-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous Systems
Certain microbes invade brain microvascular endothelial cells (BMECs) to breach the blood-brain barrier (BBB) and establish central nervous system (CNS) infection. Here we use the leading meningitis pathogen group B Streptococcus (GBS) together with insect and mammalian infection models to probe a potential role of glycosaminoglycan (GAG) interactions in the pathogenesis of CNS entry. Site-directed mutagenesis of a GAG-binding domain of the surface GBS alpha C protein impeded GBS penetration of the Drosophila BBB in vivo and diminished GBS adherence to and invasion of human BMECs in vitro. Conversely, genetic impairment of GAG expression in flies or mice reduced GBS dissemination into the brain. These complementary approaches identify a role for bacterial-GAG interactions in the pathogenesis of CNS infection. Our results also highlight how the simpler yet genetically conserved Drosophila GAG pathways can provide a model organism to screen candidate molecules that can interrupt pathogen-GAG interactions for future therapeutic applications.
Published in the journal: . PLoS Pathog 7(6): e32767. doi:10.1371/journal.ppat.1002082
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002082Summary
Certain microbes invade brain microvascular endothelial cells (BMECs) to breach the blood-brain barrier (BBB) and establish central nervous system (CNS) infection. Here we use the leading meningitis pathogen group B Streptococcus (GBS) together with insect and mammalian infection models to probe a potential role of glycosaminoglycan (GAG) interactions in the pathogenesis of CNS entry. Site-directed mutagenesis of a GAG-binding domain of the surface GBS alpha C protein impeded GBS penetration of the Drosophila BBB in vivo and diminished GBS adherence to and invasion of human BMECs in vitro. Conversely, genetic impairment of GAG expression in flies or mice reduced GBS dissemination into the brain. These complementary approaches identify a role for bacterial-GAG interactions in the pathogenesis of CNS infection. Our results also highlight how the simpler yet genetically conserved Drosophila GAG pathways can provide a model organism to screen candidate molecules that can interrupt pathogen-GAG interactions for future therapeutic applications.
Introduction
Bacterial meningitis is one of the top ten causes of infection-related mortality worldwide [1]. Meningitis is particularly devastating in the newborn infant, and 20–50% of survivors can suffer permanent neurological sequelae including deafness, seizures, hydrocephalus, cerebral palsy and/or cognitive deficits [2]–[5]. The most common agent of neonatal bacterial meningitis in the United States, Europe and Asia is group B Streptococcus (GBS). In recent years, GBS has also emerged as a cause of serious infections including meningitis in nonpregnant adult populations, with an invasive disease incidence approaching that reported for the neonate [6]–[8]. In gaining access to the central nervous system (CNS), GBS reveals an ability to cross the blood-brain barrier (BBB), a specialized layer of brain microvascular endothelial cells (BMECs) that regulates macromolecular traffic to maintain biochemical homeostasis in brain tissues. BBB penetration by a bacterial pathogen reflects a complex interplay between host endothelium and microbial products [9]. The fundamental mechanisms by which GBS establishes CNS infection remain incompletely understood [10].
The plasma membrane of mammalian endothelial cells is prominently decorated with linear, negatively charged sugar chains known as glycosaminoglycans (GAGs). The sulfated GAGs, chondroitin/dermatan sulfate and heparan sulfate, occur as proteoglycans that consist of one or more GAG chains covalently linked to a core protein. Cells elaborate several membrane heparan sulfate proteoglycans (HSPGs) including syndecans and the glycosylphosphatidylinositol-linked glypicans. The heparan sulfate chains assemble by copolymerization of alternating residues of N-acetylglucosamine and glucuronic acid, which then can undergo variable sulfation and uronic acid epimerization. This process occurs in a non-template driven manner, resulting in sections of the chains with variably modified sugars of variable length interspersed with domains of unmodified residues. The modified regions make up binding sites for growth factors, membrane receptors, and extracellular matrix constituents, imparting to the chains important biological activities, such as roles in cell adhesion, receptor signaling, and leukocyte trafficking across endothelium [11]. The ability of an individual cell to interact with and respond to ligands appears to depend on the array of expressed proteoglycans and the pattern of modifications of the heparan sulfate chains [12]–[14]. Proteoglycan synthesis in mammals is a complex process involving a diverse array of gene products including core proteins, enzymes that initiate and elongate the polysaccharide chain, and enzymes that modify the polymer by sulfation and other processes [15], [16]. Drosophila melanogaster contains a repertoire of GAG structures similar to that of mammals [17] through a relatively small number of genes highly homologous to those found in mammals [18], [19].
A number of viral and bacterial pathogens functionally interact with cell surface GAGs [14]. Examples include the initial attachment phase of herpes simplex virus mediated through heparan sulfate [20], [21], Mycobacterium tuberculosis lung epithelial invasion through expression of a heparin-binding protein [22], and heparan sulfate-dependent attachment and entry of intestinal epithelial cells by Listeria monocytogenes [23]. We hypothesized that direct interactions with GAGs expressed on BMECs could influence the propensity of bloodborne bacteria to breach the BBB and produce CNS infection. In the specific case of GBS, a candidate ligand for this process exists in the surface-anchored Alpha C protein (ACP), which has the capacity to bind GAGs and promote bacterial entry into cervical epithelial cells in vitro [24]–[26].
Drosophila can serve as a useful model organism for analysis of mechanisms of bacterial pathogenesis and resistance [27]–[30]. We have recently shown that GBS infection can be established in Drosophila, and that overall mortality and bacterial loads are reduced in fly strains with diminished GAG expression [31]. In the present study, we use bacterial, Drosophila, and mouse mutants to achieve the aim of examining the role of bacterial-GAG interactions as mediators of BBB translocation and CNS infection. The study highlights an emerging recognition that specialized surface glial cells regulate the flow of substances into and out of the fly brain and present a functional equivalent of the BBB in Drosophila [32]–[35]. Our findings indicate that the specific heparan sulfate-binding properties of ACP promote BBB interactions and contribute to the establishment of GBS meningitis.
Results
GAG binding by ACP facilitates GBS dissemination into fly heads
We previously demonstrated that pricking the Drosophila thorax with a needle that had been dipped into a concentrated slurry of GBS leads to fly death [31]. To assess the nature of bacterial dissemination from the prick site, we performed histologic examination of GBS-infected Drosophila. As shown (Figure S1 A and B), wild-type (WT) GBS A909 spreads systemically after localized injection in the thorax. H&E stain reveals bacterial cocci in multiple sites, including muscle, fat, and the lining of the brain. These data indicate that tissue architecture is largely preserved despite widespread GBS dissemination during infection. The mutant GBS strain A909/R185A harbors a single amino acid change in ACP that significantly reduces GAG binding without affecting overall ACP structure, bacterial growth rate, or surface polysaccharide capsule expression [26], [31]. Following pinprick inoculation of an identical dose of the A909/R185A mutant into the fly thorax, bacteria disseminate less broadly from the local site of infection, and fewer cocci are visualized at the brain lining (Figure S1 C and D) To specifically address whether ACP-GAG binding promotes GBS dissemination into fly heads, we compared the bacterial burden (colony forming units, cfu) in the heads and bodies of WT flies after infection with WT GBS A909 and A909/R185A mutant. Flies infected with mutant bacteria had a significantly lower ratio of head cfu to total (head+body) cfu than those infected with the WT strain (Figure 1A; Supplemental Table S1), indicating that disruption of ACP-GAG binding decreases GBS penetration into the fly head.
Fig. 1. ACP-GAG binding promotes GBS invasion into fly heads; glial GAG polymerase knock-down decreases lethal infection. 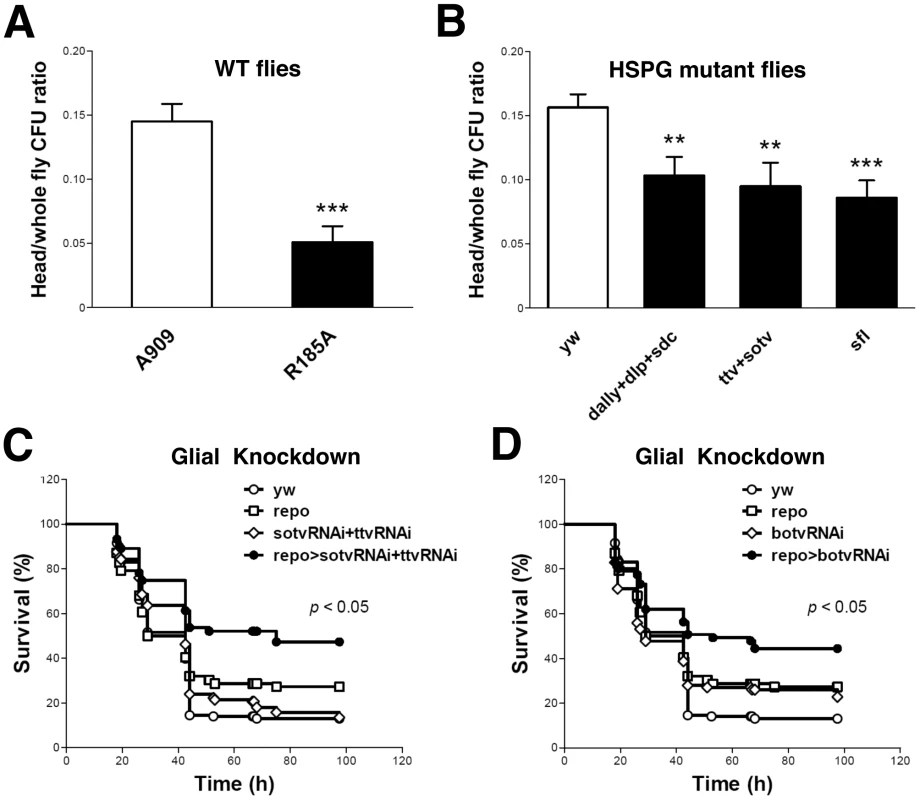
(A) The ratio of head/whole fly cfu in WT yw flies 24 h after infection with GBS mutant R185A (filled bar, n = 13 groups of 10 flies) was significantly lower than after infection with WT GBS strain A909 (open bar, n = 14 groups of 10 flies). (B) The ratios of head to whole fly cfu in HSPG mutant flies (filled bars) were lower than those in the WT control flies (open bar, n = 22 groups of 10 flies). The HSPG mutant flies studied here carry mutations in the genes encoding core proteins (dally+dlp+sdc, n = 15 groups of 10 flies), GAG polymerases (ttv+sotv, n = 12 groups of 10 flies) or the GAG NDST (sfl, n = 10 groups of 10 flies. Infected flies were heterozygotes from crosses of these mutants with yw flies, as homozygous mutants in these genes are nonviable. Each bar represents the mean and SEM. The student's t-test was used for statistical analysis in each comparison. ** p<0.01; *** p<0.001. Repo-Gal4 is a pan-glial Gal4 driver [66]. Flies expressing shRNA targeting both ttv and sotv (C) or botv alone (D) driven by repo-Gal4 show resistance to lethal A909 infection. Plots show a pool of data from at least 60 flies per sample. The genotypes of the flies used here are repo-Gal4/+ (repo), UAS-sotvRNAi/+;UAS-ttvRNAi/+ (sotvRNAi+ttvRNAi), UAS-sotvRNAi/+;UAS-ttvRNAi/repo-Gal4 (repo>sotvRNAi+ttvRNAi), UAS-botvRNAi/+ (botvRNAi) and UAS-botvRNAi/repo-Gal4 (repo>botvRNAi). The Gal4 and UAS lines were crossed with yw flies as controls. The survival curves of yw, Gal4 controls, UAS controls, and shRNA expressing groups are indicated as open circles, open squares, open diamonds, and filled circles respectively. A log rank test was used for statistical analysis in comparing shRNA-expressing groups and control groups. Impaired Drosophila GAG expression reduces GBS dissemination into fly heads
The Drosophila genome contains genes encoding the HSPG core proteins dally, dally-like protein [dlp] and syndecan [sdc]; three genes encoding heparan sulfate polymerases, tout-velu [ttv] (homolog of mammalian Ext1), sister of ttv [sotv] (homolog of Ext2) and brother of ttv [botv] (homolog of Extl3); and one gene encoding an N-deacetylase-N-sulfotransferase (sulfateless [sfl], a homolog of Ndst1). After chain initiation by Botv, polymerization occurs through a copolymerase complex consisting of Ttv and Sotv [36]. The GAG chains then undergo a series of modifications such as N-deacetylation and N-sulfation of N-acetylglucosamine residues by Sfl. Mutations in these genes lead to altered content or sulfation of heparan sulfate on all Drosophila heparan sulfate proteoglycans [37], [38].
To corroborate the role of GAG binding in GBS dissemination into the fly head, we used the pinprick method to establish infection in three different HSPG mutant fly strains deficient in membrane HSPG core proteins (dally+dlp+sdc), heparan sulfate polymerases (ttv+sotv), or NDST (sfl), respectively. After infection with WT GBS A909, all three HSPG mutant fly strains exhibited lower head/total body cfu ratios than did the WT control flies (Figure 1B; Supplemental Table S1). The extent of reduction in head/total body cfu probably underestimates the importance of HSPGs in this model since each of the host mutations had to be tested as heterozygotes due to the requirement for HSPGs in fly embryogenesis.
We next sought to specifically link the observed GAG-dependent phenotype to glial cells that form tight junctions and restrict paracellular diffusion between the fly circulatory system and brain, thereby representing the Drosphila BBB equivalent [33]. The glial cell-specific driver repo-Gal4 was used to suppress polymerase expression in fly BBB cells through in vivo shRNA knockdown of ttv, sotv, and botv. After infection with WT GBS A909, flies with glial cell-specific knockdown of either botv alone, or both ttv and sotv displayed higher survival rates than the corresponding repo and Gal4-UAS controls (Figure 1 C and D).
GAG binding by GBS ACP promotes human BMECs adherence and invasion
Immortalized human BMECs have provided a widely used and well-defined in vitro model for understanding microbial interactions with the human BBB [9], [39]. Previous studies have shown that fetal or adult primary BMECs expressed high levels of heparan and chondroitin sulfates [40], supporting earlier conclusions that proteoglycans are richly expressed on the surface of primary human endothelial cells derived from all age groups, including the fetus [41]–[43]. We found that WT GBS A909 efficiently adhered to and invaded hBMECs; however the A909/R185A mutant with decreased GAG-binding ability showed significantly reduced adhesion and invasion of these cells (Figure 2 A and B). Similar results were obtained when infection and invasion were measured in A549 lung adenocarcinoma cells (Figure S2 A and B). To further establish a role for GAG binding in GBS interaction with hBMECs, we used RNA interference to silence the expression of EXT2. The efficiency of silencing was over 80% by qRT-PCR analysis of mRNA from hBMECs infected with EXT2 shRNA lentiviruses compared to hBMECs infected with a control lentivirus (Figure 2C). Although no apparent difference in WT GBS A909 adherence to control vs. EXT2 knockdown hBMECs was discerned (Figure 2D), GBS invasion of hBMECs following EXT2 knockdown was significantly decreased (Figure 2E).
Fig. 2. Contribution of GAG-binding activity of GBS to adherence and invasion of hBMECs. 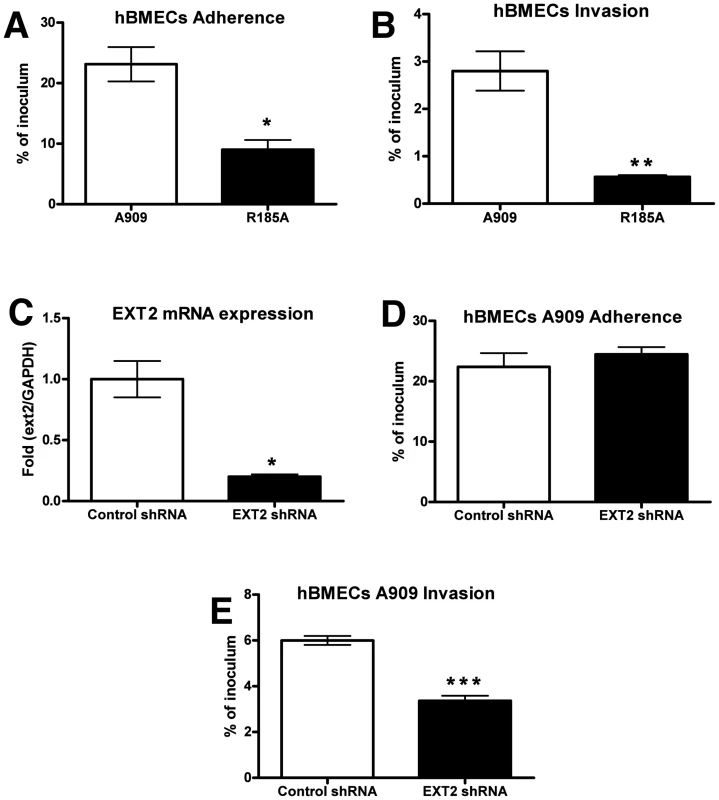
For adherence (A), bacteria were enumerated after 30 min of incubation, whereas invasion (B) was quantified after 2 h of incubation with human brain microvascular endothelial cells (hBMECs) and 2 h of incubation with antibiotics to kill extracellular bacteria. (C) Knockdown of EXT2 expression by RNA interference. hBMECs were infected with lentiviruses carrying control shRNA or EXT2 targeting shRNA and the knockdown efficiency was determined by quantitative RT-PCR. GAPDH was used as an internal control. Attenuation of A909 invasion (E) but not adherence (D) after knocking down EXT2 expression in hBMECs. Adherence and invasion assays were performed in triplicate and repeated three times with similar results, and representative experiments are shown. Statistical analysis was performed by Student's t test and error bars SEM. * p<0.05; ** p<0.01; *** p<0.001. Reduced GAG expression diminishes GBS BBB penetration in a murine meningitis model
Mice heterozygous for Ext2 provide a functional in vivo model in which the chain length of GAGs expressed by the host is significantly reduced [44]. To confirm the utility of this model for analysis of GBS-BBB interactions, we isolated and cultured primary murine BMECs (mBMECs) from Ext2+/− mice (Ext2 hets) and WT littermate controls and infected them ex vivo with WT GBS A909. We documented an approximate 40% reduction in heparan sulfate content of endothelium isolated from these Ext2 het mice vs. WT controls (Figure S3). GBS adherence and invasion were significantly reduced in mBMECs from Ext2 hets compared to those isolated from WT mice (Figure 3 A and B). Several factors may explain why adherence was altered in Ext2 het mBMECs but not in Ext2-silenced hBMECs, including differences in residual GAG quantity or structure or overall surface charge, other effects of using transformed vs. primary cells, or species-specific effects. Of note, Ext2-silencing was associated with reduced hBMEC growth rate, while Ext2 het mBMECs grew similarly to WT mBMECs.
Fig. 3. GAG contributes to blood-brain barrier penetration in mice in vivo. 
Attenuated GBS adhesion (A, C) and invasion (B, D) to primary mBMECs from Ext2 heterozygous mice (ext2 hets) and Ndst1-deficient mice. For adherence, bacteria were enumerated after 30 min of incubation, whereas invasion was quantified after 2 h of incubation with mBMECs and 2 h of incubation with antibiotics to kill extracellular bacteria. Comparison of bacterial counts (expressed in cfu) recovered from the brain (E), blood (F), and spleen (G) of WT mice and Ext2 hets 24 h after intravenous challenge with 108 cfu of A909. (H) Brain bacterial counts were corrected for blood contamination (brain/blood ratio) using the blood concentration and a conservative estimate of the mouse cerebral blood volume (2.5 ml per 100 g tissue). Results pool the data from two independent experiment with final numbers of n = 12 for WT and n = 11 for Ext2 het. Each circle denotes 1 mouse. Statistics analysis was performed by Student's t test (A–D) or Mann-Whitney test (E–H). *p<0.05. Moreover, impaired GBS adherence and invasion was also observed in Ndst1-deficient mBMECs that exhibit overall reduction in sulfation of the chains (Figure 3 C and D) [36], [37]. Comparable results were obtained in Ndst1-deficient mouse lung endothelial cells (Figure S2 C and D). To explore a potential contribution of mammalian GAG expression to GBS BBB penetration in vivo, Ext2 hets and WT littermate controls were infected intravenously with WT GBS A909 and sacrificed 24 h later. Significantly fewer bacterial cfu (9-fold decrease) were recovered from the brains of GBS-infected Ext2 hets compared to brains of WT controls (Figure 3E) and some animals showed dramatic 103–104 fold reduction in brain cfu. In contrast, lesser decreases in bacterial cfu were observed in the blood (Figure 3F) and spleen (Figure 3G) of Ext2 hets. Consequently, the mean ratio of brain∶blood cfu in Ext2 hets (10.6) was significantly lower than that observed in WT controls (116.0) (Figure 3H). In a small pilot experiment, we noted a trend of reduced CFU in the lungs of Ext2 hets compared to WT mice (Figure S4), suggested that the finding may extend to other endothelium, but such conclusions require future corroboration. Bone-marrow derived macrophages from Ext2 hets and WT controls did not differ in assays of phagocytosis, total bacterial killing, intracellular bacterial killing, or release of tumor necrosis factor-alpha when challenged with GBS ex vivo (Figure S5), indicating that reduced GAG expression does not globally compromise phagocyte innate immune function. In sum, the reduction of GAG expression resulting from EXT2 heterozygosity diminishes GBS interactions with BMECs in vitro and decreases GBS CNS entry in vivo.
Discussion
Our combined analyses in the Drosophila and mammalian systems indicate that GBS interaction with GAGs promotes BBB attachment and bacterial entry into the CNS. The GAG binding property of the surface-anchored ACP is one contributor to this invasive phenotype. Meningitis is a dangerous complication of neonatal GBS infection and understanding the predilection of the pathogen to breach the BBB is a critical goal of molecular pathogenesis investigations. To date, a number GBS factors have been shown to promote its interaction with human BMECs in vitro, including fibrinogen adhesin FbsA [38], laminin-binding protein Lmb [45], major pilus backbone subunit PilB [46], lipoteichoic acid anchoring enzyme IagA [47], and the serine-rich repeat 1 glycoprotein Srr1 [48]. In the case of IagA, Srr1, and the hBMEC-disrupting GBS ß-hemolysin/cytolysin, a contribution to meningitis in the murine model has further been demonstrated [47]–[49]. However, the present study is the first to genetically manipulate the host to identify the corresponding host receptor molecules (GAGs) that the GBS virulence factor (ACP) exploits to adhere to and invade the BBB endothelium.
Mammalian heparin sulfate chain polymerization reactions are carried out by the exostosin proteins EXT1 and EXT2 [50], [51] and subsequent modification of the chains by the bifunctional NDSTs. We found decreased GBS invasion in both hBMECs with shRNA-reduced EXT2 levels (Figure 2E) and primary Ext2 heterozygous mBMECs (Figure 3B). In additional studies, we found reduced GBS adherence to and invasion of Ndst1-deficient mBMECs (Figure 3 C and D) and lung endothelial cells (Figure S2 C and D). Adherence and invasion of the A909/R185A mutant to A549 human lung epithelial cells were also markedly attenuated compared to the WT GBS A909 (Figure S2 A and B). Thus the GAG-ACP interaction may contribute to GBS penetration of a broader spectrum of host cell barriers, and GAG expression patterns may determine the nature and efficiency of bacterial dissemination during infection. Moreover, our findings suggest that the chain lengths and negative charges on the GAG chains are both important to provide the binding forces between ACP and GAGs. These findings are consistent with prior data demonstrating that specific positively charged residues of ACP are required for full ACP-GAG binding affinity [26]. Whether ACP binds to a specific sequence of sulfated sugars within heparan sulfate remains to be determined. The availability of mutants altered in 2-O-sulfation of uronic acids, and 3-O - and 6-O-sulfation of glucosamine residues will allow further studies on the structural specificity of the interaction in flies [52], [53] and in mice [54], [55].
Our parallel findings in flies and in mammals both in vitro and in vivo support the relevance of a Drosophila infection model for the study of human CNS infections. Others have reported that the Drosophila humoral/CNS barrier conserves the xenobiotic exclusion properties of vertebrate vascular endothelium. Specifically, the exclusion process is mediated in part by a fly ATP binding cassette (ABC) transporter, Mdr65, that functions similarly to mammalian xenobiotic blood-brain barrier transporters. Thus, CNS chemoprotection involves both conserved molecular structures and functionally analogous anatomic spaces that together promote CNS selective drug partition [34]. Our data extend these findings by demonstrating that CNS penetration of microorganisms may also occur via conserved molecular structures (GAGs), and that these structures can be studied effectively in a Drosophila infection model.
Many microorganisms display GAG-binding ability, including the two leading bacterial pathogens associated with meningitis in older children and adults: Streptococcus pneumoniae utilizes heparin, heparan sulfate and chondroitin/dermatan sulfate in colonization of respiratory mucosal epithelial cells [56], and Neisseria meningitidis surface protein OpC binds HS proteoglycans to initiate epithelial cell invasion [57]. Sulfated GAGs also promote adherence of the veterinary meningeal pathogen Haemophilus somnus to bovine BMEC in vitro [58]. However, each of these GAG-binding interactions has uncertain functions in the pathogenesis of CNS infection that could be difficult to pinpoint because of functional redundancy of bacterial adhesins/invasins and the challenges of manipulating GAG structure/expression in complex mammalian host systems. The importance of positively charged residues is a common theme among GAG-binding proteins. For example, the C-terminal regions of mycobacterial heparin-binding hemagglutinin [59] and histone-like Hlp protein [60] contain Arg/Lys-rich repeats important for heparin binding. In some instances, common heparin-binding motifs, such as BBXB, BBBXXB (B representing a basic amino acid residue), and a 20 Å spacing of basic residues have been reported [61], [62]. Positively charged residues of ACP that were confirmed to contribute to GAG binding by site-directed mutagenesis were R172, R185 and K196; two of these (R185 and K196) are completely conserved in other members of the alpha-like protein (Alp) including Rib, Alp1, Alp2, Alp3 and Alp4 of GBS and R28 of group A Streptococcus [26].
The Drosophila infection model offers the advantages of a simpler genome and well-developed molecular approaches that will facilitate interrogation of host-pathogen GAG-binding interactions, and allow testing of candidate inhibitors of these interactions with an eye toward future therapeutic applications. The design of inhibitors targeting particular pathogen virulence mechanisms represents an attractive strategy in the era of increasing resistance to conventional antibiotics. In attempting to block bacterial-GAG interactions, a major limitation to competitive inhibition by heparin itself is its potency as an anticoagulant and the risk of hemorrhagic complications. However, synthetic low-molecular weight heparins or analogs devoid of anticoagulant activity could be contemplated in this context as potential adjunctive agents for infectious disease therapeutics.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the University of California, San Diego (Animal Welfare Assurance Number: A3033-01). All efforts were made to minimize suffering of animals employed in this study.
Bacteria and cell lines
The ACP-expressing human serotype Ia GBS neonatal isolate, A909, was used in this study. A909/R185A is an A909 point mutant strain that has an ACP variant with diminished GAG binding affinity [26]. GBS were grown in Todd-Hewitt broth (THB, Difco) at 37°C. SV40 large T antigen immortalized human brain endothelial cell line (hBMEC) was obtained from Kwang Sik Kim (Johns Hopkins University, Baltimore, MD). hBMECs were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS, 10% NuSerum (BD), and 1% MEM nonessential amino acids, and were incubated at 37°C in 5% CO2.
Mice and murine primary brain microvascular endothelial cell isolation
Murine brain microvascular endothelial cells (mBMEC) were isolated from cerebral cortex as described [63], except that cells were selected with 5 µg/ml puromycin for 4 days in low-glucose DMEM medium supplemented with 20% FBS (Atlanta Biologicals), 50 µg/ml endothelial growth supplement (BTI), 50 µg/ml heparin, nonessential amino acids, penicillin and streptomycin. Cell purity was higher than 98% as assessed by blood endothelial markers including CD31, CD34, CD105 and CD166. Primary cells were cultured for 5 days and passaged once for experiments. Lung microvascular endothelial cells were isolated as described previously [64]. Ext2+/− mice were described previously [44]. Endothelial cells lacking Ndst1 were derived from Ndst1f/fTie2Cre+ mice [64], [65].
Fly infections
In infection experiments, bacteria were grown to an optical density at 650 nm of 0.3, and then concentrated 10-fold to approximately 2×109 cfu/ml; the exact bacterial concentration was confirmed in each experiment. Adult male flies (2 to 5 days old) were anesthetized with CO2 and then pricked in the dorsal thorax underneath the wing with a fine needle previously dipped in THB broth or a concentrated solution of GBS in THB. After infection, flies were incubated at 29°C in vials with food, and fly survival was monitored over the following 4 days. Cumulative survival curves were derived, and the median survival time for each group was determined using Kaplan-Meier survival analysis. A log rank test was performed to compare survival curves.
To determine the bacterial load in fly heads and bodies at 24 h after infection, flies were placed on ice, and fly heads were separated from fly bodies by a sterile surgical blade. The heads and bodies of 10 flies per group were homogenized in 500 µl and 1000 µl of phosphate-buffered saline (PBS) with 0.025% Triton X-100 respectively. The homogenates were diluted in series (usually 10−1 to 10−3), and the dilutions were plated on THB plates and incubated overnight at 37°C for cfu counting.
Adherence and invasion assay
BMECs were split into 24-well plates and allowed to grow to confluence for 48 h prior to assays, to ensure similar cell numbers for each experiment. Confluent monolayers were incubated with log-phase grown bacteria at an MOI of 1 or 10, and centrifuged at 1600 rpm for 5 min to initiate contact. After 2 h incubation, the monolayers were washed, and 1 mL of media containing 100 µg of gentamicin and 5 µg of penicillin G was added for an additional 2 h. After washing, monolayers were disrupted by 0.025% Triton X-100, and the number of invasive bacteria was quantified by serial dilution plating. To assess the level of surface-adherent (total cell-associated) bacteria, bacteria were quantified after 30 min of incubation without addition of antibiotics. All cellular adherence and invasion assays were performed in triplicate and repeated at least 2–3 times.
RNA interference
Human ext2 lentiviral shRNA construct (TRCN0000039849) was purchased from Open Biosystems. The resulting viruses were produced by cotransfection of 293T cells with the shRNA plasmid and packaging vectors (Open Biosystems) according to the vendor's instruction. The knockdown efficiency was determined by qRT-PCR analysis of ext2 expression. Primers used for qRT-PCR were EXT2 forward, 5′-AAGCACCAGGTCTTCGATTACC-3′ and reverse, 5′-GAAGTACGCTTCCCAGAACCA-3′. and GAPDH forward 5′-GAAGGTGAAGGTCGGAGTCAACG-3′ and reverse 5′-TCCTGGAGGATGGTGATGGAAT-3′.
Mouse infection model
Ext2 heterozygous and littermate controls (10–12 weeks) were injected via the tail vein with 108 cfu A909. Twenty-four h after injection, samples of blood, brain/meninges, and spleen were collected aseptically from mice after euthanasia. Bacterial counts in blood and tissue homogenates were determined by plating serial dilutions. Bacterial counts in brain and spleen samples were corrected for differences in organ weight. Brain bacterial counts were corrected for blood contamination using the blood concentration and a conservative estimate of the mouse cerebral blood volume [47]. In a pilot experiment, additional Ext2 heterozygous and littermate controls were injected intravenously with 1×108 cfu of WT GBS and lungs harvested at 24 h for cfu determination. The significance of differences between treatment groups was determined using the unpaired Student t test.
Supporting Information
Zdroje
1. OrganizationWH 2004 The World Health Report 2004: changing history
2. EdwardsMSRenchMAHaffarAAMurphyMADesmondMM 1985 Long-term sequelae of group B streptococcal meningitis in infants. J Pediatr 106 717 722
3. StevensJPEamesMKentAHalketSHoltD 2003 Long term outcome of neonatal meningitis. Arch Dis Child Fetal Neonatal Ed 88 F179 184
4. GalizaEPHeathPT 2009 Improving the outcome of neonatal meningitis. Curr Opin Infect Dis 22 229 234
5. BerardiALugliLRossiCChinaMCVellaniG 2010 Neonatal bacterial meningitis. Minerva Pediatr 62 51 54
6. SchwartzBSchuchatAOxtobyMJCochiSLHightowerA 1991 Invasive group B streptococcal disease in adults. A population-based study in metropolitan Atlanta. JAMA 266 1112 1114
7. PharesCRLynfieldRFarleyMMMohle-BoetaniJHarrisonLH 2008 Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 299 2056 2065
8. SkoffTHFarleyMMPetitSCraigASSchaffnerW 2009 Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin Infect Dis 49 85 92
9. KimKS 2008 Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol 6 625 634
10. MaiseyHCDoranKSNizetV 2008 Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev Mol Med 10 e27
11. BishopJRSchukszMEskoJD 2007 Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446 1030 1037
12. BernfieldMGotteMParkPWReizesOFitzgeraldML 1999 Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68 729 777
13. EskoJDLindahlU 2001 Molecular diversity of heparan sulfate. J Clin Invest 108 169 173
14. RostandKSEskoJD 1997 Microbial adherence to and invasion through proteoglycans. Infect Immun 65 1 8
15. BulowHEHobertO 2006 The molecular diversity of glycosaminoglycans shapes animal development. Annu Rev Cell Dev Biol 22 375 407
16. EskoJDSelleckSB 2002 Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 71 435 471
17. LawrenceROlsonSKSteeleREWangLWarriorR 2008 Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling. J Biol Chem 283 33674 33684
18. NakatoHKimataK 2002 Heparan sulfate fine structure and specificity of proteoglycan functions. Biochim Biophys Acta 1573 312 318
19. NybakkenKPerrimonN 2002 Heparan sulfate proteoglycan modulation of developmental signaling in Drosophila. Biochim Biophys Acta 1573 280 291
20. ShiehMTWuDunnDMontgomeryRIEskoJDSpearPG 1992 Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol 116 1273 1281
21. WuDunnDSpearPG 1989 Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol 63 52 58
22. PetheKAlonsoSBietFDeloguGBrennanMJ 2001 The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 412 190 194
23. Alvarez-DominguezCVazquez-BolandJACarrasco-MarinELopez-MatoPLeyva-CobianF 1997 Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect Immun 65 78 88
24. BolducGRBaronMJGravekampCLachenauerCSMadoffLC 2002 The alpha C protein mediates internalization of group B Streptococcus within human cervical epithelial cells. Cell Microbiol 4 751 758
25. BaronMJBolducGRGoldbergMBAuperinTCMadoffLC 2004 Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. J Biol Chem 279 24714 24723
26. BaronMJFilmanDJPropheteGAHogleJMMadoffLC 2007 Identification of a glycosaminoglycan binding region of the alpha C protein that mediates entry of group B streptococci into host cells. J Biol Chem 282 10526 10536
27. KocksCChoJHNehmeNUlvilaJPearsonAM 2005 Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 123 335 346
28. AyresJSFreitagNSchneiderDS 2008 Identification of Drosophila mutants altering defense of and endurance to Listeria monocytogenes infection. Genetics 178 1807 1815
29. StoltzDAOzerEATaftPJBarryMLiuL 2008 Drosophila are protected from Pseudomonas aeruginosa lethality by transgenic expression of paraoxonase-1. J Clin Invest 118 3123 3131
30. SibleyCDDuanKFischerCParkinsMDStoreyDG 2008 Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog 4 e1000184
31. BaronMJWongSLNybakkenKCareyVJMadoffLC 2009 Host glycosaminoglycan confers susceptibility to bacterial infection in Drosophila melanogaster. Infect Immun 77 860 866
32. ParkerRJAuldVJ 2006 Roles of glia in the Drosophila nervous system. Semin Cell Dev Biol 17 66 77
33. StorkTEngelenDKrudewigASiliesMBaintonRJ 2008 Organization and function of the blood-brain barrier in Drosophila. J Neurosci 28 587 597
34. MayerFMayerNChinnLPinsonneaultRLKroetzD 2009 Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J Neurosci 29 3538 3550
35. EdwardsTNMeinertzhagenIA 2010 The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol 90 471 497
36. IzumikawaTEgusaNTaniguchiFSugaharaKKitagawaH 2006 Heparan sulfate polymerization in Drosophila. J Biol Chem 281 1929 1934
37. TakeiYOzawaYSatoMWatanabeATabataT 2004 Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development 131 73 82
38. ToyodaHKinoshita-ToyodaAFoxBSelleckSB 2000 Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu. Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. J Biol Chem 275 21856 21861
39. KimKS 2006 Microbial translocation of the blood-brain barrier. Int J Parasitol 36 607 614
40. BobardtMDSalmonPWangLEskoJDGabuzdaD 2004 Contribution of proteoglycans to human immunodeficiency virus type 1 brain invasion. J Virol 78 6567 6584
41. FlorisSvan den BornJvan der PolSMDijkstraCDDe VriesHE 2003 Heparan sulfate proteoglycans modulate monocyte migration across cerebral endothelium. J Neuropathol Exp Neurol 62 780 790
42. KimCWGoldbergerOAGalloRLBernfieldM 1994 Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell 5 797 805
43. LeongJMWangHMagounLFieldJAMorrisseyPE 1998 Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect Immun 66 994 999
44. StickensDZakBMRougierNEskoJDWerbZ 2005 Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development 132 5055 5068
45. TenenbaumTSpellerbergBAdamRVogelMKimKS 2007 Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect 9 714 720
46. MaiseyHCHenslerMNizetVDoranKS 2007 Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J Bacteriol 189 1464 1467
47. DoranKSEngelsonEJKhosraviAMaiseyHCFedtkeI 2005 Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J Clin Invest 115 2499 2507
48. van SorgeNMQuachDGurneyMASullamPMNizetV 2009 The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the blood-brain barrier. J Infect Dis 199 1479 1487
49. DoranKSLiuGYNizetV 2003 Group B streptococcal β-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest 112 736 744
50. McCormickCDuncanGGoutsosKTTufaroF 2000 The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc Natl Acad Sci U S A 97 668 673
51. SenayCLindTMugurumaKToneYKitagawaH 2000 The EXT1/EXT2 tumor suppressors: catalytic activities and role in heparan sulfate biosynthesis. EMBO Rep 1 282 286
52. KamimuraKKoyamaTHabuchiHUedaRMasuM 2006 Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. J Cell Biol 174 773 778
53. KamimuraKRhodesJMUedaRMcNeelyMShuklaD 2004 Regulation of Notch signaling by Drosophila heparan sulfate 3-O sulfotransferase. J Cell Biol 166 1069 1079
54. StanfordKIWangLCastagnolaJSongDBishopJR 2010 Heparan sulfate 2-O-sulfotransferase is required for triglyceride-rich lipoprotein clearance. J Biol Chem 285 286 294
55. SugayaNHabuchiHNagaiNAshikari-HadaSKimataK 2008 6-O-sulfation of heparan sulfate differentially regulates various fibroblast growth factor-dependent signalings in culture. J Biol Chem 283 10366 10376
56. TonnaerELHafmansTGVan KuppeveltTHSandersEAVerweijPE 2006 Involvement of glycosaminoglycans in the attachment of pneumococci to nasopharyngeal epithelial cells. Microbes Infect 8 316 322
57. de VriesFPColeRDankertJFroschMvan PuttenJP 1998 Neisseria meningitidis producing the Opc adhesin binds epithelial cell proteoglycan receptors. Mol Microbiol 27 1203 1212
58. Behling-KellyEVonderheidHKimKSCorbeilLBCzuprynskiCJ 2006 Roles of cellular activation and sulfated glycans in Haemophilus somnus adherence to bovine brain microvascular endothelial cells. Infect Immun 74 5311 5318
59. MenozziFDBischoffRFortEBrennanMJLochtC 1998 Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc Natl Acad Sci U S A 95 12625 12630
60. PortugalMITodeschiniARde LimaCSSilvaCAMohana-BorgesR 2008 Characterization of two heparan sulphate-binding sites in the mycobacterial adhesin Hlp. BMC Microbiol 8 75
61. CapilaILinhardtRJ 2002 Heparin-protein interactions. Angew Chem Int Ed Engl 41 391 412
62. HilemanREFrommJRWeilerJMLinhardtRJ 1998 Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays 20 156 167
63. PerriereNDemeusePGarciaEReginaADebrayM 2005 Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J Neurochem 93 279 289
64. WangLFusterMSriramaraoPEskoJD 2005 Endothelial heparan sulfate deficiency impairs L-selectin - and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol 6 902 910
65. FusterMMWangLCastagnolaJSikoraLReddiK 2007 Genetic alteration of endothelial heparan sulfate selectively inhibits tumor angiogenesis. J Cell Biol 177 539 549
66. SeppKJAuldVJ 2003 Reciprocal interactions between neurons and glia are required for Drosophila peripheral nervous system development. J Neurosci 23 8221 8230
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral ReleaseČlánek Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1Článek High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 6- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- The N-Terminus of the RNA Polymerase from Infectious Pancreatic Necrosis Virus Is the Determinant of Genome Attachment
- Evolutionary Analysis of Inter-Farm Transmission Dynamics in a Highly Pathogenic Avian Influenza Epidemic
- Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous Systems
- Endemic Dengue Associated with the Co-Circulation of Multiple Viral Lineages and Localized Density-Dependent Transmission
- The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral Release
- The -glycan Glycoprotein Deglycosylation Complex (Gpd) from Deglycosylates Human IgG
- The Lipid Transfer Protein CERT Interacts with the Inclusion Protein IncD and Participates to ER- Inclusion Membrane Contact Sites
- Induction of Noxa-Mediated Apoptosis by Modified Vaccinia Virus Ankara Depends on Viral Recognition by Cytosolic Helicases, Leading to IRF-3/IFN-β-Dependent Induction of Pro-Apoptotic Noxa
- Environmental Constraints Guide Migration of Malaria Parasites during Transmission
- HIV-1 Efficient Entry in Inner Foreskin Is Mediated by Elevated CCL5/RANTES that Recruits T Cells and Fuels Conjugate Formation with Langerhans Cells
- Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis
- Highly Pathogenic Avian Influenza Virus H5N1 Infects Alveolar Macrophages without Virus Production or Excessive TNF-Alpha Induction
- Uses Host Triacylglycerol to Accumulate Lipid Droplets and Acquires a Dormancy-Like Phenotype in Lipid-Loaded Macrophages
- Compensatory T Cell Responses in IRG-Deficient Mice Prevent Sustained Infections
- Detection of Inferred CCR5- and CXCR4-Using HIV-1 Variants and Evolutionary Intermediates Using Ultra-Deep Pyrosequencing
- A Dynamic Landscape for Antibody Binding Modulates Antibody-Mediated Neutralization of West Nile Virus
- HSV-2 Infection of Dendritic Cells Amplifies a Highly Susceptible HIV-1 Cell Target
- “Rational Vaccine Design” for HIV Should Take into Account the Adaptive Potential of Polyreactive Antibodies
- Impact of Endofungal Bacteria on Infection Biology, Food Safety, and Drug Development
- Infection Reduces B Lymphopoiesis in Bone Marrow and Truncates Compensatory Splenic Lymphopoiesis through Transitional B-Cell Apoptosis
- The Intrinsic Antiviral Defense to Incoming HSV-1 Genomes Includes Specific DNA Repair Proteins and Is Counteracted by the Viral Protein ICP0
- Molecular Interactions that Enable Movement of the Lyme Disease Agent from the Tick Gut into the Hemolymph
- Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1
- A Freeze Frame View of Vesicular Stomatitis Virus Transcription Defines a Minimal Length of RNA for 5′ Processing
- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Tipping the Balance: Secreted Oxalic Acid Suppresses Host Defenses by Manipulating the Host Redox Environment
- Bacteria-Induced Dscam Isoforms of the Crustacean,
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Insertion of an Esterase Gene into a Specific Locust Pathogen () Enables It to Infect Caterpillars
- A Role for TLR4 in Infection and the Recognition of Surface Layer Proteins
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
- Low CCR7-Mediated Migration of Human Monocyte Derived Dendritic Cells in Response to Human Respiratory Syncytial Virus and Human Metapneumovirus
- HIV/SIV Infection Primes Monocytes and Dendritic Cells for Apoptosis
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- Productive Parvovirus B19 Infection of Primary Human Erythroid Progenitor Cells at Hypoxia Is Regulated by STAT5A and MEK Signaling but not HIFα
- Identification of DNA-Damage DNA-Binding Protein 1 as a Conditional Essential Factor for Cytomegalovirus Replication in Interferon-γ-Stimulated Cells
- The Influenza Virus Protein PB1-F2 Inhibits the Induction of Type I Interferon at the Level of the MAVS Adaptor Protein
- Passively Administered Pooled Human Immunoglobulins Exert IL-10 Dependent Anti-Inflammatory Effects that Protect against Fatal HSV Encephalitis
- Infection of Induces Antifungal Immune Defenses
- Merozoite Invasion Is Inhibited by Antibodies that Target the PfRh2a and b Binding Domains
- Cyclic di-GMP is Essential for the Survival of the Lyme Disease Spirochete in Ticks
- Cross-Neutralizing Antibodies to Pandemic 2009 H1N1 and Recent Seasonal H1N1 Influenza A Strains Influenced by a Mutation in Hemagglutinin Subunit 2
- Spatial Dynamics of Human-Origin H1 Influenza A Virus in North American Swine
- Coronavirus Gene 7 Counteracts Host Defenses and Modulates Virus Virulence
- Clathrin Facilitates the Morphogenesis of Retrovirus Particles
- Contribution of Intrinsic Reactivity of the HIV-1 Envelope Glycoproteins to CD4-Independent Infection and Global Inhibitor Sensitivity
- Functional Analysis of Host Factors that Mediate the Intracellular Lifestyle of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



