-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Influence of Microbiota on Viral Infections
article has not abstract
Published in the journal: . PLoS Pathog 8(5): e32767. doi:10.1371/journal.ppat.1002681
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002681Summary
article has not abstract
Most pathogens gain access to the host through surfaces of the body that are exposed to the surrounding environment and rife with resident microorganisms, termed microbiota. Microbiota play an integral role in modulating host health. One significant benefit of the microbiota is that they provide protection against incoming bacterial pathogens [1]. Commensals make their immediate environment inhospitable to many pathogens by producing biosurfactants, by competing for sites of attachment and nutrients, and by excreting metabolites with antimicrobial effects [1]. Furthermore, the presence of commensals promotes maturation of secondary lymphoid organs in the intestine, which are the first line of defense in the intestinal mucosa [2]. Therefore, when a pathogen infiltrates the host, it is not entering a sterile environment, but one that has been shaped by a dynamic commensal community. Although many interactions between bacterial pathogens and the microbiota have been characterized [1], little is known about the interplay between viral pathogens and the natural flora of the host. Are viral pathogens blind to the commensal microbes surrounding them? Judging from recent publications, this appears not to be the case. There is strong evidence that the microbiota can either protect the host from virally induced disease or promote viral propagation/transmission, through direct or indirect mechanisms.
Beneficial Influence of Microbiota on Antiviral Immunity
Because the microbiota are present at the sites used by viruses to gain entry to their host, they can potentially alter the outcome of infection. For example, the commensal microbiota of the insect vector Aedes aegypti indirectly mitigate Dengue virus transmission [3]. Mosquitoes whose commensals are ablated by antibiotics have higher viral titers than those that are left untreated. Moreover, mosquitoes possessing their natural flora show elevated expression of several immune-related genes, including those encoding antimicrobial peptides regulated by Toll-like receptor (TLR) pathways [3]. The authors hypothesize that the endogenous bacterial flora stimulate the mosquitoes' antiviral immune system through basal-level activation of innate immune pathways. Likewise, ablation of the natural flora of mice via antibiotic treatment increases the animals susceptibility to influenza A virus (Figure 1). Again, the mechanism of microbiota-mediated protection against the virus appears to be indirect—the microbiota are responsible for activation of the inflammasome [4], which is required for defense against influenza [5]. Inflammasome activation induces migration of dendritic cells from the lung to the draining lymph node, to prime influenza-specific T-cell responses [4]. Interestingly, a TLR agonist such as lipopolysaccharide (LPS) added intranasally or intrarectally restores the immune response to influenza in antibiotic-treated animals [4].
Fig. 1. An overview of how the commensal microbiota influence viral pathogenesis. 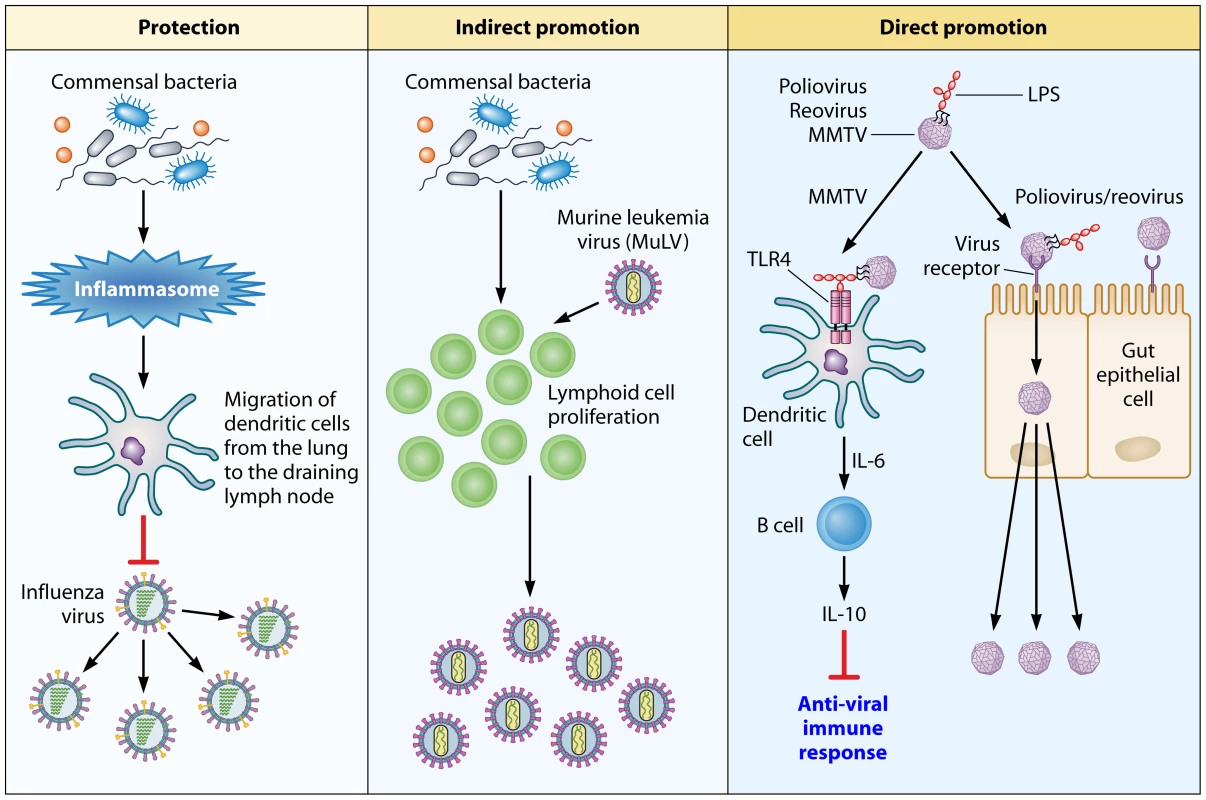
Protection: The microbiota of the host activates the inflammasome by priming signal 1 for IL-1β and IL-18 secretion. The secretion of these cytokines induces migration of dendritic cells from the lung to the draining lymph node, where they prime T cells. The downstream effect of T-cell priming is protection of the host against influenza virus-induced pathology. Indirect promotion: In the case of MuLV, the microbiota of the host stimulate proliferation of lymphoid cells that are targeted by the virus. Direct promotion: Microbial ligands, such as LPS, are utilized by viruses to enhance their attachment to target cells (polio virus and reovirus) or to counteract the antivirus immune response by activating the TLR4 pathway, which leads to IL-10 production (MMTV). It should be stressed that both of the aforementioned studies used antibiotics to alter the microbiota of the host. However, antibiotic treatment can lead to changes in the host's physiology that are independent of microbiota disturbance. Additionally, antibiotic treatment may not eliminate all microorganisms from the host—many microbes are known to be resistant to antimicrobial therapies [6], and the majority of commensal species are unculturable, making it difficult to prove the existence of an antibiotic-induced sterile environment [7]. To subvert this problem, one can use germ-free (GF) organisms. These organisms are completely sterile, and exhibit normal developmental patterns overall. However, both the gut associated lymphoid tissue and the intestinal immune cells of these animals are underdeveloped. Consequently, when studying the interaction between the host's microbiota and a given pathogen, it is imperative to use both antibiotic-treated and GF animals to account for the limitations of both experimental systems.
Indirect Promotion of Viral Infections by Commensal Microbiota
Although microbiota can help the host fight viral infections, as in the case of influenza, it may also enhance viral infection, either indirectly or directly. One example of the indirect beneficial effects of microbiota on virus replication is the promotion of viral infection by stimulating the proliferation or activation of target cells (Figure 1). This is particularly true of retroviruses that target proliferating cells. For example, GF mice infected with murine leukemia virus (MuLV) are relatively resistant to virally induced leukemia compared to conventionally housed or specific pathogen free (SPF) mice [8], [9]. Immunization of MuLV-infected GF mice with sheep red blood cells results in a significant increase in leukemia development comparable to that of infected SPF mice [9]. The authors hypothesize that the decrease of MuLV pathogenicity in GF mice could be due to microbiota-stimulated division of lymphoid cells, which would cause an increase in virus replication and, thus, a higher frequency of leukemia. It should be noted that other studies demonstrate that GF mice are more susceptible than SPF mice to MuLV-induced leukemia [10], which conflicts with the aforementioned findings. One potential explanation for this discrepancy is that the studies showing increased susceptibility of GF mice to MuLV were conducted before it was revealed that some MuLV isolates contain a contaminating lactate dehydrogenase-elevating virus (LDV). LDV induces systemic lymphocyte activation [11] and could have skewed the results of the investigations.
Direct Assistance of Viral Infections by Microbiota
To date, two studies, including our own, indicate that viruses from three distinct families rely on commensal organisms for efficient replication/transmission [12], [13]. In the first study, Kuss et al. found that antibiotic-treated, poliovirus-susceptible mice show lower mortality following oral poliovirus infection compared to untreated mice (Figure 1). Importantly, replication of the virus in the mouse intestines is dependent on the microbiota, as GF or antibiotic-treated mice secrete poorly infectious virus. Gram-negative or Gram-positive bacteria incubated with poliovirus greatly promote virus infectivity in tissue culture cells. This enhancement did not require live bacteria, as bacterial surface polysaccharides, including LPS and peptidoglycan (PG), have the same effect on virus infectivity [12]. Importantly, these findings were not unique to poliovirus; the pathogenesis of reovirus, an unrelated enteric virus, is also more severe in the presence of intestinal microbes [12].
We discovered that Mouse Mammary Tumor Virus (MMTV), a retrovirus transmitted through the milk, utilizes the innate immune Toll-like receptor TLR4 to induce tolerance to itself, and thus to evade the antiviral response (Figure 1) [13]. Triggering of TLR4 by the virus results in interleukin 6 (IL-6)-mediated production of the immunosuppressive cytokine IL-10, which is required for blockage of the antiviral response [13]. MMTV does not signal directly through TLR4 but uses LPS, a well-characterized TLR4 ligand, to trigger the receptor, as LPS-free MMTV stocks fail to induce IL-10 production. Furthermore, GF mice infected with MMTV by intraperitoneal injection are unable to transmit infectious virus to their offspring. Therefore, MMTV exploits tolerogenic properties of commensal bacteria to induce unresponsiveness to itself. Together, the two studies reveal that orally transmitted viruses from three diverse families take advantage of the gut microbiota for successful propagation. Exploitation of the microbiota of the host can now be added to the list of innovative evasion strategies used by viruses.
Do Lentiviruses Utilize Microbiota for Their Benefit?
Like the viruses described in the preceding section, HIV-1 is also transmitted across mucosal surfaces, which are rich in microbiota. This prompts the question—do the microbiota contribute to infection with HIV-1? People chronically infected with HIV exhibit raised plasma levels of LPS [14]. Moreover, the peptide derived from the V3 loop of gp120 specifically interacts with the lipid A moiety of LPS, as does the full gp120 protein [15]. In addition, glycerol monolaurate, a widely used antimicrobial compound, protects rhesus macaques from acute infection of simian immunodeficiency virus (SIV) [16]. Therefore, it is possible that HIV and SIV may also take advantage of commensal bacteria to assure successful propagation and spread.
Concluding Remarks
With the advent of the Human Microbiome Project, we are now aware of the number and diversity of microbes that make the human body their primary place of residence. Consequently, the microbiota can no longer be ignored when studying host–pathogen interactions. The influences of microbiota on virus infections could be either protective or detrimental for the host. Whereas the microbiota positively regulate adaptive immune responses against influenza, they suppress antivirus adaptive responses against MMTV and facilitate replication of poliovirus and reovirus by enhancing virus attachment to target cells. Thus, microbiota play a dual role in virus–host interactions. An open question that currently drives research related to microbiota is how the microbiota can be manipulated so that the host is protected from deleterious infections. In the case of pathogens that take advantage of the microbiota, one can hope to find a way to ablate these interactions, thus preventing pathogen spread/propagation. This could be done either by manipulating the composition of the microbiota (ablation of a specific microbe exploited by a virus) or by blocking interactions between the viral pathogen and specific bacterial compounds that benefit the pathogen. Future discoveries in the area of microbiota–pathogen interactions will undoubtedly unveil new opportunities for therapeutic interventions in infectious disease.
Zdroje
1. SekirovIFinlayBB 2009 The role of the intestinal microbiota in enteric infection. J Physiol 587 4159 4167
2. LeeYKMazmanianSK 2010 Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 330 1768 1773
3. XiZRamirezJLDimopoulosG 2008 The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 4 e1000098 doi:10.1371/journal.ppat.1000098
4. IchinoheTPangIKKumamotoYPeaperDRHoJH 2011 Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 108 5354 5359
5. IchinoheTLeeHKOguraYFlavellRIwasakiA 2009 Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med 206 79 87
6. PogueJMMarchaimDKayeDKayeKS 2011 Revisiting “older” antimicrobials in the era of multidrug resistance. Pharmacotherapy 31 912 921
7. SchmeisserCSteeleHStreitWR 2007 Metagenomics, biotechnology with non-culturable microbes. Appl Microbiol Biotechnol 75 955 962
8. IsaakDDBartizalKFCaulfieldMJ 1988 Decreased pathogenicity of murine leukemia virus-Moloney in gnotobiotic mice. Leukemia 2 540 544
9. KouttabNMJutilaJW 1972 Friend leukemia virus infection in germfree mice following antigen stimulation. J Immunol 108 591 595
10. MirandEAGraceJTJr 1963 Responses of germ-free mice to friend virus. Nature 200 92 93
11. AmmannCGMesserRJPetersonKEHasenkrugKJ 2009 Lactate dehydrogenase-elevating virus induces systemic lymphocyte activation via TLR7-dependent IFNalpha responses by plasmacytoid dendritic cells. PLoS One 4 e6105 doi:10.1371/journal.pone.0006105
12. KussSKBestGTEtheredgeCAPruijssersAJFriersonJM 2011 Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334 249 252
13. KaneMCaseLKKopaskieKKozlovaAMacDearmidC 2011 Successful transmission of a retrovirus depends on the commensal microbiota. Science 334 245 249
14. BrenchleyJMPriceDASchackerTWAsherTESilvestriG 2006 Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12 1365 1371
15. MajerleAPristovsekPMancek-KeberMJeralaR 2011 Interaction of the HIV-1 gp120 viral protein V3 loop with bacterial lipopolysaccharide: a pattern recognition inhibition. J Biol Chem 286 26228 26237
16. LiQEstesJDSchlievertPMDuanLBrosnahanAJ 2009 Glycerol monolaurate prevents mucosal SIV transmission. Nature 458 1034 1038
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
-
Všechny články tohoto čísla
- Five Questions on Prion Diseases
- Type III Secretion in : Injectisome or Not?
- In Vitro and In Vivo Isolation and Characterization of Duvenhage Virus
- CD200 Receptor Controls Sex-Specific TLR7 Responses to Viral Infection
- From Molecular Genetics to Phylodynamics: Evolutionary Relevance of Mutation Rates Across Viruses
- Evolution of an Eurasian Avian-like Influenza Virus in Naïve and Vaccinated Pigs
- Vitamin D Inhibits Human Immunodeficiency Virus Type 1 and Infection in Macrophages through the Induction of Autophagy
- Influence of Microbiota on Viral Infections
- Hydrophobins—Unique Fungal Proteins
- Interferon-Induced Protects Mice from Lethal VSV Neuropathogenesis
- A New Evolutionary Model for Hepatitis C Virus Chronic Infection
- The [Het-s] Prion, an Amyloid Fold as a Cell Death Activation Trigger
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Type III Secretion in : Injectisome or Not?
- Hydrophobins—Unique Fungal Proteins
- In Vitro and In Vivo Isolation and Characterization of Duvenhage Virus
- The [Het-s] Prion, an Amyloid Fold as a Cell Death Activation Trigger
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



