-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
CLINICAL EFFICACY OF A NEW CHITIN NANOFIBRILS-BASED GEL IN WOUND HEALING
Autoři: P. Mezzana
Působiště autorů: Gestione Servizi Medici e Chirurgici, Laser Centre Rome, Italy
Vyšlo v časopise: ACTA CHIRURGIAE PLASTICAE, 50, 3, 2008, pp. 81-84
INTRODUCTION
Acute wounds normally heal in a very orderly and efficient manner characterized by four distinct but overlapping phases: hemostasis, inflammation, proliferation and remodeling.
Many factors have an impact on the healing process, from the initial inflammatory reaction to the final maturation of the fibrous scar. Any impairment in the normal reparative process may lead to either delayed healing or excess fibrosis (1). Skin ulcers, including diabetic foot ulcers, venous ulcers and pressure ulcers are among the most frequent and characteristic type of chronic non-healing wounds. One of the major causes of delayed healing is the persistence of inflammation or an inadequate angiogenic response (2, 3). In contrast, overhealing, or excessive fibrosis of wounds, is observed in fibroproliferative disorders such as keloids and hypertrophic scars. The precise etiologic factors associated with keloid formation are elusive, with the exception of tissue trauma; however, keloids have been reported in the absence of trauma. Adverse wound healing characteristics, such as infection and excessive wound tension, are associated with keloids and hypertrophic scars.These conditions are characterized by abnormal accumulation of collagen within the wound site as a result of failure to eliminate granulation tissue cells (1). The expression of vascular endothelial growth factor (VEGF) and several enzyme systems, including nitric oxide synthase (NOS), cyclooxygenase (COX) and Arginase, are vital for maintaining the different phases of wound healing (2). Research in wound healing has been concerned primarily with one of three clinical objectives. The first involves the search for agents that could improve the end result of the healing process. The second concerns the search for treatments that could accelerate the early phases of the healing process under normal conditions. The third involves the development of protocols to close difficult or non-healing wounds.
Recent progress in wound management is mainly in terms of physiological support of healing. Since infections delay healing and worsen scar formation, there is a desire to achieve closure as soon as possible. The main goals of wound care are prevention of infection, maintenance of a moist environment, protection of the wound, and minimum scar formation (4).
Chitin is the second most abundant polysaccharide in nature (after cellulose). At least 10 gigatons of chitin are synthesised and degraded each year in the biosphere. Chitin mainly consists of the aminosugar N-acetylglucosamine, which is partially deacetylated. The mostly deacetylated form of chitin is called chitosan. Chitin is present in nature usually complexed with other polysaccharides and with proteins. Chitin is a renewable resource and is isolated from crab and shrimp waste. It is used for waste water clearing, for cosmetics and for medical and veterinary applications. Chitin has some unusual properties which accelerate healing of wounds in humans. Recently a novel method for the production of nanofibrillar chitin has been developed, which is sustainable from an industrial manufacturing standpoint and suitable for producing chitin nanofibrils with improved properties which are free from other crystalline components (5). Nanofibrils are extremely small objects, each of which is composed of fewer than 20 polysaccharide chains that recognize each other. From a biological point of view, the crystalline nanofibrils themselves stimulate keratinocytes to grow quickly and encourage fibroblasts to produce the right amount and quality of collagen fibrils. Chitin is recognized easily by the cutaneous enzymes and hydrolized in N-acetyl glucosamine (5).
The main biochemical activities of chitin and chitosan-based materials are: polymorphonuclear cell activation, fibroblast activation, cytokine production, giant cell migration and stimulation of type IV collagen synthesis (6, 7).
In this study the effects of a new chitin nanofibrils-based gel on the rate and quality of wound healing were investigated in a clinical model.
MATERIALS AND METHODS
Forty-eight patients (20 males and 28 females, mean age 50.2 years) were treated and controlled according to a standard protocol for 3 months and were divided in three groups. Members of the first group, 20 patients, were treated for wounds of different origin (trauma, surgery, lasers, etc.) in different parts of the body. Those in the second group, 19 patients, were treated for difficult wounds which showed severe slowing of the healing process (delays in excess of one month). Patients in the third group, 9 in number, were treated “split manner” (after written informed consent had been obtained): one part of the wound, or in case of two adjacent wounds only one of these, was medicated with the new gel, the other part or the other wound was medicated traditionally.
The chitin nanofibrils-based gel used in this study was Mavimed Gel® produced by MAVI SUD s.r.l. Aprilia (LT), Italy.
The product was applied in a home-based protocol twice a day in every case. Before every application the wound was washed with 0.9% isotonic saline solution. No other local antiseptic was employed. The patients in the second group – these with difficult wounds – were also treated with other appropriate systemic therapies (i.e. correct insulin administration in those with diabetes), removable elastic bandages (i.e. venous ulcers), mechanical or erbium:YAG laser debridment of the fibrin coat excess if present. In none of the patients there were signs of acute infections. The wound surface did not exceed 90 cm2 in any case. The assessment of the wounds progress was made for every group at 2, 6, 10, 14 and 30 days, and at 3 months.
Linear surgical wounds were evaluated for early phases of the healing process and the end result of it (i.e. hypertrophic scarring or keloids).
Non-linear wounds (i.e. laser surgery, traumas or ulcers) were also evaluated with the computation of the percentage of wound healing. A semi-transparent paper was placed on the location of the wound and its shape drawn on the paper. The percentage of wound healing was calculated by Walker formula (Tab. 1) after measurement of the wound area (8). Percentage of wound healing was computed at the beginning of the treatment and then at 2, 6, 10, 14 and 30 days. In the second group the percentage of wound healing was also computed the week before starting the application, at day 1 and 7.
Student t-test and ANOVA were used to test differences in the healing speed between different areas of the same wound or different wounds in the same patient.
Differences were considered significant when P<0.05. Results are given as means of values.
The 3-month scar assessment was made with a modified Singer&Hollander (2007) evaluation scale (9). Scars were assigned 0 or 1 point each for the presence or absence of the following:
width greater than 2 mm, elevation or depression, colour alterations, paresthesias, and overall poor appearance. A total score was then calculated by adding the individual scores on each of the five categories ranging from 0 (worst) to 5 (best).
RESULTS
Every patient treated with the new chitin nanofibrils-based gel had complete healing. The assessment at 3 months for the first group of patients, based on scar features, registered only 3 cases (15%) of serious hypertrophic trend (badly oriented surgical wounds). Moreover, in two patients with keloid scar history was observed normal healing of the treated wounds. The average score was 3.95 (modified Singer&Hollander evaluation scale).
In the second group every patient had a resumption of healing at day 6, and the difference between the percentage of wound healing calculated by Walker formula (see Tab. 1) the week before starting the application of the gel and a week after application was statistically significant (P<0.05) (Graph 1, Fig. 1).
Tab. 1. The percentage of wound healing was calculated by Walker formula after measurement of the wound area 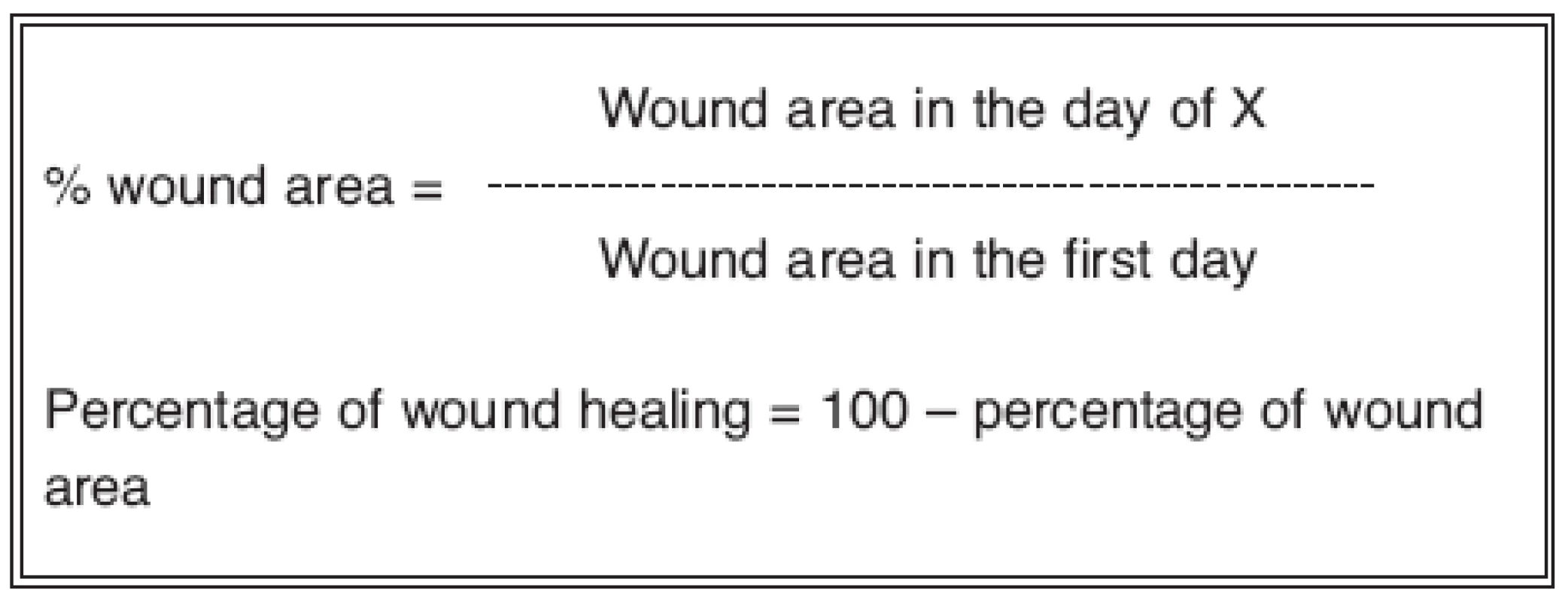
Graph 1. Group 2: The difference between the percentage of wound healing the week before starting the application of the gel and a week after application was statistically significant (P<0.05) 
Fig. 1. Chronic wound of the leg: left before treatment, center 6 days after the application of the new gel, right 25 days after complete healing 
In the third group, in 7 patients (77.7%), a statistically significant higher percentage of wound healing of the treated area than the non-treated one was seen, starting at day 6 (P<0.05) (Fig. 2, Fig. 3). In this group the assessment at 3 months also showed a better average score of the treated areas than the non-treated ones: 4.2 treated ones vs 3 non treated ones. In one patient, with hypertrophic scarring history, different Er:YAG laser abrasions of the shoulders were treated. The treated ones at 3 months showed normal healing, the non-treated ones hypertrophic trend (Fig. 4).
Fig. 2. Three months after reductive breast surgery: left part of the image wounds treated with the new gel, right part control 
Fig. 3. Three months after tummy tuck: left side part of the wound treated with the new gel, right side control 
Fig. 4. Laser treatment for actinic keratosis of the shoulder in a patient prone to hypertrophic scar formation: green circles areas treated with the new gel 3 months after laser application, red circles control areas 
No major or minor complications were associated with the use of the product.
DISCUSSION
Wound repair must occur in a physiological environment conducive to tissue repair and regeneration. Alteration in the sequence of wound healing may result in the development of an abnormal wound, leading to either delayed healing or excessive fibrosis (10). The most common examples of delayed healing include diabetic foot ulcers, venous leg ulcers and pressure ulcers. Overhealing is observed in fibroproliferative disorders such as hypertrophic scars and keloids, which are characterized by excessive accumulation of collagen fibers within the wound site (11). Several clinically significant factors are known to hinder wound healing, including hypoxia, infections, metabolic disorders such as diabetes mellitus, the presence of debris and necrotic tissue, certain medications, etc. The objective in wound management is to heal the wound in the shortest time possible, with minimal pain, discomfort, and scarring to the patient. At the site of wound closure a flexible and fine scar with high tensile strength is desired.
Crystalline chitin nanofibrils have a microstructure typical of crustacean and insect cuticles. From a biological point of view, when a gel containing these structures is applied to a wound it is able to form a protective biofilm, thin, elastic and water soluble, which promotes tissue healing. It was demonstrated that these extremely small objects (each of them is composed of fewer than 20 polysaccharide chains that recognize each other) stimulate keratinocytes and fibroblasts to proliferate faster and lead the fibroblasts to produce the right amount and quality of collagen (5). Because of the chemical bonds that can be established with many molecules, the chitin and chitosan induce fast blood coagulation following adsorption of some enzymes and blood platelets on its surface. Moreover, chitin nanofibrils act on the cells by modulating the cytokines production; hence they have an interesting anti-inflammatory action (5).
Even if the first group of this study is not considered representative of the general population, the frequency of hypertrophic scarring observed – 15%, 3 in 20 – is much less than the frequency in previous reports (in skin surgery it is about 35.3%) (12). Moreover, in the patient of the third group with hypertrophic scars history the laser abrasions treated with the chitin nanofibrils gel showed normal healing, while the abrasions which were not treated showed hypertrophic trend. Finally, the two patients with keloid scars history and a surgical wound treated with the gel had normal wound healing.
The chitin nanofibrils-based gel seems to prevent hypertrophic scarring and keloid scarring, probably through two different mechanisms: the first promotes prevention of infections, the maintenance of a moist environment and the protection of the wound; the second involves modulation of fibroblasts proliferation and collagen production, also through its antinflammatory activity. The modulation of fibroblasts proliferation is probably also related to the mechanical inhibition due to the presence of the chitin network in the wound environment (13).
The results of this clinical study confirm that chitin nanofibrils-based gel promotes rapid and physiological healing of different types of wounds.
CONCLUSIONS
Because of its protective activities the nanofibrils based gel can be used for the treatment of superficial wounds, abrasions, sores (I–IV) and surgical wounds, and it is useful in all cases of delayed or difficult healing. It seems to prevent hypertrophic scarring and keloid scarring. The natural film that it forms on the skin is well tolerated and devoid of side effects.
Address for correspondence:
Paolo Mezzana, M.D.
Via Merulana 61A
00185 Rome, Italy
E-mail: pmezzana@yahoo.it
Zdroje
1. Desmouliere A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol., 146, 1995, p. 1029–1039.
2. Appleton I. Wound healing: future directions. Invest. Drugs, 6, 2003, p. 1067–1072.
3. Norrby K. Angiogenesis: new aspects relating to its initiation and control. Acta Pathol. Microbiol. Immunol. Scand., 105, 1997, p. 417–437.
4. Clark RAF. (Ed.). The molecular and cellular biology of wound repair. New York: Plenum, 1996.
5. Muzzarelli RAA., Morganti, P., Morganti G. et al. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydrate Polymers, 70, 2007, p. 274–284.
6. Kojima K., Okamoto Y., Miyatake K., Fujise H., Shigemasa Y. et al. Effects of chitin and chitosan on collagen synthesis in wound healing. J. Vet. Med. Sci., 66, 2004, p. 1595–1598.
7. Minami S., Okamoto Y., Hamada K., Fukumoto Y., Shigemasa Y. (1999). Veterinary practice with chitin and chitosan. In Jolle`s P., Muzzarelli RAA. (Eds.), Chitin and chitinases, Basel: Birkhauser Verlag, 1999, p. 251–264.
8. Walker HL., Mason AD. A standard animal burn. J. Trauma, 8, 1969, p. 1049-1051.
9. Singer AJ., Arora B., Dagum A., Valentine S., Hollander JE. Development and validation of a novel scar evaluation scale. Plast. Reconstr. Surg., 120, 2007, p. 1892–1897.
10. Greenhalgh DG. The role of apoptosis in wound healing. Int. J. Biochem. Cell Biol., 30, 1998, p. 1019–1030.
11. Tredget EE., Nedelec B., Scott PG., Ghahary A. Hypertrophic scars, keloids, and contractures: the cellular and molecular basis for therapy. Surg. Clin. North Am., 77, 1997, p. 701–730.
12. Niessen FB., Spawen P., Robinson P., Fidler V., Moshe K. The use of silicone occlusive sheeting (Sil-K) and silicone occlusive gel (Epiderm) in the prevention of hypertrophic scar formation. Plast. Reconstr. Surg., 102, 1998, p. 1962–1972.
13. Boateng S., Hartman TJ., Ahluwalia N. et al. Inhibition of fibroblast proliferation in cardiac myocyte cultures by surface microtopography. Am. J. Physiol. Cell Physiol., 10, 2003, p. 1152–1180.
Štítky
Chirurgie plastická Ortopedie Popáleninová medicína Traumatologie
Článek ČESKÉ SOUHRNY
Článek vyšel v časopiseActa chirurgiae plasticae
Nejčtenější tento týden
2008 Číslo 3- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Metamizol v léčbě různých bolestivých stavů – kazuistiky
- Neodolpasse je bezpečný přípravek v krátkodobé léčbě bolesti
- Léčba akutní pooperační bolesti z pohledu ortopeda
-
Všechny články tohoto čísla
- THE PRESERVATION OF A SPARE HEMI-ABDOMINAL FLAP IN UNILATERAL BREAST RECONSTRUCTION WITH ABDOMINAL FREE FLAP IN HIGH RISK PATIENTS
- PRIMARY RECONSTRUCTION OF ARTERIAL SUPPLY IN THE PALM AFTER AN INJURY: A CASE STUDY
- CLINICAL EFFICACY OF A NEW CHITIN NANOFIBRILS-BASED GEL IN WOUND HEALING
- SYNMASTIA – AN UNUSUAL COMPLICATION OF AUGMENTATION MAMMAPLASTY
- NEUROCUTANEOUS METACARPAL FLAPS
- PERFORATOR-BASED FOREARM ISLAND FLAP
- ČESKÉ SOUHRNY
- Acta chirurgiae plasticae
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- ČESKÉ SOUHRNY
- SYNMASTIA – AN UNUSUAL COMPLICATION OF AUGMENTATION MAMMAPLASTY
- NEUROCUTANEOUS METACARPAL FLAPS
- CLINICAL EFFICACY OF A NEW CHITIN NANOFIBRILS-BASED GEL IN WOUND HEALING
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



