-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Hybrid thoracoscopic esophagectomy for cancer – retrospective analysis and comparison with transhiatal resection
Hybridní torakoskopická resekce jícnu pro karcinom – retrospektivní analýza a srovnání s transhiatální ezofagektomií
Úvod:
Využití miniinvazivních metod při ezofagektomii bylo zavedeno s cílem snížení pooperačních komplikací. Práce srovnává otevřenou transhiatální ezofagektomii a minimálně invazivní hybridní ezofagektomii. Obě metody mají rozdílný rozsah lymfadenektomie a transhiatální ezofagektomie je považována za méně radikální.
Metoda:
Do unicentrické retrospektivní studie bylo zařazeno 39 pacientů s transhiatální ezofagektomií a 25 pacientů s hybridní ezofagektomií, která kombinuje torakoskopické uvolnění jícnu s laparotomií a krční anastomózou. Všichni pacienti byli operování pro karcinom střední a distální třetiny jícnu a kardie (Siewert II) v období 2006–2016 na chirurgickém oddělení Nemocnice Nový Jičín, a. s. Data obou skupin, zejména výskyty časných pooperačních komplikací a množství disekovaných uzlin, byla statisticky srovnána. K hodnocení komplikací byla využita doporučení dle Mezinárodního konsenzu ke standardizaci a sběru dat komplikací spojených s resekcí jícnu.
Výsledky:
Délka výkonu byla signifikantně delší ve skupině hybridních resekcí (345 vs. 240 min., p <0,001). Počet disekovaných uzlin byl srovnatelný v obou skupinách (15 vs. 16, p=0,072). Pooperační plicní komplikace byly nižší u hybridních operací (16 % vs. 30,8 %, p=0,243). Nejčastější komplikací byl hrudní výpotek s nutností drenáže u transhiatální ezofagektomie, který se vyskytl u 7 pacientů. Nejčastější plicní komplikací u hybridního výkonu bylo respirační selhání u 3 nemocných. Anastomotický leak vznikl u 5 pacientů po transhiatálním výkonu a u 1 pacienta po torakoskopické resekci (12,8 % vs. 4 %, p=0,391). 30denní a 90denní letalita byla nesignifikantně nižší u hybridní resekce s torakoskopií (0 % vs. 5,1 %, p=0,516 a 4 % vs. 10,3 %, p=0,64). Ve skupině transhiatální resekce zemřeli 2 nemocní na následky respirační komplikace, 1 pro nekrózu žaludečního tubusu a 1 na akutní infarkt myokardu. Ve skupině hybridních výkonů zemřel 1 na respirační selhání. Morbidita hybridních výkonů byla nižší (36 % vs. 59 %, p=0,123). Statisticky významný byl počet celkových komplikací bez rozdílu v jejich závažnosti dle Clavien-Dindovy klasifikace ve prospěch hybridní resekce (11 vs. 30, p=0,015).
Závěr:
Hybridní ezofagektomie je dobře proveditelnou metodu. V našem souboru byla spojena s nižším počtem časných komplikací ve srovnání s transhiatální resekcí. Stejně jako torakotomie umožňuje dostatečnou lymfadenektomii. Velký počet a různé kombinace užívaných technik ezofagektomie ztěžují vyhodnocení a srovnání výsledků jednotlivých metod. Preference chirurgického pracoviště k technice resekce zůstává klíčová a jednoznačná doporučení zatím neexistují. Využití minimálně invazivních technik u resekcí jícnu se postupně stává standardem.
Klíčová slova:
minimálně invazivní ezofagektomie – torakoskopie – pooperační komplikace – lymfadenektomi
Authors: T. Jínek 1; L. Adamcik 1; M. Duda 1,2; M. Skrovina 1,3
Authors place of work: Department of Surgery, Hospital Nový Jičín, a. s., Center of highly specialized oncological care for adults Nový Jičín, AGEL research and Training Institute 1; 2nd Department of Surgery, University Hospital Olomouc and Faculty of Medicine and Dentistry, Palacky University Olomouc 2; 1st Department of Surgery, University Hospital Olomouc and Faculty of Medicine and Dentistry, Palacky University Olomouc 3
Published in the journal: Rozhl. Chir., 2018, roč. 97, č. 7, s. 320-327.
Category: Původní práce
Summary
Introduction:
Minimally invasive methods for esophagectomy have been introduced to reduce postoperative complications. This paper compares open transhiatal esophagectomy and minimally invasive hybrid esophagectomy. Both methods have different extents of lymphadenectomy, transhiatal esophagectomy being considered less radical.
Method:
A single-centre retrospective study comprised 39 patients subjected to transhiatal esophagectomy and 25 patients subjected to hybrid esophagectomy combining thoracoscopy with laparotomy and cervical anastomosis. All patients were operated for middle and distal third carcinoma of the esophagus, including cardia (Siewert II), in the period of 2006−2016 at the Surgery department of Nový Jičín hospital. The data of both groups, in particular the incidence of early postoperative complications and the number of dissected lymph nodes, were statistically compared. Complications are reported according to the International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy.
Results:
The duration of operation was significantly longer in the group that underwent hybrid resections (345 vs. 240 min, p<0.001). The number of dissected lymph nodes was comparable in both groups (15 vs. 16, p=0.072). Postoperative pulmonary complications were lower for hybrid operations (16% vs. 30.8%, p=0.243). The most common complication of transhiatal esophagectomy was pleural effusion requiring drainage, which occurred in 7 patients. The most common pulmonary complication of hybrid procedures was respiratory failure, which occurred in 3 patients. Anastomotic leak occurred in 5 patients after transhiatal esophagectomy and in one after thoracoscopic resection (12.8% vs. 4%, p=0.391). 30-day and 90-day mortality was nonsignificantly lower for hybrid resections (0% vs. 5.1%, p=0.516 and 4% vs. 10.3%, p=0.64). Following transhiatal esophagectomy, two patients died as a result of respiratory complications, one died from necrosis of the gastric tube and one from acute myocardial infarction. In the hybrid group, one patient died from respiratory failure. Hybrid resection exhibited lower morbidity (36% vs. 59%, p=0.123). The number of overall complications, irrespective of their severity according to the Clavien-Dindo classification, was statistically in favor of hybrid resection (11 vs. 30, p=0.015).
Conclusion:
In our study, we found that thoracoscopic hybrid resection was a feasible and well-executable method, with a statistically lower incidence of postoperative complications. Thoracoscopy allows lymphadenectomy to be performed to sufficient extent. The large number and various combinations of esophagectomy techniques make it difficult to evaluate and compare the outcomes of individual methods. Preference for a specific resection technique within a given surgical department remains an important factor as clear recommendations for esophageal resections do not yet exist. However, the use of minimally invasive techniques in esophageal resections is gradually becoming a standard.
Key words:
minimally invasive esophagectomy – thoracoscopy – postoperative complications – lymphadenectomy
INTRODUCTION
Esophageal cancer is a cancer, whose incidence in the Czech Republic has risen three times in the last three decades (3,38/100 000)[1]. Surgical treatment with neoadjuvant radiochemotherapy is now standard in advanced tumors of the middle and distal third of the esophagus [2]. The esophageal resection is performed by various surgical approaches. Mortality is 1−5% in specialized centers and morbidity is 30−60% [3,4]. Classical open resection can be performed with a thoracotomy (transthoracic “Ivor-Lewis” surgery with intrathoracic anastomosis or McKeown resection with cervical anastomosis) and no thoracotomy with neck anastomosis (transhiatal resection − THE). Localization of anastomosis and the possibility of minimally invasive implementation of some phases of the operation contribute to various variations of esophagectomy, making it difficult to compare. Traditionally, there is a lesser incidence of respiratory complications and a higher incidence of anastomotic leakage and subsequent benign strictures of cervical anastomosis in patients undergoing THE. The disadvantage of THE is also inadequate access to middle esophageal tumors with lesser radicality and higher incidence of recurrent nerve palsy [5,6]. The type of resection preference depends on the center experience and localization of the cancer itself. THE is prefered for less advanced tumors of distal esophagus and cardia in patients with limited node involvement and pulmonary comorbidities [7]. In 2009, the International Society for Diseases of the Esophagus (ISDE) and the European Society of Esophagology (ESE − GEEMO) questionnaire showed that with growing center experience a more radical transthoracic esophagectomy (TTE) is being preferred. TTE is nowadays the gold standard mainly in Asian countries [8].
With the development of minimally invasive approaches, these methods have also been used in esophageal surgery. In 1992, Cushieri performed a thoracoscopic mobilization of the esophagus which, with the same radicularity as TTE [9]. The thoracoscopic approach (VATS) can be combined with laparoscopy (complete minimally invasive esophagectomy – MIE) or open laparotomy (hybrid esophagectomy). Systemic review or meta-analyses confirm the safety of minimally invasive operations and comparable oncological results of thoracoscopic resection, possibly MIE with open techniques [10,11].
The aim of our study is to compare classical THE and hybrid minimally invasive esophagectomy using thoracoscopy (MIHE) as two techniques seeking to reduce the incidence of postoperative complications with a different extent of lymphadenectomy. The cervical phase of the operation and the construction of the esophagogastric anastomosis was performed by the same technique.
METHOD
In the period 2006−2016 a total of 110 esophageal resections were performed at the Surgery department of Nový Jičín hospital. Patients with a surgery for benign diseases, non-standard esophageal resections, thoracotomy or thoracoscopic procedures converted to open surgery, were excluded from the study. The two most common types of resections for middle and distal third esophageal carcinoma including cardia (Siewert II) were compared, focusing on early post-operative complications: transhiatal esophagectomy (THE) and minimally invasive hybrid esophagectomy (MIHE), which combines VATS with laparotomy and cervical anastomosis. Gastric conduit was used for GI tract reconstruction in all cases. The data was obtained retrospectively from the hospital database. In the THE group there were 36 men and 3 women, median age was 60 (range 39−76). In the MIHE group there were 20 men and 5 women, median age was 61 (range 43−78). Postoperative complications are classified according to the current International consensus for data collection after esophagectomy, focusing on pulmonary complications [12]. The severity of the complication was assessed by Clavien-Dindo’s classification. The stage of the malignancy was ranked according to the recommendations for pathological staging (including neoadjuvant therapy) for the 8th edition of the TNM AJCC/UICC classification for Esophageal cancer [13,14]. The nutritional status of the patients was evaluated according to the Nutritional Risk Score (NRS 2002) [15]. Subsequent sub-analysis included patients with a score of ≥3, i.e. patients with a weight loss of 10−15% over the last 3 months.
The technique of hybrid thoracoscopic resection of esophagus (MIHE)
VATS resection was performed as a three − phase operation. All operations were performed by two surgeons with experience in upper gastrointestinal surgery and thoracic surgery. Selective pulmonary ventilation with double luminal endobronchial cannula was used. At first we operated in a position on the left side with three thoracic ports (1x5 mm, 1x10 mm, 1x12 mm camera) in the posterior axillary line and a 3 cm minithoracotomy in the anterior axillary line below the nipple level. Later we moved to a prone position with three thoracic ports. The patient is positioned with 100 degree abduction of the right upper limb on the abdomen. Only one assistant was needed in this positioning. The surgeon is on the right side of the patient and the assistant to the left of him. The esophagus is mobilized with periesophageal fat-lymphatic tissue along the pericardium, the aorta and the thoracic spine up to the upper thoracic aperture (Fig. 1).
Fig. 1. A) esophageal mobilization and elevation (left lateral decubitus position); B) transsection of azygos vein with endostapler (prone position); C) lymphadenectomy along azygos vein (prone position); D) lymphadenectomy along trachea and bronchi (prone position) 
In the abdominal phase, the patient is in a supine position with abducted lower limbs in a 30 degree antiTrendelenburg position with a slight head inclination to the back and to the right. The surgeon stands between the patient’s feet and the first assistant on his left. We chose the upper middle or more often Chevron laparotomy. We mobilize the esophagus and create a gastric tube with nutrition from right gastroepiploic artery. Part of the performance is also a dissection of the abdominal compartment node in the modified D2 range (stations 7, 8, 9 and 11) according to Japanese classification of lymphatic stations for gastric cancer. The tube is attached to a sterile packaging used for laparoscopy to cover the optical cable.
Approach in the cervical phase is selected by incision along the medial margin of the left sternocleidomastoideus muscle. The covered stomach tube is pulled up from the abdomen to the neck. The anastomosis is performed by the end-to-side circular stapler (21 mm or 25 mm circular stapler, Ethicon, USA).
Statistical analysis
Numerical data are expressed as averages with standard deviation or medians with range of values. Relation of dichotomous variables to groups were evaluated by Fisher’s exact test, while metric variables to groups by Welch’s t-test following Box-Cox transformation of original data to symmetric data distribution and homoscedasticity. Symmetry of distribution and homogeneity of transformed data was verified using quantile-quantile (Q-Q) plot for normal distribution and histograms. Statistical analysis of the data was performed in R Statistics 3.3.3 (2017-03-06) - “Another Canoe” from “R Foundation for Statistical Computing”, using the R-commander, fBasics and AID libraries. Tests were performed at a significance level of 0.05.
RESULTS
Comparison of patients
Comparing the comorbidities, ASA score and the nutritional status, we did not record a statistically significant difference (Table 1). Comparison of oncological data is shown in Tab. 2. In the THE group we statistically confirmed a higher incidence of adenocarcinoma (THE 66.7% vs. MIHE 36%, p=0.022). In the localization of tumors there was a statistically higher incidence of distal third tumors in the THE group (THE 71.8% vs. MIHE 36%, p=0.009). In the MIHE group lower stages of the disease were more common (stage 0 – 1 MIHE 32% vs. THE 10.3%, p=0.048, stage 2 MIHE 32.6% vs. THE 25.6%, p=0.584). No statistically significant difference was observed for neoadjuvant treatment, but more neoadjuvant patients were in the MIHE group (MIHE 40% vs. THE 25.6%, p=0.275).
Tab. 1. Statistical comparison of demographic characteristics in groups subjected to THE and MIHE 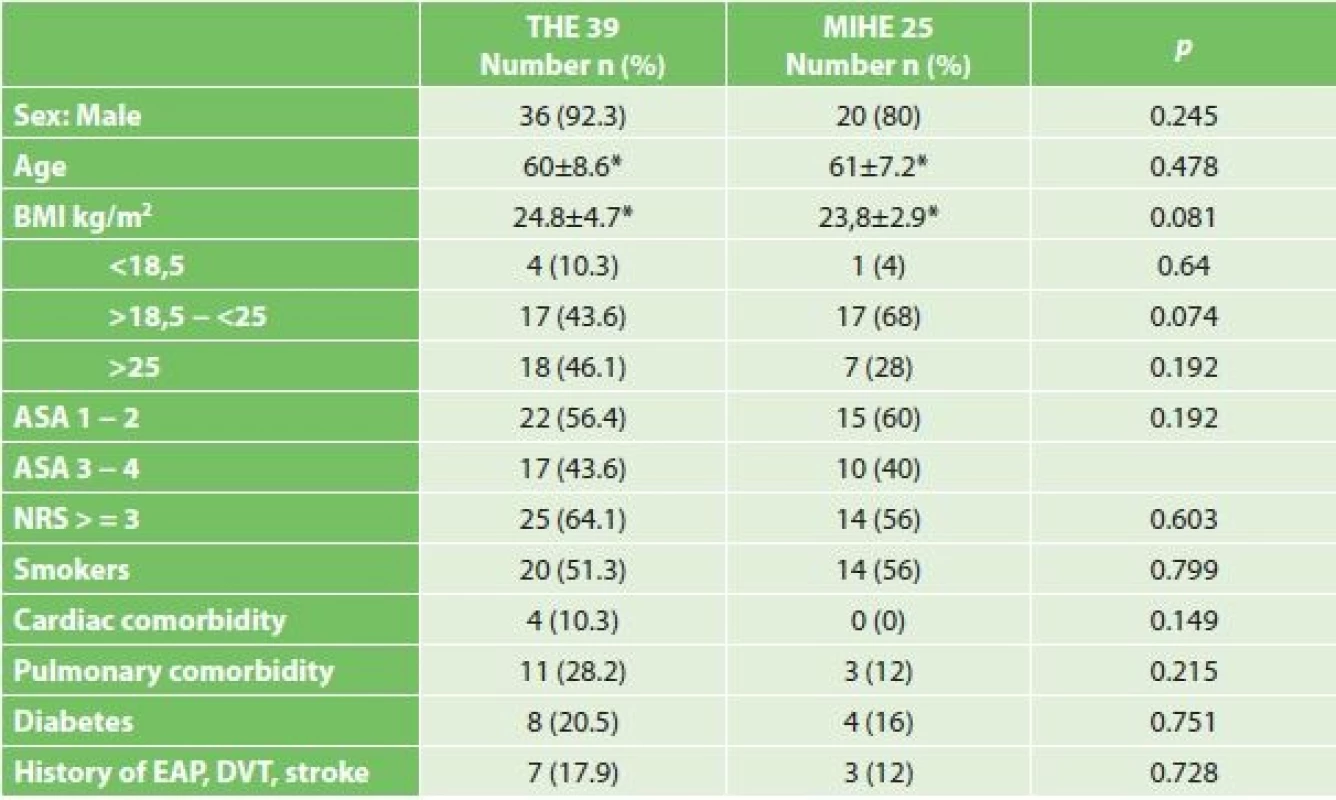
Note: * average ± standard deviation Tab. 2. Comparison of oncological data in both resected groups 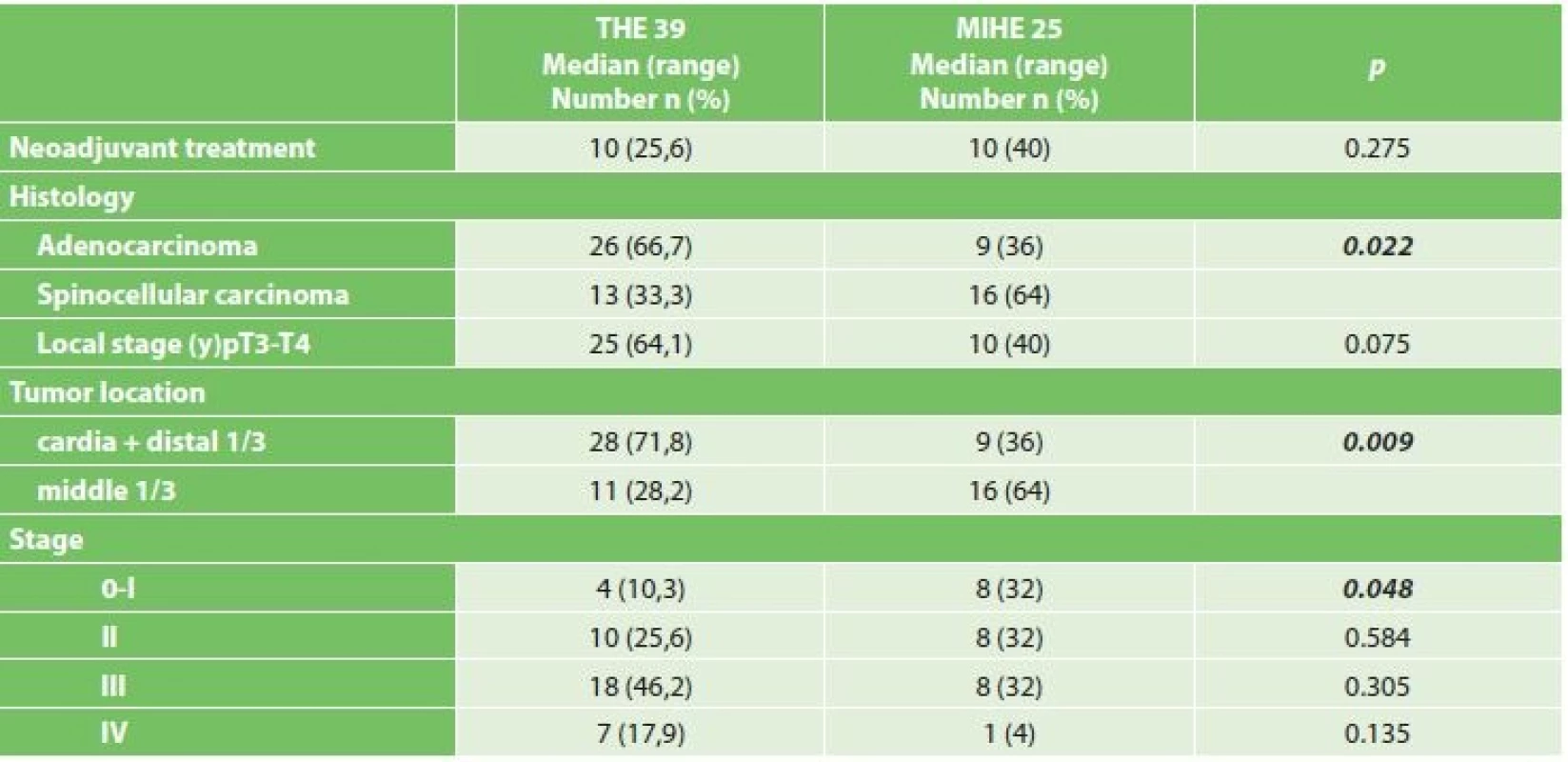
Operational results
The duration of operation was significantly longer in the group that underwent hybrid resections (345 vs. 240 min, p<0.001). The number of dissected lymph nodes was comparable in both groups (15 vs. 16, p=0.072). A positive circumferential margin was found in three THE patients (7.7%) with tumors on the distal third of the esophagus. When comparing individual types of complications, we observed a trend of better results for MIHE, although this difference was statistically not significant (Tab. 3). The most common postoperative complications were pulmonary and anastomosis insufficiency. Pulmonary complications occurred in 16% of the MIHE and 30.8% in the THE group (MIHE 16% vs. THE 30.8%, p=0.243). The most frequent complication was pleural effusion requiring drainage in the THE group in 7 patients (C−D 3a). In the MIHE group respiratory failure in three patients (C−D 4a and C−D 5) was the most frequent complication. We observed anastomotic leak (AL) in 5 patients in the THE and one in the MIHE group (THE 12.8% vs. MIHE 4%, p=0.391). In the THE group there were two insufficiencies treated by the reoperation (AL grade 3). By the upper GI tract x-ray exam, routinely performed on the 7th postoperative day, in another patient leakage from the area of the stapler line on the gastric tube was confirmed with the formation of a gastropleural fistula, which we have solved by introducing a stent (AL grade 2). At one patient in each group disconnection had to be performed due to gastric tube necrosis (CN 3). Recurent nerve palsy, with left larynx transit paresis (type 1A) occurred in both groups (THE 10.3% vs. MIHE 8%, n=1).
Tab. 3. Comparison of perioperative results and incidence of postoperative complications 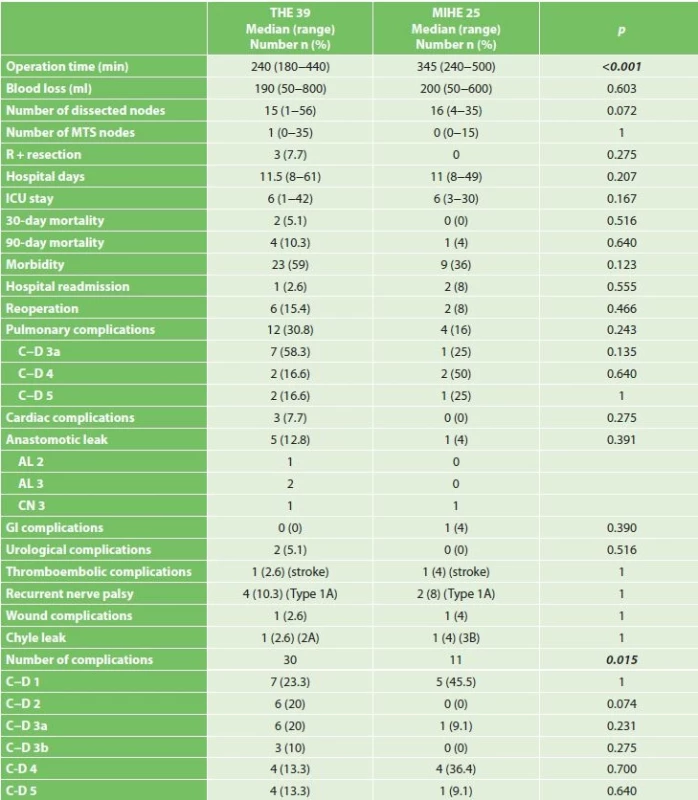
Note: Reported according to the International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy (Low 2015),(* median and range), Clavien-Dindo classification: C-D 1 a C-D 2 complications requiring pharmacological treatment, C-D 3a complications requiring intervention without general anesthesia, C-D 3b complications requiring intervention under general anesthesia, C-D 4 complication with single or multi organ dysfunction, C-D 5 death of patient In both groups, we did not see a significant difference in the 30-day and 90-day mortality (30-day mortality, THE 5.1% vs. MIHE 0%, p=0.516, 90-day mortality 10.3% vs. MIHE 4%, p=0.64). In the MIHE group one death occurred in the 48th postoperative day, when a diabetic patient died due to respiratory failure and ARDS. Two patients died in the THE group as a result of respiratory complications due to ARDS and pneumonia. One patient died on the 32nd postoperative day due to gastric tube necrosis (CN) and one patient died of cardial arrest during the first day after esophagectomy. We recorded a lower morbidity in MIHE (36% vs. 59%, p=0.123). However, the total number of postoperative complications was significantly lower for MIHE (11 vs. 30, p=0.015).
DISCUSSION
Esophagectomy is an invasive procedure with significant postoperative mortality and morbidity. For opened performances, the mortality of THE and TTE was about 10% [16,17]. Over the last two decades it has declined significantly, especially in high-volume centers [3,4]. The decrease in mortality involves improvements in perioperative care, patient selection and the introduction of minimally invasive methods. Current studies suggest a significant impact of the center experience on reducing mortality [18]. In an analysis of the US national database with more than 23,000 esophagectomies the average 30-day mortality was 7%. This study demonstrated a significant effect of the number of esophagectomies performed on postoperative mortality and the influence of minimally invasive methods, particularly laparoscopy, on morbidity [19]. There is little information about the mortality of hybrid methods after thoracoscopic resection. Decker summarized data from 14 retrospective studies with 743 MIHE patients according to which the 30-day mortality was 2.4% and the overall morbidity was 51.6% [20]. Most studies report only a 30-day mortality that underestimates hospital, or 90-day mortality by up to 4 times. For this reason the 90-day mortality is more indicative [12]. In our group MIHE was associated with lower mortality and lower total postoperative complications (11 vs. 30, p=0.015). Our 30-day mortality is comparable to medium-volume centers [19,20]. However, the mortality doubled after 90-days, confirming the need for its reporting(4% for MIHE, 10.3% for THE).
The extent of lymphadenectomy, its significance and the way it is performed are still the subject of discussions between Western countries and Asia. To determine the pathological staging, at least 15 nodes should be removed [2]. THE is limited by its technical reach through the diaphragmatic hiatus and dissects the paraesophageal tissue by standard lymphadenectomy in the lower posterior mediastinum. Orringer initially reserved this method for patients without lymphatic disorder and relied on adjuvant therapy [21]. The potential benefits of TTE as well as MIHE can be seen in the possibility of sufficient access to dissection of lymph nodes, including the upper mediastinum. Studies show that the number of nodes taken and the ratio of metastatic nodes to the total number of dissected nodes correlates with survival of patients in esophageal cancer. Other studies confirm that overall survival correlates with the total number of dissected nodes regardless of their positivity [22,23]. The meta-analysis comparing classical TTE and THE from 2011 showed significantly higher numbers of dissected nodes in TTE (an average of 8). In 5-year survival, it did not show the difference between THE and TTE. The authors of this meta-analysis point to a higher proportion of later stages of esophageal carcinoma in TTE [24]. The benefit of the thoracic lymphadenectomy in relation to 5-year survival was demonstrated in a randomized study of middle esophageal carcinoma and cardia Siewert type I. carcinoma [25]. Most of these analyzes were based on primary surgical treatment without neoadjuvant radiochemotherapy. Koen in a prospective study confirmed a lower number of dissected nodes in neoadjuvant patients and refuted a similar correlation of survival rate of dissected nodes [26]. What these effects will have on the recommendation of the extent of lymphadenectomy and the amount of dissected nodes in the era of neoadjuvant treatment, we do not yet know. Which approach to resection of the esophagus should be chosen is, however, still controversial [27]. In our set, the median of the MIHE was 16, no difference compared to THE. However, we must take into account a larger number of patients after neoadjuvant treatment in the MIHE group that may have affected this number. The number of dissected nodes in our set is comparable to most published papers [20, 24].
Pulmonary complications are the most common postoperative complications in esophageal resection with an incidence of 20−40%. Approximately 50% of deaths are associated with respiratory failure and pneumonia [28]. The high incidence of pulmonary complications is explained by the extent of surgery, the need for unilateral ventilation and the disruption of bronchial innervation. Some meta-analyzes and reviews show a reduction in MIE incidence compared to open procedures [29,30]. However, the relevance of VATS has not been demonstrated in the Mocan review with 1190 patients or in the national English esophageal resection database. Different definitions of pulmonary postoperative complications were considered as a reason for not making adequate comparisons [31,32]. Palanivelu in a group of 130 MIE patients in the prone position achieved excellent results with a low incidence of postoperative complications (pneumonia 1.5% and pulmonary effusions 0.77%) [33]. In a multicenter prospective randomized study of postoperative pulmonary complications in the first two postoperative weeks from 2012 Bier confirmed significant reductions at MIE (9% vs. 29%, p=0.005) [34]. In our set there were fewer pulmonary complications after MIHE. In this group, however, we operated less patients with pulmonary comorbidity, which could have affected these results.
The hospitalization after esophagectomy is affected by nutritional and performance status, comorbidity or the presence of a rehabilitation team with the ERAS protocol. In our report the length of hospitalization in both groups was almost the same. In MIHE, the median of hospitalization was 11 days and was shorter than in most published hybrid esophagectomies in Decker’s review (18 days). For THE hospitalization was the same (11 days) [20]. We recorded a significantly longer operating time for MIHE. Hybrid procedures required a perioperative change in patient position and mobilization of the esophagus was longer at the beginning of the “learning curve”. All hybrid performances were performed by one surgeon and the operating times were shortened.
Cushieri thoracoscopically mobilized the esophagus first in the left lateral decubitus position and later described the technique in the prone position and highlighted the advantages of a good operating field image, fall of the right lung caused by gravity allowing for both bilateral lung ventilation [9,35]. The disadvantage is the difficulty of performing an acute thoracotomy and a less clear view of the upper mediastinum. In the position on the left side we had to push the lung tissue through by a hook, which required another assistant. In the prone position one assistant was required and dissection towards the thoracic aorta seemed more profitable and safer. In all cases the thoracic duct was visualized and currently we preferred this position. The thoracoscopy can be operated as radically as in the thoracotomy. Contraindications to VATS can be extensive adhesions or locally advanced tumor near the tracheobronchial tree where palpability is beneficial.
The goal of minimally invasive methods is to reduce the incidence of complications and to speed up recovery without worsening oncological results. A large number of combinations of esophagectomy techniques make it difficult to evaluate and compare the results of individual methods. Meta-analyzes comparing MIE and open resections were often limited by non-randomized retrospective data, but they indicated lower blood loss, shorter postoperative hospitalization time and favored minimally invasive methods in terms of lower incidence of pulmonary complications [10,29,30]. Similar findings were subsequently confirmed in a multicenter prospective randomized trial [34]. Minimally invasive techniques are used in Japan and the UK for almost half of the esophagectomies. In high-volume centers MIE numbers exceeded the number of open procedures [36,37]. Several czech papers on the implementation of MIE have been published largely using a laparoscopic approach or a complete MIE [38,39,40]. However, the hybrid method combining VATS with laparotomy has not yet been described in the Czech Republic.
CONCLUSION
Based on our experience MIHE is a well feasible method with a lower number of early complications compared to THE. Like thoracotomy it allows for sufficient lymphadenectomy. The effect of neoadjuvant treatment on the recommended extent of lymphadenectomy remains unclear. The large number and various combinations of esophagectomy techniques make it difficult to evaluate and compare the results of individual methods. The center experience to esophagectomy remains crucial and unambiguous recommendations for the esophageal resection technique do not yet exist. A definitive assessment of the role of the hybrid esophageal resection will only be possible once the long-term outcomes have been evaluated. Consistency of reporting postoperative complications to current standards may lead to clearer conclusions in the future. The use of minimally invasive techniques for esophageal resections is gradually increasing.
List of abbreviations:
AL − anastomotic leak
ARDS − acute respiratory distress syndrome
ASA − American Society of Anaesthesiologists
BMI − body mass Index
C−D − Clavien-Dindo classification
DVT − deep venous thrombosis
CN − conduit necrosis
EAP − pulmonary artery embolisation
ERAS − enhanced recovery after surgery
ESE-GEEMO − European Society of Esophagology − Group d‘Etude Europeen des Maladies de l‘Oesophage (GEEMO)
ICU – intensive care unit
ISDE − International Society for Diseases of the Esophagus
MIE − minimally invasive esophagectomy
MIHE − minimally invasive hybrid esophagectomy
MTS − metastases
NRS − nutritional risk score
THE − transhiatal esophagectomy
TTE − transthoracic esophagectomy
VATS − video-assisted thoracoscopic surgery
We thank to ing. Martin Hill, DrSc. for statistical data processing.
Conflict of interests
The authors declare that they have not conflict of interest in connection with the emergence of and that the article was not published in any other journal.
MUDr. Tomáš Jínek
Nemocnice Nový Jičín, a.s.
Purkyňova 2138/16
741 01 Nový Jičín
e-mail: jinek.tomas@gmail.com
Zdroje
- Duda M, Adamčík L, Dušek L, et al. Zhoubné nádory jícnu v České republice. Rozhl Chir 2012;91 : 132−40.
- NCCN Clinical Practice Guidelines in Oncology, Esophageal and Esophagogastric Junction Cancers, Version I.2017. Availeble from: www: <https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf>.
- Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg 2007;246 : 363−72; discussion 372−4.
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256 : 95−103.
- Pennathur A, Zhang J, Chen H, et al. The best operation for esophageal cancer? Ann Thorac Surg 2010;89 : 2163−7.
- Barreto JC, Posner MC. Transhiatal versus transthoracic esophagectomy for esophageal cancer. World J Gastroenterol 2010;16 : 3804−10.
- Donohoe CL, O´Farrell NJ, Ravi N, et al. Evidence-based selective application of transhiatal esophagectomy in a high-volume esophageal center. World J Surg 2012;36 : 98−103.
- Boone J, Livestro DP, Elias SG, et al. International survey on esophageal cancer: part I surgical techniques. Dis Esophagus 2009;22 : 195−202.
- Cuschieri A, Shimi S, Banting S. Endoscopic Esophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37 : 7−11.
- Kim T, Hochwald SN, Sarosi GA, et al. Review of minimally invasive esophagectomy and current controversies. Gastroenterol Res Pract 2012. Available from: doi: 1155/2012/683213.
- Dantoc MM, Cox MR, Eslick GD. Does minimally invasive esophagectomy (MIE) provide for comparable oncologic outcomes to open techniques? A systematic review. J Gastrointest Surg 2012;16 : 486−94.
- Low DE, Alderson D, Cecconello I, et al. International Consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262 : 286−94.
- Rice TW, Ishwaran H, Kelsen DP, et al. Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus 2016;29 : 906−12.
- Rice TW, Ishwaran H, Hofstetter WL, et al. Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus 2016;29 : 897−905.
- Kondrup J, Rasmussen HH, Hamberg O, et al. Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22 : 321−36.
- Rindani R, Martin CJ, Cox MR. Transhiatal versus Ivor-Lewis Esophagectomy: is there a difference? Aust N Z J Surg 1999;69 : 187−94.
- Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72 : 306−13.
- Fuchs HF, Harnsberger CR, Broderick RC, et al. Simple preoperative risk scale accurately predicts perioperative mortality following esophagectomy for malignancy. Dis Esophagus 2017;30 : 1−6.
- Fuchs HF, Harnsberger CR, Broderick RC, et al. Mortality after esophagectomy is heavily impacted by center volume: retrospective analysis of the Nationwide Inpatient Sample. Surg Endosc 2017;31 : 2491−7.
- Decker G, Coosemans W, De Leyn P, et al. Minimally invasive esophagectomy for cancer. Eur J Cardiothorac Surg 2009;35 : 13−
- Dubecz A, Kun L, Stadlhuber RJ, et al. The origins of an operation: a brief history of transhiatal esophagectomy. Ann Surg 2009;249 : 535−40.
- Peyre CG, Hagen JA, DeMeester SR, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008;248 : 979−85.
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248 : 221−6.
- Boshier PR, Anderson O, Hanna GB. Transthoracic versus transhiatal esophagectomy for the treatment of esophagogastric cancer: a meta-analysis. Ann Surg 2011;254 : 894−906.
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246 : 992−1000.
- Koen Talsma A, Shapiro J, Looman CW, et al. Lymph node retrieval during esophagectomy with and without neoadjuvant chemoradiotherapy: prognostic and therapeutic impact on survival. Ann Surg 2014;260 : 786−92.
- Noordman BJ, Wijnhoven BP, van Lanschot JJ. Optimal surgical approach for esophageal cancer in the era of minimally invasive esophagectomy and neoadjuvant therapy. Dis Esophagus 2016;29 : 773−9.
- Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg 2011;91 : 1494v1500.
- Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir 2009;64 : 135−46.
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24 : 1621−9.
- Mocanu SN, Balagué Ponz MC, Targarona Soler EM, et al. Influence of the type of thoracic access on postesophagectomy respiratory complications. Cir Esp 2013;91 : 563−73.
- Sihag S, Kosinski AS, Gaissert HA, et al. Minimally invasive versus open esophagectomy for esophageal cancer: A comparison of early surgical outcomes from The Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016;101 : 1281−8.
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position--experience of 130 patients. J Am Coll Surg 2006;203 : 7−16.
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open Esophagectomy for patients with Esophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379 : 1887−92.
- Cuschieri A. Thoracoscopic subtotal Esophagectomy. Endosc Surg Allied Technol 1994;2 : 21−5.
- Mamidanna R, Bottle A, Aylin P, et al. Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population-based national study. Ann Surg 2012;255 : 197−203.
- Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014;260 : 259−66.
- Aujeský R, Neoral Č, Král V, et al. Videoasistovaná resekce jícnu pro karcinom – desetileté zkušenosti. Rozhl Chir 2010;89 : 746−9.
- Vrba R, Aujeský R, Vomáčková K, et al. Minimally invasive esophagectomy for esophageal cancer – results of surgical therapy. Wideochir Inne Tech Maloinwazyjne 2015;10 : 189−96.
- Zonča P, Peteja M, Richter V, et al. Kompletní miniinvazivní Ivor-Lewisova resekce jícnu. Rozhl Chir 2017;96 : 114−9.
Štítky
Chirurgie všeobecná Ortopedie Urgentní medicína
Článek vyšel v časopiseRozhledy v chirurgii
Nejčtenější tento týden
2018 Číslo 7- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Hojení análních fisur urychlí čípky a gel
-
Všechny články tohoto čísla
- Chirurgie horní části zažívací trubice (chirurgie foregutu)
- The use of retrosternally placed colon in esophageal replacement
- Current state of surgical treatment of cancer of the stomach and gastro-esophageal junction in the Czech Republic
- Hybrid thoracoscopic esophagectomy for cancer – retrospective analysis and comparison with transhiatal resection
- K životnímu jubileu profesora Jana Wechslera
- Prophylactic ligation of the thoracic duct in the prevention of chylothorax after esophagectomy
- Results of minimally invasive esophagectomy for esophageal cancer performed after ischemic gastric conditioning
- Esophageal cancer − results of surgical treatment at the Department of Surgery I. at the University Hospital Olomouc
- Successfull therapy of grade III leak after thoracic oesophagectomy using endoscopic vacuum assisted closure therapy – a case study
- Aktuální stav přípravy nového Vzdělávacího programu oboru chirurgie
- Rozhledy v chirurgii
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The use of retrosternally placed colon in esophageal replacement
- Prophylactic ligation of the thoracic duct in the prevention of chylothorax after esophagectomy
- Esophageal cancer − results of surgical treatment at the Department of Surgery I. at the University Hospital Olomouc
- Current state of surgical treatment of cancer of the stomach and gastro-esophageal junction in the Czech Republic
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



