingJResnickMAGordeninDABurgersPM 2008 Flexibility of eukaryotic Okazaki fragment maturation through regulated strand displacement synthesis. J Biol Chem 283 34129 34140
96. PavlovYIMakiSMakiHKunkelTA 2004 Evidence for interplay among yeast replicative DNA polymerases alpha, delta and epsilon from studies of exonuclease and polymerase active site mutations. BMC Biol 2 11
97. PrestonBDAlbertsonTMHerrAJ 2010 DNA replication fidelity and cancer. Semin Cancer Biol 20 281 293
98. ShermanF 2002 Getting started with yeast. GuthrieCFinkGR Part B: Guide to Yeast Genetics and Molecular and Cell Biology San Diego Academic Press 3 41 Volume 350 ed
99. ToynJHGunyuzluPLWhiteWHThompsonLAHollisGF 2000 A counterselection for the tryptophan pathway in yeast: 5-fluoroanthranilic acid resistance. Yeast 16 553 560
100. RoseMDNovickPThomasJHBotsteinDFinkGR 1987 A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene 60 237 243
101. GiotLSimonMDuboisCFayeG 1995 Suppressors of thermosensitive mutations in the DNA polymerase δ gene of Saccharomyces cerevisiae. Mol Gen Genet 246 212 222
102. GietzRDSuginoA 1988 New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74 527 534
103. BrachmannCBDaviesACostGJCaputoELiJ 1998 Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14 115 132
104. WachABrachatAPohlmannRPhilippsenP 1994 New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10 1793 1808
105. VenkatesanRNHsuJJLawrenceNAPrestonBDLoebLA 2005 Mutator phenotypes caused by substitution at a conserved motif A residue in eukaryotic DNA polymerase δ. J Biol Chem 281 4486 4494
106. GuldenerUHeckSFielderTBeinhauerJHegemannJH 1996 A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res 24 2519 2524
107. PavlovYINewlonCSKunkelTA 2002 Yeast origins establish a strand bias for replicational mutagenesis. Mol Cell 10 207 213
108. LangGIMurrayAW 2008 Estimating the per-base-pair mutation rate in the yeast Saccharomyces cerevisiae. Genetics 178 67 82
109. RoscheWAFosterPL 2000 Determining mutation rates in bacterial populations. Methods 20 4 17
110. ZhengQ 2002 Statistical and algorithmic methods for fluctuation analysis with SALVADOR as an implementation. Math Biosci 176 237 252
111. MorganCLewisPD 2006 iMARS--mutation analysis reporting software: an analysis of spontaneous cII mutation spectra. Mutat Res 603 15 26
112. DrakeJW 1991 A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci USA 88 7160 7164
113. GroganDWCarverGTDrakeJW 2001 Genetic fidelity under harsh conditions: analysis of spontaneous mutation in the thermoacidophilic archaeon Sulfolobus acidocaldarius. Proc Natl Acad Sci USA 98 7928 7933
114. DrakeJW 2009 Avoiding dangerous missense: thermophiles display especially low mutation rates. PLoS Genet 5 e1000520 doi:10.1371/journal.pgen.1000520
115. CroyleMLWooALLingrelJB 1997 Extensive random mutagenesis analysis of the Na+/K+-ATPase alpha subunit identifies known and previously unidentified amino acid residues that alter ouabain sensitivity--implications for ouabain binding. Eur J Biochem 248 488 495
116. FallowsDKentRBNelsonDLEmanuelJRLevensonR 1987 Chromosome-mediated transfer of the murine Na,K-ATPase alpha subunit confers ouabain resistance. Mol Cell Biol 7 2985 2987
117. PriceEMLingrelJB 1988 Structure-function relationships in the Na,K-ATPase alpha subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry 27 8400 8408
118. CantleyLGCunhaMJZhouXM 1994 Ouabain-resistant OR6 cells express the murine alpha 1-subunit of the Na,K-ATPase with a T797-I797 substitution. J Biol Chem 269 15358 15361
119. DhillonKKSidorovaJMAlbertsonTMAndersonJBLadigesWC 2010 Divergent cellular phenotypes of human and mouse cells lacking the Werner syndrome RecQ helicase. DNA Repair 9 11 22
120. WangTS-FWongSWKornD 1989 Human DNA polymerase α: predicted functional domains and relationships with viral DNA polymerases. FASEB J 3 14 21
121. TranHTDegtyarevaNPGordeninDAResnickMA 1999 Genetic factors affecting the impact of DNA polymerase δ proofreading activity on mutation avoidance in yeast. Genetics 152 47 59
122. HadjimarcouMIKokoskaRJPetesTDReha-KrantzLJ 2001 Identification of a mutant DNA polymerase δ in Saccharomyces cerevisiae with an antimutator phenotype for frameshift mutations. Genetics 158 177 186
123. TianWHwangYTLuQHwangCBC 2009 Finger domain mutation affects enzyme activity, DNA replication efficiency, and fidelity of an exonuclease-deficient DNA polymerase of herpes simplex virus type 1. J Virol 83 7194 7201
124. LynchMSungWMorrisKCoffeyNLandryCR 2008 A genome-wide view of the spectrum of spontaneous mutations in yeast. Proc Natl Acad Sci USA 105 9272 9277
Štítky
Genetika Reprodukční medicína
Článek Macroautophagy Is Regulated by the UPR–Mediator CHOP and Accentuates the Phenotype of SBMA MiceČlánek Dynamic Replacement of Histone H3 Variants Reprograms Epigenetic Marks in Early Mouse EmbryosČlánek Mutations in a Guanylate Cyclase GCY-35/GCY-36 Modify Bardet-Biedl Syndrome–Associated Phenotypes in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 10- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Transcriptional Robustness Complements Nonsense-Mediated Decay in Humans
- Identification, Replication, and Fine-Mapping of Loci Associated with Adult Height in Individuals of African Ancestry
- Genetic Determinants of Serum Testosterone Concentrations in Men
- A One Base Pair Deletion in the Canine Gene Causes Exon Skipping and Late-Onset Neuronal Ceroid Lipofuscinosis in the Tibetan Terrier
- Three Structure-Selective Endonucleases Are Essential in the Absence of BLM Helicase in
- Identification of Widespread Ultra-Edited Human RNAs
- Multiple Wnts Redundantly Control Polarity Orientation in Epithelial Stem Cells
- The Bicoid Stability Factor Controls Polyadenylation and Expression of Specific Mitochondrial mRNAs in
- Transcriptome-Wide Binding Sites for Components of the Non-Poly(A) Termination Pathway: Nrd1, Nab3, and Sen1
- Macroautophagy Is Regulated by the UPR–Mediator CHOP and Accentuates the Phenotype of SBMA Mice
- Genetic Rearrangements Can Modify Chromatin Features at Epialleles
- Novel Function of as a Gap Gene during Spider Segmentation
- A Genome-Wide Screen for Interactions Reveals a New Locus on 4p15 Modifying the Effect of Waist-to-Hip Ratio on Total Cholesterol
- Comparative Genomic Analysis of Human Fungal Pathogens Causing Paracoccidioidomycosis
- Genetic Diversity in Cytokines Associated with Immune Variation and Resistance to Multiple Pathogens in a Natural Rodent Population
- Mutator Suppression and Escape from Replication Error–Induced Extinction in Yeast
- Dynamic Replacement of Histone H3 Variants Reprograms Epigenetic Marks in Early Mouse Embryos
- A Barcode Screen for Epigenetic Regulators Reveals a Role for the NuB4/HAT-B Histone Acetyltransferase Complex in Histone Turnover
- HIF–VEGF Pathways Are Critical for Chronic Otitis Media in and Mouse Mutants
- A Conserved Developmental Patterning Network Produces Quantitatively Different Output in Multiple Species of Drosophila
- Role of Exonic Variation in Chemokine Receptor Genes on AIDS: Association with Pneumocystis Pneumonia
- Whole-Exome Sequencing Identifies Homozygous Mutations in a Spastic Ataxia-Neuropathy Syndrome Linked to Mitochondrial -AAA Proteases
- Von Hippel-Lindau () Inactivation in Sporadic Clear Cell Renal Cancer: Associations with Germline Polymorphisms and Etiologic Risk Factors
- A Systems Biology Approach Reveals the Role of a Novel Methyltransferase in Response to Chemical Stress and Lipid Homeostasis
- Identification of Genomic Regions Associated with Phenotypic Variation between Dog Breeds using Selection Mapping
- Global Mapping of Cell Type–Specific Open Chromatin by FAIRE-seq Reveals the Regulatory Role of the NFI Family in Adipocyte Differentiation
- Natural Selection Affects Multiple Aspects of Genetic Variation at Putatively Neutral Sites across the Human Genome
- MicroRNA Expression and Regulation in Human, Chimpanzee, and Macaque Brains
- An Adaptive Allelic Series Featuring Complex Gene Rearrangements
- Feed-Forward Microprocessing and Splicing Activities at a MicroRNA–Containing Intron
- Developmental Stability: A Major Role for in
- A Phenomics-Based Strategy Identifies Loci on , , and Associated with Metabolic Syndrome Phenotype Domains
- Association of , , , , and with Systemic Lupus Erythematosus
- Small RNAs Prevent Transcription-Coupled Loss of Histone H3 Lysine 9 Methylation in
- Successive Increases in the Resistance of to Viral Infection through a Transposon Insertion Followed by a Duplication
- Mutations in a Guanylate Cyclase GCY-35/GCY-36 Modify Bardet-Biedl Syndrome–Associated Phenotypes in
- The Glycobiome Reveals Mechanisms of Pentose and Hexose Co-Utilization in Bacteria
- Insights into Hox Protein Function from a Large Scale Combinatorial Analysis of Protein Domains
- Mutations Cause Seckel and Jawad Syndromes
- Zelda Binding in the Early Embryo Marks Regions Subsequently Activated at the Maternal-to-Zygotic Transition
- Temporal Coordination of Gene Networks by Zelda in the Early Embryo
- Genetic Interaction between MTMR2 and FIG4 Phospholipid Phosphatases Involved in Charcot-Marie-Tooth Neuropathies
- Oxr1 Is Essential for Protection against Oxidative Stress-Induced Neurodegeneration
- Transforming Growth Factor β Receptor Type 1 Is Essential for Female Reproductive Tract Integrity and Function
- Positional Cloning of a Type 2 Diabetes Quantitative Trait Locus; , a Negative Regulator of Insulin Secretion
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Glycobiome Reveals Mechanisms of Pentose and Hexose Co-Utilization in Bacteria
- Global Mapping of Cell Type–Specific Open Chromatin by FAIRE-seq Reveals the Regulatory Role of the NFI Family in Adipocyte Differentiation
- Genetic Determinants of Serum Testosterone Concentrations in Men
- MicroRNA Expression and Regulation in Human, Chimpanzee, and Macaque Brains
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



