-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Experimental Treatment of Ebola Virus Disease with TKM-130803: A Single-Arm Phase 2 Clinical Trial
In a single-arm trial, Peter Horby and colleagues probe the effectiveness of a small interfering RNA-based drug intended to treat Ebola virus disease.
Published in the journal: . PLoS Med 13(4): e32767. doi:10.1371/journal.pmed.1001997
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001997Summary
In a single-arm trial, Peter Horby and colleagues probe the effectiveness of a small interfering RNA-based drug intended to treat Ebola virus disease.
Introduction
An advisory panel convened in August 2014 by the World Health Organization (WHO) concluded that unregistered experimental products that showed promising results in laboratory and animal models should be evaluated in humans with Ebola virus disease (EVD) [1]. The pathophysiology of severe EVD in humans is characterised by vascular leakage, shock, coagulopathy, and multi-organ injury, with severity closely correlated with Ebola virus (EBOV) RNA levels in blood (henceforth referred to as “viral load”) [2–7]. Antiviral agents have therefore been a main focus of drug development for EVD. One of the lead experimental therapies prioritised for evaluation by WHO was TKM-100802 (Tekmira Pharmaceuticals, British Columbia, Canada), a lipid nanoparticle (LNP) formulation of small interfering RNAs (siRNAs) directed against the gene products encoding two viral proteins: L polymerase (Lpol), involved in transcription and replication of Zaire ebolavirus, and Viral Protein-35 (VP35), involved in suppression of the host immune response [8].
TKM-100802 has been evaluated in guinea pig, non-human primate (NHP), and human phase 1 clinical trials [9]. The TKM-100802 Investigational New Drug Application (IND) is currently on partial clinical hold by the US Food and Drug Administration, whereby TKM-100802 may be used in individuals with confirmed or suspected EBOV infection but may not be administered to normal healthy individuals. The basis for the clinical hold in normal healthy individuals was concern about cytokine release syndrome or infusion-related reactions. Cytokine release syndrome is a pro-inflammatory reaction that occurs when activated lymphocytes and/or myeloid cells release soluble immune mediators following administration of certain therapeutic agents, especially monoclonal antibodies. Onset can be rapid (within hours of administration) and can be life-threatening [10]. TKM-100802 has been administered to five patients with EVD medically evacuated to the US and Europe, and to one individual as post-exposure prophylaxis (personal communication, Mark Kowalski, Tekmira Pharmaceuticals). Since the product was administered on a compassionate basis to these individuals and because the patients simultaneously received other experimental products, it has not been possible to assess the efficacy or safety of TKM-100802 in the treatment of EVD [11,12].
TKM-130803 is a new formulation of TKM-100802 in which the siRNA component has been adapted by two nucleotide substitutions in the VP35 siRNA and a single nucleotide substitution in the L-polymerase siRNA to ensure specificity to the West African Makona variant of Zaire ebolavirus causing the 2014–2015 West African outbreak. The siRNA drug component of TKM-130803 is termed siEbola-3 and is formulated with lipid excipients (LNP1 composition) to form LNPs. In rhesus monkeys infected with a lethal challenge of Makona variant EBOV, administration of siEbola-3 formulated with a different LNP (LNP2) and dosed at 0.5 mg/kg/d for 7 d resulted in 100% (3/3) survival when commenced 72 h post-inoculation, a point in the disease course where viral RNA levels up to 106 RNA copies/ml can be detectable by blood sampling [13].
The Rapid Assessment of Potential Interventions and Drugs for Ebola (RAPIDE) clinical trial platform was developed in 2014 to assess potential treatments for EVD. The platform allows a multi-stage approach, and the concept is described elsewhere [14]. This report describes the RAPIDE-TKM trial, an open-label, non-randomised, single-arm trial to generate early evidence of the effectiveness of TKM-130803.
Methods
Ethics Statement
The trial was approved by the Sierra Leone Ethics and Scientific Review Committee and the Oxford Tropical Research Ethics Committee. Approval to conduct the trial and import the trial drug was granted by the Pharmacy Board of Sierra Leone. The Committee for Medicinal Products for Human Use of the European Medicines Agency was asked for an opinion on the use of TKM-130803 in humans with EVD and was of the view that conducting a clinical trial of TKM-130803 in the context of the Ebola outbreak was acceptable. The UK Department for International Development and GOAL Global approved for the trial to be conducted at the Port Loko Ebola treatment centre (ETC). An independent data monitoring committee (IDMC) reviewed data on a sequential basis and reviewed any reported adverse events or other safety concerns. The trial was conducted in compliance with the International Conference on Harmonisation guidance on good clinical practice, and the Pharmacy Board of Sierra Leone conducted a good clinical practice compliance inspection during the trial. Written informed consent was obtained for all participants, including those enrolled in the observational cohort.
Trial Setting
The trial was conducted between 11 March and 15 June 2015 at the Port Loko (Mathaska) ETC in Sierra Leone. The ETC was operated by the international humanitarian organisation GOAL Global. The on-site laboratory was operated by Public Health England.
Patients
Patients with laboratory-confirmed EVD aged 18 y or older were eligible for enrolment. Exclusion criteria were as follows: underlying disease or condition that could jeopardise the safety of the participant or other individuals, patient determined by the treating physician on admission to be for end-of-life care only, intravenous access not possible, use of any other investigational or non-registered product within 30 d prior to trial enrolment or planned use during the trial period, and inability to comply with protocol requirements. There were additional criteria to receive TKM-130803 (those not meeting these criteria could participate in the observational cohort): female patients aged 18–49 y were required to have a negative beta-HCG pregnancy test prior to enrolment, women who were lactating had to agree to stop breastfeeding, sexually active participants had to agree to use condoms for at least 3 mo following discharge. Pregnant and breastfeeding women could not be included initially due to an absence of fertility and reproductive toxicity study data for TKM-130803 or its predecessor compound, TKM-100802. Children could not be included initially because TKM-130803 had never been used in children, and the bio-distribution and pharmacokinetics are not known in this population. It was planned that, after the first 15 patients had received TKM-130803, the possibility of enrolling these patient groups would be considered by the IDMC following a review of all available safety data. A patient could be enrolled anytime within 48 h of first arriving at the ETC with a confirmed diagnosis or within 48 h of being informed of a Zaire ebolavirus–positive PCR result if this occurred whilst admitted in the centre.
Study Drug
The study drug (TKM-130803) was a liquid (non-lyophilised) formulation of siEbola-3 with LNP1. TKM-130803 was administered at a dose of 0.3 mg/kg/d for 7 d by intravenous infusion at a rate of 1.25 ml/min over 2 h, for a total infused volume of 150 ml. The dose of 0.3 mg/kg/d for 7 d was selected based on the safety and pharmacokinetics of TKM-100802 in a single ascending dose study in healthy adult volunteers, in which the maximum tolerated dose was determined to be 0.3 mg/kg. In NHPs infected with EBOV Kikwit in a fatal infection model, 100% survival was observed following administration of TKM-100802 at 0.5 mg/kg/d and 66% survival was observed following administration of TKM-100802 at 0.2 mg/kg/d (personal communication, Mark Kowlaski, Tekmira Pharmaceuticals). Thus, the dose of TKM-130803 selected for this trial was determined to be appropriate in terms of balancing safety and potential clinical benefit. In the event of suspected drug-related toxicity or change in the patient’s clinical condition, the dose of TKM-130803 could be reduced to a minimum of 0.24 mg/kg/d.
Non-trial Treatment
ETC clinicians provided standard supportive care in accordance with established GOAL Global treatment guidelines for EVD. This included routine malaria testing and treatment, empirical antibiotics, antihelminthics, antiemetics, anti-diarrhoeal therapy, pain relief, oral and intravenous fluid therapy, and electrolyte supplementation, as appropriate.
Primary Outcome
The primary outcome was survival in patients who received TKM-130803 at a dose of 0.3 mg/kg/d in addition to standard care, assessed 14 d after admission, excluding patients who died within 48 h of admission. Survival at day 14 was chosen since most EVD deaths occur within 14 d of admission to an ETC, and this early time point allowed a rapid assessment of treatment effect.
Trial Design
The trial was an open-label, non-randomised, single-arm trial with a concurrent observational study.
Design Rationale
The general approach of the RAPIDE platform in evaluating potential treatments of EVD was to begin with a single-arm phase 2 trial to generate early evidence of effectiveness or ineffectiveness. If the initial phase 2 trial provided evidence of effectiveness (as assessed against a predetermined survival probability threshold at 14 d after enrolment), the phase 2 trial result would require confirmation in follow-up studies [14]. The additional ethical and practical considerations that influenced the decision to perform an initial single-arm trial have been discussed elsewhere [15].
The evaluation of TKM-130803 was, however, constrained by the fact that only 100 courses of the drug were available and that the incidence of new cases was falling dramatically in early 2015. This led to a modification of the phase 2 component of the RAPIDE approach and a realisation that, should testing be indicated beyond phase 2, it might have to take place during a subsequent outbreak of EVD. Therefore, for the TKM-130803 trial, the single-arm phase 2 design did not allow early stopping in the case of evidence of effectiveness, because enrolling the full 100 patients would maximise the precision of the final estimate of effectiveness. A futility design was used to allow early stopping in the event of evidence of futility or harm [16]. The trial would recruit up to 100 patients but would stop if the number of successes observed fell below a pre-specified threshold. To avoid early stopping due to enrolment of patients with very severe, late-stage EVD—who may not be expected to survive even with an effective antiviral therapy—the stopping rule was to be calculated after exclusion of enrolled patients who died within 48 h of admission to the ETC.
Because of the potential risk of infusion reactions, TKM-130803 was infused over a minimum 2-h period, during which clinical monitoring for infusion reactions took place. The intensity of required clinical monitoring and the challenges of care delivery within the ETC meant that the number of participants who could safely receive TKM-130803 infusions concurrently was limited. Therefore, the maximum number of patients receiving TKM-130803 on a single day was capped. Each day the clinical trial lead physician decided the maximum number of beds available for patients to receive TKM-130803 (“TKM beds”). If on any given day the number of patients eligible for and consenting to inclusion in the trial exceeded the number of available TKM beds, patients were randomly allocated to receive either TKM-130803 with standard care (as part of the TKM-130803 cohort) or standard care alone (as part of the observational cohort). Consent for inclusion in the trial included consent to randomisation and inclusion in the observational cohort if TKM bed capacity was reached. Random allocation was conducted using R (R Project for Statistical Computing).
Statistical Analysis Plan
For the purposes of determining futility, the effectiveness of TKM-130803 was judged in terms of the probability that a patient allocated to receive TKM-130803 would survive to day 14 after admission, after excluding patients who died within 48 h of admission to the ETC. If the survival probability (p) was >0.55, then TKM-130803 would be regarded as “promising”; otherwise, it would be regarded as “not promising”. The power of the study (1 − beta) to detect that TKM-130803 was promising was 0.827 if the true success rate p was equal to 0.70, and 0.973 if p was equal to 0.75. If the trial ever reached a point at which significant evidence (at the one-sided 2.5% level) that p was greater than 0.55 could not be found, then continuation of the trial would be considered futile, and it would be stopped. The choice of 0.55 as the target for p was made following an analysis of individual-level data on 1,820 adult patients with PCR-confirmed EBOV infection from the 2014–2015 outbreak (personal communication, Annick Antierens, Médecins Sans Frontières). EBOV PCR cycle threshold (Ct) values and viral load data were not available for this historic cohort. The properties of the design were calculated exactly, based on the independent Bernoulli distributions of each patient outcome.
The data management centre was informed every time a patient was enrolled and, after 14 d, whether that patient did or did not survive. Every time that a day 14 report was received, the number of patients who had survived to day 14 was plotted against the number who had been entered into the trial, and the plotted point was compared with the futility boundary. Enrolment into the trial would be stopped if the futility boundary was reached. When the trial was completed, a point estimate and a 95% confidence interval for p was computed using the method of Jovic and Whitehead [17]. Provided that all 100 patients received TKM-130803 without the futility boundary being reached, the formal conclusion of the trial would be that TKM-130803 was promising, and this would happen with probability ≤ 0.025 if in fact the success rate p was ≤0.55.
Safety Assessments
Safety assessments of patients included monitoring of vital signs (pulse rate, blood pressure, respiratory rate, temperature, and level of consciousness), symptoms, and the occurrence of serious adverse reactions (SARs) and suspected unexpected serious adverse reactions. Vital signs were assessed at the following time points: pre-infusion, during the infusion (preferably between 30 and 90 min after the start of infusion), at the end of infusion, and at approximately 1, 2, 4, and 8 h after the end of infusion, as well as at additional time points if indicated by the patient’s clinical condition. Trial staff observed patients directly throughout the entire infusion period. Trial assessments and observations were in addition to routine clinical assessments performed by the ETC’s clinicians.
To assess the feasibility and safety of dosing with TKM-130803 in an ETC in Sierra Leone, data on the first four enrolled patients were assessed by the IDMC prior to opening enrolment to additional patients. These first four patients (termed the safety cohort) were recruited sequentially, with each patient receiving at least three doses (or dying) before dosing of the next patient started. It was planned that the safety cohort could be expanded following advice from the IDMC, although this was not required.
Laboratory Methods
Diagnostic Ebola virus PCR RNA extraction
In a flexible film isolator, viral RNA was extracted from EDTA–whole blood either (1) from 80 µl of plasma using the EZ1 Virus Mini Kit v2.0 (Qiagen) in conjunction with the EZ1 platform (Qiagen) or (2) manually from 50 µl of plasma using the QIAamp Viral RNA Kit (Qiagen). Intact MS2 phage was included in all extractions as an exogenous internal control for the downstream reverse transcription PCR (RT-PCR) step. Extracts were resuspended in 60 µl of AVE buffer (Qiagen), and diagnostic EBOV RT-PCR was carried out immediately. Residual extracts were frozen pending reverse transcription quantitative PCR (RT-qPCR).
Diagnostic RT-PCR analysis
Qualitative RT-PCR for detection of Zaire ebolavirus was performed using duplex RT-PCR with primers/probes directed against the Zaire EBOV nucleoprotein (FAM channel) and the MS2 genome (Alx532 channel; in-house assay) using TaqMan Fast Virus 1-Step Master Mix (Applied Biosystems) on a SmartCycler II platform [18]. Assays were conducted with the following cycling conditions: 50°C for 5 min (one cycle), 95°C for 20 s (one cycle), 95°C for 3 s and 60°C for 30 s (45 cycles). A single fluorescence read was taken at the end of each 60°C step. Samples with Ct > 40 and a positive internal control were interpreted as EBOV negative. Samples with Ct ≤ 40, with or without a positive internal control, were interpreted as EBOV positive.
Viral load determination by RT-qPCR
Viral loads were estimated by determining the level of nucleoprotein-containing RNA per millilitre of plasma based on a previously described assay [18]. RNA extracts were diluted 1 : 5 in 100 ng/µl yeast RNA dilution buffer (Ambion) for genome quantification in triplicate by one-step RT-qPCR. Briefly, diluted RNA extracts were mixed with TaqMan Fast Virus 1-Step 4X Master Mix (Applied Biosystems), primers NP1-F (TCTGACATGGATTACCACAAGATC) and NP1-R (GGATGACTCTTTGCCGAACAATC), and the NP1 probe (6-FAM-AGGTCTGTCCGTTCAA-MGB). One-step RT-qPCR was performed on a LightCycler 96 (Roche) beginning with reverse transcription at 50°C for 5 min, followed by heat denaturation at 95°C for 20 s and 50 cycles of 95°C for 3 s and 60°C for 30 s. The genome copy number was interpolated from a standard curve generated by serial dilution of a plasmid containing the NP1 amplicon, and was calculated per millilitre of plasma. The limit of detection for diluted RNA extracts was 1 × 103 copies. For samples falling below the limit of detection, genome quantification was repeated using undiluted RNA.
Biochemistry and haematology
Biochemistry and haematology testing was introduced for all patients at the ETC only midway through the trial, due to constraints beyond the control of the study team. Haematology and blood chemistry assays were performed using an ABX Micros ES 60 haematology analyser (Horiba) and the Fuji DRI-CHEM NX500i platform (Fujifilm), respectively, according to the manufacturers’ instructions. Coagulation tests (activated partial thromboplastin time [APTT and APTT citrate] and prothrombin time [PT and PT citrate]) were carried out using a Hemochron Signature Elite Whole Blood Microcoagulation System (International Technidyne), according to the manufacturer’s instructions. All patients were tested for malaria using the SD Bioline Malaria Ag P.f test (Standard Diagnostics).
Results
Trial Patients
Thirty-four patients with confirmed EVD were admitted to the ETC during the 3-mo recruitment period, and 17 patients were enrolled (Fig 1). Fourteen patients were enrolled into the TKM-130803 cohort, and three were enrolled into the observational cohort. The three observational cohort patients were recruited during the initial safety cohort phase. TKM bed capacity was never exceeded following the initial safety cohort phase, and randomisation of patients for operational reasons was therefore not required after that initial phase. None of the enrolled patients shared a known close genetic relationship.
Fig. 1. Patient screening and enrolment. 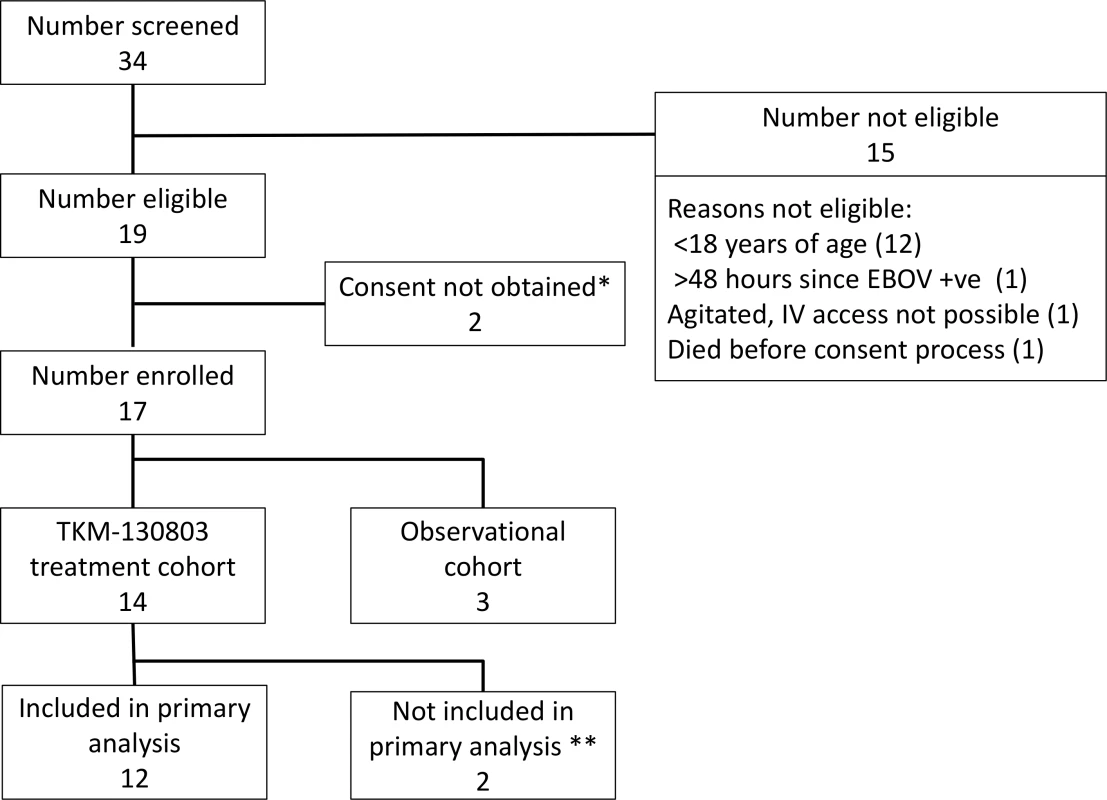
*One patient did not give consent. One patient was not competent to give consent, and a suitable proxy to provide consent could not be identified within inclusion time limits. **Two patients who died within 48 h of admission were excluded from the primary outcome analysis, as specified in the protocol. Baseline Characteristics
The median age of the 17 patients was 36 y (range 20 to 85 y), and the median number of days from illness onset to admission was 2 d (range 0–4 d) for the TKM-130803 recipients and 5 d (range 4–6 d) for the observational patients (Table 1). Baseline values for vital signs and blood results were determined from the first set of post-enrolment collections in observational cohort patients and from the collections taken immediately before the first infusion in the TKM-130803 cohort. At baseline, the geometric mean EBOV load in all 17 participants was 1.56 × 109 RNA copies/ml plasma (95% CI 5.75 × 108, 4.23 × 109). The pre-treatment geometric mean EBOV load in the 14 TKM-130803 recipients was 2.24 × 109 RNA copies/ml plasma (95% CI 7.52 × 108, 6.66 × 109). The median time from admission to the ETC to receiving study drug was 23 h. Diarrhoea or vomiting was reported or observed at baseline in 11/17 patients, and 5/17 patients had evidence of bleeding complications on admission. Three patients had a positive malaria rapid test on admission and were treated with artesunate/amodiaquine. In six patients in whom renal function could be determined on admission, three had evidence of renal failure (serum creatinine ≥ 3-fold the upper limit of normal). All five patients in whom hepatic function could be determined on admission had results consistent with liver injury (aspartate aminotransferase ≥ 3-fold the upper limit of normal). Three of four patients in whom coagulation studies were performed on admission had abnormal coagulation profiles.
Tab. 1. Baseline demographic and clinical characteristics of trial population. 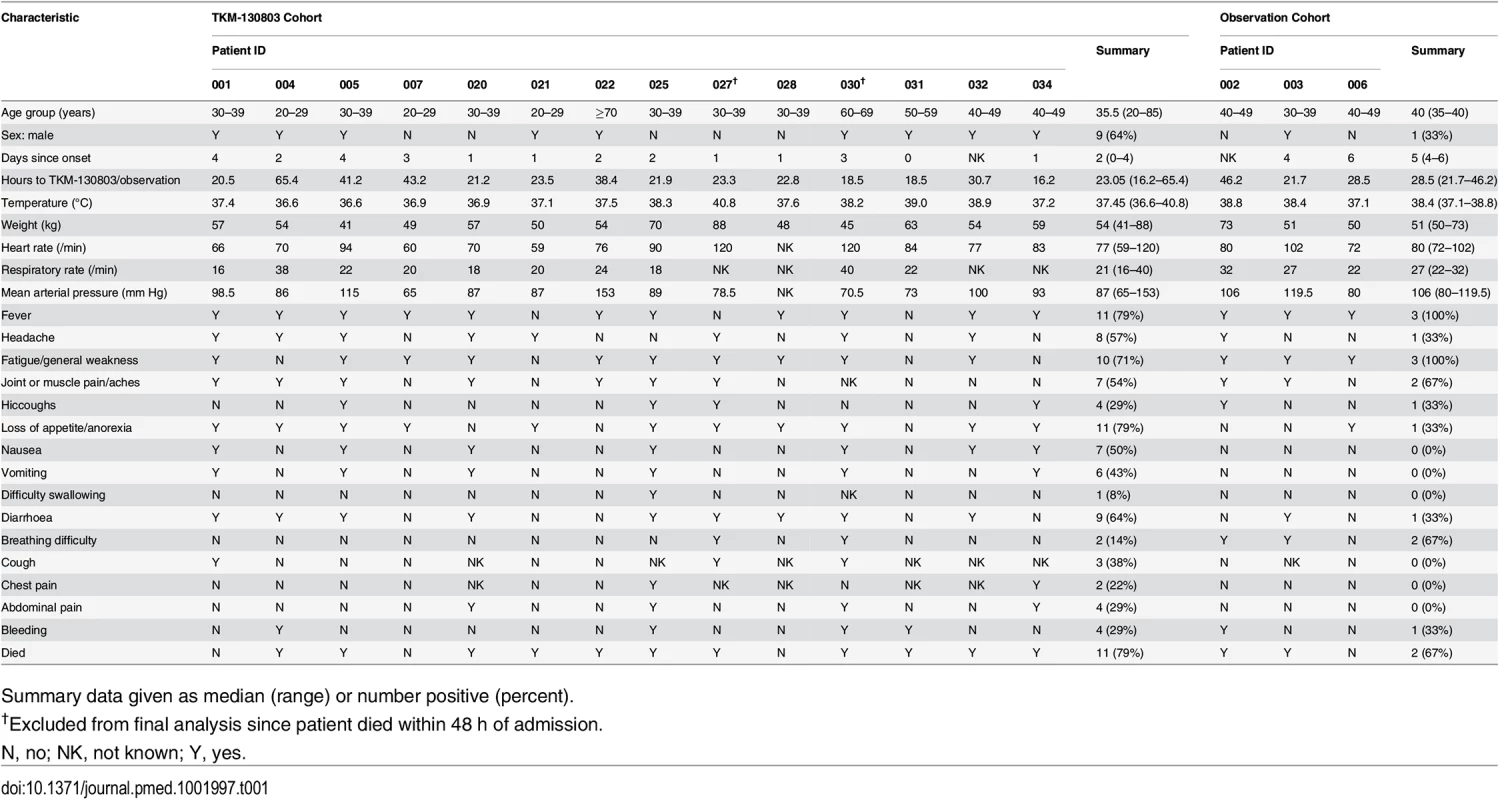
Summary data given as median (range) or number positive (percent). Study Drug Received and Outcomes
The 14 patients enrolled into the TKM-130803 cohort received between one and seven infusions of TKM-130803 (Table 2). Of these 14 patients, three survived to day 14 and were discharged from the ETC, and 11 died. Two patients died within 48 h of admission and were excluded from the primary outcome analysis (and the ongoing futility plot). Two patients died on 15 June 2015, at which point enrolment to the trial was stopped since the futility boundary had been reached, with only three of the twelve patients eligible for inclusion in the primary outcome analysis surviving to day 14 (Fig 2). All deaths were considered to be consistent with severe EVD. In participants who died, viral loads were high at admission and remained high over time (Fig 3); correspondingly, EBOV PCR Ct values were low at admission and remained low over time (Ct values are inversely proportional to viral load). Serial viral load and Ct data were available for two patients in the observational cohort; viral load steadily decreased in the survivor, whereas viral load increased in the patient who died. The final point estimate of the probability that a patient receiving TKM-130803 who survives for 48 h will subsequently survive to day 14 was 0.27 (95% CI 0.06, 0.58). Two of the three patients in the observational cohort died.
Fig. 2. Survival plot with futility boundary for TKM-130803 recipients. 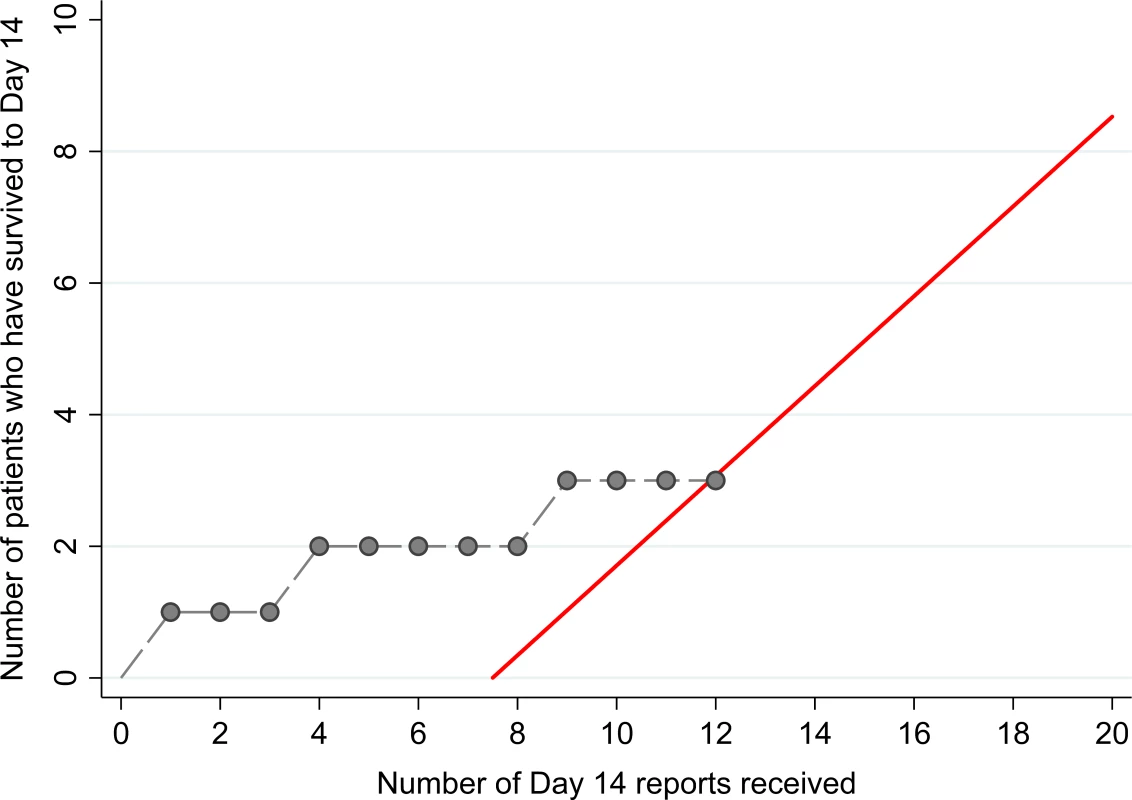
The red line denotes the futility boundary. The points and dashed line denote the number of survivors at day 14 plotted against the number of day 14 reports. Fig. 3. Ebola virus RT-PCR cycle threshold values and RNA copies/ml over time. 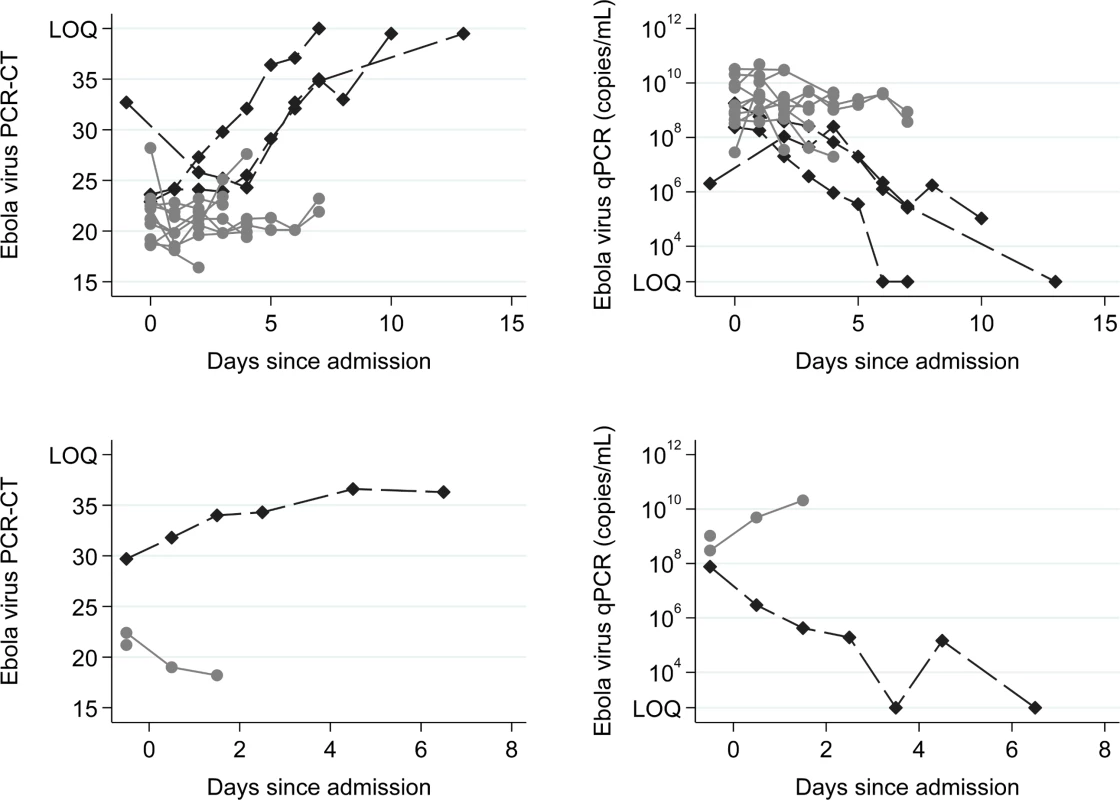
Top row: TKM-130803 recipients. Bottom row: Observational patients. RT-PCR Ct upper limit of quantitation (LOQ) = 40. RT-qPCR lower limit of quantitation = 1,000 genome copies. The Ebola virus RT-qPCR quantification is expressed as the number of genome copies/millilitre of plasma. Black diamonds denote results for survivors. Grey circles denote results for non-survivors. Tab. 2. Timelines, TKM-130803 doses received, and outcomes. 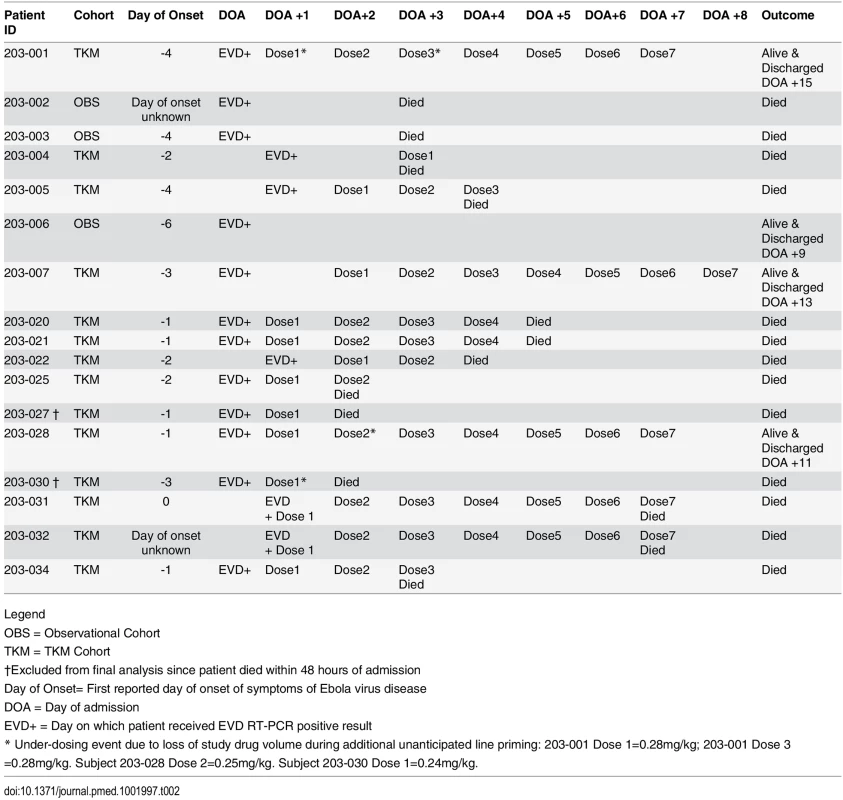
Legend Adverse Events
A total of 56 infusions of TKM-130803 were administered. Adverse reactions consistent with acute cytokine release syndrome were not seen during or following any of the infusions, and none of the infusions required termination or slowing of the infusion rate (Fig 4) [10]. As such, the infusions of TKM-130803 were well tolerated. One patient (203–025) was observed to have worsening tachypnoea in the 48 h following the second TKM-130803 infusion, but new onset or worsening of additional symptoms or signs that might indicate infusion-related cytokine release syndrome (tachycardia, flushing, headache, hypotension, chills, nausea, and vomiting) were not observed in this patient. The event was reported to the IDMC as a SAR because of the temporal relationship with the administration of the study drug, but it was also felt the event was compatible with progression of EVD.
Fig. 4. Box and whisker plot of vital signs in TKM-130803 recipients, before, during, and after TKM-130803 infusions. 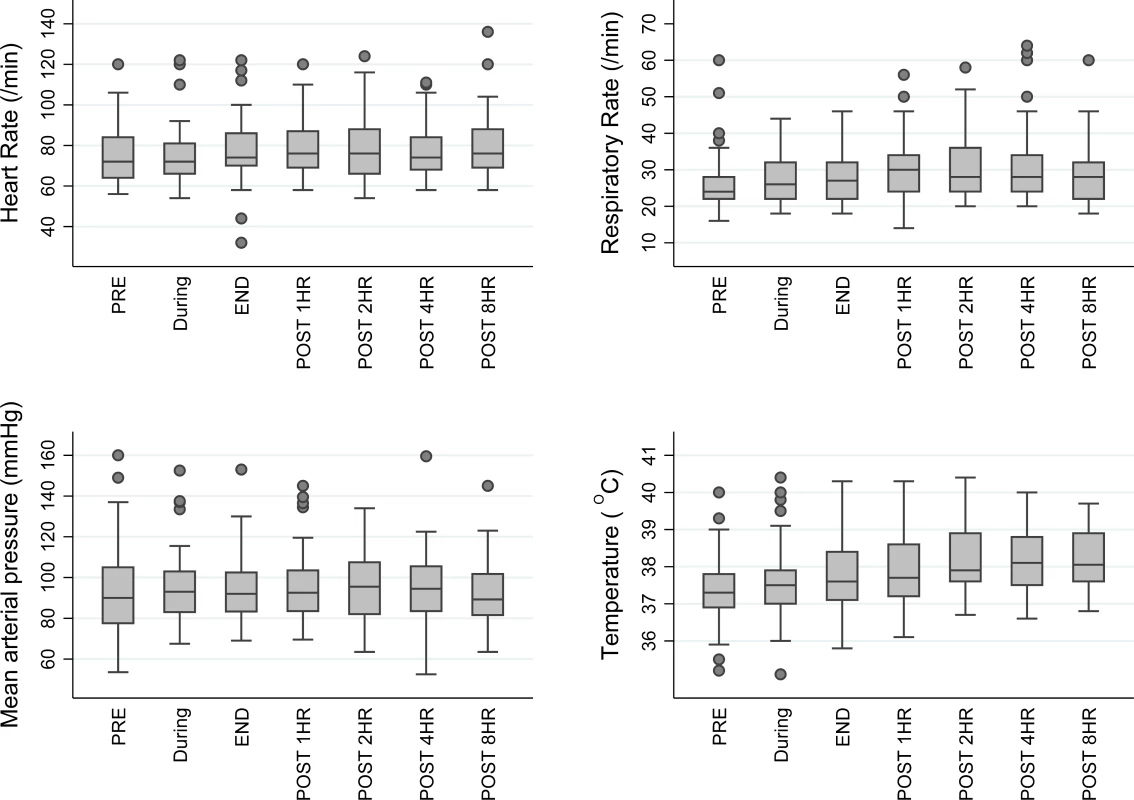
Heart rate, respiratory rate, mean arterial blood pressure, and tympanic temperature in patients administered TKM-130803 at the following time points: immediately prior to TKM-130803 infusion (PRE), during the infusion, immediately at the end of the infusion (END), and at 1, 2, 4, and 8 h after the end of the infusion. The middle line shows the median value, the box shows the interquartile range, and the whiskers spread from the lower to the upper adjacent values. Outside values, that is, observations that are larger/smaller than the upper/lower adjacent values, are shown as circles. Discussion
Our trial has shown that the administration of TKM-130803 at a dose of 0.3 mg/kg/d in adults with predominantly severe EVD and high viral loads does not improve survival compared to historic survival rates. This result contrasts with the protective efficacy of various formulations of this product observed in NHPs challenged with a lethal dose of EBOV [9,13]. There are a number of potential reasons for the difference between the NHP study results and our results. In the animal challenge studies of siEbola-3, the first infusion was administered between 30 min and 3 d after the lethal viral challenge, with day three roughly corresponding to the first day of clinical illness and viral RNA detection (ranging from just detectable to 106 RNA copies/ml) in serum in the NHP challenge model used (1,000 plaque-forming units administered intramuscularly) [19,20]. Although survival was 100% when the first siEbola-3 infusion was commenced 72 h post-infection, studies of the earlier formulation TKM-100802 found that survival declined with time from infection to first infusion, with 83% survival when the first infusion was started 24–48 h post-infection, declining to 67% survival at 72 h, and 0% survival at 96 h [13,21]. In our trial, the mean number of days from reported illness onset to first infusion was 2 d (range 0–4 d), although there is uncertainty about the reliability of the onset dates reported by participants. This is shorter than the time from illness onset to admission to ETC in several large analyses of patients with EVD in West Africa, which was 5–6 d, but assuming a mean incubation period of 10 d, our patients were still presenting approximately 12 d after exposure [2,4,22–26]. As such, we were administering the study drug to patients later in the infection and disease course than in the NHP models.
All of the 14 patients who received TKM-130803 had ≥1 × 108 RNA copies/ml plasma prior to their first infusion. Although data are currently limited, this level has been associated in other studies with a fatal outcome in >90% of patients [2,3]. Of the 11 TKM-130803 recipients who died, nine had ≥1 × 109 RNA copies/ml prior to their first infusion, and seven presented with symptoms or signs reported by other studies to be associated with a high probability of death (haemorrhagic signs, hiccough, tachypnoea) [2,4,22,23,27]. Therefore, the failure of TKM-130803 to achieve a survival rate exceeding 0.55 may have arisen from an insufficient antiviral effect in the face of high viral loads and existing organ injury in patients presenting with advanced disease. Although data on viral load in our historic patient dataset are not available, it seems likely that the severity of illness of the patients recruited into this trial was greater than the average severity of illness of the historic dataset. As such, in this patient group, a target survival rate of 0.55 is likely to have been too high to allow the study to detect a small or moderate beneficial effect. Nevertheless, the survival probability of 0.27 (95% CI 0.06, 0.58) that we estimated is not encouraging. Whilst a randomised controlled trial could potentially determine whether a survival probability of 0.27 in the experimental treatment arm represents a survival improvement compared to concurrent controls of similar severity, a sample size of around 90 patients in each arm would be required to have 80% power to detect a difference in survival of 0.10 versus 0.27. There were insufficient patients to have conducted such a study, with only one additional patient with confirmed EBOV infection admitted to the treatment centre following closure of the trial. At the location and time our study was conducted, there was also no possibility to determine effectiveness in less severe cases, since less severe cases were not presenting to the ETC. Although the fatal cases in our trial on average presented sooner in their illness than the survivors, this paradox of better survival in patients presenting later has been reported by others, and likely reflects a survival bias, whereby those with the most severe disease do not survive long enough to present late [4].
The optimal dose of TKM-100802 in NHP studies was 0.5 mg/kg/d for 7 d, whereas we administered a dose of 0.3 mg/kg/d based on observed tolerability data in healthy adult volunteers and an assumption that the pharmacokinetics of TKM-100802/TKM-130803 (which share the same LNP composition) in NHPs, healthy human volunteers, and patients with EVD was similar [13]. We did not have sufficient product, patients, or time to conduct dose ranging or dose comparative studies. We do not know whether the dosing regimen used in this trial resulted in adequate drug concentrations or whether higher doses may have resulted in a therapeutic benefit. In addition, the formulation we used differs in the LNP component from that reported in the NHP work of Thi et al. [13]. The lipid excipients used in TKM-130803 are the same as those used in previous healthy human volunteer studies (formulated as TKM-100802), whereas the LNPs used by Thi et al. in NHP studies have not been assessed for safety in healthy human volunteers and were therefore not available for use in this trial.
The trial cannot identify whether the drug is both ineffective and harmful since the futility rule terminated the trial when there was evidence, as pre-specified, that the survival in those receiving TKM-130803 was no better than historic survival rates. Fifty-six separate TKM-130803 infusions were administered, and the patients were monitored closely for adverse events. Contrary to our expectations based on healthy volunteers and repatriated EVD patients given TKM-100802, the infusions were well tolerated, and no obvious cytokine release reactions were observed. Only one SAR, worsening tachypnoea, was reported. This patient had severe EVD, as evidenced by a high viral load, a coagulation disorder, and bloody diarrhoea on admission. Since tachypnoea was present prior to the first TKM-130803 infusion, and because tachypnoea is common in severe EVD (possibly related to metabolic acidosis or pulmonary oedema secondary to vascular leakage), the SAR may not have been causally related to drug administration [5,12,27–31]. Use of TKM-100802 in three medically evacuated patients has been reported, with infusion-related reactions of fever and rigors noted in two, and with the drug being discontinued in one of these patients after six doses due to concerns that the drug may have been contributing to clinical deterioration [11,12]. TKM-100802 and TKM-130803 share the same lipid excipient, and the siRNA components are identical except for minor nucleotide sequence differences. However, TKM-100802 is a lyophilised product, and TKM-130803 is a ready-to-use liquid formulation; therefore, the lyoprotectant excipients present in TKM-100802 are absent from TKM-130803. This might explain the observed differences in the incidence of infusion-related reactions and is consistent with the finding that in human whole-blood cultures TKM-130803 had similar or less capacity than TKM-100802 to cause release of IL-6 and MCP-1 pro-inflammatory cytokines (personal communication, Mark Kowalski, Tekmira Pharmaceuticals). It is also possible that the immune response is dampened in patients with later-stage EVD infection. Overall, TKM-130803 was well tolerated in this study, and the clinical progression of all of the 12 patients who died in the TKM-130803 cohort was consistent with severe EVD with sustained high viral loads.
The RAPIDE studies were set up to rapidly triage potential therapies for EVD, to eliminate agents that are not effective and allow resources to be concentrated on agents with greater promise. The single-arm design with a pre-specified futility boundary and sequential analysis was able to rapidly identify a low probability of survival and cease recruitment in this study, thus minimising harm to patients and risks to healthcare workers and improving the probability that ongoing trials of other interventions could recruit sufficient EVD patients to reach a conclusion.
In summary, administration of TKM-130803 at a dose of 0.3 mg/kg/d to adult patients with EVD and predominantly high levels of viral RNA in blood was well tolerated but did not improve survival compared to historic controls. Further work is needed to assess whether the lack of observed effectiveness is generalisable to other patient subgroups in other treatment settings. Additionally, the potential influence of drug formulation and dose requires further investigation.
Supporting Information
Zdroje
1. World Health Organization. Ethical considerations for use of unregistered interventions for Ebola viral disease. WHO/HIS/KER/GHE/14.1. Geneva: World Health Organization; 2014.
2. Schieffelin JS, Shaffer JG, Goba A, Gbakie M, Gire SK, Colubri A, et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371 : 2092–2100. doi: 10.1056/NEJMoa1411680 25353969
3. Towner JS, Rollin PE, Bausch DG, Sanchez A, Crary SM, Vincent M, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78 : 4330–4341. 15047846
4. Fitzpatrick G, Vogt F, Gbabai OB, Decroo T, Keane M, De Clerck H, et al. The contribution of Ebola viral load at admission and other patient characteristics to mortality in a Medecins Sans Frontieres (MSF) Ebola Case Management Centre (CMC), Kailahun, Sierra Leone, June–October, 2014. J Infect Dis. 2015;12 : 1752–1758. doi: 10.1093/infdis/jiv304
5. Wolf T, Kann G, Becker S, Stephan C, Brodt HR, de Leuw P, et al. Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet. 2015;385 : 1428–1435. doi: 10.1016/S0140-6736(14)62384-9 25534190
6. Takada A, Kawaoka Y. The pathogenesis of Ebola hemorrhagic fever. Trends Microbiol. 2001;9 : 506–511. 11597453
7. Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4 : 487–498. doi: 10.1016/S1473-3099(04)01103-X 15288821
8. World Health Organization. Potential Ebola therapies and vaccines. WHO/EVD/HIS/EMP/14.1. Geneva: World Health Organization; 2014.
9. Geisbert TW, Lee AC, Robbins M, Geisbert JB, Honko AN, Sood V, et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375 : 1896–1905. doi: 10.1016/s0140-6736(10)60357-1 20511019
10. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124 : 188–195. doi: 10.1182/blood-2014-05-552729 24876563
11. Liddell AM, Davey RT Jr, Mehta AK, Varkey JB, Kraft CS, Tseggay GK, et al. Characteristics and clinical management of a cluster of 3 patients with Ebola virus disease, including the first domestically acquired cases in the United States. Ann Intern Med. 2015;163 : 81–90. doi: 10.7326/M15-0530 25961438
12. Kraft CS, Hewlett AL, Koepsell S, Winkler AM, Kratochvil CJ, Larson L, et al. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis. 2015;61 : 496–502. doi: 10.1093/cid/civ334 25904375
13. Thi EP, Mire CE, Lee AC, Geisbert JB, Zhou JZ, Agans KN, et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature. 2015;521 : 362–365. doi: 10.1038/nature14442 25901685
14. Cooper BS, Boni MF, Pan-Ngum W, Day NP, Horby PW, Olliaro P, et al. Evaluating clinical trial designs for investigational treatments of Ebola virus disease. PLoS Med. 2015;12:e1001815. doi: 10.1371/journal.pmed.1001815 25874579
15. Adebamowo C, Bah-Sow O, Binka F, Bruzzone R, Caplan A, Delfraissy JF, et al. Randomised controlled trials for Ebola: practical and ethical issues. Lancet. 2014;384 : 1423–1424. doi: 10.1016/S0140-6736(14)61734-7 25390318
16. Whitehead J, Matsushita T. Stopping clinical trials because of treatment ineffectiveness: a comparison of a futility design with a method of stochastic curtailment. Stat Med. 2003;22 : 677–687. doi: 10.1002/sim.1429 12587099
17. Jovic G, Whitehead J. An exact method for analysis following a two-stage phase II cancer clinical trial. Stat Med. 2010;29 : 3118–3125. doi: 10.1002/sim.3837 21170906
18. Trombley AR, Wachter L, Garrison J, Buckley-Beason VA, Jahrling J, Hensley LE, et al. Comprehensive panel of real-time TaqMan polymerase chain reaction assays for detection and absolute quantification of filoviruses, arenaviruses, and New World hantaviruses. Am J Trop Med Hyg. 2010;82 : 954–960. doi: 10.4269/ajtmh.2010.09–0636 20439981
19. Nakayama E, Saijo M. Animal models for Ebola and Marburg virus infections. Front Microbiol. 2013;4 : 267. doi: 10.3389/fmicb.2013.00267 24046765
20. Martins K, Cooper C, Warren T, Wells J, Bell T, Raymond J, et al. Characterization of clinical and immunological parameters during Ebola virus infection of rhesus macaques. Viral Immunol. 2015;28 : 32–41. doi: 10.1089/vim.2014.0085 25514385
21. Arbutus Biopharma. Tekmira announces new preclinical data from anti-viral programs. 2013 Nov 12 [cited 16 Oct 2015]. Burnaby (Canada); Arbutus Biopharma. Available: http://investor.arbutusbio.com/releasedetail.cfm?ReleaseID=806769.
22. Qin E, Bi J, Zhao M, Wang Y, Guo T, Yan T, et al. Clinical features of patients with Ebola Virus disease in Sierra Leone. Clin Infect Dis. 2015;61 : 491–495. doi: 10.1093/cid/civ319 25995207
23. Qureshi AI, Chughtai M, Bah EI, Barry M, Beavogui K, Loua TO, et al. High survival rates and associated factors among Ebola virus disease patients hospitalized at Donka National Hospital, Conakry, Guinea. J Vasc Interv Neurol. 2015;8:S4–S11. 25992182
24. Bah EI, Lamah MC, Fletcher T, Jacob ST, Brett-Major DM, Sall AA, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372 : 40–47. doi: 10.1056/NEJMoa1411249 25372658
25. WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371 : 1481–1495. doi: 10.1056/NEJMoa1411100 25244186
26. WHO Ebola Response Team, Agua-Agum J, Ariyarajah A, Blake IM, Cori A, Donnelly CA, et al. Ebola virus disease among children in West Africa. N Engl J Med. 2015;372 : 1274–1277. doi: 10.1056/NEJMc1415318
27. Barry M, Toure A, Traore FA, Sako FB, Sylla D, Kpamy DO, et al. Clinical predictors of mortality in patients with Ebola virus disease. Clin Infect Dis. 2015;60 : 1821–1824. doi: 10.1093/cid/civ202 25770172
28. Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204 (Suppl 3):S810–S816. doi: 10.1093/infdis/jir299 21987756
29. Julien B, Ludovic K, Marie CJ, Nicolas G, Magali B, Claire R, et al. Dyspnea and risk of death in Ebola infected patients. Is lung really involved? Clin Infect Dis. 2015; 61 : 852. doi: 10.1093/cid/civ441
30. Florescu DF, Kalil AC, Hewlett AL, Schuh AJ, Stroher U, Uyeki TM, et al. Administration of brincidofovir and convalescent plasma in a patient with Ebola virus disease. Clin Infect Dis. 2015;61 : 969–973. doi: 10.1093/cid/civ395 25991468
31. Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A. Ebola virus disease in West Africa—clinical manifestations and management. N Engl J Med. 2014;371 : 2054–2057. doi: 10.1056/NEJMp1413084 25372854
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 4- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Why Are Some Population Interventions for Diet and Obesity More Equitable and Effective Than Others? The Role of Individual Agency
- Risk of Bias in Systematic Reviews of Non-Randomized Studies of Adverse Cardiovascular Effects of Thiazolidinediones and Cyclooxygenase-2 Inhibitors: Application of a New Cochrane Risk of Bias Tool
- The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control
- The Association between Sulfonylurea Use and All-Cause and Cardiovascular Mortality: A Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials
- Disentangling the Association between Statins, Cholesterol, and Colorectal Cancer: A Nested Case-Control Study
- Gender Differences in Homicide of Neonates, Infants, and Children under 5 y in South Africa: Results from the Cross-Sectional 2009 National Child Homicide Study
- Mobile Phones As Surveillance Tools: Implementing and Evaluating a Large-Scale Intersectoral Surveillance System for Rabies in Tanzania
- Building Learning Health Systems to Accelerate Research and Improve Outcomes of Clinical Care in Low- and Middle-Income Countries
- The Future of the RTS,S/AS01 Malaria Vaccine: An Alternative Development Plan
- Birth “Out-of-Hours”: An Evaluation of Obstetric Practice and Outcome According to the Presence of Senior Obstetricians on the Labour Ward
- A Nested Case–Control Study of Metabolically Defined Body Size Phenotypes and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC)
- Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma
- Underweight, Markers of Cachexia, and Mortality in Acute Myocardial Infarction: A Prospective Cohort Study of Elderly Medicare Beneficiaries
- The Impact of Hotspot-Targeted Interventions on Malaria Transmission in Rachuonyo South District in the Western Kenyan Highlands: A Cluster-Randomized Controlled Trial
- Experimental Treatment of Ebola Virus Disease with TKM-130803: A Single-Arm Phase 2 Clinical Trial
- Is There Evidence of Poorer Birth Outcomes for Mothers and Babies When the Most Senior Obstetrician Is Not On Site?
- Clinical Implications of Cancer Genomics: A Call for Papers
- The ITA.LI.CA Staging System: A Novel Staging System for Hepatocellular Carcinoma
- Observational Evidence of For-Profit Delivery and Inferior Nursing Home Care: When Is There Enough Evidence for Policy Change?
- Child Homicide: A Global Public Health Concern
- The Chernobyl Disaster and Beyond: Implications of the Sendai Framework for Disaster Risk Reduction 2015–2030
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Observational Evidence of For-Profit Delivery and Inferior Nursing Home Care: When Is There Enough Evidence for Policy Change?
- Experimental Treatment of Ebola Virus Disease with TKM-130803: A Single-Arm Phase 2 Clinical Trial
- The Chernobyl Disaster and Beyond: Implications of the Sendai Framework for Disaster Risk Reduction 2015–2030
- Is There Evidence of Poorer Birth Outcomes for Mothers and Babies When the Most Senior Obstetrician Is Not On Site?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



