-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Lack of Efficacy of Ticlopidine Pre-Treatment in the Reduction of Troponin I Release Following Percutaneous Intervention in Stable Angina Patients
Protidestičková léčba snižuje pravděpodobnost postprocedurálních kardiálních příhod u pacientů s provedenou perkutánní koronární intervencí. Cílem této studie bylo určit efekt předléčení tiklopidinem plus aspirinem ve srovnání s podáváním aspirinu samotného. Analyzovali jsme data 360 konsekutivních pacientů se stabilní anginou pectoris nebo inducibilní ischemií myokardu při zátěžovém testu. Při použití nerandomizovaného designu studie jsme srovnávali 3denní předléčení tiklopidinem plus aspirinem s podáváním aspirinu a začátkem léčby tiklopidinem až po provedení intervence. Primárním výsledným ukazatelem byla patologická hodnota troponinu I (TnI) za 24 hodin po katetrizační proceduře. Sekundárním cílovým ukazatelem byl výskyt úmrtí, infarktu myokardu a revaskularizace během 21měsíčního sledování. Patologický vzestup TnI jsme pozorovali u 16 (13 %) tiklopidinem předléčených pacientů a 27 (11 %) nepředléčených pacientů (p = 0,84). Nebyl nalezen významný rozdíl hodnoty TnI mezi pacienty v obou skupinách, kteří prodělali postprocedurálně nekrózu myokardu [medián: 4 ng/mL (3,1-8,3) vs 4,3 ng/mL (2,1-12,3); p = 0,39]. Na analýzu neměly vliv další zvažované parametry. V tiklopidinem předléčené skupině bylo 106 pacientů (85 %) v dlouhodobém sledování a z nich 24 (23 %) dosáhlo sekundárního cílového ukazatele; v nepředléčené skupině bylo hodnoceno 190 pacientů (81 %) a z nich 37 (19 %) dosáhlo sekundárního cílového ukazatele [p = 0,62; (OR 0,83; 95 % CI 0,46 až 1,48)]. U pacientů se stabilní anginou pectoris předléčených tiklopidinem plus aspirinem vs aspirinem samotným jsme nenalezli významné snížení postintervenčního myokardiálního poškození. Tento nález perzistoval i během dlouhodobého klinického sledování. K definitivnímu potvrzení platnosti těchto výsledků by však bylo nutno provést velkou randomizovanou studii.
Klíčová slova:
tiklopidin - klopidogrel - stent - angioplastika
Authors: J. Veselka 1; Š. Procházková 1; R. Duchoňová 1; I. Bolomová-Homolová 1; L. Fědorová 1; D. Tesař 2
Authors place of work: Department of Cardiology, Medical School II Charles University and University Hospital Motol, Prague, Czech Republic, Head Josef Veselka, MD, PhD, FESC, FSCAI 1; Department of Imaging Methods, Medical School II Charles University and University Hospital Motol, Prague, Czech Republic, Head Jiří Neuwirth, MD, PhD 2
Published in the journal: Vnitř Lék 2005; 51(9): 940-944
Category: Původní práce
Summary
Anti-platelet therapy reduces postprocedural cardiac events in patients referred to percutaneous coronary intervention. The object of this study was to determine the effect of pre-treatment with ticlopidine plus aspirin compared to aspirin alone. Prospectively collected data on 360 consecutive patients with stable angina pectoris or evidence of inducible myocardial ischemia were analyzed. In a non-randomized trial, three days of pre-treatment with ticlopidine plus aspirin was compared with standard post-procedural ticlopidine therapy started one hour after percutaneous intervention. The primary end point was the incidence of pathological troponin I (TnI) release 24 hours after procedure. The secondary end point was the incidence of death, myocardial infarction and revascularization at 21-month follow-up. A pathological rise of TnI was found in 16 pts. (13%) pre-treated with ticlopidine and 27 pts. (11%) in those non-pre-treated with ticlopidine (p = 0.84). Of the patients experiencing a post-procedural myocardial infarction, those pre-treated with ticlopidine had a non-significant difference in TnI elevation compared with those not receiving ticlopidine pre-PCI [median: 4 ng/mL (3.1-8.3) vs 4.3 ng/mL (2.1-12.3); p = 0.39]. Analyses were repeated with adjustment for significant baseline variables, which did not change the findings. Among the 106 patients (85%) who had received pre-procedural ticlopidine therapy and had available follow-up data, 24 (23%) experienced a secondary end point; among the 190 patients (81%) who had not received pre-PCI therapy and had available followed-up data, 37 (19%) experienced a secondary end point [p = 0.62; (OR 0.83; 95% CI 0.46 to 1.48)]. In stable angina pectoris patients, we did not detect a significant reduction in myocardial injury in patients pre-treated with aspirin plus ticlopidine compared to aspirin alone. This result persisted in long-term follow-up. Although our results are suggestive, a large, randomized clinical trial evaluating the benefit of ticlopidine pre-treatment prior to PCI in stable angina patients would be needed to verify our results.
Key words:
ticlopidine - clopidogrel - stent - angioplastyIntroduction
Pre-procedural therapy in elective percutaneous coronary interventions (PCI) is mainly based on the administration of antiplatelet agents to minimize procedural and post-procedural complications. The standard combination of aspirin with clopidogrel or ticlopidine is used to reduce the risk of sub-acute thrombosis when started prior to or soon after a stenting procedure [1-6]. In the clinical practice, some patients referred to coronary angiography are pretreated with combination anti-platelet therapy to achieve maximum platelet inhibition during PCI performed at the same time as coronary intervention. However, this strategy should be balanced against the increased risk of surgical complications seen in patients receiving combination therapy compared with aspirin alone when acute coronary surgery is performed [7]. Moreover, there is a larger treatment effect of anti-platelet agents in patients presenting with acute coronary syndromes than those who have a stable angina pectoris [8].
A recent study by van der Heijden et al [9] has shown the lack of efficacy of clopidogrel pre-treatment in the prevention of myocardial biomarker release following PCI for stable angina pectoris. Additionally, this small randomized controlled study showed no significant difference between the treatment groups in adverse cardiovascular events at one and six month follow-up.
In this study, we performed evaluation of the occurrence of pathological elevation of troponin I (TnI) within 24 hours after PCI for stable angina pectoris in patients pre-treated for three days with ticlopidine plus aspirin or aspirin alone. Additionally, we evaluated the association between pre-PCI anti-platelet therapy and long-term outcomes following PCI.
Materials and Methods
Patients
Prospectively collected data on 360 consecutive patients with stable angina pectoris or evidence of inducible myocardial ischemia on a cardiac stress test who were referred for percutaneous coronary intervention (PCI) of a lesion greater than 50% of luminal diameter were analyzed in this observational study. Patients suffering from acute coronary syndromes in the last three weeks were excluded. Patients meeting the criteria for inclusion in this analysis were stratified into one of two groups based on anti-platelet therapy (lasting > 72 hours) prescribed by the referring cardiologist. Written informed consent was obtained from each patient.
Antiplatelet Therapy
The antithrombotic protocol called for administration of 100 mg/d of aspirin for several days before PCI, continued indefinitely. In group A, ticlopidine treatment was started at least 3 days before the procedure (ticlopidine 750 mg/d) and after coronary stent implantation was maintained for 1 month (ticlopidine 500 mg/d). In the controlled group (group B), the loading dose of ticlopidine was started one hour after coronary stent implantation (ticlopidine 1000 mg/d on the day of the procedure) and maintained for one month at a decreased dosage (ticlopidine 500 mg/d). Routine blood count analyses were performed two weeks after PCI.
Percutaneous Intervention
Stenoses were visually assessed independently by two cardiologists. Quantitative analysis was used only when visual assessment of individual lesions approximated 50% stenosis or when the assessment of both examiners was disparate in regard to categorization of lesion severity greater than 50% of cross-sectional luminal diameter. Stenoses that reduced the lumen diameter by 50% or more were considered significant. The coronary artery tree was divided into three compartments (left anterior descending artery, left circumflex artery, and right coronary artery) for categorization of one, two, and three vessel coronary artery disease. The left main stenosis was considered two vessels disease.
Number of treated lesions, inflations, and inflation pressures were determined by the operators as clinically indicated. Stent delivery was routinely followed by high-pressure balloon inflations (>16 atm). In selected cases, direct coronary stenting or other stent implantation techniques were used. Neither drug eluting stents nor GP IIb/IIIa inhibitors were used in this study. Angiographic success was defined as residual diameter stenosis < 20% by visual estimation and the ultimate achievement of thrombolysis in myocardial infarction (TIMI) flow grade 3.
End Points Definitions
The primary end point evaluated was the incidence of myocardial infarction (MI) based on post-interventional release of TnI. The cut-off level for diagnosis of acute MI suggested by both the manufacturer and the biochemical laboratory of our institution was TnI > 1.5 ng/mL at 24 hours following PCI. Blood samples for TnI measurements were taken 24 hours after PCI. TnI was assayed in the plasma using immunometric assay (IMMULITE Turbo Troponin I, Diagnostic Products Corporation, Los Angeles, CA).
The secondary end point was the composite incidence of death (all-cause), MI and repeat revascularization during follow-up. To assess clinical outcomes following hospital discharge, all patients were contacted via telephone or mail using a standardized questionnaire at least 12 months after the procedure. All adverse events were confirmed by reviewing the medical records.
Statistical Analysis
Continuous variables are expressed as mean ± SD, discrete variables as counts and percentages. Differences between group characteristics were compared using the chi-square test Fisher exact test for categorical variables and Student’s t test for continuous variables. Since TnI data were skewed, these values are expressed as median with interquartile ranges (IQR). The Mann-Whitney U test was used to analyze the difference between the medians of TnI in both groups of patients. Analyses were repeated with additional adjustment for age, history of myocardial infarction, cholesterol level > 5 mmol/L, triglyceride level > 2 mmol/L, presence of smoking and diabetes mellitus by way of logistic regression analysis. A p-value of <0.05 was considered statistically significant.
Results
Baseline Characteristics
Three hundred and sixty consecutive patients meeting the criteria for inclusion underwent PCI. There were no significant differences in baseline characteristics and procedural variables between groups. Clinical and procedural characteristics are shown in Tables 1 and 2. No major complications related to ticlopidine treatment (clinically significant neutropenia or major bleeding) were detected.
Tab. 1. Baseline clinical characteristics. 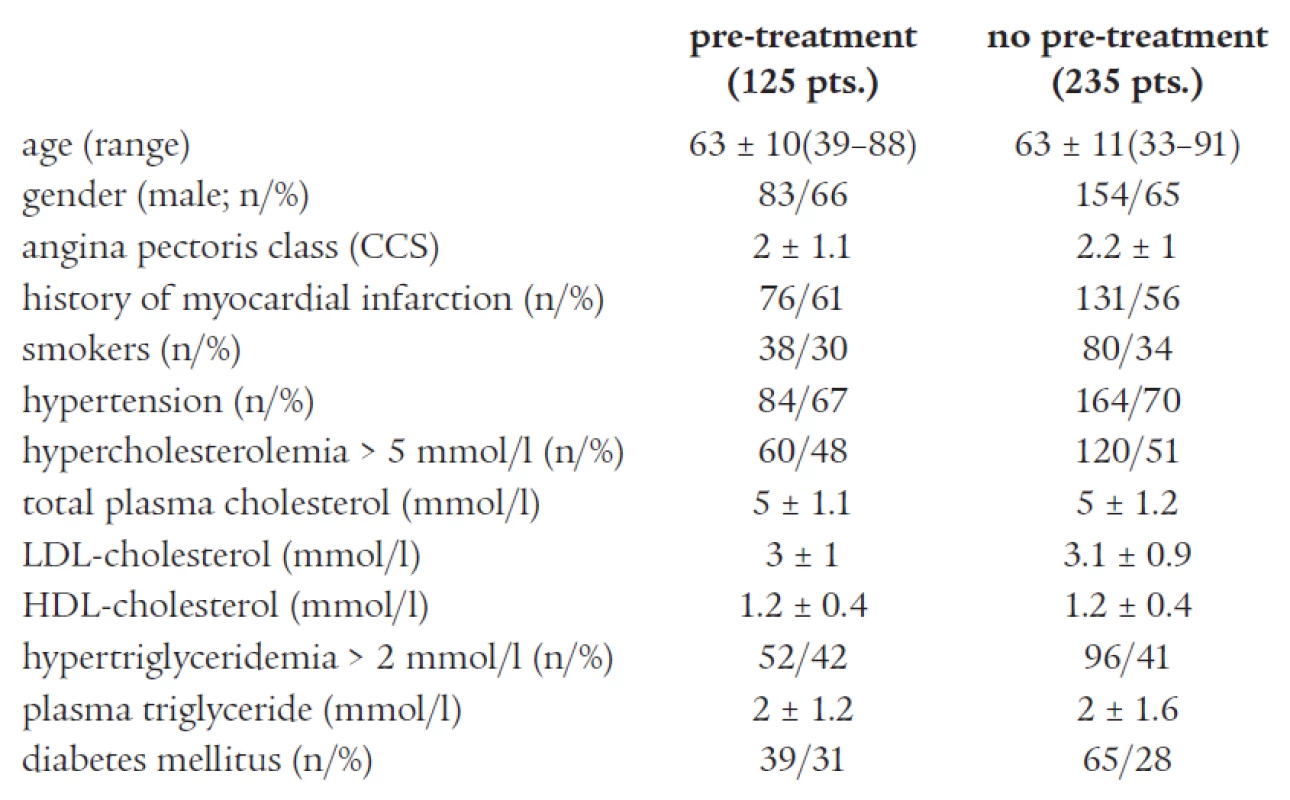
Tab. 2. Angiographic and interventional characteristics of the study population. 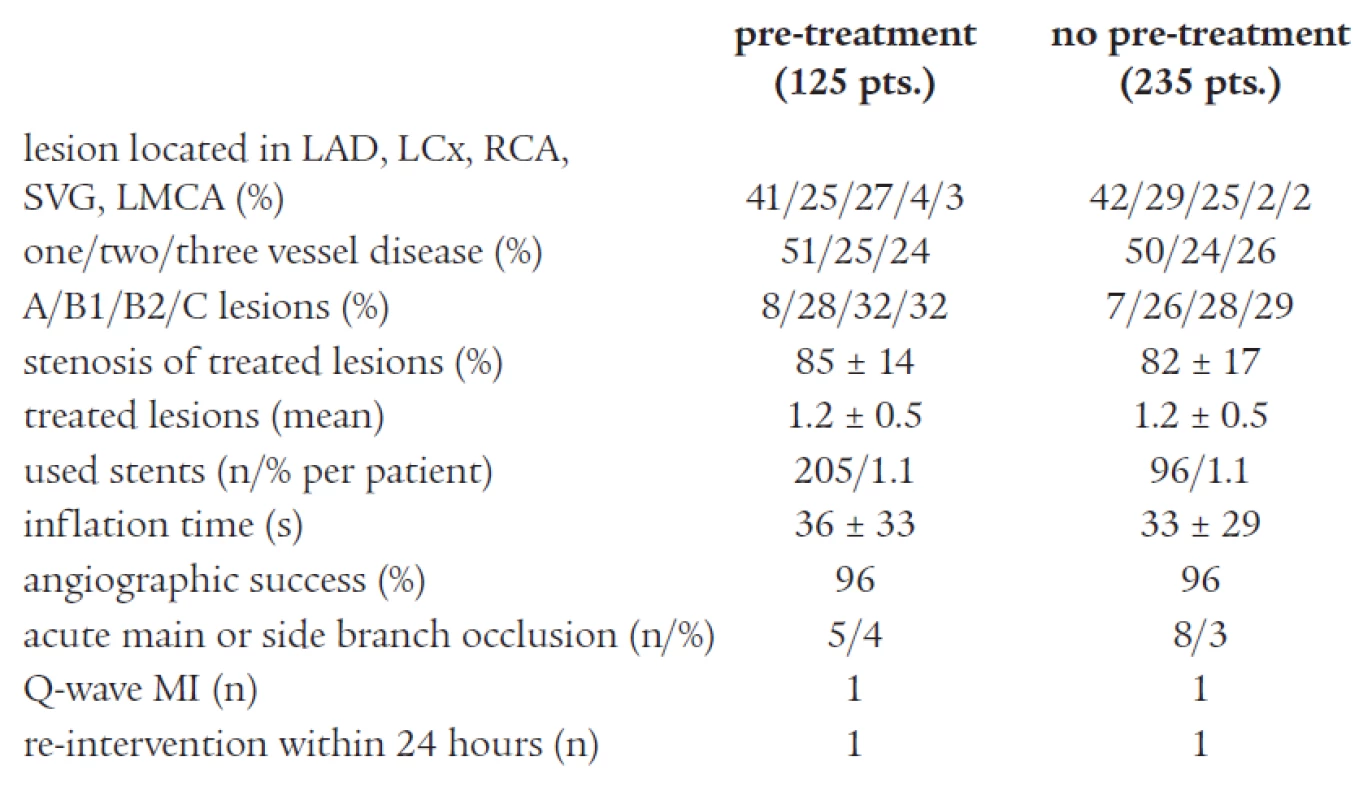
Primary End Point
A pathological rise of TnI > 1.5 ng/mL was found in 16 pts. (13%) pre-treated with ticlopidine and 27 pts. (11%) in those non-pre-treated with ticlopidine (p = 0.84). Of the patients experiencing a post-PCI troponin elevation >1.5 ng/mL, those pre-treated with ticlopidine had a non-significant difference in TnI elevation compared with those not receiving ticlopidine pre-PCI [median: 4 ng/mL (3.1-8.3) vs 4.3 ng/mL (2.1-12.3); p = 0.39]. Analyses were repeated using a TnI cut-off point of 1 ng/mL, which did not change the results (14% vs 17%; p = 0.70). Also, analyses were repeated with adjustment for significant baseline variables, which did not change our findings.
Secondary End Point
Of the 360 patients treated by PCI, long-term follow-up (21 ± 9 months in both groups) was available in 296 patients (82%). Among the 106 patients (85%) who had received pre-procedural ticlopidine therapy and had available follow-up data, 24 (23%) experienced a secondary end point. Among the 190 patients (81%) who had not received pre-PCI therapy and had available followed-up data, 37 (19%) experienced a secondary end point [p = 0.62; (OR 0.83; 95% CI 0.46 to 1.48)], data are summarized in Table 3.
Tab. 3. Adverse coronary events during follow-up. 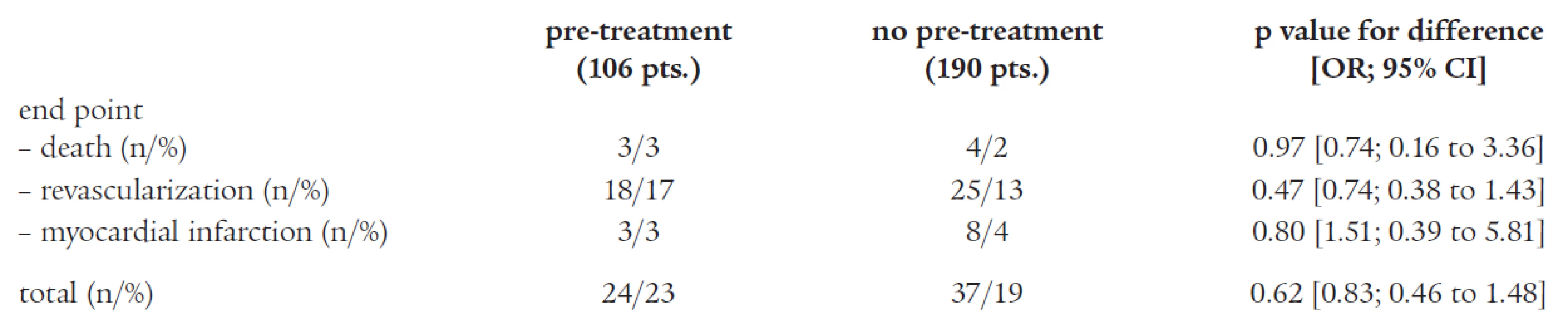
Discussion
The results of this study demonstrate that in patients undergoing PCI for stable coronary disease, pre-treatment with a ticlopidine for at least 72 hours preceding PCI is not associated with a lower risk and extent of myocardial injury measured by TnI release; adjustment for possible confounding baseline factors do not alter these results. In the 21-month follow-up period, we did not detect any difference in clinical events. In the other words, there was no obvious benefit to administering ticlopidine prior to PCI when biomarker release and clinical end points were assessed.
Antiplatelet Therapy
Several studies sought to determine the clinical impact of pre-treatment with combination anti-platelet therapy in patients undergoing elective PCI for angina pectoris [1-6, 9-14]. Steinhubl et al [11] showed that longer duration of ticlopidine pretreatment was strongly associated with a lower incidence of procedure related non-Q-wave MI. However, the population of this study was heterogeneous and included patients with both stable and acute coronary syndromes. The PCI-CURE study showed a reduction of pathological biomarker release and urgent revascularization in patients pre-treated with clopidogrel [3]. However, again, the study population comprised patients with unstable angina pectoris. Similarly, Atmaca et al [6] found that antiplatelet treatment with ticlopidine prior to the coronary stenting is associated with decreased incidence of procedure-related minor myocardial injury. Unfortunately, that study was small, retrospective, and nonrandomized, and patients were followed up only during the short hospital stay. Recently, van der Heijden et al [9] published randomized comparisons of pre-treatment with clopidogrel versus clopidogrel treatment started soon after elective PCI for stable angina pectoris. They did not find any difference between non-pre-treated and pre-treated patients in the occurrence of elevation of TnI or CK-MB. Similarly, in the CREDO trial [12], clopidogrel pre-treatment was not associated with a statistically significant reduction in the composite end point at the 28-day follow-up, although there was a trend toward benefit (p = 0.23).
Thus, the strategy of delaying PCI to allow combined pretreatment is controversial at present. Pretreatment with aspirin before PCI is essential to decrease ischemic complications [15,16]. In high-risk patients, combined therapy given several hours prior to procedure decreases the risk of procedure-related biomarkers release. On the other hand, the potential benefit of combined pretreatment is merely minimal in low-risk patients. In this sense, clopidogrel given several hours before PCI may obviate the need for glycoprotein IIb/IIIa inhibitors [17].
Our findings are important and add to the medical literature. First, we prospectively selected patients with stable angina pectoris, who represent the majority of patients undergoing PCI in our institution; the early risk of procedure-related minor myocardial injury is low in this group of patients and it is likely to be dependent on the operator’s skills and technique than antiplatelet therapy. Second, no difference between both groups persisted during a long-term follow-up. On the other hand, we believe that other interventionalists may find one or more of the other antiplatelet pre-treatment choices having a significant impact on PCI procedural risk in stable patients in their own practice settings. Moreover, from the clinical point of view is very important optimizing anti-platelet therapy prior to PCI in patients with acute coronary syndromes [1-3,15,18].
Limitations
This study was an observational analysis and, as such, pre-procedural ticlopidine therapy was not randomly assigned. Therefore, unrecognized confounding factors may exist. Nevertheless, we have controlled for an extensive list of potentially confounding variables. Patients in the ticlopidine and non-ticlopidine pre-treatment groups had similar severity of coronary artery disease assessed by angiography, cardiovascular risk factors and lipid profiles, and similar complexity of coronary lesions. To achieve more robust statistical data, we would need more patients, mainly in ticlopidine pre-treated group. Follow-up information was not available in 18% of patients, which could impact the results of our secondary outcomes and this analysis likely lacked the statistical power needed to detect a statistically significant difference in the secondary clinical outcome measures. Finally, this study included patients who had received ticlopidine (that is fully covered by Health Insurance Companies in our country) as an anti-platelet agent. Thus, our conclusions should not be extrapolated to patients receiving clopidogrel that replaced ticlopidine in most countries.
Conclusion
In stable angina pectoris patients, we did not detect a significant reduction in myocardial injury in patients pre-treated with aspirin plus ticlopidine compared to aspirin alone. This result persisted in long-term follow-up. Although our results are suggestive, a large, randomized clinical trial evaluating the benefit of ticlopidine pre-treatment prior to PCI in stable angina patients would be needed to verify our results.
Josef Veselka, MD, PhD, FESC, FSCAI
www.motol.cz
e-mail: veselka.josef@seznam.cz
Doručeno do redakce: 14. 10. 2004
Přijato po recenzi: 8. 1. 2005
Zdroje
1. Berglund U, Richter A. Clopidogrel treatment before percutaneous coronary intervention reduces adverse cardiac events. J Invas Cardiol 2002; 14 : 243-246.
2. Gregorini L, Marco J, Fajadet J et al. Ticlopidine and aspirin pretreatment reduces coagulation and platelet activation during coronary dilatation procedures. J Am Coll Cardiol 1997; 29 : 13-20.
3. Mehta SR, Yusuf S, Peters RJ et al. Effects of pretreatment with clopidogrel and aspirin follow by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358 : 527-533.
4. Schuhlen H, Kastrati A, Pache J et al. Incidence of thrombotic occlusion and major adverse cardiac events between two and four weeks after coronary stent placement: analysis of 5,678 patients with a four-week ticlopidine regimen. J Am Coll Cardiol 2001; 37 : 2066-2073.
5. Orford JL, Lennon R, Melby S et al. Frequency and correlates of coronary stent thrombosis in the modern era. J Am Coll Cardiol 2002; 40 : 1567-1572.
6. Atmaca Y, Gulec S, Ertas F et al. The prevention of minor myocardial injury with ticlopidine pretreatment in patients undergoing elective stenting. Int J Cardiol 2003; 87 : 151-157.
7. Hongo RH, Ley J, Dick SE et al. The effect of clopidogrel in combination with aspirin when given before coronary artery bypass grafting. J Am Coll Cardiol 2002; 40 : 231-237.
8. Stadius ML. Diminishing returns... and too many choices... The saga of pharmacologic therapy to reduce the complications of percutaneous coronary intervention. J Am Coll Cardiol 2004; 44 : 25-27.
9. van der Heijden DJ, Westendorp ICD, Riezebos RK et al. Lack of efficacy of clopidogrel pre-treatment in the prevention of myocardial damage after elective stent implantation. J Am Coll Cardiol 2004; 44 : 20-24.
10. Bonz AW, Lengenfelder B, Strotmann J et al. Effect of additional temporary glycoprotein IIb/IIIa receptor inhibition on troponin release in elective percutaneous coronary interventions after pretreatment with aspirin and clopidogrel (TOPSTAR trial). J Am Coll Cardiol 2002; 40 : 662-668.
11. Steinhubl S, Lauer MS, Mukherjee DP et al. The duration of pretreatment with ticlopidine prior to stenting is associated with the risk of procedure-related non-Q-wave myocardial infarctions. J Am Coll Cardiol 1998; 32 : 1366-1370.
12. Steinhubl SR, Berger PB, Mann T et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention, a randomized controlled trial (CREDO). JAMA 2002; 288 : 2411-2420.
13. Berglund U, Lindahl T. Enhanced onset of platelet inhibition with loading dose of ticlopidine in ASA-treated stable coronary patients. Int J Cardiol 1998; 64 : 215-217.
14. Bertinchant JP, Polge A, Ledermann B et al. Relation of minor troponin I elevation to late cardiac events after uncomplicated elective successful percutaneous transluminal coronary angioplasty for angina pectoris. Am J Cardiol 1999; 84 : 51-57.
15. Blankenship JC, Klein LW, Laskey WK et al. SCAI statement on ad hoc versus the separate performance of diagnostic cardiac catheterization and coronary intervention. Cathet Cardiovasc Interv 2004; 63 : 444-451.
16. Malý M, Vojáček J, Hadačová I et al. Stanovení rychlosti nástrupu protidestičkového vlivu dvou různých dávek kyseliny acetylsalicylové agregometrickou metodou. Vnitř Lék 2004; 50(6): 428-433.
17. Kastrati A, Mehilli J, Schuhlen H et al. A clinical trial of abciximab in elective percutaneous coronary intervention after npretreatment with clopidogrel. N Engl J Med 2004; 350 : 232-238.
18. Chan AW, Moliterno DJ, Berger PB et al. Triple antiplatelet therapy during percutaneous coronary intervention is associated with improved outcomes including one-year survival. J Am Coll Cardiol 2003; 42 : 1188-1199.
Štítky
Diabetologie Endokrinologie Interní lékařství
Článek Význam kombinovaného rehabilitačního programu u nemocných s chronickou ischemickou chorobou srdečníČlánek Nekomplikovaný priebeh“ hypertenzie, paroxyzmálnej atriálnej fibrilácie a esenciálnej trombocytémieČlánek KarcinoidČlánek Stoleté výročíČlánek Dopis redakciČlánek „Editorialmánie“Článek Z odborné literaturyČlánek Karcinoid – editorial
Článek vyšel v časopiseVnitřní lékařství
Nejčtenější tento týden
2005 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
-
Všechny články tohoto čísla
- Nekomplikovaný priebeh“ hypertenzie, paroxyzmálnej atriálnej fibrilácie a esenciálnej trombocytémie
- Karcinoid
-
Prevence kardiovaskulárních onemocnění v dospělém věku
Společné doporučení českých odborných společností -
Diskusní příspěvek k článku: Pospíšilová Y, Adam Z, Vorlíček J.
Postavení výuky interní propedeutiky (a oboru vnitřního lékařství obecně) v době stále pokračující specializace v oblasti interní medicíny.
Vnitř Lék 2005; 51: 479-481. - Stoleté výročí
- Dopis redakci
- „Editorialmánie“
- Z odborné literatury
- Je tělesný trénink nezbytnou součástí léčby chronické ICHS i v 21. století? – editorial
- Radiofrekvenční ablace v terapii arytmií – editorial
- Migraeflux v akutní léčbě migrény – editorial
- Karcinoid – editorial
- Lack of Efficacy of Ticlopidine Pre-Treatment in the Reduction of Troponin I Release Following Percutaneous Intervention in Stable Angina Patients
- Esenciální hypertenze a Arg16Gly polymorfizmus genu pro β2-adrenergní receptor
- Význam vyšetření kontraktilní rezervy levé komory dobutaminovou echokardiografií u nemocných s pokročilým chronickým srdečním selháním
- Význam kombinovaného rehabilitačního programu u nemocných s chronickou ischemickou chorobou srdeční
- Big endotelin, interleukin 6 a funkce pravé komory
- Léčba symptomatické intermitentní fibrilace síní katetrovou ablací v levé srdeční síni. Bezprostřední a dlouhodobé výsledky u 150 pacientů
- Migraeflux v akutní léčbě migrény
- Účinnost a komplikace léčby snímatelnými kontaktními fixacemi u pacientů s neuropatickými ulceracemi, akutní Charcotovou osteoartropatií a neuropatickými frakturami
- Poruchy metabolizmu železa II.
- Vnitřní lékařství
- Archiv čísel
- Aktuální číslo
- Pouze online
- Informace o časopisu
Nejčtenější v tomto čísle- Karcinoid
- Poruchy metabolizmu železa II.
- Migraeflux v akutní léčbě migrény
- Význam vyšetření kontraktilní rezervy levé komory dobutaminovou echokardiografií u nemocných s pokročilým chronickým srdečním selháním
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



