-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The [Het-s] Prion, an Amyloid Fold as a Cell Death Activation Trigger
article has not abstract
Published in the journal: . PLoS Pathog 8(5): e32767. doi:10.1371/journal.ppat.1002687
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002687Summary
article has not abstract
Prions Are Amyloids
Prions are self-propagating amyloids. Amyloids are protein polymers with a cross-β structure, in which short β-strands from the monomers stack one on top of each other to make up a fibrillar polymer [1], [2]. These amyloids act as templates that convert monomers to the amyloid polymerised state. Spontaneous or chaperone-assisted fragmentation of this amyloid fibril allows multiplication of the prion particle by generating novel fibril ends where templating occurs.
Prions have initially been identified in the context of mammalian spongiform encephalopathies such as scrapie in sheep, Creutzfeld-Jacob disease in humans, or bovine spongiform encephalopathy (BSE) in cattle [3]. In these diseases, the host encoded GPI-anchored PrP prion protein turns into prion aggregates, leading to incurable lethal neurodegenerative diseases. The prion phenomenon is not restricted to this sole example. In particular, nine prion proteins have been identified in yeast and correspond to proteins with a wide range of cellular functions [4]. Generally, prion formation leads to loss of the cellular function of the protein. Yeast prions are thus detected as non-Mendelian genetic elements, leading to infectious and inheritable protein inactivation.
More than 30 human diseases, including Alzheimer disease, are caused by accumulation of amyloid aggregates of various proteins and peptides in the brain or systemic locations [2]. Many proteins can form amyloids, and the amyloid fold has been envisioned as a default conformational state that is formed when the native state of the protein cannot be efficiently attained or maintained. This view is, for instance, illustrated by the finding that inclusion bodies formed during recombinant protein over-expression in E. coli have an amyloid-like structure [5]. But in nature, the amyloid fold is not only found in abnormal or pathological situations. The specific physico-chemical properties of this fold have also been exploited to perform various biological functions. Amyloids constitute various cell surface structures in bacteria, fungi, and insects. Amyloids allow storage and delayed release of various peptide hormones in mammals [6]. Then, it has been proposed that this ability of a protein to exist under two states (a functional and a self-inactivating form) represents an epigenetic mechanism of gene regulation [7]. In that perspective, yeast prions have been suggested to represent a benefit [8], but this view remains debated [9].
The [Het-s] Prion Has a Role in Non-Self Recognition
[Het-s] is a prion of the filamentous fungus Podospora anserina and is involved in a non-self recognition process termed heterokaryon incompatibility [10]. In filamentous fungi, cell fusions between different strains occur spontaneously and lead to the formation of heterokaryons, containing nuclei from both fusion partners. Yet, almost invariably, these heterokaryotic cells undergo cell death soon after fusion. This rejection of non-self is due to genetic differences between the fusion partners at certain specific loci designated het loci. The biological significance of incompatibility is not fully understood. Incompatibility could serve to limit the cytoplasmic mixing between unlike individuals to restrict transmission of mycoviruses between strains or to prevent conspeficic parasitism of one nuclear type by another. It has also been proposed that incompatibility in fungi could represent an evolutionary by-product of pathogen-driven divergence in genes whose primary function lies in host defense against microbial pathogens [11]. In this view, het genes were identified because they cause rejection of conspecific non-self, but their original function lies in the recognition of heterospecific non-self, a situation analogous to the MHC complex in mammals.
Nine het loci have been identified in Podospora anserina and het-s, the gene encoding the [Het-s] prion is one of them. The het-s locus has two alternative incompatible alleles, het-s and het-S. When a het-s strain fuses with a het-S strain, the fusion cell undergoes cell death. But this cell death reaction only occurs when the HET-s protein is in the prion conformation. In other words, [Het-s] prion-infected strains are incompatible with het-S, while prion-free strains (designated [Het-s*]) are compatible with het-S. The [Het-s] prion is transmitted from one strain to another after cell fusion and is transmitted from the maternal parent to the meiotic progeny [10].
The Structure of the HET-s Prion Forming Domain Is Well Defined and Conserved in Evolution
The HET-s prion protein contains two distinct domains: an N-terminal α-helical globular domain designated HeLo and the C-terminal prion forming domain (PFD), which is necessary and sufficient for prion propagation and amyloid formation [12], [13]. This domain is natively unfolded in the soluble conformation of the protein.
The structure of the HET-s PFD in its prion conformation was solved by solid state NMR [14]. The PFD adopts a β-solenoid structure with two repeated motifs of 21 amino acids delimiting a triangular hydrophobic core. This structure is exceptionally well resolved and organised, in contrast to many other amyloid proteins, which often exist as a mixture of structural variants. A number of HET-s orthologs have been identified in other pezizomycotina fungi, and sequence comparisons and functional studies reveal a conservation of key residues, critical for formation of the β-solenoid fold, and indicate a selective pressure for the maintenance of the ability to form that specific amyloid fold [15], [16].
HET-s Is the Trigger for Activation of the HET-S Toxicity Domain
The HET-s and HET-S proteins differ by 13 residues, and the HET-S protein displays the same two-domain organisation as HET-s. The HET-s and HET-S PFD regions are functionally equivalent and interchangeable; the functional difference between HET-s and HET-S is determined by the amino acid differences in their HeLo domains [12]. The HeLo domain of HET-S represents the cell death execution domain in the het-s/het-S system. It is proposed that in the incompatibility reaction, the prion form of HET-s interacts with the PFD region of HET-S, and the conversion of the HET-S PFD region into the β-solenoid fold induces a conformational change in the HeLo domain, leading to its activation and triggering of cell death by incompatibility (Figure 1) [13]. Upon interaction with [Het-s], HET-S relocates to the cell periphery and this cell periphery localisation correlates with cell death [17]. In contrast, the HeLo domain of HET-s does not exert any toxicity. Thus, in the incompatibility reaction, HET-s and HET-S do not have equivalent roles, the HET-S HeLo domain represents the cell death execution entity, and the prion form of [Het-s] acts as a trigger for activation of this toxicity domain. Sequence analyses suggest that het-s-homologs in other fungal species are actually HET-S rather than HET-s homologs.
Fig. 1. Two proposed mode of activation of the HeLo toxicity domain of HET-S. 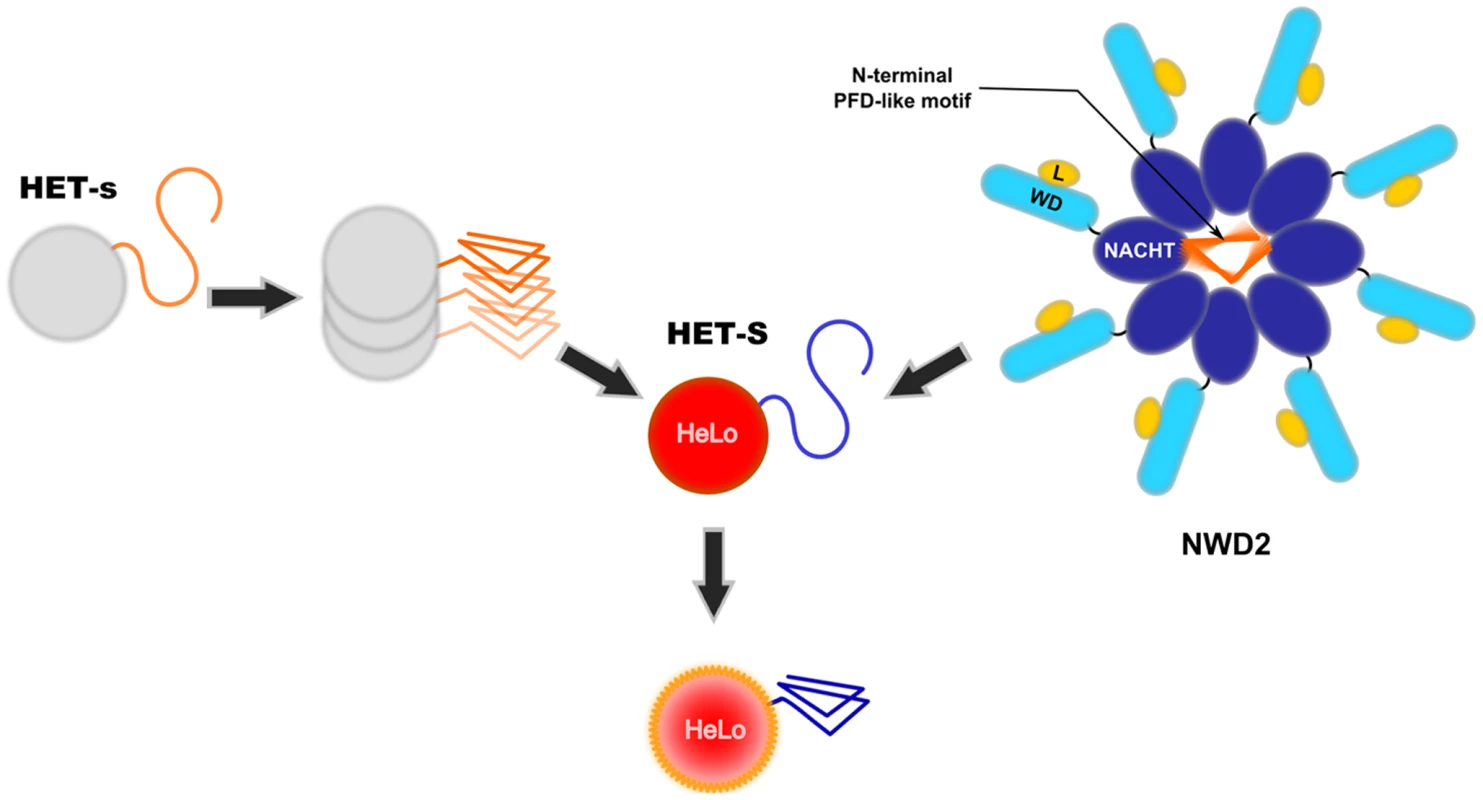
The current model envisioned for activation of the HeLo toxicity domain of HET-S is depicted. Upon prion formation, the C-terminal HET-s prion forming domain (PFD) adopts the β-solenoid amyloid fold. The β-solenoid fold of HET-s then serves as a template to transconform the corresponding region in the HET-S protein. Refolding of the HET-S PFD region leads to a refolding of the globular HeLo domain, and this refolding renders the protein toxic. The NWD2 protein displays three domains: an N-terminal motif homologous to the HET-s PFD, a central NACHT oligomerisation domain, and a C-terminal WD-repeat domain (WD). It is proposed that upon binding of a ligand (L), NWD2 undergoes oligomerisation and that this oligomerisation allows the N-terminal region of the NWD2 protein to adopt a HET-s-like β-solenoid fold. This N-terminal extension would then lead to templating and activation of the HET-S HeLo domain (in a way analogous to [Het-s]). NWD2 as a Functional Partner of HET-S
Recently, a potential additional functional partner of HET-S was identified. A search for proteins displaying homology to the HET-s PFD led to the identification of a protein termed NWD2 encoded by the gene immediately adjacent to het-S [18]. NWD2 is part of an NWD gene family comprising other het genes. NWD proteins are STAND proteins resembling Nod-like receptors and are thought to represent the fungal counterparts of pathogen recognition receptors described in plants and metazoans [19]. STAND proteins are signal transducing NTPases that undergo ligand-induced oligomerisation. They typically display three domains: a central nucleotide-binding oligomerisation domain (NOD) flanked by a C-terminal ligand-binding domain and an N-terminal effector domain. NWD2 lacks a defined effector domain and in its place displays at the N-terminal end a short region of homology with the elementary HET-s repeat motif [18]. Based on homology modelling, this region is predicted to adopt the β-solenoid fold. A model postulating the existence of a functional interaction between NWD2 and HET-S was proposed [18]. In that model, NWD2 recognises a ligand via its C-terminal WD-40 repeat domain and oligomerises in response to this binding. This oligomerisation step would put the N-terminal extensions of NWD2 proteins into close proximity and allow their cooperative folding into the β-solenoid fold. Once formed, this fold would be used as a template for transconformation of the HET-S PFD and activation of the HeLo toxicity domain. Thus, two modes of activation of the HET-S HeLo domain are now envisioned, either by interaction with the prion form of HET-s (as occurs during incompatibility) or by interaction with the oligomeric form of NWD2 (Figure 1).
This model postulates the existence of a mechanism of signal transduction STAND proteins where oligomerisation of a STAND protein induces formation of an amyloid-like fold in a short region N-terminal to the NOD domain. This amyloid-fold would in turn trigger transconformation of the protein responsible for the execution of the cell death reaction. Fungal genome analyses suggest that this mode of interaction between STAND signal transducing proteins and prion or prion-like proteins is not an isolated occurrence [18]. Several other analogous systems were identified in genome searches, suggesting that this mode of activation of effector domains is both widespread and evolutionarily conserved in fungi. What is emerging is the notion that transmission of an amyloid fold can be used as integrated part of a signal transduction pathway.
In the end, the focus needs to be redirected from [Het-s] to HET-S. [Het-s] turns out to be but a trigger to activate the HeLo toxicity domain of HET-S. HET-S is actually doing the job and represents the central player in the system. It will be of interest to elucidate the actual mechanism of toxicity that is associated with relocalisation of HET-S to the cell membrane. Other current questions regarding this system deal with the biological meaning of NWD2/HET-S pathway: what is the ligand recognised by NWD2, and what is the purpose of the HET-S-induced cell death reaction in response to this ligand binding?
Zdroje
1. ToyamaBHWeissmanJS 2011 Amyloid structure: conformational diversity and consequences. Annu Rev Biochem 80 557 585
2. EichnerTRadfordSE 2011 A diversity of assembly mechanisms of a generic amyloid fold. Mol Cell 43 8 18
3. ColbyDWPrusinerSB 2011 Prions. Cold Spring Harb Perspect Biol 3 a006833
4. CrowETLiL 2011 Newly identified prions in budding yeast, and their possible functions. Semin Cell Dev Biol 22 452 459
5. Garcia-FruitosESabateRde GrootNSVillaverdeAVenturaS 2011 Biological role of bacterial inclusion bodies: a model for amyloid aggregation. Febs J 278 2419 2427
6. GreenwaldJRiekR 2010 Biology of amyloid: structure, function, and regulation. Structure 18 1244 1260
7. HalfmannRAlbertiSLindquistS 2010 Prions, protein homeostasis, and phenotypic diversity. Trends Cell Biol 20 125 133
8. HalfmannRJaroszDFJonesSKChangALancasterAK 2012 Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature 482 363 368
9. NakayashikiTKurtzmanCPEdskesHKWicknerRB 2005 Yeast prions [URE3] and [PSI+] are diseases. Proc Natl Acad Sci U S A 102 10575 10580
10. SaupeSJ 2011 The [Het-s] prion of Podospora anserina and its role in heterokaryon incompatibility. Semin Cell Dev Biol 22 460 468
11. PaolettiMSaupeSJ 2009 Fungal incompatibility: evolutionary origin in pathogen defense? Bioessays 31 1201 1210
12. BalguerieADos ReisSRitterCChaignepainSCoulary-SalinB 2003 Domain organization and structure-function relationship of the HET-s prion protein of Podospora anserina. Embo J 22 2071 2081
13. GreenwaldJBuhtzCRitterCKwiatkowskiWChoeS 2010 The mechanism of prion inhibition by HET-S. Mol Cell 38 889 899
14. WasmerCLangeAVan MelckebekeHSiemerABRiekR 2008 Amyloid fibrils of the HET-s(218–289) prion form a beta solenoid with a triangular hydrophobic core. Science 319 1523 1526
15. BenkemounLNessFSabateRCeschinJBretonA 2011 Two structurally similar fungal prions efficiently cross-seed in vivo but form distinct polymers when coexpressed. Molecular Microbiology 82 1392 1405
16. WasmerCZimmerASabateRSoragniASaupeSJ 2010 Structural similarity between the prion domain of HET-s and a homologue can explain amyloid cross-seeding in spite of limited sequence identity. J Mol Biol 402 311 325
17. MathurVSeuringCRiekRSaupeSJLiebmanSW 2012 Localization of HET-S to the cell periphery, not to [Het-s] aggregates, is associated with [Het-s]-HET-S toxicity. Mol Cell Biol 32 139 153
18. DaskalovAPaolettiMNessFSaupeSJ 2012 Genomic clustering and homology between HET-S and the NWD2 STAND protein in various fungal genomes. PLoS ONE 7 e34854 doi:10.1371/journal.pone.0034854
19. SalehM 2011 The machinery of Nod-like receptors: refining the paths to immunity and cell death. Immunol Rev 243 235 246
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Five Questions on Prion Diseases
- Type III Secretion in : Injectisome or Not?
- In Vitro and In Vivo Isolation and Characterization of Duvenhage Virus
- CD200 Receptor Controls Sex-Specific TLR7 Responses to Viral Infection
- From Molecular Genetics to Phylodynamics: Evolutionary Relevance of Mutation Rates Across Viruses
- Evolution of an Eurasian Avian-like Influenza Virus in Naïve and Vaccinated Pigs
- Vitamin D Inhibits Human Immunodeficiency Virus Type 1 and Infection in Macrophages through the Induction of Autophagy
- Influence of Microbiota on Viral Infections
- Hydrophobins—Unique Fungal Proteins
- Interferon-Induced Protects Mice from Lethal VSV Neuropathogenesis
- A New Evolutionary Model for Hepatitis C Virus Chronic Infection
- The [Het-s] Prion, an Amyloid Fold as a Cell Death Activation Trigger
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Type III Secretion in : Injectisome or Not?
- Hydrophobins—Unique Fungal Proteins
- In Vitro and In Vivo Isolation and Characterization of Duvenhage Virus
- The [Het-s] Prion, an Amyloid Fold as a Cell Death Activation Trigger
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



