-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Capsid-Encoded PPxY-Motif Facilitates Adenovirus Entry
Viruses use cellular machinery to enter and infect cells. In this study we address the cell entry mechanisms of nonenveloped adenoviruses (Ads). We show that protein VI, an internal capsid protein, is rapidly exposed after cell surface attachment and internalization and remains partially associated with the capsid during intracellular transport. We found that a PPxY motif within protein VI recruits Nedd4 E3 ubiquitin ligases to bind and ubiquitylate protein VI. We further show that this PPxY motif is involved in rapid, microtubule-dependent intracellular movement of protein VI. Ads with a mutated PPxY motif can efficiently escape endosomes but are defective in microtubule-dependent trafficking toward the nucleus. Likewise, depletion of Nedd4 ligases attenuates nuclear accumulation of incoming Ad particles and infection. Our data provide the first evidence that virus-encoded PPxY motifs are required during virus entry, which may be of significance for several other pathogens.
Published in the journal: . PLoS Pathog 6(3): e32767. doi:10.1371/journal.ppat.1000808
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000808Summary
Viruses use cellular machinery to enter and infect cells. In this study we address the cell entry mechanisms of nonenveloped adenoviruses (Ads). We show that protein VI, an internal capsid protein, is rapidly exposed after cell surface attachment and internalization and remains partially associated with the capsid during intracellular transport. We found that a PPxY motif within protein VI recruits Nedd4 E3 ubiquitin ligases to bind and ubiquitylate protein VI. We further show that this PPxY motif is involved in rapid, microtubule-dependent intracellular movement of protein VI. Ads with a mutated PPxY motif can efficiently escape endosomes but are defective in microtubule-dependent trafficking toward the nucleus. Likewise, depletion of Nedd4 ligases attenuates nuclear accumulation of incoming Ad particles and infection. Our data provide the first evidence that virus-encoded PPxY motifs are required during virus entry, which may be of significance for several other pathogens.
Introduction
Many viruses use the microtubule network of the host cell for transport to their site of replication (i.e. the nucleus) [1]. Access to the microtubule network is achieved through recruitment of cytoplasmic dynein motor proteins followed by efficient retrograde transport towards the nucleus [2],[3]. Virus-induced cellular signaling cascades help stimulate the directionality and efficacy of the transport [4]. Viral interaction with dynein motor proteins occurs either directly through capsid proteins or indirectly via hijacking of adapters from existing transport pathways [5]. Most DNA viruses accumulate transiently at the microtubule organizing center (MTOC) prior to nuclear translocation [1],[3],[6]. How they release from the microtubules or the MTOC and transport to nuclear pores is poorly understood. MTOC release may involve a switch from dynein to kinesin mediated transport, the cellular ubiquitin/proteasome system and/or nuclear transport receptors [1], [3], [5]–[8].
Indirect evidence that the host's ubiquitylation machinery participates in parts of the viral entry process comes from studies using pharmacological inhibitors of the ubiquitin/proteasome system. For example, translocation of a murine coronavirus from the endosome to the cytoplasm is facilitated by the ubiquitin-proteasome system [9]. Similarly, influenza viruses appear to be trapped in an endosomal compartment upon pharmacological inhibition of the proteasome [10]. In contrast, blocking the proteasome increases the transduction efficiency of adeno-associated virus vectors and this correlates with ubiquitylation of capsid proteins [11],[12]. The Semliki forest and the vesicular stomatitis virus, however, do not seem to be affected by proteasome inhibition during their entry suggesting different host factor requirements [10].
A role for the ubiquitylation machinery during egress of enveloped viruses is better understood. Egress involves the transport of assembled capsids, subviral structures or individual capsid proteins to assembly and budding sites at the cell surface or at intracellular membranes [1]. Budding, and potentially trafficking, to the egress site requires an intact class E vesicular sorting pathway (VSP, [13],[14]. The VSP is believed to involve the consecutive activity of three distinct heteromeric complexes termed endosomal sorting complexes required for transport (ESCRT-I, -II and –III, [15]). The capsid proteins of several enveloped viruses encode ‘late domains’ that specifically interact with ESCRT components and redirect them towards the site of viral egress [13]. Some late domains of the PPxY motif type (where x can be any amino acid) require the binding of ubiquitin ligases of the Nedd4 family of HECT-E3 ubiquitin ligases (Homologous to E6-AP Carboxyl Terminus) for efficient ESCRT recruitment [13].
Nedd4.1 and its close relative Nedd4.2 are prototypic members of this family, which is conserved from yeast to mammals [16]. They encode a N-terminal C2 domain for Ca2+-dependent lipid interaction, a catalytical HECT domain and three to four WW-domains for protein-protein interactions with proline-rich domains such as the PPxY motif [16],[17]. The exact role of PPxY-recruitment of Nedd4 ligases in VSP-mediated viral budding is still unclear. A possible link was recently shown by enhanced ESCRT ubiquitylation through Nedd4.2 overexpression [18],[19].
Late domains, including the PPxY type, have also been found in some nonenveloped reoviruses but a general function in virus release remains to be shown [20]. PPxY type late domains where also described for the Ad capsid protein penton, which can interact with ubiquitin ligases of the Nedd4 family. However, its role in Ad infection is unclear [21].
At least in vitro, Ad infects cells by first attaching to primary receptors, including CAR, CD46 and sialic acid, via the fiber protein [22]. In some cells endocytosis of Ad may be triggered by penton base-mediated signaling through alpha(v) integrins [23]–[25].
In epithelial cells, Ad serotype 5 (Ad5) particles undergo stepwise disassembly during entry, starting with detachment of the fiber at or near the cell surface and followed by a passage through endosomal compartments in which acidification serves as additional disassembly trigger for membrane penetration and cytosolic translocation [26],[27]. Partial disassembly releases the internal capsid protein VI, which can lyse membranes in vitro via its predicted N-terminal amphipathic helix [28]. In the cytosol, the particle engages in microtubule-directed transport towards the MTOC and is translocated to the nuclear pore complex (NPC) for nuclear import of the genome [29]–[31].
In this study we address the mechanisms of Ad cell entry. We demonstrate that the internal capsid protein VI is rapidly exposed to antibodies during cell entry, possibly at the cell surface or immediately after endocytosis. We further determine that protein VI remains partially associated with Ad capsids as they traffic to MTOCs and the NPC. We identify a functional PPxY motif within protein VI that mediates the association of protein VI with Nedd4 E3 ubiquitin ligases and facilitates its ubiquitylation. Recombinant Ad5 in which the protein VI PPxY motif is mutated have normal capsid morphology, escape from endosomes with similar efficiency as wildtype viruses, but are defective in genome delivery to the nucleus. We show that the PPxY motif in protein VI is involved in its efficient microtubule-mediated transport and mutating it in the virus alters the intracellular targeting of Ads towards the MTOC region concomitant with a post-entry block in viral infectivity. Furthermore, Nedd4.1 and Nedd4.2 are involved in Ad infection and intracellular targeting of incoming virions to the MTOC. We propose that the PPxY motif, in other viral systems, may also function during entry and interact with novel cellular pathways for efficient viral entry.
Results
Protein VI is exposed during Ad infection and partially remains associated with the viral particle
The fate of Ad particles immediately after internalization is only partially characterized. In the context of endosome escape of Ad5, very little is known about how this occurs and which, if any, cellular proteins are involved. From in vitro studies it was proposed that the internal capsid protein VI mediates Ad endosome escape [28]. Previous reports showed that protein VI dissociates from the Ad capsid very early after attachment [26],[27].
To delineate the fate of protein VI during Ad entry we performed infection assays and followed the intracellular distribution of the viral capsid and protein VI as a function of time. To this end, fluorescently labeled Ad particles were adsorbed to either human retina epithelia pigment cells (hTERT-RPE1, Figure 1) or human osteosarcoma cells (U2OS, Figure S1) at 4°C and then transferred to 37°C to synchronize internalization. Cells were fixed at various times and analyzed by confocal microscopy (Figure 1). Protein VI was detected using an affinity purified polyclonal-antiserum and the location of the MTOC was marked by detecting the primary cilia, which originates at the MTOC using antibodies against acetylated tubulin [32]. At 4°C viral particles accumulated at the cell periphery showing sporadic positive staining with the protein VI antiserum (∼1%) possibly due to the recognition of protein VI from damaged particles (Figure 1 first row). In contrast, 5 min after the temperature shift, Ad particles were still localized close to the cell periphery but approximately 40% of them gave a signal with the protein VI antibody indicating that more protein VI was accessible (Figure 1, second row). After 15 min, particles had entered the cell with some localized at the MTOC region (Figure 1, third row) and some at the nuclear rim as described previously [6]. About 10% of the particles remained protein VI positive, including particles at the nuclear rim. After 45 min the majority of the particles were concentrated at the MTOC region (Figure 1, bottom row) as previously reported [3]. Protein VI staining was also concentrated at the MTOC region but most of the signal was not particle associated. Similar results were obtained in U2OS cells (Figure S1). Together these data suggested that structural rearrangements leading to protein VI exposure take place at or close to the cell surface during Ad entry. In addition, the data showed that protein VI trafficked to the MTOC region and partially remained associated with the capsid.
Fig. 1. Timecourse of protein VI release during Ad entry. 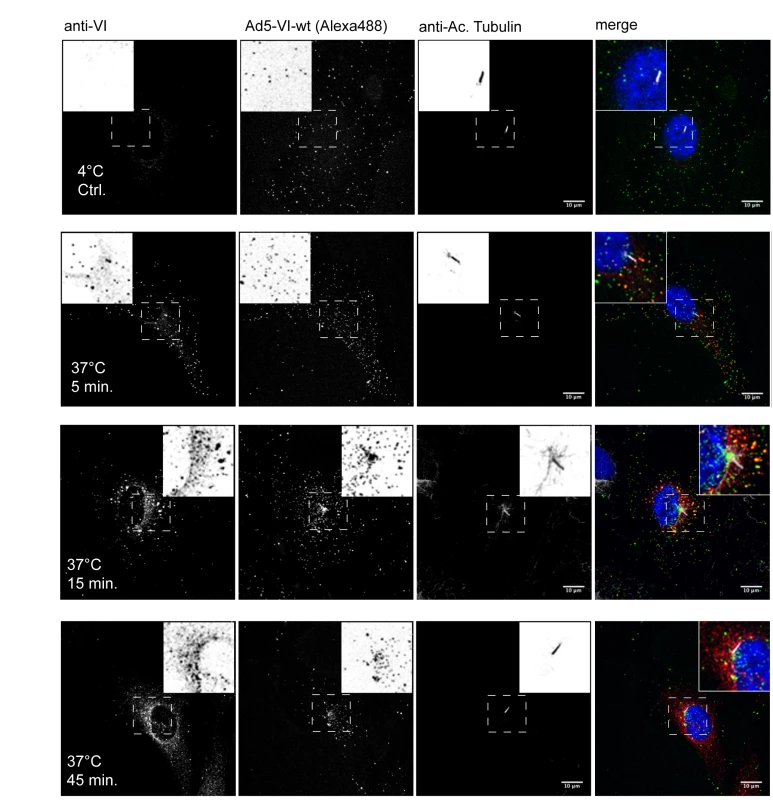
Ad5-488 was pre-bound to hTERT-RPE1 cells at 4°C (top row) and shifted to 37°C for 5 min (second row), 15 min (third row) and 45 min (bottom row) as indicated. Protein VI was detected using anti-protein VI antibodies (left column), adenoviral particles by detecting the Alexa-488 fluorescent signal (second column) and the MTOC by staining the primary cilia (third column). A composite of all three signals including the nucleus (in grayscale) is shown to the left. The inset shows an inverted magnification of representative virus, protein VI and the primary cilia signals from the small dashed box. In the composite protein VI signals are shown in red, Ad is shown in green, the primary cilia in grey and the nucleus in blue. Colocalization of protein VI and Ad appears as yellow. The scale bar is 10 µm. Ad5 protein VI encodes a conserved PPxY motif determining viral infectivity
To identify possible trafficking determinants, we analyzed the sequences of protein VI from several Ad serotypes and identified a highly conserved ubiquitin-ligase interacting motif present in PPxY-type viral late domains (PPxY, Figure S2). To examine the role of this PPxY motif in Ad cell entry, we used an E1/E3-deleted Ad5 that had the protein VI PPSY motif mutated to PGAA (Ad5-VI-M1, detailed in Figure S3) [33],[34]. This mutation, when introduced into Mason-Pfizer monkey virus, was previously shown to abolish late domain functions with no apparent structural changes, which would impair virus assembly [35],[36]. To control for unintended mutations introduced during the cloning, we reverted the PGAA sequence back to PPSY (Ad5-VI-wt). Because the ∼360 copies of protein VI appears to be in contact with several proteins in the mature capsid, modifications that disrupt the tertiary structure could also affect the capsid composition. In large-scale preparations, mutant and wt virus banded at identical densities and gave similar yields of particles as determined by genome and protein quantification. A biochemical analysis of the capsid composition of purified viral particles showed no apparent differences between wt and mutant viruses (Figure 2A and data not shown). To confirm that viral capsid integrity between mutant and wt virus remained unchanged, we used negative stain electron microscopy. As shown in Figure 2B capsid integrity and morphology of the mutant virus was indistinguishable from the wt virus. In contrast, the infectious versus physical particle ratio of Ad5-VI-M1 was ∼20-fold lower than Ad5-VI-wt as assayed by plaque formation on monolayers of 911 cells (Figure 2C). Because infectious vs. physical particles can vary between preparations, we assayed plaque size, which is more informative measurement of propagation rate. Plaques were significantly smaller for Ad5-VI-M1 versus Ad5-VI-wt (see below), suggesting that the altered PPxY domain affects some stages of virus propagation.
Fig. 2. Reduced infectivity for an Ad with an altered PPxY motif. 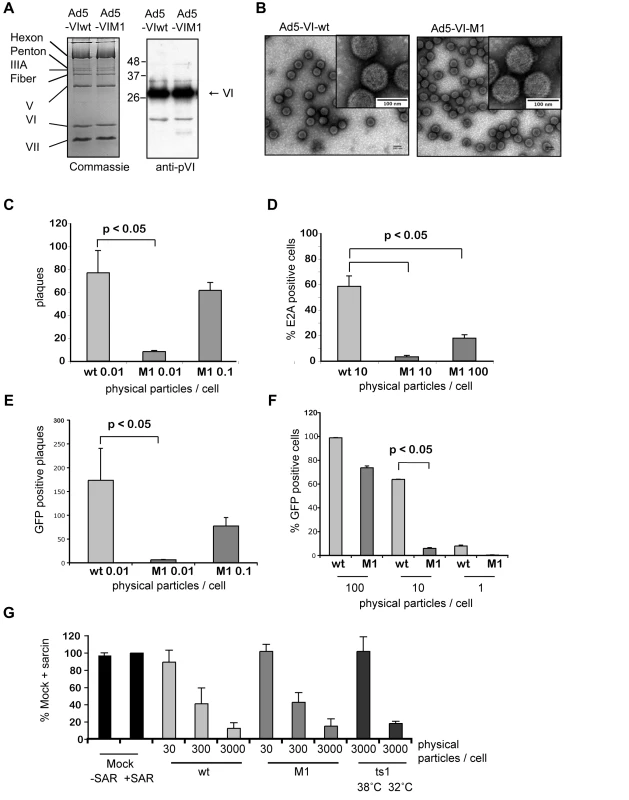
A) Biochemical comparison of Ad5-VI-wt vs. Ad5-VI-M1. Left panel; Coomassie gel comparison of wt Ad (Ad5-VI-wt, lane 1) and Ad with PPSY in protein VI mutated to PGAA (Ad5-VI-M1, lane 2). Individual capsid proteins are shown on the left. Right panel; western blot analysis of protein VI for Ad5-VI-wt (lane 1) and Ad5-VI-M1 (lane 2). B) Negative stain of purified Ad capsids. Electronic microscopy images of purified Ad5-VI-wt capsids (left panel) and purified Ad5-VI-M1 (right panel) are shown. The inset in each figure shows a magnification of individual capsids. The scale bar is 100nm. C) Plaque assay comparison of Ad5-VI-wt vs. Ad5-VI-M1. Quantification of plaques at day 12 for different physical particle per cell ratios for Ad5-VI-wt and Ad5-VI-M1. Shown is the average plaque number per 6 well (+/− standard deviation) of three individual experiments. D) Focus forming assay. E1 complementing 911 cells were infected with Ad5-VI-wt or Ad5-VI-M1 and replication centers were stained with E2A antibodies 24 h post-infection and at different particle per cell ratios. >5 random fields with >200 cells were counted, standard deviation represents field-to-field variations. E) Plaque forming assay comparison of Ad5GFP-VI-wt vs. Ad5GFP-VI-M1. Quantification of GFP positive plaques at day 12 at different physical particle to cell ratios. The values show the average number of GFP positive plaques per 6-well (+/− standard deviation) of two independent experiments. F) Single round infection assay comparison of Ad5GFP-VI-wt vs. Ad5GFP-VI-M1. U2OS cells were infected with increasing amounts of physical particle to cell ratios as indicated. GFP expression was quantified using FACS. Values correspond to the average percentage of GFP positive cells of two experiments done in triplicates (+/− standard deviation). Note that the ∼100% infection of wt infected cells at 100 physical particles per cell is saturated and not comparable to M1. G) Analysis of membrane penetration. A549 cells were infected with Ad5-VI-wt, Ad5-VI-M1, Ad2ts1 grown at 32°C or at 38°C at different physical particle per cell ratios as indicated and in the presence of alpha-Sarcin and translational efficiency was determined by measuring the incorporation of radiolabeled amino acids over time. Values are given in percentage normalized to 100% translation measured in the presence of the toxin but in absence of virus. Conditions are indicated below each bar and are the mean of at least three independent experiments done in triplicates. To determine whether the M1 mutation influences Ad cell entry, we performed a fluorescent focus forming assay and stained cells at 8, 12 and 24 h post-infection for expression of the E2A protein, which marks the appearance of viral replication centers (Figure 2D and data not shown). Compared to Ad5-VI-wt, the Ad5-VI-M1 virus produced approximately 20-fold fewer fluorescent foci when equivalent numbers of viral particles were used for infections. This suggested that steps prior to replication (i.e. internalization) require an intact PPxY motif in protein VI.
To address trafficking using a different approach, we inserted a GFP expression cassette into the FRT site in the E1-deleted region of the wt and the M1 mutant virus (see Figure S3 for details). The GFP expressing viruses showed no difference in the quantitative yield and biochemical composition after large-scale purification. We repeated plaque forming assays (Figure 2E) and single round infection assays (Figure 2F), this time using GFP expression as the quantification method in non-complementing U2OS cells. We observed a reduction in infectivity in the same order of magnitude as previously (compare Figures 2C with 2E and 2D with 2F). In addition the GFP expression allowed us to follow the plaque formation over time. As shown in Figure S4 the spread of the M1 mutant virus was significantly slower and led to fewer and much smaller plaques (Figure 2E and Figure S4).
Next we asked whether Ad5 endosomal lysis efficiency following internalization is affected by the mutation in the PPxY motif of protein VI. We infected A549 cells with 30, 300 or 3000 particles per cell of either Ad5-VI-wt or Ad5-VI-M1 in the presence of alpha-sarcin, a membrane impermeable toxin that inhibits translation when it enters the cytoplasm. Alpha-sarcin enters the cell by virus-mediated endosomal membrane lysis thus providing a quantifiable marker for endsome membrane lysis (Figure 2G, [28]). We measured the incorporation of radiolabeled amino acids over time as a means to determine translational efficiency. As control we used the temperature sensitive Ad mutant Ad2ts1 that is closely related to Ad5. Ad2ts1 mutants poorly lyse the endosomal membrane when the virus is grown at non-permissive temperatures due to an increased capsid stability that lacks intra-endosomal disassembly. Ad2ts1 mutants grown at permissive temperatures lyse the endosome with the same efficiency as the wt virus. Incorporation of radiolabeled amino acids was compared to alpha-sarcin treated cells. When we added alpha-Sarcin and Ad5-VI-wt, Ad5-VI-M1 or the Ad2ts1 mutant grown at a permissive temperature translation was diminished in a dose-dependent manner over at least two orders of magnitude, showing efficient cytoplasmic delivery of the toxin. Ad2ts1 grown at the non-permissive temperature did not inhibit translation in this assay (Figure 2G). We observed no difference between the PPxY-mutant virus and the wt controls. This indicated that an attenuating effect of the PPxY-mutation occurs after endosomal lysis and prior to the onset of replication.
We next characterized the release of protein VI from fluorescently labeled Ad-VI-M1 and Ad5-VI-wt following synchronous infections of U2OS cells using the infection assay as described for Figure 1. Ad5-VI-wt released protein VI within 5 min (Figure 3A, left panel). In contrast, we observed a delayed release of protein VI from Ad5-VI-M1 (15% of M1 after 5 min compared to 38% of wt, Figure 3A) and an increase in colocalization of viral particles with protein VI at the nucleus (Figure 3A). This observation suggests a delayed accessibility of protein VI within the virus or a defect in protein VI dissociation from the virion (Figure 3A, images to the right). In addition to the increase of protein VI capsid association, Ad5-VI-M1 appeared to be more evenly distributed throughout the cell and did not efficiently accumulate at the MTOC region (Figure 3B, images to the left). Quantification revealed that 45 min post-infection approximately 60% of the wt viral particles in proximity of the MTOC could be found within a 10 µm radius around the MTOC and 40% within 10–20 µm. In contrast, the localization for the M1 virus was 50% for each region showing a decreased targeting towards the MTOC (Figure 3B, right panel). In summary these data suggest that the PPxY motif in protein VI is required for proper uncoating and normal nuclear targeting.
Fig. 3. Adenovirus with altered PPxY motif lacks efficient protein VI release and shows reduced MTOC accumulation. 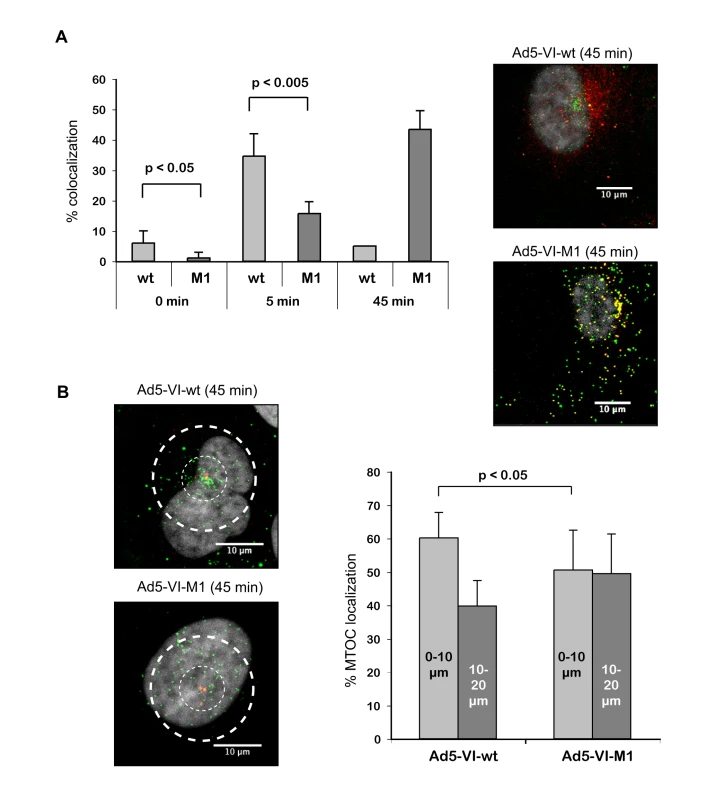
A) Protein VI release from Ad5-VI-wt vs. Ad5-VI-M1 particles. Left panel; Shown is the percentage of viral particles that are positive for protein VI co-stain (+/− standard deviation) at different time points. Over 700 particles were counted for each condition. Due to the strong overlap of the signals 45 min after infection at the MTOC region for the wt, colocalization is given as an approximate value. Right panel; Intracellular localization of protein VI and capsids 45 min after infection. The panel shows Alexa488 labeled Ad5-VI-wt (top panel) or Ad5-VI-M1 (bottom panel) capsids in green and protein VI detected with protein VI specific antibodies in red 45 min after infection of U2OS cells. Colocalization of protein VI and the capsid appear as yellow. The scale bar is 10 µm. B) Ad-VI-M1 has a reduced MTOC accumulation. Left panel; Cells were infected with fluorescently labeled Ad5-VI-wt (top) or Ad5-VI-M1 (bottom). Subcellular localization in a representative cell is shown after 45 min. The scale bar is 10 µm. A quantification of the MTOC localization is shown to the right. Cells were synchronous infected with Alexa488 labeled Ad5-VI-wt or Ad5-VI-M1 respectively after preadsorption in the cold. 45 min post infection cells were fixed and stained for the MTOC using a rabbit anti-pericentrin antibody. To quantify the targeting of the virus towards the MTOC, confocal 0.4 µm sections were taken around the MTOC stain (∼3–5 sections), and combined using maximum image projection. Two concentric circles with 10 µm and 20 µm in diameter were positioned around the MTOC as shown in the left panel. Virus particles were then counted inside the 10 µm radius and in the region between 10–20 µm. The graph to the right shows the relative abundance within the two regions. Distribution for the wild type virus is shown on the left of the graph and for the PPxY mutated virus to the right. The analysis shows that mutated particles are less likely to accumulate at the MTOC then wt particles. The error bar represents cell-to-cell variation (n>15, p<0.05). Intracellular dynamics of protein VI depend on the PPxY motif and microtubules
To understand the accumulation defect of Ad5-VI-M1 at the MTOC, we expressed wt (VI-wt), mutant (VI-M1) and protein VI deleted in the amphipathic helix (VI-ΔΦ) fused to mRFP in cells and analyzed protein VI localization in relation to microtubules (Figure 4). We found VI-wt in a punctuated distribution throughout the cell suggesting association with a vesicular compartment or tubulo-vesicular structures associated with microtubules (Figure 4, top row). In contrast, the PPxY motif mutant VI-M1 localized to a more central compartment and was rarely associated with the microtubules (Figure 4, middle row). Deletion of the amphipathic helix, in contrast, abrogated membrane association, causing nuclear targeting of protein VI and redistribution of protein VI from membrane fractions into soluble fractions ([37], and Figure 4 bottom row, data not shown).
Fig. 4. Subcellular localization of protein VI. 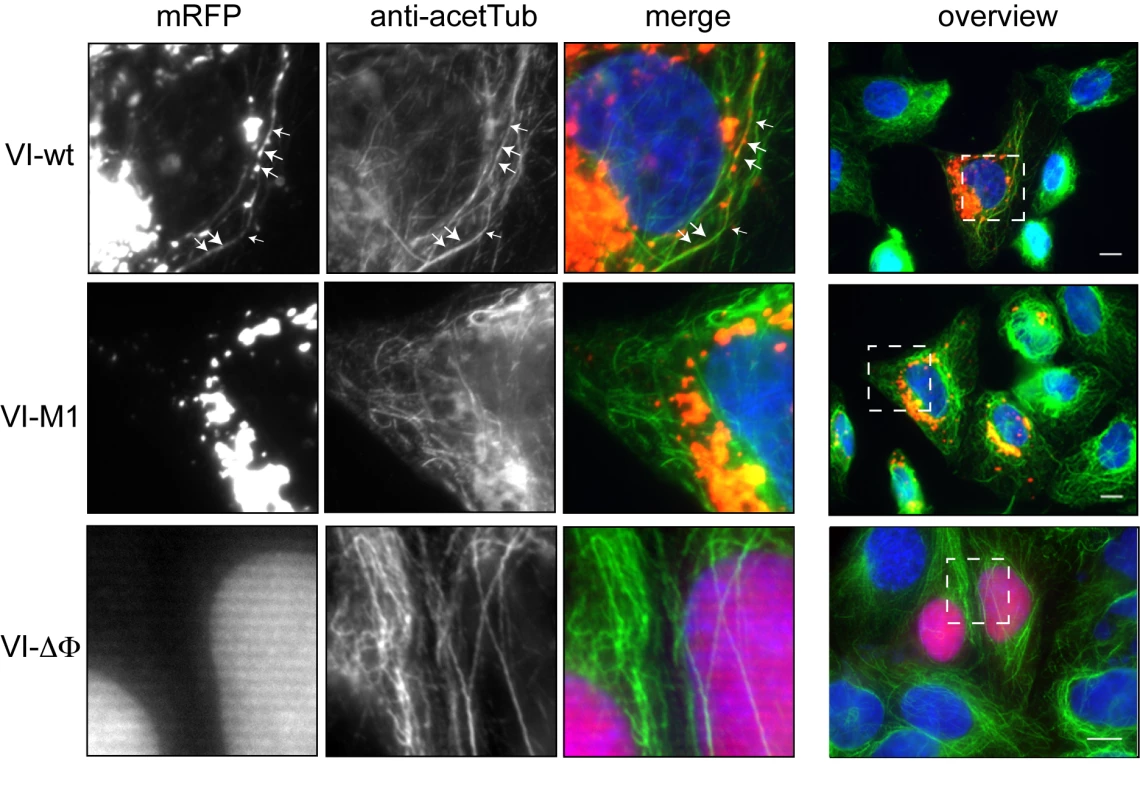
U2OS cells were transfected with protein VI fused to mRFP, either using wt protein VI (VI-wt, top row), with mutated protein VI (VI-M1, middle row) or with deleted amphipathic helix (VI-ΔΦ, bottom row). Cells were co-stained for microtubules. The protein VI-signal is shown in the left column, the microtubule stain is shown in the second column and an overlay of the signals is shown in the third column. Protein VI fusions appear in red, microtubules in green and the nucleus in blue. The right column shows the field of cells from which the insets (small white square) was taken. The scale bar is 10 µm, Please note that the lower panel is a higher magnification. Association of protein VI with microtubules in tubulo-vesicular structures is indicated by arrows in the top row. Further examples of tubulo-vesicular structures can also be seen in Video S1 showing life-cell imaging of VI-wt transfected cells. Owing to its association with membranes, microtubules and the viral capsid, we next asked whether protein VI displayed intracellular dynamics that could explain virus trafficking. We therefore performed live-cell imaging (LCI) using cells expressing mRFP-VI-wt or mRFP-VI-M1. We found that VI-wt was fast moving with short - and long-range movements whereas VI-M1 was essentially motionless (Figure 5A and Video S1). The length of the trajectories and the movement of >300 particles were plotted (Figure 5B). We found that protein VI-M1 motility was greatly reduced compared to protein VI-wt. We next asked whether VI-wt motility depends on intact microtubules and/or actin filaments. Disrupting actin filaments with cytochalasin B had no apparent effect on VI-wt localization or motility (Figure 5B, right panel) suggesting that actin was not involved in the movement. In contrast, protein VI motility in nocodazole-treated cells was strongly reduced resembling the reduced motion observed for the M1 mutant (Figure 5B, right panel, see also Video S2 in the supporting information).
Fig. 5. Subcellular dynamics of protein VI. 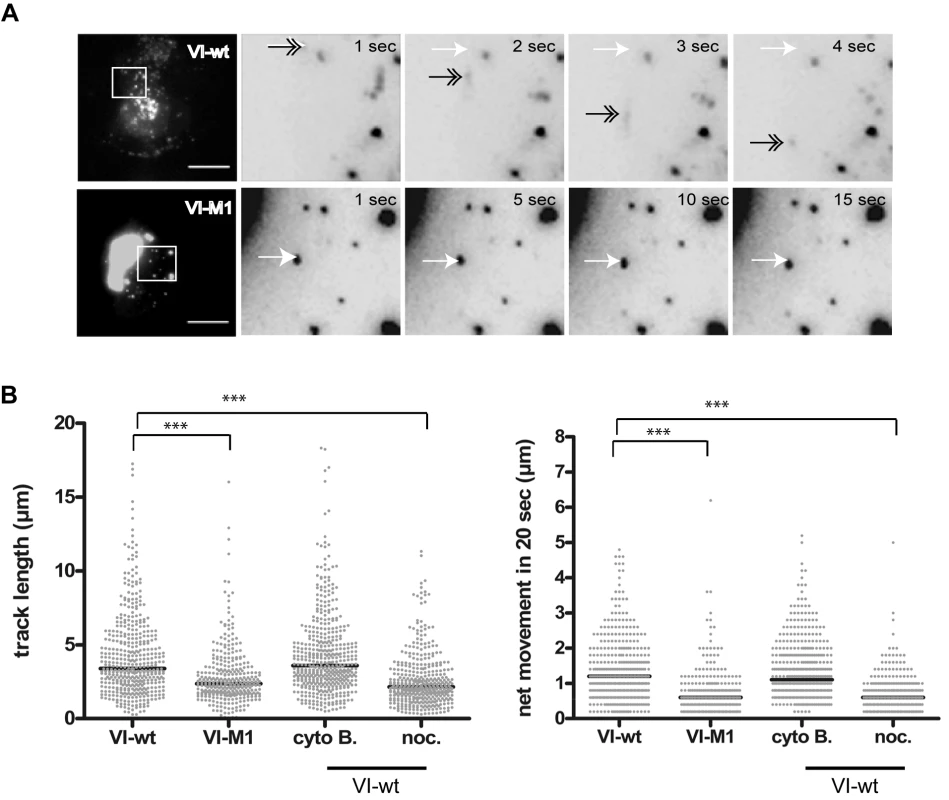
A) U2OS cells were transfected with VI-wt (top row) or VI-M1 (bottom row) fused to mRFP and analyzed by live-cell imaging. Frames were taken at 1 sec intervals using a Cascade 512B 2 camera. The left image in all rows shows maximum image projections of the full frame depicting the cell with scale bar of 10 µm. The four inverted images to the right show magnifications from the boxed inset of four consecutive frames (in the case of VI-wt) and every fifths frame (in the case of VI-M1). In the top panel a moving particle is depicted by a grey arrow within each frame using the departure point depicted by a white arrow as reference. The lower panel shows VI-M1 not moving. B) Analysis of trajectory length and relative particle speed of VI-wt or VI-M1 (left side of both panels) or trajectory length and relative particle speed of VI-wt using drugs as indicated (right side of both panels). Movies were processed and all recorded trajectories were analyzed for length and relative speed using the Imaris™ software package. Individual particles were plotted for length of trajectories (in µm, left panel) and the net movement (in µm/20sec, right panel) under the conditions tested (indicated below each chart). Significant differences are indicated by bars. We asked whether VI-M1 showed only plus-end microtubule directed movement. We applied nocodazole to VI-M1 transfected cells, followed by washout of nocodazole. During treatment and after removal of nocodazole no movement or relocalization towards the cell center was observed for VI-M1. In contrast VI-wt rapidly stopped and restarted bidirectional movement under these conditions (data not shown). Together these data suggested that protein VI is a highly mobile protein that moves along microtubules, presumably in association with vesicular structures whose motion depends on the PPxY motif.
PPxY motif is essential for protein VI ubiquitylation upon partial Ad disassembly
PPxY domains are the physiological targets of ubiquitin ligases of the Nedd4 family [16]. Therefore we asked whether protein VI ubiquitylation depends on the PPxY motif. We adapted an in vitro Ad disassembly assay mimicking the partial capsid disassembly believed to occur during Ad entry by exposing virions to 48°C. This assay dissociates the vertices including fiber, penton, protein VI and peripentonal hexons, but leaves the remainder of the capsid intact (Figure S5; [28]). Heat and mock-treated samples were subjected to in vitro ubiquitylation reactions, using free ubiquitin, recombinant E1 and E2 enzymes and purified cytosol as source for the E3 ubiquitin ligase(s), and analyzed by western blot (Figure 6, [28]). Western blot analysis showed that partial capsid disassembly resulted in the appearance of protein VI reactive signals with discrete size increments suggesting predominant modification with two to three ubiquitin as well as some higher molecular weight bands (lane 2, Figure 6A). In contrast, the lack of capsid disassembly (lane 1) or cytosol (lane 3) showed no additional protein VI reactive bands. We also tested ubiquitylation of the capsid proteins fiber, protein IIIA and penton base as internal control. We only detected ubiquitylation of the penton base (which also harbors two PPxY domains at its N-terminus), while the fiber and protein IIIA (which lack PPxY motifs) were not modified (Figure S5). The ubiquitylation of protein VI was also confirmed by using GST-ubiquitin in the above assay, followed by GST-pulldown to show covalent modification of protein VI with ubiquitin confirming the predominant modification with two to three ubiquitin-moieties (data not shown).
Fig. 6. Protein VI is ubiquitylated following capsid disassembly. 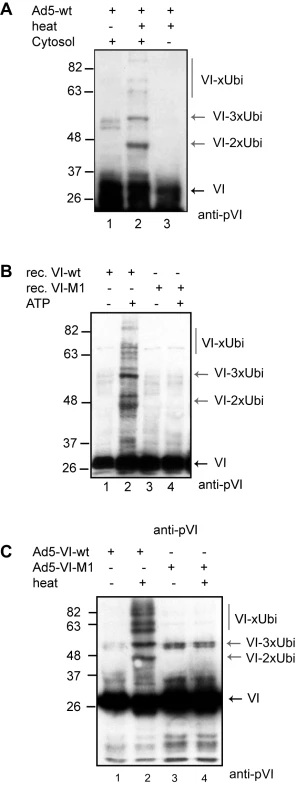
A) Purified Ad5 particles where used in in vitro ubiquitylation reactions, using ubiquitin, recombinant E1 and E2, an energy regenerating system and purified cytosol as source of the E3-ligase, and analyzed by western blot using antibodies against protein VI. Partial disassembly was induced by heat (lane 2 and 3). Cytosol was added to the reactions in lane 1 and lane 2. Protein VI-reactive bands are indicated by the black arrow and modified protein VI is indicated by grey arrows. B) Reactions as in A) using bacterially expressed protein VI-wt (lane 1 and 2) or VI-M1 (lane 3 and 4) with or without energy regenerating system as indicated (ATP). Western blot analysis using anti-protein VI antibodies is shown with ubiquitylated proteins marked with grey arrows (VI-Ubi) and unmodified protein VI (VI) is marked with a black arrow. C) Reactions as in A) followed by detection of protein VI by western blot using Ad5-VI-wt (lane 1 and 2) and Ad5-VI-M1 capsids (lane 3 and 4). Disassembly of capsids is indicated above each lane and protein VI reactive bands are marked as in B to the right. To address the role of the PPxY motif in protein VI ubiquitylation, we repeated the in vitro ubiquitylation assay using wt or M1 mutant protein VI purified from E. coli followed by western blot analysis. We detected protein VI-reactive bands, consistent with protein VI modified with two to three ubiquitin (Figure 6B, lane 2). In contrast, no modification was observed when the PPxY motif was mutated (Figure 6B, lane 1) or in the absence of ATP (Figure 6B, lane 3 and 4). Using viral particles in in vitro ubiquitylation reactions (following partial capsid disassembly) protein VI of Ad5-VI-M1 was not ubiquitylated (Figure 6C, lane 3 and 4), while protein VI from Ad5-VI-wt was (Figure 6C, lane 1 and 2). Thus, the PPxY motif in protein VI is inaccessible in intact capsids but can recruit ubiquitin ligase activity from cytosol when protein VI is released from the capsid interior. Together our results show that protein VI ubiquitylation depends on i) virus disassembly, ii) an intact PPxY domain and iii) the presence of a cytosolic ubiquitylation activity.
Protein VI binds to Nedd4 ligases via the PPxY motif
To identify the ligase responsible for protein VI ubiquitylation, we focused on the Nedd4-family members Nedd4.1, Nedd4.2, AIP4/Itch, WWP1 and WWP2 because they can interact with viral late domains that harbor PPxY motifs [38]. We first co-expressed the VI-wt or VI-M1 mRFP fusion protein together with each of the E3 ligases fused to GFP in U2OS cells. When expressed alone, most ligases localized primarily to the cytoplasm (data not shown, WWP1 localized to the plasma membrane and WWP2 accumulated in an uncharacterized intracellular membrane compartment). In contrast, when VI-wt is coexpressed with Nedd4.1, Nedd4.2 or AIP4/Itch, the ligases are recruited to the same membrane compartment as protein VI (Figure 7A, row 1–3). WWP1 appears to sequester protein VI at the plasma membrane (Figure 7A, row 4). WWP2 does not colocalize with VI-wt (Figure 7A, row 5). We did not detect significant colocalization between VI-M1 and the E3 ligases, consistent with a PPxY-dependent interaction (Figure S6).
Fig. 7. Protein VI interacts with Nedd4 ligases via the PPxY motif. 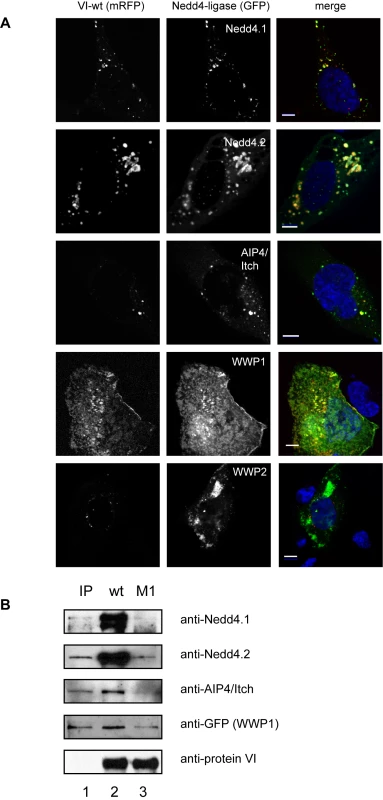
A) Protein VI (VI-wt) was N-terminally fused to mRFP and co-transfected with GFP-Nedd4.1 (first row), GFP-Nedd4.2 (second row), GFP-AIP4/Itch (third row), GFP-WWP1 (fourth row) and GFP-WWP2 (last row). Confocal images of representative cells are shown and the mRFP signal for protein VI (left column), the GFP signal for the ligases (center column) and the merged signals together with DAPI stain of the nucleus (right column) is indicated above each column. Transfected plasmids are indicated. Colocalization of Nedd4 and protein VI results in a yellow signal. The scale bar is 10µm. B) Diverse GFP-tagged Nedd4 ligases were over-expressed in cells and cytosolic extracts were used for pulldown experiments using recombinant protein VI-wt or VI-M1 coupled to beads. 10% of the input material (IP) is shown in the first lane. Bound material for protein VI-wt (lane 2) and VI-M1 (lane 3) was detected with respective antibodies as indicated to the right. Co-eluted protein VI detected with a protein VI specific antibody is shown as loading control in the lower lane. To determine whether any of the ligases specifically interact with protein VI, we used purified cytosol from cells overexpressing GFP-tagged ligases and performed pull-downs with beads coated with recombinant protein VI-wt or VI-M1. Two ligases, Nedd4.1 and Nedd4.2, were highly enriched on VI-wt beads while none of the other ligases showed strong binding to VI-wt - or to VI-M1-beads (Figure 7B). Taken together, these data suggest a preferential interaction between Nedd4.1 and Nedd4.2 and the PPxY motif in protein VI, which leads to relocalization of the ligases from the cytoplasm to a membrane compartment.
Nedd4 ligases ubiquitylate protein VI via the PPxY and reduce Ad transduction and MTOC accumulation
To further characterize the interaction between protein VI and the ligases, we knocked down Nedd4.1, Nedd4.2, AIP4/Itch, WWP1 and WWP2 using siRNAs (Figure 8A). The cells were then incubated with an Ad5 vector harboring a GFP expression cassette (AdGFP) at a low multiplicity of infection (30 physical particles per cell) for 3 h to achieve approximately 20% transduced cells and limit the time of virus exposure. The following day the percentage of GFP-positive cells was quantified by flow cytometry. Most ligase knockdowns had no significant effects on transduction, but Nedd4.2 knockdown diminished transduction by 50% (Figure 8A).
Fig. 8. Nedd4.2 ubiquitylates protein VI and is required for efficient Ad transduction and MTOC accumulation. 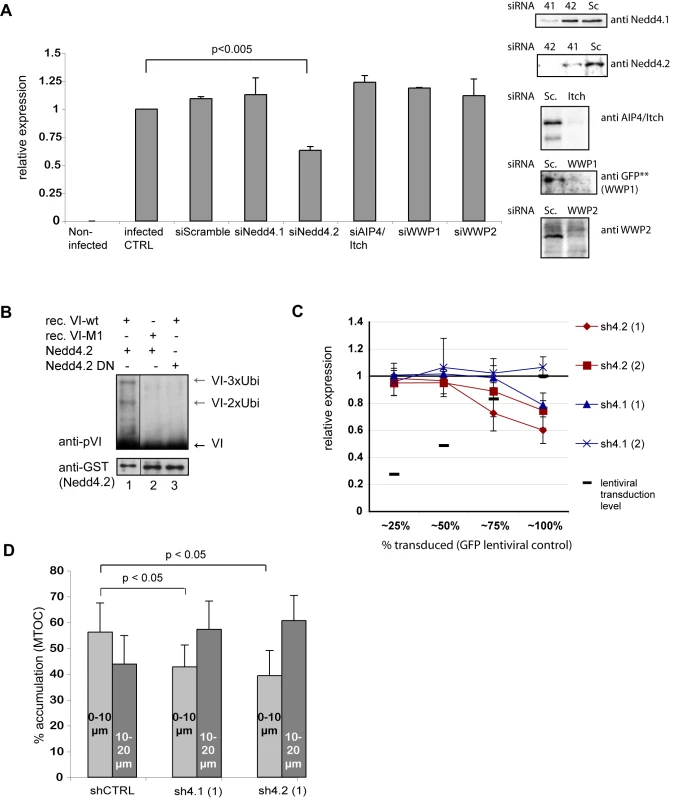
A) Effects of Nedd4 depletion on Ad transduction. U2OS cells were transfected with siRNAs specific for Nedd4.1, Nedd4.2, AIP4/Itch, WWP1 and WWP2 or scrambled siRNAs. Western blot controls for depletion are shown to the left (except for WWP1 where depletion of transfected WWP1-GFP was detected using GFP specific antibodies because no specific antibodies were available for detection of the endogenous protein). Following depletion, cells were transduced with AdGFP. Relative GFP expression is shown. Statistical analysis was performed using pairwise comparison using the Mann & Whitney-test showing significant reduction for Nedd4.2 depleted cells. B) In vitro ubiquitylation reaction using recombinant active Nedd4.2 (lane 1 and 2) or inactive Nedd4.2 (lane 3). Recombinant VI-wt (lane 1 and 3) or VI-M1 (lane 2) was used as substrate. Modified protein VI (grey arrows) or unmodified protein VI (black arrows) was detected following western blot analysis. Antibodies are indicated to the left of each blot. C) Dose dependent depletion of Nedd4.2 and Nedd4.1. U2OS cells were transduced with increasing amounts of shRNA expressing lentiviral vectors achieving transduction levels as indicated (from 25–100%) and transduced with AdGFP. GFP expression levels were determined 24 h later using flow cytometry. GFP expression levels within each condition were normalized to transduction controls of cells treated with shRNA expressing lentiviral vectors against luciferase (arbitrarily set to 1). Values are the mean (+/− standard deviation) of at least two experiments done in triplicates D) Accumulation of Ad at the MTOC region following Nedd4 depletion. Fluorescently labeled Ad was used to infect cells following control depletions (shLuc) or depletion of Nedd4.1 or Nedd4.2 using (sh4.1 (1) and sh4.2 (1) as in C). Subcellular localization of viral particles was determined at 45 min. post infections. Cells were fixed and stained for the MTOC using a pericentrin antibody. Particles were counted and scored for their relative proximity to the MTOC using two concentric circles with 10 and 20 µm diameter around the MTOC (compare also Figure 3). The graph shows percentage of viral particles within 10µm or 10–20 µm proximity to the MTOC as indicated. Note that in Nedd4.1 and Nedd4.2 depleted cells the relation is inverted compared to control depleted cells showing less viral particles accumulating at the MTOC. The error bar represents cell-to-cell variation (n>15, p<0.05). Because Nedd4.2 showed the strongest effect on Ad transduction we determined whether bacterially expressed and purified Nedd4.2 could ubiquitylate purified protein VI in vitro. A minimal system where cytosol was replaced by recombinant Nedd4.2 was sufficient for protein VI ubiquitylation (Figure 8B, lane 1). The ubiquitylation pattern was similar to that obtained with cytosol (Figure 6) and required an intact PPxY and a catalytically active Nedd4.2 (Figure 8B, lane 2 and 3).
Nedd4.1 and Nedd4.2 both showed strong interaction with protein VI in pulldown assays. To further investigate the role of each ligase we transduced cells with lentiviral vector expressing shRNAs against either Nedd4.1 or Nedd4.2 or luciferase as a control. We transduced cells in a dose-dependent manner to achieve different levels of knockdown. Transduction efficiency was monitored using a GFP expressing lentiviral vector in control cells. Seven days post-transduction shRNA treated cells were infected with AdGFP virus as described above and the transduction rate was determined by flow cytometry. We observed a dose-dependent decrease in infectivity for two different shRNAs against Nedd4.2, which was similar to what we observed when we used siRNAs (Figure 8C). The results for shRNAs against Nedd4.1 were less clear. One shRNA also reduced viral infection at very high transduction rates but to a lesser extent than shRNAs against Nedd4.2 while a second shRNA showed no effect on Ad transduction (Figure 8C). A combined treatment of cells with either siRNAs or shRNAs against Nedd4.1 and Nedd4.2 did not further decrease Ad-transduction indicating that the effects of Nedd4-ligase knockdowns on Ad transduction may be complex (data not shown).
A hallmark of Ad5 infection is its transient accumulation at the MTOC during entry [5]. The mutation of the PPxY in the Ad5-VI-M1 virus seemed to alter this localization (Figure 3). Similarly, the PPxY motif was required for Nedd4.2 dependent ubiquitylation of protein VI. Because knockdown of Nedd4 ligases also diminished transduction with AdGFP vectors we examined accumulation at the MTOC region in cells depleted with control shRNA and Nedd4.1 and Nedd4.2 specific shRNAs. We used the same strategy as in Figure 3 by quantifying viral particles in proximity to the MTOC, which was identified by stain for pericentrin. MTOC accumulation for Nedd4.1 and Nedd4.2 shRNA treated cells was reduced when compared to cells treated with control shRNA indicating that both ligases might be involved in proper targeting of viruses towards the MTOC (Figure 8D).
In summary, these data provide evidence that release of protein VI during entry and a possible interaction between the PPxY motif of protein VI and Nedd4-family ligases are determinants of Ad5 trafficking during infection.
Discussion
In this study we show that the Ads internal capsid protein VI harbors a PPxY-motif that is involved in virus entry and infectivity. For Ads, reaching the nucleus requires a series of sequential steps: receptor-mediated endocytic uptake, partial capsid disassembly, endosomal rupture, microtubule based transport to the MTOC and nuclear trafficking. The link between these steps has been best exemplified in the case of the thermostable temperature-sensitive mutant Ad2ts1. This mutant enters cells by receptor-mediated endocytosis, but remains in an endosomal compartment due to increased capsid stability. Therefore, Ad2ts1 particles are directed to lysosomes for destruction and/or recycled back to the surface thus precluding accumulation at the nuclear pore complex [26],[28],[39]. The role of facilitating endosomal escape during Ad entry was initially assigned to the penton base [40]. Later, Wiethoff and co-workers showed that most membrane lytic activity of Ad viral capsids comes from the predicted N-terminal amphipathic helix of the internal capsid protein VI, and that membrane lytic activity required partial capsid disassembly to release protein VI [28].
Here we present several lines of evidence that protein VI plays an additional and previously unidentified role in nuclear targeting of the Ad capsid. We show that protein VI exposure from Ad5 capsids occurs within minutes when pre-adsorbed Ad5 is shifted from 4°C to 37°C. This is consistent with the loss of the fiber prior to internalization and the rapid accumulation of Ad5 in the cytosol [41],[42]. We found that significant amounts of protein VI remains partially associated with the viral capsid after the initial exposure and until the viral particle accumulates at the MTOC or the nuclear rim.
We show that protein VI is engaged in rapid intracellular trafficking that depends on intact microtubules and requires the N-terminal amphipathic helix for microtubule association and the PPxY motif for motion. To our knowledge protein VI is the first Ad capsid protein described that possesses its own microtubule-dependent dynamics and future work has to address if other capsid proteins have similar properties. Inactivation of the PPxY in the viral context (Ad5-VI-M1) resulted in a post-entry delay that reduced infectivity and prevented efficient accumulation of the entering virus particles at the MTOC. While we cannot exclude the possibility that the PPxY motif in protein VI also plays a role in adenoviral replication, assembly or egress, our single round infection assays showed that the majority of the titer reduction for the mutant was related to steps prior to the initiation of replication or the delivery and expression of a reporter gene. Moreover, our data show that the efficiency with which a toxin is delivered to the cytosol during Ad infection is similar for mutant and wt virus. This provides strong evidence that the PPSY motif in protein VI has a function during cell entry, but probably only after endosomal membrane lysis has occurred. Current structural data place protein VI inside the assembled capsid, therefore potentially precluding it from functions during egress at least when capsid associated [43]–[45]. Our observations show that protein VI is exposed after entry and that capsid disassembly is required for its ubiquitylation, which is consistent with our hypothesis that the PPxY motif is accessible only during Ad entry following partial capsid disassembly. Furthermore, it is currently not clear whether late domains containing PPxY motifs present in other viral systems, which are required for viral egress, have additional functions. Mutational inactivation of PPxY motifs in the VP40 structural protein of Ebola virus and matrix protein of rabies virus both showed an attenuation of the virus and a reduction of infectivity [20],[46]. Interestingly, for Ebola virus, the PPxY mutants showed no budding defect, but virus production was reduced, which could indicate a disruption earlier on in the life cycle than previously thought [46]. For rabies virus, Wirblich et al. describe a budding defect of the late-domain mutant but also noted a reduction of early mRNA production [20].
Earlier studies have shown that PPxY type late domains bind to ubiquitin ligases of the Nedd4 family rather then directly to class E VSP proteins [13]. Our study showed that the PPxY motif in protein VI can interact with all Nedd4 ligases tested but preferentially binds to Nedd4.1 and Nedd4.2 and re-targets them to membranes. Owing to topological constrains, recognition of the PPxY domain should require membrane rupture because of the cytosolic localization of Nedd4 ligases, which according to our data does not seem to be affected by the PPxY mutation in the M1 virus.
The PPxY motif in protein VI seems to favor interaction with Nedd4.1 and Nedd4.2 although we observed interactions with AIP4/Itch and WWP1 as well. It is possible that an interaction between protein VI and WWP1, as we observe in transient transfections, is circumvented by exposure of the PPxY domain after the virus has entered the endosomal compartment. A role for Nedd4.1 or Nedd4.2 in Ad entry is underscored by our observation that Nedd4.2 can directly ubiquitylate protein VI via the PPxY motif and its depletion (and to a lesser extent also depletion of Nedd4.1) reduces Ad transduction. In addition this depletion also reduced MTOC accumulation of viral particles following infection, which is similar to the effect of the PPxY mutation in the M1 virus. However the effects were modest, indicating that additional mechanisms contribute to Ad entry. Further studies will be needed to identify a specific role for each ligase in Ad entry and trafficking towards the MTOC and to determine whether other ligases like AIP4/Itch and WWP1 are involved.
How ubiquitylation of protein VI or interaction with Nedd4 ligases directs accumulation of Ads at MTOCs remains unknown. Ubiquitylated protein VI could be specifically recognized by cellular factors. Alternatively, recruitment of Nedd4.1 and/or Nedd4.2 by protein VI could result in the ubiquitylation of other cellular factors that constitute an efficient transport means used by the virus. Recent work has shown that some members of ESCRT-I become ubiquitylated when Nedd4.2 is overexpressed [18],[19]. Therefore, it is possible that Nedd4.2 (or other Nedd4-ligases) binding to protein VI could activate the ESCRT pathway via ubiquitylation. Whether membrane compartments or the ESCRT pathway plays a direct role in Ad virus transport during entry remains to be addressed. However, ESCRT components can be found at the endosomal compartments as well as associated with the centromeric region [47]. It is noteworthy that endosomal escape is also required for interferon induction by Ad via yet unknown mechanisms [48]. This pathway may also be related to protein interaction between protein VI and Nedd4-family ligases.
Release and separation of protein VI from the capsid is clearly defective in the Ad5-VI-M1 mutant virus. A lack of functional PPxY may therefore block disassembly steps, preclude efficient endosomal escape following the membrane lysis event or fail in the recruitment of subsequent factors required to efficiently link the virus with retrograde transport pathways. Penton base also encodes PPxY motifs [49]. Because eliminating the PPxY motif from protein VI had only modest effects on MTOC accumulation, there may be functional overlap between the PPxY in protein VI and penton base. This is further supported by the observation that ubiquitylation of penton could be abolished by depleting the cytosol with protein VI indicating that maybe the same ligases are involved (Figure S5).
In conclusion, our study is the first demonstration of a PPxY motif, previously described for viral late domains, present in a non enveloped DNA virus that exerts a function during entry. Conservation of the amphipathic helix and PPxY motif in all Mastadenovirus suggests that protein VI fulfils a key function. We suggest that after endocytosis cell or serotype specific capsid disassembly cues regulate exposure, membrane-interaction and ubiquitylation of protein VI (and possibly other capsid proteins). This could facilitate the recruitment of a common cellular microtubule-dependent pathway for retrograde trafficking. This mechanism could be more important in vivo in highly polarized epithelia or neurons where long-range movement is crucial and might be less important in cell culture models [50]. Given the prevalence of viral late domains, ubiquitylation and trafficking towards the MTOC our results may have uncovered a more general mechanism by which viruses and other cargos achieve intracellular delivery and provide a rational to look for further “early” functions of PPxY motif containing late-domains in other viral systems.
Materials and Methods
Cell lines, cell culture and virus production
Immunofluorescence experiments and infection experiments were performed using U2OS cells (human bone osteosarcoma epithelial cells), hTERT-RPE1 cells (human retina epithelia pigment cells, Clontech) or A549 cells (human alveolar basic cells). Cytoplasmic extracts for pulldowns were prepared from 293T cells (human embryonic kidney cells). All cells except hTERT-RPE were grown in DMEM Glutamax™ (Gibco) supplemented with 10% of fetal calf serum (FCS) (Biowest). hTERT-RPE1 cells were a kind gift from M. Bonhivers (University of Bordeaux 2) and grown in DMEM/HamsF12 media supplemented with 10% FCS according to the suppliers instructions. Prior to infection experiments, cells were serum starved for 24h to induce primary cilia growth [32]. Recombinant Ad5-VI-wt and Ad5-VI-M1 viruses and their GFP expressing counterparts were constructed as described in the supplemental material. Amplification of viruses was done in 293 cells and purified using double CsCl2-banding. Virus particle to cell ratios were calculated based on the estimated copy numbers of viral genomes. Copy numbers were calculated according to Mittereder et al. [51]. Briefly, purified particles were diluted 1∶10 in virus lysis buffer (0.1% SDS, 10 mM Tris/HCl pH 7.4, 1 mM EDTA) and incubated for 10 min at 56°C to release the viral genomes and the OD260 was determined. Calculations were based on 1 OD260 = 1.1×1012 particles/ml [51] .
Lentiviral vector production for shRNA encoding vectors was done by the service platform for lentiviral vector production of the Institute Federative de Recherche 66 of the Bordeaux 2 University. Prevalidated lentiviral vectors encoding shRNAs in the vector backbone pLKO.1 against Nedd4.1 and Nedd4.2 were purchased from the Mission™ shRNA collection from Sigma. For downregulation of Nedd4.1 we used NM_006154.1-1753s1c1 (sh4.1 (1), CCGGCCGGAGAATTATGGGTGTCAACTCGAGTTGACACCCATAATTCTCCGGTTTTT) against the coding sequence and NM_006154.1-3522s1c1 (sh4.1 (2), CCGGGCCTTTCTCTTGCCTGCATATCTCGAGATATGCAGGCAAGAGAAAGGCTTTTT) against the 3′ UTR. For downregulation of Nedd4.2 we used NM_015277.x-2772s1c1 (sh4.2 (1), CCGGGCGAGTACCTATGAATGGATTCTCGAGAATCCATTCATAGGTACTCGCTTTTT) against the coding sequence and NM_015277.x-3959s1c1 (sh4.2 (2), CCGGCCTGTTTGTATGCGTTTGCTACTCGAGTAGCAAACGCATACAAACAGGTTTTT) against the 3′ UTR. Control vectors for shRNAs encoded for shRNA against luciferase (Sigma) and control vectors for transduction and estimation of the titer encoded for GFP.
Plasmids, siRNA
All sequences for protein VI were derived from Ad serotype 5 (Ad5) and cloned into the Gateway™ compatible entry vector pDONR221. Sequence verified DONR plasmids were used for recombination into Gateway™ compatible destination vectors for N-terminal fusion of mRFP (L30-mRFP, kindly provided by E. Bertrand). Bacterial expression vectors for protein VI are based on pET15b. Site-directed mutagenesis was used to change amino acids 148-PPSY-151 to 148-PGAA-151 in protein VI. N-terminal tagged expression vectors for Nedd4.1, and Nedd4.2 were provided by E. Bertrand [52] and tagged expression vectors for AIP4/Itch and WWP1 were a kind gift of Paul Bieniasz (Rockefeller University, New York). Bacterial expression vectors for catalytically active murine GST-Nedd4.2 and the inactive GST-Nedd4.2-DN was kindly provided by S. Kumar [53].
siRNAs were purchased as duplexes from EuroGentec (only the reverse strand is shown): Scramble (5′-CGCAAUUCGAUGUCCCGUGdTdT), Nedd4.1 (5′-AAACAACCCAGCCAGGCUCdTdA), Nedd4.2 (5′-CUGUGACUUUGUGUUGUGGdTdA), were previously described by Segura-Morales et al. (2005), AIP4/Itch siRNAs (5′-UCAUCAUUCUGAGAAGCACdTdT, [54] , and WWP1 siRNA (5′-CUUCUACGAUCAUCAACUCdTdT) was previously described by Chen et al. (2005). The WWP2 siRNA was a Smartpool from Dharmacon.
RNA interference, adenoviral transduction and FACS analysis
Depletions were performed in 12-well dishes using 2×105 U2OS cells. Cells were transfected after 24 and 48 h with 20 pmol of each siRNA duplex per well. Forty-eight hours after the first transfection cells were transduced using 30 physical particles per cell of Ad5-GFP virus for 3 h without prior pre-adsorption. Cells were harvested 24 h later and analyzed by flow cytometry for GFP expression and further processed for western blot analysis to verify knock-down efficiency. Acquisitions were done with FACSCalibur® or FACSCantoII® cytometer (BD Biosciences) and the data were processed and analyzed by the CellQuest® Pro and FACSDIVA® software (BD Biosciences).
Labeling and infection assays
Purified Ad particles were labeled using the Alexa-488 microscale protein labeling kit (Invitrogen) using the manufacturers protocol. Infectivity of labeled virus preparations was determined by quantification of GFP-transduction. Only preparations with >90% activity where used. For time course experiments, U2OS cells were seeded at semiconfluency on coverslips. Pre-binding was done with 5000 physical particles per cell in 100 µl at 4°C on a shaking platform for 1 h. At t0 coverslips where rinsed in cold DMEM and transferred to pre-equilibrated (37°C, 5% CO2) DMEM. At indicated time points the cells where fixed and processed for IF analysis.
Analysis of membrane penetration (Sarcin assay)
A549 lung epithelial cells were plated in 96-well plates at a density of 10,000 cells/well on the day before infection. The cells were washed once with DMEM without cysteine or methionine and supplemented, 2 mM glutamine, 10% dialyzed FCS, penicillin and streptomycin (DMEM-SA) and infected with the respective viruses in 50 µl DMEM-SA containing 0.1 mg/well α-sarcin (Sigma). The infected cells were incubated 30 min at 4°C to facilitate virus attachment and 90 min at 37°C to facilitate virus internalization. After this 50 µl of DMEM-SA containing 0.1 µCi of [35S]L-methionine (Hartmann Analytic) was added to each well and the cells were incubated for an additional 60 min at 37°C for labeling. The cells were then washed with 100 µl PBS and extracted in 150 µl lyses buffer containing 1% Triton-X100, 150 mM NaCl, 10 mM MgCl2, 20 mM Tris-HCl (pH 7.5) supplemented with 1× Complete™ protease inhibitor cocktail (Roche). The lysates were clarified by centrifugation at 20,000 g for 12 min. To remove the residual [35S]L-methionine, 100 µl cleared lysates were further purified with Zeba Desalt Spin Columns (Pierce). The incorporation [35S]L-methionine into the extracted fraction of newly synthesized proteins was measured by liquid scintillation using TRI-CARB 1900CA counter (Packard).
Immunofluorescence (IF)
Cells grown on coverslips were rinsed in PBS and fixed with 4% PFA in PBS and blocked/permeabilized with IF-buffer (10% FCS in PBS and 0.1% Saponin). Primary and secondary antibodies where applied to the coverslip in IF-buffer for 1 h each. Cells were mounted in DAKO mounting media containing DAPI and analyzed by confocal - or epifluorescence microscopy. For IF involving microtubule staining cells were treated with crosslinkers prior to fixation. The following primary antibodies were used in this study: mouse anti-AcTubulin (kind gift from C. Janke, Montpellier), Mouse anti E2A (kind gift of T. Dobner, Hamburg), rabbit anti pericentrin (Abcam) and rabbit anti-protein VI antibodies raised against recombinant protein VI and affinity purified (see supplemental material). Secondary antibodies Alexa546 anti mouse was from Affinity Research and Atto647 anti rabbit was from Sigma.
Microscopy and image analysis
Confocal pictures were taken on a Zeiss LSM 510 Meta confocal microscope or a Leica SP5 confocal microscope and epifluorescence pictures were taken on a Zeiss Axiolmager Z1 microscope with CoolSnap HQ Photometrics camera both equipped with Metamorph™ software. Confocal stacks where taken every 0.5 µm with a pinhole setting of 1 for all channels to achieve high local resolution. Images were processed using ImageJ and Adobe Photoshop™. Counting of viral particles was performed using the semi-automated cell counting tool from ImageJ. Colocalization analysis: Stacks from confocal images where combined as Z-projection using maximum intensity, converted into 8-bit images for each channel. Colocalization between protein VI and Ad was then determined using the colocalization finder plugin from ImageJ. Live-Cell Imaging: U2OS cells were seeded in 3.5cm glass-bottom dishes and transfected with 1.5 µg of protein VI-expressing vectors. Twenty-four h later the medium was replaced by pre-warmed OPTI-MEM (Gibco). Movies were acquired on a Nikon TE 2000 microscope with Cascade 512B 2 camera using Metamorph™ software for data acquisition (120 frames, 1 frame/sec). In some cases, 2 h before the acquisition, cells were incubated with either Nocodazole (Sigma) or Cytochalasin B (Sigma) diluted respectively at 0.4 µg/ml and 5 µg/ml in OPTI-MEM. Drugs were not removed during acquisition. Acquired movies were further processed using ImageJ to enhance protein VI particle detection by background subtraction and bleach correction. Particles were tracked using the spot-tracking tool of Imaris™ software to determine the length of their trajectories and the speed of their movement. Particle detection size was scaled to 0.75 µm and tracks were built with a maximum displacement of 1.5 µm between consecutive frames, a maximum gap size of 3 frames and a minimal track length of 20 s. At least 5 cells were analyzed for each condition that equals a minimum of 300 analyzed tracks per condition.
Transmission electron microscopy
Three microliter of purified sample virus was adsorbed to a carbon-coated film (200 mesh grids). The grids with adsorbed virus were floated onto a solution of the negative stain (1% solution of uranyl acetate). The film was picked up by a copper EM grid and then air-dried. Specimens were examined under a HITACHI H7650 electron microscope operating at 80 kV and images were further processed using ImageJ software.
Western blot and antibodies
Affinity purified rabbit anti-protein VI antibodies were used at a dilution of 1∶2000. Other antibodies used for the study were: rabbit polyclonal anti-Nedd4.1 and anti-Nedd4.2 antibodies that were a kind gift of O. Staub (Lausanne, Switzerland) (dilution 1∶1000), rabbit polyclonal anti-WWP2 antibody (sc-30052, Santa Cruz Biotechnology) (dilution 1∶200), goat polyclonal anti-WWP1 antibody (sc-11893, Santa Cruz Biotechnology) (dilution 1∶200), mouse monoclonal anti-AIP4/Itch antibody (sc-28367, Santa Cruz Biotechnology) (dilution 1∶100) and mouse monoclonal anti-GFP antibody (Roche) (dilution 1∶500). SDS-PAGE was done using 12% poly-acrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked in TBS containing 10% of dry-milk and 0.01% of Tween 20 (Sigma), followed by over-night detection of antigens using primary antibodies diluted in TBS containing 10% of dry-milk and 0.01% of Tween 20 (Sigma). Primary antibodies were detected using HRP-conjugated secondary antibodies against rabbit, goat or mouse (Sigma) at a dilution of 1∶5000. Specific signals were revealed using the enhanced chemiluminescence detection system (ECL) (PerkinElmer).
Statistical analysis
Data are presented as mean, error bars as STD. Statistical analysis if not indicated otherwise was done using unpaired students t-test (*:P<0.05; **:P<0.01; ***:P<0.005).
Supporting Information
Zdroje
1. BrandenburgB
ZhuangX
2007 Virus trafficking - learning from single-virus tracking. Nat Rev Microbiol 5 197 208
2. GreberUF
WayM
2006 A superhighway to virus infection. Cell 124 741 754
3. LeopoldPL
CrystalRG
2007 Intracellular trafficking of adenovirus: many means to many ends. Adv Drug Deliv Rev 59 810 821
4. GreberUF
2002 Signalling in viral entry. Cell Mol Life Sci 59 608 626
5. LeopoldPL
PfisterKK
2006 Viral strategies for intracellular trafficking: motors and microtubules. Traffic 7 516 523
6. BaileyCJ
CrystalRG
LeopoldPL
2003 Association of adenovirus with the microtubule organizing center. J Virol 77 13275 13287
7. SuomalainenM
NakanoMY
KellerS
BouckeK
StidwillRP
1999 Microtubule-dependent plus - and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol 144 657 672
8. StrunzeS
TrotmanLC
BouckeK
GreberUF
2005 Nuclear targeting of adenovirus type 2 requires CRM1-mediated nuclear export. Mol Biol Cell 16 2999 3009
9. YuGY
LaiMM
2005 The ubiquitin-proteasome system facilitates the transfer of murine coronavirus from endosome to cytoplasm during virus entry. J Virol 79 644 648
10. KhorR
McElroyLJ
WhittakerGR
2003 The ubiquitin-vacuolar protein sorting system is selectively required during entry of influenza virus into host cells. Traffic 4 857 868
11. DuanD
YueY
YanZ
YangJ
EngelhardtJF
2000 Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest 105 1573 1587
12. YanZ
ZakR
LuxtonGW
RitchieTC
Bantel-SchaalU
2002 Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol 76 2043 2053
13. BieniaszPD
2006 Late budding domains and host proteins in enveloped virus release. Virology 344 55 63
14. WilliamsRL
UrbeS
2007 The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 8 355 368
15. SaksenaS
WahlmanJ
TeisD
JohnsonAE
EmrSD
2009 Functional reconstitution of ESCRT-III assembly and disassembly. Cell 136 97 109
16. InghamRJ
GishG
PawsonT
2004 The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene 23 1972 1984
17. Shearwin-WhyattL
DaltonHE
FootN
KumarS
2006 Regulation of functional diversity within the Nedd4 family by accessory and adaptor proteins. Bioessays 28 617 628
18. UsamiY
PopovS
PopovaE
GottlingerHG
2008 Efficient and specific rescue of human immunodeficiency virus type 1 budding defects by a Nedd4-like ubiquitin ligase. J Virol 82 4898 4907
19. ChungHY
MoritaE
von SchwedlerU
MullerB
KrausslichHG
2008 NEDD4L overexpression rescues the release and infectivity of human immunodeficiency virus type 1 constructs lacking PTAP and YPXL late domains. J Virol 82 4884 4897
20. WirblichC
TanGS
PapaneriA
GodlewskiPJ
OrensteinJM
2008 PPEY motif within the rabies virus (RV) matrix protein is essential for efficient virion release and RV pathogenicity. J Virol 82 9730 9738
21. GalinierR
GoutE
Lortat-JacobH
WoodJ
ChroboczekJ
2002 Adenovirus protein involved in virus internalization recruits ubiquitin-protein ligases. Biochemistry 41 14299 14305
22. ZhangY
BergelsonJM
2005 Adenovirus receptors. J Virol 79 12125 12131
23. MeierO
GreberUF
2004 Adenovirus endocytosis. J Gene Med 6 Suppl 1 S152 163
24. RaumaT
TuukkanenJ
BergelsonJM
DenningG
HautalaT
1999 rab5 GTPase regulates adenovirus endocytosis. J Virol 73 9664 9668
25. WickhamTJ
MathiasP
ChereshDA
NemerowGR
1993 Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73 309 319
26. GreberUF
WillettsM
WebsterP
HeleniusA
1993 Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75 477 486
27. GreberUF
WebsterP
WeberJ
HeleniusA
1996 The role of the adenovirus protease on virus entry into cells. Embo J 15 1766 1777
28. WiethoffCM
WodrichH
GeraceL
NemerowGR
2005 Adenovirus protein VI mediates membrane disruption following capsid disassembly. J Virol 79 1992 2000
29. SaphireAC
GuanT
SchirmerEC
NemerowGR
GeraceL
2000 Nuclear import of adenovirus DNA in vitro involves the nuclear protein import pathway and hsc70. J Biol Chem 275 4298 4304
30. TrotmanLC
MosbergerN
FornerodM
StidwillRP
GreberUF
2001 Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat Cell Biol 3 1092 1100
31. WodrichH
CassanyA
D'AngeloMA
GuanT
NemerowG
2006 Adenovirus core protein pVII is translocated into the nucleus by multiple import receptor pathways. J Virol 80 9608 9618
32. LoktevAV
ZhangQ
BeckJS
SearbyCC
ScheetzTE
2008 A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell 15 854 865
33. CopelandNG
JenkinsNA
CourtDL
2001 Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet 2 769 779
34. RuzsicsZ
WagnerM
OsterlehnerA
CookJ
KoszinowskiU
2006 Transposon-assisted cloning and traceless mutagenesis of adenoviruses: Development of a novel vector based on species D. J Virol 80 8100 8113
35. YasudaJ
HunterE
1998 A proline-rich motif (PPPY) in the Gag polyprotein of Mason-Pfizer monkey virus plays a maturation-independent role in virion release. J Virol 72 4095 4103
36. GottweinE
BodemJ
MullerB
SchmechelA
ZentgrafH
2003 The Mason-Pfizer monkey virus PPPY and PSAP motifs both contribute to virus release. J Virol 77 9474 9485
37. WodrichH
GuanT
CingolaniG
Von SeggernD
NemerowG
2003 Switch from capsid protein import to adenovirus assembly by cleavage of nuclear transport signals. Embo J 22 6245 6255
38. Martin-SerranoJ
EastmanSW
ChungW
BieniaszPD
2005 HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J Cell Biol 168 89 101
39. GastaldelliM
ImelliN
BouckeK
AmstutzB
MeierO
2008 Infectious Adenovirus Type 2 Transport Through Early but not Late Endosomes. Traffic
40. Medina-KauweLK
2003 Endocytosis of adenovirus and adenovirus capsid proteins. Adv Drug Deliv Rev 55 1485 1496
41. SvenssonU
1985 Role of vesicles during adenovirus 2 internalization into HeLa cells. J Virol 55 442 449
42. NakanoMY
BouckeK
SuomalainenM
StidwillRP
GreberUF
2000 The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J Virol 74 7085 7095
43. SabanSD
SilvestryM
NemerowGR
StewartPL
2006 Visualization of alpha-helices in a 6-angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J Virol 80 12049 12059
44. RussellWC
2009 Adenoviruses: update on structure and function. J Gen Virol 90 1 20
45. FabryCM
Rosa-CalatravaM
ConwayJF
ZubietaC
CusackS
2005 A quasi-atomic model of human adenovirus type 5 capsid. Embo J 24 1645 1654
46. NeumannG
EbiharaH
TakadaA
NodaT
KobasaD
2005 Ebola virus VP40 late domains are not essential for viral replication in cell culture. J Virol 79 10300 10307
47. MoritaE
SandrinV
ChungHY
MorhamSG
GygiSP
2007 Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. Embo J 26 4215 4227
48. FejerG
DrechselL
LieseJ
SchleicherU
RuzsicsZ
2008 Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog 4 e1000208 doi:10.1371/journal.ppat.1000208
49. ChroboczekJ
GoutE
FavierAL
GalinierR
2003 Novel partner proteins of adenovirus penton. Curr Top Microbiol Immunol 272 37 55
50. SalinasS
BilslandLG
HenaffD
WestonAE
KerielA
2009 CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog 5 e1000442
51. MitterederN
MarchKL
TrapnellBC
1996 Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 70 7498 7509
52. Segura-MoralesC
PesciaC
Chatellard-CausseC
SadoulR
BertrandE
2005 Tsg101 and Alix interact with murine leukemia virus Gag and cooperate with Nedd4 ubiquitin ligases during budding. J Biol Chem 280 27004 27012
53. FotiaAB
EkbergJ
AdamsDJ
CookDI
PoronnikP
2004 Regulation of neuronal voltage-gated sodium channels by the ubiquitin-protein ligases Nedd4 and Nedd4-2. J Biol Chem 279 28930 28935
54. RossiM
AqeilanRI
NealeM
CandiE
SalomoniP
2006 The E3 ubiquitin ligase Itch controls the protein stability of p63. Proc Natl Acad Sci U S A 103 12753 12758
55. BubeckA
WagnerM
RuzsicsZ
LotzerichM
IglesiasM
2004 Comprehensive mutational analysis of a herpesvirus gene in the viral genome context reveals a region essential for virus replication. J Virol 78(15) 8026 8035
56. CherepanovPP
WackernagelW
1995 Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158(1) 9 14
57. WarmingS
CostantinoN
CourtDL
JenkinsNA
CopelandNG
2005 Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33(4) e36
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- All Mold Is Not Alike: The Importance of Intraspecific Diversity in Necrotrophic Plant Pathogens
- Tsetse EP Protein Protects the Fly Midgut from Trypanosome Establishment
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- N-Acetylglucosamine Induces White to Opaque Switching, a Mating Prerequisite in
- Origin and Evolution of Sulfadoxine Resistant
- Rapid Evolution of Pandemic Noroviruses of the GII.4 Lineage
- Natural Strain Variation and Antibody Neutralization of Dengue Serotype 3 Viruses
- Fine-Tuning Translation Kinetics Selection as the Driving Force of Codon Usage Bias in the Hepatitis A Virus Capsid
- Structural Basis of Cell Wall Cleavage by a Staphylococcal Autolysin
- Direct Visualization by Cryo-EM of the Mycobacterial Capsular Layer: A Labile Structure Containing ESX-1-Secreted Proteins
- Lipopolysaccharide Is Synthesized via a Novel Pathway with an Evolutionary Connection to Protein -Glycosylation
- MicroRNA Antagonism of the Picornaviral Life Cycle: Alternative Mechanisms of Interference
- Limited Trafficking of a Neurotropic Virus Through Inefficient Retrograde Axonal Transport and the Type I Interferon Response
- Direct Restriction of Virus Release and Incorporation of the Interferon-Induced Protein BST-2 into HIV-1 Particles
- RNAIII Binds to Two Distant Regions of mRNA to Arrest Translation and Promote mRNA Degradation
- Direct TLR2 Signaling Is Critical for NK Cell Activation and Function in Response to Vaccinia Viral Infection
- The Essentials of Protein Import in the Degenerate Mitochondrion of
- Dynamic Imaging of Experimental Induced Hepatic Granulomas Detects Kupffer Cell-Restricted Antigen Presentation to Antigen-Specific CD8 T Cells
- An Accessory to the ‘Trinity’: SR-As Are Essential Pathogen Sensors of Extracellular dsRNA, Mediating Entry and Leading to Subsequent Type I IFN Responses
- Innate Killing of by Macrophages of the Splenic Marginal Zone Requires IRF-7
- Exoerythrocytic Parasites Secrete a Cysteine Protease Inhibitor Involved in Sporozoite Invasion and Capable of Blocking Cell Death of Host Hepatocytes
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
- Membrane Damage Elicits an Immunomodulatory Program in
- Fatal Transmissible Amyloid Encephalopathy: A New Type of Prion Disease Associated with Lack of Prion Protein Membrane Anchoring
- Nucleophosmin Phosphorylation by v-Cyclin-CDK6 Controls KSHV Latency
- A Combination of Independent Transcriptional Regulators Shapes Bacterial Virulence Gene Expression during Infection
- Inhibition of Host Vacuolar H-ATPase Activity by a Effector
- Human Cytomegalovirus Protein pUL117 Targets the Mini-Chromosome Maintenance Complex and Suppresses Cellular DNA Synthesis
- Dispersion as an Important Step in the Biofilm Developmental Cycle
- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Differential Regulation of Effector- and Central-Memory Responses to Infection by IL-12 Revealed by Tracking of Tgd057-Specific CD8+ T Cells
- The Human Polyoma JC Virus Agnoprotein Acts as a Viroporin
- Expansion, Maintenance, and Memory in NK and T Cells during Viral Infections: Responding to Pressures for Defense and Regulation
- T Cell-Dependence of Lassa Fever Pathogenesis
- HIV and Mature Dendritic Cells: Trojan Exosomes Riding the Trojan Horse?
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- A Capsid-Encoded PPxY-Motif Facilitates Adenovirus Entry
- Homeostatic Interplay between Bacterial Cell-Cell Signaling and Iron in Virulence
- Serological Profiling of a Protein Microarray Reveals Permanent Host-Pathogen Interplay and Stage-Specific Responses during Candidemia
- YfiBNR Mediates Cyclic di-GMP Dependent Small Colony Variant Formation and Persistence in
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



