-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
Timothy Henrich and colleagues study the effect of very early antiretroviral treatment on the reservoir of HIV-infected cells in two patients.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002417
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002417Summary
Timothy Henrich and colleagues study the effect of very early antiretroviral treatment on the reservoir of HIV-infected cells in two patients.
Introduction
The development of a cure for HIV infection is a major public health objective [1]. Despite the ability of antiretroviral therapy (ART) to significantly reduce disease-related morbidity and mortality in HIV-1 infection, viral reservoirs persist indefinitely in latently infected cells [2]. HIV persists during ART primarily within circulating and tissue-resident, long-lived memory CD4+ T cells that harbor integrated HIV DNA; these cells are not cleared with ART and are a source of viral rebound when treatment is discontinued [3]. A major HIV eradication strategy involves aborting the initial seeding of these long-lived reservoirs by the very early initiation of ART [4,5]. For example, initiation of ART in a perinatally infected infant at 31 hours of life led to significant reductions in the viral reservoir and a significant time off ART (>2 years) before eventual viral recrudescence [6,7]. However, the impact of extremely early ART on HIV persistence and seeding the viral reservoir with the potential to prevent establishment of lifelong infection in adults is unknown.
Antiretroviral drugs initiated before HIV exposure (pre-exposure prophylaxis [PrEP]) can be an effective method of preventing HIV acquisition [8–11]. PrEP programs involve HIV antibody testing before the initiation of prophylactic ART in individuals at high risk for acquiring HIV. PrEP is typically started following negative HIV antibody or combined antibody/antigen screening in the absence of clinical symptoms [8,11]. Because there is a delay between HIV infection and when an HIV antibody or combined antibody/antigen test is reactive, PrEP may be unknowingly started in an individual who has very recently been infected with HIV. As a result, a small number of individuals may begin 2-drug ART just prior to or after the development of detectable plasma HIV-1 RNA (the transition from the "eclipse phase" to Fiebig stage I of infection) and prior to the detection of HIV antigen or antibody [12,13]. PrEP programs are therefore ideal settings in which to identify individuals treated extremely early during infection for the longitudinal study of HIV-1 reservoir persistence in blood and various tissues [4,5,14,15].
The aims of this study were to determine the impact of extremely early initiation of ART on the size of the HIV reservoir in blood and various tissues and the potential for long-term ART-free remission. As a result, we studied 2 PrEP study participants who initiated ART during emergent, unrecognized HIV infection in Fiebig stage I, with 1 individual treated just as he was transitioning out of the “eclipse phase” of HIV infection [12,13]. We describe the results of extensive tissue and blood sampling in these individuals and the result of a highly monitored treatment interruption. Using this case and our previously described recipient of an allogeneic bone marrow transplant (hematopoietic stem cell transplantation [HSCT] Participant B) [16,17], we also describe potential biomarkers for HIV reactivation during prolonged states of viremia post-ART interruption.
Methods and materials
Study design and population
The PrEP Demo Project was a prospective study of PrEP for men who have sex with men (MSM) in which participants were tested for HIV both by HIV antibody/antigen combination assay and by HIV RNA on the day of PrEP initiation [18]. There was no prespecified plan for the present analysis at that time. Two participants were identified in the study who had positive viral load tests performed on the day of initiation of PrEP (truvada/emtricitabine). PrEP was converted to standard ART once HIV infection was confirmed. The participants were enrolled in the longitudinal University of California San Francisco (UCSF) SCOPE study after providing written informed consent. The study was approved by the UCSF Committee on Human Research. A protocol and an analysis plan were in place prior to analytical treatment interruption (ATI). The protocol and STROBE checklist are included in the supporting information (S1 Protocol and S1 STROBE Checklist).
To more fully explore biomarkers associated with HIV reactivation after the interruption of ART, we also accessed peripheral blood mononuclear cells (PBMCs) from a previously described case of an HIV-infected adult who underwent an allogenic hematopoietic stem-cell transplantation (HSCT Participant B) [16]. All HIV reservoir studies for HSCT Participant B during ATI were conducted at commercial laboratories and cryopreserved PBMCs were not available.
Tissue collection, apheresis, and sample processing
PBMCs and plasma were collected longitudinally either by large-volume peripheral blood draws or by leukapheresis and purified by Ficoll-Hypaque (Sigma-Aldrich) density gradient centrifugation. Colorectal and ileal tissue were collected and processed as previously described [19,20]. A whole, inguinal lymph node was obtained from each participant by surgical excision for mononuclear cell isolation and downstream HIV reservoir characterization months following combination ART initiation. Total PBMCs, purified CD4+ T cells, or CD4+ T cell subsets [naive (TN), central memory (TCM), transitional memory (TTM), and effector memory (TEM)] from blood and tissues were collected via bead-based separation (Stem Cell Technologies) or by fluorescence-activated cell sorting using previously described methods [21]. Bone marrow biopsies were performed, followed by sorting and collection of 4 cell populations: myeloid cells (CD33+Lin+/−), CD3+CD4+ T cells (CD33−Lin+CD4+), multilineage CD4+ cells (CD33−Lin−CD4dim), and Lin − cells that are not CD4+ or myeloid cells (CD33−Lin−CD4−CD34+/−). Cerebrospinal fluid (CSF) was collected by lumbar puncture and centrifuged to separate the liquid and cellular fraction for downstream HIV-1 detection, and quantification was carried out as previously described [22,23].
HIV testing and viral reservoir characterization
HIV-1 RNA was isolated from baseline (pre-PrEP) and subsequent plasma samples followed by single-genome sequencing (SGS) of HIV-1 protease-pol (pro-pol) or a 1.5 kb portion of the envelope gene as previously described [24,25]. Population sequencing on subsequent timepoints was performed using TRUGENE (Siemens, Tarrytown NY). PBMCs and tissue-derived cells were analyzed for HIV-1 persistence using a variety of highly sensitive assays in up to 10 independent laboratories located at the UCSF, University of Montreal, University of Pittsburgh, University of California San Diego (UCSD), and Johns Hopkins University. Assays utilized were previously described and included highly sensitive quantitative PCR for total and integrated HIV-1 DNA (unspliced and total RNA) [26,27], droplet digital PCR (ddPCR) (HIV pol DNA and 2-LTR circles) [28], total virus recovery assay, quantitative viral outgrowth assay (qVOA) [29,30], Tat/Rev Induced Limiting Dilution Assay (TILDA) [31], and whole HIV genome sequencing [32]. In addition, large volume plasma was tested using the MEGA iSCA [33].
Murine viral outgrowth assay
CD4+ T cells obtained by leukapheresis from both participants approximately 18 months following the initiation of ART were tested using the murine (humanized mouse) viral outgrowth assay (mVOA) as previously described [34]. Briefly, 530 million and 379 million CD4+ T cells were divided and injected intraperitoneally into 8–10 humanized female young adult NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice from Jackson Labs (53 million and 50 million cells per mouse from the 2 participants). Plasma HIV-1 RNA testing was then performed following up to 5.5 weeks after engraftment on blood obtained by weekly mandibular sinus bleed not exceeding 0.5% of body weight. Mice were euthanized by CO2 inhalation and HIV-1 sequencing was attempted from plasma and spleen cells using cDNA and methods optimized for low HIV-1 RNA copy numbers [34]. The Johns Hopkins University Institutional Animal Care and Use Committee approved this research and it was conducted in accordance with the 8th edition of the Guide for the Care and Use of Laboratory Animals within fully AAALACi accredited animal facilities. Mice were group-housed with other mice xenografted from the same donor in microisolator caging (Allentown) and fed a commercial rodent chow (Harlan) and hyperchlorinated water ad libitum.
ATI
A carefully monitored ATI was performed on 1 participant (PrEP Participant A) who initiated PrEP an estimated 10 days following HIV infection and had no definitive HIV detected in blood or tissues for 32 months on ART. After ATI, viral load testing was performed twice weekly using the COBAS AmpliPrep/COBAS TaqMan V.2 assay (Roche) for the first 3 months, followed by weekly testing thereafter with close clinical observation. Large-volume blood draws were also obtained monthly for the isolation of PBMCs, and plasma was obtained for further reservoir and flow cytometric testing. ART was initiated immediately after confirmation of detectable plasma HIV-1 RNA.
Flow cytometric analysis of lymphocyte phenotypes and markers of activation, exhaustion, and proliferation
Flow cytometric testing was performed on cryopreserved PBMCs isolated longitudinally just before, during, and after ATI for PrEP Participant A and HSCT Participant B. Thawed PBMCs were stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (ThermoFisher Scientific), Brilliant Violet 711-conjugated anti-CD4 (SK3) (BD Biosciences), Brilliant Violet 650-conjugated anti-CD3 (SP34-2) (BD Biosciences), allophycocyanin (APC)-conjugated anti-CD38 (HB7) (BD Bioscience), PE-conjugated anti-CD30 (BERH8) (BD Biosciences), APC-H7-conjugated anti-HLA-DR (L243) (BD Bioscience), CD8 Alexa Fluor 700-conjugated anti-CD8 (RPA-T8) (BD Bioscience), BV786-conjugated anti-CD16 (3G8) (BD Bioscience), PE-Cy7-conjugated anti-CD107a (H4A3) (BD Bioscience), FITC-conjugated anti-CD56 (NCAM 16.2) (BD Bioscience), and PerCP-conjugated anti-CD69 (L78) (BD Bioscience). Cells were then analyzed on a BD LSRII flow cytometer (BD Biosciences). Single stained beads (Life Technologies) were used for compensation, and fluorescence minus one (FMO) controls were used to set gates. Data for phenotyping were acquired on all events and analyzed in FlowJo V10 (TreeStar). Examples of gating strategies are shown in S1 Fig.
CD4+ and CD8+ T cell phenotypes were further characterized on live cells for PrEP Participant B by flow cytometry after exclusion of dead cells (Fixable Aqua dye, Molecular Probes) using the following fluorescently labeled antibodies: CD3 BV650 clone SK7, CD4 BV711 clone OKT4, CCR7 BV785 clone G043H7, CD45RA APC-Cy7 clone H100, Tbet PE clone 4B10, Eomesodermin PE-e610, PD-1 BV421 or BV605 clone EH12.2H7, CD160 PE-Cy7 clone BY55, Ki-67 FITC clone Ki-67, Granzyme B FITC clone GB11, Perforin PE-Cy7 clone B-D48 (all Biolegend), and CD8α APC-R700 clone RPA-T8 (BD Biosciences). Positive gates for activation markers and effector cell transcription factors were drawn based on expression of these markers in peripheral blood naive CD8+ T cells isolated from an HIV-uninfected donor. To limit experimental variability, flow cytometry was performed on the same day.
Flow cytometric analysis of HIV-1–specific immune responses by intracellular cytokine staining
After thawing, PBMCs were incubated at 37°C overnight at a concentration of 2 × 106 cells/mL in RPMI medium containing 10% FBS and penicillin/streptomycin. The next day, 1 × 106 cells were stimulated with Gag pooled peptides (final concentration 1 μg/mL in DMSO provided by the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 Con B Gag Peptide Pool cat #12425), for 6 hours in the presence of brefeldin A and monensin (BD). The percentage of CD8+ or CD4+ T cells producing interferon gamma (PE-dazzle, clone 4S.B3, Biolegend), tumor necrosis factor alpha (Alexa fluor 700, clone mAb11, eBioscience) or interleukin-2 (BV785, clone MQ1-17H12, Biolegend), or degranulation as detected by CD107a expression (APC, clone H4A3, Biolegend), was measured by flow cytometry, and the frequency of positive cells was determined after subtraction of the frequency measured in wells incubated with DMSO alone. Examples of gating strategies are shown in S2 Fig.
Data analysis and mathematical modeling
Graphical analyses were performed using Prism v.6 (GraphPad software). Mathematical modeling of reservoir size, rebound rate, and probability of cure were performed using our previously described methods [35]. A summary of these methods and the input variables used is provided in S1 Text.
Results
Reduced HIV reservoir seeding following very early initiation of ART
Participant A, a 54-year-old male, was HIV-uninfected at 2 PrEP pre-enrollment visits but continued to have ongoing sexual risk for HIV infection. He then initiated tenofovir/emtricitabine PrEP and usage was confirmed by testing drug levels. Seven days after PrEP initiation, results from the baseline (day 0 of PrEP) visit revealed a plasma HIV RNA level of 220 copies/mL (Abbott RealTime HIV-1, lower limit of detection [LLD] <40 copies/mL), and 69 copies/mL by the Single-Copy Assay (LLD 1 copy/mL); 4th generation EIA (Abbott) and rapid HIV-antibody (Clearview HIV 1/2 Stat-Pak) tests were negative. Based upon these results (and a negative pooled HIV RNA, 4th generation EIA, and rapid HIV-antibody tests at 2 pre-enrollment visits 21 and 13 days prior), it was determined that he had been in the transition from the eclipse phase to Fiebig stage I HIV infection (estimated 10 days after HIV infection [12,13]) at the time of initiating PrEP. PrEP was substituted with a 4-drug ART regimen (darunavir/ritonavir/raltegravir/tenofovir/emtricitabine) 7 days after the initiation of PrEP. This ART regimen was chosen due to concern about the potential for the development of drug resistance to tenofovir/emtricitabine. The participant was asymptomatic at the time. HIV western blot assays (BioRad) were repeatedly indeterminate (p55 only) and became nonreactive at an estimated 130 days after time of infection. SGS from the plasma sample from the date when PrEP was initiated confirmed that the individual was infected with subtype B virus without known drug resistance mutations.
Plasma HIV RNA levels subsequently declined following PrEP and combination ART initiation: 220 copies/mL (PrEP baseline), 120 copies/mL (7 days after initiation of PrEP), and <40 copies/mL but detectable (estimated 22 days after starting PrEP). All subsequent plasma HIV RNA levels were undetectable. Low-level cell-associated HIV RNA (3.2 copies/million CD4+ T cells) was detected 32 days after infection. However, sorted rectal CD4+ T cells were negative for HIV RNA and DNA (collected 1.9 months after infection), and leukapheresis-collected PBMCs enriched for total CD4+ T cells and sorted CD4+ T cell subsets (TN, TCM, TTM, and TEM) were negative for cellular HIV RNA, total HIV DNA (confirmed in 2 independent laboratories), integrated HIV DNA, and 2-LTR circles (collected 2.1 months after infection). Over the following 2 years, no further HIV (nucleic acid, viral outgrowth, total virus recovery, or whole genome sequences) could be detected, despite sampling from ileum, rectum, lymph nodes, bone marrow, CSF, and circulating CD4+ T cell subsets. A detailed timeline of clinical viral load measurements and results of longitudinal HIV-1 reservoir assays are shown in Fig 1 and Table 1. Chemokine receptor 5 (CCR5) genotyping revealed that the individual did not carry any CCR5 Δ32 mutations, and HLA typing revealed that he was HLAB5701 negative. We estimated that greater than 1 billion CD4+ T cells were eventually examined without any evidence of HIV infection.
Fig. 1. Extremely early ART reduces HIV reservoir seeding. 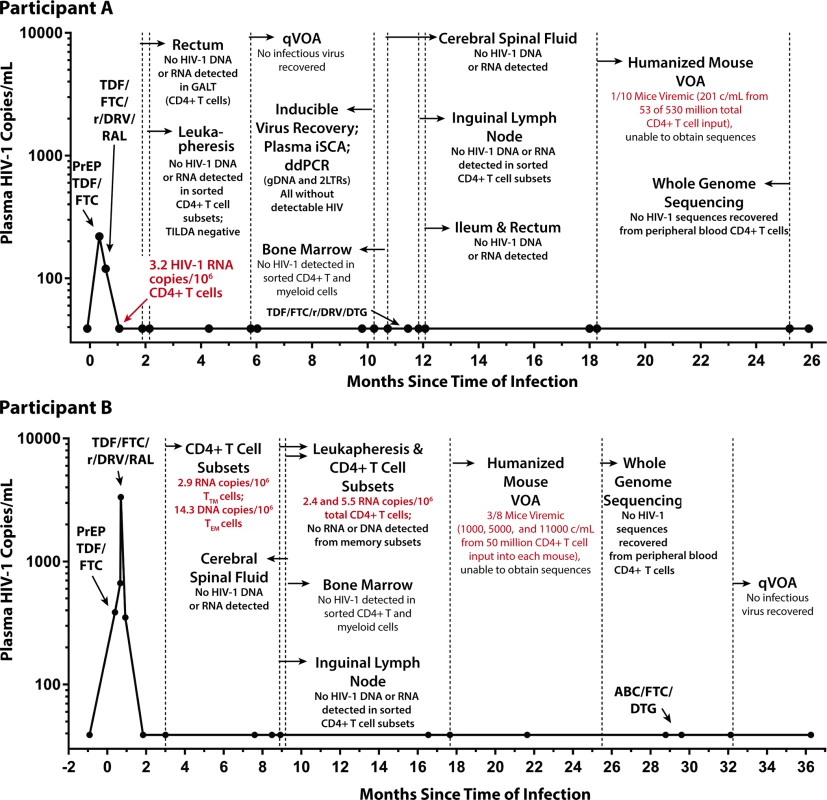
No HIV was detected in colorectal and lymph node tissues, bone marrow, CSF, plasma, and very large numbers of PBMCs obtained longitudinally from Participant A (top panel) who started PrEP 10 days following HIV infection. In contrast, low-level HIV RNA and DNA were intermittently detected in PBMCs from Participant B (bottom panel) who initiated PrEP 12 days following infection prior to starting a 4-drug ART regimen. One of 10 humanized mice given 53 million input cells from Participant A developed low-level HIV RNA in plasma (201 copies/mL), but sequencing of HIV from plasma was unsuccessful. Abbreviations: ABC, abacavir; CSF, cerebral spinal fluid; ddPCR, droplet digital PCR; DTG, dolutegravir; FTC, emtricitabine; GALT, gut-associated lymphoid tissue; iSCA, HIV integrase single copy plasma RNA assay; PBMC, peripheral blood mononuclear cell; PrEP, pre-exposure prophylaxis; qVOA, quantitative viral outgrowth assay; RAL, raltegravir; r/DRV, ritonavir-boosted darunavir; TDF, tenofovir; TILDA, Tat/Rev Induced Limiting Dilution Assay; VOA, viral outgrowth assay. Tab. 1. HIV-1 assay results from Participant A longitudinal pre-ATI blood and tissue sampling. 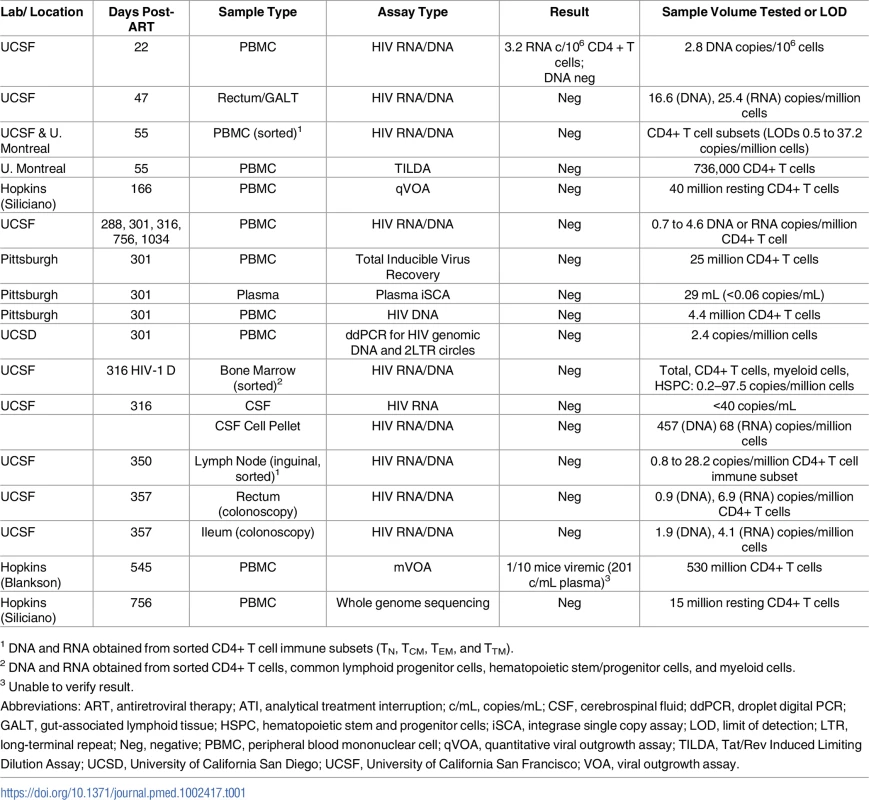
1 DNA and RNA obtained from sorted CD4+ T cell immune subsets (TN, TCM, TEM, and TTM). PrEP Participant B, a 31-year-old male, was HIV-uninfected at a pre-enrollment visit 6 weeks before initiating tenofovir/emtricitabine PrEP but had ongoing sexual risk for HIV infection. On the date of initiation of PrEP his Clearview HIV 1/2 Stat-Pak, HIV-1 CMIA (Abbott Ag/Ab combo assay), HIV-1 IFA, western blot, and HIV 1/2 MultiSpot Rapid Test were all non-reactive. Six days after initiation of PrEP, results from the baseline (day 0 of PrEP) visit revealed a plasma HIV of 359 copies/mL and PrEP was discontinued. Based on these results and clinical information, it was determined that he was infected approximately 12 days prior to starting PrEP (Fiebig stage I). Eight days after starting PrEP, the plasma HIV-1 RNA level increased to 668 copies/mL. HIV genotyping was positive for the M184M/I resistance mutation (no mutation was detected by genotyping on PrEP day 0), and 3,343 copies/mL were measured 9 days after the initiation of PrEP. He started combination ART (darunavir/ritonavir/raltegravir/tenofovir/emtricitabine) 12 days after starting PrEP, with subsequent decline of HIV plasma RNA to 351 copies/mL and 39 copies/mL on post-PrEP days 16 and 44, respectively. All subsequent plasma HIV RNA levels were undetectable starting at post-infection day 91. He changed ART to tenofovir/emtricitabine/dolutegravir/rilpiverine 56 days after starting PrEP and then to abacavir/lamivudine/dolutegravir 29 months following initial PrEP (Fig 1). HIV antibody testing remained negative through day 219 after the initiation of PrEP, but a western blot was indeterminate (p24 weekly positive, all other bands negative) on day 258.
Three months following infection, Participant B had very low level detectable HIV-1 RNA (2.9 copies/106 cell) and DNA (14.3 copies/106 cells) in TTM and TEM CD4+ T cell subsets, respectively, and again 2.4 and 5.5 HIV RNA copies/106 total CD4+ T cells approximately 9 months after infection. No HIV could be detected from CSF, bone marrow cells or CD4+ T cell subsets from a whole excised inguinal lymph node 8–9 months following infection. No definitive HIV outgrowth could be detected with a qVOA performed 32 months after infection utilizing 20 million input total CD4+ T cells. Very low levels of HIV-1 RNA (<50 copies/mL) were detected from several of the viral outgrowth assay (VOA) wells, but no increases were observed in RNA over time, and no bulk or single genome HIV-1 sequences could be obtained (see Fig 1 and Table 2).
Tab. 2. HIV-1 assay results from PrEP Participant B longitudinal blood and tissue sampling. 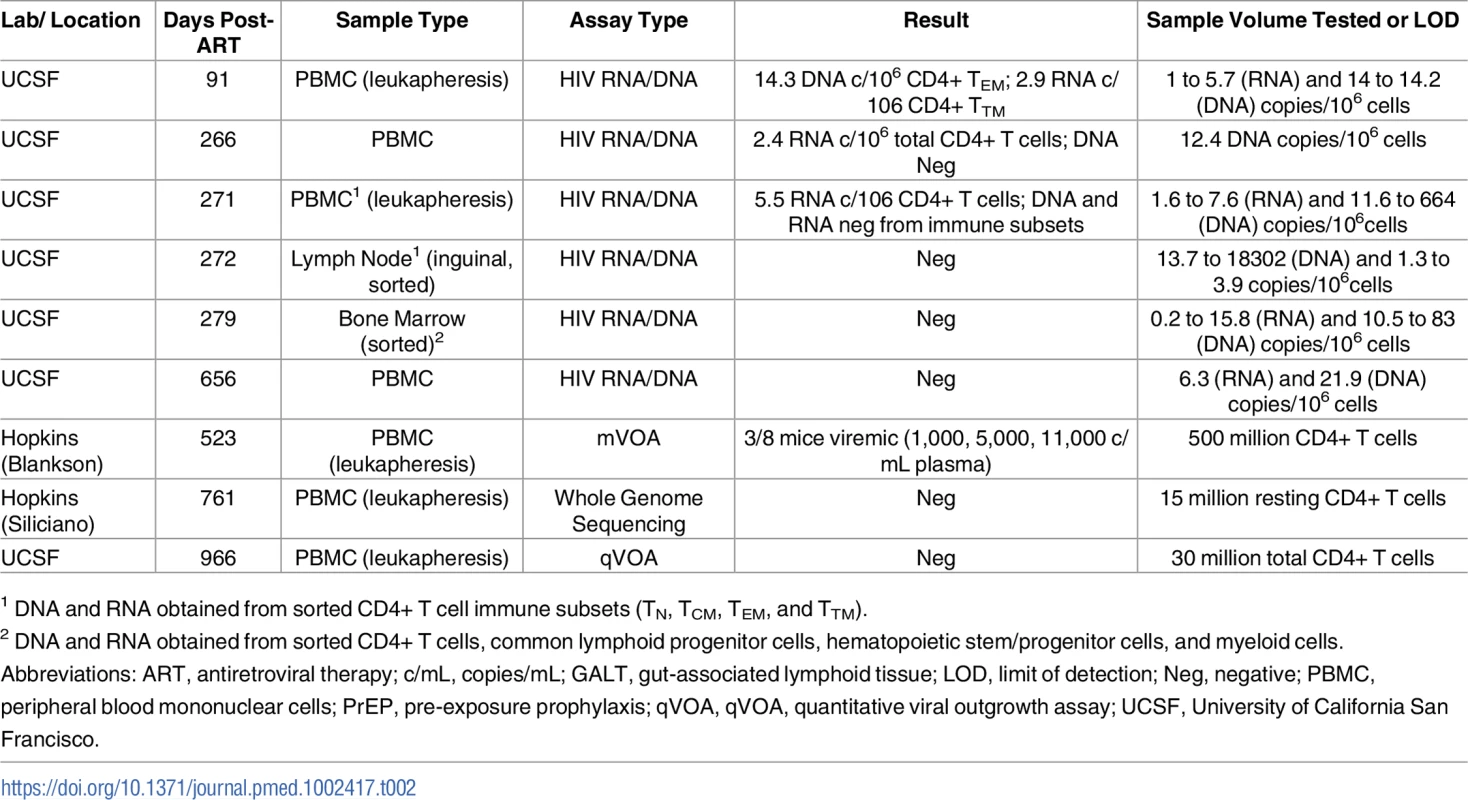
1 DNA and RNA obtained from sorted CD4+ T cell immune subsets (TN, TCM, TEM, and TTM). mVOA
Given the very low level or lack of detectable HIV-1 from large numbers of cells from various tissues, large quantity plasma, or CSF, we performed a leukapheresis on both participants and used large numbers of CD4+ T cells for input into a previously reported mVOA [34]. We obtained 530 million total CD4+ T cells from Participant A approximately 18 months following infection on ART. One of 10 mice given 53 million input cells developed low-level HIV RNA in plasma (201 copies/mL) following in vivo activation with anti-CD3 antibody approximately 5.5 weeks after engraftment, but HIV was not detected in spleen tissue (<1 million spleen cells present). However, HIV-1 RNA and cDNA sequencing at an independent laboratory was unsuccessful from plasma and spleen cells, and true infection in cells from Participant A could not be verified. We obtained 500 million total CD4+ T cells from Participant B. Three of 8 mice developed detectable HIV RNA starting approximately 4.5 weeks following cell transfer (1,000; 5,000; and 11,000 copies/mL, respectively, from a total of 50 million CD4+ T cells per mouse).
ATI
Following 34 months of continuous ART, Participant A provided informed consent to undergo a carefully monitored ATI (Fig 2). The patient remained clinically well with undetectable plasma viral load measurements through 225 days of observation post-interruption. On day 225, he had a detectable, low-level plasma HIV-1 RNA of 36 copies/mL. Repeat testing 6 days later (day 231) confirmed rebound with virus increasing to 77,397 copies/mL. He initiated therapy (tenofovir/emtricitabine/ritonavir/darunavir/dolutegravir) on post-ATI day 231 prior to receiving the confirmatory test. He then had viral loads of 158 and 19 copies RNA/mL on days 245 and 252, respectively. Subsequent plasma viral load testing revealed no detectable HIV RNA, and he has remained clinically well. Single genome sequences of a 1,190 base-pair region of HIV-1 Pol were monoclonal and identical to the PrEP baseline sequences. The level of intra-sequence diversity was extremely low at both time points (<0.02%) but distinct from the consensus ancestral subtype B sequence. HIV-1 envelope single genome (1,557 base-pair region) analysis at the time of recrudescence revealed 2 unique but highly related sequences; HIV envelope sequences were not obtained at PrEP baseline (S3 Fig).
Fig. 2. Summary timeline of plasma viral load and CD4+ T cell counts following analytical ART treatment interruption in Participant A. 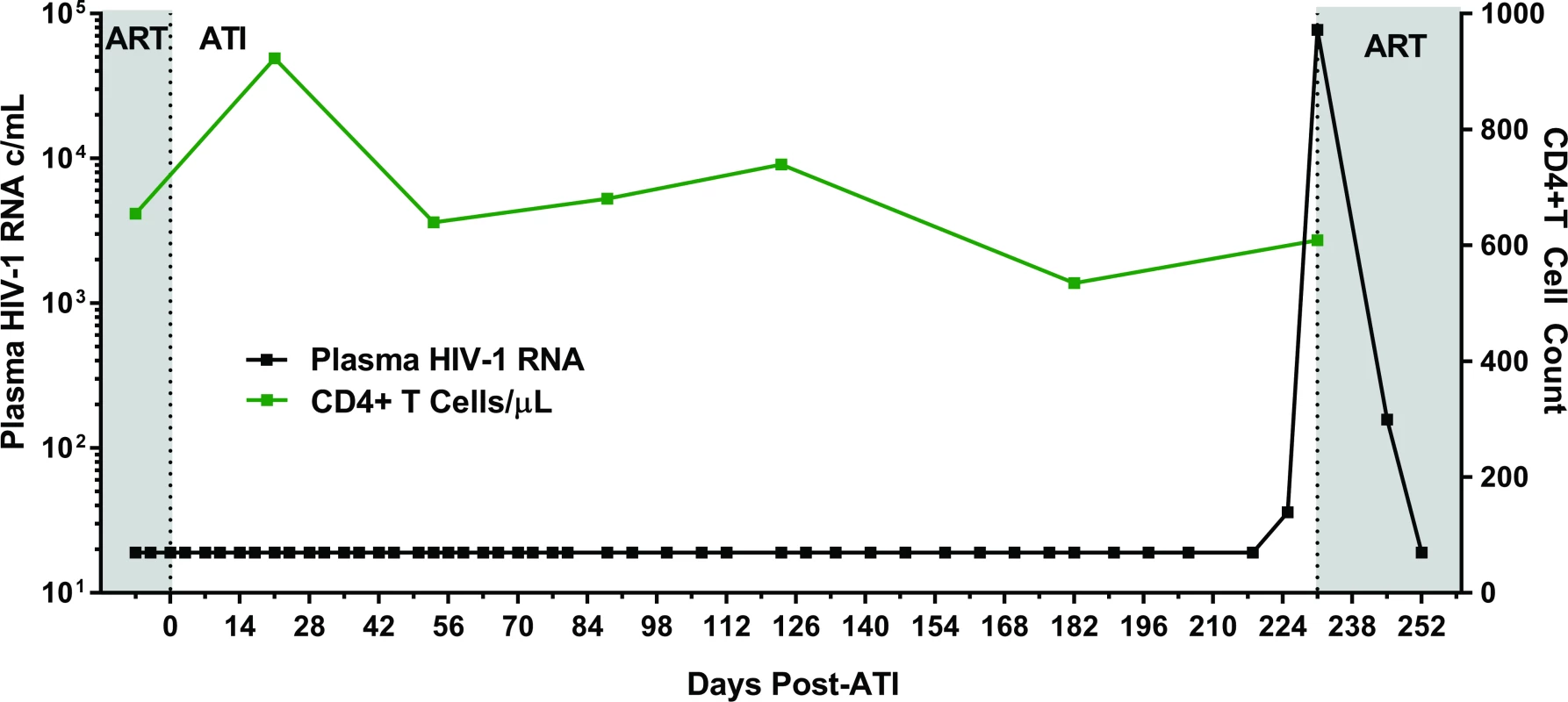
Abbreviations: ART, antiretroviral therapy; ATI, analytical treatment interruption; c/mL, copies/mL. Estimates of HIV reservoir size following extremely early initiation of ART
We used a collection of mathematical modeling approaches (see S1 Text) to better understand the dynamics of reservoir seeding and rebound in these participants. For PrEP Participant A, the estimated latent reservoir size immediately before treatment interruption, based only on the observed time of rebound (226 days) [36] was 0.0020 [0.00045, 0.0063] infectious units per million cells (IUPM) (brackets give 95% credible intervals). With this estimated reservoir size (including uncertainty), about 1% of identical individuals (i.e., having similar restrictions in reservoir size) would be expected to achieve lifelong (>70 year) ART-free HIV remission. These results are consistent with estimates of reservoir size from various assays described in Fig 1 and Tables 1 and 2. Based on the viral load level at initial diagnosis and a calibrated model of reservoir seeding during acute infection [37], we estimate that the reservoir size prior to the initiation of ART was 0.02 IUPM, with about 1 log uncertainty in either direction. qVOA results on day 166 suggest that there is a 95% probability that the reservoir size is below 0.075 IUPM [38]. If we assume the positive outgrowth in the mVOA is a true positive signal, then there is a 95% probability that the reservoir size is below 0.0057 IUPM. If we assume the outgrowth was not real, then the central estimate for the reservoir size is 0.0020 IUPM (95% credible interval 0.00028–0.014 IUPM). Assuming there are around 1011 total body CD4+ T cells [39,40], these estimates collectively suggest there were only approximately a few hundred cells infected with replication-competent HIV provirus prior to treatment interruption. Separately, we estimated the exponential growth rate of viral load during rebound to be 1.3/day, very similar to that seen in the HSCT Boston participants [36] and near the central value seen in acute infection [41–43], but much higher than that seen in rebound following chronic infection (excluding the prolonged time off ART prior to first detection of HIV-1) [44–46].
Surface marker expression and HIV-1–specific responses prior to and following ATI
Flow cytometric characterization of surface markers of T and natural killer (NK) cell activation was performed on samples from PrEP Participant A before and during ATI and following HIV rebound in order to identify potential predictors of viral recrudescence. In addition, pre - and post-ATI samples were investigated from HSCT Participant B, an individual who lost detectable HIV-1 in blood and gut tissue following allogeneic HSCT for malignancy. Similar to PrEP Participant A, the HSCT participant experienced confirmed HIV rebound 225 days after interrupting ART and had similar exponential growth rate during recrudescence [16]. Interestingly, surface expression of CD30, a member of the tumor necrosis factor (TNF) super-receptor family and lymphoma tumor marker, increased on CD4+ and CD8+ T cells months prior to detectable plasma HIV in both of these participants (Fig 3). The frequency of CD30+ and CD69-expressing cells also increased on CD4+ and CD8+ T cells and CD56+ and CD16+ NK cells prior to viral recrudescence in PrEP Participant A. Overall, increases in the frequency of CD30-expressing cells appeared to be larger than those expressing CD69. However, no distinct patterns in lymphocyte HLA-DR/CD38 were observed in either participant, and only CD30 expression increased prior to rebound in the HSCT recipient.
Fig. 3. Changes in lymphocyte surface markers following ATI in PrEP Participant A and HSCT Participant B. 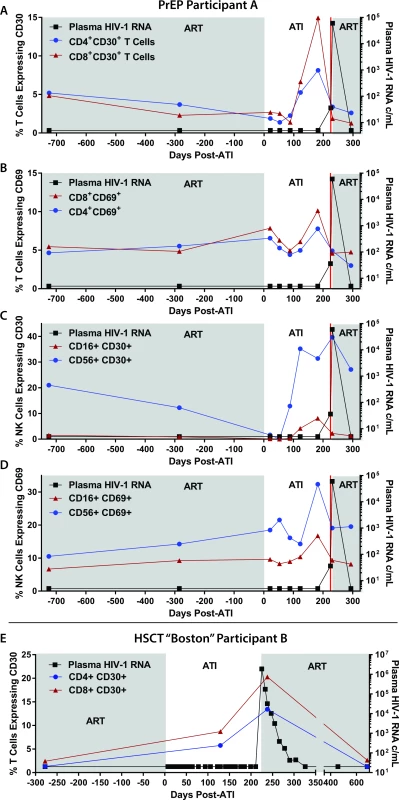
The percentage of CD8+ and CD4+ T cells expressing CD30 and CD69 are shown in (A) and (B). The percentage of CD16+ and CD56+ NK cells expressing CD30 and CD69 are shown in (C) and (D). T cell expression of CD30 from the allogeneic HSCT recipient who also underwent ATI is shown in (E). Abbreviations: ATI, analytical treatment interruption; c/mL, copies/mL; HSCT, hematopoietic stem cell transplantation; NK, natural killer; PrEP, pre-exposure prophylaxis. Sufficient cells were available for further immunological phenotyping from PrEP Participant A, and we identified an increase in the frequency of eomesodermin expressing CD8+ T cells prior to viral rebound. However, no patterns emerged during ATI for expression of PD1, TIGIT, or Ki67. There were no changes in CD8 responses in CD107a expression, and no significant intracellular INF-gamma, TNF-alpha, or IL1 production was detected in response to overlapping HIV-1 gag peptide pools prior to, during, or following ATI.
Discussion
We report 2 cases of extremely early HIV diagnosis and initiation of ART at the threshold when plasma viremia begins to expand exponentially (the end of the so-called “eclipse phase” and beginning of Fiebig stage I). These stages of HIV infection precede the time when acute HIV infection becomes clinically apparent and are theoretically the earliest time when ART can be initiated in an adult [12,13]. To the best of our knowledge, PrEP Participant A is the earliest documented case of adult HIV infection followed by immediate initiation of ART with the exception of successful post-exposure ART prophylaxis, and it would be very challenging to initiate therapy any earlier. Despite the complete or near-complete loss of detectable HIV in blood and a variety of tissues, HIV rebounded in this person 225 days following cessation of ART. Although identifying hyperacute infection is rare, our group has previously reported that 15.6% of patients referred to our HIV clinic for newly diagnosed infection had detectable plasma HIV-1 RNA but negative HIV-specific antibody test results [47]. These data include individuals taking part in a rapid treatment initiation study and may overestimate the incidence of acute infection identified during Fiebig stage I. Excluding participants in this study, 6.3% of individuals presented with positive plasma HIV-1 RNA and no detectable HIV-specific antibodies at the time of diagnosis [47].
The delayed timing of viral rebound in PrEP Participant A was similar to that observed in an HIV-infected individual who lost detectable HIV in blood and tissue following allogeneic HSCT (Boston Participant B). In each case, modeling predicted a residual HIV replication-competent reservoir of perhaps hundreds of infected CD4+ T cells throughout the body, which explains the lack of detectable HIV from blood or tissues despite massive sampling. Overall, allogeneic HSCT and extremely early ART initiation appear to have similar long-term effects on reducing both the residual HIV reservoir and immune responses. Nonetheless, small numbers of latently infected cells likely persisted in these individuals and became activated, leading to HIV rebound in the absence of ART. Modeling of these types of participants leads to wide confidence intervals, which are likely due to the varying size of the reservoir in different patients, uncertainty around model parameters, and the stochastic nature of reactivation of latently infected cells in vivo. It is also possible that a non-CD4+ T cell source of HIV persistence contributed the viral rebound; a majority of cells tested for persistence in blood and tissue were CD4+ lymphocytes. Of note, the median time to viral rebound in 8 Thai individuals treated with ART during later phases of Fiebig stage I was recently reported to be 26 days [48]. Although sample size is limited, these and our data suggest that differences in initiation of ART time of just a few days during Fiebig stage I infection may have a noticeable impact on the duration of ART-free remission.
After this delay, the rapid HIV rebound dynamics in PrEP Participant A were again similar to those observed in HSCT Participant B [16], consistent with rapid exponential growth seen with primary infection. Unlike the HSCT participant, however, the PrEP recipient was asymptomatic and the peak viral load may have been mitigated by the earlier reinitiation of ART. The observed rapid rebound kinetics also differ from those in individuals who achieved post-treatment HIV-1 control following early ART initiation [49].The rapid rebound kinetics and lack of post-treatment control observed in this study are likely secondary to the lack of HIV-specific immunity since exponential growth is lower in the setting of withdrawal of ART initiated during chronic infection when HIV-specific immunity is present. Concomitant immune-modifying therapies may be necessary in order to achieve ART-free HIV control in very-early treated individuals.
PrEP Participant B initiated ART later than PrEP Participant A and had higher levels of viremia at his baseline visit. In many ways, he is similar to the “Mississippi baby” [6,7] in that he started ART with moderate levels of viremia and subsequently had a difficult-to-detect reservoir. Individuals who initiate therapy during the earliest stages of infection will almost certainly need other “curative” interventions before ART might be interrupted with the expectation that a viral relapse will be avoided. It is also possible that higher peak plasma HIV RNA levels and subsequent low-level viral reservoir detection in samples from PrEP Participant B were a result of the appearance of an emtricitabine-resistant associated mutation and exposure to a single active antiretroviral drug during initial PrEP.
It is likely that there are rare individuals who start PrEP during the true eclipse phase, when HIV has the potential to establish a long-lived reservoir but before the development of detectable HIV in blood or tissues. Detecting a potential “cure” in such a setting will be challenging but such efforts are ongoing. Of note, non-human primate studies have demonstrated that, while ART initiated within 48 hours of SIV challenge is able to prevent infection [50–52], ART started 3 days following SIV infection leads to viral rebound following cessation of therapy [53]. As a result, the "curative window" between infection and the potential to abort infection after exposure is likely very small. It is also possible that there may be individuals infected just before the start of PrEP, but in whom infection would not be known for some time until they stop ART or until a 2-drug regimen is unable to suppress the virus.
Our study is one of the first to incorporate an mVOA to measure potential HIV persistence following an intervention that leads to loss of detectable HIV in blood and various tissues. One mouse became viremic (at low levels) and 3 mice became viremic following receipt of cells from PrEP Participants A and B, respectively. This positive finding with PrEP Participant A's PBMCs may be the only evidence that this individual had persistent HIV prior to ART interruption. Unfortunately, sequence verification of plasma and splenic HIV in mice from both PrEP participants could not confirm definitive viral outgrowth from participant cells. Sample volumes are limited in murine models of HIV infection and were exhausted during testing. Nonetheless, 2 published studies suggest that mVOAs may be more sensitive than traditional outgrowth assays to detect replication-competent HIV in individuals with undetectable HIV-1 DNA or RNA by traditional means [34,54]. If this is the case, our study suggests that sampling of hundreds of millions of PBMCs may, at times, be more sensitive than tissue-based studies for the detection of residual HIV infection since a much larger number of cells can be interrogated. Further studies comparing mVOAs with traditional ex vivo co-culture assays utilizing rigorous positive and negative controls are certainly warranted.
A major emphasis of HIV curative science has been to identify potential markers or correlates of HIV rebound before or after treatment cessation. CD30, a member of the TNF receptor superfamily, is expressed on very small percentages of lymphocytes and myeloid cells but is dramatically upregulated on Hodgkin and other lymphoma cells [55–59]. CD30 has been implicated in the activation, proliferation, and cell death of selected cell populations [55,57,60,61]; and infections with human T-cell lymphotropic virus (HTLV), Epstein-Barr virus (EBV), and poxviruses can lead to increases in CD30 expression [57,62,63]. In addition, increases in the plasma concentration of the soluble form of CD30 have been associated with HIV disease progression prior to ART initiation [57,58,60,64–71]. Although anecdotal, our data in PrEP Participant A and the HSCT participant suggest that upregulation of CD30 may occur prior to detectable plasma HIV-1 RNA. Furthermore, given a similar but somewhat less pronounced pre-rebound expression pattern of CD69 in the PrEP Participant A, it is possible that these markers represent more global lymphocyte activation, proliferation, or stress responses that are detectable prior to viral recrudescence. Further investigation of CD30 and related cell-surface markers as potential predictors of HIV rebound is urgently needed.
These cases also suggest that PrEP programs should test individuals for HIV using an HIV test with the narrowest window period possible, just before initiating PrEP. If cost permits and in settings with a high incidence of acute HIV (e.g., STI clinics), it is reasonable to test with a plasma HIV RNA before PrEP initiation and when reinitiating PrEP after an interruption. Fourth generation combination p24 antigen/antibody tests will identify most patients with acute HIV but miss those in the earliest Fiebig stages, such as those presented in this analysis. Plasma HIV RNA testing should also be considered when reinitiating PrEP after interruption [9]. PrEP programs should recommend an immediate switch to conventional ART if an individual is found to be newly HIV-positive. Potential benefits of such programmatic changes include (1) lower rates of missed acute HIV diagnoses, (2) decreased acquired drug resistance to tenofovir/emtricitabine, (3) individual clinical benefit [72], and (4) fewer subsequent transmission events [73].
This study was limited by its small sample size. The identification of individuals treated during hyperacute infection is rare given the rapid increase in plasma HIV-1 RNA during the early phase of Fiebig stage I infection and the fact that hyperacute infection may be asymptomatic. The limited number of individuals included in the analysis make it difficult to draw definitive conclusions about the impact of very early ART on restricting HIV reservoir size or prolonging ART-free remission. In addition, a larger number of individuals are required to validate potential cell-surface markers of HIV persistence or predictors of HIV rebound. Despite the challenges with including very early treated individuals in prospective studies, these cases provide valuable information as to the rapidity of seeding of the HIV reservoir and the impact of extremely early ART on HIV persistence.
In summary, we report 2 cases of extremely early initiation of prophylactic ART immediately following the “eclipse phase” in Fiebig stage I (at approximately 10 days of HIV infection). A very small residual HIV reservoir size was observed in these participants who started very early PrEP and subsequently converted to full ART. In 1 individual, we observed a prolonged period of ART-free remission similar in duration to the allogeneic HSCT Boston Participant B. However, although HIV persisted indefinitely in both of these PrEP cases, a continuum may exist across PrEP, post-exposure prophylaxis and curative early ART strategies. Further investigation in larger cohorts of individuals treated extremely early following HIV infection is warranted.
Supporting Information
Zdroje
1. Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med. 2016 Aug;22(8):839–50. doi: 10.1038/nm.4108 27400264
2. Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4(+) T cells. Nat Med. 2003 Jun;9(6):727–8. doi: 10.1038/nm880 12754504
3. Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009 Mar 6;323(5919):1304–7. doi: 10.1126/science.1165706 19265012
4. Ananworanich J, Puthanakit T, Suntarattiwong P, Chokephaibulkit K, Kerr SJ, Fromentin R, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014 Apr 24;28(7):1015–20. doi: 10.1097/QAD.0000000000000178 24384692
5. Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014 Dec;10(12):e1004543. doi: 10.1371/journal.ppat.1004543 25503054
6. Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M Jr., Chun TW, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013 Nov 7;369(19):1828–35. doi: 10.1056/NEJMoa1302976 24152233
7. Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015 Feb 19;372(8):786–8. doi: 10.1056/NEJMc1413931 25693029
8. Announcement: Updated Guidelines for Antiretroviral Postexposure Prophylaxis after Sexual, Injection-Drug Use, or Other Nonoccupational Exposure to HIV—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016 May 06;65(17):458. doi: 10.15585/mmwr.mm6517a5 27149423
9. Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–99. doi: 10.1056/NEJMoa1011205 21091279
10. Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012 Aug 02;367(5):423–34. doi: 10.1056/NEJMoa1110711 22784038
11. Smith DK, Grohskopf LA, Black RJ, Auerbach JD, Veronese F, Struble KA, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005 Jan 21;54(RR-2):1–20. 15660015
12. Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003 Sep 05;17(13):1871–9. doi: 10.1097/01.aids.0000076308.76477.b8 12960819
13. McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010 Jan;10(1):11–23. doi: 10.1038/nri2674 20010788
14. de Souza MS, Pinyakorn S, Akapirat S, Pattanachaiwit S, Fletcher JL, Chomchey N, et al. Initiation of Antiretroviral Therapy During Acute HIV-1 Infection Leads to a High Rate of Nonreactive HIV Serology. Clin Infect Dis. 2016 Aug 15;63(4):555–61. doi: 10.1093/cid/ciw365 27317797
15. Ananworanich J, Chomont N, Eller LA, Kroon E, Tovanabutra S, Bose M, et al. HIV DNA Set Point is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. EBioMedicine. 2016 Sep;11 : 68–72. doi: 10.1016/j.ebiom.2016.07.024 27460436
16. Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, et al. Antiretroviral-Free HIV-1 Remission and Viral Rebound After Allogeneic Stem Cell Transplantation: Report of 2 Cases. Ann Intern Med. 2014 Jul 22;161(5):319–27. doi: 10.7326/M14-1027 25047577
17. Henrich TJ, Hu Z, Li JZ, Sciaranghella G, Busch MP, Keating SM, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013 Jun 1;207(11):1694–702. doi: 10.1093/infdis/jit086 23460751
18. Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal - and Community-Based Sexual Health Services. JAMA Intern Med. 2016 Jan;176(1):75–84. doi: 10.1001/jamainternmed.2015.4683 26571482
19. Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010 Nov 15;202(10):1553–61. doi: 10.1086/656722 20939732
20. Yukl SA, Shergill AK, Ho T, Killian M, Girling V, Epling L, et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. J Infect Dis. 2013 Oct 15;208(8):1212–20. doi: 10.1093/infdis/jit308 23852128
21. Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009 Aug;15(8):893–900. doi: 10.1038/nm.1972 19543283
22. Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014 Sep 24;28(15):2251–8. doi: 10.1097/QAD.0000000000000400 25022595
23. Probasco JC, Deeks SG, Lee E, Hoh R, Hunt PW, Liegler T, et al. Cerebrospinal fluid in HIV-1 systemic viral controllers: absence of HIV-1 RNA and intrathecal inflammation. AIDS. 2010 Apr 24;24(7):1001–5. doi: 10.1097/QAD.0b013e328331e15b 20299968
24. Kearney M, Maldarelli F, Shao W, Margolick JB, Daar ES, Mellors JW, et al. Human immunodeficiency virus type 1 population genetics and adaptation in newly infected individuals. J Virol. 2009 Mar;83(6):2715–27. doi: 10.1128/JVI.01960-08 19116249
25. Henrich TJ, Hanhauser E, Hu Z, Stellbrink HJ, Noah C, Martin JN, et al. Viremic control and viral coreceptor usage in two HIV-1-infected persons homozygous for CCR5 Delta32. AIDS. 2015 Feb 27;29(8):867–76. doi: 10.1097/QAD.0000000000000629 25730507
26. Vandergeeten C, Fromentin R, Merlini E, Lawani MB, DaFonseca S, Bakeman W, et al. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J Virol. 2014 Nov;88(21):12385–96. doi: 10.1128/JVI.00609-14 25122785
27. Kumar AM, Borodowsky I, Fernandez B, Gonzalez L, Kumar M. Human immunodeficiency virus type 1 RNA Levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. J Neurovirol. 2007 Jun;13(3):210–24. doi: 10.1080/13550280701327038 17613711
28. Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, et al. Highly Precise Measurement of HIV DNA by Droplet Digital PCR. PLoS ONE. 2013;8(4):e55943. doi: 10.1371/journal.pone.0055943 23573183
29. Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304 : 3–15. doi: 10.1385/1-59259-907-9 : 003 16061962
30. Laird GM, Eisele EE, Rabi SA, Lai J, Chioma S, Blankson JN, et al. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Pathog. 2013;9(5):e1003398. doi: 10.1371/journal.ppat.1003398 23737751
31. Procopio FA, Fromentin R, Kulpa DA, Brehm JH, Bebin AG, Strain MC, et al. A Novel Assay to Measure the Magnitude of the Inducible Viral Reservoir in HIV-infected Individuals. EBioMedicine. 2015 Aug;2(8):874–83. doi: 10.1016/j.ebiom.2015.06.019 26425694
32. Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med. 2016 Aug 8;22(9):1043–9. doi: 10.1038/nm.4156 27500724
33. Cillo AR, Vagratian D, Bedison MA, Anderson EM, Kearney MF, Fyne E, et al. Improved single-copy assays for quantification of persistent HIV-1 viremia in patients on suppressive antiretroviral therapy. J Clin Microbiol. 2014 Nov;52(11):3944–51. doi: 10.1128/JCM.02060-14 25187636
34. Metcalf Pate KA, Pohlmeyer CW, Walker-Sperling VE, Foote JB, Najarro KM, Cryer CG, et al. A Murine Viral Outgrowth Assay to Detect Residual HIV Type 1 in Patients With Undetectable Viral Loads. J Infect Dis. 2015 Nov 1;212(9):1387–96. doi: 10.1093/infdis/jiv230 25883388
35. Hill AL, Rosenbloom DI, Fu F, Nowak MA, Siliciano RF. Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):13475–80. doi: 10.1073/pnas.1406663111 25097264
36. Hill AL, Rosenbloom DI, Goldstein E, Hanhauser E, Kuritzkes DR, Siliciano RF, et al. Real-Time Predictions of Reservoir Size and Rebound Time during Antiretroviral Therapy Interruption Trials for HIV. PLoS Pathog. 2016;12(4):e1005535. doi: 10.1371/journal.ppat.1005535 27119536
37. Archin NM, Vaidya NK, Kuruc JD, Liberty AL, Wiegand A, Kearney MF, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A. 2012 Jun 12;109(24):9523–8. doi: 10.1073/pnas.1120248109 22645358
38. Rosenbloom DI, Elliott O, Hill AL, Henrich TJ, Siliciano JM, Siliciano RF. Designing and Interpreting Limiting Dilution Assays: General Principles and Applications to the Latent Reservoir for Human Immunodeficiency Virus-1. Open Forum Infect Dis. 2015 Dec;2(4):ofv123. doi: 10.1093/ofid/ofv123 26478893
39. Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533 27541692
40. Ganusov VV, De Boer RJ. Do most lymphocytes in humans really reside in the gut? Trends Immunol. 2007 Dec;28(12):514–8. doi: 10.1016/j.it.2007.08.009 17964854
41. Ribeiro RM, Qin L, Chavez LL, Li D, Self SG, Perelson AS. Estimation of the initial viral growth rate and basic reproductive number during acute HIV-1 infection. J Virol. 2010 Jun;84(12):6096–102. doi: 10.1128/JVI.00127-10 20357090
42. Pinkevych M, Cromer D, Tolstrup M, Grimm AJ, Cooper DA, Lewin SR, et al. Correction: HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5–8 Days-Implications for HIV Remission. PLoS Pathog. 2016;12(8):e1005745. doi: 10.1371/journal.ppat.1005745 27561082
43. Bloch MT, Smith DE, Quan D, Kaldor JM, Zaunders JJ, Petoumenos K, et al. The role of hydroxyurea in enhancing the virologic control achieved through structured treatment interruption in primary HIV infection: final results from a randomized clinical trial (Pulse). J Acquir Immune Defic Syndr. 2006 Jun;42(2):192–202. doi: 10.1097/01.qai.0000219779.50668.e6 16688094
44. Ruiz L, Martinez-Picado J, Romeu J, Paredes R, Zayat MK, Marfil S, et al. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. AIDS. 2000 Mar 10;14(4):397–403. 10770542
45. Frost SD, Martinez-Picado J, Ruiz L, Clotet B, Brown AJ. Viral dynamics during structured treatment interruptions of chronic human immunodeficiency virus type 1 infection. J Virol. 2002 Feb;76(3):968–79. doi: 10.1128/JVI.76.3.968-979.2002 11773372
46. Davey RT Jr., Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A. 1999 Dec 21;96(26):15109–14. 10611346
47. Pilcher CD, Ospina-Norvell C, Dasgupta A, Jones D, Hartogensis W, Torres S, et al. The Effect of Same-Day Observed Initiation of Antiretroviral Therapy on HIV Viral Load and Treatment Outcomes in a US Public Health Setting. J Acquir Immune Defic Syndr. 2017 Jan 01;74(1):44–51. doi: 10.1097/QAI.0000000000001134 27434707
48. Colby D, Chomont N, Kroon E, Leyre L, Pinyakorn S, Michael NL, et al. HIV RNA Rebound Post-Interruption in Persons Suppressed in Fiegiv I Acute HIV. Abstract #124, Conference on Retroviruses and Opportunistic Infections, Seattle, WA, February 13–16. 2017.
49. Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. doi: 10.1371/journal.ppat.1003211 23516360
50. Lifson JD, Rossio JL, Arnaout R, Li L, Parks TL, Schneider DK, et al. Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J Virol. 2000 Mar;74(6):2584–93. 10684272
51. Lifson JD, Rossio JL, Piatak M Jr., Parks T, Li L, Kiser R, et al. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001 Nov;75(21):10187–99. doi: 10.1128/JVI.75.21.10187-10199.2001 11581387
52. Lifson JD, Piatak M Jr., Cline AN, Rossio JL, Purcell J, Pandrea I, et al. Transient early post-inoculation anti-retroviral treatment facilitates controlled infection with sparing of CD4+ T cells in gut-associated lymphoid tissues in SIVmac239-infected rhesus macaques, but not resistance to rechallenge. J Med Primatol. 2003 Aug;32(4–5):201–10. 14498980
53. Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014 Aug 07;512(7512):74–7. doi: 10.1038/nature13594 25042999
54. Charlins P, Schmitt K, Remling-Mulder L, Hogan LE, Hanhauser E, Hobbs KS, et al. A humanized mouse-based HIV-1 viral outgrowth assay with higher sensitivity than in vitro qVOA in detecting latently infected cells from individuals on ART with undetectable viral loads. Virology. 2017 Jul;507 : 135–9. doi: 10.1016/j.virol.2017.04.011 28432928
55. Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995 Jan 1;85(1):1–14. 7803786
56. Chiarle R, Podda A, Prolla G, Gong J, Thorbecke GJ, Inghirami G. CD30 in normal and neoplastic cells. Clin Immunol. 1999 Feb;90(2):157–64. doi: 10.1006/clim.1998.4636 10080826
57. Biswas P, Mantelli B, Delfanti F, Ferrarini M, Poli G, Lazzarin A. CD30 ligation differentially affects CXCR4-dependent HIV-1 replication and soluble CD30 secretion in non-Hodgkin cell lines and in gamma delta T lymphocytes. Eur J Immunol. 2003 Nov;33(11):3136–45. doi: 10.1002/eji.200324344 14579282
58. Biswas P, Smith CA, Goletti D, Hardy EC, Jackson RW, Fauci AS. Cross-linking of CD30 induces HIV expression in chronically infected T cells. Immunity. 1995 Jun;2(6):587–96. 7540942
59. Agrawal B, Reddish M, Longenecker BM. CD30 expression on human CD8+ T cells isolated from peripheral blood lymphocytes of normal donors. J Immunol. 1996 Oct 15;157(8):3229–34. 8871616
60. Biswas P, Cozzi-Lepri A, Delfanti F, Galli A, Colangeli V, Moioli MC, et al. Significant link between sCD30 changes and HIV viremia in patients treated with HAART. J Med Virol. 2006 Dec;78(12):1513–9. doi: 10.1002/jmv.20733 17063513
61. Gilfillan MC, Noel PJ, Podack ER, Reiner SL, Thompson CB. Expression of the costimulatory receptor CD30 is regulated by both CD28 and cytokines. J Immunol. 1998 Mar 1;160(5):2180–7. 9498756
62. Higuchi M, Matsuda T, Mori N, Yamada Y, Horie R, Watanabe T, et al. Elevated expression of CD30 in adult T-cell leukemia cell lines: possible role in constitutive NF-kappaB activation. Retrovirology. 2005;2 : 29. doi: 10.1186/1742-4690-2-29 15876358
63. Makino M, Yashiki S, Fujiyoshi T, Baba M, Sonoda S. An expression of anaplastic large cell lymphoma-associated antigens on HTLV-I-infected CD4+ T cells. Ann Hematol. 1998 Jan;76(1):31–5. 9486922
64. Smith KJ, Barrett TL, Neafie R, Tomaszewski MM, Yeager J, Nelson A, et al. Is CD30 (Ki-1) immunostaining in cutaneous eruptions useful as a marker of Th1 to Th2 cytokine switching and/or as a marker of advanced HIV-1 disease? Br J Dermatol. 1998 May;138(5):774–9. 9666821
65. Pizzolo G, Vinante F, Nadali G, Krampera M, Morosato L, Chilosi M, et al. High serum level of soluble CD30 in acute primary HIV-1 infection. Clin Exp Immunol. 1997 May;108(2):251–3. doi: 10.1046/j.1365-2249.1997.d01-1005.x 9158093
66. Tsitsikov EN, Wright DA, Geha RS. CD30 induction of human immunodeficiency virus gene transcription is mediated by TRAF2. Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1390–5. 9037063
67. Silvestri G, Munoz C, Butini L, Bagnarelli P, Montroni M. Changes in CD8 cell subpopulations induced by antiretroviral therapy in human immunodeficiency virus infected patients. Viral Immunol. 1997;10(4):207–12. doi: 10.1089/vim.1997.10.207 9473151
68. Rizzardi GP, Barcellini W, Tambussi G, Lillo F, Malnati M, Perrin L, et al. Plasma levels of soluble CD30, tumour necrosis factor (TNF)-alpha and TNF receptors during primary HIV-1 infection: correlation with HIV-1 RNA and the clinical outcome. AIDS. 1996 Nov;10(13):F45–50. 8931778
69. Manetti R, Annunziato F, Biagiotti R, Giudizi MG, Piccinni MP, Giannarini L, et al. CD30 expression by CD8+ T cells producing type 2 helper cytokines. Evidence for large numbers of CD8+CD30+ T cell clones in human immunodeficiency virus infection. J Exp Med. 1994 Dec 1;180(6):2407–11. 7964515
70. Pizzolo G, Vinante F, Morosato L, Nadali G, Chilosi M, Gandini G, et al. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994 Jun;8(6):741–5. 8086130
71. Sadeghi M, Susal C, Daniel V, Naujokat C, Zimmermann R, Huth-Kuhne A, et al. Short communication: decreasing soluble CD30 and increasing IFN-gamma plasma levels are indicators of effective highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2007 Jul;23(7):886–90. doi: 10.1089/aid.2006.0228 17678471
72. Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013 Oct 15;208(8):1202–11. doi: 10.1093/infdis/jit311 23852127
73. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243 21767103
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



