-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis
In this systematic review and meta-analysis, Nathan Ford and colleagues assess the most frequently reported barriers by patients experiencing challenges adhering to antiretroviral therapy.
Published in the journal: . PLoS Med 13(11): e32767. doi:10.1371/journal.pmed.1002183
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002183Summary
In this systematic review and meta-analysis, Nathan Ford and colleagues assess the most frequently reported barriers by patients experiencing challenges adhering to antiretroviral therapy.
Introduction
Global targets for scaling up antiretroviral therapy (ART) include ensuring that 90% of patients on ART achieve viral suppression. This gives a renewed emphasis to ensuring optimal levels of adherence. Negative outcomes of longer-term suboptimal adherence include increased risk of disease progression [1], drug resistance [2], high viral load and consequent risk of transmission [3,4], and death [5,6].
Maintaining high levels of adherence is a challenge across settings. Suboptimal adherence to antiretroviral medication has been reported for specific patient groups such as adolescents [7], pregnant women [8], and others in high-, middle-, and low-income countries. A broad range of context-specific barriers to adherence have been reported, including forgetfulness, stigma, adverse drug reactions, and competing responsibilities [9,10]. These challenges have been categorized as individual, interpersonal, community, and structural factors [11].
Several interventions have been found to improve adherence in randomized trials, including adherence counselling, text messaging, and reminder devices [12], and these are recommended by the WHO [13]. However, there remains a need to understand the relative importance of different barriers to adherence in order to inform the targeting of different interventions and inform future research.
We conducted this systematic review to assess patient reported barriers to adherence among HIV-infected adults, adolescents and children in high-, middle-, and low-income countries.
Methods
Search Strategy and Selection Criteria
This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [14]. The study protocol and PRISMA statement are available in the Supporting Information (S1 Text and S2 Text) [14].
To be included, studies had to provide information about barriers to adherence reported by at least 50 adult patients or 20 children or their caregivers who were non-adherent to ART according to study definitions. These cut offs were chosen in practical consideration of the high number of studies identified through preliminary searches and the limited and sometimes unreliable information to be gained from small studies [15]. Using a search strategy that combined terms for ART, adherence, and commonly reported reasons for non-adherence (See S1 Text), two investigators (ZS, NF) screened MEDLINE via PubMed, Embase, Web of Science, and PsychINFO from 01 January 1997 to 31 March 2016. We also screened abstracts from all International AIDS Society conferences, all conferences on HIV Treatment and Prevention Adherence, and all conferences of the European Society for Patient Adherence, Compliance, and Persistence from 2012 to 2015, and electronically available abstracts of the Conference on Retroviruses and Opportunistic Infections (2014–2016) to identify recent studies that may not yet have been published in full. We supplemented database searches by screening bibliographies of review articles and all included full-text articles. Studies that included patients mainly receiving dual - or mono-therapy (>20% of cohort), antiretroviral interventions other than treatment (PEP, PrEP, or PMTCT), or reported adherence to medication other than ART were excluded from review. We only extracted data on non-adherent patients, following the definition of non-adherence provided by the studies; if studies included both adherent and non-adherent patients and data could not be disaggregated, the study was excluded. Studies assessing adherence interventions were also excluded unless baseline (i.e., pre-intervention) information was relevant to our analysis. Qualitative studies were excluded unless relevant quantitative data were also provided. No language restriction was applied.
Data Extraction and Analysis
We extracted data independently in duplicate (ZS, NF) using a standardized, piloted data extraction form and following the predefined protocol. Barriers to adherence were initially categorized according to AIDS Clinical Trials Group adherence instrument items, with additional categories developed to capture challenges common in resource-limited settings [16]. Using categories adapted from a qualitative review of adherence barriers [11], responses were grouped into individual, contextual, and health service–related barriers; where there was uncertainty, investigators discussed to achieve consensus. Information about study design and setting, age of patients, and adherence measure was also extracted. The following variables were extracted to assess study quality: use of a previously piloted and/or validated questionnaire to assess barriers to adherence; random sampling; and use of objective adherence measures. These indicators were identified following a review of the first ten eligible studies.
We calculated proportions and corresponding 95% CIs for all reported barriers to adherence and pooled data following transformation [17,18], using random effects models stratified by age (adults, adolescents, and children, as defined by the studies) [19]. Because statistical tests for heterogeneity are not reliable for pooled proportions [20], we assessed heterogeneity through visual inspection of forest plots. We ran prespecified subgroup analyses to assess the potential influence of study quality indicators and explored changes over time (assessed using date of study completion) using meta-regression. All analyses were conducted in Stata version 13.0.
Results
From an initial screen of 5,560 abstracts, 125 studies met our inclusion criteria (Fig 1). These studies provided information about barriers to adherence for 19,016 patients—17,061 adults, 1,099 children, and 856 adolescents—with documented non-adherence to ART. Studies were carried out across 38 countries, with the majority carried out in the Africa region (58 studies, 16 countries), the European region (14 studies, 10 countries), and the Western Pacific region (8 studies, 6 countries). Study quality was rated to be moderate overall. The majority of studies (78/125, 62%) used a validated questionnaire to assess barriers to adherence and piloted the questionnaire (89/125, 71%); however, less than half of studies (38/125, 30%) used random sampling, and objective adherence measures (pill count, pharmacy refill, and viral load) were only used in the minority (18/125, 14%) of studies; these limitations are important potential sources of selection and information bias. The most common definitions of adherence were no missed doses (55 studies) and >95% adherence (37 studies). Average duration on ART ranged from 4 wk to 239 wk (median 78 wk). Characteristics of included studies are summarized in S1 Table.
Fig. 1. Study selection process. 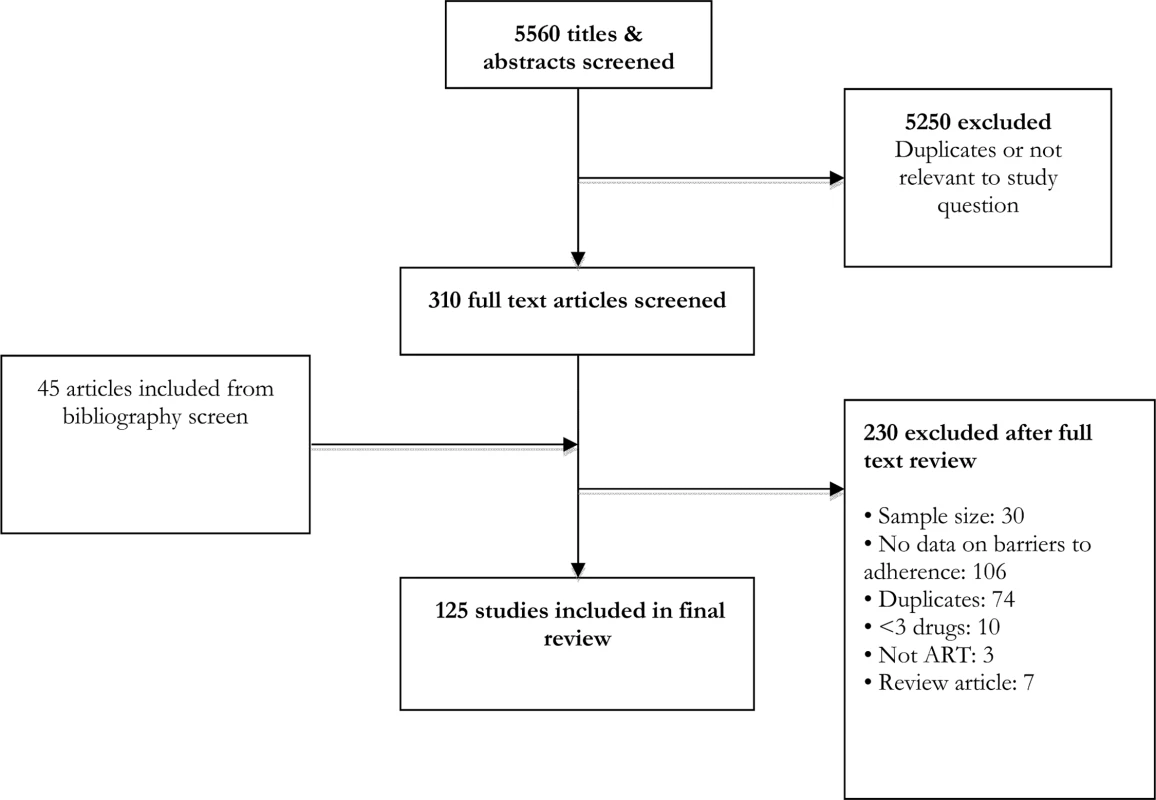
The most frequently reported barriers to adherence for adults, adolescents, and children are summarized in Figs 2–4. The most frequently reported individual barriers across all age groups included forgetting, being away from home, and a change to daily routine. Depression was reported as a barrier to adherence by more than 15% of patients across all age categories, while alcohol/substance misuse was commonly reported as a barrier by adults and adolescents. Among adults, feeling sick was a more commonly cited barrier to adherence than feeling well (relative risk 1.68, 95% CI 1.23–2.30). The proportion of adolescents reporting barriers to adherence was higher per barrier compared to adults, but data are limited and confidence intervals are wide for most estimates.
Fig. 2. Barriers to adherence among adults on ART. 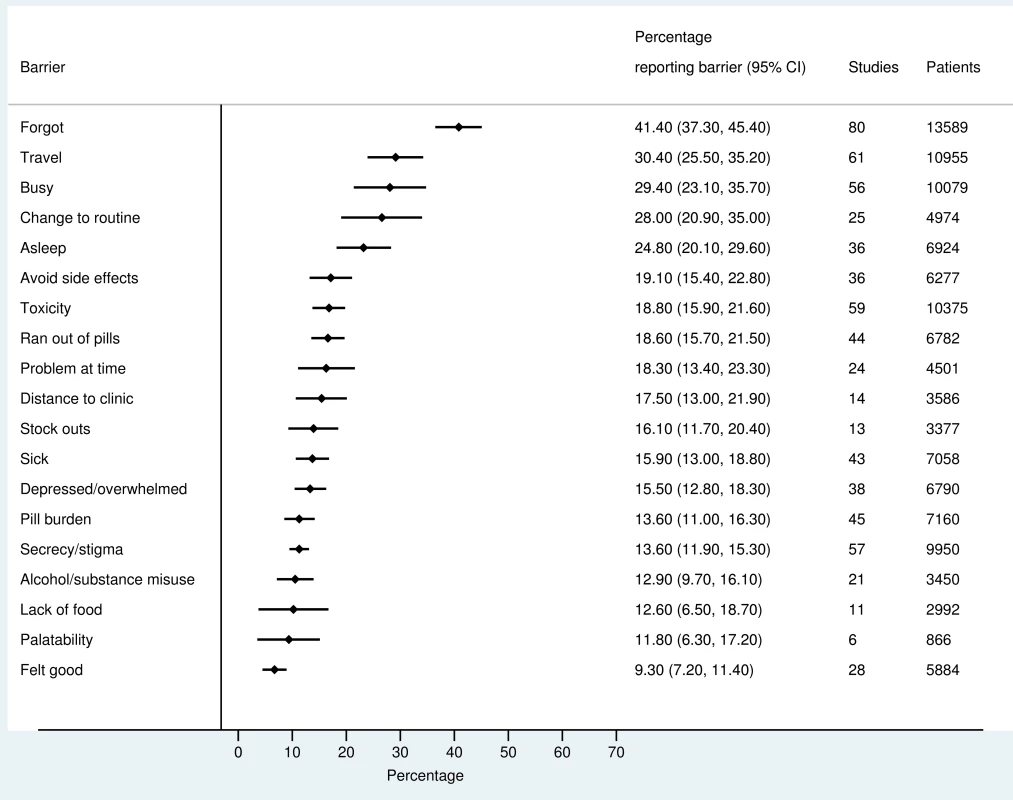
Fig. 3. Barriers to adherence among children on ART. 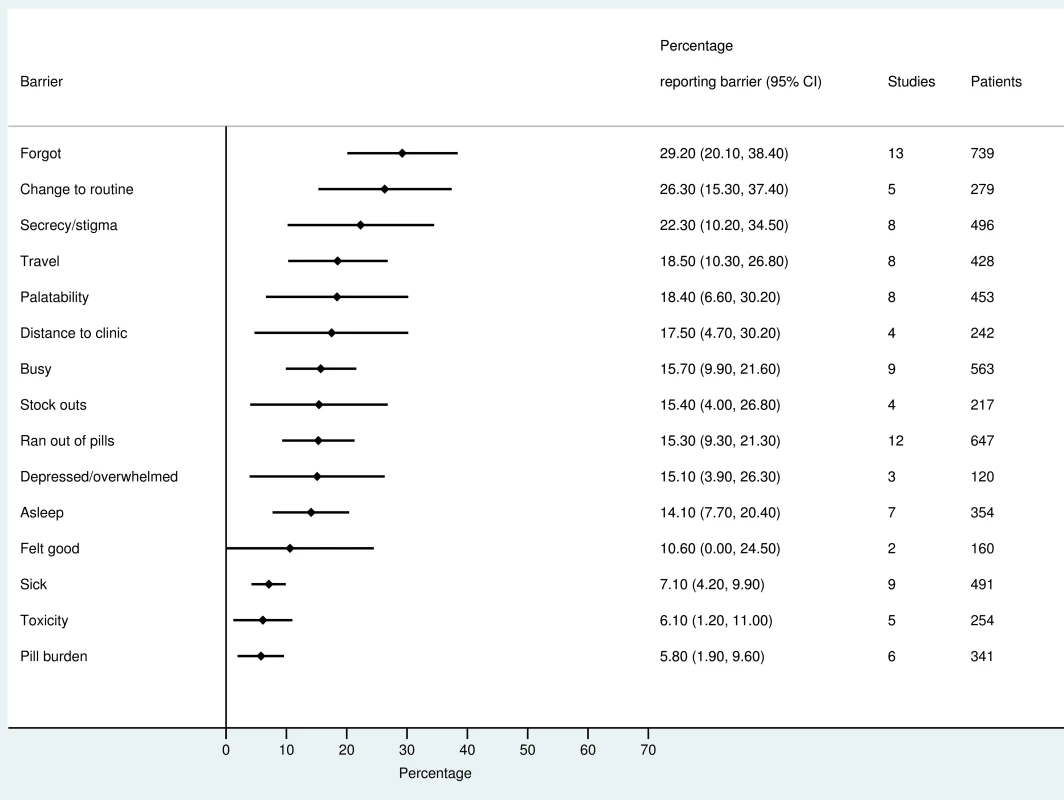
Fig. 4. Barriers to adherence among adolescents on ART. 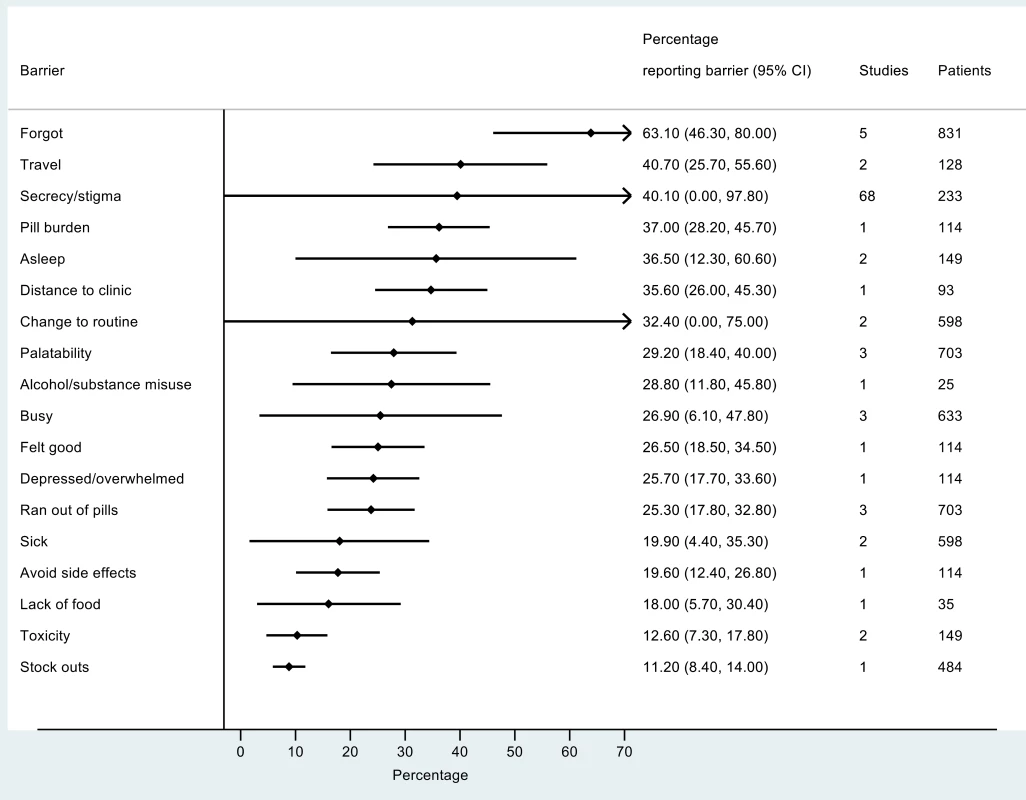
With respect to contextual barriers, secrecy/stigma was a commonly cited barrier to adherence, reported by more than 10% of patients across all regions (S2 Table). Notably, secrecy/stigma was more commonly reported as a barrier to adherence among children/caregivers compared to adults.
Health service–related barriers were frequently reported, including distance to clinic and stock outs. Distance to clinic was reported as a barrier for all age groups across 11 low - and middle-income countries in the Africa, South East Asia, and Western Pacific regions. Stock outs were reported across 13 countries for adults, all in low - and middle-income countries. For children, two studies reported stock outs in the United States. Barriers related to drug toxicity were frequently reported among adults and adolescents, while for children and adolescents, palatability was an important concern. In meta-regression, there was evidence that the frequency of reporting toxicity, pill burden, and being sick as barriers to adherence have reduced over time (Table 1).
Tab. 1. Random effects meta-regression. 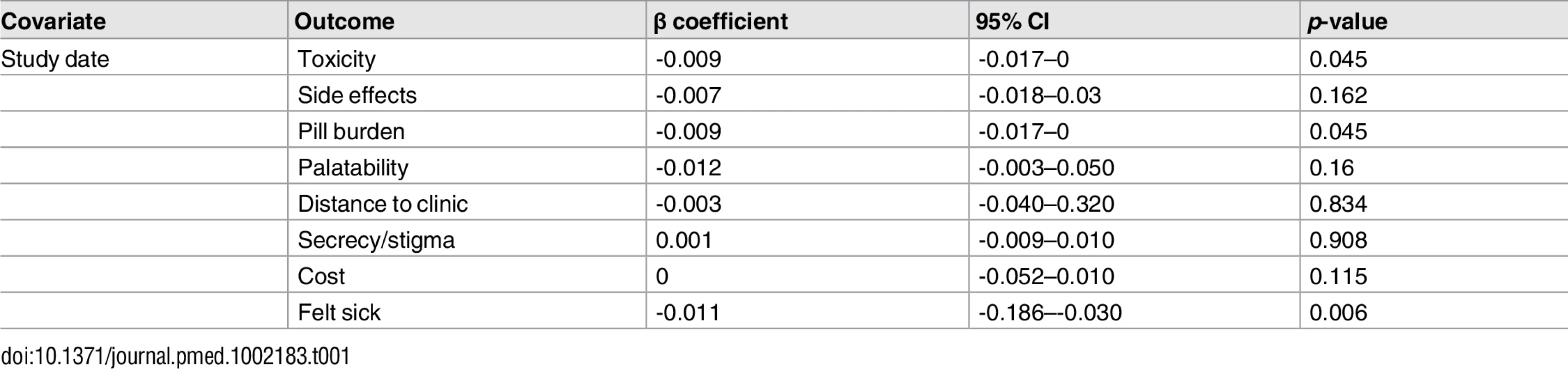
Discussion
Our review highlights the diversity of patient-reported barriers to adherence across age groups. The most frequently reported individual barriers across all age groups included forgetting, being away from home, depression, and a change to daily routine; alcohol and/or substance misuse was commonly reported by adults and adolescents. Health service–related barriers, including distance to clinic and stock outs, were also frequently reported.
Most barriers to adherence are amenable to interventions that have been evaluated in randomized trials (Table 2) [12,21–30]. Notably, forgetting was the most frequently cited barrier to adherence across all age groups. Challenges relating to timing of medication, including being asleep, could be overcome through text messaging, reminder devices, and individual counseling that seeks to routinize medication taking in a way that fits in with other daily activities [31].
Tab. 2. Common barriers to adherence and corresponding interventions supported by evidence from randomized trials. 
We found that health service barriers played an important role in frustrating efforts to maintain high levels of adherence to treatment. Long distance to clinics is a risk factor for loss to care [32], and decentralization of HIV services is associated with better retention [23]. Recent stock outs of antiretroviral medication have been recorded in several countries in Africa [33], and there is a pressing need for increased vigilance as countries move to adopt the policy of treating all HIV-positive individuals and consider transitioning from established first-line medications to newer regimens.
Previously, concern has been expressed that people who receive ART early in their disease progression may be less adherent to treatment [34,35]. The finding that feeling sick was a more commonly reported barrier to adherence than feeling well suggests that this may not be the case and supports the recent recommendation by WHO to treat all HIV-positive individuals regardless of immune status [36]. As HIV programs start to provide early ART to people earlier in their HIV infection, it will be important to prospectively collect data to further evaluate this concern.
Toxicity and pill burden have both been found to be associated with poor adherence in other reviews [37,38]. This review found that the frequency of reporting these factors as barriers to adherence has reduced over time, which is consistent with efforts by WHO and other agencies to promote fixed-dose combinations and rationalize treatment guidelines towards the use of antiretroviral drugs associated with a better safety profile [39].
The main adherence barriers identified by our review are consistent with a recent review by Langenbeek et al that found substance use, concerns about ART, satisfaction with care providers, stigma, social support, and self-efficacy to be strongly associated with adherence [40]. In contrast to the Langenbeek review, which assessed the influence between baseline patient characteristics and adherence, our review assesses adherence barriers that are reported by patients and, as such, was able to identify a number of additional frequently reported barriers that could not be gleaned from clinic records; because of this difference in approach, the number of studies in our review that were included in the previous review is small (19%). This approach builds on a previous review of patient-reported barriers to adherence that was published in 2006 [10]. There have been considerable changes in ART delivery over the past decade: the number of patients on ART globally has increased; drug regimens have improved with respect to tolerability and simplicity; and service provision has been decentralized. In updating this review, we have been able to include a larger sample size that allowed for a ranking in the frequency of reporting of barriers, disaggregated by age, and an understanding of how these barriers differ by geographical region and over time. This can allow for a better understanding about where resources need to be focused in order to improve adherence among different patient populations.
Several recent studies have indicated that adolescents face challenges across the continuum of HIV care, and outcomes of ART are worse for adolescents compared to adults [41]. HIV programs should pay particular attention to the adherence challenges faced by this vulnerable population and target adherence interventions accordingly; such an approach would be facilitated by the development of better ways to measure adherence. To date, few adolescents have been enrolled into trials of interventions to improve adherence, and this is an important area of future research [42]. A pilot feasibility study found that personalized, interactive, daily text message reminders were feasible and acceptable, and significantly improved self-reported adherence. However, larger controlled studies are needed to determine the impact of this intervention on ART adherence and other related health outcomes for youth living with HIV/AIDS globally [43,44].
Pregnant and postpartum women are another group who face challenges in maintaining high levels of adherence to medication [8]. Despite the critical need for ART during pregnancy and the postpartum period, evidence-based interventions to promote ART adherence during this period are lacking. A recent exploratory study of 109 HIV-positive pregnant South African women found that mobile phone access (>90%) and interest in text messaging for adherence support (88.1%) was high, and the majority (95%) of women were willing to disclose their status to a treatment buddy/supporter [45].
More generally, the fact that most adherence intervention studies are only able to show a modest effect in randomized trials is likely in part a consequence of the multiple challenges patients face in adhering to treatment as indicated by the findings of our review (i.e., within studies the percentages of reported barriers added up to more than 100%). Future research is encouraged to evaluate the effectiveness (effect size and interaction effects) of more than one intervention on virological suppression, using a factorial or adaptive clinical trial design to precisely determine the specific interventions and components of interventions that work best. [46].
Our review has several strengths and limitations. Strengths include our broad search strategy and inclusion criteria that allowed for the identification of a substantial number of studies and synthesis of a large dataset. Limitations are mainly related to study quality and include the variable definitions of adherence used by the different studies, different approaches to assessing adherence barriers and time on ART and it is possible that these and other unreported factors may have influenced outcomes. Information about drug toxicity is limited by the possibility that not all experienced adverse events are related to ART, even if they were perceived as a reason to stop taking the medication. Caution is also needed in the interpretation of results as some reasons for poor adherence (e.g., forgetting) may be put forward because they are perceived to be more socially acceptable than others (e.g., chaotic lifestyle or substance misuse). Although several analyses were undertaken to identify potential explanations for variance in findings, we could not thoroughly explore all possible differences in covariates (e.g., geographic region, income) due to the need to avoid spurious associations that may arise from large numbers of outcomes and covariates. We searched multiple databases and conferences, which allowed us to include data from over 100 studies for analysis; however, we did not include regional databases, and this may have limited identification of potentially eligible studies. An additional limitation to note with respect to children is that barriers were mainly reported by caregivers, and these may not represent the most important barriers faced by children themselves [47]. Finally, any study that looks at self-reported adherence will likely underestimate the frequency of non-adherence, and studies that assessed objective measures of adherence are more likely to be accurate in terms of reflecting true adherence rates.
In conclusion, this review highlights that patients on ART face multiple barriers to adherence and no single intervention will be sufficient to ensure that high levels of adherence to treatment and virological suppression are sustained. Rather than introducing single interventions into HIV programs, health providers should consider a more triaged approach that first identifies patients at risk of poor adherence and then seeks to establish the support that is needed to overcome the most important barriers to adherence. For maximum efficacy, adherence support strategies should be targeted to those individuals who require support. Finally, although the majority of the most commonly reported barriers are amenable to intervention at the individual level, several key health service improvements are also required to ensure that patients are able to access ART.
Supporting Information
Zdroje
1. Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–3. 11416722.
2. Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191(3):339–47. doi: 10.1086/427192 15633092.
3. Lingappa JR, Hughes JP, Wang RS, Baeten JM, Celum C, Gray GE, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS ONE. 2010;5(9):e12598. doi: 10.1371/journal.pone.0012598 20856886; PubMed Central PMCID: PMCPMC2938354.
4. Martin M, Del Cacho E, Codina C, Tuset M, De Lazzari E, Mallolas J, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses. 2008;24(10):1263–8. doi: 10.1089/aid.2008.0141 18834323.
5. Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, Regensberg L, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43(1):78–84. doi: 10.1097/01.qai.0000225015.43266.46 16878045.
6. Hogg RS, Heath K, Bangsberg D, Yip B, Press N, O'Shaughnessy MV, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16(7):1051–8. 11953472.
7. Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS. 2014;28(13):1945–56. doi: 10.1097/QAD.0000000000000316 24845154; PubMed Central PMCID: PMCPMC4162330.
8. Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26(16):2039–52. doi: 10.1097/QAD.0b013e328359590f 22951634.
9. Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296(6):679–90. doi: 10.1001/jama.296.6.679 16896111.
10. Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438 17121449; PubMed Central PMCID: PMCPMC1637123.
11. Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E, Albertini J, et al. A systematic review of individual and contextual factors affecting ART initiation, adherence, and retention for HIV-infected pregnant and postpartum women. PLoS ONE. 2014;9(11):e111421. doi: 10.1371/journal.pone.0111421 25372479; PubMed Central PMCID: PMCPMC4221025.
12. Kanters S, Park JH, Chan K, Socias ME, Ford N, Forrest J, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and meta-analysis. Lancet HIV. 2016. Published online November 15.
13. WHO. Consolidated guideliens on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. WHO, Geneva: 2nd Edition. 2016.
14. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 19621072; PubMed Central PMCID: PMC2707599.
15. Roberts I, Kerr K. How systematic reviews cause research waste. Lancet. 2015 Oct 17;386(10003):1536. doi: 10.1016/S0140-6736(15)00489-4 26530621.
16. Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12(3):255–66. doi: 10.1080/09540120050042891 10928201.
17. Freeman MF TJ. Transformations Related to the Angular and the Square Root. Annals of Mathematical Statistics 1950. 21 : 607–611.
18. Miller J. The Inverse of the Freeman-Tukey Double Arcsine Transformation. The American Statistician. 1978. 32 : 138.
19. Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121–45. Epub 1993/01/01. 8261254
20. Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC medical research methodology. 2008; 8 : 79. doi: 10.1186/1471-2288-8-79 19036172
21. Petersen ML, Wang Y, van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis. 2007;45(7):908–15. doi: 10.1086/521250 17806060
22. Patel DA, Snedecor SJ, Tang WY, Sudharshan L, Lim JW, Cuffe R, et al. 48-week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-naive HIV-1-infected patients: a systematic review and network meta-analysis. PLoS ONE. 2014;9(9):e105653. doi: 10.1371/journal.pone.0105653 25188312; PubMed Central PMCID: PMCPMC4154896.
23. Kredo T, Ford N, Adeniyi FB, Garner P. Decentralising HIV treatment in lower - and middle-income countries. Cochrane Database Syst Rev. 2013;6:CD009987. doi: 10.1002/14651858.CD009987.pub2 23807693.
24. Apollo T, Ford N, Eugenia S, Mathew W, Mills EJ, Kanters S. Effect of frequency of clinic visits and medication pick-up on antiretroviral therapy outcomes: a systematic review and meta-analysis. Abstract THUAE0804. 18th International cnference on AIDS and STIs in Africa Harare, November 29th—December 4th. 2015.
25. Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46(4):443–50. 18077833; PubMed Central PMCID: PMCPMC2666542.
26. Sin NL, DiMatteo MR. Depression treatment enhances adherence to antiretroviral therapy: a meta-analysis. Ann Behav Med. 2014;47(3):259–69. doi: 10.1007/s12160-013-9559-6 24234601; PubMed Central PMCID: PMCPMC4021003.
27. Honagodu AR, Krishna M, Sundarachar R, Lepping P. Group psychotherapies for depression in persons with HIV: A systematic review. Indian J Psychiatry. 2013;55(4):323–30. doi: 10.4103/0019-5545.120541 24459301; PubMed Central PMCID: PMCPMC3890933.
28. Crepaz N, Passin WF, Herbst JH, Rama SM, Malow RM, Purcell DW, et al. Meta-analysis of cognitive-behavioral interventions on HIV-positive persons' mental health and immune functioning. Health Psychol. 2008;27(1):4–14. doi: 10.1037/0278-6133.27.1.4 18230008.
29. Musiime V, Fillekes Q, Kekitiinwa A, Kendall L, Keishanyu R, Namuddu R, et al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr. 2014;66(2):148–54. doi: 10.1097/QAI.0000000000000135 24828266.
30. Ramjan R, Calmy A, Vitoria M, Mills EJ, Hill A, Cooke G, et al. Systematic review and meta-analysis: Patient and programme impact of fixed-dose combination antiretroviral therapy. Trop Med Int Health. 2014;19(5):501–13. doi: 10.1111/tmi.12297 24628918.
31. Ryan GW, Wagner GJ. Pill taking 'routinization': a critical factor to understanding episodic medication adherence. AIDS Care. 2003;15(6):795–806. doi: 10.1080/09540120310001618649 14617501.
32. Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15(2):17383. doi: 10.7448/IAS.15.2.17383 23199799; PubMed Central PMCID: PMCPMC3503237.
33. Anon. Empty Shelves, Come Back Tomorrow–ARV Stock Outs Undermine Efforts to Fight HIV. MSF, Johannesburg, 2015 Available http://wwwmsforg/sites/msforg/files/msf_out_of_stocks_low_respdf.
34. Goldman JD, Cantrell RA, Mulenga LB, Tambatamba BC, Reid SE, Levy JW, et al. Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS research and human retroviruses. 2008;24(8):1031–5. 18724803. doi: 10.1089/aid.2008.0035
35. Glass TR, Rotger M, Telenti A, Decosterd L, Csajka C, Bucher HC, et al. Determinants of sustained viral suppression in HIV-infected patients with self-reported poor adherence to antiretroviral therapy. PLoS ONE. 2012;7(1):e29186. 22235271. doi: 10.1371/journal.pone.0029186
36. WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. World Health Organization: Geneva. Available at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. 2015.
37. Al-Dakkak I1, Patel S, McCann E, Gadkari A, Prajapati G, Maiese EM. The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: a systematic review and meta-analysis. AIDS Care. 2013;25(4):400–14. doi: 10.1080/09540121.2012.712667 22908886.
38. Nachega JB, Parienti JJ, Uthman OA, Gross R, Dowdy DW, Sax PE, Gallant JE, Mugavero MJ, Mills EJ, Giordano TP. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis. 2014 May;58(9):1297–307.24457345. doi: 10.1093/cid/ciu046
39. Ford N, Flexner C, Vella S, Ripin D, Vitoria M. Optimization and simplification of antiretroviral therapy for adults and children. Curr Opin HIV AIDS. 2013 Nov;8(6):591–9. doi: 10.1097/COH.0000000000000010 24100871.
40. Langebeek N, Gisolf EH, Reiss P, Vervoort SC, Hafsteinsdottir TB, Richter C, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12 : 142. doi: 10.1186/PREACCEPT-1453408941291432 25145556; PubMed Central PMCID: PMCPMC4148019.
41. Ferrand RA, Briggs D, Ferguson J, Penazzato M, Armstrong A, MacPherson P, et al. Viral suppression in adolescents on antiretroviral treatment: review of the literature and critical appraisal of methodological challenges. Trop Med Int Health. 2016;21(3):325–33. doi: 10.1111/tmi.12656 26681359; PubMed Central PMCID: PMCPMC4776345.
42. Shaw S, Amico KR. Antiretroviral therapy adherence enhancing interventions for adolescents and young adults 13 to 24 years of age: A review of the evidence base. J Acquir Immune Defic Syndr. 2016. doi: 10.1097/QAI.0000000000000977 26959190.
43. Dowshen N, Kuhns LM, Johnson A, Holoyda BJ, Garofalo R. Improving adherence to antiretroviral therapy for youth living with HIV/AIDS: a pilot study using personalized, interactive, daily text message reminders. J Med Internet Res. 2012;14(2):e51. doi: 10.2196/jmir.2015 22481246; PubMed Central PMCID: PMCPMC3376506.
44. Dowshen N, Kuhns LM, Gray C, Lee S, Garofalo R. Feasibility of interactive text message response (ITR) as a novel, real-time measure of adherence to antiretroviral therapy for HIV+ youth. AIDS Behav. 2013;17(6):2237–43. doi: 10.1007/s10461-013-0464-6 23546844.
45. Nachega JB J, Skinner D, Jennings L, Magidson JF, Altice FL, Burke JG, Lester RT, Uthman OA, Knowlton AR, Cotton MF, Anderson JR, Theron GB. Acceptability and feasibility of mHealth and community-based patient nominated treatment supporter to prevent mother-to-child HIV transmission in South African pregnant women under Option B+: an exploratory study. Patient Preference and Adherence. 2016; 28(10):683–90.
46. Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Barnighausen T. Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. AIDS. 2014;28 Suppl 2:S187–204. doi: 10.1097/QAD.0000000000000252 24849479.
47. Buchanan AL, Montepiedra G, Sirois PA, Kammerer B, Garvie PA, Storm DS, et al. Barriers to medication adherence in HIV-infected children and youth based on self - and caregiver report. Pediatrics. 2012;129(5):e1244–51. doi: 10.1542/peds.2011-1740 22508915; PubMed Central PMCID: PMCPMC3340587.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 11- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Effectiveness of Seasonal Malaria Chemoprevention in Children under Ten Years of Age in Senegal: A Stepped-Wedge Cluster-Randomised Trial
- Lifestyle Advice Combined with Personalized Estimates of Genetic or Phenotypic Risk of Type 2 Diabetes, and Objectively Measured Physical Activity: A Randomized Controlled Trial
- Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
- Projected Impact of Mexico’s Sugar-Sweetened Beverage Tax Policy on Diabetes and Cardiovascular Disease: A Modeling Study
- Promoting Partner Testing and Couples Testing through Secondary Distribution of HIV Self-Tests: A Randomized Clinical Trial
- Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
- Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050?
- Educational Outreach with an Integrated Clinical Tool for Nurse-Led Non-communicable Chronic Disease Management in Primary Care in South Africa: A Pragmatic Cluster Randomised Controlled Trial
- Measures of Malaria Burden after Long-Lasting Insecticidal Net Distribution and Indoor Residual Spraying at Three Sites in Uganda: A Prospective Observational Study
- Leukocyte Telomere Length in Relation to 17 Biomarkers of Cardiovascular Disease Risk: A Cross-Sectional Study of US Adults
- Under-prescribing of Prevention Drugs and Primary Prevention of Stroke and Transient Ischaemic Attack in UK General Practice: A Retrospective Analysis
- The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study
- Three Steps to Improve Management of Noncommunicable Diseases in Humanitarian Crises
- A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project
- Improving the Pipeline for Developing and Testing Pharmacological Treatments in Pregnancy
- Seasonal Malaria Chemoprevention: An Evolving Research Paradigm
- Exposure Patterns Driving Ebola Transmission in West Africa: A Retrospective Observational Study
- Minimally Invasive Autopsy: A New Paradigm for Understanding Global Health?
- Towards Equity in Health: Researchers Take Stock
- Willingness to Know the Cause of Death and Hypothetical Acceptability of the Minimally Invasive Autopsy in Six Diverse African and Asian Settings: A Mixed Methods Socio-Behavioural Study
- Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels
- Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis
- Validity of a Minimally Invasive Autopsy for Cause of Death Determination in Adults in Mozambique: An Observational Study
- The Dengue Vaccine Dilemma: Balancing the Individual and Population Risks and Benefits
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
- Three Steps to Improve Management of Noncommunicable Diseases in Humanitarian Crises
- Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
- A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



