-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Dementia incidence trend over 1992-2014 in the Netherlands: Analysis of primary care data
Using primary care data, Emma van Bussel and colleagues examine trends in dementia incidence trend over 1992-2014 in the Netherlands.
Published in the journal: . PLoS Med 14(3): e32767. doi:10.1371/journal.pmed.1002235
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002235Summary
Using primary care data, Emma van Bussel and colleagues examine trends in dementia incidence trend over 1992-2014 in the Netherlands.
Introduction
Since dementia care places a heavy social and economic burden on society, future projections of dementia prevalence rates are important for health care planning. In view of a growing and ageing population, an increasing number of older people are at risk for dementia [1]. It is estimated that the prevalence of dementia will nearly double every 20 y, to 132 million in 2050 worldwide [2]. Recently, cohort studies from Europe and the United States have suggested a declining trend in age-specific dementia incidence rates over the last 30 y [3–12]. This putative decline is mostly attributed to better education and vascular risk factor treatment [5,9], and fuels hope that the absolute increase in dementia prevalence might be more moderate than previously anticipated.
Until recently, studies on trends in dementia occurrence have been surprisingly rare. European studies that attempted to quantify changes in incidence or prevalence over time often suffered from decreasing response rates and changing methods for dementia case identification between time points [13]. Furthermore, most studies were based on local or regional data using population-based research cohorts, rather than on nationwide registries within real-world settings [4,13].
Data from electronic health records (EHRs) may facilitate time-trend analyses, provided that the populations studied are representative and that diagnostic algorithms and procedures are relatively stable over time. In the Netherlands, nearly all non-institutionalized inhabitants are registered with a single general practitioner (GP), and morbidity is recorded through EHRs [14]. In 1988, the first national dementia guideline appeared, followed by a primary care guideline 10 y later, both of which have been amended since. The diagnostic criteria for most dementia types (e.g., Alzheimer disease and vascular dementia) have not substantially changed over the last decades, but there have been increases in awareness and attention to dementia in the population. The aim of this study was to estimate age-specific dementia incidence rates and dementia incidence trends among community-dwelling older people (≥60 y) in the Netherlands over the last decades, based on GP registry data.
Methods
Ethics statement
In the Netherlands, no approval from an ethical committee or individual participant consent is necessary for analyzing anonymized data from general practitioner registration networks (GPRNs).
Database characteristics and selection
In the Netherlands, routinely collected data from GPRNs are often used to monitor the incidence and prevalence of diseases in the general population [14]. GPs use the International Classification of Primary Care (ICPC) to code all diagnoses in the patient’s EHR [15], including those made by specialists after referral [16]. GPRNs collect and manage the EHR data of large numbers of associated general practices. Most operate regionally, some nationally. For this study, all eleven GPRNs that routinely and continuously collected data on morbidity and mortality in the Netherlands over the last decades were invited to participate. We aimed to include as many consecutive years per GPRN as possible. Databases of GPRNs were considered eligible if data were available for at least 5 y and registration or extraction methods had not substantially changed over time (Table 1).
Tab. 1. Characteristics of the Dutch general practice registration networks included in this study. 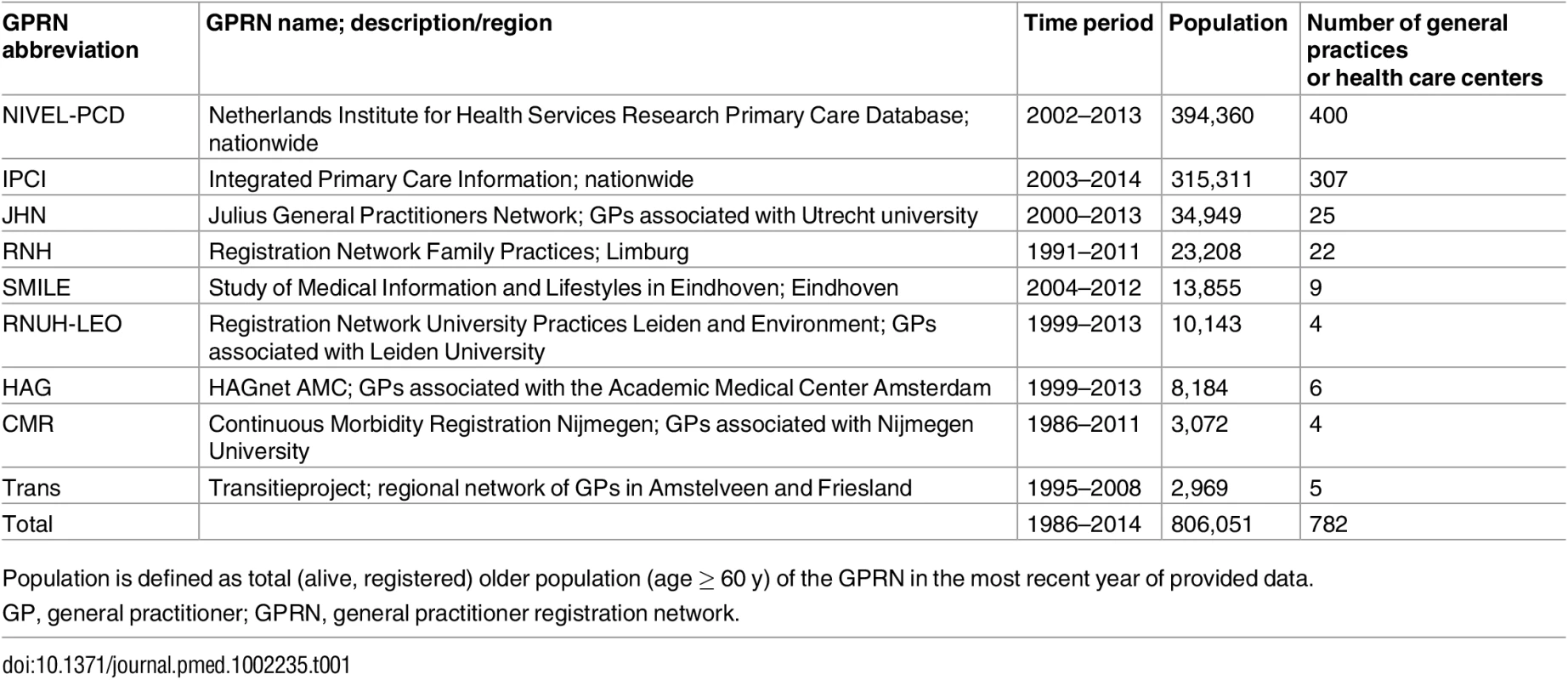
Population is defined as total (alive, registered) older population (age ≥ 60 y) of the GPRN in the most recent year of provided data. Data extraction
For all databases, count data of incident cases and person-years at risk per year, sex, and age group were directly obtained or calculated from the anonymized data. For each calendar year, data on all people aged 60 y and over were used. Dementia was defined as P70 (senile dementia/Alzheimer disease), the only code within the ICPC for dementia. Another code slightly related is P20 (memory/concentration/orientation impairment), but it is nonspecific and was disregarded. The numerator was defined as all new dementia cases (the first date the ICPC code P70 had been recorded in a patient’s EHR) per year. For the denominator, for each calendar year the number of registered person-years was calculated (NIVEL-PCD, IPCI, RNUH-LEO, CMR) or, if person-years could not be calculated, the number of registered persons (JHN, RNH, SMILE, HAG, Trans). At the start of each calendar year, prevalent dementia cases were excluded from both the numerator and denominator.
Methods of data collection are episode-based in some registries and problem-based in others. Problem-based data (“problem list”) contain information about health problems that are permanent, chronic (duration longer than 6 mo), or recurrent. Thus, for dementia, recording on the problem list is clearly expected. Episode-based data (“episode list”) have information about all health problems. In two databases, new dementia cases were identified when the ICPC code P70 was entered on the problem list (RNUH-LEO and RNH). In the seven other databases, new dementia cases were identified when the ICPC code P70 was recorded in the episode list for a patient contact [16]. Within each database, coding and selection criteria were stable over the whole time frame of the study. Although differences in data recording and data selection may cause variation in incidence or prevalence rates between GPRNs, they do not impact trends or variation within GPRNs [14].
Statistical analysis
Data for all available years with at least 10,000 observed person-years were used for data analysis, in order to avoid unrepresentative sample years, resulting in data for the years 1992 through 2014.
To model the observed rates over time, a prespecified analysis plan was followed, and both linear and nonlinear relations were considered. Fitting count models using splines with varying degrees of freedom for the continuous variables did not reveal significant departure from linearity. Regarding the linear structures and using the log link function for the mean, both the Poisson and the negative binomial distribution were considered, the latter being able to account for overdispersed count data. Based on Akaike’s Information Criterion, it was concluded that the negative binomial distribution fit our data best. During the study, GPRNs appeared to differ individually in terms of methods of data collection and denominator calculation; therefore, we incorporated a GPRN random intercept and slope term into the negative binomial regression models to allow for GPRN-specific trends of dementia over time. The most parsimonious random structure was chosen based on the likelihood ratio test (model 1).
The time—rate relation was adjusted for age (in 5-y age groups) and sex. We also investigated whether the time—rate relation differed across age groups and sex by adding the appropriate interaction terms to the model (models 3 and 4).
Furthermore, to test the hypothesis that the recorded dementia incidence increased as a result of increased awareness and case-manager-led integrated dementia care, a piecewise linear spline was included in the model, with an internal knot at year 2003 (when a national dementia care program was launched in the Netherlands) (model 2) [17]. Additional sensitivity analyses were performed including all available data (1986–2014) in the negative binomial regression (model 5) and using Poisson regression instead of negative binomial regression (model 6).
Database preparation was performed in IBM SPSS Statistics 22; statistical analyses were performed in R version 3.1.2 using packages plyr, R2admb, and glmmADMB [18–21]. The programming codes can be requested from the corresponding author.
Results
All eleven Dutch GPRNs were willing to participate. However, one GPRN (in Amsterdam) was able to deliver coherent incidence data only for the years 2010 to 2013 and was excluded from participation. Another GPRN (in Groningen) was excluded because its data were already part of another database within this study (NIVEL-PCD). The other nine databases, representing over 806,051 older persons, were eligible and were used for this trend study (Table 1).
Registration periods were between 1986 and 2014 and ranged from 9 to 26 y across the networks. Populations covered by the individual GPRNs ranged from 2,969 to 394,360 older people, with the two largest networks operating nationally. From 1992 onwards, at least 10,000 person-years were available for each year; thus, the years 1992–2014 were used for data analysis.
Fig 1 shows the number of person-years at risk and the number of incident dementia cases for each calendar year. Between 1992 and 2014, a total of 4,020,550 person-years were available, during which 23,186 incident cases of dementia were recorded. Table 2 shows the crude mean incidence rate per age group and its range across GPRNs. The incidence of dementia increased with age in all of the individual databases.
Fig. 1. Absolute number of person-years at risk and incident dementia cases per calendar year (logarithmic <i>y</i>-axis). 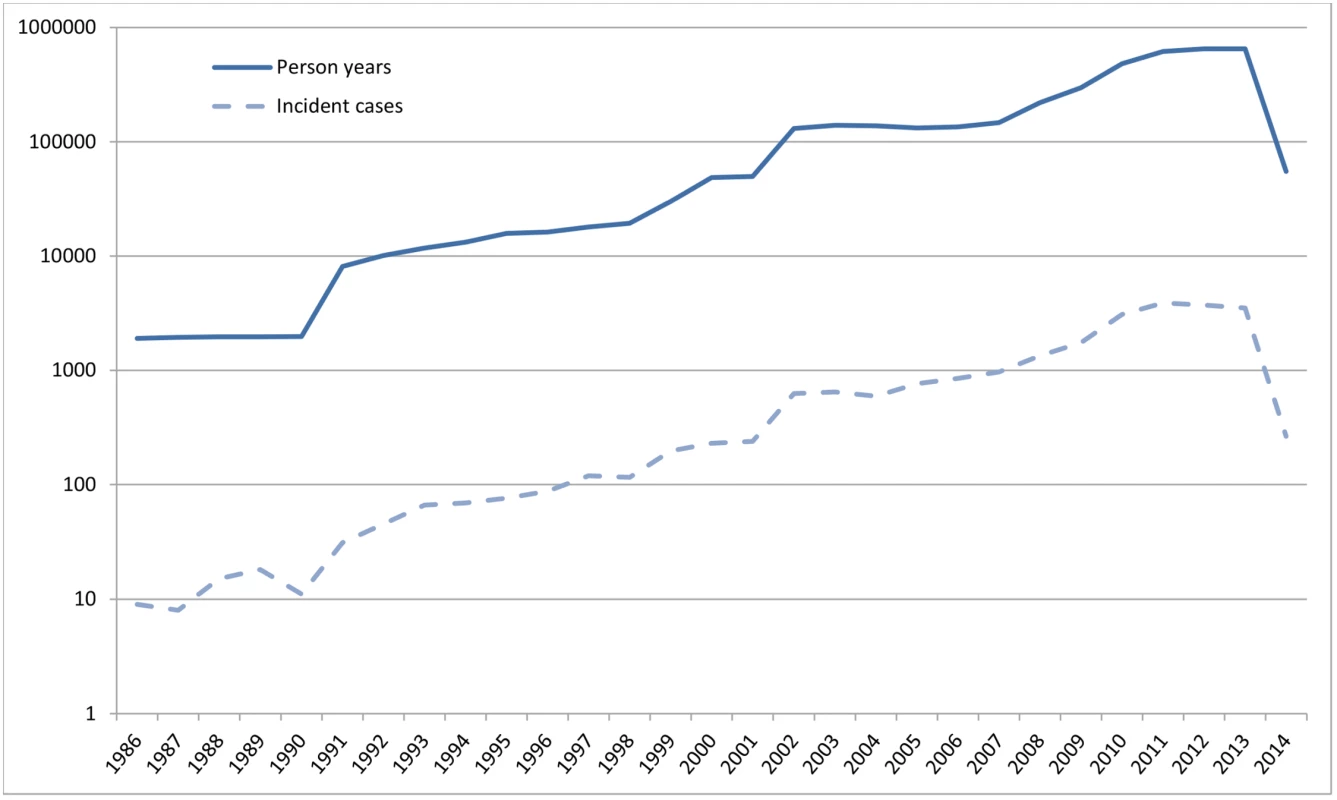
Tab. 2. Crude mean and range of dementia incidence rate for all nine general practitioner registration networks (1992–2014), and number and percentage of person-years per age group. 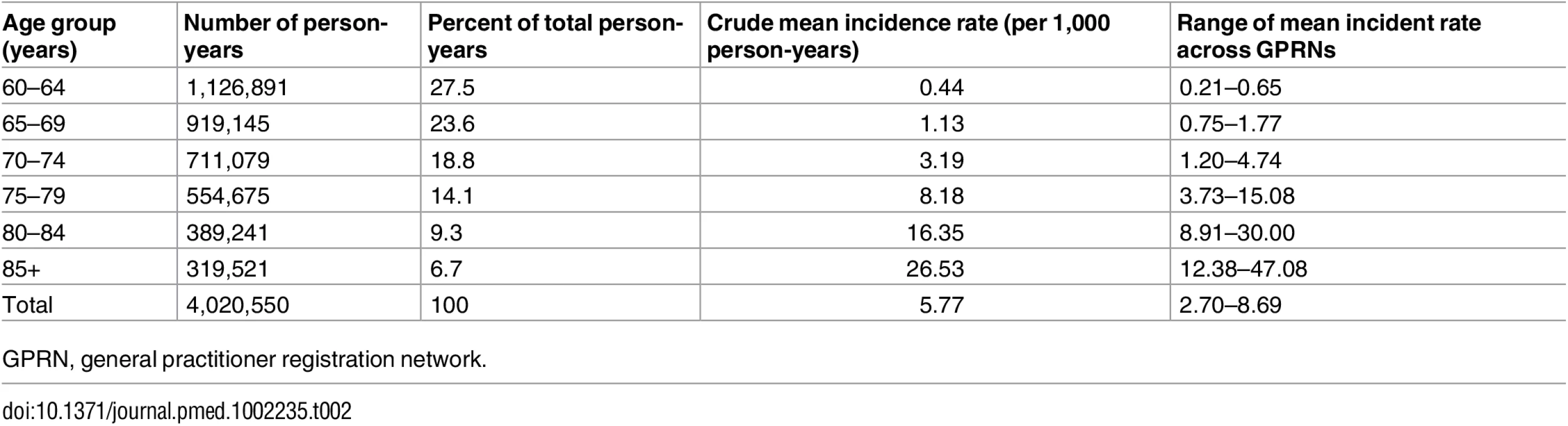
GPRN, general practitioner registration network. The observed and estimated trend of the incidence rate per age group are shown in Fig 2. The dementia incidence rate ratio was 1.021 (95% CI 1.005 to 1.038), reflecting an annual growth in dementia incidence rate of 2.1% (95% CI 0.5% to 3.8%) (Table 3). Considering an overall mean incidence rate of 5.77/1,000 person-years, incidence increased from 4.59/1,000 person-years in 1992 to 7.25/1,000 person-years in 2014. This estimate was based on the best-fitting model, adjusting for age and sex and with a random intercept and slope term for GPRN. Despite their relatively low numbers of person-years, the highest age groups contributed most to the positive trend, showing the strongest increase in dementia incidence over time. Between the GPRNs we found variation in the trend, with estimated standard deviations of 0.38 and 0.02 for the random intercept and slope terms, respectively. Table 4 shows the variation: the incidence rate ratio per year indicates the individual slope per GPRN, as fitted through model 1. The estimate for the negative binomial dispersion parameter was 31.73 (standard error 4.92), indicating substantial extra variation in the counts. Furthermore, there were no differential trends according to age and sex, as can be seen from the analysis of interaction terms (models 3 and 4; S1 Table). However, independent of time, the incidence rate for females was estimated to be 1.08 (95% CI 1.04 to 1.13) times higher than for males. Also independent of time, rates increased with age, approximately doubling with each 5-y increment in age, with a slower increase towards older age groups (Table 3).
Fig. 2. Dementia incidence rate by age group. 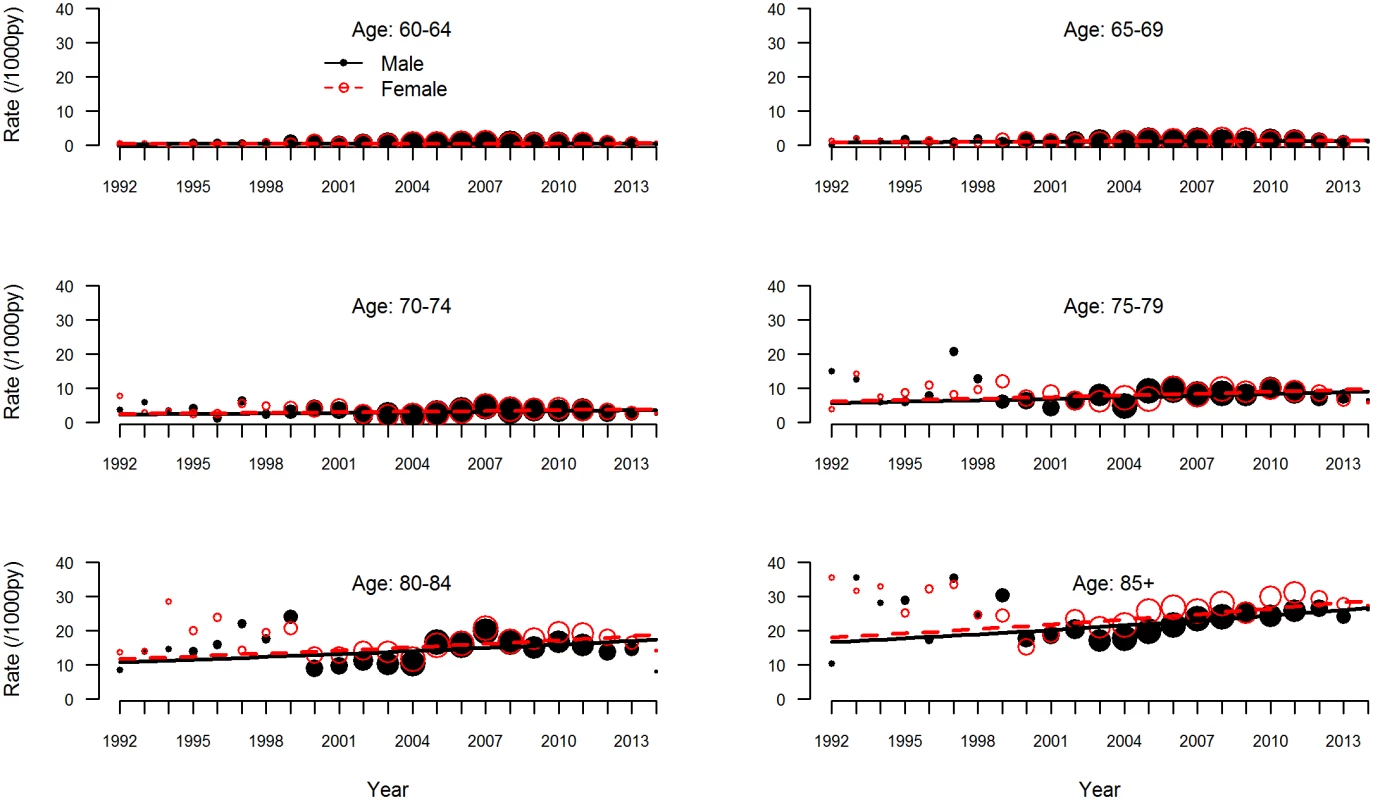
Observed (circles) and estimated (lines) dementia incidence rate per 1,000 person-years (py) by age group for men (solid black circles and lines) and women (open red circles, dashed lines). The sizes of the circles indicate the number of general practitioner registration networks that provided data for the respective years. Tab. 3. Results of negative binomial regression analysis, giving incidence rate ratios of change in dementia incidence. 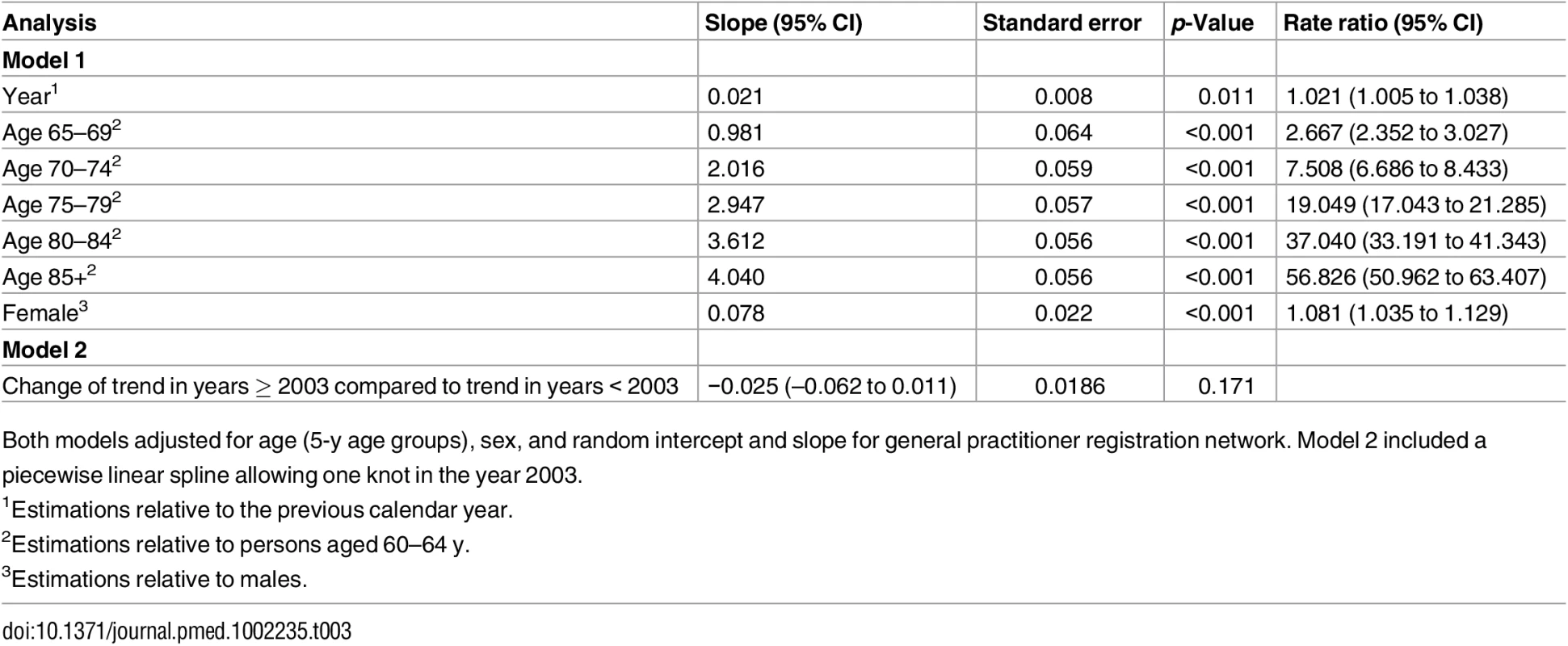
Both models adjusted for age (5-y age groups), sex, and random intercept and slope for general practitioner registration network. Model 2 included a piecewise linear spline allowing one knot in the year 2003. Tab. 4. Variation in trends between general practitioner registration networks as estimated using model 1. 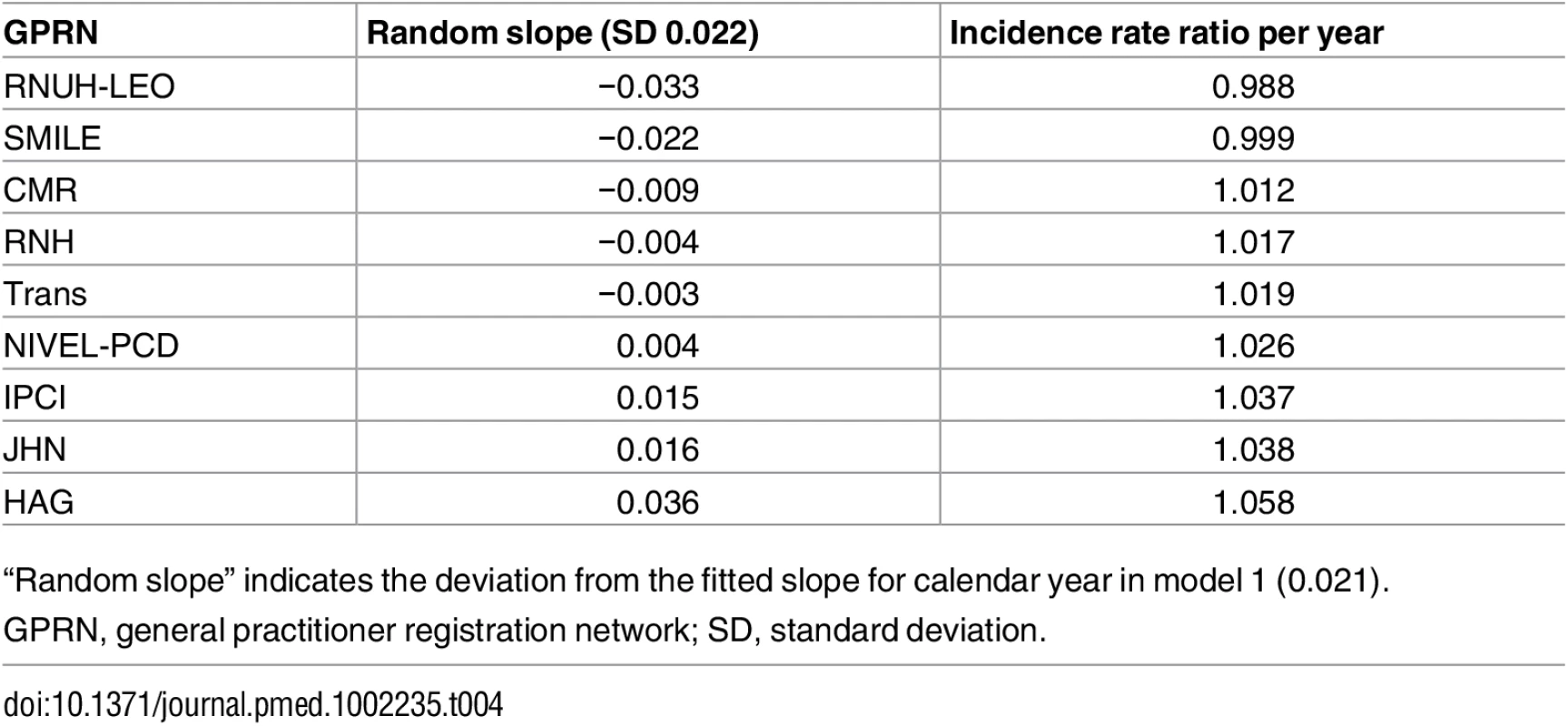
“Random slope” indicates the deviation from the fitted slope for calendar year in model 1 (0.021). In the piecewise linear spline model, the trend over the years from 2003 to 2014 showed a small, nonsignificant change compared to the trend over the years prior to 2003 (−0.025; 95% CI −0.062 to 0.011) (Table 3). Thus, there was no significant change in the trend of recorded dementia incidence rate since the Dutch national dementia care program was launched in 2003.
When taking into account all available years, including the years between 1986 and 1992 with fewer than 10,000 person-years, findings did not notably change (incidence rate ratio 1.022; 95% CI 1.006 to 1.039) (model 5, S1 Table). Also, when we used Poisson regression instead of negative binomial regression, we observed a comparable increase in dementia incidence over time (model 6, S1 Table).
Discussion
This study evaluated whether there was a declining trend in dementia incidence rate in the Netherlands, using a real-world sample of routinely collected data from primary care networks comprising over 800,000 people aged 60 y and over. Pooled data from nine GPRNs showed a dementia incidence rate ratio of 1.021 (95% CI 1.005 to 1.038) per year between 1992 and 2014, with higher incidence rates among women than among men and no significant change since the start of a national dementia program in 2003.
This study is unique in that it combines data from virtually all of the GPRNs of one country. Strengths are the relatively long period of observation (23 y) and the large numbers of observed person-years at risk (over 4 million) and incident dementia cases (over 23,000). Other strengths are the representativeness of the studied population: nearly all Dutch inhabitants are registered with one general practice, and the included GPRNs cover inhabitants from all geographical areas [14]; thus, there was no selection or attrition bias in this dynamic cohort. A limitation may be the potential underestimation of dementia diagnoses, especially for mild dementia [22,23]. Although diagnostic criteria for dementia have not substantially changed over the last decades, in early phases of the disease the diagnosis is often not formally made by GPs, even if suspected [24]. However, this leads to high specificity, and therefore high internal validity of diagnostic labels by the GPs [25]. Despite a potentially low sensitivity, the long period of observation ensures that patients with moderate to severe dementia are likely to eventually receive a diagnostic label in their EHR. Nevertheless, patient and GP awareness of dementia and individual GPs’ perspectives of disease may have changed and thus inflated recorded incidence rates over time. For example, in 2003 a national dementia care program was launched [17], followed by programs to finance and facilitate integrated dementia care [26,27], which may have supported both diagnosis and care in primary care. However, our analyses did not show any change in the overall incidence rate trend following initiation of these programs compared to the years prior to their introduction, nor did including a longer time period affect the overall incidence rate trend. Nevertheless, secular trends towards diagnosis in earlier stages of dementia are suggested by studies that compared clinical diagnosis with Mini-Mental State Examination (MMSE) scores over time, and found higher scores on the MMSE in patients diagnosed with dementia in more recent years [11,28]. Another limitation might be the inability to fully correct for increased overall life expectancy over time and the national development towards an increasing share of non-institutionalized older people [29]. Since people living in nursing homes are not registered with a GP, this may have contributed to increased numbers of dementia diagnoses in GP registries. Although we corrected for age in 5-y groups, these phenomena could have affected the incidence rates in the highest age group, which lacked an upper age limit (85 y and over). However, a differing trend by age was not confirmed by an analysis allowing different time trends across age groups (model 3, S1 Table). We cannot exclude the possibility that the trend towards a small increase in dementia incidence rate that was found in our study reflects a balance of increased awareness, earlier diagnosis, and an increasing percentage of community-dwelling older people on the one hand and stable or even declining dementia incidence rates on the other hand. Another limitation of the study might be the difference in available data between GPRNs to calculate dementia rates. Although calculation methods did not differ essentially within GPRNs, differences in establishing incident cases (using episode lists in some and problem lists in others) and defining the denominator (using the number of person-years at risk or the number of registered persons at risk) might explain some of the variation in morbidity estimates across the studied GPRNs. Nevertheless, additional analyses comparing trends within one GPRN that provided both the number of person-years and the number of persons per year showed no differences in trend between the two methods (estimates differed only from the fourth decimal; S2 Table). Also, previous analyses of morbidity data from all Dutch GPRNs showed that neither population nor practice characteristics could explain the variation in incidence and prevalence estimates between practices or GPRNs [14,30]. Finally, a disadvantage of studying incidence rates is that this approach cannot directly be used for future projections of dementia prevalence, since this also requires integration with dementia-specific mortality rates. On the other hand, age-specific incidence rates are not influenced by demographic ageing and show less variation than prevalence rates within GPRNs [14].
The dementia incidence rates reported here are similar to those found in a literature review on rates in Europe and the US that reported estimates of 7.1 to 19.2 and 12.8 to 36.2 per 1,000 person-years for people aged 75–79 and 80–84 y, respectively [31]. Different trends have been reported for men and women, though results are conflicting [11,32,33]. We found similar trends for both sexes, even though overall rates were higher among women. So far, few studies have presented incidence data using consistent research methods across multiple time points [12]. The Rotterdam Study reported a nonsignificant decline in dementia occurrence between 1990 and 2005 [9]. A recent study in the UK found a decline in incidence between 1989 and 2011 that was significant among men [33]. In the same cohort, a significant 22% decrease in prevalence was found [5]. In the Framingham Heart Study, four epochs between 1977 and 2008 showed a decline in the incidence of dementia, especially for vascular dementia and in those who had a high school diploma. The decline in incidence rate was mainly seen between the first two epochs, while rates stabilized from the 1990s onwards, suggesting that the overall decline was driven mainly by data from the years prior to this period [10]. Compared to our findings, age-specific rates were similar, and considering the time period since the 1990s, only small changes in the rates over time were found in both studies. Other studies in Sweden, France, and the US found small, nonsignificant changes in incidence rates over time [7,8,11,34]. Based on integration of prevalence and mortality figures, a study conducted in Stockholm, Sweden, suggested the possibility of reduced dementia incidence [35], and findings from a study in Zaragoza, Spain, showed a significant reduction in dementia prevalence only in men [32]. Main limitations of these studies concerned decreasing response rates [5,11,32], with varying ability to assess the potential effects of such changes on the findings. Inflation of estimates may have taken place if nonstandardized diagnostic criteria were used [7,34] or when medical records were retrospectively used to supplement incomplete information [9,32], because of the increased inclusiveness of broader diagnostic criteria across time [5,32].
The overall increase in diagnosis of dementia of 2.1% in general practice registries reported here differs from the declining dementia incidence rate in some population-based studies. These studies were specifically designed to measure dementia incidence in fixed cohorts, rather than in dynamic populations such as the ones reported here. At the same time, our findings do not preclude the possibility that age-specific prevalence rates are stabilizing, depending on dementia-specific mortality rates [13]. Although improved vascular risk management has been linked to the alleged decrease in dementia incidence in previous studies, favorable trends with respect to smoking and hypertension may have been reversed by increasing rates of obesity and type 2 diabetes mellitus [10]. Perhaps the gains from improved cardiovascular prevention were capitalized in the 1970s and 1980s, yielding relatively stable trends over the last decades. The complex interplay between these and other factors, like survival after cardiovascular disease, will require further study to determine their net effect on dementia occurrence. Irrespective of the question of to what extent the figures presented here exactly reflect incidence rates of dementia in a Western population, our data indicate that the burden of work for physicians and nurses in general practice associated with newly diagnosed dementia has not declined in the past two decades, although there may have been a shift to milder spectrum disorder. With an ever increasing older population, the absolute capacity required for the care of dementia patients in general practice can still be expected to double every 20 y, despite observed decreasing dementia incidence rates in some specific populations, especially before the 1990s.
Results from other population registries or public health records in high-income countries are needed to confirm our findings, and to study demographics and the impact of dementia risk factors on incidence trends in ageing societies. Direct comparison of such registries with epidemiologic studies performed simultaneously in the same area may help to explain the apparent discrepancy between the current findings and those in specific cohort populations. In this study on longitudinal, real-world primary care data, we have found a small absolute increase in dementia incidence rates over the last two decades. Although this finding appears to be in contrast with recent reports of attenuating incidence rates and dementia occurrence, the exact reasons remain to be explored, highlighting the need for greater understanding of complex time trends in dementia incidence.
Supporting Information
Zdroje
1. Prince M, Guerchet M, Prina M. Policy brief for heads of government: the global impact of dementia 2013–2050. London: Alzheimer’s Disease International; 2013.
2. Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer report 2015: the global impact of dementia, an analysis of prevalence, incidence, costs and trends. London: Alzheimer’s Disease International; 2015.
3. Christensen K, Thinggaard M, Oksuzyan A, Steenstrup T, Andersen-Ranberg K, Jeune B, et al. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet. 2013;382(9903):1507–13. doi: 10.1016/S0140-6736(13)60777-1 23849796
4. Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med. 2013;369(24):2275–7. doi: 10.1056/NEJMp1311405 24283198
5. Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382(9902):1405–12. doi: 10.1016/S0140-6736(13)61570-6 23871492
6. Bogren M, Mattisson C, Horstmann V, Bhugra D, Munk-Jorgensen P, Nettelbladt P. Lundby revisited: first incidence of mental disorders 1947–1997. Aust N Z J Psychiatry. 2007;41(2):178–86. doi: 10.1080/00048670601109964 17464697
7. Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80–93. doi: 10.1016/j.jalz.2010.11.002 21255746
8. Sacuiu S, Gustafson D, Sjogren M, Guo X, Ostling S, Johansson B, et al. Secular changes in cognitive predictors of dementia and mortality in 70-year-olds. Neurology. 2010;75(9):779–85. doi: 10.1212/WNL.0b013e3181f0737c 20805523
9. Schrijvers EMC, Verhaaren BFJ, Koudstaal PJ, Hofman A, Ikram MA, Breteler MMB. Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78(19):1456–63. doi: 10.1212/WNL.0b013e3182553be6 22551732
10. Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523–32. doi: 10.1056/NEJMoa1504327 26863354
11. Grasset L, Brayne C, Joly P, Jacqmin-Gadda H, Peres K, Foubert-Samier A, et al. Trends in dementia incidence: evolution over a 10-year period in France. Alzheimers Dement. 2016;12(3):272–80. doi: 10.1016/j.jalz.2015.11.001 26693893
12. Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8 27473681
13. Wu YT, Fratiglioni L, Matthews FE, Lobo A, Breteler MM, Skoog I, et al. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 2016;15(1):116–24. doi: 10.1016/S1474-4422(15)00092-7 26300044
14. van den Dungen C, Hoeymans N, van den Akker M, Biermans MC, van BK, Joosten JH, et al. Do practice characteristics explain differences in morbidity estimates between electronic health record based general practice registration networks? BMC Fam Pract. 2014;15 : 176. doi: 10.1186/s12875-014-0176-7 25358247
15. Lamberts H, Wood M. The birth of the International Classification of Primary Care (ICPC). Serendipity at the border of Lac Leman. Fam Pract. 2002;19(5):433–5. 12356688
16. van den Dungen C, Hoeymans N, Gijsen R, van den Akker M, Boesten J, Brouwer H, et al. What factors explain the differences in morbidity estimations among general practice registration networks in the Netherlands? A first analysis. Eur J Gen Pract. 2008;14(Suppl 1):53–62.
17. Meerveld J, Schumacher J, Krijger E, Bal R, Nies H. Landelijk Dementieprogramma: werkboek. Utrecht: Nederlands Instituut voor Zorg en Welzijn; 2004.
18. R Core Team. R: a language and environment for statistical computing. Version 3.1.2. Vienna: R Foundation for Statistical Computing. https://www.r-project.org/.
19. Wickham H. The split-apply-combine strategy for data analysis. J Stat Softw. 2011;40(1):1–29.
20. Bolker B, Skaug H, Laake J. R2admb: ‘ADMB’ to R interface functions. Version 0.7.13. https://cran.r-project.org/web/packages/R2admb/index.html.
21. Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder M, et al. AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Methods Softw. 2012;27(2):233–49.
22. Iliffe S, Robinson L, Brayne C, Goodman C, Rait G, Manthorpe J, et al. Primary care and dementia: 1. diagnosis, screening and disclosure. Int J Geriatr Psychiatry. 2009;24(9):895–901. doi: 10.1002/gps.2204 19226529
23. van Baal PH, Engelfriet PM, Hoogenveen RT, Poos MJ, van den Dungen C, Boshuizen HC. Estimating and comparing incidence and prevalence of chronic diseases by combining GP registry data: the role of uncertainty. BMC Public Health. 2011;11 : 163. doi: 10.1186/1471-2458-11-163 21406092
24. Bamford C, Eccles M, Steen N, Robinson L. Can primary care record review facilitate earlier diagnosis of dementia? Fam Pract. 2007;24(2):108–16. doi: 10.1093/fampra/cml068 17237496
25. van den Dungen P, van Marwijk HW, van der Horst HE, Moll van Charante EP, MacNeil VJ, van de Ven PM, et al. The accuracy of family physicians’ dementia diagnoses at different stages of dementia: a systematic review. Int J Geriatr Psychiatry. 2012;27(4):342–54. doi: 10.1002/gps.2726 21626568
26. Alzheimer Europe. Dutch dementia care plan. Luxembourg: Alzheimer Europe; 2014 [cited 2016 May 1]. http://www.alzheimer-europe.org/Policy-in-Practice2/National-Dementia-Strategies/Netherlands#fragment2.
27. BeterOud. The national care for the elderly programme. Utrecht: BeterOud; 2016 [cited 2016 May 1]. http://www.beteroud.nl/ouderen/dutch-national-care-programme-for-the-elderly.html.
28. Grimmer T, Beringer S, Kehl V, Alexopoulos P, Busche A, Forstl H, et al. Trends of patient referral to a memory clinic and towards earlier diagnosis from 1985–2009. Int Psychogeriatr. 2015;27(12):1939–44. doi: 10.1017/S104161021500157X 26424328
29. Garssen J, Harmsen C. Elderly people live independently to increasingly older ages. The Hague: Statistics Netherlands; 2011 Jul 12 [cited 2016 May 1]. https://www.cbs.nl/en-gb/news/2011/28/elderly-people-live-independently-to-increasingly-older-ages.
30. van den Dungen C, Hoeymans N, Boshuizen HC, van den Akker M, Biermans MC, van Boven K, et al. The influence of population characteristics on variation in general practice based morbidity estimations. BMC Public Health. 2011;11 : 887. doi: 10.1186/1471-2458-11-887 22111707
31. Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51(3):728–33. 9748017
32. Lobo A, Saz P, Marcos G, Dia JL, De la Camara C, Ventura T, et al. Prevalence of dementia in a southern European population in two different time periods: the ZARADEMP Project. Acta Psychiatr Scand. 2007;116(4):299–307. doi: 10.1111/j.1600-0447.2007.01006.x 17803760
33. Matthews FE, Stephan BC, Robinson L, Jagger C, Barnes LE, Arthur A, et al. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun. 2016;7 : 11398. doi: 10.1038/ncomms11398 27092707
34. Sauvaget C, Tsuji I, Haan MN, Hisamichi S. Trends in dementia-free life expectancy among elderly members of a large health maintenance organization. Int J Epidemiol. 1999;28(6):1110–8. 10661655
35. Qiu C, von Strauss E, Backman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80(20):1888–94. doi: 10.1212/WNL.0b013e318292a2f9 23596063
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 3- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- 2016 Reviewer and Editorial Board Thank You
- , , and mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases
- Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C Study: A longitudinal, population-based prospective cohort study
- Mixed pathologies and neural reserve: Implications of complexity for Alzheimer disease drug discovery
- -related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts
- Neuropathological diagnoses and clinical correlates in older adults in Brazil: A cross-sectional study
- Early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool
- Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study
- Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study
- Fine-mapping of the human leukocyte antigen locus as a risk factor for Alzheimer disease: A case–control study
- What’s the “Take Home” from Research on Dementia Trends?
- Cultural representations of dementia
- Dementia and aging populations—A global priority for contextualized research and health policy
- Dementia in the oldest old: Beyond Alzheimer disease
- Rehabilitation for people living with dementia: A practical framework of positive support
- Dementia in low-income and middle-income countries: Different realities mandate tailored solutions
- Challenges and opportunities in understanding dementia and delirium in the acute hospital
- Dementia incidence trend over 1992-2014 in the Netherlands: Analysis of primary care data
- Association between delirium superimposed on dementia and mortality in hospitalized older adults: A prospective cohort study
- Development of an adaptive, personalized, and scalable dementia care program: Early findings from the Care Ecosystem
- Genetic assessment of age-associated Alzheimer disease risk: Development and validation of a polygenic hazard score
- Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study
- The impact of individual Cognitive Stimulation Therapy (iCST) on cognition, quality of life, caregiver health, and family relationships in dementia: A randomised controlled trial
- Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: Cluster randomised controlled trial
- Subjective and objective cognitive function among older adults with a history of traumatic brain injury: A population-based cohort study
- Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: A community-based cohort study
- Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: A retrospective analysis of a population-based cohort
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: Cluster randomised controlled trial
- , , and mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases
- Challenges and opportunities in understanding dementia and delirium in the acute hospital
- Early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



