-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaExploring New Biological Functions of Amyloids: Bacteria Cell Agglutination Mediated by Host Protein Aggregation
Antimicrobial proteins and peptides (AMPs) are important effectors of the innate immune system that play a vital role in the prevention of infections. Recent advances have highlighted the similarity between AMPs and amyloid proteins. Using the Eosinophil Cationic Protein as a model, we have rationalized the structure-activity relationships between amyloid aggregation and antimicrobial activity. Our results show how protein aggregation can induce bacteria agglutination and cell death. Using confocal and total internal reflection fluorescence microscopy we have tracked the formation in situ of protein amyloid-like aggregates at the bacteria surface and on membrane models. In both cases, fibrillar aggregates able to bind to amyloid diagnostic dyes were detected. Additionally, a single point mutation (Ile13 to Ala) can suppress the protein amyloid behavior, abolishing the agglutinating activity and impairing the antimicrobial action. The mutant is also defective in triggering both leakage and lipid vesicle aggregation. We conclude that ECP aggregation at the bacterial surface is essential for its cytotoxicity. Hence, we propose here a new prospective biological function for amyloid-like aggregates with potential biological relevance.
Published in the journal: . PLoS Pathog 8(11): e32767. doi:10.1371/journal.ppat.1003005
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003005Summary
Antimicrobial proteins and peptides (AMPs) are important effectors of the innate immune system that play a vital role in the prevention of infections. Recent advances have highlighted the similarity between AMPs and amyloid proteins. Using the Eosinophil Cationic Protein as a model, we have rationalized the structure-activity relationships between amyloid aggregation and antimicrobial activity. Our results show how protein aggregation can induce bacteria agglutination and cell death. Using confocal and total internal reflection fluorescence microscopy we have tracked the formation in situ of protein amyloid-like aggregates at the bacteria surface and on membrane models. In both cases, fibrillar aggregates able to bind to amyloid diagnostic dyes were detected. Additionally, a single point mutation (Ile13 to Ala) can suppress the protein amyloid behavior, abolishing the agglutinating activity and impairing the antimicrobial action. The mutant is also defective in triggering both leakage and lipid vesicle aggregation. We conclude that ECP aggregation at the bacterial surface is essential for its cytotoxicity. Hence, we propose here a new prospective biological function for amyloid-like aggregates with potential biological relevance.
Introduction
Antimicrobial proteins and peptides (AMPs) represent a wide family that contributes to the host defense system with multiple pathogen killing strategies [1]–[3]. Their fast and multitarget mechanism of action reduces the emergence of bacteria resistance and represents a valuable alternative for common antibiotics [4], [5].
The mechanism of action of AMPs has been systematically investigated, suggesting that AMPs bind to bacteria cell membranes and disrupt cell homeostasis. However, more investigations are needed to completely understand how different structures determine the function of AMPs [6]–[12]. Membrane damage is a multifaceted mechanism that can involve different peptide assemblies and ultimately promotes membrane permeabilization when achieving a critical concentration [13], [14]. Several authors have highlighted the striking resemblance of membrane disrupting mechanisms with those observed for amyloid peptides and proteins [15]–[17]. In both cases, membrane composition (e.g. cholesterol content) and biophysical properties (e.g. membrane fluidity and curvature) were found critical for the peptide action [13], [15], [18]–[26]. Furthermore, we have recently suggested that antimicrobial activity could have arisen through cationization of amyloid-prone regions [27]. In this light, some AMPs have been described to form amyloid structures in vitro [28], [29] and some amyloid peptides have also been considered as putative AMPs [30], [31]. In fact, we have proposed that inherent AMP aggregation properties can modulate antimicrobial activity [32].
Interestingly, some antimicrobial proteins and peptides have been found to agglutinate bacteria cells. In this sense, bacteria agglutination has been ascribed to unspecific adhesion through hydrophobic interactions, as observed for synthetic peptides derived from the parotid secretory protein [33]. Comparative analysis on those peptides highlighted the contributions of both hydrophobic and cationic residues in the agglutination activity [33]. These results suggest that some AMPs could exploit their intrinsic aggregation properties, by triggering bacteria agglutination as part of its mechanism of action as observed for a wealth source of AMPs in saliva, which provides a first barrier to bacteria adherence in the oral cavity [34]. Agglutinating activity has been reported crucial for the antimicrobial function of Eosinophil Cationic Protein (ECP) [35], a small cationic protein specifically secreted by eosinophil granules during inflammation processes with diverse antipathogen activities [36]–[38]. ECP displays high antimicrobial action, with a specific bacteria agglutination activity reported for Gram-negative bacteria, at a concentration range close to the minimal inhibitory concentration, a behavior that may represent an effective bactericidal mechanism in vivo [39].
In order to characterize the relation between AMPs, bacteria agglutination and amyloid aggregation, we have used ECP as a model of study. We present here a detailed characterization of protein-mediated bacteria agglutination and prove the contribution of an aggregation prone domain to the protein antimicrobial action. Complementary studies on model membranes provide a further understanding of the membrane damage process promoted by protein aggregation.
Results/Discussion
ECP was previously reported to aggregate in vivo on both bacterial and eukaryotic cell surface without detectable internalization [39], [40]. Though these findings were essential to explain the antimicrobial and cytotoxic properties of ECP, the real nature of the aggregation process remained unknown. Besides, the protein has a high affinity towards lipopolysaccharides (LPS) [41] and agglutinates all tested Gram-negative strains [42]. On the other hand, ECP has been reported to form amyloid-like aggregates in vitro at specific conditions due to a hydrophobic patch located at the N-terminus. Remarkably, protein amyloid-like aggregation was efficiently abolished by mutating Ile 13 to Ala [28]. The screening of the protein primary structure [43]–[45] and the design of derived peptides [42], [46] also allocated the antimicrobial region at the N-terminus. As the antimicrobial and amyloid active segments of the protein colocalize [28], [35], [42], [46], it is tempting to hypothesize that bacteria agglutination by ECP could be directly dependent on an amyloid-like aggregation process. This hypothesis raises some exciting questions: (i) Is cell agglutination required for antimicrobial activity? (ii) Is cell agglutination mediated by protein aggregation at the bacteria surface? (iii) Are aggregates formed on the surface of bacteria of amyloid nature?
Bacteria cell agglutination and antimicrobial activities
To address the first question we compared the antimicrobial action of wild type ECP (wtECP) with the I13A mutant, previously described to be unable to form aggregates in vitro [28]. The antimicrobial assays reveal that, while wtECP has an average minimal inhibitory concentration (MIC) value around 0.5–1 µM, the I13A mutant is unable to kill bacteria even at 5 µM concentration (Table 1). To further correlate ECP antimicrobial and agglutination activities we studied bacteria cell cultures by confocal microscopy using the SYTO9/Propidium iodide nucleic acid fluorescent labels that allow registering both cell agglutination and viability over time. Interestingly, wtECP can agglutinate Gram-negative bacteria before a viability decrease is observed (Figure 1A), however no cell agglutination takes place when bacteria are incubated with the I13A variant, even after 4 hours (Supporting Information Figure S1). These results are also supported by minimal agglutination concentrations (MAC) close to the MIC values (Table 1) and by FACS experiments showing that wtECP but not I13A mutant is able to agglutinate E. coli cells (Figure 1B). Thus, ECP antimicrobial activity on Gram-negative strains is strongly affected when abolishing the agglutination behavior (Ile13 to Ala mutation).
Fig. 1. ECP but not I13A is able to agglutinate bacteria. 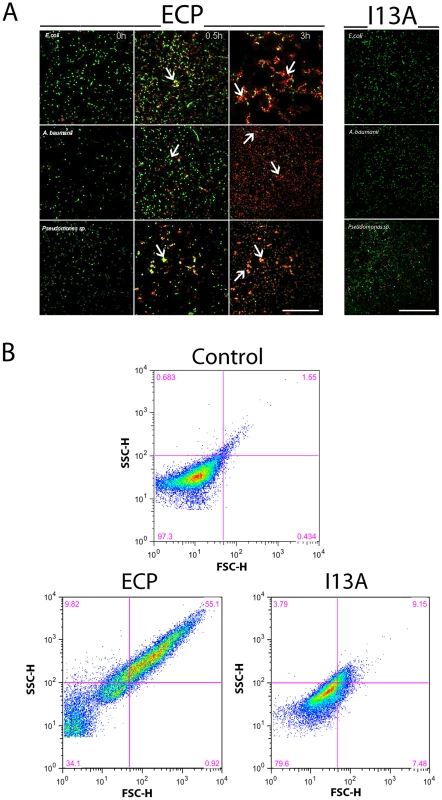
(A) E. coli, P. aeruginosa and A. baumanii cells were incubated with 5 µM of ECP or I13A mutant in microscopy plates during 4 h and stained with syto9 (live cells, green) and propidium iodide (dead cells, red). Images were taken at 0, 0.5 and 3 h using a Leica SP2 confocal microscopy as described in the Materials and Methods section. Scale bar represents 50 µm. Arrows were depicted to show cell agglutination. Images depicted are representative from two independent experiments. (B) E. coli cells were incubated with 5 µM of ECP or I13A mutant during 4 h and samples were analyzed using a FACSCalibur cytometer. FSC-H is the low-angle forward scattering, which is roughly proportional to the diameter of the cell and SSC-H is the orthogonal or side scattering, which is proportional to cell granularity or complexity. Agglutination is registered as an increase in both scattering measures. In all experiments cell cultures were grown at exponential phase (OD600 = 0.2) and incubated with proteins in 10 mM sodium phosphate buffer, 100 mM NaCl, pH 7.4. The plots are representative of three independent experiments. Tab. 1. Antimicrobial (MIC100) and agglutinating (MAC) activities of wtECP and I13A mutant in Gram-negative strains. 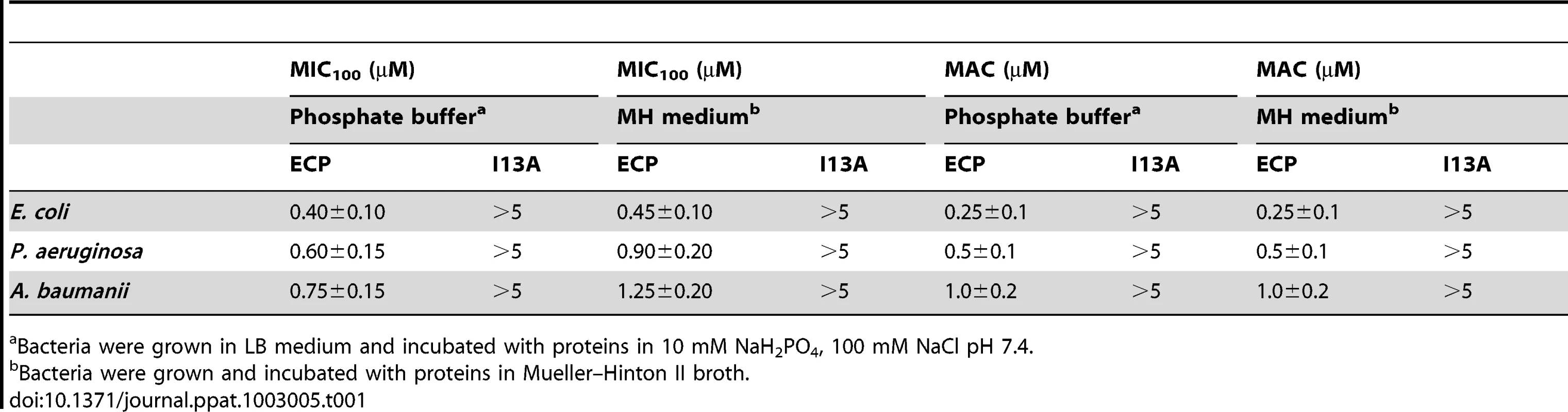
Bacteria were grown in LB medium and incubated with proteins in 10 mM NaH2PO4, 100 mM NaCl pH 7.4. Protein aggregation on membrane models
To further analyze the protein agglutination mechanism, we tested the wtECP and I13A mutant action on a simpler biophysical system such as phospholipid membranes where liposome agglutination is registered as a function of protein concentration. In contrast to wtECP, I13A mutant completely looses the ability to agglutinate membranes (Figure 2A). In particular, when following wtECP agglutinating activity as a function of ionic strength, we observe that liposome agglutination is enhanced at high NaCl concentration (Supporting Information, Figure S2). These results suggest that vesicle agglutination is promoted by hydrophobic interactions. Even more, leakage activity in model membranes is also lost for I13A mutant (Figure 2B), meaning that protein aggregation on the membrane surface is important not only for agglutination but also for later membrane permeabilization. These results are entirely consistent with those described above for bacteria cell cultures where the Ile to Ala mutation not only abolishes the cell agglutinating activity of ECP but also its bactericidal action.
Fig. 2. Liposome agglutination and leakage activity. 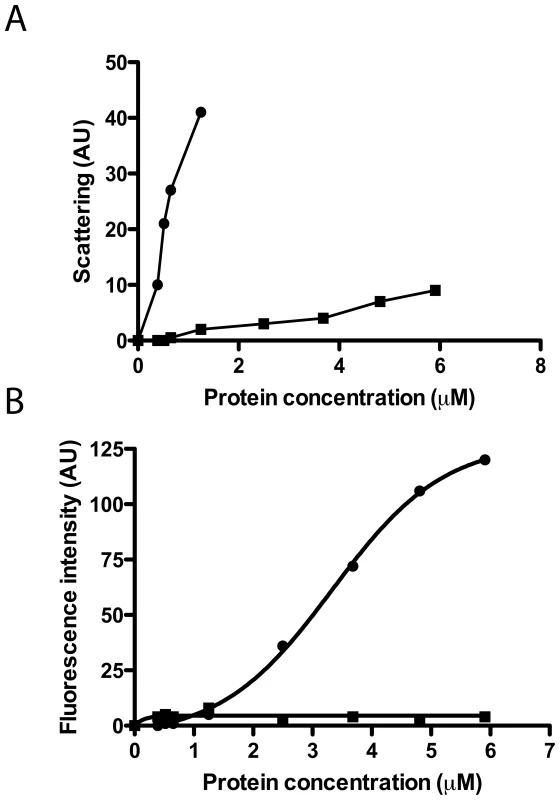
wtECP (circles) and I13A mutant (squares) were incubated with liposomes and the agglutination (A) and leakage (B) were followed at increasing protein concentrations (1–6 µM). Agglutination was measured as light scattering (470 nm) at 90° from the beam source in a 10 mM Tris-HCl, 100 mM NaCl, pH 7.4 buffer and leakage was followed using the ANTS/DPX assay in the same buffer as described in the Materials and Methods section. Agglutination mediated by protein aggregation
Next, to address the question whether cell agglutination is consistently driven by protein aggregation at the bacteria surface, we incubated bacteria cultures with ECP and visualized the samples using confocal microscopy. Our results show that wtECP binds to the bacteria surface and a strong protein signal is registered at the aggregation zones (Figure 3A). On the contrary, though cell interaction is maintained for the I13A mutant, agglutination is observed neither in bacteria cell cultures nor in model membranes (Figures 3A and 3B). As expected, for model membranes we show that only wtECP is able to promote agglutination (Figure 3B). Therefore, we conclude that protein aggregation on the cellular surface is required for bacteria agglutination, which turns to be essential for the antimicrobial action. Agglutination is also observed in the presence of 20% plasma in a similar extent, suggesting that ECP agglutination is likely to take place in the physiological context (Supplementary Information Figure S3). As previously mentioned, ECP binding to bacteria is favored by interactions with the LPS outer membrane [35], [41], [47]. Consistently, we show here that LPS binding activity is lost for the I13A mutant, when compared with wtECP (Supplementary Information Figure S4).
Fig. 3. ECP and I13A mutant bind to the surface of bacteria and membranes. 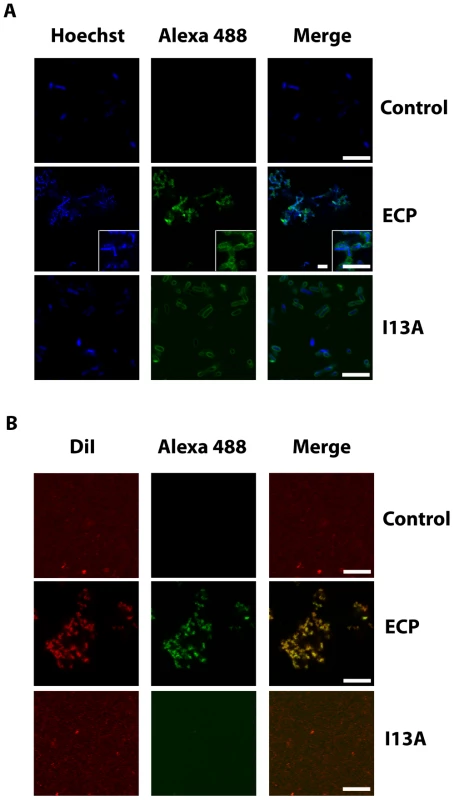
(A) E. coli bacteria cells stained with Hoechst (blue signal) were incubated with 5 µM of either wtECP or I13A mutant (both labeled with Alexa Fluor 488; green signal) for 4 h and visualized by confocal microscopy. In all experiments cell cultures were grown at exponential phase (OD600 = 0.2) and incubated with proteins in 10 mM sodium phosphate buffer, 100 mM NaCl, pH 7.4. (B) 500 µl of 200 µM LUV liposomes stained with DiI (red signal) were incubated with 5 µM of either wtECP or I13A mutant (both labeled with Alexa Fluor 488; green signal) for 4 h and visualized using a Leica SP2 confocal microscope. Scale bars are 5 µm length in all images and insight captions. Images depicted are representative from two independent experiments. In situ follow-up of amyloid aggregates
At this point however, the nature of the protein aggregates remained unknown. Thus, having previously shown that ECP is able to form amyloid-like aggregates in vitro, we decided to test if the observed aggregates have an amyloid-like structure using the amyloid-diagnostic dyes Thioflavin-T and Congo Red. When bacteria cultures are incubated with non-labeled wtECP, stained with ThT and visualized by total internal reflection fluorescence (TIRF) microscopy, we show that wtECP amyloid-like aggregates are located also at the cell surface (Figure 4A) similarly as what we observe for Alexa labeled wtECP (Figure 3A). Consistently, no staining is observed for non-incubated cultures and for the I13A mutant (Figure 4A). Moreover, upon bacteria incubation with wtECP, a red shift in the Congo Red spectrum is observed (Supplementary Information Figure S5A), revealing that the protein amyloid-like aggregation is triggered upon incubation with bacteria cultures.
Fig. 4. ECP but not I13A form amyloid-like aggregates on the surface of bacteria and membranes. 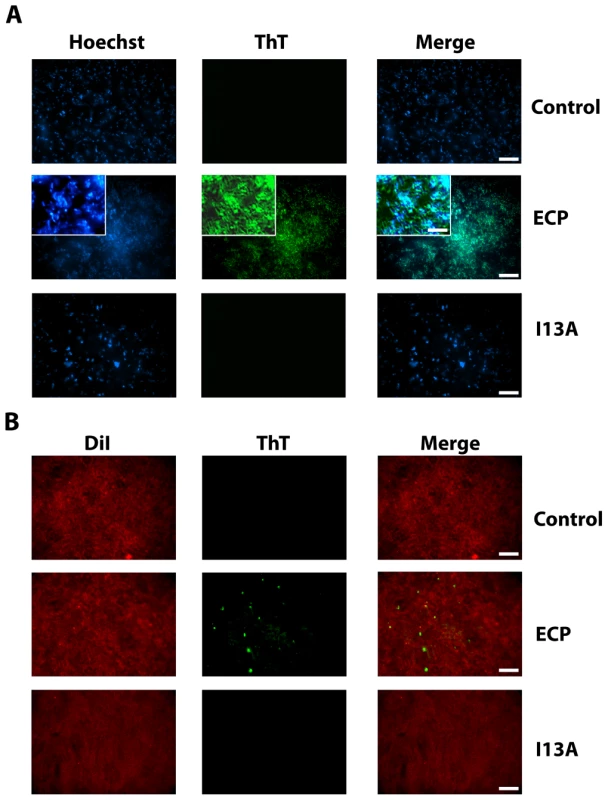
(A) E. coli bacteria cells stained with Hoechst (blue signal) were incubated with unlabeled wtECP or I13A mutant for 4 h, stained with ThT (green signal) and visualized by TIRF microscopy. In all experiments cell cultures were grown at exponential phase (OD600 = 0.2) and incubated with proteins in 10 mM sodium phosphate buffer, 100 mM NaCl, pH 7.4. (B) Planar lipid bilayers prepared as described in the Materials and Methods section (stained with DiI; red signal) were incubated with 5 µM of either unlabeled wtECP or I13A mutant for 4 h, stained with 25 µM ThT (green signal) and visualized using a Olympus FluoView 1000 TIRF microscope. Scale bar represents 20 µm (5 µm in the insight caption). Images depicted are representative from two independent experiments. Though ECP was previously shown to form amyloid-like aggregates in vitro only at low pH after a long incubation time (1–2 weeks), amyloid-like structures observed here are detected after only 4 hours of incubation. However, it is well known that some proteins can accelerate its aggregation kinetics in the presence of membrane-like environments [48]–[50]. Our results show that wtECP is able to form fibrillar-like aggregates on model membranes with an average size of 845±150 nm (Figure 4B), comparable in size with the wtECP aggregates observed in vitro in the absence of lipid membranes (∼150 nm) [28]. In fact, when tested for ThT binding, we observe aggregates with similar size (Figure 4B). When wtECP is incubated with model membranes and tested for Congo Red binding, we obtain again a noticeable spectral shift (Supplementary Information Figure S5B). To complete these results we have also performed all the experiments detailed above using the I13A mutant and found it to be unable to form amyloid-like aggregates (Figure 4).
Conclusions
The results presented here for ECP reinforce the hypothesis that an amyloid-like aggregation process is taking place in the bacteria surface that drives bacteria cell agglutination, which is essential for the antimicrobial activity of the protein. In summary, after binding to the bacteria surface, a rearrangement of the protein could take place, exposing the hydrophobic N-terminal patch of the protein. Following, the aggregation process would start promoting the agglutination of the bacteria cells through the aggregation of the surface-attached protein molecules. The formation of aggregates on the bacteria surface will disrupt the lipopolysaccharide bilayer of Gram-negative cells exposing the internal cytoplasmatic membrane to the protein action, promoting the membrane disruption and eventually the bacteria killing.
Cell agglutinating activity provides a particularly appealing feature that may contribute to the clearance of bacteria at the infectious focus. In this sense, bacteria agglutination would prepare the field before host phagocytic cells enter in the scene [33]. However, despite the interest in the pharmaceutical industry to identify the structural determinants for bacteria cell agglutination, bibliography on that subject is scarce and only few agglutinating antimicrobial proteins are described in the literature. Excitingly, there may be other proteins and peptides with similar characteristics that also follow the proposed model. Hence, the agglutinating mechanism may represent a more generalized process that may derivate in amyloid deposit formation at bacterial infection focuses.
Besides, it has been reported that systematic exposure to inflammation may represent a risk factor on developing Alzheimer's disease [51], [52] and other types of dementia [53]. Some studies have also demonstrated that the release of inflammatory mediators can also cause generalized cytotoxicity. In particular, ECP has been discovered to be cytotoxic [40], [54] and neurotoxic, causing the Gordon phenomenon after injection intratechally in rabbits [55]. Therefore, our results suggest that the release of inflammatory mediators after infection (like AMPs) may either seed the aggregation processes in the brain and/or influence the membrane biophysical properties to trigger neurotoxicity and aggregation events.
Materials and Methods
MIC (Minimal Inhibitory Concentration) and MAC (Minimal Agglutination Concentration) determination
Antimicrobial activity was expressed as the MIC100, defined as the lowest protein concentration that completely inhibits microbial growth. MIC of each protein was determined from two independent experiments performed in triplicate for each concentration. Bacteria were incubated at 37°C overnight in Mueller-Hinton II (MHII) broth and diluted to give approximately 5·105 CFU/mL. Bacterial suspension was incubated with proteins at various concentrations (0.1–5 µM) at 37°C for 4 h either in MHII or 10 mM sodium phosphate buffer, 100 mM NaCl, pH 7.4. Samples were plated onto Petri dishes and incubated at 37°C overnight.
For MAC determination, bacteria cells were grown at 37°C to mid-exponential phase (OD600 = 0.6), centrifuged at 5000×g for 2 min, and resuspended in 10 mM sodium phosphate buffer, 100 mM NaCl, pH 7.4, in order to give an absorbance of 0.2 at 600 nm. A 200 µL aliquot of the bacterial suspension was incubated with proteins at various (0.1–10 µM) concentrations at 25°C for 4 h. Aggregation behavior was observed by visual inspection and minimal agglutinating concentration expressed as previously described [42].
Fluorescence-Assisted Cell Sorting (FACS) assay
Bacteria cells were grown at 37°C to mid-exponential phase (OD600 = 0.6), centrifuged at 5000×g for 2 min, resuspended in 10 mM sodium phosphate buffer, 100 mM NaCl, pH 7.4 or the same buffer supplemented with 20% plasma to give a final OD600 = 0.2 and preincubated for 20 min. A 500 µL aliquot of the bacterial suspension was incubated with 5 µM of wtECP or I13A mutant during 4 h. After incubation, 25000 cells were subjected to FACS analysis using a FACSCalibur cytometer (BD Biosciences, New Jersey) and a dot-plot was generated by representing the low-angle forward scattering (FSC-H) in the x-axis and the side scattering (SSC-H) in the y-axis to analyze the size and complexity of the cell cultures. Results were analyzed using FlowJo (Tree Star, Ashland, OR).
Bacteria viability assay
Bacteria viability assays were performed as described before [39]. Briefly, bacteria were incubated in 10 mM sodium phosphate buffer, 100 mM NaCl, pH 7.4 with 5 µM of wtECP or I13A mutant and then stained using a syto 9/propidium iodide 1∶1 mixture. The viability kinetics were monitored using a Cary Eclipse Spectrofluorimeter (Varian Inc., Palo Alto, CA, USA). To calculate bacterial viability, the signal in the range 510–540 nm was integrated to obtain the syto 9 signal (live bacteria) and from 620–650 nm to obtain the propidium iodide signal (dead bacteria). Then, the percentage of live bacteria was represented as a function of time.
Liposome agglutination and leakage assay
The ANTS/DPX liposome leakage fluorescence assay was performed as previously described [56]. Briefly, a unique population of LUVs of DOPC/DOPG (3∶2 molar ratio) lipids was obtained containing 12.5 mM ANTS, 45 mM DPX, 20 mM NaCl, and 10 mM Tris/HCl, pH 7.4. The ANTS/DPX liposome suspension was diluted to 30 µM concentration and incubated at 25°C in the presence of wtECP or I13A mutant. Leakage activity was followed by monitoring the increase of the fluorescence at 535 nm.
For liposome agglutination, 200 µM LUV liposomes were incubated in 10 mM phosphate buffer, pH 7.4, containing 5 to 100 mM NaCl, in the presence of 5 µM wtECP or I13A mutant and the scattering signal at 470 nm was collected at 90° from the beam source using a Cary Eclipse Spectrofluorimeter (Varian Inc., Palo Alto, CA, USA) [57].
Confocal microscopy
Experiments were carried out in 35 cm2 plates with a glass coverslip. For phospholipid membranes, 500 µl of 200 µM LUV liposomes (prepared as described in Supplementary Information) were incubated with 5 µM wtECP or I13A mutant for 4 h in 10 mM sodium phosphate buffer, 100 mM NaCl, pH 7.4. For bacteria, 500 µl of E. coli cells (OD600 = 0.2) were incubated with 5 µM wtECP or I13A mutant for 4 h in 10 mM sodium phosphate buffer, 100 mM NaCl, pH 7.4. RNase A was used always as a negative control. Samples of both liposomes and bacteria were imaged using a laser scanning confocal microscope (Olympus FluoView 1000 equipped with a UPlansApo 60× objective in 1.4 oil immersion objective, United Kingdom). wtECP and I13A mutant labeled with Alexa Fluor 488 were excited using a 488-nm argon laser (515–540 nm emission collected) and Vibrant DiI was excited using an orange diode (588–715 nm emission collected).
TIRF microscopy
To study the interaction of proteins with lipid membranes, planar supported lipid bilayers were used (Supplementary Information). When using bacteria, glass coverslips were previously treated with 0.1% poly-L-lysine to ensure that samples will adhere to the surface. 500 µl of E. coli cells (OD600 = 0.2) were incubated with 5 µM wtECP or I13A mutant for 4 h and then transferred to poly-L-lysine treated microscopy plates and incubated for 15 minutes. To remove unattached cells, plates were washed twice with 10 mM sodium phosphate, 100 mM NaCl, pH 7.4 buffer. RNase A was used always as a negative control. Images were captured using a laser scanning confocal microscope (Olympus FluoView 1000 equipped with a PlansApo 60× TIRF objective in 1.4 oil immersion objective, United Kingdom) using the same conditions as described for confocal microscopy experiments. Thioflavin T (ThT) was used to detect amyloid aggregates. In this case, samples were incubated for 4 h with unlabeled proteins as described before and then incubated with ThT at 25 µM final concentration for 15 minutes. Then, plates were washed twice with 10 mM sodium phosphate, 100 mM NaCl buffer, pH 7.4 to remove unattached cells and ThT excess.
Supporting Information
Zdroje
1. OtvosLJr (2005) Antibacterial peptides and proteins with multiple cellular targets. J Pept Sci 11 : 697–706.
2. YountNY, BayerAS, XiongYQ, YeamanMR (2006) Advances in antimicrobial peptide immunobiology. Biopolymers 84 : 435–458.
3. ZasloffM (2002) Antimicrobial peptides of multicellular organisms. Nature 415 : 389–395.
4. HancockRE, SahlHG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24 : 1551–1557.
5. ZhangL, FallaTJ (2010) Potential therapeutic application of host defense peptides. Methods Mol Biol 618 : 303–327.
6. BrogdenKA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3 : 238–250.
7. GottlerLM, RamamoorthyA (2009) Structure, membrane orientation, mechanism, and function of pexiganan–a highly potent antimicrobial peptide designed from magainin. Biochim Biophys Acta 1788 : 1680–1686.
8. MarshEN, BuerBC, RamamoorthyA (2009) Fluorine–a new element in the design of membrane-active peptides. Mol Biosyst 5 : 1143–1147.
9. RamamoorthyA (2009) Beyond NMR spectra of antimicrobial peptides: dynamical images at atomic resolution and functional insights. Solid State Nucl Magn Reson 35 : 201–207.
10. ThennarasuS, HuangR, LeeDK, YangP, MaloyL, et al. (2010) Limiting an antimicrobial peptide to the lipid-water interface enhances its bacterial membrane selectivity: a case study of MSI-367. Biochemistry 49 : 10595–10605.
11. PulidoD, NoguesMV, BoixE, TorrentM (2012) Lipopolysaccharide neutralization by antimicrobial peptides: a gambit in the innate host defense strategy. J Innate Immun 4 : 327–336.
12. TorrentM, NoguesMV, BoixE (2012) Discovering new in silico tools for antimicrobial Peptide prediction. Curr Drug Targets 13 : 1148–1157.
13. WimleyWC (2010) Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5 : 905–917.
14. NguyenLT, HaneyEF, VogelHJ (2011) The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol 29 : 464–472.
15. ButterfieldSM, LashuelHA (2010) Amyloidogenic protein-membrane interactions: mechanistic insight from model systems. Angew Chem Int Ed Engl 49 : 5628–5654.
16. MahalkaAK, KinnunenPK (2009) Binding of amphipathic alpha-helical antimicrobial peptides to lipid membranes: lessons from temporins B and L. Biochim Biophys Acta. 1788 : 1600–1609.
17. KaganBL, JangH, CaponeR, Teran ArceF, RamachandranS, et al. (2011) Antimicrobial Properties of Amyloid Peptides. Mol Pharm 9 : 708–717.
18. BrenderJR, DurrUH, HeylD, BudarapuMB, RamamoorthyA (2007) Membrane fragmentation by an amyloidogenic fragment of human Islet Amyloid Polypeptide detected by solid-state NMR spectroscopy of membrane nanotubes. Biochim Biophys Acta 1768 : 2026–2029.
19. BrenderJR, HartmanK, GottlerLM, CavittME, YoungstromDW, et al. (2009) Helical conformation of the SEVI precursor peptide PAP248–286, a dramatic enhancer of HIV infectivity, promotes lipid aggregation and fusion. Biophys J 97 : 2474–2483.
20. BrenderJR, HartmanK, ReidKR, KennedyRT, RamamoorthyA (2008) A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity. Biochemistry 47 : 12680–12688.
21. BrenderJR, LeeEL, CavittMA, GafniA, SteelDG, et al. (2008) Amyloid fiber formation and membrane disruption are separate processes localized in two distinct regions of IAPP, the type-2-diabetes-related peptide. J Am Chem Soc 130 : 6424–6429.
22. NangaRP, BrenderJR, VivekanandanS, PopovychN, RamamoorthyA (2009) NMR structure in a membrane environment reveals putative amyloidogenic regions of the SEVI precursor peptide PAP(248–286). J Am Chem Soc 131 : 17972–17979.
23. NangaRP, BrenderJR, XuJ, HartmanK, SubramanianV, et al. (2009) Three-dimensional structure and orientation of rat islet amyloid polypeptide protein in a membrane environment by solution NMR spectroscopy. J Am Chem Soc 131 : 8252–8261.
24. PopovychN, BrenderJR, SoongR, VivekanandanS, HartmanK, et al. (2012) Site specific interaction of the polyphenol EGCG with the SEVI amyloid precursor peptide PAP(248–286). J Phys Chem B 116 : 3650–3658.
25. JelinekR, KolushevaS (2005) Membrane interactions of host-defense peptides studied in model systems. Curr Protein Pept Sci 6 : 103–114.
26. TangM, HongM (2009) Structure and mechanism of beta-hairpin antimicrobial peptides in lipid bilayers from solid-state NMR spectroscopy. Mol Biosyst 5 : 317–322.
27. TorrentM, ValleJ, NoguesMV, BoixE, AndreuD (2011) The generation of antimicrobial peptide activity: a trade-off between charge and aggregation? Angew Chem Int Ed Engl 50 : 10686–10689.
28. TorrentM, OdorizziF, NoguesMV, BoixE (2010) Eosinophil cationic protein aggregation: identification of an N-terminus amyloid prone region. Biomacromolecules 11 : 1983–1990.
29. JangH, ArceFT, MustataM, RamachandranS, CaponeR, et al. (2011) Antimicrobial protegrin-1 forms amyloid-like fibrils with rapid kinetics suggesting a functional link. Biophys J 100 : 1775–1783.
30. SosciaSJ, KirbyJE, WashicoskyKJ, TuckerSM, IngelssonM, et al. (2010) The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One 5: e9505.
31. HarrisF, DennisonSR, PhoenixDA (2012) Aberrant action of amyloidogenic host defense peptides: a new paradigm to investigate neurodegenerative disorders? FASEB J 26 : 1776–1781.
32. TorrentM, AndreuD, NoguesVM, BoixE (2011) Connecting peptide physicochemical and antimicrobial properties by a rational prediction model. PLoS One 6: e16968.
33. GorrSU, SotskyJB, ShelarAP, DemuthDR (2008) Design of bacteria-agglutinating peptides derived from parotid secretory protein, a member of the bactericidal/permeability increasing-like protein family. Peptides 29 : 2118–2127.
34. Van Nieuw AmerongenA, BolscherJG, VeermanEC (2004) Salivary proteins: protective and diagnostic value in cariology? Caries Res 38 : 247–253.
35. PulidoD, MoussaouiM, AndreuD, NoguesMV, TorrentM, et al. (2012) Antimicrobial Action and Cell Agglutination by Eosinophil Cationic Protein Is Modulated by the Cell Wall Lipopolysaccharide Structure. Antimicrob Agents Chemother 56 : 2378–2385.
36. BoixE, TorrentM, SánchezD, NoguésMV (2008) The Antipathogen Activities of Eosinophil Cationic Protein. Current Pharm Biotec 9 : 141–152.
37. VengeP, BystromJ, CarlsonM, HakanssonL, KarawacjzykM, et al. (1999) Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy 29 : 1172–1186.
38. BoixE, SalazarVA, TorrentM, PulidoD, NoguesMV, et al. (2012) Structural determinants of the eosinophil cationic protein antimicrobial activity. Biol Chem 393 : 801–815.
39. TorrentM, BadiaM, MoussaouiM, SanchezD, NoguesMV, et al. (2010) Comparison of human RNase 3 and RNase 7 bactericidal action at the Gram-negative and Gram-positive bacterial cell wall. FEBS J 277 : 1713–1725.
40. NavarroS, AleuJ, JimenezM, BoixE, CuchilloCM, et al. (2008) The cytotoxicity of eosinophil cationic protein/ribonuclease 3 on eukaryotic cell lines takes place through its aggregation on the cell membrane. Cell Mol Life Sci 65 : 324–337.
41. TorrentM, NavarroS, MoussaouiM, NoguesMV, BoixE (2008) Eosinophil cationic protein high-affinity binding to bacteria-wall lipopolysaccharides and peptidoglycans. Biochemistry 47 : 3544–3555.
42. TorrentM, PulidoD, de la TorreBG, Garcia-MayoralMF, NoguesMV, et al. (2011) Refining the eosinophil cationic protein antibacterial pharmacophore by rational structure minimization. J Med Chem 54 : 5237–5244.
43. SanchezD, MoussaouiM, CarrerasE, TorrentM, NoguesV, et al. (2011) Mapping the eosinophil cationic protein antimicrobial activity by chemical and enzymatic cleavage. Biochimie 93 : 331–338.
44. TorrentM, Di TommasoP, PulidoD, NoguesMV, NotredameC, et al. (2012) AMPA: an automated web server for prediction of protein antimicrobial regions. Bioinformatics 28 : 130–131.
45. TorrentM, NoguesVM, BoixE (2009) A theoretical approach to spot active regions in antimicrobial proteins. BMC Bioinformatics 10 : 373.
46. TorrentM, de la TorreBG, NoguesVM, AndreuD, BoixE (2009) Bactericidal and membrane disruption activities of the eosinophil cationic protein are largely retained in an N-terminal fragment. Biochem J 421 : 425–434.
47. TorrentM, NoguesMV, BoixE (2011) Eosinophil cationic protein (ECP) can bind heparin and other glycosaminoglycans through its RNase active site. J Mol Recognit 24 : 90–100.
48. KayedR, SokolovY, EdmondsB, McIntireTM, MiltonSC, et al. (2004) Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem 279 : 46363–46366.
49. AmbroggioEE, KimDH, SeparovicF, BarrowCJ, BarnhamKJ, et al. (2005) Surface behavior and lipid interaction of Alzheimer beta-amyloid peptide 1–42: a membrane-disrupting peptide. Biophys J 88 : 2706–2713.
50. AuvynetC, El AmriC, LacombeC, BrustonF, BourdaisJ, et al. (2008) Structural requirements for antimicrobial versus chemoattractant activities for dermaseptin S9. FEBS J 275 : 4134–4151.
51. KrsticD, MadhusudanA, DoehnerJ, VogelP, NotterT, et al. (2012) Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J Neuroinflammation 9 : 151.
52. MiklossyJ (2011) Emerging roles of pathogens in Alzheimer disease. Expert Rev Mol Med 13: e30.
53. AlmeidaOP, LautenschlagerNT (2005) Dementia associated with infectious diseases. Int Psychogeriatr 17Suppl 1: S65–77.
54. NavarroS, BoixE, CuchilloCM, NoguesMV (2010) Eosinophil-induced neurotoxicity: the role of eosinophil cationic protein/RNase 3. J Neuroimmunol 227 : 60–70.
55. FredensK, DahlR, VengeP (1982) The Gordon phenomenon induced by the eosinophil cationic protein and eosinophil protein X. J Allergy Clin Immunol 70 : 361–366.
56. TorrentM, CuyasE, CarrerasE, NavarroS, LopezO, et al. (2007) Topography studies on the membrane interaction mechanism of the eosinophil cationic protein. Biochemistry 46 : 720–733.
57. TorrentM, SanchezD, BuzonV, NoguesMV, CladeraJ, et al. (2009) Comparison of the membrane interaction mechanism of two antimicrobial RNases: RNase 3/ECP and RNase 7. Biochim Biophys Acta 1788 : 1116–1125.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Egyptian H5N1 Influenza Viruses—Cause for Concern?
- Epigenetics of Host–Pathogen Interactions: The Road Ahead and the Road Behind
- Environmental Regulation of Prions in Yeast
- : Dissecting the Molecular Interface between Pathogen and Plant
- A Wolf in Sheep's Clothing: SV40 Co-opts Host Genome Maintenance Proteins to Replicate Viral DNA
- Exploring New Biological Functions of Amyloids: Bacteria Cell Agglutination Mediated by Host Protein Aggregation
- A Trade-off between the Fitness Cost of Functional Integrases and Long-term Stability of Integrons
- Intracellular Vesicle Acidification Promotes Maturation of Infectious Poliovirus Particles
- Distinct Effects on Diversifying Selection by Two Mechanisms of Immunity against
- Whole Genome Sequencing Reveals Local Transmission Patterns of in Sympatric Cattle and Badger Populations
- Early Mechanisms of Pathobiology Are Revealed by Transcriptional Temporal Dynamics in Hippocampal CA1 Neurons of Prion Infected Mice
- Revised Phylogeny and Novel Horizontally Acquired Virulence Determinants of the Model Soft Rot Phytopathogen SCC3193
- : Where Does It Live?
- The Role of Auxin-Cytokinin Antagonism in Plant-Pathogen Interactions
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Wolf in Sheep's Clothing: SV40 Co-opts Host Genome Maintenance Proteins to Replicate Viral DNA
- Intracellular Vesicle Acidification Promotes Maturation of Infectious Poliovirus Particles
- Revised Phylogeny and Novel Horizontally Acquired Virulence Determinants of the Model Soft Rot Phytopathogen SCC3193
- The Role of Auxin-Cytokinin Antagonism in Plant-Pathogen Interactions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



