-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Metaeffector Exploits Host Proteasome to Temporally Regulate Cognate Effector
Pathogen-associated secretion systems translocate numerous effector proteins into eukaryotic host cells to coordinate cellular processes important for infection. Spatiotemporal regulation is therefore important for modulating distinct activities of effectors at different stages of infection. Here we provide the first evidence of “metaeffector,” a designation for an effector protein that regulates the function of another effector within the host cell. Legionella LubX protein functions as an E3 ubiquitin ligase that hijacks the host proteasome to specifically target the bacterial effector protein SidH for degradation. Delayed delivery of LubX to the host cytoplasm leads to the shutdown of SidH within the host cells at later stages of infection. This demonstrates a sophisticated level of coevolution between eukaryotic cells and L. pneumophila involving an effector that functions as a key regulator to temporally coordinate the function of a cognate effector protein.
Published in the journal: . PLoS Pathog 6(12): e32767. doi:10.1371/journal.ppat.1001216
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001216Summary
Pathogen-associated secretion systems translocate numerous effector proteins into eukaryotic host cells to coordinate cellular processes important for infection. Spatiotemporal regulation is therefore important for modulating distinct activities of effectors at different stages of infection. Here we provide the first evidence of “metaeffector,” a designation for an effector protein that regulates the function of another effector within the host cell. Legionella LubX protein functions as an E3 ubiquitin ligase that hijacks the host proteasome to specifically target the bacterial effector protein SidH for degradation. Delayed delivery of LubX to the host cytoplasm leads to the shutdown of SidH within the host cells at later stages of infection. This demonstrates a sophisticated level of coevolution between eukaryotic cells and L. pneumophila involving an effector that functions as a key regulator to temporally coordinate the function of a cognate effector protein.
Introduction
Many bacterial pathogens encode a large array of “effector proteins,” that manipulate host cellular processes during infection. Effector proteins are translocated from bacteria directly into the cytosol of host cells. This process is mediated by dedicated bacterial protein delivery systems, including the type III and the type IV secretion systems. In some cases, effector proteins delivered into host cells by a bacterium have opposing functions on a single host protein. For example, Legionella pneumophila DrrA (SidM) and LepB are effector proteins with opposing effects on the host Rab1 GTPase, with DrrA functioning as a guanine nucleotide exchange factor (GEF) and guanine nucleotide dissociation inhibitor-displacement factor (GDF), and LepB having GTPase-activating protein (GAP) activity[1], [2], [3], [4]. Similarly, the Salmonella enterica serovar typhimurium effectors SopE and SptP have GEF and GAP activities for the Rho family of GTPases[5], [6], respectively. Although the GEF activity of SopE is dominant in the host cell immediately after infection, degradation of SopE by the host proteasome alters the balance of these effectors, resulting in the GAP activity of SptP to be dominant later in infection [7]. Although differential regulation of gene transcription and post-translational modifications of effectors have also been shown to regulate their activities in host cells[8], [9], details on how these processes are controlled remain largely unknown; other effector-regulating mechanisms probably also exist.
L. pneumophila is a gram-negative bacterium ubiquitously found in freshwater environments [10]. When phagocytosed by eukaryotic cells, L. pneumophila remodels the Legionella-containing phagosome to form a compartment that allows its intracellular replication [11], [12], [13]. As a result, L. pneumophila is able to replicate in a wide variety of phagocytic cells, from amoebae to macrophages; human infections can result in a severe pneumonia called Legionnaires' disease [14]. The Dot/Icm type IV secretion system is an essential virulence determinant that translocates L. pneumophila effector proteins into host cells during infection [15], [16]. These effector proteins control host cell functions to initiate trafficking of the L. pneumophila vacuole, promote host cell survival, modulate innate immune responses, and promote bacterial egress [17]. Although over 100 L. pneumophila effector proteins have been identified, the biochemical and cellular functions of most effector proteins remain unknown [17], [18].
Ubiquitin is a small, well-conserved peptide of 76 amino acids, present in all eukaryotes [19]. Ubiquitination of substrate proteins involves a cascade of reactions. At the last step of the cascade, an E3 ligase recognizes a substrate protein and transfers ubiquitins to the substrate from an E2 conjugating enzyme [20]. Ubiquitinated proteins are subjected to further cellular processes, most notably proteasomal degradation [21]. E3 ligases can be divided into several major families; HECT-type, RING-type, U-box-type and NEL-type [22], [23], [24]. The each family has distinct structural feature, while RING and U-box domains are closely related. Many but not all RING-type E3 ligases work as multi-subunit complexes called SCF complexes containing Skp1, Cullin and F-box proteins. U-box-type E3 ligases contain single U-box domain that serves as an E2-binding site, while L. pneumophila effector protein LubX carries two U-box domains, one of which functions as a substrate-binding site (Figure 1A, see below). NEL-type is the most recent addition to E3 ubiquitin ligase families [24]. NEL family is composed of IpaH/SspH family proteins from bacterial pathogens Shigella flexneri and Salmonella enterica [25], [26], [27] and more than 30 homologous proteins found in bacterial pathogens[24]. Most notably NEL-type E3 ligases seem to be prevalent among bacterial pathogens, whereas no homologous protein has found in eukaryotic cells.
Fig. 1. LubX is an ubiquitin ligase that regulates SidH degradation in the host cytosol. 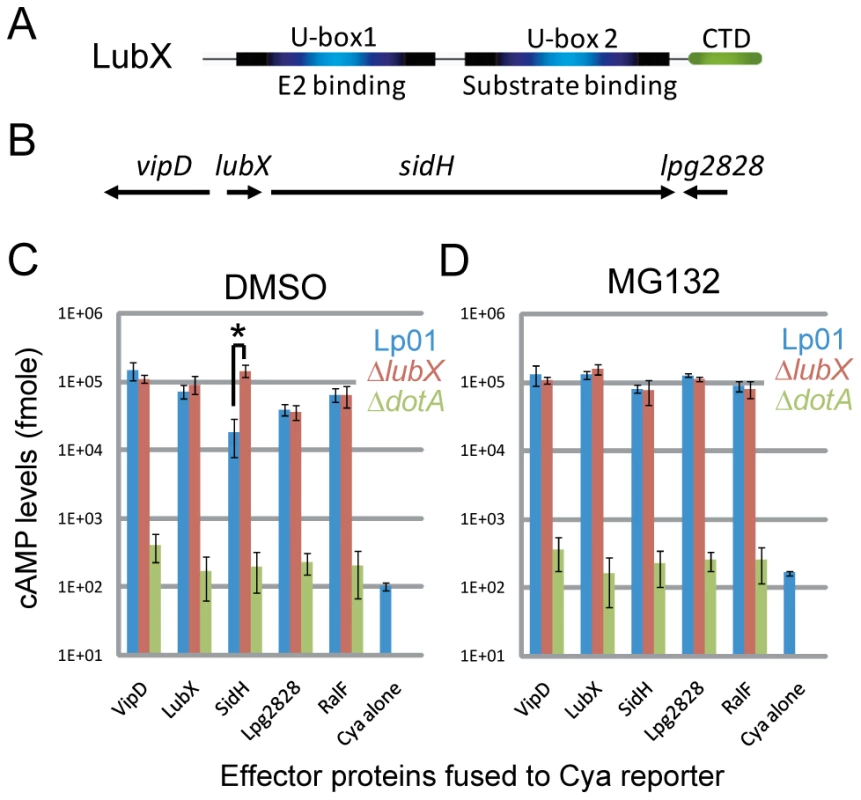
(A) Schematic representation of functional regions in LubX. (B) Schematic representation of lubX and neighboring genes encoding Dot/Icm type IV secretion system substrates. (C, D) CHO-FcγRII cells were infected with indicated L. pneumophila strains carrying a plasmid encoding the indicated Cya fusion proteins or Cya alone under the control of a constitutive promoter. Medium contained solvent alone 0.1% DMSO (panel C) or 10 µM MG132 (panel D) added 30 minutes prior to infection. The y axis indicates cAMP levels in cells infected for 8 hours plotted on a logarithmic scale. Data are mean ± SD from three independent samples. *P = 0.005 (t-test). It is well documented that bacterial pathogens exploit the host ubiquitin-proteasome pathway by delivering effectors that function as E3 ubiquitin ligases or as deubiquitinating enzymes [24], [28], [29]. L. pneumophila encode one U-box protein (LubX) [30] and several F-box-containing proteins including AnkB/LegAU13/Lpg2144/Lpp2082 [31], [32], [33], [34], [35]. We previously reported that LubX functions as a U-box-type E3 ubiquitin ligase in vitro and in host cells [30]. LubX mediates polyubiquitination of a host kinase Clk1, but its consequence remains unknown. Interestingly, the expression and translocation of LubX is induced upon infection and the levels of LubX within host cells come to maximum at later stages of infection, compared to other L. pneumophila effectors so far characterized. The gene encoding LubX is in close proximity to genes that encode several other type IV effectors, including VipD [36] and SidH [37] (Figure 1B). Surprisingly, analyses of these effector proteins led us to identify SidH as a target of LubX. LubX acts as a negative temporal regulator of SidH within host cells. This is the first example of the bacterial effector that targets and regulates a cognate effector within the host cells, and we propose the designation “metaeffector” for this class of bacterial effectors.
Results
SidH level within host cells is affected by LubX and host proteasome
Translocation of putative effector proteins encoded in vicinity of the lubX gene was assessed by measuring cAMP production in the host cytosol generated by an effector containing an amino-terminal fusion to an adenylate cyclase (Cya) domain that is only active in the cytosol of eukaryotic cells[38], [39]. These measurements appear to indicate that translocation of the Cya-SidH fusion protein by wild-type L. pneumophila was significantly less than that by an isogenic lubX mutant producing the same fusion protein at eight hours post infection (Figure 1C—the asterisk [*], Lp01 vs. ΔlubX). The difference was the most potent at late stages of infection, while we did not see significant difference at one hour post infection (Figure S1). Translocation of other Cya-tagged effectors such as RalF [40], however, was not affected by the lubX mutation (Figure 1C) suggesting that the lubX mutation does not have a general effect on type IV secretion. Because LubX has ubiquitin ligase activity, we investigated whether LubX affected SidH translocation through a process requiring host proteasome activity, by treating cells with the proteasome inhibitor MG132. Remarkably, wild type L. pneumophila and the lubX mutant appeared to translocate Cya-SidH equally in cells treated with the proteasome inhibitor (Figure 1C vs. 1D), indicating that inhibition of the host proteasome mimics a bacterial mutant deficient in LubX. These results suggested that LubX-mediated proteasomal degradation of a factor within the host cytosol was required for the reduced levels of Cya-SidH activity. It should be noted though that the Cya fusion assay is not an ideal system to examine the dynamics of intracellular effector levels in infection context, partly because the Cya fusions are under control of a non-authentic constitutive promoter and expressed in trans.
LubX directly binds to SidH
Because LubX is able to directly target proteins for degradation, we next examined whether LubX-mediated degradation of SidH in the host cytosol was due to a direct interaction between LubX and SidH. Purified proteins were used to test for direct interactions between SidH and LubX in vitro (Figure 2A). His-SidH became bound to the purified GST-LubXΔC, a deletion derivative lacking the C-terminal domain of LubX, but not with GST alone, suggesting interaction between SidH and LubX. The SidH interaction was detected using a fusion protein containing the LubX U-box2 region (GST-U-box2), but not using a fusion protein containing the LubX U-box1 region (GST-U-box1). Another Legionella effector RalF became bound neither to GST-LubXΔC nor to GST-U-box2 (Figure 2B), suggesting that LubX U-box2 is a specific protein binding domain. Collectively the U-box2 region of LubX binds specifically and directly to the effector protein SidH.
Fig. 2. LubX directly binds to SidH but not to RalF. 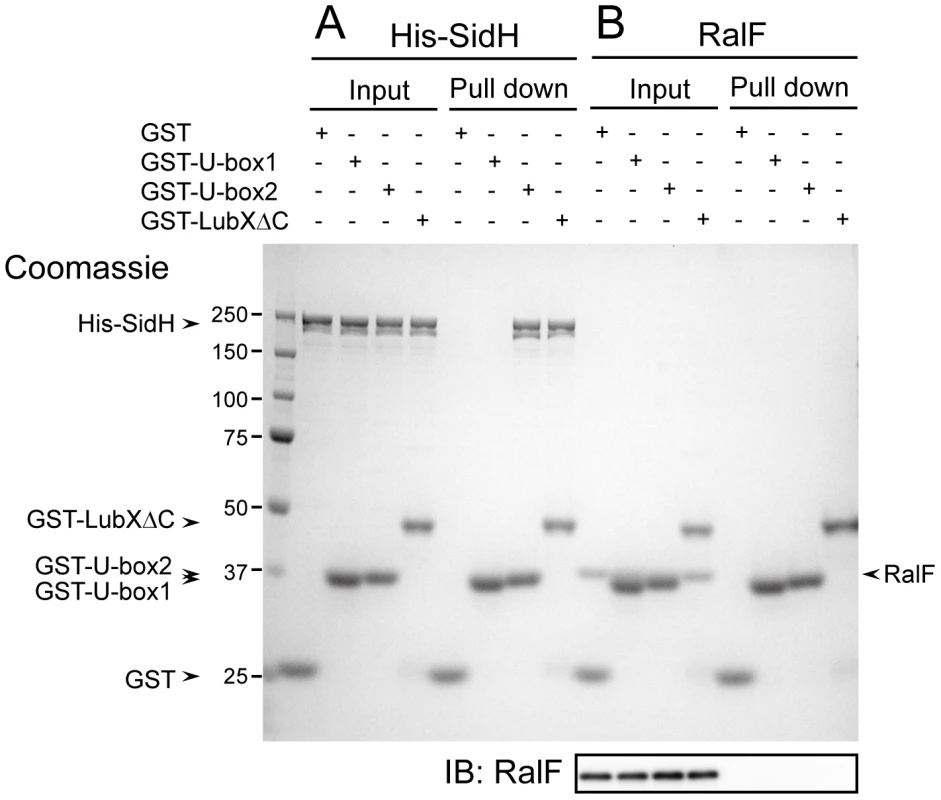
Purified GST or indicated GST fusion proteins (10 µg) was mixed with purified His-SidH (panel A) or RalF (panel B) (5 µg), and protein complexes were isolated by glutathione sepharose. Equivalent amounts of input and pulled down samples were analyzed by 10% SDS-PAGE followed by Coomassie Brilliant Blue staining. Numbers at the left side of the image designate positions of molecular weight markers (in kDa). Because the mobility in the gel of RalF is so close to those of GST-U-box1 and GST-U-box2, the western immunoblotting of the same samples using anti-RalF antibody (IB: RalF) was provided in order to clearly demonstrate that RalF was not pulled-down with any GST derivatives. SidH is a substrate of LubX E3 ubiquitin ligase
An in vitro ubiquitination assay was used to determine if LubX binding to SidH could target SidH for ubiquitination. Purified components used were ubiquitin, E1, UbcH5c (E2), LubXΔC or its inactive derivative LubXΔCI39A (E3), His-SidH or another effector protein RalF, and ATP, and the reactions were conducted as described previously[30]. In a functional LubX dependent manner, His-SidH shifted to a very high molecular weight species (Figure 3A, IB: αSidH). Another effector protein RalF was not affected (Figure 3A, IB: αRalF), suggesting the specificity of the reaction. To examine whether the retarded His-SidH species contain ubiquitin, His-SidH was isolated from the reaction mixtures by pull-down using nickel resin and analyzed by western immunibloting using anti-polyubiquitin antibodies (Figure 3A, PD: His-SidH IB: αUbiquitin). The results indicated that the retarded His-SidH species were polyubiquitinated. These results clearly demonstrate that His-SidH is polyubiquitinated by LubXΔC in vitro.
Fig. 3. LubX promotes ubiquitination of SidH in vitro and within host cell. 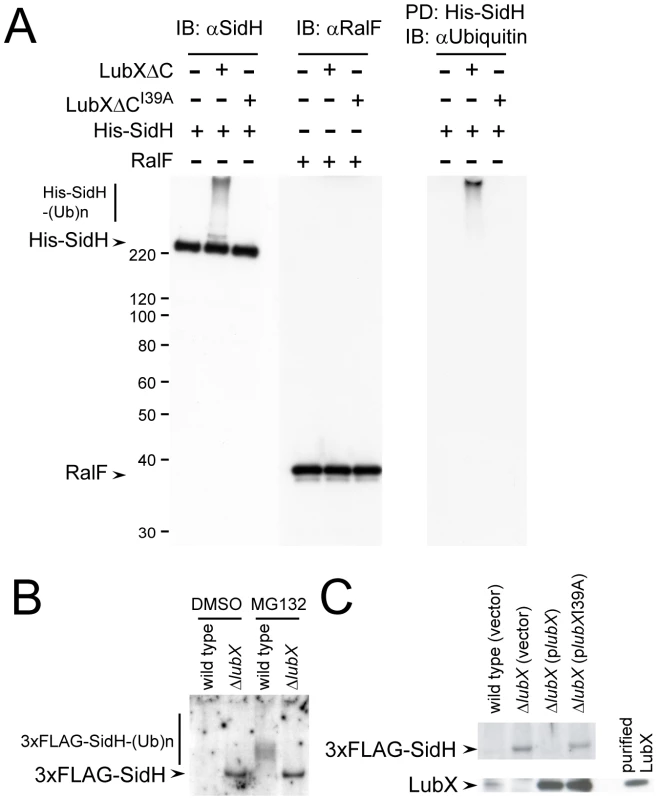
(A) LubX polyubiquitinates SidH in vitro. In vitro ubiquitination reactions containing the indicated E3 and substrate proteins were carried out as described in Materials and Methods. The reaction mixtures were analyzed by western immunoblotting using anti-SidH or anti-RalF antibodies (IB: αSidH or IB: αRalF, respectively). In the right panel, His-SidH derivatives in the reaction mixtures were pulled down with nickel resins. Pulled-down materials were analyzed by immunoblotting using anti-Ubiquitin antibody (PD: His-SidH, IB: αUbiquitin). Numbers at the left side of the images designate positions of molecular weight markers (in kDa). (B) LubX-mediated ubiquitination and proteasomal degradation of SidH within the host cells. CHO-FcγRII cells were infected for 8 hours with wild-type or lubX deletion strains carrying the triple FLAG-tagged sidH gene. DMSO or MG132 was employed as in Figure 1CD. One percent digitonin extracts of the infected cells were subjected to immunoprecipitation with anti-FLAG antibody and the immunoprecipitates were analyzed by immunoblotting using anti-FLAG antibody. (C) The defect in SidH degradation in cells infected with the lubX mutant was rescued by producing LubX in trans. Indicated strains were used for infection. LubX-mediated proteasomal degradation of SidH
To determine whether SidH is polyubiquitinated by LubX in the cytosol of infected host cells, nucleotide sequences encoding an amino-terminal triple-FLAG (3× FLAG) epitope tag were appended to the sidH gene on the L. pneumophila chromosome. The strain encoding the 3× FLAG-SidH protein expressed SidH at similar levels and the regulation of LubX expression was not affected (Figure S2). Chinese hamster ovary (CHO)-FcγRII cells infected for eight hours with L. pneumophila strains producing 3× FLAG-SidH were extracted with a buffer containing 1% digitonin. L. pneumophila proteins recovered in the extracts contain proteins translocated into the host cells, but not proteins in the bacterial cells[30], [41]. The 3× FLAG-SidH protein was not detected in cells infected with wild-type L. pneumophila, while it was readily detectable in cells infected with the lubX mutant (Figure 3B: DMSO, wild type vs ΔlubX). Importantly, when host cells were treated with MG132 to inhibit proteasome-mediated degradation, polyubiquitinated 3× FLAG-SidH derivatives were detected from cells infected with L. pneumophila producing a functional LubX protein, but only unmodified SidH was detected from cells infected with the isogenic lubX mutant (Figure 3B: MG132). The defect in SidH degradation in cells infected with the lubX mutant was rescued by producing LubX in trans (Figure 3C). Thus LubX is essential for polyubiquitination of SidH in host cells, and polyubiquitinated SidH is degraded by the host proteasome.
SidH levels are temporally regulated within host cells
LubX expression by L. pneumophila is induced intracellularly. L. pneumophila grown extracellularly on laboratory media does not produce LubX, and the levels of LubX increase gradually upon host cell infection, peaking at 10 hours post infection [30] (and Figure S2). In contrast, expression of SidH is induced at the stationary phase of growth in laboratory media, which means SidH levels in the bacterial cell are high when infection is initiated (Figure S2). Accordingly, we hypothesized that SidH levels are regulated intracellularly by the temporal expression and intracellular activities of LubX during infection. SidH levels were measured over time in CHO-FcγRII cells infected with L. pneumophila producing 3xFLAG-SidH to test this hypothesis. Full-length 3× FLAG-SidH was detected within the host cells shortly after infection (Figure 4A, 15 m). At one hour post infection, polyubiquitinated 3× FLAG-SidH was detected. Intracellular levels of 3xFLAG-SidH declined over time, and by eight hours post infection, 3× FLAG-SidH was no longer detected. By contrast, LubX levels within the host cells increased over time (Figure 4A), consistent with LubX mediating the intracellular ubiquitination and degradation of SidH. In cells infected with the L. pneumophila lubXI39A mutant, similar levels of 3× FLAG-SidH were detected in host cells at all time points and the protein was not polyubiquitinated (Figure 4B: lubXI39A), indicating that LubX E3 ubiquitin ligase activity is required for the temporal degradation of SidH. When cells were treated with MG132, we observed the increased levels of polyubiquitinated 3× FLAG-SidH derivatives over time by a process requiring LubX activity (Figure 4C: MG132). Lastly, pretreatment of L. pneumophila with the irreversible bacterial translation inhibitor gentamicin abrogated the shutdown of SidH, indicating that intracellular production of LubX was necessary for SidH degradation (Figure 4D: Gm and Figure S3). These results clearly indicate that SidH transiently accumulates within host cells at an early stage of infection, and that the eventual disappearance of SidH in host cells results from LubX-mediated proteasomal degradation (Figure 4E).
Fig. 4. Temporal regulation of SidH is mediated by ubiquitin ligase LubX and host proteasome. 
(A) Time course of intracellular SidH and LubX levels after infection with L. pneumophila strain producing the triple FLAG-tagged SidH. CHO-FcγRII cells were infected and digitonin extracts were prepared at indicated time after infection as in Figure 3B. The Ni lane denotes the non-infected control. Immunoprecipitation and immunoblotting was carried out as in Figure 3B. The asterisks denote a non-specific signal. (B) Shutdown of SidH requires ubiquitin ligase activity of LubX. CHO-FcγRII cells infected with L. pneumophila strains carrying wild-type lubX gene or lubXI39A U-box1 dead mutant were analyzed as in panel A. (C) Shutdown of SidH requires host proteasome. CHO-FcγRII cells were treated with 10 µM MG132 or 0.1% DMSO from 30 minutes before infection with L. pneumophila. (D) Shutdown of SidH requires bacterial protein synthesis after infection. L. pneumophila was pretreated with 100 µg/ml gentamicin (Gm) or water for 30 minutes and used for infection. (E) Delayed delivery of LubX results in proteasomal degradation of SidH at later stages of infection. Biological significance of the down regulation of SidH
The temporal regulation model predicts that the persistence of intracellular SidH led by lubX disruption adversely affects L. pneumophila fitness in hosts. To address the prediction, we utilized the Drosophila infection model [42]. It has been shown that L. pneumophila infect and grow to high levels within Drosophila cells, and their replication depends on the Dot/Icm type IV secretion system [43]. Correspondingly, although most flies infected with wild-type Legionella died within twelve days, ∼80% of flies infected with Legionella defective in the Dot/Icm type IV secretion system (ΔdotA) survived (Figure 5A). Numbers of the surviving flies infected with the sidH mutant were similar to or slightly higher than those infected with the wild-type strain (Figure 5A). The lubX mutant consistently showed hyper-lethality to flies compared with the sidH mutant or the lubX sidH double mutant (Figure 5A, P<0.01 for both comparisons). Viable bacterial counts in survived flies infected with the lubX mutant were consistently lower than those in flies infected with the wild-type strain (Figure 5B). This apparent defect of replication of the lubX mutant in flies was rescued by further introduction of the sidH mutant (Figure 5B). Thus, the loss of lubX in sidH+ L. pneumophila gives a disadvantage in multiplication within the model host. It should be noted that the hyper-lethality of the lubX mutant did not stem from increased number of viable bacteria (Figure 5B). The lubX mutant might be more toxic to a specific type of fly cells (e.g. phagocyte) important for survival.
Fig. 5. Phenotypes of lubX, sidH and double knockout strains in fly model. 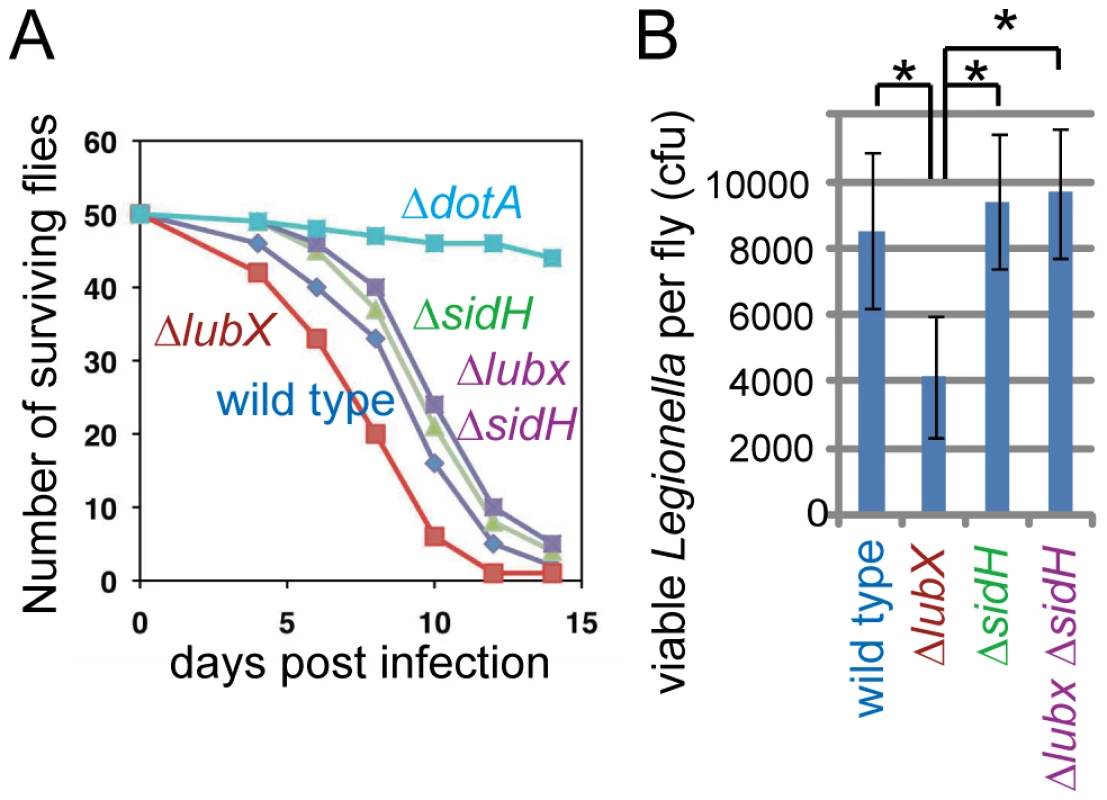
(A) Survival curves of Drosophila flies after infection with indicated L. pneumophila strains. A representative experiment from at least four independent experiments was shown. Statistics is discussed in the text. (B) Viable bacterial counts in infected flies at 10 days post infection. *P = 0.01 (t-test). Discussion
The temporal regulation of SidH mediated by L. pneumophila LubX E3 ligase and the host proteasome system illustrates a novel mechanism by which bacterial effectors are regulated. In the previously reported temporal regulation of the induced membrane ruffling by Salmonella effectors SopE and SptP, it was shown that these two effectors have distinct susceptibility towards ubiquitin-mediated proteasomal degradation [7]. Importantly, the determinants of the susceptibility are encoded in the effectors themselves. By contrast, the L. pneumophila SidH is an intrinsically stable protein within host cells; the effector protein LubX controls SidH instability in the host cytosol by directly targeting this protein for host ubiquitination. Thus LubX represents a bona fide metaeffector—a designation for an effector that regulates the function of another effector within the host cell. Discovery of an effector having a regulatory role on another effector provides unique insight into the sophisticated mechanisms that underlie the ability of L. pneumophila to coordinate the function of such a large array of effector proteins with diverse activities.
As shown previously [30] and partly in Figure 4 as well, the intracellular level of LubX increases over time and reaches to the maximum at 10–12 hours post infection, which is far late stages compared to the critical time window in which SidH is polyubiquitinated and targeted to proteasomal degradation. It appears that relatively small amount of LubX detected within first couple of hours post infection is sufficient for the polyubiquitination and degradation of SidH (Figure 4). This raised the question on the role of LubX at late stages of infection. We previously reported that host kinase Clk1 is a substrate of LubX [30]. Together with the current findings, one of reasonable explanations would be that LubX has multiple targets within host cells including bacterial SidH and host Clk1.
Accumulating lines of evidence suggest that effector proteins functioning as an E3 ubiquitin ligase are prevalent among many plant and animal bacterial pathogens. Some effector proteins which have been reported to possess E3 ligase activity do not show similarity to eukaryotic E3 ligases at sequence level. AvrPtoB of a plant pathogen Pseudomonas syringae shows structural similarity to U-box E3 ligase, whereas they do not share sequence level homology [44]. This suggests that there could be more bacterial E3 ligases than predicted by simple homology search. Structural analyses demonstrated that IpaH/SspH family proteins Shigella flexneri IpaH1.4, IpaH3 and Salmonella enterica SspH2 show no similarity to eukaryotic E3 ligases and represents a new class of E3 ligase [25], [26], [27]. This class of E3 ligase, called NEL, seems to comprise a large family of bacterial E3 ligases, because more than 30 homologous proteins are found in a subset of bacterial pathogens [24]. Furthermore, there are more than 30 bacterial F-box proteins which are expected to be a key component of SCF E3 ligase complexes [28]. However, the targets of these bacterial E3 ligases remain largely unknown, and all previous studies have aimed to identify host target proteins. SidH is the first example of bacterial targets of the bacterial E3 ligase effector. Future studies without prejudice will reveal that some of bacterial effectors implicated in manipulation of the host ubiquitin system possess regulatory functions as metaeffector that coordinate the expression of effector functions spatiotemporally.
Materials and Methods
Bacterial strains, media, plasmids and cell culture
All Legionella strains used in this study were derivatives of L. pneumophila strain Lp01 [45] and were grown on charcoal-yeast extract (CYE) plates or in ACES-buffered yeast extract (AYE) broth as described previously[46]. The strains defective in lubX and dotA genes have been previously described [30], [47]. Legionella strains defective in sidH and lubX-sidH genes as well as Legionella strains carrying 3xFLAG-sidH or the lubX I39A mutation were constructed by allelic exchange[47]. E. coli strain BL21 (strain B lon ompT) was used as an expression host for protein purification. Plasmids used in this study and details of plasmid construction are provided in the Tables S1 and S2. Chinese hamster ovary (CHO)-FcγRII cells were cultured at 37°C in 5% CO2 in α-MEM, supplemented with heat-inactivated 10% FBS, as described[48].
Antibodies
Custom made anti-serum against SidH peptide CQNIKGPEPVATPMETPE (SidH 2196–2212) was purchased from MBL. Antibodies were purified from the anti-serum by affinity chromatography using peptide-conjugated SulfoLink resins (Pierce). The affinity-purified rabbit polyclonal antibodies against RalF, LubX and GroEL were described previously [30], [40]. Mouse monoclonal antibody against polyubiquitin (clone FK1) was purchased from BIOMOL. Monoclonal antibody against the FLAG tag (M2) was purchased from Sigma-Aldrich.
Cya reporter assay
Translocation of Cya-fused proteins into CHO-FcγRII cells after infection with Legionella was assayed as described previously [30], [38] with minor modifications. Briefly, CHO-FcγRII cells were replated in 24-well plates, and challenged by Legionella strains expressing Cya fusions at a multiplicity of infection (moi) of 30 in the presence of opsonizing antibody (1∶3000 dilution). Eight hours later, infected cells were lysed in 500 µl of lysis reagent 1B provided from a cAMP Biotrak EIA System (GE Healthcare, RPN2251); cAMP levels were determined according to manufacturer's instructions.
Protein purification
Purification of LubXΔC, RalF and GST fusion proteins has been described previously [30], [40]. For purification of His-SidH, BL21 cell pellets from a 2-liter culture expressing His-SidH were suspended with 80 ml PBS containing Complete Protease Inhibitor Cocktail (Roche) and 20 mg lysozyme (Wako Chemical). After incubation with stirring for 30 minutes at 4°C, the lysozyme-treated cells were lysed by sonication. After centrifugation (16,000× g for 20 minutes) to remove unsolubilized materials, the supernatant fraction was mixed with ammonium sulfate (final 40% saturation) and incubated for 30 minutes at 4°C. After centrifugation to remove precipitates, the supernatant fraction was mixed with ammonium sulfate (final 60% saturation) and incubated for 30 minutes further at 4°C. After centrifugation, the precipitates were dissolved in 20 ml PBS containing Complete Protease Inhibitor Cocktail (Roche). This solution was dialyzed against PBS to remove residual ammonium sulfate. After centrifugation to remove insoluble materials, the supernatant fraction was mixed with 6 ml (bed volume) HIS-Select Resin (Sigma-Aldrich) and incubated for 30 minutes at 4°C. The resins were washed 5 times with PBS containing 5 mM imidazole, and the bound proteins were eluted with 6 ml PBS containing 100 mM imidazole. The elution step was repeated once more, and the resulting two eluate fractions were pooled. The pooled fraction was mixed with half its volume of 20 mM Tris HCl, pH 7.5 to reduce salt concentration (final 0.1 M NaCl). The resulting solution was applied to a MonoQ 5/50GL chromatography column (GE healthcare). After elution by NaCl gradient (0.1 M to 0.5 M in 20 mM Tris HCl, pH. 7.5), the peak fractions were further subjected to a Superose 6 10/300GL column (GE healthcare) equilibrated with 20 mM Tris HCl pH 7.5, 150 mM NaCl. The peak fractions were pooled and concentrated using a Microcon device (Millipore).
GST pull-down assays
For a GST pull-down using purified proteins, GST, GST-LubXΔC, GST-U-box1 or GST-U-box 2 (10 µg) were mixed with 5 µg of His-SidH or RalF in 500 µl PBS containing 1 mM EDTA, 1 mM DTT, and 1% (w/v) Triton X-100. The resulting solutions were mixed with 25 µl of a 50% suspension of Glutathione-Sepharose and incubated for three hours with gentle rotation at 4°C. Unbound proteins were removed by centrifugation, and resins were washed four times with the same buffer, and once with a buffer omitting TritonX-100. GST and interacting proteins were eluted with 50 µl of SDS sample buffer containing reducing agent.
Ubiquitin ligase assays
The in vitro ubiquitin polymerization assay was performed essentially as described [30] with a couple of modifications; 300 nM E3 enzyme (LubX derivatives) were employed; where indicated, 240 nM of purified His-SidH or RalF was included. Where indicated, His-SidH derivatives were pulled down with His-Select resin (Sigma-Aldrich) and washed in the presence of 2.5 mM imidazole to suppress nonspecific interaction between reaction component proteins and the resin. Pulled-down materials were eluted with 250 mM imidazole. Samples were subjected to 10% SDS-PAGE and analyzed by immunoblotting using antibodies against SidH, RalF or polyubiquitin.
Fractionation of infected cells
CHO-FcγRII cells were replated in a 6-well culture dish, and challenged by Legionella strains at a moi of 30 in the presence of opsonizing antibody (1∶3000 dilution). One hour after infection, the cells were washed three times with PBS (pre-warmed to 37°C) to remove non-internalized bacteria, then further incubated in cell culture medium. When indicated, 10 µM of MG132 (Calbiochem) or equivalent amount of the solvent DMSO was added to the cell medium 30 min prior to infection as well as to the replacing medium. At the indicated time points, the cells were washed three times with cold PBS and lysed in 150 µl of PBS containing 1% (w/v) of digitonin (Calbiochem), 10 mM of N-ethylmaleimide (Sigma) to prevent deubiquitination, and protease inhibitor cocktail (1∶100 dilution, Sigma-Aldrich). The cells were scraped off, collected into microfuge tubes and centrifuged at 16,000× g for 10 min at 4°C to separate the digitonin-soluble fraction containing translocated proteins from the digitonin-insoluble fraction containing internalized bacteria. The digitonin-soluble fractions were filtrated through a 0.45 µm filter unit (Millex-HV, Millipore). Immunoprecipitates with anti-FLAG or anti-LubX antibodies, extracted using nProteinA Separose (GE Healthcare), were analyzed by SDS-PAGE followed by immunoblotting using anti-FLAG or anti-LubX antibodies, respectively.
Infection to fruit flies and colony forming assay
Five to seven days olds yw male Drosophila melanogaster flies were used for infection experiments. Before injection, the bacteria-containing medium was adjusted to 0.1 OD using Gene Quant pro (Amersham) with distilled water. Flies were anesthetized with CO2 and injected with each strain of bacteria in 65 nl of water (approximately 500 colony-forming units). Injection was carried out by using an individually calibrated pulled glass needle attached to IM-300 microinjector (Narishige). Flies were always injected in the abdomen, close to the junction with thorax and just ventral to the junction between the ventral and dorsal cuticles. After injection, flies were transferred to fresh vials once a week. For colony-forming assay, 10 days after bacterial injection flies were homogenized in 10 mM MgSO4 solution and the diluted series of the homogenized samples were plated on CYE media containing 100 µg/ml streptomycin.
Supporting Information
Zdroje
1. IngmundsonA
DelpratoA
LambrightDG
RoyCR
2007 Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature 450 365 369
2. MachnerMP
IsbergRR
2006 Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell 11 47 56
3. MachnerMP
IsbergRR
2007 A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science 318 974 977
4. MurataT
DelpratoA
IngmundsonA
ToomreDK
LambrightDG
2006 The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol 8 971 977
5. FuY
GalanJE
1999 A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401 293 297
6. HardtWD
ChenLM
SchuebelKE
BusteloXR
GalanJE
1998 S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93 815 826
7. KuboriT
GalanJE
2003 Temporal regulation of salmonella virulence effector function by proteasome-dependent protein degradation. Cell 115 333 342
8. BackertS
SelbachM
2005 Tyrosine-phosphorylated bacterial effector proteins: the enemies within. Trends Microbiol 13 476 484
9. PetterssonJ
NordfelthR
DubininaE
BergmanT
GustafssonM
1996 Modulation of virulence factor expression by pathogen target cell contact. Science 273 1231 1233
10. FieldsBS
1996 The molecular ecology of legionellae. Trends Microbiol 4 286 290
11. HorwitzMA
1983 The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome lysosome fusion in human monocytes. J Exp Med 158 2108 2126
12. SwansonMS
IsbergRR
1995 Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun 63 3609 3620
13. TilneyLG
HarbOS
ConnellyPS
RobinsonCG
RoyCR
2001 How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J Cell Sci 114 4637 4650
14. FieldsBS
BensonRF
BesserRE
2002 Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev 15 506 526
15. SegalG
PurcellM
ShumanHA
1998 Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA 95 1669 1674
16. VogelJP
AndrewsHL
WongSK
IsbergRR
1998 Conjugative transfer by the virulence system of Legionella pneumophila. Science 279 873 876
17. ShinS
RoyCR
2008 Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol 10 1209 1220
18. FrancoIS
ShumanHA
CharpentierX
2009 The perplexing functions and surprising origins of Legionella pneumophila type IV secretion effectors. Cell Microbiol 11 1435 1443
19. GoldsteinG
ScheidM
HammerlingU
SchlesingerDH
NiallHD
1975 Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A 72 11 15
20. HershkoA
HellerH
EliasS
CiechanoverA
1983 Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem 258 8206 8214
21. VogesD
ZwicklP
BaumeisterW
1999 The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem 68 1015 1068
22. JacksonPK
EldridgeAG
FreedE
FurstenthalL
HsuJY
2000 The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol 10 429 439
23. PattersonC
2002 A new gun in town: the U box is a ubiquitin ligase domain. Sci STKE 2002 PE4
24. HicksSW
GalanJE
2009 Hijacking the host ubiquitin pathway: structural strategies of bacterial E3 ubiquitin ligases. Curr Opin Microbiol
25. QuezadaCM
HicksSW
GalanJE
StebbinsCE
2009 A family of Salmonella virulence factors functions as a distinct class of autoregulated E3 ubiquitin ligases. Proc Natl Acad Sci U S A 106 4864 4869
26. SingerAU
RohdeJR
LamR
SkarinaT
KaganO
2008 Structure of the Shigella T3SS effector IpaH defines a new class of E3 ubiquitin ligases. Nat Struct Mol Biol 15 1293 1301
27. ZhuY
LiH
HuL
WangJ
ZhouY
2008 Structure of a Shigella effector reveals a new class of ubiquitin ligases. Nat Struct Mol Biol 15 1302 1308
28. AngotA
VergunstA
GeninS
PeetersN
2007 Exploitation of eukaryotic ubiquitin signaling pathways by effectors translocated by bacterial type III and type IV secretion systems. PLoS Pathog 3 e3
29. RytkonenA
HoldenDW
2007 Bacterial interference of ubiquitination and deubiquitination. Cell Host & Microbe 1 13 22
30. KuboriT
HyakutakeA
NagaiH
2008 Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol Microbiol 67 1307 1319
31. Al-KhodorS
PriceCT
HabyarimanaF
KaliaA
Abu KwaikY
2008 A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol Microbiol 70 908 923
32. EnsmingerAW
IsbergRR
2010 E3 ubiquitin ligase activity and targeting of BAT3 by multiple Legionella pneumophila translocated substrates. Infect Immun 78 3905 3919
33. LommaM
Dervins-RavaultD
RolandoM
NoraT
NewtonHJ
2010 The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell Microbiol 12 1272 1291
34. PriceCT
Al-KhodorS
Al-QuadanT
Abu KwaikY
2010 Indispensable role for the eukaryotic-like ankyrin domains of the ankyrin B effector of Legionella pneumophila within macrophages and amoebae. Infect Immun 78 2079 2088
35. PriceCT
Al-QuadanT
SanticM
JonesSC
Abu KwaikY
2010 Exploitation of conserved eukaryotic host cell farnesylation machinery by an F-box effector of Legionella pneumophila. J Exp Med 207 1713 1726
36. ShohdyN
EfeJA
EmrSD
ShumanHA
ChenJ
2005 Pathogen effector protein screening in yeast identifies Legionella factors that interfere with membrane trafficking. Proc Natl Acad Sci U S A 102 4866 4871
37. LuoZQ
IsbergRR
2004 Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci U S A 101 841 846
38. NagaiH
CambronneED
KaganJC
AmorJC
KahnRA
2005 A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc Natl Acad Sci U S A 102 826 831
39. SoryMP
CornelisGR
1994 Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol 14 583 594
40. NagaiH
KaganJC
ZhuJ
KahnRA
RoyCR
2002 A Bacterial Guanine Nucleotide Exchange Factor Activates ARF on Legionella Phagosomes. Science 295 679 682
41. DerreI
IsbergRR
2005 LidA, a translocated substrate of the Legionella pneumophila type IV secretion system, interferes with the early secretory pathway. Infect Immun 73 4370 4380
42. ShinzawaN
NelsonB
AonumaH
OkadoK
FukumotoS
2009 p38 MAPK-Dependent Phagocytic Encapsulation Confers Infection Tolerance in Drosophila. Cell Host & Microbe 6 244 252
43. DorerMS
KirtonD
BaderJS
IsbergRR
2006 RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog 2 e34
44. JanjusevicR
AbramovitchRB
MartinGB
StebbinsCE
2006 A bacterial inhibitor of host programmed cell death defenses is an E3 ubiquitin ligase. Science 311 222 226
45. BergerKH
IsbergRR
1993 Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol 7 7 19
46. FeeleyJC
GibsonRJ
GormanGW
LangfordNC
RasheedJK
1979 Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol 10 437 441
47. ZuckmanDM
HungJB
RoyCR
1999 Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol Microbiol 32 990 1001
48. KaganJC
RoyCR
2002 Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol 4 945 954
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Is Adherence to Erythrocytes a Factor in Extrapulmonary Dissemination?
- Identifying the Age Cohort Responsible for Transmission in a Natural Outbreak of
- Blockade of Immunosuppressive Cytokines Restores NK Cell Antiviral Function in Chronic Hepatitis B Virus Infection
- Metaeffector Exploits Host Proteasome to Temporally Regulate Cognate Effector
- The p53-Target Gene Drives Neutrophil-Mediated Protection against Lethal Bacterial Sepsis
- Development of an RNAi Protocol to Investigate Gene Function in the Filarial Nematode,
- Lysine Acetyltransferase GCN5-A Functions in the Cellular Response to Alkaline Stress and Expression of Cyst Genes
- Structural Basis for Apoptosis Inhibition by Epstein-Barr Virus BHRF1
- Molecular Architectures of Trimeric SIV and HIV-1 Envelope Glycoproteins on Intact Viruses: Strain-Dependent Variation in Quaternary Structure
- Interaction of c-Cbl with Myosin IIA Regulates Bleb Associated Macropinocytosis of Kaposi's Sarcoma-Associated Herpesvirus
- Glacial Refugia in Pathogens: European Genetic Structure of Anther Smut Pathogens on and
- Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Larvae
- Inflammasome Sensor Nlrp1b-Dependent Resistance to Anthrax Is Mediated by Caspase-1, IL-1 Signaling and Neutrophil Recruitment
- Noise Cancellation: Viral Fine Tuning of the Cellular Environment for Its Own Genome Replication
- Infectious Speciation Revisited: Impact of Symbiont-Depletion on Female Fitness and Mating Behavior of
- Eis Regulates Autophagy, Inflammation, and Cell Death through Redox-dependent Signaling
- NleC, a Type III Secretion Protease, Compromises NF-κB Activation by Targeting p65/RelA
- Early Myeloid Dendritic Cell Dysregulation is Predictive of Disease Progression in Simian Immunodeficiency Virus Infection
- HIV Capsid is a Tractable Target for Small Molecule Therapeutic Intervention
- Structural and Functional Studies of Nonstructural Protein 2 of the Hepatitis C Virus Reveal Its Key Role as Organizer of Virion Assembly
- Coming of Age—Sexual Reproduction in Species
- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Compartmentation of Redox Metabolism in Malaria Parasites
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Rapid End-Point Quantitation of Prion Seeding Activity with Sensitivity Comparable to Bioassays
- Dimeric 2G12 as a Potent Protection against HIV-1
- Hypoxia Induces an Immunodominant Target of Tuberculosis Specific T Cells Absent from Common BCG Vaccines
- CD4 Natural Regulatory T Cells Prevent Experimental Cerebral Malaria via CTLA-4 When Expanded In Vivo
- The Killing of African Trypanosomes by Ethidium Bromide
- H2A.Z Demarcates Intergenic Regions of the Epigenome That Are Dynamically Marked by H3K9ac and H3K4me3
- Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (, Hymenoptera: Apidae)
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Coming of Age—Sexual Reproduction in Species
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Compartmentation of Redox Metabolism in Malaria Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



