-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Lysine Acetyltransferase GCN5-A Functions in the Cellular Response to Alkaline Stress and Expression of Cyst Genes
Parasitic protozoa such as the apicomplexan Toxoplasma gondii progress through their life cycle in response to stimuli in the environment or host organism. Very little is known about how proliferating tachyzoites reprogram their expressed genome in response to stresses that prompt development into latent bradyzoite cysts. We have previously linked histone acetylation with the expression of stage-specific genes, but the factors involved remain to be determined. We sought to determine if GCN5, which operates as a transcriptional co-activator by virtue of its histone acetyltransferase (HAT) activity, contributed to stress-induced changes in gene expression in Toxoplasma. In contrast to other lower eukaryotes, Toxoplasma has duplicated its GCN5 lysine acetyltransferase (KAT). Disruption of the gene encoding for TgGCN5-A in type I RH strain did not produce a severe phenotype under normal culture conditions, but here we show that the TgGCN5-A null mutant is deficient in responding to alkaline pH, a common stress used to induce bradyzoite differentiation in vitro. We performed a genome-wide analysis of the Toxoplasma transcriptional response to alkaline pH stress, finding that parasites deleted for TgGCN5-A fail to up-regulate 74% of the stress response genes that are induced 2-fold or more in wild-type. Using chromatin immunoprecipitation, we verify an enrichment of TgGCN5-A at the upstream regions of genes activated by alkaline pH exposure. The TgGCN5-A knockout is also incapable of up-regulating key marker genes expressed during development of the latent cyst form, and is impaired in its ability to recover from alkaline stress. Complementation of the TgGCN5-A knockout restores the expression of these stress-induced genes and reverses the stress recovery defect. These results establish TgGCN5-A as a major contributor to the alkaline stress response in RH strain Toxoplasma.
Published in the journal: . PLoS Pathog 6(12): e32767. doi:10.1371/journal.ppat.1001232
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001232Summary
Parasitic protozoa such as the apicomplexan Toxoplasma gondii progress through their life cycle in response to stimuli in the environment or host organism. Very little is known about how proliferating tachyzoites reprogram their expressed genome in response to stresses that prompt development into latent bradyzoite cysts. We have previously linked histone acetylation with the expression of stage-specific genes, but the factors involved remain to be determined. We sought to determine if GCN5, which operates as a transcriptional co-activator by virtue of its histone acetyltransferase (HAT) activity, contributed to stress-induced changes in gene expression in Toxoplasma. In contrast to other lower eukaryotes, Toxoplasma has duplicated its GCN5 lysine acetyltransferase (KAT). Disruption of the gene encoding for TgGCN5-A in type I RH strain did not produce a severe phenotype under normal culture conditions, but here we show that the TgGCN5-A null mutant is deficient in responding to alkaline pH, a common stress used to induce bradyzoite differentiation in vitro. We performed a genome-wide analysis of the Toxoplasma transcriptional response to alkaline pH stress, finding that parasites deleted for TgGCN5-A fail to up-regulate 74% of the stress response genes that are induced 2-fold or more in wild-type. Using chromatin immunoprecipitation, we verify an enrichment of TgGCN5-A at the upstream regions of genes activated by alkaline pH exposure. The TgGCN5-A knockout is also incapable of up-regulating key marker genes expressed during development of the latent cyst form, and is impaired in its ability to recover from alkaline stress. Complementation of the TgGCN5-A knockout restores the expression of these stress-induced genes and reverses the stress recovery defect. These results establish TgGCN5-A as a major contributor to the alkaline stress response in RH strain Toxoplasma.
Introduction
Stress responses are critical to cell survival, allowing cells to adapt to changing environmental conditions. In certain pathogenic eukaryotes, such as the protozoan Toxoplasma gondii (phylum Apicomplexa), the stress response takes on added significance as it triggers a developmental change into a latent cyst form. Parasitic protozoa often rely on stimuli in the environment or host organism in order to progress through the parasite life cycle. The study of stress-induced developmental changes in Toxoplasma is significant as this process underlies pathogenesis. This obligate intracellular protist develops from a rapidly growing form (tachyzoite) into a latent cyst form (bradyzoite) in response to stress [1]. In human hosts, the cyst forms can re-emerge as destructive tachyzoites if immunity wanes, causing recurring bouts of toxoplasmosis that may endanger immunocompromised individuals [2]. A major gap in our knowledge that impedes the development of novel therapeutics against Toxoplasma infection is our poor understanding of how tachyzoites reprogram their expressed genome in response to stresses that prompt cyst development. The identification of proteins that contribute to stress response and bradyzoite formation would be a significant step towards the design of new therapies to treat toxoplasmosis.
How the parasite coordinates the changes in gene expression that accompany stress-induced bradyzoite development is not clear, but epigenetic mechanisms, including histone modifications, have been implicated as contributing to this process [3], [4]. Formerly referred to as histone acetyltransferases (HATs), lysine acetyltransferases (KATs) of the general control nonderepressible-5 (GCN5/KAT2) family are well-conserved among eukaryotes [5]. While invertebrates generally possess a single GCN5, vertebrate species harbor two: GCN5 and the highly similar homologue called PCAF (p300/CBP-Associating Factor) [6]. The GCN5 KAT family has been implicated in cell-cycle progression [7], chromatin remodeling at specific promoters [8], transcription elongation [9], cellular differentiation [10], and the cellular stress response [11]. Microarray analyses of knockouts made in yeast suggest that GCN5 is a gene-specific coactivator, regulating 1.1% of genes in Schizosaccharomyces pombe and up to 4% in Saccharomyces cerevisiae [12], [13]. The GCN5 deletion mutant in S. cerevisiae is viable, but grows poorly on minimal media [14]. Similarly, GCN5 is not essential for growth under normal conditions in S. pombe, but is required for mounting an appropriate response to KCl and CaCl2-mediated stresses [15], [16]. In Arabidopsis plants, GCN5 controls ∼5% of genes and is important for normal growth and development, as well as the response to cold stress [17]. GCN5 was shown to be instrumental in the control of specific morphogenetic cascades during developmental transitions in Drosophila [18]. GCN5-null mouse embryos fail to form dorsal mesoderm lineages due to a high incidence of apoptosis and die 10.5 days post conception, suggesting a critical role for GCN5 in mammalian development [10], [19], [20]. In contrast, PCAF appears to be dispensable in mice as its loss confers no distinct phenotype [10]. Collectively, these studies support the idea that GCN5 KATs modulate gene expression during stress, or exposure to other environmental stimuli, to elicit the appropriate response or developmental change.
Toxoplasma is unique among fellow apicomplexan parasites and other invertebrates in possessing two GCN5 HATs, designated TgGCN5-A and –B [21], [22]. We sought to delineate the function of TgGCN5-A by creating a genetic knockout using homologous recombination in haploid RH strain tachyzoites. As in other lower eukaryotes, Toxoplasma lacking TgGCN5-A (ΔGCN5-A) showed no discernible phenotype under normal culture conditions [21], but its response to stress was not addressed. Here, we analyzed wild-type and ΔGCN5-A parasites under normal and alkaline pH growth conditions using Toxoplasma genome microarrays. The results illuminate the parasite's response to alkaline pH and demonstrate that TgGCN5-A is required for most of these changes in gene expression. We also show that ΔGCN5-A parasites exhibit greater sensitivity to alkaline pH stress - a novel role for GCN5 KATs. Moreover, Toxoplasma lacking TgGCN5-A cannot activate bradyzoite-specific genes that are normally up-regulated during alkaline pH-induced cyst development. These studies establish a novel function for GCN5 KATs in the eukaryotic response to alkaline stress and support the idea that TgGCN5-A is a key contributor to gene expression pertinent to the development of the latent cyst form of Toxoplasma.
Results/Discussion
Differential gene expression in Toxoplasma during alkaline pH stress
We have previously created a null mutation of the TgGCN5-A gene by replacing the majority of the genomic locus with a hypoxanthine-xanthine-guanine phosphoribosyltransferase (HXGPRT) minigene in type I RH strain parasites lacking HXGPRT, designated ΔGCN5-A [21]. The loss of TgGCN5-A had no overt effect on tachyzoites grown in standard culture conditions [21], mirroring phenotypes reported for other lower eukaryotes such as yeast [14]. In other species, GCN5 has been shown to be important for stress responses and developmental changes [23]. In Toxoplasma, stress can lead to expression of bradyzoite-specific genes and the eventual formation of tissue cysts. We predicted that if TgGCN5-A played a role in stress-induced bradyzoite gene expression, then it may be up-regulated in response to a stress agent. After 3 days in alkaline culture conditions (pH 8.2), message levels for TgGCN5-A increase >5-fold in wild-type RH Toxoplasma (Fig. 1). Actin was monitored as a control to show that alkaline pH stress does not globally increase gene expression (Fig. 1).
Fig. 1. TgGCN5-A mRNA is up-regulated in response to alkaline stress. 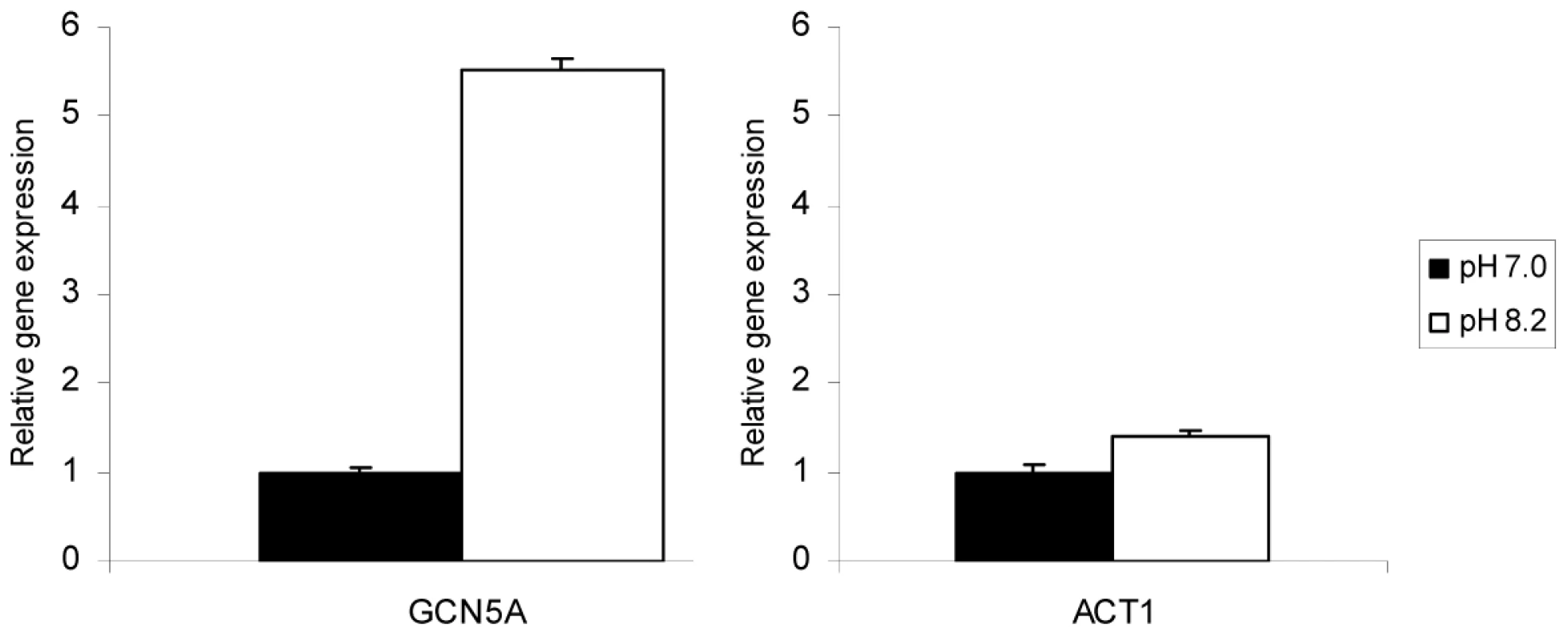
Wild-type (WT) parasites were incubated in control (pH 7.0) or alkaline media (pH 8.2) for three days. The levels of mRNA expression for TgGCN5-A or ACT1 were monitored by quantitative real-time PCR. Gene expression relative to control is shown. Error bars indicate the S.D. To further assess if TgGCN5-A played a role in the stress response in Toxoplasma tachyzoites, we performed whole genome expression profiling to compare ΔGCN5-A and wild-type parasites grown for 3 days either in alkaline pH (8.2) medium or control medium. Gene profiling of certain Toxoplasma strains under stress has been reported previously, but the effects of alkaline pH stress on RH strain has not been examined [24]. Affymetrix ToxoGeneChip microarrays, which contain probe sets for ∼8000 predicted Toxoplasma genes, were used for this study. The differentially regulated genes were grouped into functional categories based on Gene Ontology (GO) annotations in the Toxoplasma genome database at ToxoDB.org. The entire dataset of Toxoplasma genes affected by alkaline pH stress is available as supplemental data (Dataset S1). TgGCN5-A was present in wild-type and absent in ΔGCN5-A, validating the identity of the samples and supporting the fidelity of the microarray analysis. The fidelity of select microarray results was further confirmed through independent qPCR (Table S1).
Results show that ∼14% (1,114) of genes are differentially regulated (p value<0.001) in intracellular wild-type parasites after 3 days of alkaline pH exposure. Similar to findings in S. cerevisiae [25], a broad range of genes have altered expression in Toxoplasma during alkaline pH exposure, including genes involved in invasion, metabolism, protein processing, signaling and gene expression, and membrane transport. Of the 1,114 genes affected, 592 genes were up-regulated (Fig. 2A) and 522 genes were down-regulated (Fig. 2B). Among the genes with largest changes in response to alkaline stress (an arbitrary cut-off of 2-fold or more), 177 were up-regulated (Table S2) and 84 were down-regulated (Table S3).
Fig. 2. Genes modulated during alkaline pH stress in wild-type Toxoplasma. 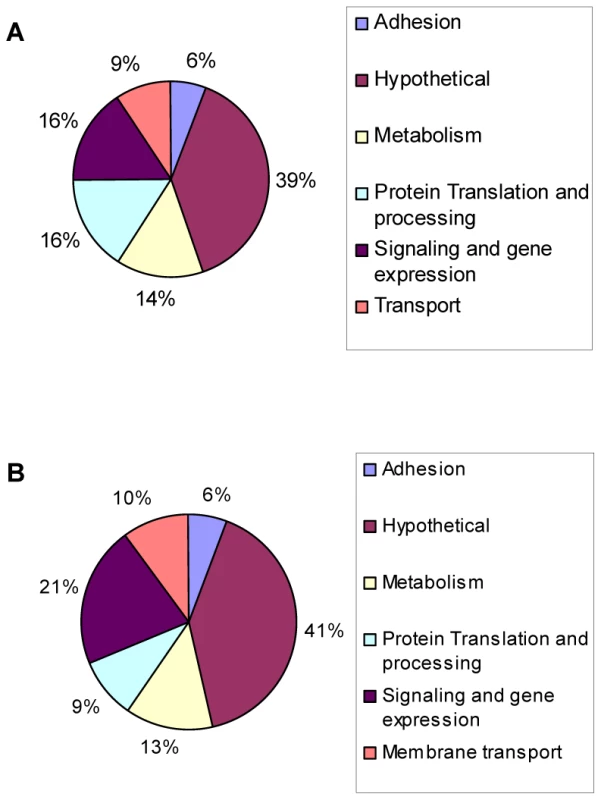
Microarray analysis was performed on wild-type parasites incubated in HFFs cells under normal culture conditions (pH 7.0) or alkaline conditions (pH 8.2) for three days. Pie chart displays the GO categories of genes reported to be (A) up-regulated or (B) down-regulated in response to alkaline conditions (p<0.001). Common Environmental Response/Environmental Stress Response genes
Many of the genes altered during pH stress in Toxoplasma have been previously described as being part of the Common Environmental Response (CER) or Environmental Stress Response (ESR) [26], [27] while others appear specific to the alkaline stress response.
CER/ESR is characterized by increased expression of genes involved in a wide array of cellular processes including metabolism, detoxification, protein folding and degradation, metabolite transport, vacuolar and mitochondrial function, and intracellular signaling [26], [27]. Our genome-wide analysis revealed that intracellular tachyzoites exposed to alkaline pH have increased levels of messages encoding enzymes involved in glycogen synthesis, such as 1,4-alpha-glucan branching enzyme, glycan synthase, and other gluconeogenic enzymes that facilitate glycogen synthesis (Table S4). Moreover, the 2.3-fold increase in trehalose-6-phosphate synthase transcript suggests trehalose synthesis increases during alkaline stress in Toxoplasma (Table S4). Glycogen and trehalose act as stores of energy reserves, stabilize proteins, and provide osmolyte balance during stress [28], [29].
Another classical feature of CER/ESR is the up-regulation of protein folding enzymes and protein degrading enzymes. Our microarray study showed that pH-stressed tachyzoites generally have increased levels of chaperones and protein folding enzymes such as TCP-1/cpn chaperones and DnaJ domain containing proteins. Sixteen genes involved in protein folding increased while 7 showed reduced expression (Table S4 and Dataset S1). Similarly, the pH-stressed parasites up-regulate factors involved with proteosomal degradation, suggesting that they are preparing to remove malfolded proteins. During pH stress in Toxoplasma, ribosomal genes tend to be slightly depressed or unchanged, consistent with the cell shifting to an energy saving mode.
Another feature of CER/ESR is the induction of genes that provide protection from oxidizing agents, including transporters that maintain ion homeostasis and an intracellular reducing environment. In alkaline pH-stressed tachyzoites, thioredoxins, glutraredoxins, and glutathione reductase genes are up-regulated, as well as genes encoding a cation transporter, solute transporter, and sodium/hydrogen exchangers (Table S4). We conclude that tachyzoites exposed to alkaline stress exhibit many parallels to the CER/ESR reported in yeast [26], [27], supporting the idea that this type of core stress response arose very early in eukaryotic cell evolution.
pH-specific changes in gene expression
Very few studies have examined the alkaline stress response of eukaryotic cells in detail. Studies in S. cerevisiae have demonstrated up-regulation of several phosphate transport and metabolism genes in response to alkaline pH, including PHO84, PHO11/PHO12, and PHO89, as well as ion transport and homeostasis genes such as CTR3, FRE1, and ARN4 [27], [30], [31]. We found a potential homologue of PHO84 in Toxoplasma (46.m01634) that is increased nearly 2-fold in wild-type parasites exposed to alkaline stress. Our data also shows that a ctr copper transporter domain-containing protein increases 2-fold (55.m05007), two calcium-transporting ATPase increase ∼2-fold (44.m02594 and 583.m00010), and a cation-transporting ATPase (80.m00077) increases 2.8-fold.
A number of C2H2 zinc finger transcription factors are known to be up-regulated during alkaline pH exposure in S. cerevisiae, such as Rim101 and NRG2 [30]. Only one predicted C2H2 zinc finger protein was slightly up-regulated in Toxoplasma, 55.m04837 (1.3-fold), and it is not clear if it is orthologous to Rim101 or NRG2. TIS11 is a CCCH type zinc finger protein up-regulated during alkaline stress in yeast [30]; two zinc fingers of this class were up-regulated >2-fold in Toxoplasma exposed to alkaline stress. Genes encoding several other zinc finger proteins were also increased during alkaline stress (Table 1). A number of transcription factors in Apicomplexa containing plant-like Apetela-2 domains (AP2) also increased during alkaline pH stress; those increasing 2-fold or more include 20.m03817, 80.m02236, 37.m00767, and 641.m01483 (Table 2). No AP2 domain proteins were down-regulated 2-fold or more. The data suggest that the Toxoplasma and yeast alkaline stress response share some common features, but there are clearly parasite-specific changes taking place, as evidenced by the up-regulation of 75 hypothetical genes of unknown function at least 2-fold in alkaline pH-stressed Toxoplasma (Table S5).
Tab. 1. Predicted zinc finger proteins up-regulated during alkaline stress in Toxoplasma. 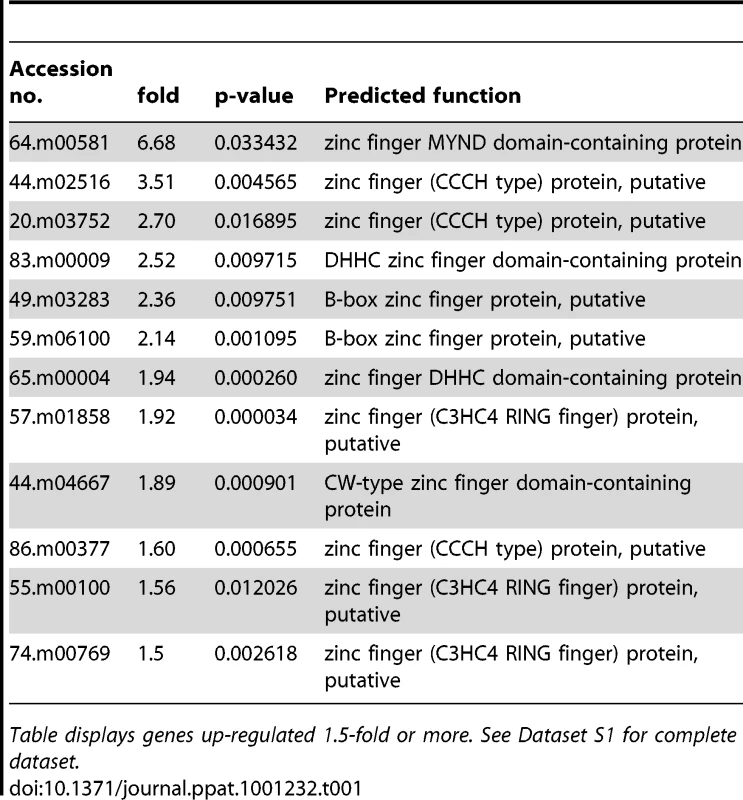
Table displays genes up-regulated 1.5-fold or more. See Dataset S1 for complete dataset. Tab. 2. Predicted AP2 proteins up-regulated during alkaline stress in Toxoplasma. 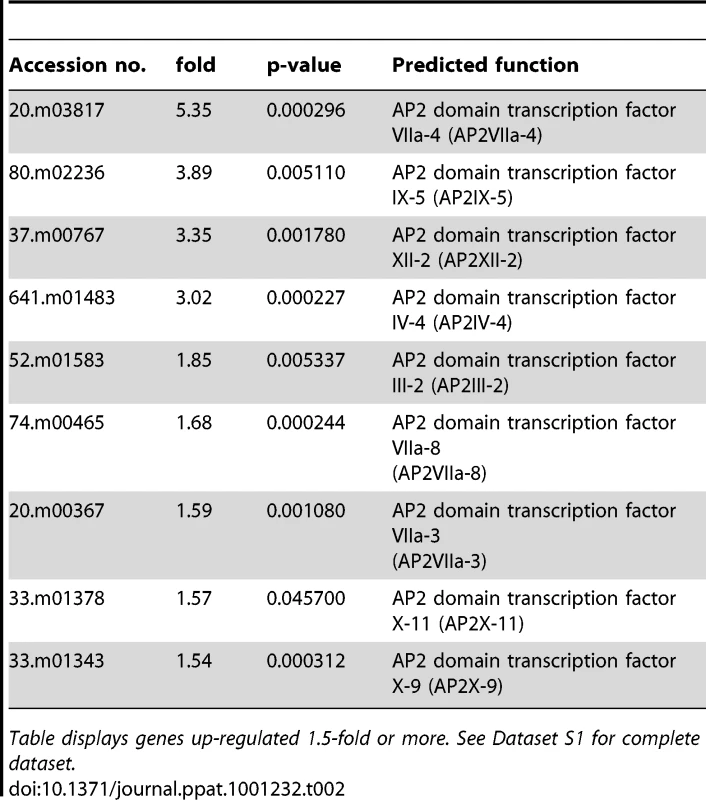
Table displays genes up-regulated 1.5-fold or more. See Dataset S1 for complete dataset. TgGCN5-A itself was not observed to increase during alkaline stress by microarray analysis, appearing to contradict the highly reproducible findings in Fig. 1. However, only a small portion of the 5′ end of the TgGCN5-A gene is represented on the ToxoGeneChip array, which likely prevents accurate measurements of TgGCN5-A. In contrast, the qPCR primers used for Fig. 1 were designed to the 3′ end of the open reading frame, providing much more accurate measurements of TgGCN5-A message levels.
Deficient gene regulation in ΔGCN5-A parasites during alkaline stress
A scatter plot of the microarray data reveals that there are virtually no differences (R2 = 0.99) in gene expression patterns between wild-type and ΔGCN5-A parasites grown under normal culture conditions (Fig. 3A), which is consistent with the indiscernible phenotype of ΔGCN5-A cultured in normal conditions. However, relative to wild-type, the ΔGCN5-A parasites differ dramatically in their ability to regulate gene expression when grown in alkaline medium (R2 = 0.43) (Fig. 3B). While 1,114 genes are altered in wild-type parasites exposed to alkaline stress, only 502 genes were changed in alkaline-stressed parasites lacking TgGCN5-A (p<0.001). Since TgGCN5-A is an activator of gene expression we focused on the genes up-regulated during alkaline stress. Further examination of up-regulated genes reveals that ΔGCN5-A parasites fail to up-regulate 439 of the 592 genes up-regulated by wild-type parasites grown in alkaline pH. In other words, TgGCN5-A is required for increased expression of 74% of the genes activated in response to alkaline pH. TgGCN5-A-dependent genes have diverse roles in signaling and gene expression (18%), protein processing and translation (17%), metabolism (13%), membrane transport (8%), and adhesion (7%) (Fig. 4). TgGCN5-A also controls a substantive number of hypothetical proteins with no known function (37%). Hypothetical genes with a change of 2-fold or more are listed in Table S5.
Fig. 3. ΔGCN5-A parasites fail to up-regulate alkaline pH response genes. 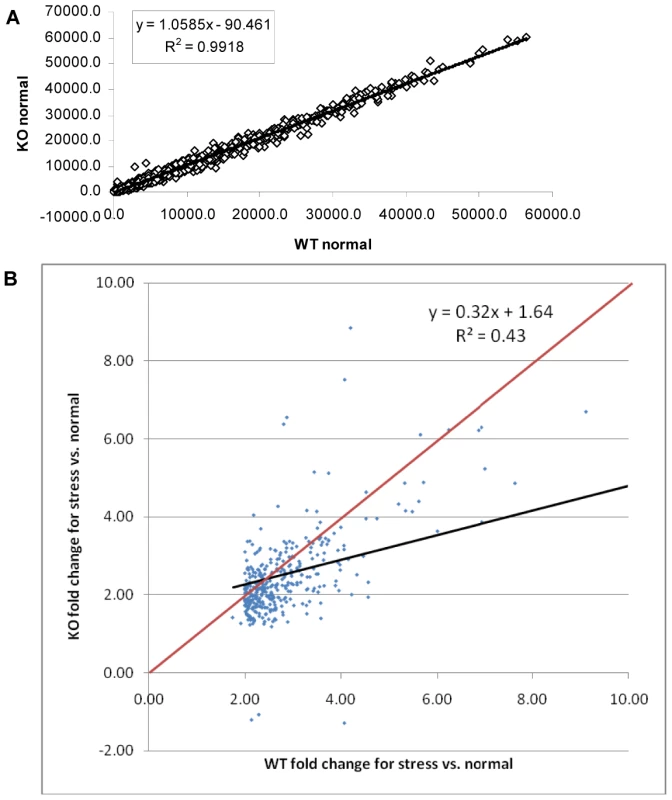
A. Scatter plot of expression levels for ΔGCN5-A (KO – vertical axis) vs. wild-type (WT –horizontal axis) under normal conditions. Data limited to those genes with fraction present ≥0.5 in WT or KO. B. Scatter plot of wild-type (WT) vs. ΔGCN5-A knockout (KO) fold changes. Probe sets limited to those with maximum fraction present ≥0.5, p<0.01 and fold ≥2.0 for WT stress vs. normal culture conditions. Regression line is in black. All points below the red line are genes with smaller fold changes in KO than in WT for stress vs. normal culture conditions. Fig. 4. Types of genes modulated by TgGCN5-A during alkaline stress. 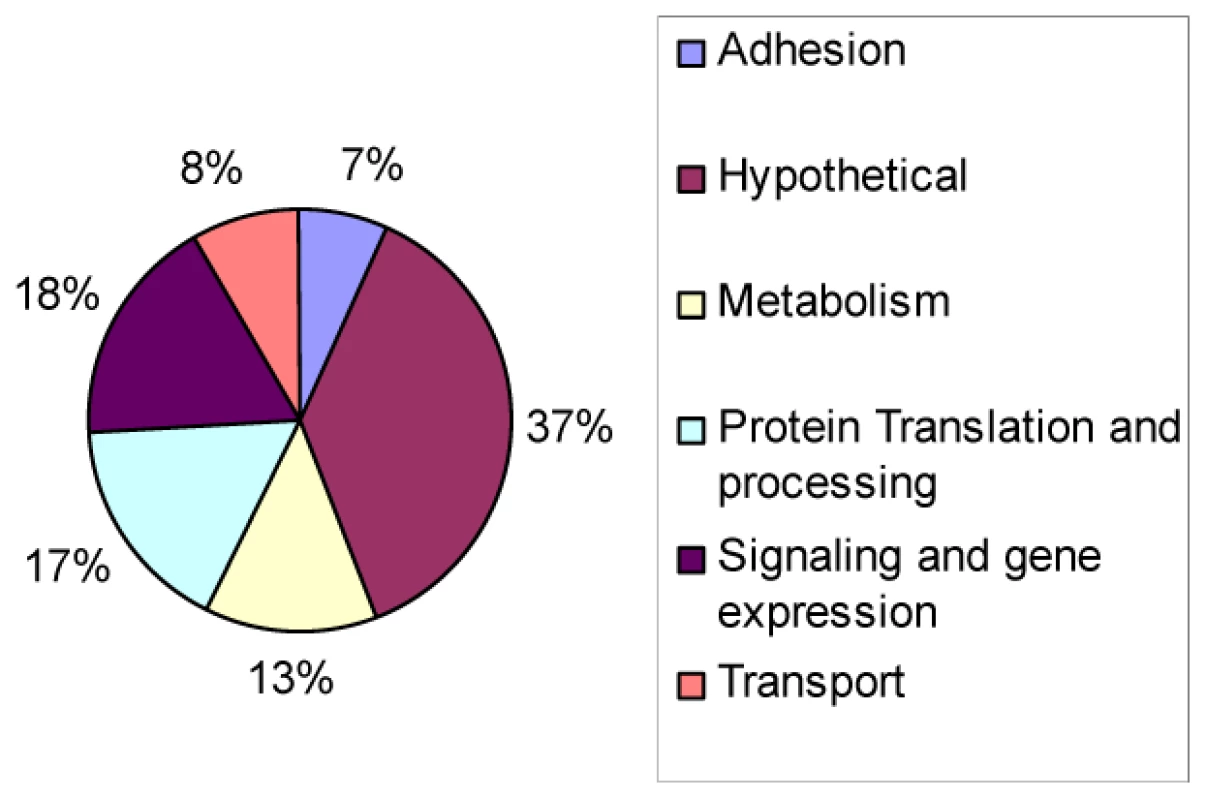
Pie chart displays GO categories of genes that were not up-regulated in ΔGCN5-A parasites relative to wild-type (p<0.001) after culture in alkaline (pH 8.2) conditions. Our microarray data (p<0.05) identifies numerous genes related to Ca2+ signaling that were not up-regulated normally in parasites lacking TgGCN5-A. These include calcium-dependent protein kinase (86.m00003), calmodulin beta (42.m03474), calmodulin genes (50.m03141, 59.m03587), and calcium/calmodulin-dependent 3′, 5′-cyclic nucleotide phosphodiesterases (583.m05366, 59.m03644) (Dataset S1). Although the eukaryotic response to alkaline exposure is poorly understood, transient increases in intracellular calcium occur, possibly activating calcineurin and leading to a signaling cascade that results in mobilization of transcription factors [25], [31].
TgGCN5-A is enriched at genes up-regulated during alkaline stress
Previously we have demonstrated that increased acetylation accompanies TgGCN5-A promoter occupancy [3]. To obtain in vivo confirmation that TgGCN5-A plays a direct role in the co-activation of genes shown to be up-regulated during alkaline culture, we used chromatin immunoprecipitation (ChIP). RH parasites expressing FLAG-tagged TgGCN5-A (fGCN5-A) were employed in ChIP experiments to purify DNA in association with fGCN5-A using anti-FLAG [3], [32]. We examined a region ∼1.0 kb upstream of the start ATG for phosphatidylinositol 3 - and 4-kinase (PI3-4K, 76.m01548) and protein kinase (PK, 641.m01507) genes, both of which are up-regulated during pH stress (p<0.05, Dataset S1). Two housekeeping genes, actin (25.m00007) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 80.m00003), whose expression was not altered during pH stress, were included as controls. ChIP data show an enrichment of fGCN5-A at a region upstream of PI3K and PK during alkaline stress (Fig. 5A). The levels of fGCN5-A remained unchanged at housekeeping genes, demonstrating that the increase of fGCN5-A at pH-responsive genes above is not random (Fig. 5B). We performed an additional negative control for each ChIP sample using a nonspecific antibody (anti-TgIF2K-A) as described previously [33], none of which produced a signal (data not shown). Combined with the microarray analysis, these data support the idea that TgGCN5-A is required for proper gene activation in response to alkaline stress.
Fig. 5. TgGCN5-A is enriched at genes up-regulated during alkaline pH stress. 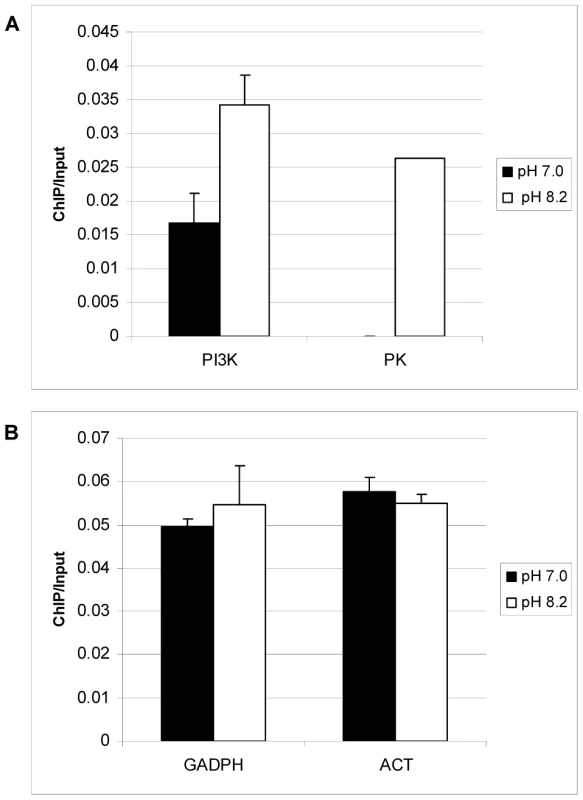
Toxoplasma parasites stably expressing FLAG-tagged TgGCN5-A were maintained in HFFs with normal (pH 7.0) or alkaline (pH 8.2) medium prior to harvesting for qChIP using anti-FLAG antibody. Immunoprecipitated DNA was examined using primers to pH responsive genes phosphatidylinositol 3- and 4-kinase (PI3K), and protein kinase (PK) (A), or housekeeping gene controls, GAPDH and actin, ACT (B). Results are represented as a ratio of ChIP/Input DNA. The difference between pH 7 and pH 8 for PI3K is statistically significant (p = 0.02, Student's t-test). ΔGCN5-A parasites fail to activate bradyzoite genes in response to alkaline stress
The impaired response to alkaline pH stress suggests that ΔGCN5-A parasites may also be defective in bradyzoite development. The knockout was made in the hypervirulent type I RH strain, which does not fully develop into bradyzoites at high frequency. RH strain will generally not form cyst walls, but will express detectable levels of bradyzoite-specific marker genes in response to stresses that induce cyst formation [34]. In our hands, we can detect BAG1 and LDH2 mRNAs by day 4 of alkaline pH treatment. To test if TgGCN5-A plays a role in stress-induced bradyzoite gene expression, we grew wild-type or ΔGCN5-A parasites in pH 7.0 (control) or pH 8.2 (stress) media. At day 4, intracellular parasites were harvested for quantitative real-time PCR to monitor mRNA levels for bradyzoite-specific genes BAG1 and LDH2. While wild-type parasites up-regulate both bradyzoite marker genes in response to pH 8.2, the ΔGCN5-A parasites fail to do so (Fig. 6A and 6B). Actin was monitored as a control gene that does not significantly change during bradyzoite induction (Fig. 6C). To ensure the defect was not an indirect effect, we complemented ΔGCN5-A parasites by stably expressing a recombinant copy of TgGCN5-A. Expression of BAG1 and LDH2 was restored in the complemented ΔGCN5-A parasites exposed to alkaline stress (Fig. 6A and 6B). The mRNA levels of BAG1 and LDH2 following stress in complemented ΔGCN5-A parasites are higher than wild-type, presumably because the recombinant TgGCN5-A is being expressed above wild-type levels. Interestingly, this higher level of TgGCN5-A expression in the complemented clone does not affect levels of BAG1 or LDH2 under non-stressed conditions, implying that TgGCN5-A does not activate these bradyzoite genes until a stress signal is perceived by the parasite. We used ChIP analysis to further demonstrate the involvement of TgGCN5-A in regulating developmentally expressed genes. ChIP demonstrates that TgGCN5-A is recruited to BAG1 and LDH2 promoter regions during alkaline stress (Fig. 6D). We conclude that parasites lacking TgGCN5-A are defective in up-regulating bradyzoite-specific genes in response to alkaline stress.
Fig. 6. ΔGCN5-A parasites fail to up-regulate developmental genes in response to alkaline pH. 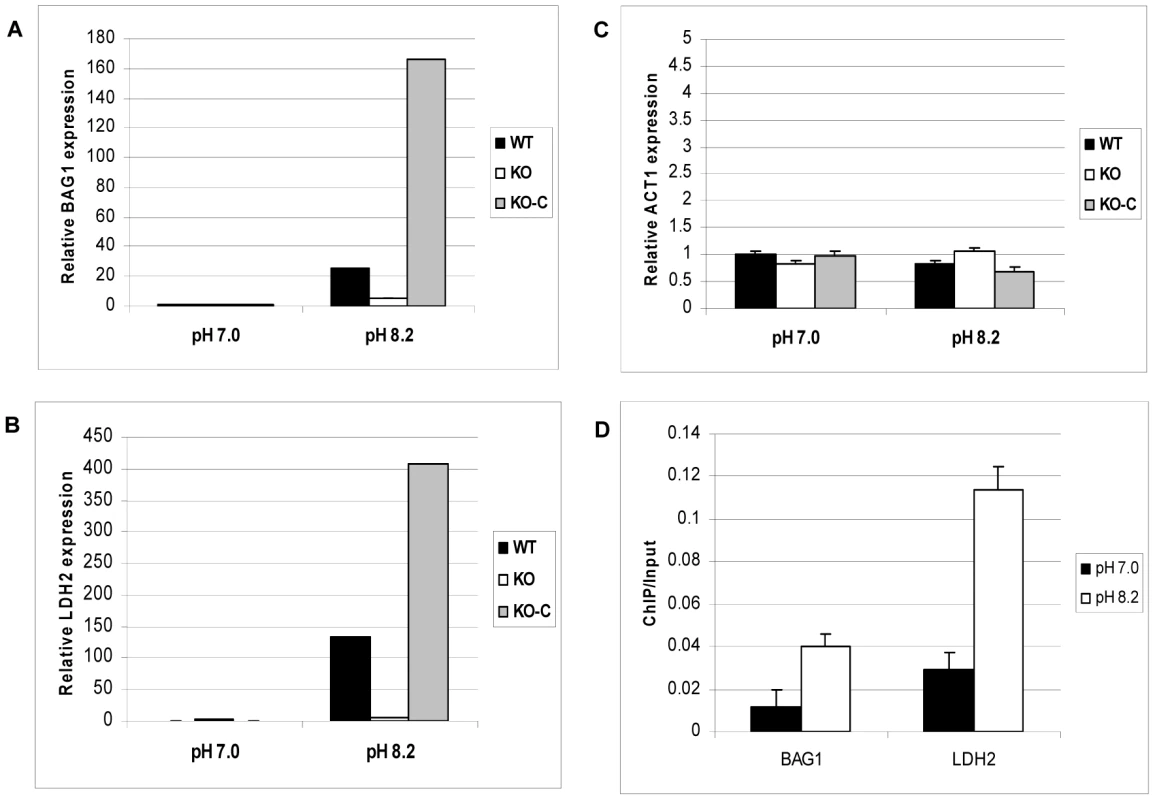
Wild-type (WT), ΔGCN5-A (KO), or complemented ΔGCN5-A (KO-C) parasites were incubated in control (pH 7.0) or alkaline media (pH 8.2) for four days. The levels of mRNA expression for stress-induced bradyzoite genes BAG1 (A) and LDH2 (B) or the constitutive gene actin, ACT1, (C) were monitored by quantitative real-time PCR. Error bars indicate the S.D. D. ChIP of FLAG-tagged TgGCN5-A performed as described in Fig. 5, but using primers for BAG1 and LDH2 (Table S6). Results are represented as a ratio of ChIP/Input DNA. P value for BAG1 sample = 0.02, P value for LDH2 sample = 0.01 (Student's t-test). TgGCN5-A knockout parasites are deficient in recovery from alkaline stress
Based on our results, it would be predicted that parasites exposed to alkaline stress would have difficulty recovering from the insult. To test this hypothesis, intracellular parasites were exposed to pH 8.2 for 3 or 5 days, harvested, and then inoculated into fresh host cells and cultured under normal (pH 7.0) conditions. Parasite proliferation was monitored using the PCR-based B1 assay. Data show that intracellular parasites lacking GCN5-A exposed to pH 8.2 for 3 days do not recover as efficiently as wild-type or the GCN5-A complemented clone (Fig. S1). The recovery defect is even more pronounced when the intracellular ΔGCN5-A parasites are subjected to pH 8.2 for 5 days (Fig. S1).
The preceding studies were performed on tachyzoite-infected host cells. We also examined if direct exposure to alkaline stress produced a phenotype in the parasites. In order to test if alkaline pH impacts ΔGCN5-A parasites directly, we monitored the ability of purified, extracellular ΔGCN5-A tachyzoites to recover from a short term exposure to alkaline pH. Equal numbers of extracellular ΔGCN5-A or wild-type tachyzoites were placed in media of pH 7.0 (control) or 8.2 for 30 min, and then allowed to infect confluent host cell monolayers under normal culture conditions. At day five, plaques in the infected monolayers were counted. The ΔGCN5-A mutant displayed increased sensitivity to alkaline pH as evidenced by its impaired ability to produce plaques following this insult (Fig. 7A). Recombinant TgGCN5-A was able to restore the ability of ΔGCN5-A parasites to recover from alkaline stress (Fig. 7A). We verified the results with a second, independent type of growth assay that monitors parasite proliferation through quantitative PCR of the parasite-specific B1 gene (Fig. 7B). Collectively, these studies establish that TgGCN5-A is a key factor that manages the Toxoplasma response to alkaline pH stress in RH strain tachyzoites. It is curious that the complemented clone, which appears to be over-expressing TgGCN5-A, does not offer greater protection from alkaline stress despite being able to up-regulate BAG1 and LDH2 greater than wild-type (Fig. 6). GCN5 HATs function in large multi-subunit complexes, the components of which vary in different cells or under different conditions. A possible explanation could be that a “minimal” GCN5 complex can up-regulate certain stress response genes, but it is not capable of providing greater protection to the cell because other components are required that are not over produced.
Fig. 7. Parasites lacking TgGCN5-A are defective in recovering from alkaline stress. 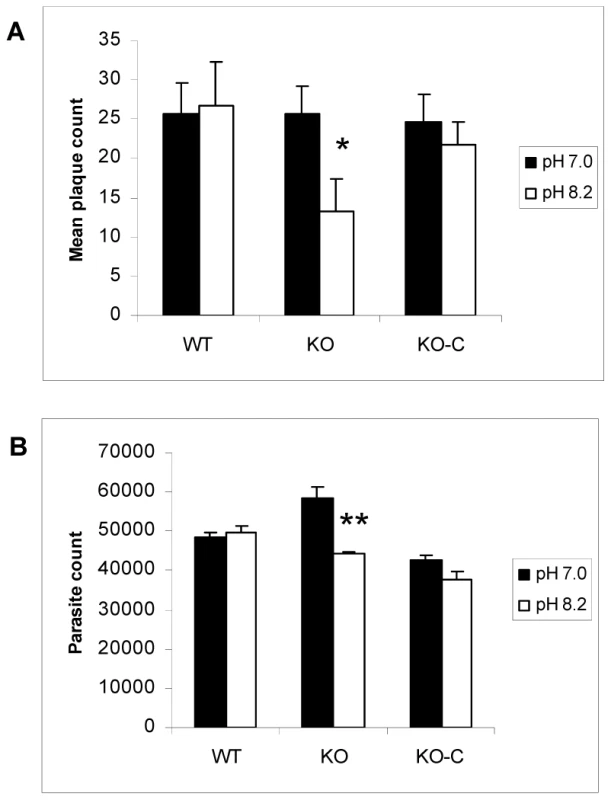
Equal numbers of extracellular wild-type (WT), ΔGCN5-A (KO), or complemented ΔGCN5-A (KO-C) parasites were subjected to alkaline (pH 8.2) or control (pH 7) media for 30 min at 37°C in 5% CO2. Following treatment, the parasites were allowed to infect HFF monolayers and incubated under normal culture conditions. A. Parasite growth was monitored with a standard plaque assay at day 5 (* denotes p = 0.017). B. Viability assay was set up as described above, but growth was monitored with the PCR-based B1 assay (** denotes p = 0.012). We examined whether ΔGCN5-A parasites were hypersensitive to other stresses, including 30 minute exposure to 0.6 M KCl, 5 µM arsenite, 1 µM ionophore, or 42°C heat shock. Upon being placed back in culture following the exposure to these stresses, the ΔGCN5-A parasites grew similarly to wild-type with exception of those exposed to KCl stress (Fig. 8A and 8B). Remarkably, TgGCN5-A appears to have a striking specificity for managing the alkaline and possibly KCl stress responses, possibly because each disrupts ion potential. Such narrow specificity in stress response has been reported for Schizosaccharomyces pombe GCN5 [15].
Fig. 8. Response of ΔGCN5-A parasites to other stress conditions. 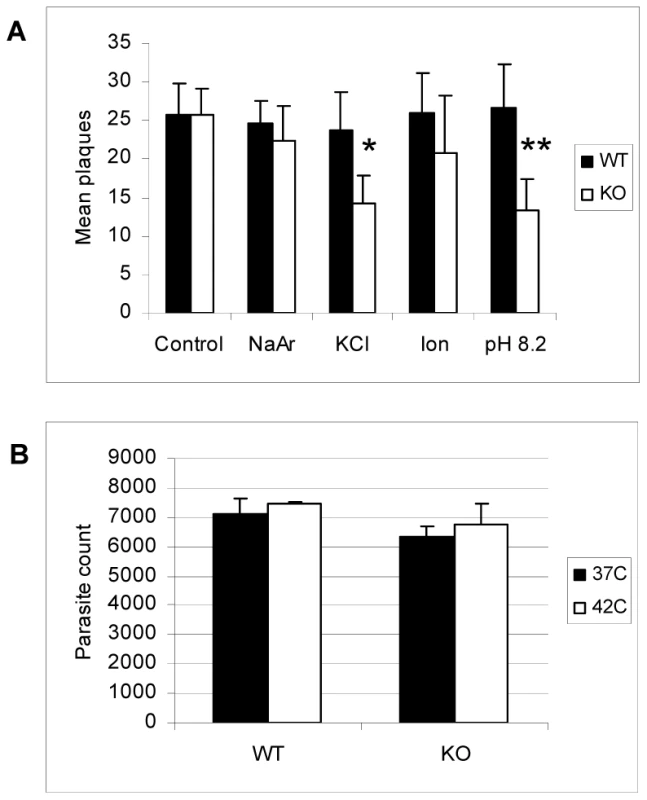
A. Equal numbers of extracellular wild-type (WT) or ΔGCN5-A (KO) were subjected to one of the following stresses for 30 min before being placed back into HFF culture under normal growth conditions: 0.6 M KCl, 5 µM sodium arsenite (NaAr), or 1 µM ionophore (Ion). Alkaline pH (8.2) stress was used as a positive control and no stress as a negative control. The mean number of plaques after five days recovery in HFF monolayer culture is shown. P values for Student's t-test are denoted as * = 0.03 and ** = 0.02. B. Equal numbers of extracellular wild-type (WT) or ΔGCN5-A (KO) were incubated at 37°C or 42°C for 30 minutes then placed in HFF culture at 37°C. The number of parasites in culture at day 5 post-infection was determined using the PCR-based B1 assay. Concluding remarks
Toxoplasma gondii possesses two GCN5 KATs, which is unusual as lower eukaryotes tend to have a single GCN5. We have sought to delineate the roles of these two GCN5s by making genetic knockouts. The ΔGCN5-A mutant is viable and does not show growth defects under normal culture conditions; however, attempts to generate a knockout of TgGCN5-B have not been successful. These results support a model that TgGCN5-B is essential for housekeeping functions while TgGCN5-A is required to overcome certain stress situations. Such a role for TgGCN5-A is established by the studies described herein. It remains possible that there is some functional overlap between the two TgGCN5s, but whatever contribution is made by TgGCN5-B is not sufficient to compensate for the loss of TgGCN5-A in terms of responding normally to alkaline pH stress, including the up-regulation of bradyzoite marker genes BAG1 and LDH2.
A key finding in this study is that TgGCN5-A is required to activate developmental genes in response to pH stress. These studies were performed in type I RH strain, which is not well suited for a more thorough characterization of bradyzoite development in vitro or in vivo. Such studies would require the generation of an analogous TgGCN5-A knockout in type II strain Toxoplasma. Our initial attempts to disrupt the TgGCN5-A knockout in type II strains have not yet succeeded, probably due to technical challenges inherent in working with the slow growing type II strain, but it is possible that TgGCN5-A is essential in type II strain.
It is intriguing that Toxoplasma possesses a duplicate GCN5 HAT that appears to be exquisitely tailored to respond to alkaline, and to a lesser extent, KCl stress. Adaptation to fluctuations in pH is likely to be relevant to proliferating tachyzoites, as pH stress almost certainly is encountered by Toxoplasma in vivo as it moves in and out of host cells throughout diverse regions of the body. Additionally, while not addressed for Toxoplasma infection, it has been reported that other infections elevate intracellular pH [35]. It is difficult to distinguish whether the observed changes in intracellular parasites are due to direct effects of high pH on Toxoplasma parasites themselves or indirect effects produced by the response of host cells. What is clear is that RH strain parasites lacking TgGCN5-A are defective in regulating changes in the transcriptome that accompany the response to alkaline pH. The data are significant as alkaline pH is considered a “gold standard” method for triggering bradyzoite development in vitro [1]. In summary, our studies establish that TgGCN5-A plays a major role in the normal response to alkaline pH stress, including the activation of developmentally regulated genes, in Toxoplasma RH strain. The conclusion is based on multiple lines of data from independent studies, including up-regulation of TgGCN5-A mRNA during pH stress, microarray analysis, TgGCN5-A enrichment at genes up-regulated during alkaline stress, and phenotypic analysis showing that the TgGCN5-A knockout has impaired alkaline stress recovery. Our microarray analysis also provides novel insight into the molecular basis of the alkaline stress response in intracellular Toxoplasma.
Materials and Methods
Parasite culture and reagents
Toxoplasma tachyzoites (wild-type (WT) RH, ΔGCN5-A, and TgGCN5-A complemented lines) were cultured in primary human foreskin fibroblasts (HFF) using Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 1.0% fetal bovine serum (FBS, Invitrogen). Parasites were grown in a humidified CO2 (5%) incubator at 37°C. Cultures were confirmed to be free of Mycoplasma contamination by MycoAlert Assay (Cambrex Bio Science). Parasites were harvested immediately following lysis of host cell monolayers and purified by filtration through a 3.0 micron filter [36]. Bradyzoite growth conditions were identical except the infection medium was replaced with alkaline medium (DMEM with 20 mM HEPBS, 2 g NaHCO3/L and 1.0% FBS, adjusted to pH 8.2 using NaOH) and changed daily.
Stress conditions and parasite growth assays
Tachyzoites were released from HFF monolayers using a 25 gauge syringe needle, and filtered to remove the host cell debris was spun out at 500×g for 5 min. Extracellular parasites were resuspended at a concentration of 105 parasites/ml in DMEM, alkaline medium (pH 8.2, adjusted with NaOH as described above), or medium containing 0.6 M KCl, 5 µM arsenite, 1 µM ionophore. This suspension was incubated at 37°C (or 42°C for heat shock experiment) in 5% CO2 for 30 min, and then 103 parasites were inoculated onto HFF monolayers in 24-well plates. After 5 day incubation at 37°C, the infected monolayers were fixed with 100% methanol and stained with crystal violet to score the number of plaques [36] or processed for B1 PCR assay [37].
RNA preparation and hybridization to microarrays
Confluent HFF monolayers grown in T25-cm2 flasks were infected with 106 parasites using normal parasite culture media (above). After 2 hr, infection medium was replaced with normal medium (pH 7.0) or alkaline medium (see above). Flasks were maintained in humidified 37°C incubator in 5% CO2. Medium for both normal and alkaline cultures was replaced each day for 3 days, at which point the infected monolayers were scraped with a rubber policeman. Samples were centrifuged (1500 rpm, 10 min) and resuspended in sterile PBS. Intracellular parasites were released from host cells by syringe passage using a 25 gauge needle and washed in PBS. Total RNA was isolated from the purified parasites using an RNeasy Mini Kit according to the manufacturer's instructions (Qiagen). To minimize genomic DNA contamination, additional treatment with DNase was performed. The cDNA and cRNA were synthesized according to the protocols recommended by Affymetrix in their GeneChip Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA). Briefly, cDNA was synthesized using T7 promoter-dT24 oligonucleotide as primer with the Invitrogen Life Technologies SuperScript Choice system. Biotinylated cRNA was made using the Affymetrix in vitro transcription (IVT) labeling kit. Fifteen µg of biotinylated cRNA was added to a total hybridization cocktail of 300 µl, and 200 µl was hybridized to an Affymetrix custom T. gondii ToxoGeneChip (http://roos-compbio2.bio.upenn.edu/~abahl/Array-Tutorial.html) after adding control oligonucleotides. Hybridization was performed at 45°C for 17 h with constant rotation. The hybridization mixture was then removed and the GeneChips were washed, stained with phycoerythrin-labeled streptavidin, washed, incubated with biotinylated anti-streptavidin, and re-stained with phycoerythrin-labeled streptavidin to amplify the signals; these steps were carried out using the Affymetrix Fluidics Station. To reduce non-random error, balanced groups of samples were handled in parallel. Arrays were then scanned using the dedicated scanner, controlled by Affymetrix GeneChip Operating Software (GCOS). The Affymetrix Microarray Suite version 5 (MAS5) algorithm was used to analyze the hybridization intensity data from GeneChip expression probe arrays and to calculate the set of metrics that describe probe set performance. The average intensity on each array was normalized by global scaling to a target intensity of 1000. For each of the two conditions (normal and alkaline), four independent preparations of WT and ΔGCN5-A RNA were prepared; each of the 16 preparations was hybridized to its own microarray to ensure a strong statistical basis for analysis.
Statistical analysis and data deposition
We analyzed only those probe sets (genes) that were called “present” by the MAS5 detection call in at least half of the arrays for at least one of the four conditions to eliminate probe sets that are not reliably detected (those at or near background or that may reflect cross-hybridization) [38]. We used a Welch's t-test on log2 transformed MAS5 signals to reveal significant differences between WT and ΔGCN5-A. The resultant p-values were used to calculate false discovery rates (FDR) using the Benjamini and Hochberg method [39]. Fold-changes were calculated by taking the ratio of the mean of the WT and ΔGCN5-A signal values, using the larger mean as the numerator; by convention we show the result as negative if the mean of the ΔGCN5-A samples was smaller. Microarray data are available at Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), accession number GSE22100.
Real-time reverse transcription-PCR (qRT-PCR)
Primers were designed using the Primer Express 2.0 software (Applied Biosystems, CA) and are listed in Table S6. The RT reaction was performed using 1.0 µg total RNA isolated from designated tachyzoites, with oligo-dT primers and Omniscript reverse transcriptase according to the manufacturer's directions (Qiagen). 1.0 µl of a 1∶10 dilution of the resulting cDNA was amplified in a 25 µl total volume containing SYBR Green PCR Master Mix (Applied Biosystems, CA) and 0.5 µM of each forward and reverse primer. All reactions were performed in triplicate using the 7500 Real-time PCR system and analyzed with relative quantification software (7500 software v2.0.1) (Applied Biosystems, CA). The Toxoplasma β-tubulin gene (Genbank accession number M20025) was used to normalize the qRT-PCRs. β-tubulin was determined to be equivalent between samples using actin and GAPDH as normalizing controls.
Chromatin immunoprecipitation (ChIP)
ChIP was performed on tachyzoites stably expressing fGCN5-A using polyclonal anti-FLAG antibody (Sigma F7425) immobilized to Dynabeads Protein A (Invitrogen). Quantitative PCR was performed as described above. Immunoprecipitated DNA samples were quantified using a standard curve created with serially diluted input DNA. 0.1 ng of total ChIP DNA was added to each reaction and reactions were performed in triplicate. Primers used are listed in Table S6, each pair designed to amplify ∼90 bp regions located ∼1.0 kb upstream of the ATG start site.
Generation of complemented ΔGCN5-A parasite clones
To complement ΔGCN5-A parasites, the ptubXFLAG::HX Toxoplasma expression vector [32] was modified to replace its HX minigene marker with a CAT minigene to confer resistance to chloramphenicol. Recombinant, tagged full-length TgGCN5-A was cloned into the vector using the NdeI and AvrII sites, referred to as ptubMYCGCN5-AFLAG::CAT. The TgGCN5-A coding sequence was amplified from Toxoplasma cDNA using Phusion® High-Fidelity DNA Polymerase (New England Biolabs) and primers containing NdeI and AvrII restriction enzyme sites (italicized below). The sense primer contained sequence encoding the MYC epitope tag (underlined) and the antisense primer lacked the TgGCN5-A stop codon to allow in-frame fusion with a FLAG tag in the vector [32]: sense, 5′-ATACCATCATATGAAAATGGCGTACCCGTACGACGTCCCGGACTACGCGGAGACTGTCGAAGTGCCTGCATTC; antisense, 5′-ATACCATCCTAGGGAAACTCCCGAGAGCCTCGACCTTGGGCC. 106 ΔGCN5-A parasites were transfected with 20 µg NotI-linearized vector and selected for resistance to 20 µM chloramphenicol before cloning by limiting dilution as previously described [36]. Multiple clones were selected and verified to express ectopic MYCGCN5-AFLAG protein by IFA using anti-FLAG (Sigma F7425). Phenotypes reported were similar for multiple independent clones.
Supporting Information
Zdroje
1. WeissLM
KimK
2000 The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci 5 D391 405
2. WongSY
RemingtonJS
1993 Biology of Toxoplasma gondii. AIDS 7 299 316
3. SaksoukN
BhattiMM
KiefferS
SmithAT
MussetK
2005 Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol Cell Biol 25 10301 10314
4. SullivanWJJr
SmithAT
JoyceBR
2009 Understanding mechanisms and the role of differentiation in pathogenesis of Toxoplasma gondii: a review. Mem Inst Oswaldo Cruz 104 155 161
5. BergerSL
SternerDE
2000 Acetylation of histones and transcription-related factors. Microbiology and Molecular Biology Review 64 435 459
6. YangXJ
OgryzkoVV
NishikawaJ
HowardBH
NakataniY
1996 A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382 319 324
7. ZhangW
BoneJR
EdmondsonDG
TurnerBM
RothSY
1998 Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. Embo J 17 3155 3167
8. GregoryPD
SchmidA
ZavariM
LuiL
BergerSL
1998 Absence of Gcn5 HAT activity defines a novel state in the opening of chromatin at the PHO5 promoter in yeast. Mol Cell 1 495 505
9. JohnssonA
Durand-DubiefM
Xue-FranzenY
RonnerbladM
EkwallK
2009 HAT-HDAC interplay modulates global histone H3K14 acetylation in gene-coding regions during stress. EMBO Rep
10. YamauchiT
YamauchiJ
KuwataT
TamuraT
YamashitaT
2000 Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc Natl Acad Sci U S A 97 11303 11306
11. HuisingaKL
PughBF
2004 A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol Cell 13 573 585
12. LeeTI
CaustonHC
HolstegeFC
ShenWC
HannettN
2000 Redundant roles for the TFIID and SAGA complexes in global transcription. Nature 405 701 704
13. HelmlingerD
MargueratS
VillenJ
GygiSP
BahlerJ
2008 The S. pombe SAGA complex controls the switch from proliferation to sexual differentiation through the opposing roles of its subunits Gcn5 and Spt8. Genes Dev 22 3184 3195
14. MarcusGA
SilvermanN
BergerSL
HoriuchiJ
GuarenteL
1994 Functional similarity and physical association between GCN5 and ADA2: putative transcriptional adaptors. Embo J 13 4807 4815
15. JohnssonA
Xue-FranzenY
LundinM
WrightAP
2006 Stress-specific role of fission yeast Gcn5 histone acetyltransferase in programming a subset of stress response genes. Eukaryot Cell 5 1337 1346
16. Xue-FranzenY
JohnssonA
BrodinD
HenrikssonJ
BurglinTR
2010 Genome-wide characterisation of the Gcn5 histone acetyltransferase in budding yeast during stress adaptation reveals evolutionarily conserved and diverged roles. BMC Genomics 11 200
17. VlachonasiosKE
ThomashowMF
TriezenbergSJ
2003 Disruption mutations of ADA2b and GCN5 transcriptional adaptor genes dramatically affect Arabidopsis growth, development, and gene expression. Plant Cell 15 626 638
18. CarreC
SzymczakD
PidouxJ
AntoniewskiC
2005 The histone H3 acetylase dGcn5 is a key player in Drosophila melanogaster metamorphosis. Mol Cell Biol 25 8228 8238
19. XuW
EdmondsonDG
EvrardYA
WakamiyaM
BehringerRR
2000 Loss of Gcn5l2 leads to increased apoptosis and mesodermal defects during mouse development. Nat Genet 26 229 232
20. BuP
EvrardYA
LozanoG
DentSY
2007 Loss of Gcn5 acetyltransferase activity leads to neural tube closure defects and exencephaly in mouse embryos. Mol Cell Biol 27 3405 3416
21. BhattiMM
LivingstonM
MullapudiN
SullivanWJJr
2006 Pair of unusual GCN5 histone acetyltransferases and ADA2 homologues in the protozoan parasite Toxoplasma gondii. Eukaryot Cell 5 62 76
22. SullivanWJJr
SmithCK2nd
2000 Cloning and characterization of a novel histone acetyltransferase homologue from the protozoan parasite Toxoplasma gondii reveals a distinct GCN5 family member. Gene 242 193 200
23. KoutelouE
HirschCL
DentSY
2010 Multiple faces of the SAGA complex. Curr Opin Cell Biol
24. BehnkeMS
RadkeJB
SmithAT
SullivanWJJr
WhiteMW
2008 The transcription of bradyzoite genes in Toxoplasma gondii is controlled by autonomous promoter elements. Mol Microbiol 68 1502 1518
25. ViladevallL
SerranoR
RuizA
DomenechG
GiraldoJ
2004 Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J Biol Chem 279 43614 43624
26. GaschAP
SpellmanPT
KaoCM
Carmel-HarelO
EisenMB
2000 Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell 11 4241 4257
27. CaustonHC
RenB
KohSS
HarbisonCT
KaninE
2001 Remodeling of yeast genome expression in response to environmental changes. Mol Biol Cell 12 323 337
28. HounsaCG
BrandtEV
TheveleinJ
HohmannS
PriorBA
1998 Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology 144 Pt 3 671 680
29. SingerMA
LindquistS
1998 Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell 1 639 648
30. LambTM
XuW
DiamondA
MitchellAP
2001 Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J Biol Chem 276 1850 1856
31. SerranoR
RuizA
BernalD
ChambersJR
ArinoJ
2002 The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol Microbiol 46 1319 1333
32. BhattiMM
SullivanWJJr
2005 Histone acetylase GCN5 enters the nucleus via importin-alpha in protozoan parasite Toxoplasma gondii. J Biol Chem 280 5902 5908
33. VonlaufenN
NaguleswaranA
CoppensI
SullivanWJJr
2010 MYST family lysine acetyltransferase facilitates ataxia telangiectasia mutated (ATM) kinase-mediated DNA damage response in Toxoplasma gondii. J Biol Chem 285 11154 11161
34. SoeteM
CamusD
DubremetzJF
1994 Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol 78 361 370
35. PattonHK
ChuWJ
HetheringtonHP
den HollanderJ
StewartKE
2001 Alkaline pH changes in the cerebellum of asymptomatic HIV-infected individuals. NMR Biomed 14 12 18
36. RoosDS
DonaldRG
MorrissetteNS
MoultonAL
1994 Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol 45 27 63
37. CostaJM
PautasC
ErnaultP
FouletF
CordonnierC
2000 Real-time PCR for diagnosis and follow-up of Toxoplasma reactivation after allogeneic stem cell transplantation using fluorescence resonance energy transfer hybridization probes. J Clin Microbiol 38 2929 2932
38. McClintickJN
EdenbergHJ
2006 Effects of filtering by Present call on analysis of microarray experiments. BMC Bioinformatics 7 49
39. BenjaminiY
DraiD
ElmerG
KafkafiN
GolaniI
2001 Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125 279 284
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Is Adherence to Erythrocytes a Factor in Extrapulmonary Dissemination?
- Identifying the Age Cohort Responsible for Transmission in a Natural Outbreak of
- Blockade of Immunosuppressive Cytokines Restores NK Cell Antiviral Function in Chronic Hepatitis B Virus Infection
- Metaeffector Exploits Host Proteasome to Temporally Regulate Cognate Effector
- The p53-Target Gene Drives Neutrophil-Mediated Protection against Lethal Bacterial Sepsis
- Development of an RNAi Protocol to Investigate Gene Function in the Filarial Nematode,
- Lysine Acetyltransferase GCN5-A Functions in the Cellular Response to Alkaline Stress and Expression of Cyst Genes
- Structural Basis for Apoptosis Inhibition by Epstein-Barr Virus BHRF1
- Molecular Architectures of Trimeric SIV and HIV-1 Envelope Glycoproteins on Intact Viruses: Strain-Dependent Variation in Quaternary Structure
- Interaction of c-Cbl with Myosin IIA Regulates Bleb Associated Macropinocytosis of Kaposi's Sarcoma-Associated Herpesvirus
- Glacial Refugia in Pathogens: European Genetic Structure of Anther Smut Pathogens on and
- Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Larvae
- Inflammasome Sensor Nlrp1b-Dependent Resistance to Anthrax Is Mediated by Caspase-1, IL-1 Signaling and Neutrophil Recruitment
- Noise Cancellation: Viral Fine Tuning of the Cellular Environment for Its Own Genome Replication
- Infectious Speciation Revisited: Impact of Symbiont-Depletion on Female Fitness and Mating Behavior of
- Eis Regulates Autophagy, Inflammation, and Cell Death through Redox-dependent Signaling
- NleC, a Type III Secretion Protease, Compromises NF-κB Activation by Targeting p65/RelA
- Early Myeloid Dendritic Cell Dysregulation is Predictive of Disease Progression in Simian Immunodeficiency Virus Infection
- HIV Capsid is a Tractable Target for Small Molecule Therapeutic Intervention
- Structural and Functional Studies of Nonstructural Protein 2 of the Hepatitis C Virus Reveal Its Key Role as Organizer of Virion Assembly
- Coming of Age—Sexual Reproduction in Species
- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Compartmentation of Redox Metabolism in Malaria Parasites
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Rapid End-Point Quantitation of Prion Seeding Activity with Sensitivity Comparable to Bioassays
- Dimeric 2G12 as a Potent Protection against HIV-1
- Hypoxia Induces an Immunodominant Target of Tuberculosis Specific T Cells Absent from Common BCG Vaccines
- CD4 Natural Regulatory T Cells Prevent Experimental Cerebral Malaria via CTLA-4 When Expanded In Vivo
- The Killing of African Trypanosomes by Ethidium Bromide
- H2A.Z Demarcates Intergenic Regions of the Epigenome That Are Dynamically Marked by H3K9ac and H3K4me3
- Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (, Hymenoptera: Apidae)
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Coming of Age—Sexual Reproduction in Species
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Compartmentation of Redox Metabolism in Malaria Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



