-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Dendritické buněčné vakcíny proti nemalobuněčnému karcinomu plic – nová terapeutická alternativa
Dendritic Cell Vaccines Against Non ‑ small Cell Lung Cancer – an Emerging Therapeutic Alternative
Many clinical trials have been carried out or are in progress to assess the therapeutic potential of dendritic cell-based vaccines on cancer patients. Herewith, we describe the clinical trials of non‑small cell lung cancer (NSCLC) published in the literature. Although the number of clinical trials and NSCLC patients enrolled in these studies is small, it is possible to conclude that the administration of dendritic cells (DCs) by any route is safe and that a clinical benefit after their administration can be observed. These initial results encourage continued investigation in clinical trials into the benefit of DCs along with different strategies to enhance their immune response in this deadly disease.
Key words:
dendritic cells – non-small cell lung cancer – cancer – immunotherapy
The author declare he has no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.Submitted:
15. 3. 2014Přijato:
7. 6. 2014
Autoři: L. Mendoza
Působiště autorů: INC Research, Prague, Czech Republic
Vyšlo v časopise: Klin Onkol 2014; 27(4): 294-298
Kategorie: Sdělení
Souhrn
Řada klinických studií byla realizována nebo v současnosti probíhá s cílem ověřit terapeutický potenciál vakcín na bázi dendritických buněk (dendritic cell – DC) u pacientů se zhoubnými nádory. V tomto sdělení se zabýváme publikovanými výsledky uvedených klinických studií u pacientů s nemalobuněčným karcinomem plic (non-small cell lung cancer – NSCLC). Přestože se nejedná o velké počty studií a i počet zařazených pacientů je limitovaný, z dosažených výsledků je možné dojít k závěru, že podávání DC, a to jakýmkoliv způsobem, je bezpečné, a že existují pacienti, u kterých byl po aplikaci pozorován klinický přínos. Tyto počáteční výsledky podporují pokračování výzkumu s DC, v rámci kterého jsou testovány různé strategie použití DC, které by vedly k co největší imunitní odpovědi proti tomuto smrtelnému onemocnění.
Klíčová slova:
dendritické buňky – nemalobuněčný karcinom plic – zhoubné novotvary – imunoterapieLung cancer is the leading cause of cancer‑related morbidity and mortality, resulting in more than 1 million deaths per year worldwide [1]. Approximately 85% of cases are non‑small cell lung cancer (NSCLC), and the majority of patients are diagnosed at an advanced stage, because there is no efficient method to improve early diagnosis [2] and this fact has a huge impact on treatment outcomes. In spite of aggressive treatment with surgery, radiation, chemotherapy and targeted therapies, long‑term survival for patients with NSCLC still remains low. Even patients with early stage disease often succumb to lung cancer due to the development of metastases (5‑year survival rates average 17% for patients with early disease and 4% for patients diagnosed with metastatic disease), indicating the need for effective and new therapeutic modalities for this condition [3].
Dendritic cells (DCs) are critical for the antigen ‑ specific priming of T cells and activate the immune system against cancer. DCs constitute a heterogeneous population of cells [1], and although the precise ontogeny of DCs remains to be elucidated, they can be differentiated from both bone marrow (BM) and peripheral blood precursors [2]. DCs are readily obtained in large numbers from peripheral blood or CD34+ bone marrow progenitors expanded in vitro in medium containing various combinations of cytokines, including GM‑CSF, interleukin 4 (IL‑4), c ‑ kit ligand and tumor necrosis factor α (TNF‑α).
The application of autologous DCs to cancer therapy has received much interest [3 – 5]. Animal models have clearly documented the ability of syngeneic BM ‑ derived DCs pulsed with tumor ‑ derived peptide epitopes or genetically engineered to express immuno-stimulatory cytokines to serve as effective immunogens of antitumor CTLs in vivo [6 – 11].
Historically, NSCLC has not been considered sensitive to immune‑based therapies; however, data show lung tumors are recognized by the immune system, and a more robust antitumor immune response is associated with better survival. Several tumor‑associated antigens (TAAs) known to be expressed in a range of tumor types are also found in patients with NSCLC. As examples of such TAAs, there are melanoma‑associated antigen A3 (MAGE ‑ A3) [12], mucinous glycoprotein‑1 (MUC1) [13], the epidermal growth factor receptor (EGFR) [14], Wilms’ tumor gene 1 (WT1) [15], and the carcinoembryonic antigen (CEA) [16] among others [17]. A reasonable approach to activate the immune system is to utilize the faculty of DCs to intake the TAAs and present them to the T cells. This situation, which naturally occurs in vivo, can be mimicked in vitro pulsing DCs with these antigens or with the tumor cells lysates, which express the tumor antigens and prepare the vaccines against NSCLC.
DCs have been tested in cancer immunotherapy clinical trials for two decades. Over this time, the methods of DC culture (or manufacture) have evolved. The early clinical trials of DC‑based cancer immunotherapy established the general safety and feasibility of this cancer vaccine strategy. The good immune response observed in vivo in patients treated with DC‑based vaccines did not correlate with modest clinical responses. This initially learned experience brought many discussions concerning three aspects: preparation of the most efficient DCs in vitro and antigen loading, dose and route of administration, and the avoidance of late‑stage metastatic patients that were heavily pretreated with conventional chemotherapeutic drugs [18]. Tab. 1 shows the accumulated experience of DC‑based vaccines in NSCLC. We will expand the information regarding them in the order they were published.
Tab. 1. Clinical experience of dendritic cells in patients with NSCLC. 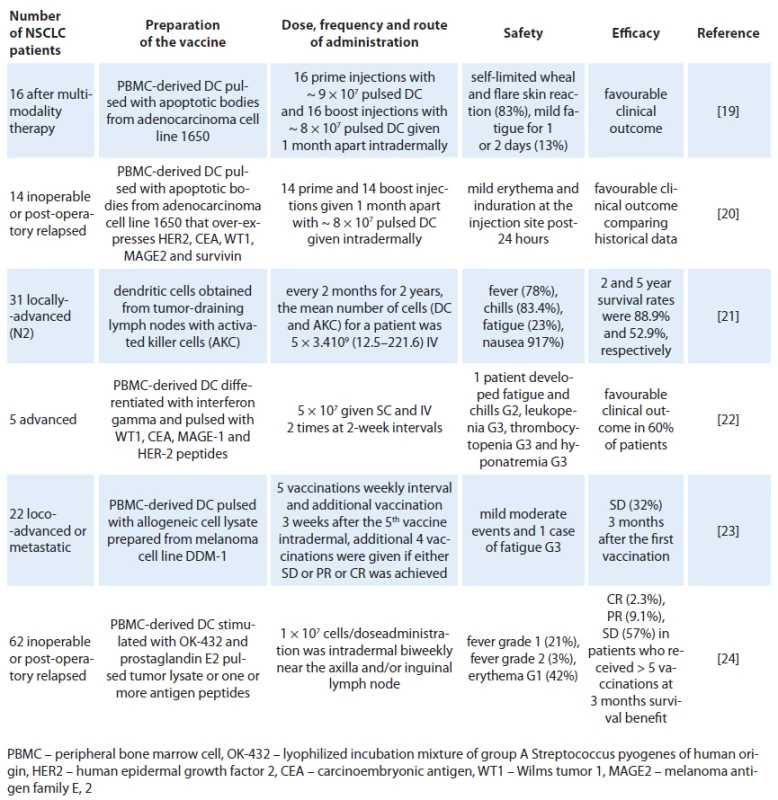
In 2004, Hirschowitz et al [19] delivered autologous matured DC vaccines in vitro with interferon ‑ gamma to 16 individuals with stage IA and IIIB NSCLC treated with multimodality therapy. To generate a pluripotent vaccine, the adenocarcinoma cell line 1650 was used as a source of TAAs. 1650 overexpresses Her ‑ 2/ neu, CEA, MAGE 2, WT ‑ 115 and survivin to pulsed DCs. A self‑limited wheal and flare skin reaction appeared at the injection site 24 – 48 hours after immunization in 10 of 16 subjects during at least one of the two immunizations. Two individuals noted a more profound reaction with the second vaccine. Maximal reaction was 6 cm. Three individuals noted minor fatigue for one to two days following the vaccine. Remarkably, it was reported that one individual with stage IB NSCLC and solitary brain metastasis survived 15 months following surgical resection of stage IV disease and 17 months postvaccine. Another two individuals with unresectable stage III NSCLC showed no signs of disease progression at 35 and 23 months from chemoradiation, respectively. One individual with resected stage IIIB bronchoalveolar cell carcinoma also remained tumor‑free 28 months post‑surgical resection and 19 months postvaccine. Years later, the same group reported a continuation study with similar inclusion criteria, immunization protocol, and analysis, using an immature DC vaccine in an additional 14 NSCLC patients [20]. There were no related adverse events. The best responses were observed in three patients with unresectable stage III disease who had radiographically stable disease at 23, 27 and 51 months following initial therapy. The data also indicate that immature DC pulsed with apoptotic tumor cells have similar activity to matured DC preparation delivered in a similar clinical protocol. In another experiment [21], NSCLC patients with positive pathologically diagnosed lymph node (N2) were treated with activated killer cells and DCs obtained from tissue cultures of tumor ‑ draining lymph nodes (TDLN) or from TDLN co ‑ cultured with peripheral blood lymphocytes, which were used for the adoptive transfer of immunotherapy. The patients received four courses of chemotherapy along with immunotherapy every two months for two years intravenous. From 28 cases treated, the main toxicities were fever (78.0%), chill (83.4%), fatigue (23.0%) and nausea (17.0%) observed on the day of cell transfer. The 2 - and 5‑year survival rates were 88.9% and 52.9%, respectively. Since this was not a randomized study, it was not possible to analyze the benefit of DCs concomitantly with activated killer cells but the prognoses of the selected N2 patients were comparable to historical data. In that experience, it was noted that prognoses of the patients who received more than 5 × 1010 cells were better than those of patients who received less, indicating that the effect is dependent on the number of administered cells. In a small pilot study with five patients with inoperable stage III or IV NSCLC after chemotherapy treatment were selected to receive two doses of 5 × 107 DC cells administered subcutaneously and intravenously two times at two week intervals [22]. The DCs were pulsed with different tumoral peptides: WT1 peptide (RMFPNAPYL), CEA peptide (YLSGANLNL), MAGE ‑ 1 peptide (KVAELVHFL), and HER ‑ 2 peptide (KIFGSLAFL) at day 9 of the culture. A prime vaccine and a single boost were given 15 days apart. For each dose of vaccine, two aliquots were prepared in separate syringes with saline solution (500 µl/ dose) containing 5 × 107 cells. First, a dose was subcutaneously administered in the arm and one hour later, a second dose was given intravenously in the other arm. No local reaction was observed at the vaccine site of application. One patient presented systemic reactions with fever and chills that required hospitalization after immunotherapy. Although it was too small a study to reach conclusions, it was observed that three of the patients had a longer survival time than expected for their TNM stage and two of these survived almost twice as long as the expected average. A recent publication, Noerregaard et al, sponsored by a Denmark biotech company (Dandrit Biotech, Copenhagen, Denmark), reported the clinical and immunological effects of autologous DCs which were pulsed with a MAGE containing allogeneic melanoma cell lysate in pre‑treated loco ‑ advanced or metastatic NSCLC patients not eligible for further treatment [23]. Twenty ‑ two patients participated in the vaccination program, consisting of 10 vaccinations. The treatment was well tolerated and only minor adverse events were reported. Seven patients remained in stable disease (SD) three months after the first vaccination. After ten vaccinations (six months), four patients still showed SD and continued vaccinations on a monthly basis. These four patients received a total of 12, 16, 26 and 35 vaccinations, respectively. Five patients showed an unexpectedly prolonged survival. Another recent publication reported that a large number of Japanese patients with inoperable disseminated or postoperative relapse NSCLC had been treated with pulsed DC vaccine intradermally and biweekly near the axillar and/ or inguinal lymph nodes. DCs were pulsed with autologous tumor lysate (50 lg/ mL) or, if that was not available, with one or more peptide antigens according to the HLA‑A pattern [24]. No serious adverse event related to the vaccination was observed. Transient Grade 1 – 2 fever within a few days after vaccination was observed in 15 patients. Transient erythema at the vaccinated sites was also observed in 26 patients within a few days, and all of them were categorized as grade 1. Clinical responses based on response evaluation criteria in solid tumors (RECIST) were found in 31 (50%) patients at three months after the first DC vaccine (complete response: 1(1.6%), partial response: 4 (6.5%), stable disease: 26 (41.9%)). Median survival time was 27 months (82% in one year and 54% in two years) from initial diagnosis, and that was 12 months (48% in one year and 22% in two years) from the first DC vaccination.
None of the above mentioned clinical trials, which studied the in vitro immunological assessment of DC efficacy, reported a correlation of such results with the clinical outcome. This observation has been reported in different DC trials in different malignancies. This is probably related to the breadth and quality of the induced T cell response, resulting in the frequent observation of induction of anti‑vaccine immune responses in the absence of an objective clinical response [25]. However, what seems clear from these trials is that DC vaccination by any route is safe and that a clinical benefit has been reported. From these studies, it is also possible to suggest that an antigen ‑ pulsed DC vaccine could demonstrate a survival benefit in patients with advanced NSCLCs and that DC‑based cancer vaccines offer the potential for an effective, non‑toxic, and outpatient‑based approach to cancer therapy. What has been shown in NSCLC patients so far is that DCs pulsed with tumor lysate and with known tumor antigen peptides are efficient. However, future clinical trials should investigate the efficacy of DCs pulsed with tumor epitopes derived from newly identified tumor‑associated peptides, RNA, apoptotic bodies or genetically modified with cDNAs encoding, for example, immunostimulatory cytokines, such as IL‑12 to augment the generation of effective anti‑tumor CTL responses, or with full ‑ length cDNAs encoding TAAs. Although there are many paradigms still to be resolved, such as the best route and schedule of DC administration, other approaches to enhance the immune therapy of DCs are being investigated, including the concomitant use of bevacizumab and metronomic chemotherapy [26]. Many academic centers around the world together with a few biotech companies are searching for the answers to these questions. We firmly believe that results from future clinical trials will confirm the benefit of DC‑based vaccines against NSCLC.
The author declare he has no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Luis Mendoza, MD, PhD
INC Research
Zeleny pruh 1560/99
140 64 Prague
Czech Republic
e-mail: Lmendoza@incresearch.com
Submitted: 15. 3. 2014
Accepted: 7. 6. 2014
Zdroje
1. O‘Mahony D, Kummar S, Gutierrez ME. Non ‑ small‑cell lung cancer vaccine therapy: a concise review. J Clin Oncol 2005; 23(35): 9022 – 9028.
2. Molina JR, Yang P, Cassivi SD et al. Non ‑ small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clinic Proceedings 2008; 83(5): 584 – 594. doi: 10.4065/ 83.5.584.
3. Baleeiro RB, Anselmo LB, Soares FA et al. High frequency of immature dendritic cells and altered in situ production of interleukin‑4 and tumor necrosis factor‑alpha in lung cancer. Cancer Immunol Immunother 2008; 57(9): 1335 – 1345. doi: 10.1007/ s00262 ‑ 008 ‑ 0468 ‑ 7.
4. Caux C, Liu YJ, Banchereau J. Recent advances in the study of dendritic cells and follicular dendritic cells. Immunol Today 1995; 16(1): 2 – 4.
5. Peters JH, Gieseler R, Thiele B et al. Dendritic cells: from ontogenic orphans to myelomonocytic descendants. Immunol Today 1996; 17(6): 273 – 278.
6. Knight SC, Hunt R, Dore C et al. Influence of dendritic cells on tumor growth. Proc Natl Acad Sci USA 1985; 82(13): 4495 – 4497.
7. Young JW, Inaba K. Dendritic cells as adjuvants for class I major histocompatibility complex ‑ restricted antitumor immunity. J Exp Med 1996; 183(1): 7 – 11.
8. Paglia P, Chiodoni C, Rodolfo M et al. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med 1996; 183(1): 317 – 322.
9. Zou JP, Shimizu J, Ikegame K et al. Tumor ‑ immunotherapy with the use of tumor ‑ antigen ‑ pulsed antigen ‑ presenting cells. Cancer Immunol Immunother 1992; 35(1): 1 – 6.
10. Mayordomo JI, Zorina T, Storkus WJ et al. Bone marrow derived dendritic cells pulsed with synthetic tumor peptides elicit protective and therapeutic anti‑tumor immunity. Nat Med 1995; 1(12): 1297 – 1302.
11. Celluzzi CM, Mayordomo JI, Storkus WJ et al. Peptide ‑ pulsed dendritic cells induce antigen ‑ specific, CTL ‑ mediated protective tumor immunity. J Exp Med 1996; 183(1): 283 – 287.
12. Sienel W, Varwerk C, Linder A et al. Melanoma associated antigen (MAGE) – A3 expression in stages I and IInon‑small cell lung cancer: results of a multi‑center study. Eur J Cardiothorac Surg 2004; 25(1): 131 – 134.
13. Reck M, Vansteenkiste J, Brahmer JR. Targeting the immune system for management of NSCLC: the revival? Curr Respir Care Rep 2013; 2(1): 22 – 39. doi: 10.1007/ s13665 ‑ 012 ‑ 0038 ‑ 5.
14. Jänne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non‑small‑cell lung cancer: implications for treatment and tumor biology. J Clin Oncol 2005; 23(14): 3227 – 3234.
15. Sugiyama H. WT1 (Wilms’ tumor gene 1): biology and cancer immunotherapy. Jpn J Clin Oncol 2010; 40(5): 377 – 387. doi: 10.1093/ jjco/ hyp194.
16. Coligan JE, Henkart PA, Todd CW et al. Heterogeneity of the carcinoembryonic antigen. Immunochemistry 1973; 10(9): 591 – 599.
17. Babiak A, Steinhauser M, Götz M et al. Frequent T cell responses against immunogenic targets in lung cancer patients for targeted immunotherapy. J Oncol Rep 2014; 31(1): 384 – 390. doi: 10.3892/ or.2013.2804.
18. Butterfield LH. Dendritic cells in cancer immunotherapy clinical trials: Are we making progress? Front Immunol 2013; 4 : 454. doi: 10.3389/ fimmu.2013.00454.
19. Edward A. Hirschowitz, Terry Foody et al. Autologous dendritic cell vaccines for non‑small‑cell lung cancer. J Clin Oncol 2004; 22(14): 2808 – 2815.
20. Hirschowitz EA, Foody T et al. Immunization of NSCLC patients with antigen ‑ pulsed immature autologous dendritic cells. Lung Cancer 2007; 57(3): 365 – 372.
21. Kimura H, Iizasa T, Ishikawa A et al. Prospective phase II study of post‑surgical adjuvant chemo ‑ immunotherapy using autologous dendritic cells and activated killer cells from tissue culture of tumor ‑ draining lymph nodes in primary lung cancer patients. Anticancer Res 2008; 28(2B): 1229 – 1238.
22. Perroud MW Jr, Honma HN, Barbeiro AS et al. Mature autologous dendritic cell vaccines in advanced non‑small cell lung cancer: a phase I pilot study. J Exp Clin Cancer Res 2011; 30 : 65. doi: 10.1186/ 1756 ‑ 9966 ‑ 30 ‑ 65.
23. Noerregaard L, Kvistborg P, Zocca M et al. Clinical and immunological effects in patients with advanced non‑small cell lung ‑ cancer after vaccination with dendritic cells exposed to an allogeneic tumor cell lysate. W J V 2013; 3(2): 68 – 76. doi: 10.4236/ wjv.2013.32011.
24. Takahashi H, Okamoto M, Shimodaira S et al. Impact of dendritic cell vaccines pulsed with Wilms’ tumour ‑ 1peptide antigen on the survival of patients with advanced non‑small cell lung cancers. Eur J Cancer 2013; 49(4): 852 – 859. doi: 10.1016/ j.ejca.2012.11.005.
25. Tuyaerts S, Aerts J, Corthals J et al. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother 2007; 56(10): 1513 – 1537.
26. Kandalaft LE, Chiang Ch, Tanyi J et al. A Phase I vaccine trial using dendritic cells pulsed with autologous oxidized lysate for recurrent ovarian cancer. J Transl Med 2013; 11 : 149. doi: 10.1186/ 1479 ‑ 5876 ‑ 11 ‑ 149.
Štítky
Dětská onkologie Chirurgie všeobecná Onkologie
Článek Aktuality z odborného tiskuČlánek Soutěž o nejlepší práciČlánek Vzťah medzi sérovou hladinou karboanhydrázy IX, hypoxiou a rádiorezistenciou nádorov hlavy a krkuČlánek Pozitronová emisní tomografie kombinovaná s počítačovou tomografií pro diagnózu synchronních nádorůČlánek Aktuality z odborného tiskuČlánek Liga proti rakovině
Článek vyšel v časopiseKlinická onkologie
Nejčtenější tento týden
2014 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Metamizol v léčbě různých bolestivých stavů – kazuistiky
- Nejasný stín na plicích – kazuistika
-
Všechny články tohoto čísla
- Aktuality z odborného tisku
- Soutěž o nejlepší práci
- Předpokládaná účinnost HPV vakcinace v profylaxi nongenitálních karcinomů
- Brazilský příběh mutace p53 R337H
- Analýza nákladů na 1. linii léčby metastatického kolorektálního karcinomu při podání režimů s bevacizumabem – data z reálné klinické praxe v České republice
- Screening rizika malnutrice versus ukazatelé nutričního stavu a systémové zánětlivé odpovědi u pacientů s nově diagnostikovaným karcinomem plic
- Vzťah medzi sérovou hladinou karboanhydrázy IX, hypoxiou a rádiorezistenciou nádorov hlavy a krku
- Soutěž na podporu autorských týmů publikujících v zahraničních odborných titulech
- Vliv anakinry na cytokinové profily a profily lymfocytů/ monocytů u pacienta s Erdheim-Chesterovou nemocí
- Pozitronová emisní tomografie kombinovaná s počítačovou tomografií pro diagnózu synchronních nádorů
- Informace z České onkologické společnosti
- Paraneoplastická vaskulitída u pacientky s karcinómom krčku maternice
- Akupunktura v léčbě symptomů onkologického onemocnění v západním světě
- Dendritické buněčné vakcíny proti nemalobuněčnému karcinomu plic – nová terapeutická alternativa
- Aktuality z odborného tisku
- Liga proti rakovině
- Pacient s Cowdenovým syndromem způsobeným mutací v genu PTEN (archiv 2. LF UK a FN v Motole)
- Klinická onkologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Brazilský příběh mutace p53 R337H
- Akupunktura v léčbě symptomů onkologického onemocnění v západním světě
- Paraneoplastická vaskulitída u pacientky s karcinómom krčku maternice
- Screening rizika malnutrice versus ukazatelé nutričního stavu a systémové zánětlivé odpovědi u pacientů s nově diagnostikovaným karcinomem plic
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



