-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Association of MTHFR 677C>T, 1298A>C and MTR 2756A>G Polymorphisms with Risk of Retinoblastoma
Asociace polymorfizmů MTHFR 677C>T, 1298A>C a MTR 2756A>G s rizikem rozvoje retinoblastomu
Úvod: U různých typů nádorových onemocnění byly studovány polymorfizmy MTHFR 677C>T, 1298A>C a MTR 2756A>G. Role těchto polymorfizmů v rozvoji retinoblastomu však zůstává nejasná. V této studie jsme hodnotili asociaci polymorfizmů MTHFR 677C>T, 1298A>C a MTR 2756A>G s rizikem rozvojem retinoblastomu u dětí v Íránu.
Metody: Pomocí Real-Time PCR systému ABI PRISM 7500 byly zachyceny polymorfizmy MTHFR 677C>T, 1298A>C a MTR 2756A>G u 66 pacientů s retinoblastomem a 99 zdravých kontrolních subjektů odpovídajícího věku a pohlaví. Asociace mezi těmito polymorfizmy a rizikem rozvoje retinoblastomu byla analyzována pomocí poměru šancí s 95% intervalem spolehlivosti.
Výsledky: Naše výsledky ukázaly významnou asociaci mezi polymorfizmem MTR 2756A>G a rizikem rozvojem retinoblastomu. V případě polymorfizmu MTR 2756A>G byly četnosti výskytu genotypu AG (39,4 %) a GG (9,1 %) statisticky významně vyšší v porovnání s kontrolním vzorkem (p < 0,05). U polymorfizmů MTHFR 677C>T a 1298A>C však nebyly pozorovány žádné významné rozdíly ve frekvenci alel nebo genotypové frekvenci mezi pacienty s retinoblastomem a kontrolními subjekty (p > 0,05).
Závěry: Naše výsledky naznačují možnou asociaci polymorfizmu MTR 2756A>G se zvýšeným rizikem rozvoje retinoblastomu u dětí v Íránu. Výsledky však také ukázaly, že polymorfizmy MTHFR 677C>T a 1298A>C nejsou se zvýšeným rizikem rozvoje retinoblastomu významně spojeny.
Klíčová slova:
retinoblastom – dětství – gen MTHFR – gen MTR – jednonukleotidový polymorfizmus
Authors: Mohsen Gohari 1; Alireza Seyed Dastgheib 2; Jamal Jafari-Nedooshan 3; Javad Mohammad Akbarian-Bafghi 4; Majid Morovati-Sharifabad 5; Reza Seyed Mirjalili 6; Hossein Neamatzadeh 7,8
Authors place of work: Department of Ophthalmology, Geriatric Ophthalmology Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 1; Department of Medical Genetics, School of Medicine, Shiraz University of Medical Sciences, Iran 2; Department of Surgery, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 3; Department of Health Care Management, Bam University of Medical Sciences, Iran 4; Department of Basic Science, Faculty of Veterinary Medicine, Ardakan University, Iran 5; Department of Pediatrics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 6; Department of Medical Genetics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 7; Mother and Newborn Health Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran 8
Published in the journal: Klin Onkol 2019; 32(5): 375-379
Category: Původní článek
doi: https://doi.org/10.14735/amko2019375Summary
Background: The MTHFR 677C>T, 1298A>C and MTR 2756A>G polymorphisms have been investigated in several different cancer types. However, the role of these polymorphisms in the development of retinoblastoma remains unclear. Here, we have evaluated the association of the MTHFR 677C>T, 1298A>C and MTR 2756A>G polymorphisms with the risk of retinoblastoma in Iranian children.
Methods: The MTHFR 677C>T, 1298A>C and MTR 2756A>G polymorphisms in 66 patients with retinoblastoma and 99 age-and gender-matched healthy controls were detected on the ABI PRISMs 7500 Real-Time PCR System. The association between these polymorphisms and the risk of retinoblastoma was analysed by an odds ratio with a 95% confidence interval.
Results: Our results showed a significant association between the MTR 2756A>G polymorphism and the risk of retinoblastoma. In the MTR 2756A>G polymorphism, the AG (39.4%) and GG (9.1%) genotype frequencies in the cases were found to be higher in comparison with the controls, showing a significant difference (p < 0.05). However, no significant difference was observed in the allelic or genotypic frequencies for both the MTHFR 677C>T and 1298A>C polymorphisms in the retinoblastoma patients of the controls (p > 0.05).
Conclusions: Our results suggested that the MTR 2756A>G polymorphism might be associated with an increased risk of retinoblastoma in Iranian children. However, the results show that the MTHFR 677C>T and 1298A>C polymorphisms are not significantly associated with an increased risk of retinoblastoma.
Keywords:
retinoblastoma – childhood – MTHFR gene – MTR gene – single-nucleotide polymorphism
Introduction
Retinoblastoma is the most common intraocular tumour in children, representing approximately 3% of all childhood cancers between the ages of 0–14 years [1,2]. It is responsible for approximately 3,000 cancer-related childhood deaths worldwide each year [3]. Although retinoblastoma can occur at any age, 95% of the cases are presented in children under the age of 5. Its incidence is approximately 1 in 15,000–28,000 live births and represents 2–4% of all paediatric malignancies [4]. Worldwide, it is estimated that there are approximately 5,000–8,000 new cases diagnosed each year [5]. However, it is an example of successful cancer treatment with the overall survival exceeding 95% in developed countries [6].
The data suggest that retinoblastoma is not a homogeneous tumour on the genomic level, and that inter-tumour heterogeneity exists between retinoblastoma tumours [7,8]. The majority of heritable retinoblastoma cases (75% of heritable patients) have no affected parents and are therefore called non-familial heritable patients with de novo mutation. Classically, retinoblastoma results from biallelic loss of the retinoblastoma susceptibility gene (RB1). However, with the continued development of medical genetics, an increasing number of studies have focused on the identification of RB genetic biomarkers such as gene polymorphisms, oncogenes (KIF14, MDM4, E2F3, MYCN and DEK), potential tumour suppressors (NGFR and CDH11), microRNA and long non-coding RNA [7,9]. Among them, the C677T and A1298C polymorphisms in the MTHFR gene and the 2756A>G polymorphism at the MTR gene have been assessed as potential candidates. MTHFR is an enzyme that catalyses the reduction of 5,10-methylenetetrahydrofolate to 5-methytetrahydrofolate, the carbon donor for the remethylation of homocysteine to methionine [10,11]. Moreover, the MTR product, a vitamin B12-dependent enzyme, plays a crucial role in the folate metabolic network [12].
The MTHFR gene is localised on chro-mosome 1 at 1p36.6, which includes 11 exons spanning 2.2 kb [13]. Two polymorphisms, 677C>T (exon 4) and 1298A>C (in exon 7) in the MTHFR gene have been shown to have reduced MTHFR activity. The C677T polymorphism is a C to T transition at base pair 677 resulting in an alanine to valine substitution and the A1298C polymorphism is an A to C transition at base pair 1298 leading to a glutamate to alanine substitution [14,15]. Moreover, the MTR gene is mapped to chromosome band 1q43, close to the telomeric region of the long arm and the MTR 2756A>G polymorphism is an extensively investigated polymorphism. These polymorphisms have been increasingly attracting attention in different cancer types. In recent years, a few studies have explored the possible association of the MTHFR 677C>T, 1298A>C and MTR 2756A>G polymorphisms with the risk of retinoblastoma and the results of these published studies remain inconsistent and inconclusive [16,17]. Therefore, the purpose of the present study was to analyse the association of the MTHFR 677C>T, 1298A>C and MTR 2756A>G polymorphisms with the risk of retinoblastoma in Iranian children.
Materials and Methods
Study Population
The study procedures were approved by the Ethics Committee for Human Research of Shahid Sadoughi University of Medical Sciences and Bam University of Medical Sciences. Moreover, informed consent had been obtained from all the study subjects. A total of 66 children diagnosed with retinoblastoma (ranging between 1 month and 6 years of age) who were referred to the different clinics between 2013 and 2017 were included in this study. Ninety-nine age-, gender-and ethnicity-matched unrelated healthy children who visited the clinic, with no family history of cancer, originating from the same geographical regions were included.
SNPs Genotyping
Genomic DNA was isolated from the donated venous blood samples using a commercial DNA extraction kit according to the manufacturer’s instructions and was stored at −20°C in a dedicated area that was only used for polymerase chain reaction (PCR). The MTHFR 677C>T (rs1801133), 1298A>C (rs1801131) and MTR 2756A>G (rs1805087) polymorphisms were detected on the ABI PRISMs 7500 Real-Time PCR System (PE Applied Biosystems, Foster City, CA, USA). The primers, probes and reaction conditions were obtained on the basis of published data and synthesised by Applied Biosystems. Briefly, the assay was performed under universal conditions, and PCR reactions were carried out in a final volume of 5 mL containing 500 nM of each primer, 200 nM each probe, 25 mM each dNTP, 1 M Tris-HCL (pH 8.3), 1 M MgCl2, 300 mM KCl, 100% glycerol, ROX reference dye, 1 U TaqMan Universal PCR Master Mix (Applied Biosystems) and 30 ng genomic DNA. The following thermal cycling condition was used: 60°C for 1 min and 95°C for 10 min (Taq activation), 50 cycles at 92°C for 15 s (denature), and at 60°C for 1 min (anneal/extend). After the PCR reaction, the plates were scanned by an allelic discrimination on the 7500 Real-Time PCR system using SDS 2.4 software for allelic discrimination (Applied Biosystems) to determine the genotypes by allelic discrimination. The genotyping accuracy was assessed by repeating 10% of the samples, which were selected randomly from both cases and healthy subjects, and a concordance of 100% was observed for both polymorphisms for all samples.
Statistical Analysis
The statistical analysis was performed on the software package SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 20.0 analysis was conducted to establish whether the MTHFR 677C>T, 1298A>C and MTR 2756A>G polymorphisms are associated with retinoblastoma in patients. Pearson’s chi-square (χ2) test was performed to assess the genotype frequency distribution for the different subject groups. The Hardy-Weinberg equilibrium (HWE) of the genotype distribution among the controls was tested by a goodness-of-fit χ2 test. The significance of the results was accepted if the p-value was less than 0.05. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the strength of the associations of the MTHFR 677C>T, 1298A>C and MTR 2756A>G polymorphisms with retinoblastoma.
Results
A total of 165 samples were included in this case-control study, including 66 childhood retinoblastoma cases and 99 healthy subjects. The characteristics of the retinoblastoma cases were summarised in Tab. 1. No significant differences were found between the retinoblastoma cases and controls in age and gender (p > 0.05). The distribution of the MTHFR 677C>T, 1298A>C and MTR 2756A>G polymorphisms in the cases and controls are presented in Tab. 2. All the allelic and genotypic frequencies in the controls were in agreement with the HWE equilibrium (p = 0.346, p = 0.613 and p = 0.239, respectively), and the minor allele frequencies in the control group were 0.384, 0.613 and 0.163, respectively.
Tab. 1. Clinical characteristics of retinoblastoma patients. 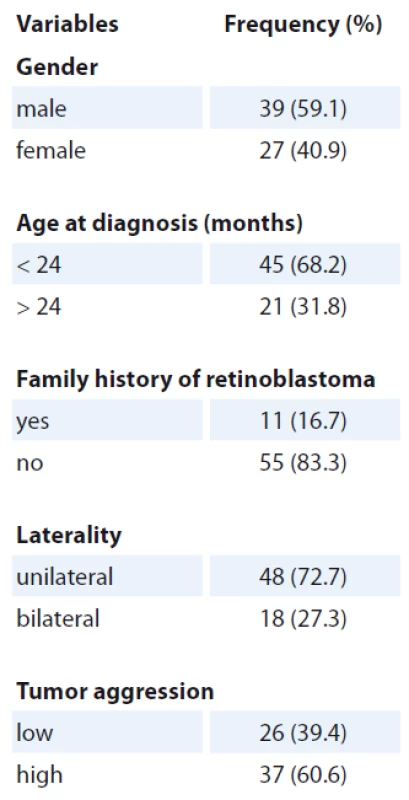
The frequencies of the MTHFR 677C>T genotypes for wild homozygotes (CC), heterozygotes (CT), and mutant homozygotes (TT) of the retinoblastoma cases were 36.7, 50.0, and 13.3%, respectively, whereas the healthy control groups showed 35.4, 52.5, and 12.1%, respectively. In the MTHFR 1298A>C polymorphism the frequency of AA, AC and CC was 59.1, 31.8 and 9.1%, respectively, in the cases and 57.6, 35.3 and 7.1%, respectively, in the controls. However, no significant risk of retinoblastoma was found to relate to the mutant genotypes of the MTHFR 677C>T and 1298A>C polymorphisms (Tab. 2). The allele frequency of both the alleles in the MTHFR 677C>T and 1298A>C polymorphisms among the cases and controls is given in Tab. 2. The values of the ORs with 95% CI for the MTHFR 677C>T and 1298A>C polymorphisms are presented in Tab. 2. Although the mutant homozygote and heterozygote genotype frequencies of both the polymorphisms in the cases were higher in comparison with the controls, the polymorphisms did not show a significant association between the cases and controls (Tab. 2).
Tab. 2. Association of MTHFR 677C>T, 1298A>C and MTR 2756A>G polymorphisms with retinoblastoma risk. 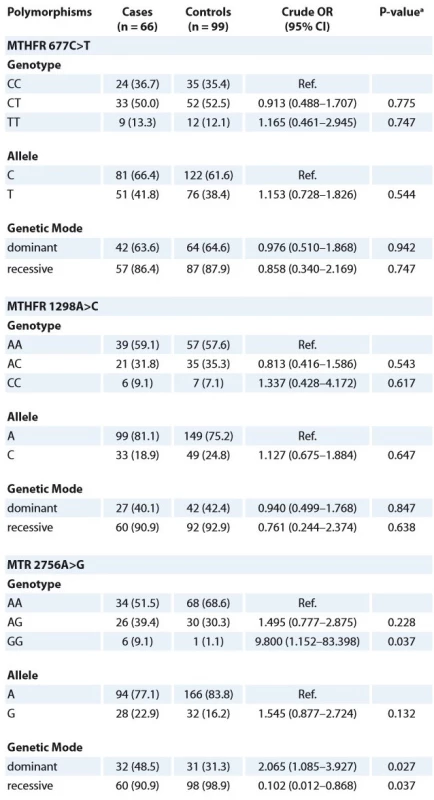
a two-sided χ2 test for the frequency distributions of genotype between cases and controls OR – odds ratios, CI – confi dence intervals In the MTR 2756A>G polymorphism, the AG (39.4%) and GG (9.1%) genotype frequencies in the cases were found to be higher in comparison with the controls, showing a significant difference (p < 0.05). However, the prevalence of the MTR 2756G allele was not significantly higher in the cases (0.132) (Tab. 2). Moreover, the values of the ORs with 95% CI were as follows: dominant model (AG/GG, OR 2.065, 95% CI 1.085–3.927, p = 0.027) and recessive model (AA/AG, OR 0.102, 95% CI 0.012–0.868, p = 0.037) (Tab. 2).
Discussion
Retinoblastoma is an embryonic malignant neoplasm of retinal origin [2,18]. Retinoblastoma tumours can be hereditary or spontaneous and they display a high degree of genetic heterogeneity [19]. Mutations in the RB1 gene have been implicated to play a role in the heritable genetic form of this disease [7]. According to the statistics, about 40% of all retinoblastoma patients have a germline mutation in the RB1 gene, although only less than 7% have a positive family history [20,21]. The risk of retinoblastoma is substantially increased when the subject has parents who have had retinoblastoma or are of an advanced parental age, as well as subjects who were conceived by in vitro fertilisation [22,23].
The genetic variants at the MTHFR gene have been implicated as risk factors for several types of tumours [24]. However, reports on the association of polymorphisms at the MTHFR gene with susceptibility of retinoblastoma are inconclusive. In the present study we evaluated the association of the MTHFR 677C>T and 1298A>C polymorphisms with the risk of retinoblastoma in central and southern Iranian children. Our results showed that the MTHFR 677C>T and 1298A>C polymorphisms were not significantly associated with an increased risk of retinoblastoma in the Iranian population. Similarly, de Lima et al. have investigated the roles of MTHFR 677C>T and 1298 A>C, MTR 2756A>G, RFC 80A>G and TYMS in retinoblastoma susceptibility in a population from north-east Brazil. They found that the MTHFR 677C>T and 1298A>C, RFC 80A>G and TYMS polymorphisms are not significantly associated with an increased risk of retinoblastoma [17]. Moreover, Bisht et al. have found a greater frequency of a mutant heterozygous genotype for both the MTHFR 677C>T and 1298A>C polymorphisms in retinoblastoma patients than in the healthy controls. Therefore, they have found a strong association of the MTHFR 677C>T and 1298A>C polymorphisms with retinoblastoma pathogenesis in an Indian population. They have suggested a possible and important role of a one-carbon metabolism pathway, methylation regulation, and an increased risk of retinoblastoma initiation [25]. Inconsistent with our results, the previous study in the Iranian population demonstrated that MTHFR 677C>T is associated with retinoblastoma. In 2016, Soleimani et al. conducted a case-control study of 96 patients with retinoblastoma and 204 healthy controls to investigate the association of the MTHFR 677C>T and 1298A>C polymorphisms with susceptibility of retinoblastoma in a northern population from Iran. Their results showed that the MTHFR 677C>T polymorphism was associated with the risk of retinoblastoma in the Iranian population. Moreover, they have reported that the T allele had a protective effect on the susceptibility of retinoblastoma [16]. Iran is a multi-national or multi-racial community with different ethnic groups, including Persians, Azeri, Kurds, Gilakis, Mazandaranis, Lurs, Tats, Talysh, Turkmen, Arabs and Baloch [26]. Thus, the differences between our results and the previous study might be describing a distinct pattern of MTHFR gene polymorphisms in different ethnic groups in Iran. Moreover, other genetic and environmental factors may modify the effect of MTHFR polymorphisms in Iranian ethnic groups.
The MTR gene encodes a protein containing 1,265 amino acids (140.5 kDa), which plays a key role in DNA repair, maintaining adequate intracellular folate, methionine and normal homocysteine concentrations [18]. The MTR gene contains a common polymorphism at nucleotide 2756 (MTR 2756A>G), which promotes the substitution of aspartic acid with glycine residue, affecting the enzyme activity and inducing hyperhomocysteinemia and DNA hypomethylation [17,27]. Plenty of studies have found that MTR A2756G polymorphism has been associated with different types of cancers [12,27]. In this case-control study, the association between MTR 2756A>G polymorphism and risk of retinoblastoma was also investigated. Our results showed that the MTR 2756A>G polymorphism is significantly associated with an increased risk of retinoblastoma. Similarly, Akbari et al. have found a significant association between the MTR 2756A>G polymorphism and the risk of retinoblastoma in the Iranian patients [18]. Moreover, de Lima et al. have showed that this polymorphism is associated with an increased risk of retinoblastoma in the Brazilian population [17]. These findings suggested that the MTR 2756A>G polymorphism may be associated with the development of retinoblastoma, possibly by reducing S-adenosyl-methionine (SAM) levels and causing DNA hypomethylation.
In summary, the current study results showed that the MTHFR 677C>T and 1298A>C polymorphisms may not be associated with an increased risk of retinoblastoma in Iranian children. However, we have found that the MTR 2756A>G polymorphism is significantly associated with an increased risk of retinoblastoma in Iranian children. Moreover, the main limitation of this study is the small population size being analysed for the MTHFR 677C>T, 1298A>C and MTR 2756A>G polymorphisms. Therefore, to validate these associations and our findings further, large and well-designed epidemiological studies are warranted.
The authors would like to thank the patients and controls who participated in the present study.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Accepted/Přijato: 15. 8. 2019
Dr. Jamal Jafari-Nedooshan
Department of General Surgery
Shahid Sadoughi Hospital
Avicenna Blvd, Shahid Ghandi Blvd
Safayeh, Yazd, Iran
e-mail: jamalnedooshan@yahoo.com
Zdroje
1. Dimaras H, Corson TW, Cobrinik D et al. Retinoblastoma. Nat Rev Dis Primers 2015; 1 : 15021. doi: 10.1038/nrdp.2015.21.
2. Francis JH, Levin AM, Abramson DH. Update on ophthalmic oncology 2014: retinoblastoma and uveal melanoma. Asia Pac J Ophthalmol (Phila) 2016; 5 (5): 368–382. doi: 10.1097/APO.0000000000000213.
3. Kivelä T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol 2009; 93 (9): 1129–1131. doi: 10.1136/bjo.2008.150292.
4. Pandey AN. Retinoblastoma: an overview. Saudi J Ophthalmol 2014; 28 (4): 310–315. doi: 10.1016/ j.sjopt.2013.11.001.
5. Abramson DH. Retinoblastoma in the 20th century: past success and future challenges the Weisenfeld lecture. Invest Ophthalmol Vis Sci 2005; 46 (8): 2683–2691. doi: 10.1167/iovs.04-1462.
6. Tamboli D, Topham A, Singh N et al. Retinoblastoma: a SEER dataset evaluation for treatment patterns, survival, and second malignant neoplasms. Am J Ophthalmol 2015; 160 (5): 953–958. doi: 10.1016/j.ajo.2015.07.037.
7. Stenfelt S, Blixt MK, All-Ericsson C et al. Heterogeneity in retinoblastoma: a tale of molecules and models. Clin Transl Med 2017; 6 (1): 42. doi: 10.1186/s40169-017-0 173-2.
8. Kooi IE, Mol BM, Massink MP et al. Somatic genomic alterations in retinoblastoma beyond RB1 are rare and limited to copy number changes. Sci Rep 2016; 6 : 25264. doi: 10.1038/srep25264.
9. Dimaras H, Khetan V, Halliday W et al. Loss of RB1 induces non-proliferative retinoma: increasing genomic instability correlates with progression to retinoblastoma. Hum Mol Genet 2008; 17 (10): 1363–1372. doi: 10.1093/hmg/ddn024.
10. Azarpira MR, Ghilian MM, Sobhan MR et al. Association of MTHFR and TNF-α genes polymorphisms with susceptibility to Legg-Calve-Perthes disease in Iranian children: a case-control study. J Orthop 2018; 15 (4): 984–987. doi: 10.1016/j.jor.2018.08.042.
11. Kamali M, Hantoushzadeh S, Borna S et al. Association between thrombophilic genes polymorphisms and recurrent pregnancy loss susceptibility in the Iranian population: a systematic review and meta-analysis. Iran Biomed J 2018; 22 (2): 78–89.
12. Shao HB, Ren K, Gao SL et al. Human methionine synthase A2756G polymorphism increases susceptibility to prostate cancer. Aging (Albany NY) 2018; 10 (7): 1776–1788. doi: 10.18632/aging.101509.
13. Abedinzadeh M, Zare-Shehneh M, Neamatzadeh H et al. Association between MTHFR C677T polymorphism and risk of prostate cancer: evidence from 22 studies with 10,832 cases and 11,993 controls. Asian Pac J Cancer Prev 2015; 16 (11): 4525–4530. doi: 10.7314/apjcp.2015.16.11.4525.
14. Gao S, Liu N, Ma Y et al. Methylenetetrahydrofolate reductase gene polymorphisms as predictive and prognostic biomarkers in ovarian cancer risk. Asian Pac J Cancer Prev 2012; 13 (2): 569–573. doi: 10.7314/apjcp. 2012.13.2.569.
15. Meguid N, Khalil R, Gebril O et al. Evaluation of MTHFR genetic polymorphism as a risk factor in Egyptian autistic children and mothers. J Psychiatry 2015; 18 (1): 14–75. doi: 10.4172/Psychiatry.1000179.
16. Soleimani E, Saliminejad K, Akbari MT et al. Association study of the common polymorphisms in the folate-methionine pathway with retinoblastoma. Ophthalmic Genet 2016; 37 (4): 384–387. doi: 10.3109/13816810.2015.1107596.
17. de Lima ELS, da Silva VC, da Silva HDA et al. MTR polymorphic variant A2756G and retinoblastoma risk in Brazilian children. Pediatr Blood Cancer 2010; 54 (7): 904–908. doi: 10.1002/pbc.22472.
18. Akbari MT, Naderi A, Saremi L et al. Methionine synthase A2756G variation is associated with the risk of retinoblastoma in Iranian children. Cancer Epidemiol 2015; 39 (6): 1023–1025. doi: 10.1016/j.canep.2015.11.002.
19. Mallipatna A, Marino M, Singh AD. Genetics of retinoblastoma. Asia Pac J Ophthalmol (Phila) 2016; 5 (4): 260–264. doi: 10.1097/APO.0000000000000219.
20. Rojanaporn D, Boontawon T, Chareonsirisuthigul T et al. Spectrum of germline RB1 mutations and clinical manifestations in retinoblastoma patients from Thailand. Mol Vis 2018; 24 : 778–788.
21. Parma D, Ferrer M, Luce L et al. RB1 gene mutations in Argentine retinoblastoma patients. Implications for genetic counseling. PLoS One 2017; 12 (12): e0189736. doi: 10.1371/journal.pone.0189736.
22. Bradbury BD, Jick H. In vitro fertilization and childhood retinoblastoma. Br J Clin Pharmacol 2004; 58 (2): 209–211. doi: 10.1111/j.1365-2125.2004.02109.x.
23. Dommering CJ, van der Hout AH, Meijers-Heijboer H et al. IVF and retinoblastoma revisited. Fertil Steril 2012; 97 (1): 79–81. doi: 10.1016/j.fertnstert.2011.10.035.
24. Pu D, Jiang SW, Wu J. Association between MTHFR gene polymorphism and the risk of ovarian cancer: a meta-analysis of the literature. Curr Pharm Des 2014; 20 (11): 1632–1638. doi: 10.2174/13816128113199990564.
25. Bisht S, Chawla B, Dada R. Oxidative stress and polymorphism in MTHFR SNPs (677 and 1298) in paternal sperm DNA is associated with an increased risk of retinoblastoma in their children: a case–control study. J Pediatr Genet 2018; 7 (3): 103–113. doi: 10.1055/s-0038-1667037.
26. Rashidvash V. Iranian people: Iranian ethnic groups. Int J Humanit Soc Sci 2013; 15 (3). [online]. Available from: http: //www.ijhssnet.com/journals/Vol_3_No_15_August_2013/24.pdf.
27. Ma LM, Yang HP, Yang XW et al. Methionine synthase A2756G polymorphism influences pediatric acute lymphoblastic leukemia risk: a meta-analysis. Biosci Rep 2019; 39 (1): BSR20181770. doi: 10.1042/BSR20181770.
Štítky
Dětská onkologie Chirurgie všeobecná Onkologie
Článek Peritoneální nádoryČlánek Pseudomyxoma PeritoneiČlánek LymphangioleiomyomatosisČlánek Aktuality z odborného tiskuČlánek Onkologie v obrazech
Článek vyšel v časopiseKlinická onkologie
Nejčtenější tento týden
2019 Číslo 5- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejasný stín na plicích – kazuistika
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Hojení análních fisur urychlí čípky a gel
-
Všechny články tohoto čísla
- Peritoneální nádory
- Malignant Peritoneal Tumors – Introduction
- Pseudomyxoma Peritonei
- Treatment of Malignant Peritoneal Mesothelioma
- Therapy and Prophylaxis of Peritoneal Metastases from Colorectal Cancer
- Peritoneal Carcinomatosis of Gastric Origin – Treatment Possibilities
- Peritoneal Carcinomatosis from Ovarian Cancer – Current Clinical Impact of Cytoreductive Surgery and Intraperitoneal Hyperthermic Chemotherapy
- Alopecia and Hair Damage Induced by Oncological Therapy
- Can Amygdalin Provide any Benefit in Integrative Anticancer Treatment?
- Lymphangioleiomyomatosis
- Association of MTHFR 677C>T, 1298A>C and MTR 2756A>G Polymorphisms with Risk of Retinoblastoma
- Tumor-Infiltrating Lymphocytes/Plasmocytes in Chemotherapeutically Non-Influenced Triple-Negative Breast Cancers – Correlation with Morphological and Clinico-Pathological Parameters
- Combined Use of Regorafenib with SBRT in Pulmonary Metastasis from Colorectal Cancer
- 68Ga-DOTA-TOC PET/CT Examination in a Patient with Gastroenteropancreatic Neuroendocrine Tumor – First Examination in the Czech Republic
- Aktuality z odborného tisku
- Onkologie v obrazech
- Klinická onkologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Alopecia and Hair Damage Induced by Oncological Therapy
- Pseudomyxoma Peritonei
- Treatment of Malignant Peritoneal Mesothelioma
- Malignant Peritoneal Tumors – Introduction
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



