-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTwo New Loci for Body-Weight Regulation Identified in a Joint Analysis of Genome-Wide Association Studies for Early-Onset Extreme Obesity in French and German Study Groups
Meta-analyses of population-based genome-wide association studies (GWAS) in adults have recently led to the detection of new genetic loci for obesity. Here we aimed to discover additional obesity loci in extremely obese children and adolescents. We also investigated if these results generalize by estimating the effects of these obesity loci in adults and in population-based samples including both children and adults. We jointly analysed two GWAS of 2,258 individuals and followed-up the best, according to lowest p-values, 44 single nucleotide polymorphisms (SNP) from 21 genomic regions in 3,141 individuals. After this DISCOVERY step, we explored if the findings derived from the extremely obese children and adolescents (10 SNPs from 5 genomic regions) generalized to (i) the population level and (ii) to adults by genotyping another 31,182 individuals (GENERALIZATION step). Apart from previously identified FTO, MC4R, and TMEM18, we detected two new loci for obesity: one in SDCCAG8 (serologically defined colon cancer antigen 8 gene; p = 1.85×10−8 in the DISCOVERY step) and one between TNKS (tankyrase, TRF1-interacting ankyrin-related ADP-ribose polymerase gene) and MSRA (methionine sulfoxide reductase A gene; p = 4.84×10−7), the latter finding being limited to children and adolescents as demonstrated in the GENERALIZATION step. The odds ratios for early-onset obesity were estimated at ∼1.10 per risk allele for both loci. Interestingly, the TNKS/MSRA locus has recently been found to be associated with adult waist circumference. In summary, we have completed a meta-analysis of two GWAS which both focus on extremely obese children and adolescents and replicated our findings in a large followed-up data set. We observed that genetic variants in or near FTO, MC4R, TMEM18, SDCCAG8, and TNKS/MSRA were robustly associated with early-onset obesity. We conclude that the currently known major common variants related to obesity overlap to a substantial degree between children and adults.
Published in the journal: . PLoS Genet 6(4): e32767. doi:10.1371/journal.pgen.1000916
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000916Summary
Meta-analyses of population-based genome-wide association studies (GWAS) in adults have recently led to the detection of new genetic loci for obesity. Here we aimed to discover additional obesity loci in extremely obese children and adolescents. We also investigated if these results generalize by estimating the effects of these obesity loci in adults and in population-based samples including both children and adults. We jointly analysed two GWAS of 2,258 individuals and followed-up the best, according to lowest p-values, 44 single nucleotide polymorphisms (SNP) from 21 genomic regions in 3,141 individuals. After this DISCOVERY step, we explored if the findings derived from the extremely obese children and adolescents (10 SNPs from 5 genomic regions) generalized to (i) the population level and (ii) to adults by genotyping another 31,182 individuals (GENERALIZATION step). Apart from previously identified FTO, MC4R, and TMEM18, we detected two new loci for obesity: one in SDCCAG8 (serologically defined colon cancer antigen 8 gene; p = 1.85×10−8 in the DISCOVERY step) and one between TNKS (tankyrase, TRF1-interacting ankyrin-related ADP-ribose polymerase gene) and MSRA (methionine sulfoxide reductase A gene; p = 4.84×10−7), the latter finding being limited to children and adolescents as demonstrated in the GENERALIZATION step. The odds ratios for early-onset obesity were estimated at ∼1.10 per risk allele for both loci. Interestingly, the TNKS/MSRA locus has recently been found to be associated with adult waist circumference. In summary, we have completed a meta-analysis of two GWAS which both focus on extremely obese children and adolescents and replicated our findings in a large followed-up data set. We observed that genetic variants in or near FTO, MC4R, TMEM18, SDCCAG8, and TNKS/MSRA were robustly associated with early-onset obesity. We conclude that the currently known major common variants related to obesity overlap to a substantial degree between children and adults.
Introduction
Recent genome-wide association studies (GWAS) conducted in adult population-based samples assessed for body mass index (BMI) or in case-control designs for extreme obesity led to the discovery of genetic loci relevant for body weight regulation. The first genetic loci were detected via variants in intron 1 of the FTO (fat mass and obesity associated gene; e.g., [1]–[4]) and variants approx. 200 kb downstream of MC4R (melanocortin 4 receptor gene; [5]–[8]) reported by the GIANT (Genetic Investigation of ANthropometric Traits) consortium. This consortium subsequently detected six additional genetic loci relevant for BMI in a meta-analysis of 15 GWAS based on 32,387 probands and large confirmation samples (>58,000 individuals; with single nucleotide polymorphisms (SNP) in or near TMEM18, transmembrane protein 18 gene; KCTD15, potassium channel tetramerization domain containing 15 gene; GNPDA2, glucosamine-6-phosphate deaminase 2 gene; SH2B1, SH2B adapter protein 1 gene; MTCH2, mitochondrial carrier homologue 2 gene; NEGR1, neuronal growth regulator 1 gene). In parallel, a combined analysis of 34,416 individuals from Iceland, the Netherlands, North America (European and African descent) and Scandinavia revealed 11 regions of genome-wide significance at ≤1.6×10−7 (in or near FTO; MC4R; TMEM18; KCTD15; SH2B1; NEGR1; SEC16B, SEC16 homologue B gene; ETV5, ets variant gene 5; BDNF, brain-derived neurotrophic factor gene and two gene rich loci on chromosome 6p21.33 and 12q13.13 with the closest genes AIF1, allograft inflammatory factor 1 gene, and BCDIN3D, BCDIN3 domain containing gene, respectively). Finally, shifting to the analysis of extremely obese subjects, Meyre et al. [9] analyzed GWAS data from 1,380 Europeans with early-onset and morbid adult obesity and 1,416 age-matched normal-weight controls and reported three new risk loci in NPC1 (Niemann-Pick disease, type C1 gene), near MAF (v-maf musculoaponeurotic fibrosarcoma oncogene homolog gene) and PTER (phosphotriesterase related gene), which were followed-up in 14,186 European subjects. Altogether, 16 genetic loci relevant for body weight regulation have been identified by these three GWAS approaches [10]–[12].
While meta-analytic combinations of multiple GWAS were highly successful in population-based samples, no such approach has up to now been applied to case-control designs for obesity. Here we combined GWAS based on two samples that were specifically ascertained for the analysis of paediatric extreme obesity [3], [9]. We aimed to identify genetic loci that are relevant for early onset extreme obesity and to determine effect sizes of such loci for obesity in adults and in population-based samples including both children and adults (see Figure 1 for the general design of the study).
Fig. 1. Study design to discover consistently associated genetic loci for (early-onset) obesity. 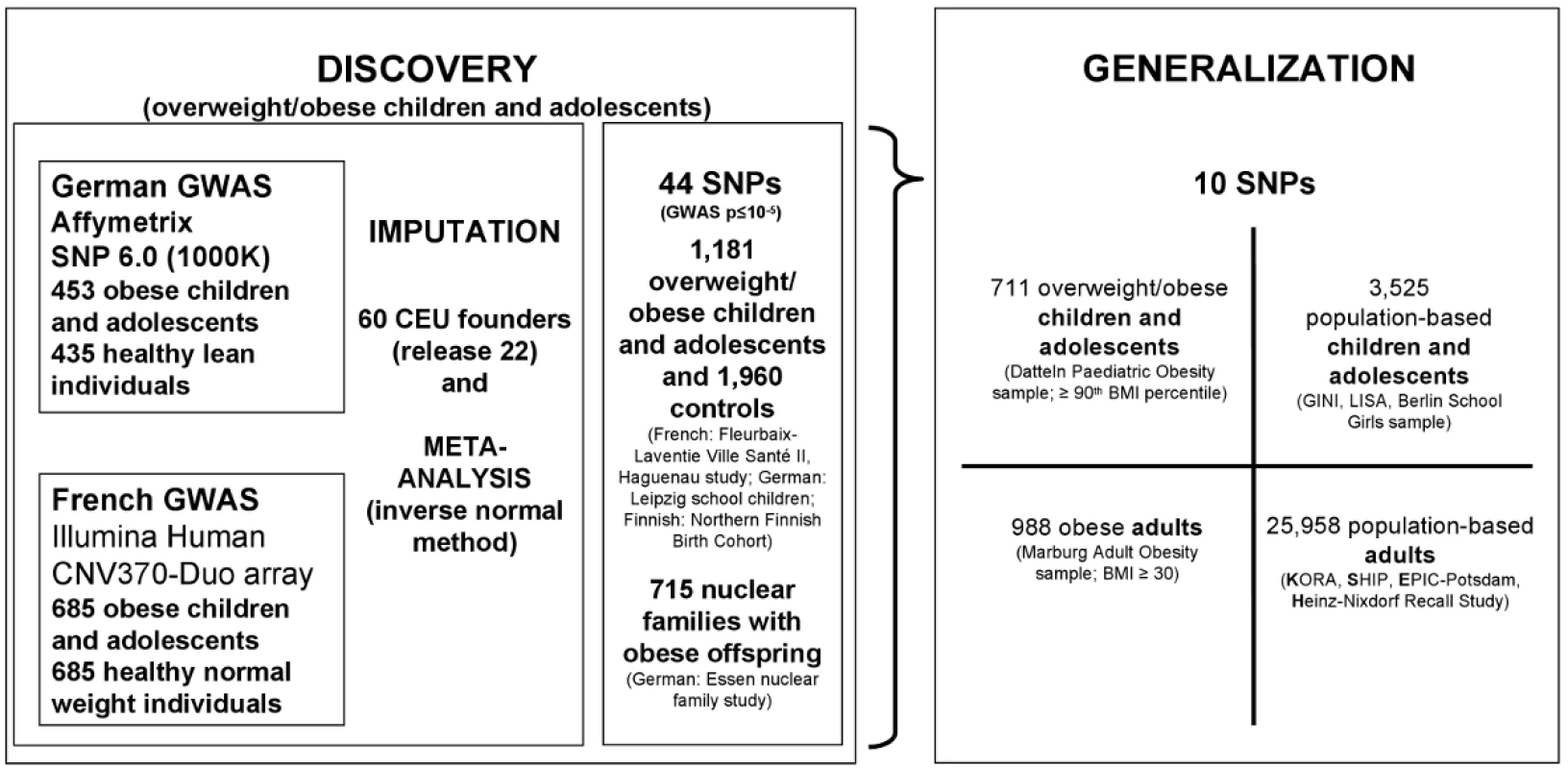
In the DISCOVERY step we jointly analysed two GWAS focussing on extremely obese children and adolescents. Markers with the smallest p-values of the GWAS were validated in independent case-control and nuclear family samples again with a focus on overweight/obese children and adolescents. Afterwards, in the GENERALIZATION step, we extended the focus in two dimensions—(i) from the extremes to the population level and (ii) from children and adolescents to adults. Note that we used controls selected from the population-based samples for the cases-control comparison with obese individuals for the GENERALIZATION (BMI quartile < median for children & BMI <25 kg/m2 for adults). In particular, our study design was based on two steps to enable hypothesis-free SNP identification and confirmation. In the DISCOVERY step, we screened 2,239,392 genotyped or imputed SNPs and tested 1,596,878 SNPs (after quality control) for association in a combined French and German sample of 1,138 extremely obese children and adolescents and 1,120 normal - or underweight controls as based on a minor allele frequency above 1%. Next, we (de novo) genotyped all SNPs with strong evidence for an association to obesity (according to p-value ranking; for details see “Materials and Methods” and Text S1) in independent samples of 1,181 obese children and adolescents and 1,960 normal - or underweight controls and in up to 715 nuclear families with at least one extremely obese offspring. In the GENERALIZATION step, we extended the focus of our study in two dimensions - (i) from children and adolescents to adults and (ii) from (extreme) obesity to the population level (in sum we (de novo) genotyped 31,182 individuals in the GENERALIZATION step).
In addition to our hypothesis-free step-wise design, we aimed to re-confirm the associations of the recently reported GWAS-based genetic loci for body weight regulation [9], [13], [14] in our paediatric extreme obesity GWAS meta-analysis.
Results
In our GWAS meta-analysis based on the German and French study groups encompassing both young obese cases and normal weight or lean controls we discovered three SNPs with genome-wide significance (Table 1 and Figure 2, Figure S1) even when applying the conservative Bonferroni correction at αBF≈3.1×10−8 for all 1,596,878 SNPs. While two markers are located in the previously reported FTO (intron 1; rs1421085; p = 2.99×10−8) and downstream of MC4R (rs17700144; p = 2.40×10−8), rs473034 indicates a new genetic locus for early onset extreme obesity located on chromosome 8p23.1 (p = 2.77×10−8) with the closest genes TNKS (tankyrase, TRF1-interacting, ankyrin-related ADP-ribose polymerase gene; ∼135 kb upstream of rs473034) and MSRA (methionine sulfoxide reductase A gene; ∼178 kb downstream of rs473034). In addition to the three genome-wide significant regions, the GWAS data revealed 18 genomic regions of interest which were defined by (i) two-sided p-values of a lead SNPs ≤10−5 and (ii) more than a single SNP within a locus (lead SNP ±500 kb) showing evidence for association as defined via a p-value rank <1,500 (roughly corresponding to p≤5×10−4; for details see Text S1).
Fig. 2. Results of the meta-analysis of two genome-wide association studies for early-onset extreme obesity. 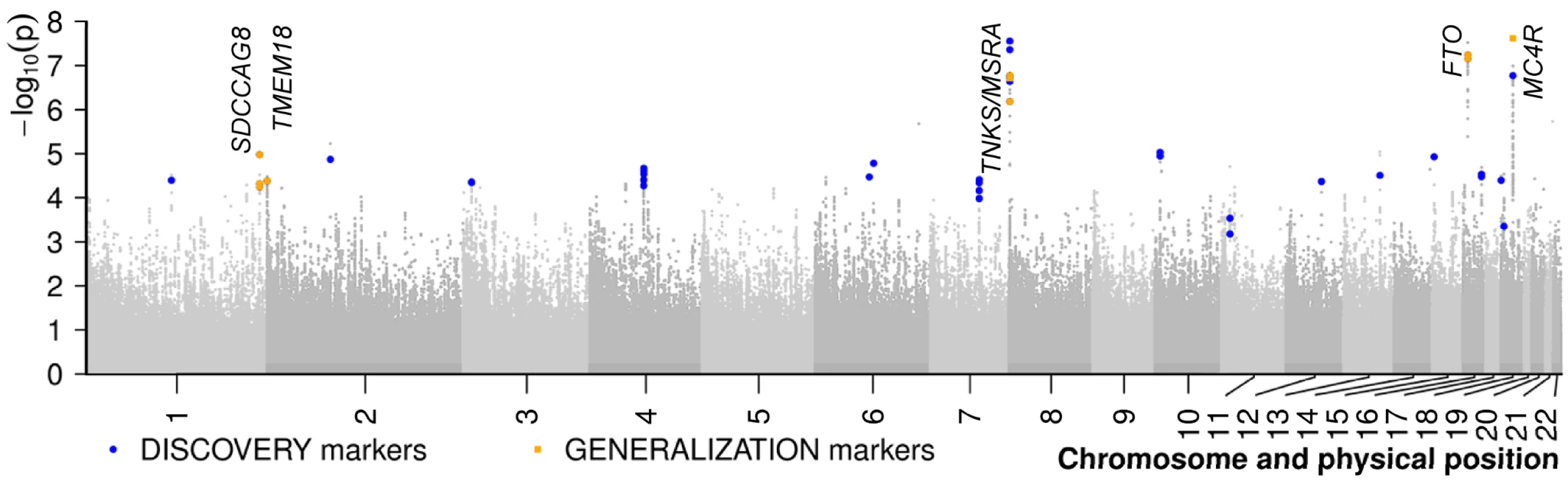
SNPs are plotted on the x-axis according to their position on each chromosome (HapMap, release 22) against the association signal on the y-axis (shown as -log10 of the two-sided (deflated/adjusted) p-value). SNPs genotyped in the independent samples of the DISCOVERY step are shown as blue circles (some of them are proxy SNPs of the best signals). SNPs followed-up in the GENERALIZATION step as well are shown as orange squares. For details on the marker selection see Text S1. Tab. 1. DISCOVERY and GENERALIZATION. 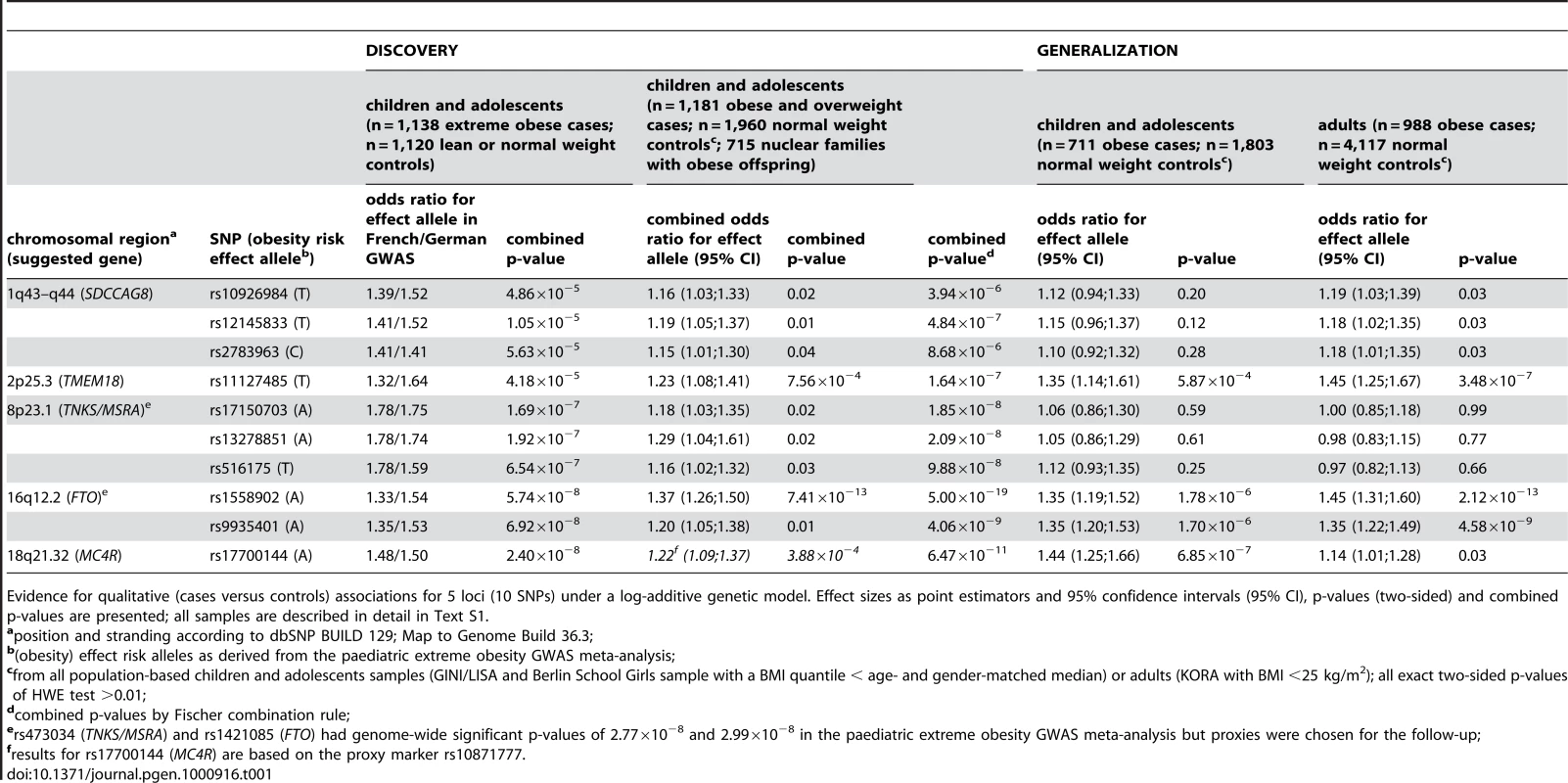
Evidence for qualitative (cases versus controls) associations for 5 loci (10 SNPs) under a log-additive genetic model. Effect sizes as point estimators and 95% confidence intervals (95% CI), p-values (two-sided) and combined p-values are presented; all samples are described in detail in Text S1. As part of our DISCOVERY step, we subsequently (de novo) genotyped 44 SNPs representing these 21 genomic regions of interest in independent 1,181 obese children and adolescents and 1,960 normal - or underweight controls and in up to 715 nuclear families with at least one extremely obese offspring (Table 1; Table S3). For 5 out of the 21 regions the association was directionally consistent (i.e. we observed the same obesity risk effect allele as in our GWAS meta-analysis) and the minimum combined p-value for each region across the samples was p≤5×10−4 (Table 1; for details see Text S1). These 5 genomic regions included three known loci on chromosome 2p25.3 (TMEM18), 16q12.2 (FTO), 18q21.32 (3′ of MC4R) as well as two new loci on chromosome 1q43-q44 and on chromosome 8p23.1 (Figure 2, Figure S2). The SNPs of the first new locus on chromosome 1q43-q44 are located within introns of the SDCCAG8 (serologically defined colon cancer antigen 8 gene) whereas the second new locus on chromosome 8p23.1 between the TNKS and MSRA had already showed evidence for an association at the genome-wide level in the initial paediatric extreme obesity GWAS meta-analysis.
Based on these results, we extended the focus of our study in two dimensions - from children and adolescents to adults and from the extremes to the population level - looking for GENERALIZATION of the replicated 5 regions represented by 10 SNPs (Table 1). Comparing children and adolescents to adults using case-control designs with overweight and obese cases vs. normal weight controls revealed directionally consistent (see above) findings for the variants of FTO, TMEM18 and the novel SDCCAG8 (Table 1). Similarly the odds ratios for the respective obesity risk effect alleles did not vary strongly by group (children and adolescents vs. adults) with point estimates ranging between 1.35–1.45 (FTO), 1.35–1.45 (TMEM18) and 1.10–1.19 (SDCCAG8). For the SNPs related to MC4R and the new TNKS/MSRA locus, however, we observed age dependent differences: For MC4R, we confirmed the findings by Loos and co-workers [6] by finding a stronger effect size estimator in children and adolescents as compared to adults (1.44 vs. 1.14 for rs17700144 of MC4R; p = 9.39×10−3 for the interaction of genotype and group). For TNKS/MSRA, we found an effect in children and adolescents but no effect in adults (e.g., 1.12 vs. 0.97 for rs516175). These differences in obesity risk effects between children and adolescents as compared to adults, however, were not due to large differences in allele frequencies as based on the population-based samples with a maximum difference of 0.82% for rs11127485 of TMEM18. We then compared (extreme) obesity assessed in case-control designs to the analyses of quantitative BMI data derived from population-based samples in the GENERALIZATION step (3,525 children and adolescents and 25,958 adults of European origin; Table 1, Table 2). BMI analyses revealed that the two SNPs in FTO and TMEM18 would have also been detectable using population-based samples of the given sizes from children/adolescents and adults (p-values 7.87×10−4 and 9.99×10−16 for FTO and 0.01 and 9.97×10−12 for TMEM18 with the values in the adults being even significant at a stringent genome-wide significance level of α = 5×10−8). The MC4R SNP, however, would have been harder to detect (p-values of 0.02 for children and adolescents and 1.10×10−4 for adults); detection of the two new loci SDCCAG8 and TNKS/MSRA would have been impossible (Table 2).
Tab. 2. GENERALIZATION. 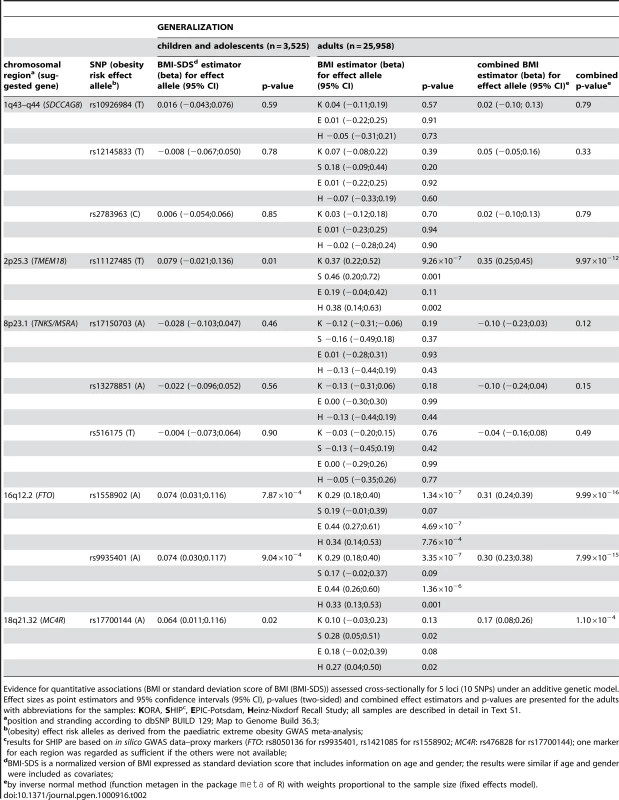
Evidence for quantitative associations (BMI or standard deviation score of BMI (BMI-SDS)) assessed cross-sectionally for 5 loci (10 SNPs) under an additive genetic model. Effect sizes as point estimators and 95% confidence intervals (95% CI), p-values (two-sided) and combined effect estimators and p-values are presented for the adults with abbreviations for the samples: KORA, SHIPc, EPIC-Potsdam, Heinz-Nixdorf Recall Study; all samples are described in detail in Text S1. In sum, our hypothesis-free step-wise design revealed three known (FTO, MC4R and TMEM18) and two new loci (SDCCAG8 and TNKS/MSRA) with estimated odds ratios that ranged from ∼1.07 to ∼1.44 in children and adolescents and from ∼1.17 to ∼1.45 in adults with the strongest overall signals related to the FTO locus. Modelling of the joint and epistatic effects revealed that <1% of the BMI (or BMI-SDS when BMI is expressed as standard deviation score) variance can be attributed to the five variants analyzed in or near TNKS/MSRA, SDCCAG8, TMEM18, FTO, and MC4R. For children and adolescents this value did not change upon inclusion of gender, age and age2 as covariates whereas it changed to 11% for the adult sample (KORA S2-S4). Applying the model including the same covariates derived in one population-based data set of adults (KORA S2-S4) to a second population-based data sets of adults (Heinz-Nixdorf Recall Study) r2 dropped from 11% to ∼2%. Proceeding similarly for epistatic effects, we found no evidence for strong epistatic effects using regression tree analyses (Figure S3, Figure S4).
In addition to our hypothesis-free step-wise design, we investigated our paediatric extreme obesity GWAS meta-analysis data focussing on recently reported GWAS-based candidate markers [9], [13], [14]. For the 16 confirmed genetic loci for which quality controlled genotyped or imputed SNPs were available, two loci on chromosome 1 (1p31.1–NEGR1, 1q25.2 - SEC16B), a locus on 11p14.1 near BDNF, and a gene-rich locus on 12q13.13 near BCDIN3D all showed directionally consistent effects of the respective SNPs (all p≤.005). Details on all analysed candidate gene SNPs are provided in Table S4 and Table S5. Note that the 16 confirmed genetic loci [9], [13], [14] correspond to 46 SNPs in our GWAS meta-analysis; in case of multiple markers at the same locus all showed evidence for strong LD (r2>.9).
Discussion
We identified two new genomic loci associated with paediatric obesity on chromosomes 1q43–q44 and 8p23.1 by a meta-analysis of two GWAS for early onset extreme obesity with a total 2,258 individuals of European origin. In addition, we confirmed the three known loci FTO, MC4R and TMEM18 using a hypothesis-free step-wise design. Leaving the hypothesis-free approach and focussing on known GWAS-based candidate markers, we were able to substantiate another four loci (NEGR1, SEC16B, BDNF and BCDIN3D) of the 16 obesity loci previously detected in GWAS [6], [9], [13], [14]. Thus, we demonstrate that the currently known major common variants related to obesity overlap to a substantial degree between children and adults confirming previous observations for FTO, MC4R, TMEM18, NEGR1 [2], [6], [14] and extending this observation to SEC16B, BDNF and BCDIN3D; [13], [14]. As our meta-analysis includes data from Meyre et al. [9] an independent well-powered replication of NPC1, MAF and PTER was not possible here.
The new chromosome 1q43–q44 locus was represented by three SNPs in strong pairwise LD (r2>.9) which are located in introns 6, 9 and 10 of SDCCAG8. There is no obvious indication for an involvement of SDCCAG8 in body weight regulation. Data on this gene are scarce. It has been shown that SDCCAG8 is located in centrosomes during interphase and mitosis in human and murine cells. N - and C - terminal truncations of the human protein alter this location; a possible role of SDCCAG8 (alternative name: NY-CO-8) in centrosomal organization has been suggested [15]. It is considered to be a naturally occurring autoantigen [16]. SDCCAG8 is ubiquitously expressed, amongst other tissues in thymus, small intestine, colon mucosa, liver and brain (http://www.genecards.org/cgi-bin/carddisp.pl?gene=SDCCAG8). Hypothalamus, pituitary and adrenals have been shown to have a particularly high transcript abundance. This pattern indicates a role of SDCCAG8 in this pivotal hormonal axis that is well-known for its impact on body weight regulation [16]. Other candidate genes in proximity of the three SNPs include CEP170 (centrosomal protein 170 kDa gene, ∼95 kb downstream of rs12145833) and AKT3 (v-akt murine thymoma viral oncogene homolog 3 (protein kinase B, gamma) gene, ∼168 kb upstream of rs12145833) with the latter being the more interesting candidate. The protein encoded by this gene is a member of the AKT family known to regulate cell signalling in response to insulin and growth factors. In particular AS160, an Akt substrate of 160 kDa, and TBC1D1 (TBC1 domain family, member 1) have been suggested to have complementary roles in regulating vesicle trafficking in response to insulin [17] with TBC1D1 being persuasively linked to body weight regulation [18]–[20]. However, we observed no evidence for strong pairwise LD (r2>.9) to any likely functional relevant variant in a region of ±1 Mb around the lead SNP (rs12145833) using Ensembl (version 56; GRCh37, 02/2009; Figure S6).
The new chromosome 8p23.1 locus, for which we observed genome-wide significance in our GWAS meta-analysis (Figure 1, ), was also represented by three SNPs with strong pairwise LD (r2>.9). TNKS and MSRA are the genes located closest to our association finding. MSRA encodes a repair enzyme for oxidative damage in proteins by enzymatic reduction of methionine sulfoxide. Oxidation of methionine residues in proteins is considered to be an important consequence of oxidative damage to cells [21]. Oxidation of proteins by reactive oxygen species (ROS) is generally associated with oxidative stress, aging and many neurodegenerative diseases such as Alzheimer's disease [21]. Also, obesity is associated with oxidative stress in the mitochondrion, with the chronic excess of ROS resulting in mitochondrial dysfunction in liver and skeletal muscle contributing to insulin resistance [22]. MSRA is mainly expressed in kidney followed by liver, brain, and adipose tissue (http://biogps.gnf.org/#goto=genereport&id=4482). The other candidate gene at the chromosome 8p23.1 locus is TNKS which is ubiquitously expressed (http://biogps.gnf.org/#goto=genereport&id=8658). Tankyrase is a Golgi-associated poly-ADP-ribose polymerase, which is involved in the regulation of GLUT4 trafficking in 3T3-L1 adipocytes. Mice lacking Tnks show increased energy expenditure, fatty-acid oxidation, and insulin-stimulated glucose utilization; they are lean even with excessive food intake [23]. In other GWAS, the 8p23.1 genomic region has been related to increased triglyceride levels [24] and to waist circumference in adults [21]. The variants with the strongest reported association signals (rs7819412; rs7826222 which is now labelled rs545854) are about 1.3 and .08 Mb downstream of our best finding (rs473034). For the former, the association to obesity was moderate in our GWAS meta-analysis data (p = 0.02) whereas for the latter no genotype data were available (with pairwise LD between rs545854 and rs473034 of r2<.01 (D' = .03) according to Ensembl version 56). Thus, further research is needed to elucidate if our finding for TNKS/MSRA detected in paediatric extremes of the quantitative trait BMI and the finding for waist circumference in adults [21] point to the same underlying genetic mechanism.
In our study we used two steps to enable hypothesis-free SNP identification and confirmation covering the extremes and the population distribution of BMI in paediatric as well as adult samples. Both dimensions of our design are related to statistical power considerations and the genetic architecture of the phenotype studied. A case-control design with highly selected individuals outperforms a design using unselected population-based individuals if the same number of individuals are genotyped and if the same alternative hypothesis holds true (see Text S1). This contrast will be aggravated the more extreme the selection and possibly also the younger the subjects [25]. In addition the selection of extremes may lead to the detection of genetic variations that are rare in the population, that accumulated in families and that might result in stronger effect sizes. Nevertheless, the power of our GWAS meta-analysis sample is still limited for small effects (see Text S1) and growing consortia like GIANT [14] will be best suited to detect them. Not surprisingly, we confirmed the strongest effects (odds ratio for the obesity risk effect alleles of ∼1.4) reported for children and adolescents near FTO, MC4R and TMEM18 [12] but also found support for variants near NEGR1, SEC16B, BDNF and BCDIN3D. Thus, one may speculate, that the genetic architecture in the paediatric extremely obese is in part similar to the BMI findings based mainly on adults from large population-based assessments (e.g. [13], [14]). On the other hand, some of the related effect sizes of these variants seem to vary longitudinally as shown here for MC4R and previously stressed by others [6], [26] while other genetic loci might only be relevant for (paediatric) extreme obesity such as TNKS/MSRA.
In conclusion, two new loci related to body weight regulation were identified using highly selected paediatric samples from the extremes of the quantitative phenotype BMI. By showing that one locus is relevant across all age groups whereas the impact of a second is limited to childhood and adolescence, our data support previous studies showing the importance of age-related aspects upon interpretation of GWAS signals.
Materials and Methods
Study samples, genotyping, and quality control
Our study design consisted of two steps (Figure 1). As first part of the DISCOVERY step we performed a meta-analysis of two genome-wide association studies (GWAS) including 1,370 individuals of French and 888 of German ancestry, defined by self-reported ethnicity. Ascertainment in both GWAS was very similar with a focus on extremely obese children and adolescents and normal weight or lean controls (Table S1). Body-mass-index (BMI in kg/m2) was calculated and the extremes were defined using percentile criteria of large population-based samples of the general population [27], [28]. We applied the cut-offs ≥97th percentile and ≥90th percentile to define ‘obesity’ and ‘overweight’ in children and adolescents; most of the cases with extreme obesity had a BMI ≥99th percentile (Table S1; [29]). Whole-genome genotyping was carried out using the Illumina Human CNV370-Duo array (French GWAS) and the Affymetrix Genome-Wide Human SNP Array 6.0 (German GWAS). Genotype data quality measures, e.g. genotype calling rates, were similar in both GWAS (Table S2). To combine both datasets, the GWAS genotypes were imputed using publicly available HapMap CEU (release 22; http://www.hapmap.org). From this GWAS meta-analysis, we selected 44 SNPs covering 21 loci (Table S3; Figure S5) which we (de novo) genotyped in 1,181 overweight and obese children and adolescents and 1,960 normal weight or lean children and adolescents and young adults (controls) of European ancestry and up to 715 nuclear families with obese offspring of European ancestry were examined. The SNP selection was based on (i) an unadjusted two-sided p-values ≤10−5 and (ii) more than a single SNP within a locus (lead SNP ±500 kb) showing evidence for association (with a p-value rank <1,500 roughly corresponding to p≤5×10−4; for details see Text S1). Sub-whole genome SNP genotyping was performed using by the MALDI-TOF mass spectrometry-based iPLEX Gold assay. In the GENERALIZATION step, 10 SNPs, for which DISCOVERY step had revealed consistent observations (Table 1; Table 2), were further investigated for generalizability to adults and to unselected population-based samples. Thus, 711 overweight and obese children and adolescents (Datteln Paediatric Obesity sample), 3,525 children and adolescents from the general population (GINI, LISA, Berlin School Girls), 988 obese adults (Marburg Adult Obesity sample) and 25,958 adults from the general population (EPIC-Potsdam Study, KORA S2-S4, SHIP, Heinz-Nixdorf Recall Study) each of European ancestry were genotyped. SNP genotyping was performed by the MALDI-TOF mass spectrometry-based iPLEX Gold assay at the Helmholtz Zentrum, München and at the Department of Genomics, Life & Brain Center, Bonn or by KBioscience, Hoddeston, UK. All were assessed for genotype calling rates and deviations from Hardy–Weinberg equilibrium (for details see Text S1).
The RefSeq accession numbers for the reported genes are: FTO: NM_001080432; MC4R: NM_005912; TNKS: NM_003747; SDCCAG8: NM_006642.2; TMEM18: NM_152834; CEP170: NM_014812; AKT3: NM_181690.
Statistical analysis
After similar quality control analyses of both GWAS, the imputed GWAS were jointly analysed using the inverse normal method to combine p-values of allele-based chi-square tests. Details on the imputation and on the marker selection for the follow-up are described in Text S1. In the paediatric extreme obesity GWAS meta-analysis data set we also explored genetic variants for obesity recently derived from other GWAS [9], [13], [14] and variants for ‘classical’ obesity candidate genes [3], [30] by testing the best SNP reported in Scuteri et al. [4].
In both the DISCOVERY and the GENERALIZATION part of the study either log-additive or additive genetic models were applied. Case-control samples were analysed using logistic regression (both with and without gender and age as covariates). The nuclear families were analysed using UNPHASED (Version 3.0.13; [31]) which addresses the correlation among sibs and provides estimators; nuclear family data and case-control data sets were combined using a method described in [32]. In the GENERALIZATION step, BMI in adults of population-based samples was analysed using linear regression with gender and age as covariates. Similarly, we used linear regression analyses for the population-based samples of children and adolescents. However, as phenotype we used a normalized version of the BMI applying Cole's least mean square method [33] to express BMI as a standard deviation score (BMI-SDS) which is comparable to the BMI z-score as e.g. used by the Center for Disease Control and Prevention (http://www.cdc.gov/). As BMI-SDS already includes information on gender and age additional sensitivity analyses were performed where these covariates were omitted. Note that the case-control analyses in GENERALIZATION step are not completely independent from the population-based analyses. In particular, controls in GENERALIZATION were individuals from the population-based samples which either had a BMI<25 for adults or a BMI percentile below the median. Due to the similarity to the original design it was nevertheless decided to report both analyses.
As secondary sensitivity analyses, we performed gender stratified analyses in all GENERALIZATION samples for the markers which we followed-up. We explored the recessive and dominant genetic model, investigated the impact of the control group cut-off for the case-control analyses (results not shown as they did not alter the conclusions drawn here) and explored joint and epistatic effects (multiple linear regression and regression trees using lm, rlm, and party of R.2.9.1) of all five loci (see Figure S3, Figure S4). To address, to some extent, problems of the ‘bias-variance trade-off’ and the ‘winners curse’ [34], the largest GENERALIZATION population-based sample KORA (n = 12,002) was chosen for this modelling. The model was tested in the Heinz-Nixdorf Recall Study sample (n = 4,646). These two samples were chosen due to their largest similarities in the recruitment and due to the availability of directly genotyped SNPs. In addition, we also explored the sample of population-based children and adolescents (GINI, LISA, Berlin School Girls; n = 3,525) separately.
Unless otherwise stated, all reported p-values are nominal, two-sided and not adjusted for multiple testing. To address multiple testing in the paediatric extreme obesity GWAS meta-analysis we applied a Bonferroni-corrected αBF≈3.1×10−8 to the quality controlled SNPs on autosomes. Confidence intervals were calculated with coverage of 95% (abbreviated 95%CI). More details on quality control and power considerations are provided in Text S1.
Ethics statement
The study, including the protocols for subject recruitment and assessment, the informed consent for participants, were reviewed and approved by all local IRB boards.
Supporting Information
Zdroje
1. DinaC
MeyreD
GallinaS
DurandE
KornerA
2007 Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 39 724 726
2. FraylingTM
TimpsonNJ
WeedonMN
ZegginiE
FreathyRM
2007 A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316 889 894
3. HinneyA
NguyenTT
ScheragA
FriedelS
BrönnerG
2007 Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS ONE 2 e1361 doi:10.1371/journal.pone.0001361
4. ScuteriA
SannaS
ChenWM
UdaM
AlbaiG
2007 Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 3 e115 doi:10.1371/journal.pgen.0030115
5. GellerF
ReichwaldK
DempfleA
IlligT
VollmertC
2004 Melanocortin-4 receptor gene variant I103 is negatively associated with obesity. Am J Hum Genet 74 572 581
6. LoosRJ
LindgrenCM
LiS
WheelerE
ZhaoJH
2008 Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40 768 775
7. StutzmannF
CauchiS
DurandE
Calvacanti-ProencaC
PigeyreM
2009 Common genetic variation near MC4R is associated with eating behaviour patterns in European populations. Int J Obes (Lond) 33 373 378
8. YoungEH
WarehamNJ
FarooqiS
HinneyA
HebebrandJ
2007 The V103I polymorphism of the MC4R gene and obesity: population based studies and meta-analysis of 29 563 individuals. Int J Obes (Lond) 31 1437 1441
9. MeyreD
DelplanqueJ
ChevreJC
LecoeurC
LobbensS
2009 Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet 41 157 159
10. HinneyA
HebebrandJ
2009 Three at One Swoop! Obesity Facts 2 3 8
11. HofkerM
WijmengaC
2009 A supersized list of obesity genes. Nat Genet 41 139 140
12. WalleyAJ
AsherJE
FroguelP
2009 The genetic contribution to non-syndromic human obesity. Nat Rev Genet 10 431 442
13. ThorleifssonG
WaltersGB
GudbjartssonDF
SteinthorsdottirV
SulemP
2009 Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 41 18 24
14. WillerCJ
SpeliotesEK
LoosRJ
LiS
LindgrenCM
2009 Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41 25 34
15. KenedyAA
CohenKJ
LoveysDA
KatoGJ
DangCV
2003 Identification and characterization of the novel centrosome-associated protein CCCAP. Gene 303 35 46
16. LeeMJ
FriedSK
2009 Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am J Physiol Endocrinol Metab 296 E1230 E1238
17. ChenS
MurphyJ
TothR
CampbellDG
MorriceNA
2008 Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409 449 459
18. ChadtA
LeichtK
DeshmukhA
JiangLQ
ScherneckS
2008 Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nat Genet 40 1354 1359
19. MeyreD
FargeM
LecoeurC
ProencaC
DurandE
2008 R125W coding variant in TBC1D1 confers risk for familial obesity and contributes to linkage on chromosome 4p14 in the French population. Hum Mol Genet 17 1798 1802
20. StoneS
AbkevichV
RussellDL
RileyR
TimmsK
2006 TBC1D1 is a candidate for a severe obesity gene and evidence for a gene/gene interaction in obesity predisposition. Hum Mol Genet 15 2709 2720
21. LindgrenCM
HeidIM
RandallJC
LaminaC
SteinthorsdottirV
2009 Genome-wide association scan meta-analysis identifies three Loci influencing adiposity and fat distribution. PLoS Genet 5 e1000508 doi:10.1371/journal.pgen.1000508
22. de FerrantiS
MozaffarianD
2008 The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem 54 945 955
23. YehTY
BeiswengerKK
LiP
BolinKE
LeeRM
2009 Hypermetabolism, hyperphagia, and reduced adiposity in tankyrase-deficient mice. Diabetes
24. KathiresanS
WillerCJ
PelosoGM
DemissieS
MusunuruK
2009 Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 41 56 65
25. PietilainenKH
KaprioJ
RissanenA
WinterT
RimpelaA
1999 Distribution and heritability of BMI in Finnish adolescents aged 16y and 17y: a study of 4884 twins and 2509 singletons. Int J Obes Relat Metab Disord 23 107 115
26. Lasky-SuJ
LyonHN
EmilssonV
HeidIM
MolonyC
2008 On the replication of genetic associations: timing can be everything! Am J Hum Genet 82 849 858
27. Rolland-CacheraMF
ColeTJ
SempeM
TichetJ
RossignolC
1991 Body Mass Index variations: centiles from birth to 87 years. Eur J Clin Nutr 45 13 21
28. Kromeyer-HauschildK
2001 Perzentilen für den Body Mass Index für das Kinder - und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd 149 807 818
29. PoskittEM
1995 Defining childhood obesity: the relative body mass index (BMI). European Childhood Obesity group. Acta Paediatr 84 961 963
30. RankinenT
ZuberiA
ChagnonYC
WeisnagelSJ
ArgyropoulosG
2006 The human obesity gene map: the 2005 update. Obesity (Silver Spring) 14 529 644
31. DudbridgeF
2008 Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum Hered 66 87 98
32. KazeemGR
FarrallM
2005 Integrating case-control and TDT studies. Ann Hum Genet 69 329 335
33. ColeTJ
FaithMS
PietrobelliA
HeoM
2005 What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr 59 419 425
34. IoannidisJP
2008 Why most discovered true associations are inflated. Epidemiology 19 640 648
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 4- Délka menstruačního cyklu jako marker ženské plodnosti
- Srdeční frekvence embrya může být faktorem užitečným v předpovídání výsledku IVF
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
-
Všechny články tohoto čísla
- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
- Fragilities Caused by Dosage Imbalance in Regulation of the Budding Yeast Cell Cycle
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Activation of Estrogen-Responsive Genes Does Not Require Their Nuclear Co-Localization
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- A Pax3/Dmrt2/Myf5 Regulatory Cascade Functions at the Onset of Myogenesis
- Two New Loci for Body-Weight Regulation Identified in a Joint Analysis of Genome-Wide Association Studies for Early-Onset Extreme Obesity in French and German Study Groups
- Hypomethylation of a LINE-1 Promoter Activates an Alternate Transcript of the MET Oncogene in Bladders with Cancer
- Candidate Causal Regulatory Effects by Integration of Expression QTLs with Complex Trait Genetic Associations
- Combined Inactivation of pRB and Hippo Pathways Induces Dedifferentiation in the Retina
- Allele-Specific Virulence Attenuation of the HopZ1a Type III Effector via the ZAR1 Resistance Protein
- Down-Regulation of Honey Bee Gene Biases Behavior toward Food Rich in Protein
- A Microarray-Based Genetic Screen for Yeast Chronological Aging Factors
- Actin-Related Protein Arp6 Influences H2A.Z-Dependent and -Independent Gene Expression and Links Ribosomal Protein Genes to Nuclear Pores
- Phosphorylation of the Conserved Transcription Factor ATF-7 by PMK-1 p38 MAPK Regulates Innate Immunity in
- A -Regulatory Signature for Chordate Anterior Neuroectodermal Genes
- Genetic Analysis of Fin Development in Zebrafish Identifies Furin and Hemicentin1 as Potential Novel Fraser Syndrome Disease Genes
- Assembly of a 40 Mb Eukaryotic Genome from Short Sequence Reads: , a Model Organism for Fungal Morphogenesis
- Trait-Associated SNPs Are More Likely to Be eQTLs: Annotation to Enhance Discovery from GWAS
- Absence of Evidence for MHC–Dependent Mate Selection within HapMap Populations
- The TALE Class Homeobox Gene Defines the Anterior Compartment for Head Regeneration
- Cyclic Expression of Lhx2 Regulates Hair Formation
- Genetic Evidence for Hybrid Trait Speciation in Butterflies
- Epigenetic Regulation of a Murine Retrotransposon by a Dual Histone Modification Mark
- Chromosome 9p21 SNPs Associated with Multiple Disease Phenotypes Correlate with Expression
- S Phase Progression in Human Cells Is Dictated by the Genetic Continuity of DNA Foci
- The Next Generation Becomes the Now Generation
- Acts as a Tumor Suppressor in a Murine Retinoblastoma Model by Facilitating Tumor Cell Death
- Genome-Wide Association Study of Lp-PLA Activity and Mass in the Framingham Heart Study
- The Five Zinc Transporters Undergo Different Evolutionary Fates towards Adaptive Evolution to Zinc Tolerance in
- MicroRNA–Directed siRNA Biogenesis in
- Deletion of the WD40 Domain of LRRK2 in Zebrafish Causes Parkinsonism-Like Loss of Neurons and Locomotive Defect
- Incipient Balancing Selection through Adaptive Loss of Aquaporins in Natural Populations
- GTPase Activity Plays a Key Role in the Pathobiology of LRRK2
- Natural Single-Nucleosome Epi-Polymorphisms in Yeast
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



