-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Low CCR7-Mediated Migration of Human Monocyte Derived Dendritic Cells in Response to Human Respiratory Syncytial Virus and Human Metapneumovirus
Human respiratory syncytial virus (HRSV) and, to a lesser extent, human metapneumovirus (HMPV) and human parainfluenza virus type 3 (HPIV3), can re-infect symptomatically throughout life without significant antigenic change, suggestive of incomplete or short-lived immunity. In contrast, re-infection by influenza A virus (IAV) largely depends on antigenic change, suggestive of more complete immunity. Antigen presentation by dendritic cells (DC) is critical in initiating the adaptive immune response. Antigen uptake by DC induces maturational changes that include decreased expression of the chemokine receptors CCR1, CCR2, and CCR5 that maintain DC residence in peripheral tissues, and increased expression of CCR7 that mediates the migration of antigen-bearing DC to lymphatic tissue. We stimulated human monocyte-derived DC (MDDC) with virus and found that, in contrast to HPIV3 and IAV, HMPV and HRSV did not efficiently decrease CCR1, 2, and 5 expression, and did not efficiently increase CCR7 expression. Consistent with the differences in CCR7 mRNA and protein expression, MDDC stimulated with HRSV or HMPV migrated less efficiently to the CCR7 ligand CCL19 than did IAV-stimulated MDDC. Using GFP-expressing recombinant virus, we showed that the subpopulation of MDDC that was robustly infected with HRSV was particularly inefficient in chemokine receptor modulation. HMPV - or HRSV-stimulated MDDC responded to secondary stimulation with bacterial lipopolysaccharide or with a cocktail of proinflammatory cytokines by increasing CCR7 and decreasing CCR1, 2 and 5 expression, and by more efficient migration to CCL19, suggesting that HMPV and HRSV suboptimally stimulate rather than irreversibly inhibit MDDC migration. This also suggests that the low concentration of proinflammatory cytokines released from HRSV - and HMPV-stimulated MDDC is partly responsible for the low CCR7-mediated migration. We propose that inefficient migration of HRSV - and HMPV-stimulated DC to lymphatic tissue contributes to reduced adaptive responses to these viruses.
Published in the journal: . PLoS Pathog 7(6): e32767. doi:10.1371/journal.ppat.1002105
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002105Summary
Human respiratory syncytial virus (HRSV) and, to a lesser extent, human metapneumovirus (HMPV) and human parainfluenza virus type 3 (HPIV3), can re-infect symptomatically throughout life without significant antigenic change, suggestive of incomplete or short-lived immunity. In contrast, re-infection by influenza A virus (IAV) largely depends on antigenic change, suggestive of more complete immunity. Antigen presentation by dendritic cells (DC) is critical in initiating the adaptive immune response. Antigen uptake by DC induces maturational changes that include decreased expression of the chemokine receptors CCR1, CCR2, and CCR5 that maintain DC residence in peripheral tissues, and increased expression of CCR7 that mediates the migration of antigen-bearing DC to lymphatic tissue. We stimulated human monocyte-derived DC (MDDC) with virus and found that, in contrast to HPIV3 and IAV, HMPV and HRSV did not efficiently decrease CCR1, 2, and 5 expression, and did not efficiently increase CCR7 expression. Consistent with the differences in CCR7 mRNA and protein expression, MDDC stimulated with HRSV or HMPV migrated less efficiently to the CCR7 ligand CCL19 than did IAV-stimulated MDDC. Using GFP-expressing recombinant virus, we showed that the subpopulation of MDDC that was robustly infected with HRSV was particularly inefficient in chemokine receptor modulation. HMPV - or HRSV-stimulated MDDC responded to secondary stimulation with bacterial lipopolysaccharide or with a cocktail of proinflammatory cytokines by increasing CCR7 and decreasing CCR1, 2 and 5 expression, and by more efficient migration to CCL19, suggesting that HMPV and HRSV suboptimally stimulate rather than irreversibly inhibit MDDC migration. This also suggests that the low concentration of proinflammatory cytokines released from HRSV - and HMPV-stimulated MDDC is partly responsible for the low CCR7-mediated migration. We propose that inefficient migration of HRSV - and HMPV-stimulated DC to lymphatic tissue contributes to reduced adaptive responses to these viruses.
Introduction
The paramyxoviruses human respiratory syncytial virus (HRSV), human metapneumovirus (HMPV) and human parainfluenza virus type 3 (HPIV3) are common respiratory pathogens. HRSV is the most important viral agent of severe pediatric respiratory tract disease worldwide [1], [2], followed by HPIV3 [3], [4] and HMPV [5], [6], [7], [8]. The orthomyxovirus influenza virus type A (IAV) infects and causes respiratory tract disease in all age groups [9], [10], [11].
These human respiratory viruses share a tropism for the respiratory epithelium and have overlapping spectra of disease, ranging from rhinitis to bronchiolitis and pneumonia [12], [13]. IAV usually induces long-term immunity following infection, such that re-infection depends on significant antigenic change [14], [15]. In contrast, HMPV, HRSV and HPIV3 are able to symptomatically re-infect humans throughout life without significant antigenic change. This is particularly common with HRSV. Glezen and colleagues followed children from birth, and found that more than two-thirds were infected with HRSV during the first year of life, and almost half of these individuals were re-infected during each of the next two years [16]. In experimental infections of adults, typically 50–80% of subjects are re-infected with HRSV, and the majority has acute illness [17]. In another study, adults were challenged at intervals of 2–6 months over a period of 26 months with the same HRSV isolate, with the result that 73% were infected at least twice and 43% at least three times, and more than half of these infections were symptomatic [18]. These observations have been widely interpreted to indicate that HRSV in particular blunts or skews the immune response, resulting in suboptimal protection.
Antigen-presenting dendritic cells (DC) are critical for a functional adaptive immune response. During a lower respiratory tract infection, the number of DC in the bronchi and lung increases by chemotactic influx of precursors that originate primarily from circulating monocytes [19], [20], [21], [22]. Migration to non-lymphoid peripheral tissues such as the lung is mediated by so called “inflammatory” chemokine receptor-ligand pairs, including CCR1-CCL3/MIP-1α, CCR2-CCL2/MCP-1 or CCR5-CCL5/RANTES. Exposure of DC to antigen in peripheral tissue initiates DC maturation. During maturation, DC increase the surface expression of co-stimulatory molecules such as CD38, CD40, CD80 CD86, and CD83 [23], [24]. DC also change their expression of cell surface chemokine receptors: expression of CCR1, CCR2, and CCR5 is reduced, reducing responsiveness to inflammatory chemokines, and expression of CCR7 is increased [25], [26]. CCR7 has two specific ligands, CCL19 and CCL21, which are expressed by endothelial cells in lymphatic venules, in high endothelial venules (HEV) in lymph nodes, and in the T cell zone of lymphoid organs [27], [28], [29]. CCL19 and CCL21 direct migration of maturing, CCR7-expressing DC through the afferent lymphatics to the draining lymph nodes, and control DC positioning within defined functional lymphoid compartments [25], [26], [30], [31] for efficient interaction with naïve and/or antigen-specific memory T lymphocytes. DC have a key role in determining the magnitude and quality of the adaptive immune response.
We previously reported that HMPV, HRSV, and HPIV3 induce low-to moderate levels of human monocyte-derived dendritic cell (MDDC) maturation, cytokine/chemokine expression, and CD4 T cell proliferation, with the magnitude increasing slightly in the order HRSV, HMPV, and HPIV3 [32], [33]. MDDC generated in vitro from primary human monocytes by treatment with IL-4 and GM-CSF represent an appropriate model for lung DC because monocytes give rise to myeloid DC in the resting lung [34] and mucosa [35], and are phenotypically and functionally similar to DC located at sites of inflammation in vivo [36]. In the present study, we expanded our previous findings by screening MDDC for expression of genes related to maturation in response to HMPV, HPIV3, HRSV and, for comparison, IAV. We found that CCR7 mRNA and protein expression were substantially increased in response to HPIV3 and IAV, but minimally increased in response to HMPV and HRSV. These differences detected by qRT-PCR and flow cytometry were functionally relevant, since MDDC stimulated with HMPV or HRSV were less efficient in their migration along a CCR7 concentration gradient than IAV - and HPIV3 stimulated MDDC. Secondary stimulation of HRSV - or HMPV-exposed MDDC with the strong DC activator LPS enhanced CCR7 expression and in vitro migration, suggesting that suboptimal stimulation, rather than inhibition, is responsible for this poor-migration phenotype. Finally, we provide evidence that low CCR7 expression by MDDC in response to HRSV and HMPV is at least partly due to the low level of expression of pro-inflammatory cytokines (TNF-α, IL-1α and IL-6).
Materials and Methods
Ethics statement
Elutriated monocytes were obtained from healthy donors at the Department of Transfusion Medicine of the National Institutes of Health, under a protocol (99-CC-0168) approved by the IRB of the Clinical Center, NIH. Written informed consent was obtained from all donors.
Virus stock preparation
Recombinant (r) HMPV (strain CAN97-83), rHRSV (strain A2) and rHPIV3 (strain JS) with or without the GFP gene were described previously [12], [37], [38]. The present study employed a genetically “stabilized” version of rHMPV, in which the SH gene was modified to silently remove tracts of A and T residues that had been sites of spontaneous mutations during passage in vitro [39]. Human Influenza/A/Udorn/72, a wildtype virus of subtype H3N2, was used as control.
All viruses were grown on Vero cells and purified by centrifugation through sucrose step gradients as described previously [32]. Sucrose purified viruses were pelleted by centrifugation to remove sucrose. Virus pellets were resuspended in Advanced RMPI 1640 (Invitrogen, Carlsbad, CA) supplemented with 2 mM L-glutamine (aRPMI), and aliquots were snap frozen and stored at −80°C until use. Virus titers were determined by immuno-plaque assay on Vero cells under methylcellulose overlay (containing trypsin for titration of rHMPV and IAV) as described previously [37]. In some experiments, UV-inactivated viruses were included as controls which were prepared using a Stratalinker UV cross-linker (Agilent, Santa Clara, CA) at 0.5 J/cm2. Complete inactivation was monitored by plaque assay (limit of detection: 5 plaque forming units per mL).
Generation of immature monocyte-derived DC
Elutriated monocytes were obtained from healthy donors at the Department of Transfusion Medicine of the National Institutes of Health, under a protocol (99-CC-0168) approved by the IRB of the Clinical Center, NIH. As previously described [32], monocytes were subjected to CD14+ sorting on an Automacs separator (Miltenyi Biotec, Auburn CA), and cultured in presence of recombinant human IL-4 (R&D Systems, Minneapolis, MN) and recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF, Bayer Healthcare, Wayne, NJ) for 7 days to generate immature MDDC. These were confirmed by flow cytometry to be CD14−, CD38−, CD80low, CD86low, CD40low, CD54low.
MDDC treatments
Immature MDDC were seeded in 12-well plates at 6×105 cells per well and were infected with live virus at an MOI of 3 PFU/cell, or with an equivalent amount of UV-inactivated virus, stimulated with 1 µg/ml of the superantigen Staphylococcus enterotoxin B (SEB; Sigma, St Louis, MO) or with 1 µg/ml of lipopolysaccharide (LPS) from Escherichia Coli O55:B5 (Sigma). The infectivity of rgHMPV, rgHRSV and rgHPIV3 for MDDC was similar (approximately 3–5% of GFP+ MDDC at 24 or 48 h post infection, no significant differences at the p≤0.05 confidence level for any of the data sets of this study). In some experiments, immature MDDC infected with rgHMPV, rgHRSV, rgHPIV3 at an input MOI of 3 PFU/cell were further stimulated 4 to 6 h later with 1 µg/ml of LPS or 150 IU of Interferon (IFN)-β (PBL Interferon source, Piscataway, NJ) or with a cocktail of pro-inflammatory cytokines of 6 ng/ml TNF-α, 10 ng/ml IL-6 and 0.36 ng/ml IL-1α (R&D systems). All inoculations or stimulations were performed in advanced RMPI 1640 (Invitrogen) supplemented with 10% heat-inactivated FBS (Hyclone, Logan, UT), 2 mM L-glutamine (Invitrogen), 200 U/ml penicillin, and 200 µg/ml streptomycin (Invitrogen) at 37°C in 5% CO2.
Reverse transcription and quantitative PCR
Cell-associated RNA was isolated using the RNeasy mini kit (Qiagen) as recommended by the manufacturer and treated with DNAse I to remove residual genomic DNA. Analysis was done in two ways. The first involved a custom made low-density Taqman gene array containing 62 genes. Here, 1 µg of isolated RNA was reverse transcribed using SuperScript II (Invitrogen) in a 50 µl mix using random primers, and each cDNA mix was loaded onto an array in triplicate. The second method involved individual RT-PCR reactions. Here, 600 ng of isolated RNA was reverse transcribed using superscript II (Invitrogen) in a 25 µl mix using random primers. The reverse transcription product was diluted three-fold, and two µl of the diluted cDNA mix were used in each quantitative TaqMan PCR (Applied Biosystems, CA) for quantification of the targets of interest, namely CCR1 (Hs00174298_m1), CCR5 (Hs00152917_m1) and CCR7 (Hs00171054_m1). qPCR results were analyzed using the comparative threshold cycle (ΔΔCT) method, normalized to 18S rRNA and expressed as fold change over mock.
Flow cytometry analysis of CCR1, 2, 5 and 7 expression
To determine the surface expression level of chemokine receptors, cells were stained with allophycocyanin (APC)-conjugated anti-human mAbs [anti-CCR1 (CD191, clone 53504), anti-CCR2 (CD192, clone 48607), anti-CCR5 (CD195, clone 2D7), anti-CCR7 (CD197, clone 2H4) (BD Biosciences, San Jose, CA)]. Isotype-matched mAbs were included as controls. Propidium iodide staining was used to exclude dead cells from further analysis. At 48 h post infection, the median viability of MDDC from six independent experiments was 85% for HMPV-, 86% for HRSV-, and 82% for HPIV3-exposed MDDC, reflecting the anti-apoptotic effects of virus-induced DC maturation [32]. In order to avoid interference, CCR1, 2, 5 and 7 expression was analyzed individually. At least 20,000 events were acquired using a FACSCalibur flow cytometer (BD Biosciences) and analyzed using FlowJo version 8.8.6 software (Tree Star, Inc., Ashland, OR).
Chemotaxis assay
After 48 h stimulation, migration of the virus-stimulated MDDC in response to a CCL19 concentration gradient was evaluated using polycarbonate 5-µm diameter pore size transwells (Corning, Lowell, MA). 1×105 live MDDC were seeded in the upper chamber, and incubated in presence or absence of CCL19 (1 µg/ml, (R&D Systems, Minneapolis, MN) in the lower chamber. Duplicate wells were used for each condition. After 3 h incubation, MDDC from the lower chamber were harvested, and the cell density of live cells was determined using a FACS Calibur flow cytometer (BD Biosciences). For each sample, data acquisition was performed for 1 min at constant flow using 200 µl final volume. Forward scatter, side scatter, live/dead staining, and GFP expression were analyzed using FlowJo version 8.8.6 software (Tree Star, Inc., Ashland, OR). The average number of MDDC specifically migrating in response to CCL19 was calculated as follows: (Average number of stimulated MDDC migrated in the presence of CCL19) – (Average number of stimulated MDDC migrated in the absence of CCL19).
Statistical analysis
Data sets were assessed for significance using parametric one-way repeated measures ANOVA with the Tukey post hoc tests for normally distributed data sets or the non-parametric Friedman test with Dunns post hoc test. A log10 transformation was applied to data sets when necessary to obtain equal standard deviation among groups, a necessary requirement of both tests. Statistics were performed using Prism 5 (GraphPad Software, Inc, San Diego, CA). Data were only considered significant at P<0.05. Analysis of CCR5 and CCR7 expression: To account for the smaller data set of the IAV control (n = 8 donors, except for IAV, n = 6 donors), data were analyzed using an unbalanced repeated measures ANOVA (JMP version 8.0.2; SAS, Cary, NC).
Results
Gene expression survey of human MDDC stimulated with rHMPV, rHRSV, rHPIV3 and IAV
We used RT-qPCR to survey maturation-related gene expression in MDDCs from 3 donors 24 h after exposure to either the superantigen SEB or to purified live or UV-inactivated rHRSV, rHMPV, rHPIV3, or IAV (Fig. S1A and B). In general, all four live viruses induced the up-regulation of the same array of genes but differed in the intensity of up-regulation increasing in the order rHRSV<rHMPV<IAV<rHPIV3. The donors also had substantial responses to UV-inactivated IAV, but weak responses to UV-inactivated rHMPV, rHRSV or rHPIV3. Donors 1 and 2 were refractory to stimulation by rHMPV and rHRSV, respectively.
Among the genes surveyed, expression of CCR7 mRNA was substantially increased in response to IAV and rHPIV3, but not in response to rHMPV and rHRSV (Fig. S1A). Based on these preliminary results, we analyzed CCR7 mRNA expression by qPCR in additional donors (total n = 9, Fig. 1), and found that, while IAV and HPIV3 induced a strong increase of CCR7 mRNA (median increases of 23-fold and 7.2 fold, respectively), HRSV and HMPV only induced a 2.2 - and 2.5-fold increase compared to mock treated cells. The effects of HMPV and HRSV on CCR7 expression were significantly smaller compared to HPIV3 and IAV (Fig. 1). By contrast, expression of CCR1 and CCR5 mRNA was increased in response to all viruses, but without any statistical difference between the viruses, except that the CCR5 mRNA expression was significantly different between rHPIV3 and IAV (Fig. 1). Because CCR7 has a unique role in DC migration towards lymph nodes and the subsequent adaptive response [26], we explored the effect of these viruses on MDDC chemokine receptor expression and migration.
Fig. 1. CCR1, 5 and 7 gene expression of MDDC stimulated with rHMPV, rHRSV, rHPIV3, or IAV. 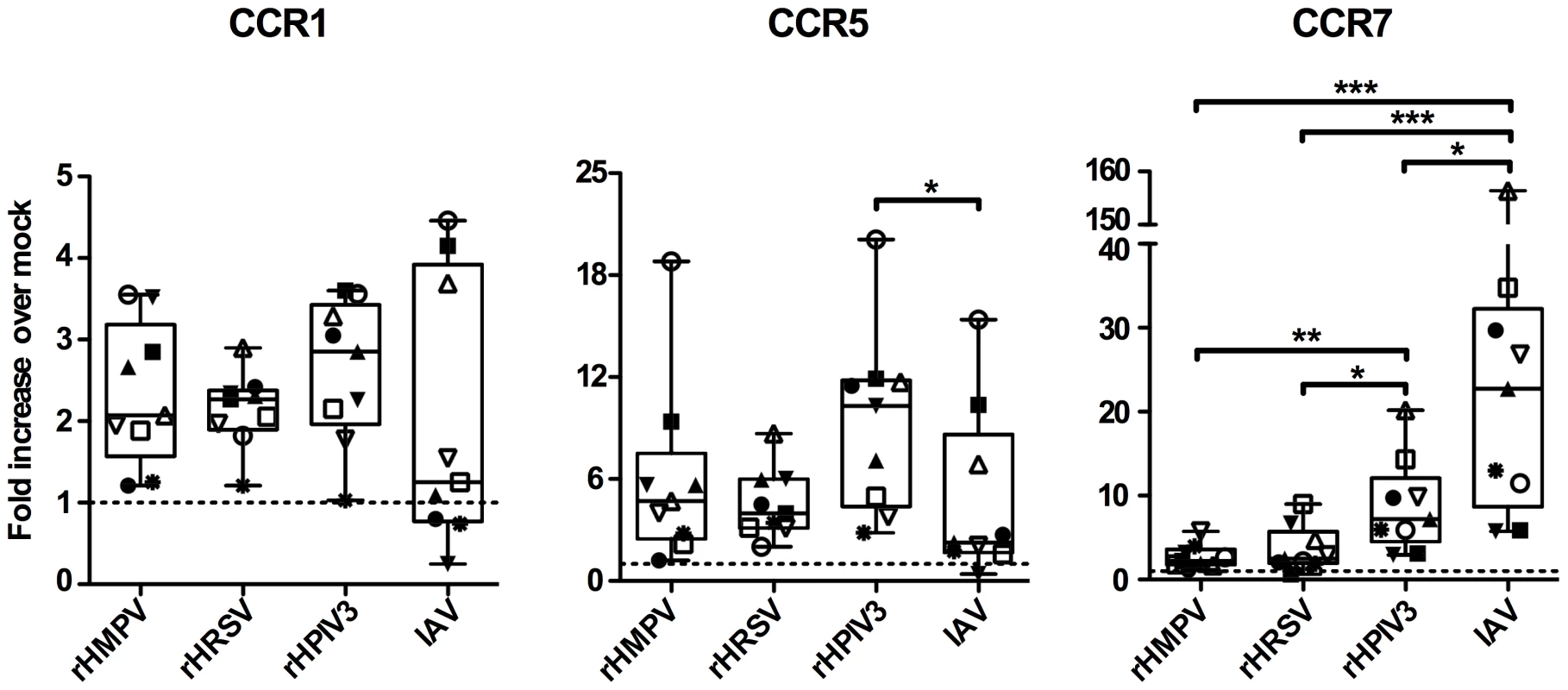
Immature MDDC (n = 9 donors) were infected with rHMPV, rHRSV, rHPIV3, or influenza/A/Udorn (IAV). Twenty-four h post infection, total cellular RNA was prepared and reverse-transcribed using random primers, and the cDNA analyzed in triplicate by qPCR using TaqMan PCR assays. qPCR results were analyzed using the comparative threshold cycle (ΔΔCT) method, normalized to 18S rRNA. The results are expressed as fold-increase over mock. Statistical differences are indicated by asterisks (* P≤0.05, ** P≤0.01, *** P≤0.001, Materials and Methods). MDDC treated with rgHMPV or rgHRSV do not efficiently change cell surface chemokine receptor expression
We next used flow cytometry to measure surface expression of CCR1, 2, 5, and 7 on MDDC 48 h after exposure to the different viruses (Fig. 2). We included CCR2 in this analysis since, like CCR1 and CCR5, it directs monocytes and DC to inflamed tissue and is down-regulated during DC maturation. LPS was used as positive control because it strongly activates DC [40], [41]. Fig. 2A shows primary data for a representative donor, and Fig. 2B–C show the compiled results for six to eight donors. In this and all subsequent experiments, we used versions of rHMPV, rHRSV, and rHPIV3 that express GFP from an added gene (rgHMPV, rgHRSV, and rgHPIV3, respectively).
Fig. 2. Cell surface expression of the chemokine receptors CCR1, 2, 5 and 7. 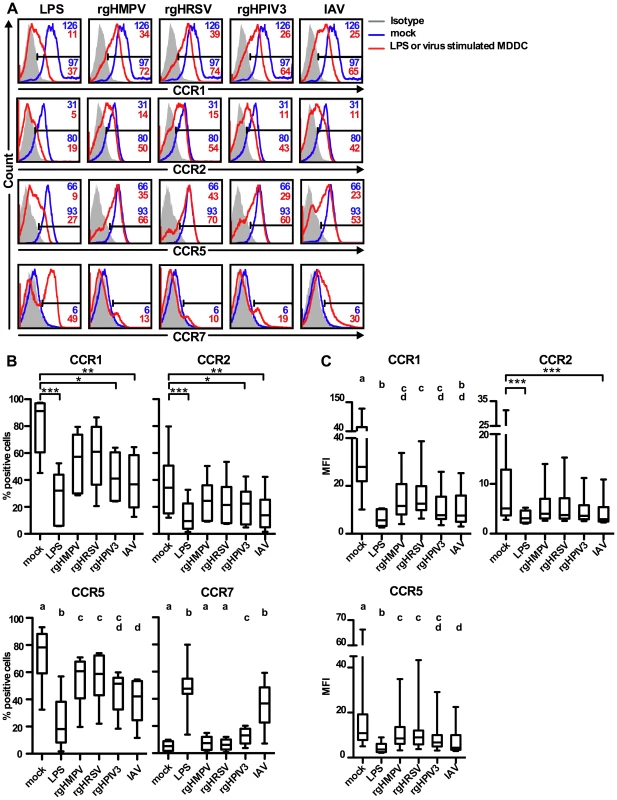
Immature MDDC were stimulated with LPS or infected with rgHMPV, rgHRSV, rgHPIV3, or influenza/A/Udorn (IAV) at an MOI of 3 PFU/cell. 48 h post-infection, the surface expression levels of CCR1, 2, 5 and 7 were assessed by flow cytometry. (A) Surface expression of CCR1, 2, 5, and 7 from a representative donor. The MFI (top right corner) and % positive (bottom right) of each cell population are indicated for each histogram, with red indicating the treated population and blue indicating the mock-treated control. Note that values of MFI are not given in the case of CCR7 because only a small percentage of treated MDDC expressed this molecule at the cell surface. (B, C) Summary of data expressed as % positive cells (B) and as MFI (C). MFI is not shown for CCR7, as explained for (A). CCR1, 2: n = 6 donors; CCR5, 7: n = 8 donors, except n = 6 donors for IAV. The box plots show the median (horizontal line), flanked by the 2nd and 3rd quartile. The outer bars show the range of values. Statistical differences are indicated by asterisks (* P≤0.05, ** P≤0.01, *** P≤0.001, Materials and Methods). CCR5 and 7 expression: To account for the smaller data set of the IAV control (n = 8 donors, except for IAV, n = 6 donors), data were analyzed using an unbalanced repeated measures ANOVA (JMP 8.0.2; SAS, Cary, NC). Treatments sharing the same lower case letters do not differ significantly at the p≤0.05 confidence level, see Materials and Methods. In mock-treated MDDC, substantial subpopulations of cells expressed CCR1 (median 91% of total), CCR2 (34%), and CCR5 (75%), and were CCR7-negative or low (Fig. 2A, B, C). High CCR1/2/5 and low CCR7 values would be typical for immature DC residing in peripheral tissue. As expected, LPS treatment induced a significant down-regulation of CCR1 (32%), CCR2 (9%), and CCR5 (24%), and up-regulation of CCR7 (48% positive cells) (Fig. 2A, B, C). Stimulation of MDDC with rgHPIV3 or IAV also induced a significant decrease in frequency of cells expressing the inflammatory chemokine receptors CCR1, 2, and 5 compared to mock-treated cells (Fig. 2B). However, only IAV significantly decreased all median fluorescence intensities (MFIs) (Fig. 2C).
In contrast, stimulation with rgHMPV or rgHRSV had only moderate effects on chemokine receptor surface expression. Cell surface expression of CCR1 and 2 decreased after stimulation with rgHRSV and rgHMPV, but the difference compared to mock-treated MDDC was not significant (Fig. 2B and C), except for the MFI of CCR1 (Fig. 2C). Stimulation with rgHMPV or rgHRSV reduced the percentage of CCR5+ MDDC significantly compared to mock-treated MDDC, but treatment with IAV reduced CCR5 expression significantly more than rgHMPV or rgHRSV treatment (Fig. 2B). CCR5 expression of rgHPIV3 stimulated MDDC was intermediate between HMPV and HRSV on the one hand, and IAV on the other hand, with no significant differences to any of the viruses (Fig. 2C).
The limited down-regulation of CCR1, 2, and 5 in response to rgHMPV and rgHRSV was coupled with a weak increase of CCR7 expression occurring on only a small subpopulation of cells (Fig. 2B, median 7% CCR7+ cells for for rgHMPV, and 6% for rgHRSV, with no statistical difference to mock). Stimulation with rgHPIV3 or IAV was associated with a significantly stronger up-regulation of CCR7 than mock, rgHMPV or rgHRSV stimulation, resulting in 13% and 37% CCR7+ cells, respectively.
Taken together, these results showed that compared to LPS, IAV, and rgHPIV3, stimulation with rgHMPV and rgHRSV induced a smaller down-regulation of surface expression of CCR1, CCR2, and CCR5, and a smaller up-regulation of CCR7 surface expression occurring on a smaller fraction of cells.
MDDC treated with HMPV or HRSV migrate poorly to the CCR7 ligand CCL19
We asked whether the lower level of CCR7 surface expression by MDDC stimulated with rgHRSV or rgHMPV affected their migration in response to the CCR7 ligand CCL19. MDDC were stimulated with LPS or the various live or UV-inactivated viruses and assayed for the ability to migrate along a CCL19 concentration gradient in a trans-well system (Fig. 3). As expected, LPS-stimulated MDDC migrated well, while mock and UV-inactivated virus-stimulated MDDC migrated poorly towards CCL19. There was a small (but not statistically significant) increase in migration for UV-IAV stimulated MDDC (between 302 and 1417 specifically migrating MDDC), commensurate with the slight increase in CCR7 mRNA expression in two out of 3 donors after exposure to UV-inactivated IAV virus (Fig. S1A). Complete UV inactivation of IAV and the other viruses had been verified by titration. Thus, the small stimulatory effect of UV-IAV could not be attributed to partial inactivation of IAV.
Fig. 3. MDDC stimulated with IAV migrate more efficiently to a CCL19 concentration gradient than HMPV- or HRSV-stimulated MDDC. 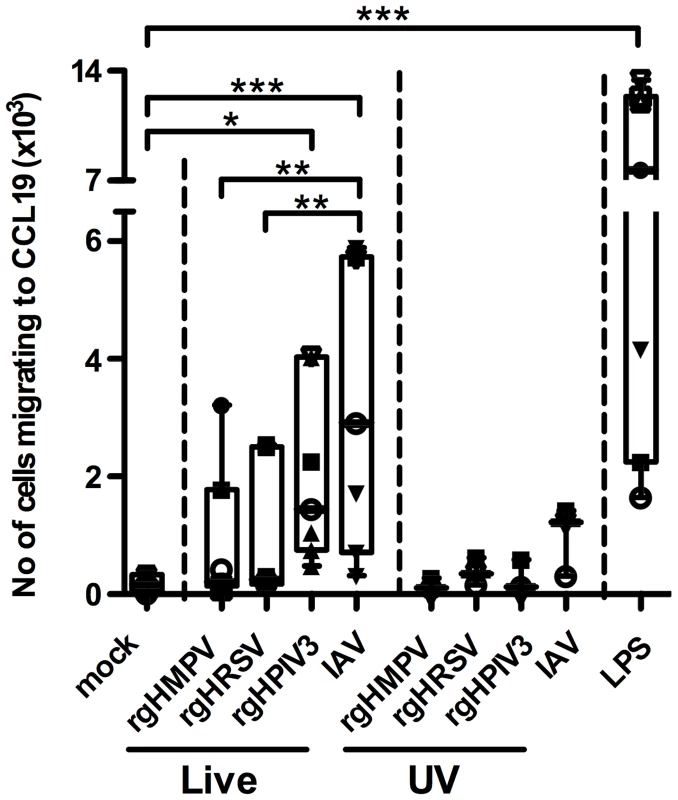
Immature MDDC were stimulated with LPS or infected with rgHMPV, rgHRSV, rgHPIV3, IAV, or with their UV-counterparts and, 48 h following stimulation, were assessed for the ability to migrate in vitro to a CCL19 concentration gradient. This was done using transwell cultures containing a polycarbonate filter with a pore diameter of 5 µm. 1×105 live MDDC were seeded in the upper chamber and incubated in presence or absence of CCL19 in the lower chamber. After 3 h, MDDC from the lower chamber were harvested, and the cell density was determined by flow cytometry. Data were expressed as the average number of MDDC migrating specifically to a CCL19 concentration gradient, calculated as follows: (Average number of stimulated MDDC migrating in the presence of CCL19) – (Average number of stimulated MDDC migrating in the absence of CCL19). The median number of MDDC migrating in absence of CCL19 for the seven analyzed donors was 207, and thus the background was low. The box plots show the median (horizontal line), flanked by the 2nd and 3rd quartile. The outer bars show the range of values. Each donor is represented by an individual symbol. n = 7 donors except for the UV-inactivated viruses, where n = 3 donors. Statistical differences are indicated by asterisks (* P≤0.05, ** P≤0.01, *** P≤0.001, Materials and Methods). Migration of MDDC towards CCL19 was increased for each of the live viruses. However, MDDC stimulated with live rgHMPV and rgHRSV migrated less efficiently towards CCL19 than cells stimulated with rgHPIV3 and IAV (medians: 202, 246, 1445, and 2903 specifically migrating MDDC, respectively). While rgHPIV3 - and IAV-stimulated MDDC migrated to CCL19 in significantly higher numbers than mock-stimulated MDDC, migration of rgHMPV - and rgHRSV-stimulated MDDC was not significantly different from that of mock-stimulated MDDC. Migration of IAV-stimulated MDDC was significantly greater than that of rgHRSV - and rgHMPV-stimulated MDDC (p<0.01; ANOVA/Tukey post hoc analysis comparing mock-stimulated MDDC with those stimulated with live virus). In summary, this assay showed that the weaker up-regulation of CCR7 mRNA and surface protein expression in response to rgHMPV and rgHRSV compared to IAV and LPS was associated with less efficient migration in response to CCL19.
Comparison of chemokine receptor expression on the GFP-positive versus GFP-negative subpopulations of virus-stimulated MDDC
We used flow cytometry to compare chemokine receptor surface expression on virus-exposed cells that were GFP-positive versus GFP-negative (Fig. 4). We previously showed that, following infection with rgHMPV, rgHRSV, or rgHPIV3 at an MOI of 3, approximately 3–5 % of MDDC were GFP+ at 24 or 48 h post-infection [32]. This was indicative of robust viral gene expression, which was confirmed by RT-qPCR. In the GFP – population, we detected a low level of viral gene expression, suggestive of abortive virus replication [32]. Thus, comparing host gene expression in GFP+ and GFP – cells provides an indication of the effects of a robust versus abortive infection. Fig. 4A shows primary data for GFP expression and CCR7 surface expression for a single donor, and Fig. 4B summarizes data for the expression of CCR1, 2, 5, and 7 for six donors.
Fig. 4. Chemokine receptor surface expression on GFP-positive versus GFP-negative MDDC following inoculation with rgHMPV, rgHRSV and rgHPIV3. 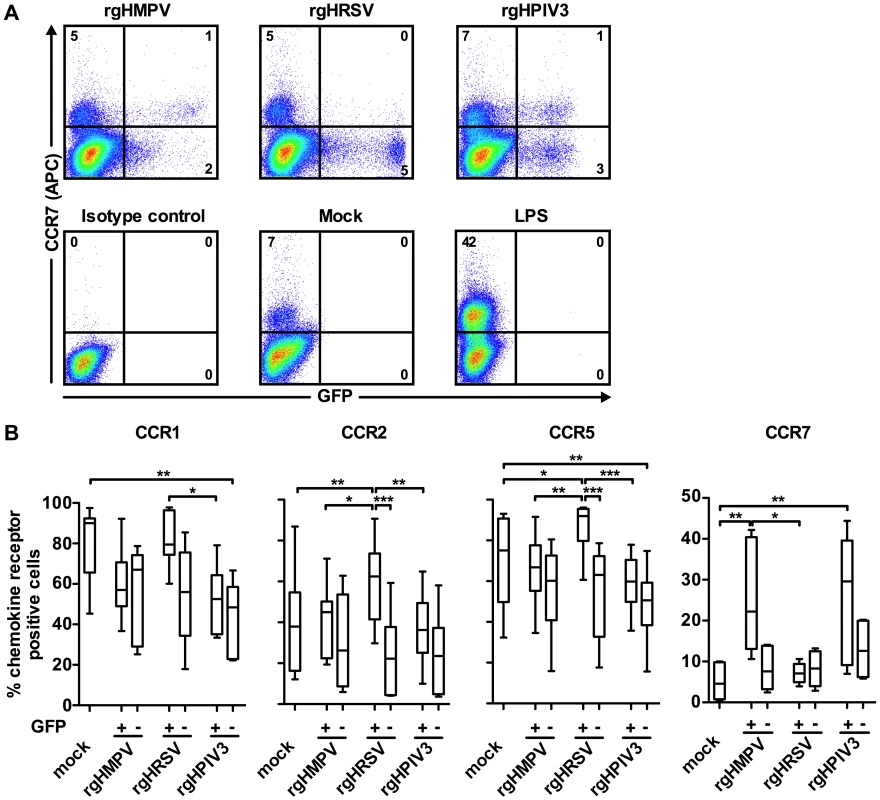
48 h after stimulation with LPS or infection with the indicated viruses, GFP+ and GFP− cells were analyzed by flow cytometry for cell surface expression of the indicated chemokine receptors. (A) Scatter plots of GFP expression and CCR7 surface staining from a typical donor and (B) summary of the data from n = 6 donors. (+) GFP+ population, (−) GFP− population. The box plots show the median (horizontal line), flanked by the 2nd and 3rd quartile. The outers bars show the range of values. Statistical differences are indicated by asterisks (* P≤0.05, ** P≤0.01, *** P≤0.001, Materials and Methods). Note that the statistical differences between the GFP+ cells from a given virus and the GFP− cells from a different virus are not shown. After treatment of MDDC with rgHMPV or rgHPIV3, the extent of down-regulation of CCR1, 2, and 5 was similar between the GFP+ and GFP − MDDC (Fig. 4). In contrast, after rgHRSV treatment, these receptors were decreased only in the GFP − cells; indeed, in the GFP+ cells, CCR2 and CCR5 expression was slightly increased compared to mock treated cells. Thus, robust rgHRSV gene expression did not induce the down-regulation of the inflammatory chemokine receptors CCR1, 2, and 5 that normally occurs as part of DC maturation.
CCR7 was expressed at higher levels in the GFP+ cells than in the GFP − cells after treatment with rgHMPV or rgHPIV3, indicating that robust infection by these viruses stimulated rather than inhibited expression (Fig. 4A and B). In contrast, CCR7 expression was not increased in either the GFP+ or the GFP − subpopulations of cells treated with rgHRSV.
The weak CCR7-driven migration of MDDC treated with rgHMPV and rgHRSV can be increased by secondary stimulation with pro-inflammatory cytokines or LPS
One possible explanation for the weak chemokine receptor modulation and migration by rgHMPV - and rgHRSV-treated MDDC was direct virus-mediated inhibition. Alternatively, it was possible that these viruses were insufficiently stimulatory, perhaps due to the low production of cytokines by virus-treated MDDC as described in our previous study [32]. We therefore investigated whether exposure of virus-stimulated MDDC to secondary stimulation with LPS or to higher concentrations of cytokines would result in more efficient chemokine receptor modulation and migration. We tested possible cytokine and IFN candidates based on the gene expression analysis described above (Fig. S1) and previously published data by ourselves and others [32], [42], [43], [44], [45], [46]. The individual additions of IFN-β, IL-28, IL-29, TNF-α, IL-1α, IL-6 and prostaglandin E2 to virus-treated MDDC had little or no effect on CCR7 mRNA levels or on the ability of MDDC to migrate to a CCL19 concentration gradient (data not shown). These preliminary results confirmed previously published data showing that CCR7 is not an IFN-regulated gene in human or mouse DC [47], [48], [49]. Thus, the poor up-regulation of CCR7 by rgHMPV and rgHRSV is unlikely to be the result of a more stringent IFN antagonism by these viruses.
We next tested the effect of a cocktail of pro-inflammatory cytokines containing TNF-α, IL-1α and IL-6 on chemokine receptor expression, with each cytokine in concentrations similar to those induced by LPS under our experimental conditions [32]. MDDC (n = 4 donors) were treated with rgHMPV or rgHRSV, and, 4–6 h later, received a secondary stimulation with the cocktail of pro-inflammatory cytokines or with LPS. The expression levels of CCR7 mRNA were quantified 24 h post-infection (Fig. 5A). The secondary treatment with LPS induced a significant (p<0.05) increase of CCR7 mRNA expression in rgHMPV - and rgHRSV-stimulated MDDC. Thus, the relatively low level of expression of CCR7 in MDDC exposed to rgHMPV or rgHRSV was not due to an irreversible block. Following treatment with the cocktail of pro-inflammatory cytokines, there was an increase of CCR7 mRNA in mock-, rgHMPV - or rgHRSV-stimulated MDDC, although there was substantial individual variation and this increase did not reach statistical significance. This suggests that the low level of expression of CCR7 mRNA in MDDC stimulated with rgHMPV or rgHRSV might be partly a consequence of the low levels of TNF-α, IL-1α and IL-6 produced after exposure to rgHMPV or rgHRSV.
Fig. 5. A cocktail of pro-inflammatory cytokines TNF-α/IL-1α/IL-6 partly restores the CCR7-driven migration of rgHMPV- or rgHRSV-stimulated MDDC. 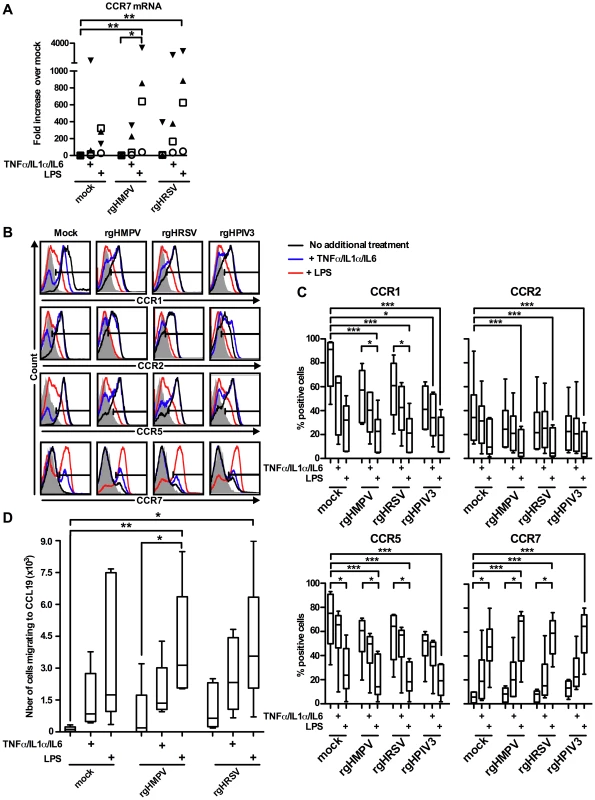
MDDC were infected with rgHMPV or rgHRSV and, 4–6 h post-infection, replica MDDC cultures were stimulated with a cocktail of the pro-inflammatory cytokines TNF-α/IL-1α/IL-6 or with LPS. (A) CCR7 mRNA levels in cells harvested at 24 h post-infection were quantified by RT-qPCR using the ΔΔCT method and expressed as fold change over mock. Each symbol represents an individual donor; n = 4 donors. (B, C) Cell surface expression of the chemokine receptors CCR1, CCR2, CCR5 and CCR7 in cells harvested 48 h post-infection were quantified by flow cytometry. Primary data from a representative donor (B) and summary of data from n = 6 donors are shown (C). The box plots show the median (horizontal line), flanked by the 2nd and 3rd quartile. The outer bars show the range of values. (D) MDDC migration to a CCL19 gradient, measured using the assay in Fig. 3, for cells harvested 48 h post-infection. The box plots show the median (horizontal line), flanked by the 2nd and 3rd quartile. The outer bars show the range of values. n = 5 donors. Statistically significant differences induced by cytokine or LPS treatment, and differences of any sample compared to untreated mock-infected cells, are indicated by asterisks (* P≤0.05, *** P≤0.001, see materials and methods for statistical analysis). To measure cell surface protein expression, MDDC that were treated with rgHMPV or rgHRSV and given a secondary stimulation with the pro-inflammatory cytokine cocktail or LPS, as described above, were analyzed by flow cytometry at 48 h post-infection. Consistent with the results at the mRNA level, stimulation with the proinflammatory cytokine cocktail induced a partial decrease in CCR1, 2 and 5 as well as a partial increase in CCR7 surface expression (Fig. 5B and C; B: 1 representative donor, and C: n = 6 donors). Secondary stimulation with LPS had stronger effects in all cases.
We also evaluated replicate samples to investigate if the profile of CCR7 mRNA and protein expression correlated with the ability of MDDC to migrate to a CCL19 concentration gradient, measured 48 h post-infection (Fig. 5D, n = 5 donors). Indeed, secondary stimulation with LPS induced a strong and significant (p≤0.05) increase of migration of rgHMPV - and rgHRSV-stimulated MDDC as compared to virus-treated cells given a mock secondary treatment. Following secondary stimulation of virus-treated cells with the cocktail of pro-inflammatory cytokines, there was an increase in migration of mock-, rgHMPV - and rgHRSV-stimulated MDDC, although this did not reach statistical significance, and did not reach the level of increase induced by LPS. Taken together, these results suggest that the low concentration of TNF-α, IL-1α and IL-6 induced by rgHMPV and rgHRSV is partly responsible for the low CCR7 mediated migration.
Chemokine receptor expression in GFP-positive versus GFP-negative virus-stimulated MDDC following secondary stimulation with a cocktail of pro-inflammatory cytokines or LPS
We next investigated possible effects of robust viral infection (indicated by intracellular GFP expression) on chemokine receptor expression following treatment with the pro-inflammatory cytokine cocktail or LPS. This was done by infecting MDDC (n = 6 donors) with rgHMPV, rgHRSV, or rgHPIV3, subjecting them to a secondary stimulation with the pro-inflammatory cytokine cocktail or LPS at 4 h post-infection, and using flow cytometry to analyze the cell surface expression of CCR1, 2, 5, and 7 in the GFP-positive versus the GFP-negative populations at 48 h post-infection (Fig. 6).
Fig. 6. Surface expression of chemokine receptors on GFP-positive and GFP-negative MDDC after inoculation with rgHMPV, rgHRSV and rgHPIV3 and secondary stimulation with the pro-inflammatory cytokines TNF-α/IL-1α/IL-6 or with LPS. 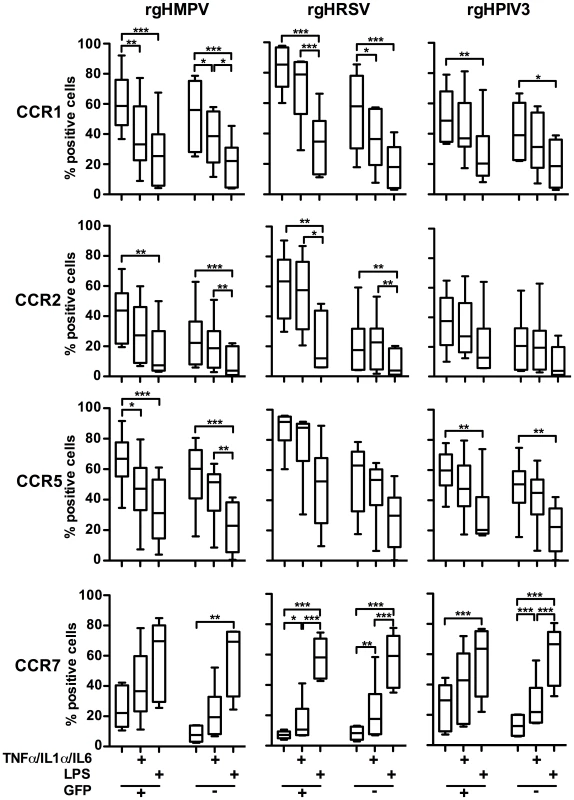
4–6 h after infection of immature MDDC with rgHMPV or rgHRSV, cells were stimulated with a cocktail of the pro-inflammatory cytokines TNF-α/IL-1α/IL-6 or LPS. 48 h after the initial infection, GFP-positive and GFP-negative cells were analyzed by flow cytometry for cell surface expression of the indicated chemokine receptors. n = 6 donors. (+) GFP+ population, (−) GFP− population. The box plots show the median (horizontal line), flanked by the 2nd and 3rd quartile. The outers bars show the range of values. Statistical differences between relevant groups, i.e. with and without cytokine treatment, are indicated by asterisks (* p≤0.05, **p≤0.01, *** P≤0.001, see materials and methods). Secondary stimulation of rgHMPV-, rgHRSV-, or rgHPIV3-stimulated MDDC with LPS decreased cell surface expression of CCR1, 2, and 5 on both GFP+ and GFP − cells. Secondary stimulation with the cocktail of pro-inflammatory cytokines also induced a decrease in surface expression of CCR1, 2, and 5. However, the magnitude of the effect usually was less than that observed with LPS.
Secondary stimulation of rgHMPV-, rHRSV-, or rgHPIV3-exposed MDDC with LPS induced an equally strong increase of CCR7 surface expression on GFP+ and GFP − cells, compared to cells that did not receive the secondary treatment (Fig. 6). Secondary stimulation of virus-infected cells with the pro-inflammatory cocktail also induced increases in CCR7 expression on both GFP − and GFP+ cells, although only in the case of rgHRSV GFP−+ and GFP − cells and rgHPIV3 GFP − cells was this difference statistically significant compared to cells receiving a mock secondary treatment. This provided further evidence that the poor expression of CCR7 in MDDC exposed to rgHRSV or rgHMPV could be overcome by secondary stimulation with LPS, and substantially overcome by secondary stimulation with the cocktail of pro-inflammatory cytokines. These increases were observed both in GFP+ and GFP − cells, indicating that robust viral infection did not irreversibly block CCR7 expression.
Discussion
Compared to HPIV3 or IAV, stimulation of human MDDC with HRSV or HMPV in vitro resulted in inefficient maturational changes in chemokine receptor usage – namely down-regulation of CCR1, CCR2, and CCR5 and up-regulation of CCR7 – that are necessary for DC migration in vivo following antigen uptake. MDDC stimulated with HRSV or HMPV did not migrate efficiently towards a CCL19 gradient in an in vitro assay, compared to HPIV3 or IAV, confirming that the poor surface expression of CCR7 had functional consequences. The weak chemokine receptor modulation and migration by MDDC exposed to HMPV and HRSV, viruses that are thought to induce incomplete immunity, was particularly evident compared to MDDC exposed to IAV, a virus that induces effective immunity.
In vivo, maturing, antigen-bearing DC migrate from peripheral tissue to secondary lymphatic tissue and localize in defined lymphoid compartments, where they present antigens to CD4+ and CD8+ T lymphocytes, initiating and polarizing the T cell response [26], [50]. DC migration to and positioning within lymphatic tissue are critical towards mounting an effective adaptive immune response [50]. While there are multiple chemokine receptors that direct immature DCs towards peripheral sites, CCR7 is the only receptor that mediates migration toward and positioning within lymphatic compartments for interaction with T lymphocytes [30], [51], [52], [53]. Thus, differential effects of pathogens on CCR7 expression in particular could be functionally relevant for differences in the immune response to these pathogens. Accordingly, the reduced migration observed in our in vitro assay for HMPV - and HRSV-treated MDDC following stimulation with HRSV and HMPV suggests that, during an HMPV or HRSV infection in vivo, maturing DC migrate with reduced efficiency from the infected mucosa towards secondary lymphatic tissues. This might lead to reduced adaptive immune responses that could explain the greater ability of HMPV and HRSV to reinfect humans throughout life without need for significant antigenic change.
The present study was done with primary human cells from multiple donors. While the use of cells from an outbred population provides data with substantial individualistic differences and reduced statistical significance compared to convenient, uniform hosts like inbred mice, it is important to note that the natural host of the viruses in the present study is the human and not the mouse. Direct in vivo studies of virus-specific effects on DC migration during respiratory infections of humans are difficult, especially in children. Gill et al [54] noted that DC persisted in the lungs of children hospitalized for HRSV infection for as long as 8 weeks following the resolution of infection [55]. Resorting to data from mice, sustained increases in pulmonary DC have also been observed following HRSV infection [56]. Lucken et al [57] tracked the migration of mouse DC following HRSV infection and showed that the increase in DC numbers in the mouse mediastinal lymph node was slower compared to IAV or Sendai virus infection [58], [59], [60]. These observations would be consistent with inefficient migration from the lung to lymphoid tissue. Our in vitro studies now provide a mechanism for these previous in vivo observations. In addition, we provided data that MDDC maturation also was reduced with HMPV compared to HPIV3 and IAV.
We previously provided data indicating that the level of MDDC maturation in response to exposure to HMPV and HRSV is lower compared to HPIV3 [32] and IAV (not shown). In vivo, the combination of these two factors, namely reduced overall maturation and inefficient CCR7-CCL19 driven migration, might result in additive net effects that could affect both the magnitude and the quality of the adaptive immune response. Compared to infection with IAV, HRSV and HMPV infections may yield lower overall numbers of virus-stimulated mature DC in the afferent lymphatics. Reduced expression of co-stimulatory surface molecules and reduced cytokine expression could affect the quality of the response as well as its magnitude. In addition, the inefficient migration of maturing DCs may also play a role in viral pathogenesis: specifically, the sustained presence of mature DC in the mouse lung has been suggested to contribute to airway inflammation [56].
Another paramyxovirus, measles virus (MeV), was recently shown to inhibit CCR7-driven DC migration. Interference with DC maturation and function is considered to be central to MeV-induced immunosuppression. Compared to LPS, MeV infection failed to promote the switch from CCR5 to CCR7 expression, and MeV-matured DC exhibited chemotactic responses to CCL3 rather than to CCL19 [61]. Inhibition of CCR7-driven migration was also described for vaccinia virus and for herpes simplex virus type 1 [45], [62], [63]. However, the effects of reduced DC maturation and migration on long-term protection might be particularly significant for respiratory viruses such as HMPV and HRSV. Both of these viruses are restricted in tropism to the superficial cell layer of the respiratory tract, and protection against re-infection has reduced effectiveness (compared to viremic viruses, for example) due to the short-lived nature of local IgA antibodies, the inefficiency with which serum antibodies access the respiratory lumen, and the down-regulation of virus-specific CD8+ T cell functionality in the respiratory tract [64]. Thus, even modest decreases in the magnitude of the adaptive response could result in decreases in viral clearance and protection against re-infection.
We used recombinant GFP-expressing viruses to distinguish between effects in robustly infected (GFP-positive) and uninfected/abortively-infected (GFP-negative cells) MDDC. This revealed additional differences between the viruses. For MDDC infected with HMPV or HPIV3, the GFP-positive population expressed significantly more surface CCR7 than the GFP-negative population. In contrast, for MDDC infected with HRSV, the GFP-positive subpopulation resembled the GFP-negative population in having very low CCR7 surface expression. Thus, whereas robust infection with HMPV and HPIV3 stimulated expression of CCR7, robust infection with HRSV did not. Furthermore, GFP-positive cells infected with HRSV showed no down-regulation of CCR1, 2, and 5 surface expression. Thus, compared to HMPV or HPIV3, even the subpopulation of DC that is robustly infected with HRSV and contains abundant intracellular antigen would not be mobilized for migration. This would impede the delivery of HRSV antigen from the periphery to lymphoid tissue. Furthermore, DC that are robustly infected with a virus can readily process newly synthesized viral antigens for display on MHC class I molecules and presentation to CD8+ T cells. Reduced migration of DC that are robustly infected with HRSV to lymphoid tissue would reduce this activity. This would make activation of CD8+ T cells more dependent on cross-presentation by non-infected DC, and could reduce the efficiency of CD8+ T cell activation during HRSV infection, reducing viral clearance and the disease-sparing regulatory effects of HRSV-specific CD8+ T cells [65].
Secondary stimulation of HRSV - or HMPV-stimulated MDDC with LPS, a strong DC activator, resulted in up-regulation of CCR7 expression on both GFP-negative and GFP-positive cells and increased in vitro migration. In contrast, with vaccinia virus or human cytomegalovirus, a secondary stimulation of the infected DC with LPS failed to up-regulate the CCR7 chemokine receptor [45], [62]. LPS is a strong NFκ-B and AP-1 dependent DC activator [66], [67]. Secondary stimulation of HRSV - and HMPV-infected MDDC with the NFκ-B/AP-1-dependent pro-inflammatory cytokines TNF-α, IL-1α and IL-6, at concentrations comparable to those induced by LPS treatment, up-regulated CCR7 expression and was pro-migratory. This suggests that, in contrast to MeV, vaccinia virus, or herpes simplex virus, suboptimal stimulation, rather than inhibition, is responsible for the poor-migration phenotype of pneumovirus-exposed MDDC.
In summary, compared to HPIV3 and, in particular, IAV, the pneumoviruses HMPV and HRSV were inefficient in inducing the maturation-related changes in cell surface chemokine receptor expression in MDDC that are necessary in vivo to re-direct DC from the periphery to lymphoid tissue. Consistent with this, both HRSV and HMPV were poor inducers of MDDC maturation and migration in vitro. These effects could be contributing factors in the incomplete nature of protection induced by HRSV infection in humans.
Supporting Information
Zdroje
1. CanqueBRosenzwajgMGluckmanJC 2003 In vitro generation of dendritic cells from cord blood CD34+ hematopoietic progenitors cells. Methods Mol Biol 215 311 325
2. NairHNokesDJGessnerBDDheraniMMadhiSA 2010 Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375 1545 1555
3. HallCB 2001 Respiratory syncytial virus and parainfluenza virus. N Engl J Med 344 1917 1928
4. WelliverRCWongDTSunMMcCarthyN 1986 Parainfluenza virus bronchiolitis. Epidemiology and pathogenesis. Am J Dis Child 140 34 40
5. FalseyAR 2008 Human metapneumovirus infection in adults. Pediatr Infect Dis J 27 S80 83
6. HamelinMEAbedYBoivinG 2004 Human metapneumovirus: a new player among respiratory viruses. Clin Infect Dis 38 983 990
7. WilliamsJVHarrisPATollefsonSJHalburnt-RushLLPingsterhausJM 2004 Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 350 443 450
8. FalseyARHennesseyPAFormicaMACoxCWalshEE 2005 Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352 1749 1759
9. ThompsonWWShayDKWeintraubEBrammerLBridgesCB 2004 Influenza-associated hospitalizations in the United States. JAMA 292 1333 1340
10. ThompsonWWShayDKWeintraubEBrammerLCoxN 2003 Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289 179 186
11. 2009 Update: influenza activity–United States, September 28, 2008-April 4, 2009, and composition of the 2009-10 influenza vaccine. MMWR Morb Mortal Wkly Rep 58 369 374
12. ZhangLBukreyevAThompsonCIWatsonBPeeplesME 2005 Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol 79 1113 1124
13. ZhangLPeeplesMEBoucherRCCollinsPLPicklesRJ 2002 Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol 76 5654 5666
14. AhmedROldstoneMBPaleseP 2007 Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol 8 1188 1193
15. CouchRBKaselJA 1983 Immunity to influenza in man. Annu Rev Microbiol 37 529 549
16. GlezenWPTaberLHFrankALKaselJA 1986 Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 140 543 546
17. JohnsonKMChanockRMRifkindDKravetzHMKnightV 1961 Respiratory syncytial virus. IV. Correlation of virus shedding, serologic response, and illness in adult volunteers. JAMA 176 663 667
18. HallCBWalshEELongCESchnabelKC 1991 Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 163 693 698
19. AuffrayCFoggDGarfaMElainGJoin-LambertO 2007 Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317 666 670
20. GeissmannF 2007 The origin of dendritic cells. Nat Immunol 8 558 560
21. GeissmannFAuffrayCPalframanRWirrigCCioccaA 2008 Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol
22. Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 143 416 429
23. FrascaLFedeleGDeaglioSCapuanoCPalazzoR 2006 CD38 orchestrates migration, survival, and Th1 immune response of human mature dendritic cells. Blood 107 2392 2399
24. Reis e SousaC 2006 Dendritic cells in a mature age. Nat Rev Immunol 6 476 483
25. AlvarezDVollmannEHvon AndrianUH 2008 Mechanisms and consequences of dendritic cell migration. Immunity 29 325 342
26. ForsterRDavalos-MisslitzACRotA 2008 CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 8 362 371
27. GunnMDTangemannKTamCCysterJGRosenSD 1998 A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A 95 258 263
28. LutherSATangHLHymanPLFarrAGCysterJG 2000 Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A 97 12694 12699
29. WillimannKLeglerDFLoetscherMRoosRSDelgadoMB 1998 The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur J Immunol 28 2025 2034
30. SozzaniS 2005 Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor Rev 16 581 592
31. Lukacs-KornekVEngelDTackeFKurtsC 2008 The role of chemokines and their receptors in dendritic cell biology. Front Biosci 13 2238 2252
32. Le NouenCMunirSLosqSWinterCCMcCartyT 2009 Infection and maturation of monocyte-derived human dendritic cells by human respiratory syncytial virus, human metapneumovirus, and human parainfluenza virus type 3. Virology
33. Le NouenCHillyerPMunirSWinterCCMcCartyT 2010 Effects of Human Respiratory Syncytial Virus, Metapneumovirus, Parainfluenza Virus 3 and Influenza Virus on CD4+ T Cell Activation by Dendritic Cells. PLoS One 5 e15017
34. JakubzickCTackeFGinhouxFWagersAJvan RooijenN 2008 Blood monocyte subsets differentially give rise to CD103+ and CD103 - pulmonary dendritic cell populations. J Immunol 180 3019 3027
35. VarolCLandsmanLFoggDKGreenshteinLGildorB 2007 Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med 204 171 180
36. XuYZhanYLewAMNaikSHKershawMH 2007 Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol 179 7577 7584
37. BiacchesiSSkiadopoulosMHTranKCMurphyBRCollinsPL 2004 Recovery of human metapneumovirus from cDNA: optimization of growth in vitro and expression of additional genes. Virology 321 247 259
38. HallakLKSpillmannDCollinsPLPeeplesME 2000 Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 74 10508 10513
39. BiacchesiSMurphyBRCollinsPLBuchholzUJ 2007 Frequent frameshift and point mutations in the SH gene of human metapneumovirus passaged in vitro. J Virol 81 6057 6067
40. De SmedtTPajakBMurailleELespagnardLHeinenE 1996 Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med 184 1413 1424
41. VerhasseltVBuelensCWillemsFDe GrooteDHaeffner-CavaillonN 1997 Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol 158 2919 2925
42. DieuMCVanbervlietBVicariABridonJMOldhamE 1998 Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med 188 373 386
43. VecchiAMassimilianoLRamponiSLuiniWBernasconiS 1999 Differential responsiveness to constitutive vs. inducible chemokines of immature and mature mouse dendritic cells. J Leukoc Biol 66 489 494
44. ZhangWChenZLiFKamencicHJuurlinkB 2003 Tumour necrosis factor-alpha (TNF-alpha) transgene-expressing dendritic cells (DCs) undergo augmented cellular maturation and induce more robust T-cell activation and anti-tumour immunity than DCs generated in recombinant TNF-alpha. Immunology 108 177 188
45. MoutaftsiMBrennanPSpectorSATabiZ 2004 Impaired lymphoid chemokine-mediated migration due to a block on the chemokine receptor switch in human cytomegalovirus-infected dendritic cells. J Virol 78 3046 3054
46. ScandellaEMenYLeglerDFGillessenSPriklerL 2004 CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood 103 1595 1601
47. RemoliMEGafaVGiacominiESeveraMLandeR 2007 IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur J Immunol 37 3499 3508
48. LehnerMStilperAMorhartPHolterW 2008 Plasticity of dendritic cell function in response to prostaglandin E2 (PGE2) and interferon-gamma (IFN-gamma). J Leukoc Biol 83 883 893
49. YenJHKongWGaneaD 2010 IFN-beta inhibits dendritic cell migration through STAT-1-mediated transcriptional suppression of CCR7 and matrix metalloproteinase 9. J Immunol 184 3478 3486
50. BanchereauJBriereFCauxCDavoustJLebecqueS 2000 Immunobiology of dendritic cells. Annu Rev Immunol 18 767 811
51. RandolphGJSanchez-SchmitzGAngeliV 2005 Factors and signals that govern the migration of dendritic cells via lymphatics: recent advances. Springer Semin Immunopathol 26 273 287
52. Sanchez-SanchezNRiol-BlancoLRodriguez-FernandezJL 2006 The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J Immunol 176 5153 5159
53. YoshidaRImaiTHieshimaKKusudaJBabaM 1997 Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem 272 13803 13809
54. GillMAPaluckaAKBartonTGhaffarFJafriH 2005 Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J Infect Dis 191 1105 1115
55. GillMALongKKwonTMunizLMejiasA 2008 Differential recruitment of dendritic cells and monocytes to respiratory mucosal sites in children with influenza virus or respiratory syncytial virus infection. J Infect Dis 198 1667 1676
56. BeyerMBartzHHornerKDothsSKoerner-RettbergC 2004 Sustained increases in numbers of pulmonary dendritic cells after respiratory syncytial virus infection. J Allergy Clin Immunol 113 127 133
57. LukensMVKruijsenDCoenjaertsFEKimpenJLvan BleekGM 2009 Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J Virol 83 7235 7243
58. McGillJVan RooijenNLeggeKL 2008 Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med 205 1635 1646
59. GraysonMHRamosMSRohlfingMMKitchensRWangHD 2007 Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol 179 1438 1448
60. LeggeKLBracialeTJ 2003 Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 18 265 277
61. AbtMGassertESchneider-SchauliesS 2009 Measles virus modulates chemokine release and chemotactic responses of dendritic cells. J Gen Virol 90 909 914
62. HumrichJYThumannPGreinerSHumrichJHAverbeckM 2007 Vaccinia virus impairs directional migration and chemokine receptor switch of human dendritic cells. Eur J Immunol 37 954 965
63. PrechtelATTurzaNMKobeltDJEisemannJICoffinRS 2005 Infection of mature dendritic cells with herpes simplex virus type 1 dramatically reduces lymphoid chemokine-mediated migration. J Gen Virol 86 1645 1657
64. CollinsPLGrahamBS 2008 Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 82 2040 2055
65. OlsonMRHartwigSMVargaSM 2008 The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J Immunol 181 7958 7968
66. KrappmannDWegenerESunamiYEsenMThielA 2004 The IkappaB kinase complex and NF-kappaB act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1. Mol Cell Biol 24 6488 6500
67. ShaWCLiouHCTuomanenEIBaltimoreD 1995 Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 80 321 330
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous SystemsČlánek The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral ReleaseČlánek Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1Článek High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 6- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- The N-Terminus of the RNA Polymerase from Infectious Pancreatic Necrosis Virus Is the Determinant of Genome Attachment
- Evolutionary Analysis of Inter-Farm Transmission Dynamics in a Highly Pathogenic Avian Influenza Epidemic
- Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous Systems
- Endemic Dengue Associated with the Co-Circulation of Multiple Viral Lineages and Localized Density-Dependent Transmission
- The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral Release
- The -glycan Glycoprotein Deglycosylation Complex (Gpd) from Deglycosylates Human IgG
- The Lipid Transfer Protein CERT Interacts with the Inclusion Protein IncD and Participates to ER- Inclusion Membrane Contact Sites
- Induction of Noxa-Mediated Apoptosis by Modified Vaccinia Virus Ankara Depends on Viral Recognition by Cytosolic Helicases, Leading to IRF-3/IFN-β-Dependent Induction of Pro-Apoptotic Noxa
- Environmental Constraints Guide Migration of Malaria Parasites during Transmission
- HIV-1 Efficient Entry in Inner Foreskin Is Mediated by Elevated CCL5/RANTES that Recruits T Cells and Fuels Conjugate Formation with Langerhans Cells
- Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis
- Highly Pathogenic Avian Influenza Virus H5N1 Infects Alveolar Macrophages without Virus Production or Excessive TNF-Alpha Induction
- Uses Host Triacylglycerol to Accumulate Lipid Droplets and Acquires a Dormancy-Like Phenotype in Lipid-Loaded Macrophages
- Compensatory T Cell Responses in IRG-Deficient Mice Prevent Sustained Infections
- Detection of Inferred CCR5- and CXCR4-Using HIV-1 Variants and Evolutionary Intermediates Using Ultra-Deep Pyrosequencing
- A Dynamic Landscape for Antibody Binding Modulates Antibody-Mediated Neutralization of West Nile Virus
- HSV-2 Infection of Dendritic Cells Amplifies a Highly Susceptible HIV-1 Cell Target
- “Rational Vaccine Design” for HIV Should Take into Account the Adaptive Potential of Polyreactive Antibodies
- Impact of Endofungal Bacteria on Infection Biology, Food Safety, and Drug Development
- Infection Reduces B Lymphopoiesis in Bone Marrow and Truncates Compensatory Splenic Lymphopoiesis through Transitional B-Cell Apoptosis
- The Intrinsic Antiviral Defense to Incoming HSV-1 Genomes Includes Specific DNA Repair Proteins and Is Counteracted by the Viral Protein ICP0
- Molecular Interactions that Enable Movement of the Lyme Disease Agent from the Tick Gut into the Hemolymph
- Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1
- A Freeze Frame View of Vesicular Stomatitis Virus Transcription Defines a Minimal Length of RNA for 5′ Processing
- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Tipping the Balance: Secreted Oxalic Acid Suppresses Host Defenses by Manipulating the Host Redox Environment
- Bacteria-Induced Dscam Isoforms of the Crustacean,
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Insertion of an Esterase Gene into a Specific Locust Pathogen () Enables It to Infect Caterpillars
- A Role for TLR4 in Infection and the Recognition of Surface Layer Proteins
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
- Low CCR7-Mediated Migration of Human Monocyte Derived Dendritic Cells in Response to Human Respiratory Syncytial Virus and Human Metapneumovirus
- HIV/SIV Infection Primes Monocytes and Dendritic Cells for Apoptosis
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- Productive Parvovirus B19 Infection of Primary Human Erythroid Progenitor Cells at Hypoxia Is Regulated by STAT5A and MEK Signaling but not HIFα
- Identification of DNA-Damage DNA-Binding Protein 1 as a Conditional Essential Factor for Cytomegalovirus Replication in Interferon-γ-Stimulated Cells
- The Influenza Virus Protein PB1-F2 Inhibits the Induction of Type I Interferon at the Level of the MAVS Adaptor Protein
- Passively Administered Pooled Human Immunoglobulins Exert IL-10 Dependent Anti-Inflammatory Effects that Protect against Fatal HSV Encephalitis
- Infection of Induces Antifungal Immune Defenses
- Merozoite Invasion Is Inhibited by Antibodies that Target the PfRh2a and b Binding Domains
- Cyclic di-GMP is Essential for the Survival of the Lyme Disease Spirochete in Ticks
- Cross-Neutralizing Antibodies to Pandemic 2009 H1N1 and Recent Seasonal H1N1 Influenza A Strains Influenced by a Mutation in Hemagglutinin Subunit 2
- Spatial Dynamics of Human-Origin H1 Influenza A Virus in North American Swine
- Coronavirus Gene 7 Counteracts Host Defenses and Modulates Virus Virulence
- Clathrin Facilitates the Morphogenesis of Retrovirus Particles
- Contribution of Intrinsic Reactivity of the HIV-1 Envelope Glycoproteins to CD4-Independent Infection and Global Inhibitor Sensitivity
- Functional Analysis of Host Factors that Mediate the Intracellular Lifestyle of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



