-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
David Kaslow and colleagues examine the progress in reasearch for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication and propose a research agenda.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002455
Category: Collection Review
doi: https://doi.org/10.1371/journal.pmed.1002455Summary
David Kaslow and colleagues examine the progress in reasearch for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication and propose a research agenda.
Summary points
Achieving malaria elimination likely requires new interventions and strategies in some settings. In addition, the effectiveness of existing tools must be preserved and tools deployed to counter the numerous challenges, key among which are the emergence and spread of drug-resistant parasites and mosquitoes with resistance to vector control measures.
The key research goal for diagnostics is the detection of populations with subclinical infections and low parasite counts. Such diagnostics enable the development of effective surveillance systems directed at malaria parasite elimination.
The availability of new transmission-blocking drugs, vaccines, and vector control products would accelerate elimination where there is refractoriness to currently available interventions. New regulatory pathways and product development models are needed to efficiently develop and assess these new interventions.
In areas endemic for Plasmodium vivax and P. ovale, the hypnozoite reservoir must be targeted with more robust tools and strategies.
In areas of declining transmission, as cases become less frequent, the contribution to transmission of the subclinical parasite reservoir needs to be quantified and addressed with transmission-blocking interventions.
For vector control, addressing continuing escalation of insecticide resistance—including through the identification of new chemical classes and longer-lasting insecticide formulations—remains a priority. Changes in vector populations and behaviours must also be addressed to restore responsiveness to existing interventions. In some areas, new paradigms may be needed to understand how to design interventions that reduce vector populations and receptivity to sufficiently low levels.
Policy and decision makers, faced with chronic resource limitations, insufficient surveillance, spatial and temporal heterogeneity of malaria parasite transmission, and multiple intervention choices, need improved strategies and guidance on how, where, and when to best combine and deploy existing and new interventions to maximise their longevity and effectiveness.
Introduction
Achieving malaria parasite elimination across all countries (i.e., malaria eradication), especially for those with a high disease burden, likely requires new tools and strategies to complement existing interventions [1,2]. Given the inevitable uncertainties in product development and given that different sets of tools will be applicable in different settings, a broad and imaginative research and development agenda needs to be pursued. The research and development agenda presented in this paper is in support of the WHO Global Technical Strategy for malaria goals from 2016 to 2030, and tracking the progress of this research and development (R&D) agenda and reevaluating the research needs will be required over time [2]. In Malaria Eradication Research Agenda (malERA) 2011, diagnostics, drugs, vaccines, and vector control were considered separately [3–6]. However, for malERA Refresh, this paper considers together the research agenda for all existing and prospective tools to accelerate progress towards achieving and maintaining malaria elimination. In this case, the relationships between the different research agendas can be more easily recognised. Other papers in this malERA Refresh series consider the related discussions regarding the implementation and combination of tools [7], implications of insecticide and antimalarial drug resistance [8], health system and policy issues [9], and advances in basic science [10].
Progress on tools for malaria elimination
Based on literature reviews and panel consultations [11], the most significant advances in the development and deployment of malaria control and elimination tools between 2011 and 2015 were identified (S1–S4 Texts). For diagnostics, advances include widespread incorporation of P. falciparum rapid diagnostic tests (RDTs) into routine malaria case management [12], development of highly sensitive tools for detecting subclinical infections, and development and deployment of combined tests that differentiate P. falciparum from P. vivax [13,14]. For drugs, advances include the deployment of hundreds of millions of artemisinin-based combination therapy (ACT) courses [12], publication of guidelines for mass drug administration (MDA) [15], the recommendation of low-dose primaquine for transmission interruption [16], progression of new antimalarial compound classes into clinical development [17–19], field trials to evaluate the potential role of medicines in killing mosquitoes (endectocides) [2], and the identification of Kelch13 as a marker for artemisinin resistance, enabling mapping of its geographic distribution [20,21]. For vaccines, advances include the Article 58 positive opinion by the European Medicines Agency and recommendations by the World Health Organization (WHO) on the first vaccine targeting malaria, RTS,S-AS01E (Box 1) [22–33]; revision of the Malaria Vaccine Technology Roadmap [34]; and new vaccines that progressed to clinical trials [35,36]. For vector control, advances include registration of 2 additional long-lasting insecticide formulations for indoor residual spraying (IRS) [37,38], field trials of dual-insecticide bed nets [39–41], development programmes for new insecticides [42–44], and publication of the larval source management operational manual by WHO [45]. Advances have also been made in the ‘tools for developing tools’—for example, controlled human malaria infection (CHMI) blood-stage parasite inoculation (Box 2) [46–56]; the human blood-stage challenge model for early-stage determination of antimalarial drug pharmacokinetics/pharmacodynamics [57]; the development of human liver chimeric mice, human erythroid chimeric mice, and dually engrafted mice allowing replication of the entire P. falciparum life cycle [58]; and validation of phenotypic assays for gametocyte screening to identify compounds with transmission-blocking activity [59]. In addition, new technologies and scientific insights are emerging [10], with notable improvements in mapping and modelling [7,60–62].
Box 1. Malaria vaccine RTS,S/A101E
In 2015, the preerythrocytic candidate vaccine RTS,S/AS01E (RTS,S) received a positive scientific opinion by European regulators through the Article 58 procedure. This was a breakthrough in malaria vaccine development, identifying a regulatory pathway and demonstrating that the large clinical trials necessary for approval could be conducted in Africa [23–25].
The target for RTS,S is the reduction of malaria incidence and severe disease in young children. A 3-dose regimen was shown to reduce the number of malaria cases by half in children 5–17 months of age during the first year following vaccination; efficacy waned over time but was prolonged by a fourth dose [25].
Despite modest efficacy, RTS,S prevented about 1,700 cases for every 1,000 children vaccinated in a phase III study over a 4-year period, and modelling studies predict a considerable public health impact for RTS,S, with the greatest benefit expected in areas with the highest malaria burden [27].
Following review of RTS,S data by the Strategic Advisory Group of Experts on Immunization and the Malaria Policy Advisory Committee, in 2016 the WHO adopted recommendations for RTS,S pilot implementations in 3–5 settings involving 100,000–200,000 children per setting (for a total of 400,000–800,000), in a staged manner to further evaluate safety (including meningitis [26]), feasibility of delivery, and impact on mortality.
Phase IV studies with a primary objective of further evaluation of safety as part of the Risk Management Plan approved by European Medicines Agency are planned to be linked to the larger pilots, with complementary design and objectives [28,29].
Research to optimize the regimen and explore additional applications of RTS,S
- ○
Optimising the RTS,S dosing regimen
Additional controlled human malaria infection (CHMI) and phase IIb studies are in progress to better define how to improve RTS,S/AS01 efficacy and how these data translate to the field, respectively.
A small study with RTS,S and an earlier adjuvant (AS02) found that fractional dosing, i.e., 2 full monthly doses plus a third low-dose at 7 months, resulted in apparent high efficacy against P. falciparum challenge (6/7 protected) [30].
A recent CHMI study in a larger number of volunteers using RTS,S/AS01 confirmed that a 0-, 1-, 7-month regimen that included a fractional third dose (Fx017M) was associated with higher efficacy (86.7% [95% confidence interval [CI], 66.8%–94.6%]; 26/30 protected) than the standard monthly full-dose regimen (62.5% [95% CI, 29.4%–80.1%]; 10/16 protected) against infection 3 weeks after the third dose [31].
- ○
Additional applications for RTS,S
Additional applications of RTS,S explored through modelling and, if indicated, evaluated in carefully designed field studies over the next 5-year period include the following [33]:
Evaluating the contribution to elimination of artemisinin-resistant parasites in the Greater Mekong Subregion, although data supporting an adult indication (dose and regimen) would be needed [32];
Combining RTS,S with other interventions or another malaria vaccine (mass drug administration [MDA] or a future VIMT, respectively), with the aim of enhancing or extending their effects;
Combining RTS,S with seasonal malaria chemoprevention (SMC), a study of which is in progress in Burkina Faso and Mali.
Box 2. Tools for developing tools: Demonstrating transmission-blocking activity
The validation of surrogate end points for transmission-blocking activity that translate into known effects in the field is necessary for the efficient development of new interventions aimed at this target.
Mosquito feeding assays
Three assays are available for assessing transmission-blocking activity:
Direct feeding assay (DFA): allowing mosquitos to feed on parasitaemic hosts; the most ‘physiologically relevant’ method [46,47].
Direct membrane feeding assay (DMFA): blood samples from parasitaemic hosts are fed to susceptible mosquitoes through an artificial membrane [48].
Standard membrane feeding assay (SMFA): laboratory-reared mosquitoes are fed a controlled number of cultured gametocytes from a single parasite strain combined with uninfected human erythrocytes and serum from human volunteers or animals. SFMA is now available as a medium throughput, reproducible, standardised assay [49].
In the context of elimination, the relevant outcome from these assays is a reduction in the number of infected mosquitoes.
Controlled human malaria infection (CHMI) model
Three CHMI techniques have been developed to determine the ability of drugs and vaccines to prevent human infection:
Sporozoite mosquito bites: infection of human volunteers via mosquito biting [50,51].
Sporozoite direct venous inoculation (SDVI): injection of sporozoites into human volunteers [52,53].
Induced blood-stage malaria parasite infection (IBSM): administration of Plasmodium-infected red blood cells to human volunteers [54,55].
Each of these techniques has advantages and disadvantages. Both sporozoite-based models allow evaluation of preerythrocytic and blood-stage drugs and vaccines, whereas IBSM can determine blood-stage efficacy only.
To evaluate transmission-blocking efficacy in preventing transmission from humans to mosquitoes, CHMI can be followed by a mosquito feeding assay using blood or serum from CHMI volunteers. Development of a regulatory pathway using mosquito feeding assays and CHMI with relevance to transmission-blocking activity in the field is ongoing. This effort would benefit from the development of new vaccines and drugs aimed specifically at this indication [56].
Diagnostics research agenda
Diagnostics for malaria treatment and elimination
To direct malaria treatment, all cases should be confirmed with a diagnostic test, either RDT or light microscopy, even in low-transmission settings [63]. Current WHO criteria for RDT procurement recommend a false positive rate of <10% [14]. However, a test with 99% specificity, when used at the elimination threshold (prevalence of parasitaemia in the community of ≤0.1%), results in ≥90% of positive tests coming from samples with no Plasmodium parasites [64]. In very low-transmission settings, addressing the challenge of false positive tests may require developing algorithms such as parallel or serial confirmation with a second test. Recently, false-negative results for P. falciparum histidine-rich protein 2 (PfHRP2)-based RDTs have been reported from several regions, caused by pfhrp2/pfhrp3 gene deletions [65–70]. Universal validity of these diagnostic tests cannot be assumed, and the WHO has issued guidance on PfHRP2-based RDT procurement [71].
Beyond P. falciparum, improved RDTs are needed for other species. Available lactate dehydrogenase (LDH)-targeting RDTs are less sensitive for P. vivax compared to P. falciparum, because P. vivax parasite densities tend to be much lower [72]. There is a paucity of information on test performance against minor species.
In the elimination context, a malaria diagnostic tool is needed for reactive or proactive detection of infectious parasite reservoirs residing in those individuals with subclinical infections and/or with parasite densities lower than those reliably detected with existing RDTs and microscopy (Fig 1) [73]. A target product profile (TPP) has been developed for a point-of-care malaria infection detection test for rapid detection of low-density, subclinical malaria infections [64]. Provided this was sufficiently sensitive, it would potentially enable targeting of populations harbouring reservoirs of parasite biomass with interventions interrupting transmission.
Fig. 1. Tools for detecting and interrupting malaria transmission and their action in the malaria transmission cycle. 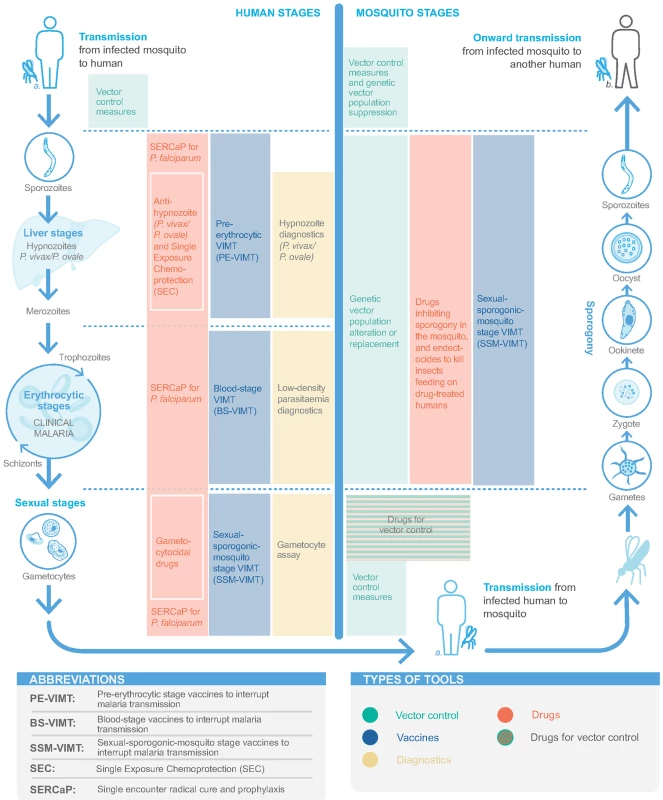
The most efficient uses for digital health are still being explored in malaria, but integrating diagnostic results generated from malaria case management into elimination programme surveillance efforts offer a near-term opportunity to fill critical data gaps in mapping malaria prevalence [2,7]. For example, 1 study combined a globally accessible database with mobile phone-based imaging of RDTs to provide an objective diagnostic readout and automated collection of surveillance data [74]. A similar approach in Kenya used digital RDT readers with upload to a cloud database [75]. However, lessons learned from digital health applied to the eradication programme for tuberculosis suggest that attaining a population-level impact are undermined by insufficient scale, coordination, and end-user engagement [76]. These issues are likely compounded in malaria given the higher prevalence of the disease globally.
Approaches to developing diagnostics
Several biomedical engineering approaches for malaria parasite detection have been investigated [77], including automated image processing [78], microfluidic systems [79], microarray chips [80], dielectrophoresis [81], and exploiting the bioelectrical properties of blood [77]. Further development of these techniques to increase sensitivity and specificity to detect clinically unapparent malaria parasite infections and allow field deployment continues.
Simplified molecular methods to detect low-level P. falciparum parasitaemia for use in low-resource settings are being developed, although improvements in throughput and cost are required [82]. Loop-mediated isothermal amplification (LAMP) is 1 promising approach, already validated in low-transmission settings [83] and as point-of-care detection of asymptomatic low-density malaria parasite carriers [84]. Further developments include noninstrumented nucleic acid amplification LAMP (NINA-LAMP) [85], achieving comparable sensitivity to P. falciparum polymerase chain reaction (PCR) detection in the field [85,86]. Another approach, using an insulated isothermal PCR (iiPCR) in a commercially available portable device, for Plasmodium detection achieved an assay efficiency of 96.9% with a lower detection limit of ≥100 copies of plasmodial DNA [87]. Nucleic acid amplification techniques can also be used for multiple pathogens in parallel, incorporating other infectious diseases (e.g., Ebola, dengue, and typhoid), depending on the setting and target population [88].
Noninvasive testing
Although a noninvasive technique is highly preferred, all currently available diagnostics require blood samples. PCR-based assays to detect Plasmodium parasites in saliva, although unsuitable for routine diagnosis, have been successfully developed [89]. Malaria detection in urine has been evaluated in field trials, but its sensitivity requires improvement [90]. Preliminary investigations indicate that malaria-specific volatile levels from breath samples correlate with parasite clearance [91], and studies are ongoing. A transdermal, noninvasive, reagent-free approach relying on the presence of iron-rich haemozoin to generate vapour nanobubbles is currently being field tested to detect parasites in skin blood vessels [92].
Detecting gametocytes
While gametocyte detection may indicate an individual’s transmission potential, further definition is required as to the most appropriate clinical sample to collect and the relevant gametocyte levels reflecting infectiousness [47]; this is complicated by a lack of correlation between gametocyte density in the blood and infectiousness following antimalarial treatment [93]. Validation of relevant target sequences is a first step towards development of molecular methods amenable for routine gametocyte detection. Circulating P. falciparum female and male and P. vivax gametocytes can now be detected using quantitative nucleic acid sequence-based amplification (QT-NASBA) or quantitative PCR (qPCR) methods with pfs25-, pfs230p-, and pvs25-based primers, respectively [94–98].
Detecting drug resistance
Detection of Kelch-propeller polymorphisms conferring artemisinin-resistance is currently restricted to sentinel surveillance [21], though more granular information is needed with continuing efforts to eliminate artemisinin-resistant parasites [99]. For example, a next-generation amplicon sequencing method suitable for use in endemic countries enables high-throughput detection of genetic mutations in 6 P. falciparum genes associated with resistance to antimalarial drugs, including artemisinins, chloroquine, and sulfadoxine-pyrimethamine [100]. For detecting P. falciparum single nucleotide polymorphisms (SNPs) associated with antifolate drug resistance, the ligase detection reaction fluorescent microsphere (LDR-FM) assay has been validated in clinical trials in Uganda [101]. As noted elsewhere in the malERA Refresh series [8], continued research on identifying markers of resistance to the other antimalarial drugs in current use (e.g., lumefantrine and piperaquine) is critical, as tools are needed to detect and manage drug resistance inevitable in elimination efforts [102,103].
Detecting hypnozoites
Hypnozoites residing in the human host is one tactic used by P. vivax and P. ovale to sustain the parasite reservoir between transmission seasons and produce multiple clinical relapses over prolonged periods, each with the potential to maintain transmission [104]. Detecting P. vivax/P. ovale hypnozoites, however, is problematic because of their low density, metabolic inactivity, and sequestration within the liver. Biomarkers that detect hypnozoites would be breakthrough tools in both case management diagnostics and elimination surveillance for P. vivax and P. ovale infections.
Glucose-6-phosphate dehydrogenase (G6PD) testing
An affordable, easy-to-use, rapid, point-of-care, semiquantitative diagnostic test is needed to identify G6PD-deficient individuals at risk of haemolysis with use of 8-aminoquinolones (i.e., primaquine or tafenoquine) to prevent P. vivax relapses. Although several tests are available [105], further refinement is needed to support greater access to these medicines. Single administration of low-dose (0.25 mg/kg) primaquine as a gametocytocidal agent is recommended after treatment for P. falciparum malaria [106], but there is still a need for more data on the optimal dose and reassurance of safety in G6PD-deficient individuals and larger populations, if used in MDA, for example [107].
Pregnancy testing
Pregnant women are excluded from receiving certain drugs or interventions, and rapid, low-cost, low-complexity, point-of-use pregnancy tests are needed, particularly for populations receiving MDA drugs with contraindications for use during pregnancy.
Challenges
Diagnostics are needed to direct treatment, support surveillance, and identify transmission reservoirs [73] and for continued progress in the development and evaluation of other tools for elimination, e.g., in settings with low-density parasitaemia and low transmission and for interventions targeting hypnozoites/prevention of relapse. The relevance of low-density parasitaemia to transmission requires further investigation to enable the design of diagnostics appropriate for these needs. Longer term, development of noninvasive assays, and field assays for detecting drug-resistant parasites should be pursued. Detecting hypnozoites remains a more profound challenge, although proteomics and metabolomics are being explored [108].
Drug research agenda
Drugs for malaria treatment, prevention, and transmission interruption
A strong portfolio of combination medicines with different or competing resistance mechanisms is required to combat resistance. It is now possible to tune the development program to advance drugs that have high barriers to resistance development and a low potential for cross resistance with other agents. In addition to classic inhibitory experiments, the propensity of drugs to induce ring-stage dormancy, characteristic of artemisinin resistance, must also be evaluated [109].
Single encounter radical cure and prophylaxis (SERCaP)
Proposed in malERA 2011, SERCaP remains a priority [5]. Radical cure means clearance of all asexual blood-stage forms, mature gametocytes, and P. vivax/P. ovale hypnozoites (Fig 1). Combination therapies of new chemical entities (NCEs) that are targeted to ‘single encounter, radical cure‘ are now in phase II clinical trials, with potential regulatory submission dates circa 2021 (S1 Table) [17].
The post-treatment prophylactic component of the SERCaP will come from the long half-life of the active pharmaceutical ingredients. Malaria parasite elimination will require new generations of single-encounter chemoprotection, to protect migrating populations and protect against epidemics in the later stages of elimination. These products would include molecules that provide chemoprotection by targeting the preerythrocytic stages (see TCP-4 specific attributes in [110]).
Reducing duration of dosing regimens, ideally to a single dose, increases adherence, be it for prevention or treatment [8]. Although better adherence improves effectiveness, it must be achieved without significantly increasing the risk of selection for drug-resistant parasites as a result of creating long periods of subtherapeutic drug levels. As a country or area approaches elimination, the remaining parasites are likely to be those most resistant to treatment, and drug classes with a low propensity to select for parasite resistance should be prioritised [111, 112]. The temptation to combine new drugs with old drugs with preexisting resistance, whilst simpler from a regulatory perspective, must be avoided to prevent novel agents being exposed as functional monotherapies when used against strains resistant to the older partner drug.
Severe malaria
Intravenous and intramuscular artesunate are currently the most effective and well-tolerated treatments for severe malaria [113,114], with rectal artesunate recommended for pre-referral treatment of children who cannot quickly access hospital care [106]. The potential spread of artemisinin resistance threatens the effectiveness of artesunate for treatment of severe malaria. Thus, new compounds with rapid activity against asexual blood stage parasites, suitable for parenteral administration, are needed for this critical indication (see TCP-1 specific attributes in [110]). The decline in the incidence of severe malaria in adults will require alternative development approaches, including the development of surrogate end points [115], as not enough patients will be available for large mortality studies [116]. However, sufficient safety data in adults would still be required for a phase III trial in African children with severe malaria.
Interrupting transmission
Drugs with activity against gametocytes in humans or that impair sporogony in the mosquito could help to interrupt transmission (Fig 1) [117]. While low-dose (0.25 mg/kg single dose) primaquine is currently recommended as a gametocytocidal following ACT treatment for P. falciparum malaria in areas of low transmission [16,118,119], NCE combination therapies with both therapeutic and transmission-blocking activity would simplify drug administration. High-throughput screening and clinical evaluation of compounds with transmission-blocking activity are now possible (Box 2) [59,120–125] and have yielded new leads, including more than a dozen from the Medicines for Malaria Venture toolbox with activity in the standard membrane feeding assay [126].
Global antimalarial drug development portfolio
There are at least 15 active projects in preclinical development or phase I or II clinical trials (S1 Table) [17]. A range of new chemotypes targeting new parasite pathways are available, with antimalarial drug development accelerated using CHMI models (Box 2). Two pairs of NCE combinations are in phase II clinical studies: the long-lasting synthetic endoperoxide artefenomel (OZ439) combined with the next-generation 4-aminoquinoline, ferroquine; and the imidazolopiperazine KAF156 combined with a new once-per-day lumefantrine formulation. This latter combination is also being explored as a 3-day regimen for use as a frontline agent in areas with ACT resistance. Single-dose effectiveness with an appropriate safety profile may require triple combination therapy. Notably, KAF156, DSM265, and MMV390048 have activity against P. falciparum liver stages and could be given as a single-dose treatment or once weekly for chemoprotection (S1 Table) [127]. TPPs and target candidate profiles with minimal essential and ideal attributes for single-encounter chemoprotection have been published [110].
Antihypnozoite drugs
In areas of high transmission, such as Papua New Guinea, relapses cause approximately 4 of every 5 P. vivax infections [128]. Modelling suggests that for rapidly relapsing tropical P. vivax strains, effective relapse prevention has the potential to significantly reduce transmission [104]. In areas of seasonal transmission, relapses allow parasites to rapidly reestablish transmission once vector populations recover [129]. The 8-aminoquinoline primaquine is the only antirelapse therapy currently available (aside from chloroquine, to which there is extensive resistance), but treatment courses are 7–14 days, and poor adherence undermines effectiveness [130]. Tafenoquine is a candidate single-dose 8-aminoquinoline, showing high antirelapse efficacy in P. vivax infections when given with chloroquine [18]. Phase III clinical trials were completed in 2016, with regulatory submission anticipated in 2017. The impact of tafenoquine on transmission remains to be evaluated in post-registration CHMI and field trials. G6PD-deficient individuals cannot be given standard doses of 8-aminoquinolones; in addition, 8-aminoquinolones are considered contraindicated during pregnancy and lactation. As such, new antihypnozoite drug classes without these contraindications are needed. Discovery should be enhanced in the next 5 years through screening campaigns against P. vivax liver stages using stable human cell systems [131,132]. Additionally, humanised mouse models are facilitating drug development against this life cycle stage that thus far has been refractory to study [133].
Seasonal malaria chemoprevention (SMC)
In areas where malaria is seasonal, providing SMC by monthly treatment with long-lasting antimalarial drugs greatly reduces malaria burden in children under 5 years of age [134,135]. Modelling studies indicate the potential for reducing transmission to very low levels if SMC is combined with long-lasting insecticidal nets (LLINs) at 80% coverage and expanded to children up to 10 years of age [136]. Sulfadoxine-pyrimethamine + amodiaquine (SPAQ) is used for SMC in the Sahel; drug resistance prevents SPAQ use in eastern and southern Africa, and there are concerns that resistance may spread to the Sahel. Thus, alternative drugs are required, ideally with simplified dosing regimens.
Endectocides
Endectocides are an alternative approach to malaria control whereby humans and/or livestock are given agents with insecticidal activity, resulting in reduced survival of the vector upon blood feeding and impairment of malaria parasite transmission [137]. Modelling studies suggest that the endectocide ivermectin could help achieve transmission interruption as an additional intervention in settings where mass treatment strategies with ACTs alone would be insufficient to accomplish elimination [138]. A research agenda was proposed in 2013 outlining the path for ivermectin use in malaria [139], with a number of studies in different settings underway. A WHO expert group recently examined this concept, with findings anticipated in 2017. The antimosquito properties of veterinary and other candidate endectocides are also being explored.
Novel formulations
An interesting possibility is the application of nanomilling and related technologies to develop long-acting drug formulations, which are being investigated for long-term HIV preexposure prophylaxis and in combination with contraception in so-called multipurpose prevention technologies (MPTs) [140–143]. Such long-acting drug formulations could potentially allow chemoprotection over several months from a single injection. Application to new generations of transmission-blocking molecules or endectocides could provide tools that reduce or prevent transmission over an entire transmission season.
Challenges
Attrition rates in antimalarial drug development are comparable with those in other infectious diseases [126]. Thus, discovery momentum needs to be maintained at high levels if new drugs are to reach licensure. A major challenge in registering NCEs for malaria is assembling the substantial clinical safety data required for regulatory approval, particularly in the key target populations of infants and pregnant women. Thus, reproductive safety should be evaluated early in preclinical development to prioritise investment in NCEs with appropriate preclinical profiles.
With the introduction of NCEs during the next 5 years, pharmacovigilance needs strengthening in malaria-endemic areas. This is also a prerequisite for safe deployment of current ACTs and next-generation treatments during mass treatment programmes targeted at populations that include individuals with subclinical malaria or who are infection free.
In the next 5–10 years, there is a need to enrich the early-stage portfolio with new antihypnozoite drugs beyond the current 8-aminoquinolones. Cell biology and the animal models supporting drug discovery for new antihypnozoite agents have progressed significantly but are still not amenable to high-throughput screening programmes [144,145]. Clinical trials for relapse prevention take 6–12 months, much longer than treatment trials. Additionally, relapses can be caused by hypnozoites that are homologous or heterologous to the initial infection and cannot, therefore, be distinguished from recrudescence or reinfection [146–148], except by the physical removal of treated patients from transmission areas, e.g., repatriated soldiers and travellers.
Although NCEs active against artemisinin-resistant isolates are in development, better strategies are needed to deploy drugs to delay or prevent the emergence of drug resistance, such as measures to tackle counterfeiting or manufacturing of poor-quality medicines, drug sequencing, multiple firstline therapies, and exploiting competing resistance mechanisms, as discussed elsewhere in the malERA Refresh series [8].
Vaccine research agenda
The Malaria Vaccines Technology Roadmap was updated in 2013 [34], with the goal of developing by 2030 vaccines for P. falciparum and P. vivax that have a protective efficacy of at least 75% against clinical malaria and/or reduce transmission of the parasite. The roadmap outlines key priorities in research, vaccine development, key capacities, policy, and commercialisation. The research issues in malaria vaccines are discussed below, but key to their success will be ensuring an efficient and cost-effective distribution system and redirection of the health system from delivering malaria treatment to prevention and transmission interruption [9].
Vaccines to prevent clinical malaria and interrupt transmission
A preerythrocytic vaccine to interrupt malaria transmission (PE-VIMT) that completely prevents liver-stage infection for a significant duration (e.g., at least 1 transmission season) would prevent parasitaemia and gametocyte generation and therefore interrupt onward transmission (Fig 1). Although RTS,S is a preerythrocytic vaccine, demonstrating modest efficacy in preventing clinical malaria, prevention of infection and transmission were not evaluated in the late-stage clinical trials (Box 1). More recently, a delayed fractional dose regimen of RTS,S with improved efficacy against a parasite transmission (mosquito-to-human) end point may be considered for transmission-blocking potential (Box 1) [31]. Several next-generation preerythrocytic candidates are in clinical development, including multistage (including asexual blood-stage and/or sexual/sporogonic/mosquito-stage targets) combinations and prime-boost strategies, as well as irradiated or genetically attenuated sporozoites (S2 Table) [35,149]. Future directions need to ensure a widely acceptable route of administration, optimised dose regimens, and lower inoculum sizes.
Blood-stage vaccines are an alternative and complementary approach to PE-VIMT. Blood-stage vaccines that interrupt malaria parasite transmission (BS-VIMTs) by efficiently clearing blood-stage infections would limit gametocyte densities and the duration that a person is infectious, thus reducing human-to-mosquito malaria parasite transmission (Fig 1). Several promising P. falciparum vaccine candidates are in clinical development [150], including the unstructured peptide P27A, the well-studied PfRH5, and the 2 placental malaria vaccine candidates PAMVAC and PRIMVAC (S2 Table). Innovative new concepts in next-generation malaria vaccine protein subunit design are being explored to develop highly effective multicomponent/multistage/multiantigen formulations [151].
Vaccines that only interrupt malaria parasite transmission
Sexual-sporogonic-mosquito-stage vaccines to interrupt transmission (SSM-VIMT) inhibit parasite transmission from human to mosquito, through reducing gametocytes’ ability to infect mosquitoes or by interfering with parasite development (sporogony) within the mosquito (Fig 1). As the potential benefit to the recipient is both delayed and indirect, the PATH Malaria Vaccine Initiative and partners are exploring potential regulatory and policy approaches with the United States Food and Drug Administration and WHO, respectively [152,153]. Progress has been made, with a design proposed for a phase III study [154]. The Pfs25 antigen is expressed on the surface of zygotes and ookinetes in the mosquito midgut, and various attempts to improve immunogenicity and transmission-blocking activity have been undertaken (S2 Table) [36,153]. The most clinically advanced is Pfs25-EPA (a detoxified form of exotoxin A from Pseudomonas aeruginosa) conjugate [155]. Most recently, Pfs25 has been fused with IMX313, a molecular adjuvant, and expressed in chimpanzee adenovirus 63 (ChAd63) and Modified Vaccinia Virus Ankara (MVA) viral vectors and as a secreted protein nanoparticle [156]. The research agenda has broadened to include other SSM-VIMT antigens, including Pfs230 and Pfs48/45 (S2 Table).
Vaccines for P. vivax/P. ovale
A vaccine that could prevent P. vivax/P. ovale infection and hypnozoite formation, target hypnozoites, or prevent disease, thereby interrupting transmission and draining the hypnozoite reservoir, would be a significant step for accelerating malaria elimination (Fig 1). P. vivax is now included in the Malaria Vaccine Technology Roadmap strategic goals [34]. While basic research in P. vivax has increased in recent years, no vaccine candidate has progressed past early human studies (S3 Table) [35,36]. Three preerythrocytic vaccines have reached clinical trials [157–159]. A blood-stage vaccine targeting the Duffy-binding protein region II has progressed to early clinical trials [160], though combination with other blood-stage antigens is likely necessary to achieve high growth inhibition. The Pvs25 antigen is also being investigated as an SSM-VIMT. The recent development of P. vivax CHMI systems allows evaluation of vaccine efficacy [157,161]. Also, publication of the P. ovale and P. malariae genomes facilitates antigen discovery for these parasites [162].
Adjuvants, delivery platforms, and desired human immune responses
Most (but not all) malaria vaccines in development are based on Plasmodium protein subunits and have shown limited immunogenicity in humans. Suitable adjuvants and delivery platforms are therefore needed to elicit the desired immune response and induce significant protection from infection and disease without unacceptable collateral inflammation [163]. There are few adjuvants licensed for human use and there is a need to (1) better define the desired human immune response; (2) facilitate access to adjuvants in development and ensure downstream availability, affordability, and acceptability; (3) develop more specific targeted adjuvants that boost desired immune responses while maintaining acceptable safety; and (4) match individual adjuvants to individual vaccine candidates depending on the postulated mechanism of action while maintaining compatibility for combination vaccines.
Prophylactic biologics
Monoclonal antibodies are another potential tool. Recently, the major barriers of cost are being overcome through improvements in manufacturing and high-expressing cell lines [164]. A recent report estimated that the cost of goods for monoclonal antibodies had reduced 10-fold, from thousands of dollars per gram to around $100 per gram, with the costs of developing these agents comparable to other therapeutic drugs and vaccines [165]. Additionally, the volume and frequency of administration of monoclonal antibodies have been reduced by improvements in potency and pharmacokinetics [166]. There has been a significant increase in the number of validated vaccine targets, and now monoclonal antibodies can be studied early in clinical development for their ability to provide immediate protection in CHMI models, either singly or in combination [167]. Antibodies are less prone to the off-target safety and toxicity issues that often plague small molecule development and thus offer significant advantages for deployment in vulnerable populations, including the immunocompromised and pregnant women. In the context of elimination, monoclonal antibodies with suitable pharmacokinetics/pharmacodynamics could represent an alternative to active immunisation by VIMTs or transmission-blocking drugs. As with any tool, prophylactic biologicals will need to be designed to meet the needs and capabilities in target settings.
Challenges
To achieve malaria elimination, vaccines would ideally be able to prevent infection by all 5 species of human malaria parasites. While humans are the major (if not only) reservoir for 4 of 5 Plasmodium ssp., zoonotic P. knowlesi presents a unique challenge for elimination given continuous sylvatic transmission [168]. If a ‘Plasmodium’ vaccine targeting all human-infecting species is not feasible, then vaccines are required against individual species. It remains to be determined whether experience gained in the development of P. falciparum vaccines can, in fact, inform approaches to other malaria species or whether new strategies are required.
Similar to drugs used in MDA, vaccines for mass inoculation need to be safe for use in pregnant women and children. Demonstrating safety across the target population is particularly important for vaccines that only prevent transmission and have an indirect benefit to the recipient.
For malaria vaccine candidates, there is limited information on immune correlates that may predict efficacy in the chosen indication. Antigenic diversity of many of the malaria vaccine targets [169,170] adds additional complexity to predicting efficacy and enables parasites to evade host immune responses, potentially leading to vaccine escape mutants [171,172].
There is also incomplete understanding of the development and maintenance of either naturally acquired or vaccine-induced human immunity to Plasmodium. A predictable ‘age shift’ in peak incidence of malaria associated with vaccines with modest and/or waning efficacy in children who have not acquired full natural immunity must be anticipated and appropriately managed [173]. The challenge of maintaining individual and population-based (herd) immunity may increase as circulating parasite prevalence declines during the later stages of elimination. Thus, rationally designing vaccines that induce long-lasting immunity in semi-immune adults and provide broad cross strain protection presents formidable challenges.
Finally, as with drugs, parasite genetic diversity and rich population structures, particularly in high-transmission settings, indicate the potential for differential parasite-specific efficacy and selection of resistant Plasmodium. The former has been observed in vaccine field studies, including a recent genetic analysis associated with a large phase III trial of RTS,S/AS01 [170]. However, there are no data regarding whether implementing malaria vaccination induces parasite resistance in the whole population of infected individuals.
Vector control research agenda
Insecticide-based interventions
LLINs are currently the single most important malaria control intervention, responsible for approximately 68% of malaria cases averted in Africa [174]. However, emerging resistance to insecticides among Anopheles mosquitoes threatens to reverse these gains [175,176]. New insecticides with different modes of action are urgently needed to deter resistance development. In response, ‘Innovation to Impact’ was initiated in 2013 with an aim to transform the process for developing and delivering life-saving vector control products for diseases caused by vector-borne pathogens. More than 30 different stakeholder groups are involved, including industry, global evaluation and regulatory bodies, procurers, local and national representatives, and donors [42,43].
Twelve insecticide products are currently available for vector control, confined to 4 chemical classes (pyrethroids, organochlorines, organophosphates, and carbamates), although only pyrethroids are widely used for LLINs. Several combination LLINs consisting of different insecticide classes or incorporating the synergist piperonyl butoxide are in late-stage development (Table 1) [39–41,177–181]. Similar to LLINs, long-lasting insecticide-treated hammocks could be effective in remote areas; however, the lifespan of these interventions has a significant impact on cost-effectiveness, and exploration of technologies to increase durability is needed [182].
Tab. 1. Insecticides for indoor residual spraying (IRS) under World Health Organization Pesticide Evaluation Scheme (WHOPES) evaluation and long-lasting insecticidal nets (LLINs) in late-stage development [39–41,177,178]*. ![Insecticides for indoor residual spraying (IRS) under World Health Organization Pesticide Evaluation Scheme (WHOPES) evaluation and long-lasting insecticidal nets (LLINs) in late-stage development [<em class="ref">39</em>–<em class="ref">41</em>,<em class="ref">177</em>,<em class="ref">178</em>]<em class="ref">*</em>.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/e82b58cea3ca0d861b04bee1c2d591d2.png)
*March/April 2016. After screening around 4 million compounds, 3 new insecticides have progressed to development, with registration typically taking 5–7 years [44,178]. These new insecticides are primarily pyrethroid alternatives for use in LLINs but also would be expected to have use in IRS. For IRS, 2 long-lasting formulations of existing compounds have become available: a microencapsulated formulation of the organophosphate insecticide pirimiphos methyl in 2012 [37] and a polymer-enhanced suspension of deltamethrin in 2013 [38]. The Next Generation IRS project is a market intervention to accelerate uptake and increase use of long-lasting IRS products [183]. Additional long-lasting insecticides suitable for IRS are in development (Table 1).
New ways of using insecticides require more extensive field evaluation, e.g., technological advances for improving spraying techniques [184], timing of insecticide deployment to coincide with seasonal transmission, slow-release polymer-based wall linings [185,186], insecticide-treated eave tubes or eave ‘bricks’ combined with house screening, and electrostatic coatings to enhance insecticide bioavailability [187].
Vector behaviour and outdoor targeting
Greater understanding of vector behaviour is needed, including the behavioural adaptations of vectors in response to control measures, such as changes in biting times, resting locations, and rates of zoophagy [188–194]. Improved targeting of specific vector behaviours—particularly sugar feeding, oviposition, mating, dry-season survival, and swarming behaviour—and zooprophylaxis are generating novel approaches to vector control, with potential application across transmission settings [195,196].
Long-standing evidence that malaria parasite transmission to humans occurs outdoors in Southeast Asia and South America and increasing evidence of outdoor transmission in sub-Saharan Africa [3,197–202] suggest a specific need for interventions that target mosquitoes outside dwellings. Attractants/traps are a potential new area of mosquito control that can be applied both indoors and outdoors. These include attractive toxic sugar baits [203,204] and sound traps, which lure male mosquitoes by broadcasting sounds similar to the wingbeats of female mosquitoes [195,203]. All major malaria parasite vectors in Africa mate in swarms [206], which are easily found and recognised, appear to be stable over time, and exist in a defined space [195]. This facilitates close targeting either with insecticides or traps [195]. Spatial repellents are another approach, releasing into the air volatile chemicals that prevent human–vector contact within the treated space (indoor or outdoor). Guidelines for efficacy testing are now available [205,207], and evaluation in outdoor settings is needed [208,209].
Environmental management and larval source management
Environmental management, such as improved housing and water management, can be highly effective in specific epidemiological and environmental settings [210]. The best of these environmental management approaches require further investigation in tropical climates and resource-poor settings to establish their epidemiological impact in these settings [210,211]. Mosquito larval source management is the management of water bodies that are potential larval habitats to prevent immature mosquitoes developing into adults, either by environmental management or application of larvicides [45]. Larval source management has been highly effective in certain situations [211], but as this is a resource-intensive activity, better definition of the appropriate requirements and approaches across a wider range of settings is needed.
Genetic approaches
There are 2 main strategies for genetically modifying mosquito populations: (1) population suppression, whereby mosquitoes are modified in such a way that upon mating with the wild type the resulting progeny are either sterile or dysfunctional, and (2) population alteration or replacement, in which the mosquitoes are modified in such a way that upon mating with the wild type, the resulting progeny are rendered refractory to malaria parasite infection. Genetic approaches now appear operationally feasible given recent advances in molecular biology, such as the efficient genome-editing techniques based on CRISPR/Cas9 and other approaches [10,212,213].
The sterile insect technique was the first attempt at genetic population suppression, whereby large numbers of irradiated sterile males are released with the hope that females mate unsuccessfully [214]. A more recent development is the release of insects carrying a dominant lethality, with the progeny of females mating with genetically modified males inheriting a lethal gene [215,216].
Gene drive systems exploit ‘homing’ endonucleases. These induce the lateral transfer of an intervening DNA sequence to a homologous allele that lacks that sequence, thereby changing a heterozygote into a homozygote. Conventional homing endonucleases have been reengineered to recognise mosquito genes [217] and can rapidly increase the frequency of desirable traits in a mosquito population [218]. Technical feasibility has been demonstrated for a CRISPR/Cas9-based gene drive system with the potential to reduce mosquito populations [219] or make them less able to transmit malaria parasites [220].
There is also the potential for symbiont-mediated biocontrol in malaria Anopheles mosquito populations, as suggested by recent successes achieved against Aedes aegypti (e.g., Wolbachia-mediated pathogen interference for dengue control). A further step is paratransgenesis, whereby a vector symbiont (virus, bacteria, or fungi) is engineered to express ‘effector’ molecules within the vector that are deleterious to the pathogen. Genetic modification of symbionts is easier than it is for mosquitoes and is independent of mosquito species, providing the symbiont can survive and colonise the host [221], and laboratory studies have shown promise [222].
There are environmental uncertainties associated with widespread distribution of technologies involving genetic manipulation of pathogens, vectors, or their symbionts [10,212]. Phased testing starting at a small scale is recommended, though the parameters for ecological risk assessment are not well understood.
Challenges
The development of new insecticides will need to outpace the expansion of insecticide-resistant alleles in mosquito populations, and new products will need to be deployed to effectively combat behavioural resistance [8]. The imperfect correlation between entomological indicators and disease incidence complicates the accurate assessment of new vector control tools. Randomised controlled trials are expensive and time consuming, and new pathways should be explored for generating evidence for large-scale implementation of new interventions. Increasing fine-scale heterogeneity, in human and vector subpopulations and in geographic space, means that no single set of interventions will be effective across large areas or districts. Notwithstanding resource availability, the challenge is to understand which combinations of vector control measures are appropriate in different settings and how their effects can be augmented with other interventions (e.g., endectocides, transmission-blocking drugs, and vaccines) [7]. Targeting mosquito dormancy remains a challenge in large part because of the paucity of mechanistic evidence by which vectors persist during the dry-season (e.g., diapause [aestivation] and long-distance migration) [223]. Finally, it is important to note that there are very few trained entomologists in national malaria control programs, especially at the district level. To develop and implement vector-targeted interventions, greater entomology capacity building is required.
Conclusions
There are overarching areas in which greater knowledge is required to understand the utility of current interventions and define which products and strategies might be required going forward. Novel tools may allow further investigation of knowledge gaps, and some may be bridgeable (Table 2). The R&D agenda for tools for elimination is summarised in Box 3.
Tab. 2. Knowledge gaps and tools to potentially bridge the gaps. 
Box 3. Research and development agenda for tools for elimination
Diagnostics
Detecting transmission potential
Malaria diagnostic tools best suited for detection of low-density, subclinical infections
Assay for detecting infectious gametocytes
P. vivax/P. ovale hypnozoite detection methods
Noninvasive diagnostic tests
Directing treatment
Stable, valid, specific, and sensitive rapid diagnostic tests (RDTs) that do not depend on histidine-rich protein 2 (HRP2)
Detection of drug-resistant parasites
RDTs that detect and differentiate all relevant human Plasmodium ssp. pathogens
Multiplexed point-of-care tests for acute febrile illness
Special populations
Affordable, simple, and accurate point-of-care tests for glucose-6-phosphate dehydrogenase (G6PD)-deficient individuals and pregnant women
Drugs
Drugs for prevention and treatment
Drugs that overcome resistance to existing drugs, particularly artemisinin resistance
A suite of combination drugs with different or competing resistance profiles
New drugs for prophylaxis
Simplifying therapy, with the potential objective of a single encounter radical cure and prophylaxis (SERCaP)
New regimens for use in seasonal malaria chemoprevention (SMC) outside the Sahel and to potentially replace sulfadoxine-pyrimethamine + amodiaquine (SPAQ)
Drugs to interrupt transmission
Investigation of the impact of low-dose primaquine in different settings
New drugs with transmission-blocking potential
Drug combinations incorporating both asexual and transmission-blocking activity
Evaluate the impact of transmission-blocking drugs on pathogen resistance development and investigate optimal deployment strategies
Endectocides for use in humans and animals
Antihypnozoite drugs
Evaluation of P. vivax transmission reduction potential with tafenoquine via relapse prevention (draining of hypnozoite reservoir)
Special populations
New small molecules or antibodies with a potential indication for use during pregnancy
Vaccines
RTS,S
Further evaluation of RTS,S to determine the potential for increased efficacy with alternative dosing regimens
Assessment of RTS,S in combination with other interventions (e.g., SMC) and in other epidemiological settings and populations
New vaccines
Defining the required attributes of preerythrocytic or blood-stage vaccines to achieve transmission-blocking activity
New preerythrocytic and or blood-stage vaccines, ideally with transmission-blocking potential
A first sexual-sporogonic-mosquito-stage vaccine to interrupt transmission (SSM-VIMT)
Vaccines against P. vivax/P. ovale
Vaccines that prevent infection and hypnozoite formation, target hypnozoites, or can interrupt transmission to eventually eliminate the hypnozoite reservoir
Adjuvants
Access to a broader choice of adjuvants with improved risk–benefit profiles
Prophylactic biologics
Development of monoclonal antibodies (mAbs) and combinations of recombinant multi-mAbs products
Vector control
Insecticides and long-lasting insecticidal nets (LLINs)
New insecticides and combinations of insecticides to overcome vector resistance
Nonpyrethroid insecticides for LLINs
Investigation of new insecticide deployment strategies
LLINs with improved durability
Environmental management
Formal investigation of larval source management in a greater variety of settings
Development of long-lasting safe larvicides
Development of cost-effective and socially acceptable environmental management interventions
Genetic approaches
Development of scalable genetic approaches
Development of environmentally and socially responsible methods for field testing transgenic organisms
Exploiting vector behaviour
Novel interventions to target populations and behaviours
Increased entomological support for key decisions by national malaria programmes
Combination and mapping
Modelling to suggest the most effective and efficient combinations of vector control for different settings
Developing operationally relevant mapping tools to identify and target residual transmission
‘Tools to develop tools’
Validating outcomes from animal and human infection models that predict a reduction in transmission in real-life settings
Robust mathematical and laboratory models of transmission and impact of combination interventions
Increased understanding of parasite–host immunity and mechanisms of acquired and vaccine-induced protective and transmission-blocking immunity
Development of high-throughput screening assays and evaluation assays for the identification and selection of compounds with neglected profiles (e.g., antihypnozoite activity)
Transmission can remain high even with high coverage of good-quality case management and vector control. Thus, products and strategies directed specifically at accelerating elimination by targeting transmission are needed. Interventions may only achieve transmission reduction when deployed in certain populations or settings. Conversely, some populations and settings may require specific measures for transmission reduction, for example, pregnant women and infants, migrant workers, subclinical parasitaemia, or addressing outdoor transmission. The availability of new interventions is expanding, but developing algorithms for their rational combination and deployment in packages to decrease transmission is a key research need that requires modelling support [7]. Cost-effectiveness is an important determinant of whether particular interventions are adopted in public health programmes [9].
New products and strategies are needed to overcome parasite drug resistance and vector resistance to insecticides [8]. Prevalence of vaccine escape mutants has been highlighted as a potential issue if vaccines become widely used [170–172]. Thus, product development must continue, and strategies for phased replacement are needed as effectiveness wanes. New product discovery and development requires investment in basic science [10], the alignment of regulatory structures to expedite product registration, and continued investment in pharmacovigilance and surveillance. Funding organisations and malaria programmes also need to be convinced that tools are impactful and cost-effective [9]. However, measuring the efficacy of tools that potentially impact transmission is problematic, particularly at the extremes of transmission [154]. Thus, new diagnostics and screening methods are required to assess tool efficacy in low-transmission settings and determine their contribution to maintaining zero transmission [73]. Moreover, the development of new diagnostics with improved sensitivity, or for specific tasks such as resistance surveillance, may fundamentally change our perception of malaria parasite transmission and our understanding of the most appropriate interventions required to interrupt transmission.
Finally, developing new tools can be expensive. When the malaria burden is significant, the economic case for innovation is clear. However, as the malaria burden decreases, the economic argument for continued development becomes more nuanced. Public–private partnerships, which first emerged 15 years ago, have demonstrated the ability to partner and drive development for a variety of tools, including diagnostics (e.g., PATH and Foundation for Innovative New Diagnostics), drugs (e.g., Medicine for Malaria Venture and formerly Drugs for Neglected Diseases Initiative), vaccines (e.g., PATH Malaria Vaccine Initiative and European Vaccine initiative), and vectors (e.g., Innovative Vector Control Consortium and more broadly Malaria No More). New business models to attract and engage industry in developing tools for the elimination should be considered as well. Interventions will be directed at increasingly smaller populations, but these populations often represent the most difficult contexts in which to achieve elimination, and multiple interventions may be required. Once a country achieves elimination, there is the temptation to scale back infrastructure and interventions for malaria. This risks triggering a potentially lethal outbreak that could be difficult to reeliminate or even contain. There are numerous examples from earlier malaria elimination campaigns in the 1950s and 1960s of initial successes that were followed by resurgence as campaigns were deprioritized or discontinued administratively, financially, and technically. Unless malaria can be completely eradicated, interventions to maintain malaria elimination and a reserve of effective measures to counter malaria outbreaks will always be needed. However, if the right products and strategies are developed, and if they are used efficiently, effectively, and consistently, malaria eradication is an achievable goal.
Supporting Information
Zdroje
1. World Health Organization. Eliminating malaria Geneva: WHO; 2016 [Available from: http://apps.who.int/iris/bitstream/10665/205565/1/WHO_HTM_GMP_2016.3_eng.pdf?ua=1]
2. World Health Organization. Global technical strategy for malaria 2016–2030 Geneva: WHO; 2015 [Available from: http://apps.who.int/iris/bitstream/10665/176712/1/9789241564991_eng.pdf]
3. malERA Consultative Group on Vector Control. A research agenda for malaria eradication: vector control. PLoS Med. 2011;8(1):e1000401. doi: 10.1371/journal.pmed.1000401 21311587
4. malERA Consultative Group on Vaccines. A research agenda for malaria eradication: vaccines. PLoS Med. 2011;8(1):e1000398. doi: 10.1371/journal.pmed.1000398 21311586
5. malERA Consultative Group on Drugs. A research agenda for malaria eradication: drugs. PLoS Med. 2011;8(1):e1000402. doi: 10.1371/journal.pmed.1000402 21311580
6. malERA Consultative Group on Diagnoses and Diagnostics. A research agenda for malaria eradication: diagnoses and diagnostics. PLoS Med. 2011;8(1):e1000396. doi: 10.1371/journal.pmed.1000396 21311583
7. malERA Refresh Consultative Panel on Combination Interventions and Modelling. malERA: An updated research agenda on combination interventions and modelling for malaria elimination and eradication. PLoS Med. 2017;14(11):e1002453. doi: 10.1371/journal.pmed.1002453
8. malERA Refresh Consultative Panel on Insecticide and Drug Resistance. malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002450. doi: 10.1371/journal.pmed.1002450
9. malERA Refresh Consultative Panel on Health Systems and Policy Research. malERA: An updated research agenda for health systems, policy and implementation research in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002454. doi: 10.1371/journal.pmed.1002454
10. malERA Refresh Consultative Panel on Basic Science and Enabling Technologies. malERA: An updated research agenda on basic science and enabling technologies in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002451. doi: 10.1371/journal.pmed.1002451
11. Rabinovich R, Drakeley C, Djimde AA, Hall BF, Hay S, Hemingway J, et al. malERA: An updated research agenda for malaria elimination and eradication. PLoS Med. 2017;14(11):e1002456. doi: 10.1371/journal.pmed.1002456
12. World Health Organization. World malaria report Geneva: WHO; 2015 [Available from: http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf?ua=1]
13. Abba K, Kirkham AJ, Olliaro PL, Deeks JJ, Donegan S, Garner P, et al. Rapid diagnostic tests for diagnosing uncomplicated non-falciparum or Plasmodium vivax malaria in endemic countries. Cochrane Database Syst Rev. 2014;12:CD011431.
14. World Health Organization. Malaria rapid diagnostic test performance Geneva: WHO; 2015 [Available from: http://apps.who.int/iris/bitstream/10665/204118/1/9789241510035_eng.pdf?ua=1]
15. World Health Organization. The role of mass drug administration, mass screening and treatment, and focal screening and treatment for malaria Geneva: WHO; 2015 [Available from: http://www.who.int/malaria/publications/atoz/role-of-mda-for-malaria.pdf?ua=1]
16. World Health Organization. Policy brief on single-dose primaquine as a gametocytocide in Plasmodium falciparum malaria Geneva: WHO; 2015 [Available from: http://www.who.int/malaria/publications/atoz/who_htm_gmp_2015.1.pdf]
17. Medicines for Malaria Venture. Interactive R&D portfolio Geneva: MMV; 2016 [Available from: http://www.mmv.org/research-development/interactive-rd-portfolio]
18. Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014;383(9922):1049–58. doi: 10.1016/S0140-6736(13)62568-4 24360369
19. Leong FJ, Zhao R, Zeng S, Magnusson B, Diagana TT, Pertel P. A first-in-human randomized, double-blind, placebo-controlled, single - and multiple-ascending oral dose study of novel Imidazolopiperazine KAF156 to assess its safety, tolerability, and pharmacokinetics in healthy adult volunteers. Antimicrob Agents Chemother. 2014;58(11):6437–43. doi: 10.1128/AAC.03478-14 25136017
20. Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505(7481):50–5. doi: 10.1038/nature12876 24352242
21. Menard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374(25):2453–64. doi: 10.1056/NEJMoa1513137 27332904
22. European Medicines Agency. Assessment report: Mosquirix™ London: EMA; 2015 [Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_for_use_outside_EU/2015/10/WC500194577.pdf]
23. Agnandji ST, Fernandes JF, Bache EB, Ramharter M. Clinical development of RTS,S/AS malaria vaccine: a systematic review of clinical Phase I-III trials. Future Microbiol. 2015;10(10):1553–78. doi: 10.2217/fmb.15.90 26437872
24. Kaslow DC, Biernaux S. RTS S: Toward a first landmark on the malaria vaccine technology roadmap. Vaccine. 2015;33(52):7425–32. doi: 10.1016/j.vaccine.2015.09.061 26431982
25. RTS S, Clinical Trials Partnership,. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8 25913272
26. World Health Organization. Global Advisory Committee on Vaccine Safety, 10–11 June 2015 Geneva: WHO; 2015 [Available from: http://www.who.int/vaccine_safety/committee/reports/Jun_2015/en/.]
27. Penny MA, Verity R, Bever CA, Sauboin C, Galactionova K, Flasche S, et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet. 2016;387(10016):367–75. doi: 10.1016/S0140-6736(15)00725-4 26549466
28. World Health Organization. Questions and answers on malaria vaccines Geneva: WHO; 2016 [Available from: http://www.who.int/immunization/research/development/malaria_vaccine_qa/en/.]
29. Greenwood B, Doumbo OK. Implementation of the malaria candidate vaccine RTS,S/AS01. Lancet. 2016;387(10016):318–9. doi: 10.1016/S0140-6736(15)00807-7 26549465
30. Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336(2):86–91. doi: 10.1056/NEJM199701093360202 8988885
31. Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria infection and immunogenicity study. J Infect Dis. 2016;214 : 762–71. doi: 10.1093/infdis/jiw237 27296848
32. Gosling R, von Seidlein L. The Future of the RTS,S/AS01 Malaria Vaccine: An Alternative Development Plan. PLoS Med. 2016;13(4):e1001994. doi: 10.1371/journal.pmed.1001994 27070151
33. White MT, Verity R, Churcher TS, Ghani AC. Vaccine approaches to malaria control and elimination: Insights from mathematical models. Vaccine. 2015;33(52):7544–50. doi: 10.1016/j.vaccine.2015.09.099 26476361
34. World Health Organization. Malaria vaccine technology roadmap: November 2013 Geneva: WHO; 2013 [Available from: http://www.who.int/immunization/topics/malaria/vaccine_roadmap/TRM_update_nov13.pdf?ua=1]
35. World Health Organization. Tables of malaria vaccine projects globally "The Rainbow Tables" Geneva: WHO; 2016 [Available from: http://www.who.int/immunization/research/development/Rainbow_tables/en/.]
36. Mueller I, Shakri AR, Chitnis CE. Development of vaccines for Plasmodium vivax malaria. Vaccine. 2015;33(52):7489–95. doi: 10.1016/j.vaccine.2015.09.060 26428453
37. International Vector Control Consortium. WHO recommends Syngenta's new long-lasting insecticide formulation Liverpool: IVCC; 2013 [Available from: http://www.ivcc.com/news-and-media/news/who-recommends-syngentas-new-long-lasting-insecticide-formulation]
38. International Vector Control Consortium. K-Othrine Polyzone Liverpool: IVCC; 2016 [Available from: http://www.ivcc.com/news-and-media/news/bayer-cropscience-and-ivcc-offer-new-tool-for-malaria-control]
39. Djenontin A, Ahoua Alou LP, Koffi A, Zogo B, Duarte E, N'Guessan R, et al. Insecticidal and sterilizing effect of Olyset Duo(R), a permethrin and pyriproxyfen mixture net against pyrethroid-susceptible and -resistant strains of Anopheles gambiae s.s.: a release-recapture assay in experimental huts. Parasite. 2015;22 : 27. doi: 10.1051/parasite/2015027 26489479
40. Koffi AA, Ahoua Alou LP, Djenontin A, Kabran JP, Dosso Y, Kone A, et al. Efficacy of Olyset(R) Duo, a permethrin and pyriproxyfen mixture net against wild pyrethroid-resistant Anopheles gambiae s.s. from Cote d'Ivoire: an experimental hut trial. Parasite. 2015;22 : 28. doi: 10.1051/parasite/2015028 26489480
41. N'Guessan R, Ngufor C, Kudom AA, Boko P, Odjo A, Malone D, et al. Mosquito nets treated with a mixture of chlorfenapyr and alphacypermethrin control pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in West Africa. PLoS ONE. 2014;9(2):e87710. doi: 10.1371/journal.pone.0087710 24498360
42. Innovation to Impact. [Available from: http://innovationtoimpact.org/.]
43. World Health Organization. Innovation to Impact–WHO change plan for strengthening innovation, quality and use of vector-control tools Geneva: WHO; 2015 [Available from: http://www.who.int/malaria/mpac/mpac-sept2015-vector-control-innovation.pdf]
44. International Vector Control Consortium. [Available from: http://www.ivcc.com/.]
45. World Health Organization. Larval source management—a supplementary measure for malaria vector control: an operational manual Geneva: WHO; 2013 [Available from: http://apps.who.int/iris/bitstream/10665/85379/1/9789241505604_eng.pdf?ua=1]
46. Bousema T, Dinglasan RR, Morlais I, Gouagna LC, van Warmerdam T, Awono-Ambene PH, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS ONE. 2012;7(8):e42821. doi: 10.1371/journal.pone.0042821 22936993
47. Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouedraogo AL, et al. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. Elife. 2013;2:e00626. doi: 10.7554/eLife.00626 23705071
48. Ouédraogo AL, Guelbéogo WM, Cohuet A, Morlais I, King JG, Gonçalves BP, et al. A protocol for membrane feeding assays to determine the infectiousness of P. falciparum naturally infected individuals to Anopheles gambiae. MWJ. 2013;4(16):1–4.
49. Li T, Eappen AG, Richman AM, Billingsley PF, Abebe Y, Li M, et al. Robust, reproducible, industrialized, standard membrane feeding assay for assessing the transmission blocking activity of vaccines and drugs against Plasmodium falciparum. Malar J. 2015;14 : 150. doi: 10.1186/s12936-015-0665-8 25890243
50. Laurens MB, Duncan CJ, Epstein JE, Hill AV, Komisar JL, Lyke KE, et al. A consultation on the optimization of controlled human malaria infection by mosquito bite for evaluation of candidate malaria vaccines. Vaccine. 2012;30(36):5302–4. doi: 10.1016/j.vaccine.2012.04.088 22659449
51. Spring M, Polhemus M, Ockenhouse C. Controlled human malaria infection. J Infect Dis. 2014;209 Suppl 2:S40–5.
52. Gomez-Perez GP, Legarda A, Munoz J, Sim BK, Ballester MR, Dobano C, et al. Controlled human malaria infection by intramuscular and direct venous inoculation of cryopreserved Plasmodium falciparum sporozoites in malaria-naive volunteers: effect of injection volume and dose on infectivity rates. Malar J. 2015;14 : 306. doi: 10.1186/s12936-015-0817-x 26245196
53. Mordmuller B, Supan C, Sim KL, Gomez-Perez GP, Ospina Salazar CL, Held J, et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malar J. 2015;14 : 117. doi: 10.1186/s12936-015-0628-0 25889522
54. Engwerda CR, Minigo G, Amante FH, McCarthy JS. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol. 2012;28(11):515–21. doi: 10.1016/j.pt.2012.09.001 23041118
55. Krause A, Dingemanse J, Mathis A, Marquart L, Mohrle JJ, McCarthy JS. Pharmacokinetic/pharmacodynamic modelling of the antimalarial effect of Actelion-451840 in an induced blood stage malaria study in healthy subjects. Br J Clin Pharmacol. 2016;82 : 412–21. doi: 10.1111/bcp.12962 27062080
56. Malaria Vaccine Initiative. Transmission-blocking vaccines (TBVs) against malaria Seattle: MVI; 2016 [Available from: http://www.malariavaccine.org/sites/www.malariavaccine.org/files/content/resource/files/MVI-TBVfactsheetFINAL_20160420.pdf]
57. McCarthy JS, Marquart L, Sekuloski S, Trenholme K, Elliott S, Griffin P, et al. Linking murine and human Plasmodium falciparum challenge models in a translational path for antimalarial drug development. Antimicrob Agents Chemother. 2016;60 : 3669–75. doi: 10.1128/AAC.02883-15 27044554
58. Siu E, Ploss A. Modeling malaria in humanized mice: opportunities and challenges. Ann N Y Acad Sci. 2015;1342 : 29–36. doi: 10.1111/nyas.12618 25678404
59. Miguel-Blanco C, Lelievre J, Delves MJ, Bardera AI, Presa JL, Lopez-Barragan MJ, et al. Imaging-based high-throughput screening assay to identify new molecules with transmission-blocking potential against Plasmodium falciparum female gamete formation. Antimicrob Agents Chemother. 2015;59(6):3298–305. doi: 10.1128/AAC.04684-14 25801574
60. Asare EO, Tompkins AM, Bomblies A. A regional model for malaria vector developmental habitats evaluated using explicit, pond-resolving surface hydrology simulations. PLoS ONE. 2016;11(3):e0150626. doi: 10.1371/journal.pone.0150626 27003834
61. Chitnis N, Schapira A, Smith T, Steketee R. Comparing the effectiveness of malaria vector-control interventions through a mathematical model. Am J Trop Med Hyg. 2010;83(2):230–40. doi: 10.4269/ajtmh.2010.09-0179 20682861
62. Ngwa GA, Wankah TT, Fomboh-Nforba MY, Ngonghala CN, Teboh-Ewungkem MI. On a reproductive stage-structured model for the population dynamics of the malaria vector. Bull Math Biol. 2014;76(10):2476–516. doi: 10.1007/s11538-014-0021-0 25234336
63. World Health Organization. Policy brief on malaria diagnostics in low-transmission settings Geneva: WHO; 2014 [Available from: http://www.who.int/malaria/publications/atoz/malaria-diagnosis-low-transmission-settings-sep2014.pdf]
64. DIAMETER (Diagnostics for Malaria Elimination Toward Eradication). Target product profile: point-of-care malaria infection detection test for rapid detection of low-density, subclinical malaria infections Seattle: PATH; 2014 [Available from: http://sites.path.org/dx/files/2012/11/DIAMETER_IDT_TPP_FINAL_forwebsite.pdf]
65. Gamboa D, Ho MF, Bendezu J, Torres K, Chiodini PL, Barnwell JW, et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS ONE. 2010;5(1):e8091. doi: 10.1371/journal.pone.0008091 20111602
66. Akinyi S, Hayden T, Gamboa D, Torres K, Bendezu J, Abdallah JF, et al. Multiple genetic origins of histidine-rich protein 2 gene deletion in Plasmodium falciparum parasites from Peru. Sci Rep. 2013;3 : 2797. doi: 10.1038/srep02797 24077522
67. Cheng Q, Gatton ML, Barnwell J, Chiodini P, McCarthy J, Bell D, et al. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J. 2014;13 : 283. doi: 10.1186/1475-2875-13-283 25052298
68. Bharti PK, Chandel HS, Ahmad A, Krishna S, Udhayakumar V, Singh N. Prevalence of pfhrp2 and/or pfhrp3 gene deletion in Plasmodium falciparum population in eight highly endemic states in india. PLoS ONE. 2016;11(8):e0157949. doi: 10.1371/journal.pone.0157949 27518538
69. Deme AB, Park DJ, Bei AK, Sarr O, Badiane AS, Gueye Pel H, et al. Analysis of pfhrp2 genetic diversity in Senegal and implications for use of rapid diagnostic tests. Malar J. 2014;13 : 34. doi: 10.1186/1475-2875-13-34 24472178
70. Parr JB, Belson C, Patel JC, Hoffman IF, Kamthunzi P, Martinson F, et al. Estimation of Plasmodium falciparum transmission intensity in lilongwe, malawi, by microscopy, rapid diagnostic testing, and nucleic acid detection. Am J Trop Med Hyg. 2016;95(2):373–7. doi: 10.4269/ajtmh.16-0156 27325802
71. World Health Organization. False-negative RDT results and implications of new reports of P. falciparum histidine-rich protein 2/3 gene deletions Geneva: WHO; 2016 [Available from: http://www.who.int/malaria/publications/atoz/who-htm-gmp-2016.4.pdf]
72. Kobayashi T, Gamboa D, Ndiaye D, Cui L, Sutton PL, Vinetz JM. Malaria diagnosis across the International Centers of Excellence for Malaria Research: platforms, performance, and standardization. Am J Trop Med Hyg. 2015;93(3 Suppl):99–109.
73. malERA Refresh Consultative Panel on Characterising the Reservoir and Measuring Transmission. malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med. 2017;14: e1002452.
74. Scherr TF, Gupta S, Wright DW, Haselton FR. Mobile phone imaging and cloud-based analysis for standardized malaria detection and reporting. Sci Rep. 2016;6 : 28645. doi: 10.1038/srep28645 27345590
75. Soti DO, Kinoti SN, Omar AH, Logedi J, Mwendwa TK, Hirji Z, et al. Feasibility of an innovative electronic mobile system to assist health workers to collect accurate, complete and timely data in a malaria control programme in a remote setting in Kenya. Malar J. 2015;14 : 430. doi: 10.1186/s12936-015-0965-z 26530237
76. Falzon D, Timimi H, Kurosinski P, Migliori GB, Van Gemert W, Denkinger C, et al. Digital health for the End TB Strategy: developing priority products and making them work. Eur Respir J. 2016;48(1):29–45. doi: 10.1183/13993003.00424-2016 27230443
77. Ibrahim F, Thio TH, Faisal T, Neuman M. The application of biomedical engineering techniques to the diagnosis and management of tropical diseases: a review. Sensors (Basel). 2015;15(3):6947–95.
78. Das DK, Mukherjee R, Chakraborty C. Computational microscopic imaging for malaria parasite detection: a systematic review. J Microsc. 2015;260(1):1–19. doi: 10.1111/jmi.12270 26047029
79. Tay A, Pavesi A, Yazdi SR, Lim CT, Warkiani ME. Advances in microfluidics in combating infectious diseases. Biotechnol Adv. 2016;34(4):404–21. doi: 10.1016/j.biotechadv.2016.02.002 26854743
80. Taylor BJ, Howell A, Martin KA, Manage DP, Gordy W, Campbell SD, et al. A lab-on-chip for malaria diagnosis and surveillance. Malar J. 2014;13 : 179. doi: 10.1186/1475-2875-13-179 24885206
81. Kasetsirikul S, Buranapong J, Srituravanich W, Kaewthamasorn M, Pimpin A. The development of malaria diagnostic techniques: a review of the approaches with focus on dielectrophoretic and magnetophoretic methods. Malar J. 2016;15(1):358. doi: 10.1186/s12936-016-1400-9 27405995
82. Britton S, Cheng Q, McCarthy JS. Novel molecular diagnostic tools for malaria elimination: a review of options from the point of view of high-throughput and applicability in resource limited settings. Malar J. 2016;15 : 88. doi: 10.1186/s12936-016-1158-0 26879936
83. Patel JC, Lucchi NW, Srivastava P, Lin JT, Sug-Aram R, Aruncharus S, et al. Field evaluation of a real-time fluorescence loop-mediated isothermal amplification assay, RealAmp, for the diagnosis of malaria in Thailand and India. J Infect Dis. 2014;210(8):1180–7. doi: 10.1093/infdis/jiu252 24795480
84. Cook J, Aydin-Schmidt B, Gonzalez IJ, Bell D, Edlund E, Nassor MH, et al. Loop-mediated isothermal amplification (LAMP) for point-of-care detection of asymptomatic low-density malaria parasite carriers in Zanzibar. Malar J. 2015;14 : 43. doi: 10.1186/s12936-015-0573-y 25627037
85. Sema M, Alemu A, Bayih AG, Getie S, Getnet G, Guelig D, et al. Evaluation of non-instrumented nucleic acid amplification by loop-mediated isothermal amplification (NINA-LAMP) for the diagnosis of malaria in Northwest Ethiopia. Malar J. 2015;14 : 44. doi: 10.1186/s12936-015-0559-9 25626339
86. Waggoner JJ, Abeynayake J, Balassiano I, Lefterova M, Sahoo MK, Liu Y, et al. Multiplex nucleic acid amplification test for diagnosis of dengue fever, malaria, and leptospirosis. J Clin Microbiol. 2014;52(6):2011–8. doi: 10.1128/JCM.00341-14 24671788
87. Chua KH, Lee PC, Chai HC. Development of insulated isothermal PCR for rapid on-site malaria detection. Malar J. 2016;15(1):134.
88. Foundation for Innovative New Diagnostics. Acute febrile syndrome strategy Geneva: FIND; 2012 [Available from: http://r4d.dfid.gov.uk/PDF/Outputs/FIND/0031-FIND-NMFI-document-print-inhouse.pdf]
89. Singh R, Singh DP, Gupta R, Savargaonkar D, Singh OP, Nanda N, et al. Comparison of three PCR-based assays for the non-invasive diagnosis of malaria: detection of Plasmodium parasites in blood and saliva. Eur J Clin Microbiol Infect Dis. 2014;33(9):1631–9. doi: 10.1007/s10096-014-2121-z 24792127
90. Oguonu T, Shu E, Ezeonwu BU, Lige B, Derrick A, Umeh RE, et al. The performance evaluation of a urine malaria test (UMT) kit for the diagnosis of malaria in individuals with fever in south-east Nigeria: cross-sectional analytical study. Malar J. 2014;13 : 403. doi: 10.1186/1475-2875-13-403 25316216
91. Berna AZ, McCarthy JS, Wang RX, Saliba KJ, Bravo FG, Cassells J, et al. Analysis of breath specimens for biomarkers of Plasmodium falciparum infection. J Infect Dis. 2015;212(7):1120–8. doi: 10.1093/infdis/jiv176 25810441
92. Lukianova-Hleb E, Bezek S, Szigeti R, Khodarev A, Kelley T, Hurrell A, et al. Transdermal diagnosis of malaria using vapor nanobubbles. Emerg Infect Dis. 2015;21(7):1122–7. doi: 10.3201/eid2107.150089 26079141
93. Djimde AA, Maiga AW, Ouologuem D, Fofana B, Sagara I, Dembele D, et al. Gametocyte clearance dynamics following oral artesunate treatment of uncomplicated falciparum malaria in Malian children. Parasite. 2016;23 : 3. doi: 10.1051/parasite/2016003 26839003
94. Mwingira F, Genton B, Kabanywanyi AN, Felger I. Comparison of detection methods to estimate asexual Plasmodium falciparum parasite prevalence and gametocyte carriage in a community survey in Tanzania. Malar J. 2014;13 : 433. doi: 10.1186/1475-2875-13-433 25404207
95. Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, et al. Strategies for detection of Plasmodium species gametocytes. PLoS ONE. 2013;8(9):e76316. doi: 10.1371/journal.pone.0076316 24312682
96. Ouedraogo AL, Schneider P, de Kruijf M, Nebie I, Verhave JP, Cuzin-Ouattara N, et al. Age-dependent distribution of Plasmodium falciparum gametocytes quantified by Pfs25 real-time QT-NASBA in a cross-sectional study in Burkina Faso. Am J Trop Med Hyg. 2007;76(4):626–30. 17426160
97. Schneider P, Reece SE, van Schaijk BC, Bousema T, Lanke KH, Meaden CS, et al. Quantification of female and male Plasmodium falciparum gametocytes by reverse transcriptase quantitative PCR. Mol Biochem Parasitol. 2015;199(1–2):29–33. doi: 10.1016/j.molbiopara.2015.03.006 25827756
98. Hisaeda H, Stowers AW, Tsuboi T, Collins WE, Sattabongkot JS, Suwanabun N, et al. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect Immun. 2000;68(12):6618–23. 11083773
99. World Health Organization. Emergency response to artemisinin resistance in the Greater Mekong subregion. Regional framework for action 2013–2015 Geneva: WHO; 2013 [Available from: http://www.who.int/malaria/publications/atoz/9789241505321/en/]
100. Rao PN, Uplekar S, Kayal S, Mallick PK, Bandyopadhyay N, Kale S, et al. A method for amplicon deep sequencing of drug resistance genes in Plasmodium falciparum clinical isolates from India. J Clin Microbiol. 2016;54(6):1500–11. doi: 10.1128/JCM.00235-16 27008882
101. Nankoberanyi S, Mbogo GW, LeClair NP, Conrad MD, Tumwebaze P, Tukwasibwe S, et al. Validation of the ligase detection reaction fluorescent microsphere assay for the detection of Plasmodium falciparum resistance mediating polymorphisms in Uganda. Malar J. 2014;13 : 95. doi: 10.1186/1475-2875-13-95 24629020
102. Amato R, Lim P, Miotto O, Amaratunga C, Dek D, Pearson RD, et al. Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect Dis. 2017;17 : 164–73. doi: 10.1016/S1473-3099(16)30409-1 27818095
103. Witkowski B, Duru V, Khim N, Ross LS, Saintpierre B, Beghain J, et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study. Lancet Infect Dis. 2017;17 : 174–83. doi: 10.1016/S1473-3099(16)30415-7 27818097
104. White MT, Karl S, Battle KE, Hay SI, Mueller I, Ghani AC. Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission. Elife. 2014;3:e04692.
105. Ley B, Luter N, Espino FE, Devine A, Kalnoky M, Lubell Y, et al. The challenges of introducing routine G6PD testing into radical cure: a workshop report. Malar J. 2015;14 : 377. doi: 10.1186/s12936-015-0896-8 26416229
106. World Health Organization. Guidelines for the treatment of malaria, third edition Geneva: WHO; 2015 [Available from: http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf]
107. Graves PM, Gelband H, Garner P. Primaquine or other 8-aminoquinoline for reducing Plasmodium falciparum transmission. Cochrane Database Syst Rev. 2015(2):CD008152. doi: 10.1002/14651858.CD008152.pub4 25693791
108. Salinas JL, Kissinger JC, Jones DP, Galinski MR. Metabolomics in the fight against malaria. Mem Inst Oswaldo Cruz. 2014;109(5):589–97. doi: 10.1590/0074-0276140043 25185001
109. Chavchich M, Van Breda K, Rowcliffe K, Diagana TT, Edstein MD. The spiroindolone KAE609 does not induce dormant ring stages in Plasmodium falciparum parasites. Antimicrob Agents Chemother. 2016;60(9):5167–74. doi: 10.1128/AAC.02838-15 27297484
110. Burrows JN, Duparc S, Gutteridge WE, Hooft van Huijsduijnen R, Kaszubska W, Macintyre F, et al. New developments in anti-malarial target candidate and product profiles. Malar J. 2017;16(1):26. doi: 10.1186/s12936-016-1675-x 28086874
111. Artzy-Randrup Y, Alonso D, Pascual M. Transmission intensity and drug resistance in malaria population dynamics: implications for climate change. PLoS ONE. 2010;5(10):e13588. doi: 10.1371/journal.pone.0013588 21060886
112. Hastings IM, Watkins WM. Intensity of malaria transmission and the evolution of drug resistance. Acta Trop. 2005;94(3):218–29. doi: 10.1016/j.actatropica.2005.04.003 15847846
113. Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376(9753):1647–57. doi: 10.1016/S0140-6736(10)61924-1 21062666
114. Dondorp A, Nosten F, Stepniewska K, Day N, White N, South East Asian Quinine Artesunate Malaria Trial g. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366(9487):717–25. doi: 10.1016/S0140-6736(05)67176-0 16125588
115. Jeeyapant A, Kingston HW, Plewes K, Maude RJ, Hanson J, Herdman MT, et al. Defining surrogate endpoints for clinical trials in severe falciparum malaria. PLoS ONE. 2017;12(1):e0169307. doi: 10.1371/journal.pone.0169307 28052109
116. Cheah PY, Parker M, Dondorp AM. Development of drugs for severe malaria in children. Int Health. 2016;8(5):313–6. doi: 10.1093/inthealth/ihw038 27620923
117. Abdul-Ghani R, Beier JC. Strategic use of antimalarial drugs that block falciparum malaria parasite transmission to mosquitoes to achieve local malaria elimination. Parasitol Res. 2014;113(10):3535–46. doi: 10.1007/s00436-014-4091-6 25185662
118. Dicko A, Brown JM, Diawara H, Baber I, Mahamar A, Soumare HM, et al. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis. 2016;16 : 674–84. doi: 10.1016/S1473-3099(15)00479-X 26906747
119. Goncalves BP, Tiono AB, Ouedraogo A, Guelbeogo WM, Bradley J, Nebie I, et al. Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial. BMC Med. 2016;14 : 40. doi: 10.1186/s12916-016-0581-y 26952094
120. Bolscher JM, Koolen KM, van Gemert GJ, van de Vegte-Bolmer MG, Bousema T, Leroy D, et al. A combination of new screening assays for prioritization of transmission-blocking antimalarials reveals distinct dynamics of marketed and experimental drugs. J Antimicrob Chemother. 2015;70(5):1357–66. doi: 10.1093/jac/dkv003 25667405
121. Plouffe DM, Wree M, Du AY, Meister S, Li F, Patra K, et al. High-throughput assay and discovery of small molecules that interrupt malaria transmission. Cell Host Microbe. 2016;19(1):114–26. doi: 10.1016/j.chom.2015.12.001 26749441
122. Lucantoni L, Fidock DA, Avery VM. Luciferase-based, high-throughput assay for screening and profiling transmission-blocking compounds against Plasmodium falciparum gametocytes. Antimicrob Agents Chemother. 2016;60(4):2097–107. doi: 10.1128/AAC.01949-15 26787698
123. Almela MJ, Lozano S, Lelievre J, Colmenarejo G, Coteron JM, Rodrigues J, et al. A new set of chemical starting points with Plasmodium falciparum transmission-blocking potential for antimalarial drug discovery. PLoS ONE. 2015;10(8):e0135139. doi: 10.1371/journal.pone.0135139 26317851
124. Lelievre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, et al. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence "transmission blocking" assay. PLoS ONE. 2012;7(4):e35019. doi: 10.1371/journal.pone.0035019 22514702
125. Roncales M, Vidal-Mas J, Leroy D, Herreros E. Comparison and optimization of different methods for the in vitro production of Plasmodium falciparum gametocytes. J Parasitol Res. 2012;2012 : 927148. doi: 10.1155/2012/927148 22523643
126. Burrows JN, van Huijsduijnen RH, Mohrle JJ, Oeuvray C, Wells TN. Designing the next generation of medicines for malaria control and eradication. Malar J. 2013;12 : 187. doi: 10.1186/1475-2875-12-187 23742293
127. Phillips MA, Lotharius J, Marsh K, White J, Dayan A, White KL, et al. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci Transl Med. 2015;7(296):296ra111. doi: 10.1126/scitranslmed.aaa6645 26180101
128. Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, et al. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12(10):e1001891. doi: 10.1371/journal.pmed.1001891 26505753
129. White NJ. Why do some primate malarias relapse? Trends Parasitol. 2016;32(12):918–20. doi: 10.1016/j.pt.2016.08.014 27743866
130. John GK, Douglas NM, von Seidlein L, Nosten F, Baird JK, White NJ, et al. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J. 2012;11 : 280. doi: 10.1186/1475-2875-11-280 22900786
131. Wells TN, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 2010;26(3):145–51. doi: 10.1016/j.pt.2009.12.005 20133198
132. March S, Ng S, Velmurugan S, Galstian A, Shan J, Logan DJ, et al. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe. 2013;14(1):104–15. doi: 10.1016/j.chom.2013.06.005 23870318
133. Vaughan AM, Kappe SH, Ploss A, Mikolajczak SA. Development of humanized mouse models to study human malaria parasite infection. Future Microbiol. 2012;7(5):657–65. doi: 10.2217/fmb.12.27 22568719
134. Cairns M, Roca-Feltrer A, Garske T, Wilson AL, Diallo D, Milligan PJ, et al. Estimating the potential public health impact of seasonal malaria chemoprevention in African children. Nat Commun. 2012;3 : 881. doi: 10.1038/ncomms1879 22673908
135. Zongo I, Milligan P, Compaore YD, Some AF, Greenwood B, Tarning J, et al. Randomized noninferiority trial of dihydroartemisinin-piperaquine compared with sulfadoxine-pyrimethamine plus amodiaquine for seasonal malaria chemoprevention in Burkina Faso. Antimicrob Agents Chemother. 2015;59(8):4387–96. doi: 10.1128/AAC.04923-14 25918149
136. Griffin JT, Bhatt S, Sinka ME, Gething PW, Lynch M, Patouillard E, et al. Potential for reduction of burden and local elimination of malaria by reducing Plasmodium falciparum malaria transmission: a mathematical modelling study. Lancet Infect Dis. 2016;16(4):465–72. doi: 10.1016/S1473-3099(15)00423-5 26809816
137. Chaccour CJ, Rabinovich NR, Slater H, Canavati SE, Bousema T, Lacerda M, et al. Establishment of the Ivermectin Research for Malaria Elimination Network: updating the research agenda. Malar J. 2015;14 : 243. doi: 10.1186/s12936-015-0691-6 26068560
138. Slater HC, Walker PG, Bousema T, Okell LC, Ghani AC. The potential impact of adding ivermectin to a mass treatment intervention to reduce malaria transmission: a modelling study. J Infect Dis. 2014;210(12):1972–80. doi: 10.1093/infdis/jiu351 24951826
139. Chaccour CJ, Kobylinski KC, Bassat Q, Bousema T, Drakeley C, Alonso P, et al. Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination. Malar J. 2013;12 : 153. doi: 10.1186/1475-2875-12-153 23647969
140. Bogers WM, Oostermeijer H, Mooij P, Koopman G, Verschoor EJ, Davis D, et al. Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J Infect Dis. 2015;211(6):947–55. doi: 10.1093/infdis/jiu522 25234719
141. Kotta S, Khan AW, Ansari SH, Sharma RK, Ali J. Anti HIV nanoemulsion formulation: optimization and in vitro-in vivo evaluation. Int J Pharm. 2014;462(1–2):129–34. doi: 10.1016/j.ijpharm.2013.12.038 24374067
142. Jain A, Thakur K, Kush P, Jain UK. Docetaxel loaded chitosan nanoparticles: formulation, characterization and cytotoxicity studies. Int J Biol Macromol. 2014;69 : 546–53. doi: 10.1016/j.ijbiomac.2014.06.029 24971551
143. Mishra P, SS R, Jerobin J, Thomas J, Mukherjee A, Chandrasekaran N. Study on antimicrobial potential of neem oil nanoemulsion against Pseudomonas aeruginosa infection in Labeo rohita. Biotechnol Appl Biochem. 2014;61(5):611–9. doi: 10.1002/bab.1213 24502533
144. Campo B, Vandal O, Wesche DL, Burrows JN. Killing the hypnozoite—drug discovery approaches to prevent relapse in Plasmodium vivax. Pathog Glob Health. 2015;109(3):107–22. doi: 10.1179/2047773215Y.0000000013 25891812
145. Mikolajczak SA, Vaughan AM, Kangwanrangsan N, Roobsoong W, Fishbaugher M, Yimamnuaychok N, et al. Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe. 2015;17(4):526–35. doi: 10.1016/j.chom.2015.02.011 25800544
146. Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, Nandy A, et al. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis. 2007;195(7):927–33. doi: 10.1086/512241 17330781
147. Chen N, Auliff A, Rieckmann K, Gatton M, Cheng Q. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J Infect Dis. 2007;195(7):934–41. doi: 10.1086/512242 17330782
148. Beck HP, Wampfler R, Carter N, Koh G, Osorio L, Rueangweerayut R, et al. Estimation of the antirelapse efficacy of tafenoquine, using Plasmodium vivax genotyping. J Infect Dis. 2016;213(5):794–9. doi: 10.1093/infdis/jiv508 26500351
149. Richie TL, Billingsley PF, Sim BK, James ER, Chakravarty S, Epstein JE, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33(52):7452–61. doi: 10.1016/j.vaccine.2015.09.096 26469720
150. Miura K. Progress and prospects for blood-stage malaria vaccines. Expert Rev Vaccines. 2016;15(6):765–81. doi: 10.1586/14760584.2016.1141680 26760062
151. Draper SJ, Angov E, Horii T, Miller LH, Srinivasan P, Theisen M, et al. Recent advances in recombinant protein-based malaria vaccines. Vaccine. 2015;33(52):7433–43. doi: 10.1016/j.vaccine.2015.09.093 26458807
152. World Health Organization. WHO Product Development for Vaccines Advisory Committee (PD-VAC) meeting – 2015 Geneva: WHO; 2015 [Available from: http://www.who.int/immunization/research/meetings_workshops/pdvac/en/.]
153. Nunes JK, Woods C, Carter T, Raphael T, Morin MJ, Diallo D, et al. Development of a transmission-blocking malaria vaccine: progress, challenges, and the path forward. Vaccine. 2014;32(43):5531–9. doi: 10.1016/j.vaccine.2014.07.030 25077422
154. Delrieu I, Leboulleux D, Ivinson K, Gessner BD, Malaria Transmission Blocking Vaccine Technical Consultation G. Design of a Phase III cluster randomized trial to assess the efficacy and safety of a malaria transmission blocking vaccine. Vaccine. 2015;33(13):1518–26. doi: 10.1016/j.vaccine.2015.01.050 25681064
155. Shimp RL Jr., Rowe C, Reiter K, Chen B, Nguyen V, Aebig J, et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine. 2013;31(28):2954–62. doi: 10.1016/j.vaccine.2013.04.034 23623858
156. Li Y, Leneghan DB, Miura K, Nikolaeva D, Brian IJ, Dicks MD, et al. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci Rep. 2016;6 : 18848. doi: 10.1038/srep18848 26743316
157. Bennett JW, Yadava A, Tosh D, Sattabongkot J, Komisar J, Ware LA, et al. Phase 1/2a Trial of Plasmodium vivax malaria vaccine candidate VMP001/AS01B in malaria-naive adults: safety, immunogenicity, and efficacy. PLoS Negl Trop Dis. 2016;10(2):e0004423. doi: 10.1371/journal.pntd.0004423 26919472
158. Herrera S, Fernandez OL, Vera O, Cardenas W, Ramirez O, Palacios R, et al. Phase I safety and immunogenicity trial of Plasmodium vivax CS derived long synthetic peptides adjuvanted with montanide ISA 720 or montanide ISA 51. Am J Trop Med Hyg. 2011;84(2 Suppl):12–20.
159. Bauza K, Malinauskas T, Pfander C, Anar B, Jones EY, Billker O, et al. Efficacy of a Plasmodium vivax malaria vaccine using ChAd63 and modified vaccinia Ankara expressing thrombospondin-related anonymous protein as assessed with transgenic Plasmodium berghei parasites. Infect Immun. 2014;82(3):1277–86. doi: 10.1128/IAI.01187-13 24379295
160. de Cassan SC, Shakri AR, Llewellyn D, Elias SC, Cho JS, Goodman AL, et al. Preclinical assessment of viral vectored and protein vaccines targeting the duffy-binding protein region ii of Plasmodium vivax. Front Immunol. 2015;6 : 348. doi: 10.3389/fimmu.2015.00348 26217340
161. McCarthy JS, Griffin PM, Sekuloski S, Bright AT, Rockett R, Looke D, et al. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis. 2013;208(10):1688–94. doi: 10.1093/infdis/jit394 23908484
162. Rutledge G, Boehme U, Sanders M, Reid A, Maiga-Ascofare O, Djimde A, et al. Elusive Plasmodium species complete the human malaria genome set bioRxiv: Cold Spring Harbor Laboratory; 2016 [Available from: http://biorxiv.org/content/early/2016/05/12/052696.full.pdf+html]
163. Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15(2):51–7. doi: 10.4110/in.2015.15.2.51 25922593
164. Kelley B. Industrialization of mAb production technology: the bioprocessing industry at a crossroads. MAbs. 2009;1(5):443–52. 20065641
165. Centre for Biosecurity of UPMC. Next generation monoclonal antibodies: challenges and opportunities Baltimore, MD: Centre for Biosecurity of UPMC; 2013 [Available from: http://www.upmchealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2013/2013-02-04-next-gen-monoclonal-antibodies.pdf]
166. Robbie GJ, Criste R, Dall'acqua WF, Jensen K, Patel NK, Losonsky GA, et al. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother. 2013;57(12):6147–53. doi: 10.1128/AAC.01285-13 24080653
167. Rasmussen SK, Naested H, Muller C, Tolstrup AB, Frandsen TP. Recombinant antibody mixtures: production strategies and cost considerations. Arch Biochem Biophys. 2012;526(2):139–45. doi: 10.1016/j.abb.2012.07.001 22820097
168. Brock PM, Fornace KM, Parmiter M, Cox J, Drakeley CJ, Ferguson HM, et al. Plasmodium knowlesi transmission: integrating quantitative approaches from epidemiology and ecology to understand malaria as a zoonosis. Parasitology. 2016;143(4):389–400. doi: 10.1017/S0031182015001821 26817785
169. Li S, Plebanski M, Smooker P, Gowans EJ. Editorial: Why vaccines to HIV, HCV, and malaria have so far failed-challenges to developing vaccines against immunoregulating pathogens. Front Microbiol. 2015;6 : 1318. doi: 10.3389/fmicb.2015.01318 26640461
170. Neafsey DE, Juraska M, Bedford T, Benkeser D, Valim C, Griggs A, et al. Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N Engl J Med. 2015;373(21):2025–37. doi: 10.1056/NEJMoa1505819 26488565
171. Plowe CV. Vaccine-Resistant Malaria. N Engl J Med. 2015;373(21):2082–3. doi: 10.1056/NEJMe1511955 26488465
172. Takala SL, Plowe CV. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming 'vaccine resistant malaria'. Parasite Immunol. 2009;31(9):560–73. doi: 10.1111/j.1365-3024.2009.01138.x 19691559
173. Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, et al. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N Engl J Med. 2016;374(26):2519–29. doi: 10.1056/NEJMoa1515257 27355532
174. Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–11. doi: 10.1038/nature15535 26375008
175. World Health Organization. Insecticide resistance Geneva: WHO; 2015 [Available from: http://www.who.int/malaria/areas/vector_control/insecticide_resistance/en/.]
176. Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet. 2016;387(10029):1785–8. doi: 10.1016/S0140-6736(15)00417-1 26880124
177. World Health Organization Pesticide Evaluation Scheme. WHO recommended long-lasting insecticidal nets 2016 [Available from: http://www.who.int/whopes/Long-lasting_insecticidal_nets_April_2016.pdf?ua=1]
178. World Health Organization Pesticide Evaluation Scheme. Pesticide products under WHOPES laboratory and or field testing and evaluation Geneva: WHOPES; 2016 [Available from: http://www.who.int/whopes/Products_Under_WHOPES_Evaluation_March_2016.pdf?ua=1]
179. N'Guessan R, Asidi A, Boko P, Odjo A, Akogbeto M, Pigeon O, et al. An experimental hut evaluation of PermaNet((R)) 3.0, a deltamethrin-piperonyl butoxide combination net, against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in southern Benin. Trans R Soc Trop Med Hyg. 2010;104(12):758–65. doi: 10.1016/j.trstmh.2010.08.008 20956008
180. Pennetier C, Bouraima A, Chandre F, Piameu M, Etang J, Rossignol M, et al. Efficacy of Olyset(R) Plus, a new long-lasting insecticidal net incorporating permethrin and piperonyl-butoxide against multi-resistant malaria vectors [corrected]. PLoS ONE. 2013;8(10):e75134. doi: 10.1371/journal.pone.0075134 24116029
181. Tungu P, Magesa S, Maxwell C, Malima R, Masue D, Sudi W, et al. Evaluation of PermaNet 3.0 a deltamethrin-PBO combination net against Anopheles gambiae and pyrethroid resistant Culex quinquefasciatus mosquitoes: an experimental hut trial in Tanzania. Malar J. 2010;9 : 21. doi: 10.1186/1475-2875-9-21 20085631
182. Morel CM, Thang ND, Erhart A, Xa NX, Peeters Grietens K, Xuan Hung L, et al. Cost-effectiveness of long-lasting insecticide-treated hammocks in preventing malaria in South-central Vietnam. PLoS ONE. 2013;8(3):e58205. doi: 10.1371/journal.pone.0058205 23536790
183. NgenIRS. 2016 [Available from: http://www.ngenirs.org/.]
184. Knapp J, Macdonald M, Malone D, Hamon N, Richardson JH. Disruptive technology for vector control: the Innovative Vector Control Consortium and the US Military join forces to explore transformative insecticide application technology for mosquito control programmes. Malar J. 2015;14 : 371. doi: 10.1186/s12936-015-0907-9 26409879
185. Sibanda M, Focke W. Development of an insecticide impregnated polymer wall lining for malaria vector control. Malar J. 2014;13 (Suppl 1):80.
186. Kruger T, Sibanda MM, Focke WW, Bornman MS, de Jager C. Acceptability and effectiveness of a monofilament, polyethylene insecticide-treated wall lining for malaria control after six months in dwellings in Vhembe District, Limpopo Province, South Africa. Malar J. 2015;14 : 485. doi: 10.1186/s12936-015-1005-8 26628275
187. Andriessen R, Snetselaar J, Suer RA, Osinga AJ, Deschietere J, Lyimo IN, et al. Electrostatic coating enhances bioavailability of insecticides and breaks pyrethroid resistance in mosquitoes. Proc Natl Acad Sci U S A. 2015;112(39):12081–6. doi: 10.1073/pnas.1510801112 26324912
188. Briet OJ, Chitnis N. Effects of changing mosquito host searching behaviour on the cost effectiveness of a mass distribution of long-lasting, insecticidal nets: a modelling study. Malar J. 2013;12 : 215. doi: 10.1186/1475-2875-12-215 23802594
189. Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HC, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67(4):1218–30. doi: 10.1111/evo.12063 23550770
190. Govella NJ, Chaki PP, Killeen GF. Entomological surveillance of behavioural resilience and resistance in residual malaria vector populations. Malar J. 2013;12 : 124. doi: 10.1186/1475-2875-12-124 23577656
191. Killeen GF, Chitnis N. Potential causes and consequences of behavioural resilience and resistance in malaria vector populations: a mathematical modelling analysis. Malar J. 2014;13 : 97. doi: 10.1186/1475-2875-13-97 24629066
192. Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12 : 56. doi: 10.1186/1475-2875-12-56 23388506
193. Sokhna C, Ndiath MO, Rogier C. The changes in mosquito vector behaviour and the emerging resistance to insecticides will challenge the decline of malaria. Clin Microbiol Infect. 2013;19(10):902–7. doi: 10.1111/1469-0691.12314 23910459
194. Sougoufara S, Diedhiou SM, Doucoure S, Diagne N, Sembene PM, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13 : 125. doi: 10.1186/1475-2875-13-125 24678587
195. Diabate A, Tripet F. Targeting male mosquito mating behaviour for malaria control. Parasit Vectors. 2015;8 : 347. doi: 10.1186/s13071-015-0961-8 26113015
196. Donnelly B, Berrang-Ford L, Ross NA, Michel P. A systematic, realist review of zooprophylaxis for malaria control. Malar J. 2015;14 : 313. doi: 10.1186/s12936-015-0822-0 26264913
197. Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches Anopheles mosquitoes. In: Manguin S, editor. Anopheles mosquitoes, new insights into malaria vectors. Rijeka: InTech; 2013. p. 671–704.
198. Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13 : 330. doi: 10.1186/1475-2875-13-330 25149656
199. Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10 : 184. doi: 10.1186/1475-2875-10-184 21736750
200. Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10 : 80. doi: 10.1186/1475-2875-10-80 21477321
201. Derua YA, Alifrangis M, Hosea KM, Meyrowitsch DW, Magesa SM, Pedersen EM, et al. Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malar J. 2012;11 : 188. doi: 10.1186/1475-2875-11-188 22681999
202. Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors. 2013;6 : 114. doi: 10.1186/1756-3305-6-114 23601146
203. Revay EE, Schlein Y, Tsabari O, Kravchenko V, Qualls W, De-Xue R, et al. Formulation of attractive toxic sugar bait (ATSB) with safe EPA-exempt substance significantly diminishes the Anopheles sergentii population in a desert oasis. Acta Trop. 2015;150 : 29–34. doi: 10.1016/j.actatropica.2015.06.018 26119042
204. Marshall JM, White MT, Ghani AC, Schlein Y, Muller GC, Beier JC. Quantifying the mosquito's sweet tooth: modelling the effectiveness of attractive toxic sugar baits (ATSB) for malaria vector control. Malar J. 2013;12 : 291. doi: 10.1186/1475-2875-12-291 23968494
205. Johnson BJ, Ritchie SA. The siren's song: Exploitation of female flight tones to passively capture male Aedes aegypti (Diptera: Culicidae). J Med Entomol. 2016;53(1):245–8. doi: 10.1093/jme/tjv165 26502754
206. Downes J. The swarming and mating flight of Diptera. Ann Rev Entomol. 1969;14 : 271–98.
207. World Health Organization. Guidelines for efficacy testing of spatial repellents: WHO; 2013 [Available from: http://apps.who.int/iris/bitstream/10665/78142/1/9789241505024_eng.pdf?ua=1]
208. Syafruddin D, Bangs MJ, Sidik D, Elyazar I, Asih PB, Chan K, et al. Impact of a spatial repellent on malaria incidence in two villages in Sumba, Indonesia. Am J Trop Med Hyg. 2014;91(6):1079–87. doi: 10.4269/ajtmh.13-0735 25311699
209. Hill N, Zhou HN, Wang P, Guo X, Carneiro I, Moore SJ. A household randomized, controlled trial of the efficacy of 0.03% transfluthrin coils alone and in combination with long-lasting insecticidal nets on the incidence of Plasmodium falciparum and Plasmodium vivax malaria in Western Yunnan Province, China. Malar J. 2014;13 : 208. doi: 10.1186/1475-2875-13-208 24885993
210. Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling RD, et al. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malar J. 2015;14 : 209. doi: 10.1186/s12936-015-0724-1 26055986
211. Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database Syst Rev. 2013;8:CD008923.
212. Committee on Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct. Gene drives on the horizon: advancing science, navigating uncertainty and aligning research with public values Washington DC: The National Academies Press; 2016 [Available from: http://www.nap.edu/catalog/23405/gene-drives-on-the-horizon-advancing-science-navigating-uncertainty-and]
213. Burt A. Heritable strategies for controlling insect vectors of disease. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130432. doi: 10.1098/rstb.2013.0432 24821918
214. Oliva CF, Vreysen MJ, Dupe S, Lees RS, Gilles JR, Gouagna LC, et al. Current status and future challenges for controlling malaria with the sterile insect technique: technical and social perspectives. Acta Trop. 2014;132 Suppl:S130–9. doi: 10.1016/j.actatropica.2013.11.019 24295892
215. Bourtzis K, Lees RS, Hendrichs J, Vreysen MJ. More than one rabbit out of the hat: Radiation, transgenic and symbiont-based approaches for sustainable management of mosquito and tsetse fly populations. Acta Trop. 2016;157 : 115–30. doi: 10.1016/j.actatropica.2016.01.009 26774684
216. Black WCt, Alphey L, James AA. Why RIDL is not SIT. Trends Parasitol. 2011;27(8):362–70. doi: 10.1016/j.pt.2011.04.004 21659002
217. Windbichler N, Menichelli M, Papathanos PA, Thyme SB, Li H, Ulge UY, et al. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature. 2011;473(7346):212–5. doi: 10.1038/nature09937 21508956
218. Esvelt KM, Smidler AL, Catteruccia F, Church GM. Concerning RNA-guided gene drives for the alteration of wild populations. Elife. 2014:e03401. doi: 10.7554/eLife.03401 25035423
219. Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol. 2016;34(1):78–83. doi: 10.1038/nbt.3439 26641531
220. Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci U S A. 2015;112(49):E6736–43. doi: 10.1073/pnas.1521077112 26598698
221. Wilke AB, Marrelli MT. Paratransgenesis: a promising new strategy for mosquito vector control. Parasit Vectors. 2015;8 : 342. doi: 10.1186/s13071-015-0959-2 26104575
222. Wang S, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc Natl Acad Sci U S A. 2012;109(31):12734–9. doi: 10.1073/pnas.1204158109 22802646
223. Dao A, Yaro AS, Diallo M, Timbine S, Huestis DL, Kassogue Y, et al. Signatures of aestivation and migration in Sahelian malaria mosquito populations. Nature. 2014;516(7531):387–90. doi: 10.1038/nature13987 25470038
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



