-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
Claudia Langenberg and colleagues show that high circulating branched chain amino acids associate with future risk of type 2 diabetes.
Published in the journal: . PLoS Med 13(11): e32767. doi:10.1371/journal.pmed.1002179
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002179Summary
Claudia Langenberg and colleagues show that high circulating branched chain amino acids associate with future risk of type 2 diabetes.
Introduction
Early evidence of impaired branched-chain amino acid (BCAA) metabolism in insulin resistance and obesity dates back to more than 40 years ago [1]. More recently, in investigations of the human metabolome, multiple research groups have described associations of higher levels of isoleucine, leucine, and valine with insulin resistance, obesity [2], and a higher risk of future type 2 diabetes [3]. These associations have since been replicated in several studies [4–6] and are corroborated by a growing body of experimental evidence [2,7–10]. However, it remains unknown whether BCAA metabolism is causally implicated in type 2 diabetes [7] or whether observational associations simply reflect reverse causality, whereby differences in BCAA levels are a consequence of pathophysiological processes that precede the development of the disease [11].
Genetic approaches to causality assessment provide an opportunity for rapid and cost-effective prioritisation of targets for interventional studies [12–20]. Therefore, genetic variants associated with BCAA levels can be used to study the aetiologic links of the BCAA metabolic pathway with type 2 diabetes. A previous smaller-scale investigation that was limited to one genetic variant associated with 3-methyl-2-oxovalerate, a by-product of BCAA metabolism, was inconclusive [21].
Therefore, in this study, we used genome-wide association studies (GWASs) coupled with large-scale metabolomic measurements to investigate the aetiologic relationship between BCAA metabolism and type 2 diabetes, an issue of high clinical and public health relevance due to the strength of the associations between BCAA levels and type 2 diabetes [2,3] and the global burden of this condition [22,23].
Methods
Study Design
We adopted a Mendelian randomisation approach to evaluate possible causal relationships between BCAA metabolism and type 2 diabetes (Fig 1). Inherited DNA variants are randomly assigned during meiosis and remain fixed throughout the lifetime. Therefore, they are unlikely to be affected by disease processes (i.e., reverse causality) and non-genetic confounding. Mendelian randomisation exploits these properties in order to estimate the relationship of modifiable risk factors with disease outcomes without the limitations of traditional observational epidemiology [12–15]. This approach postulates that if a biomarker is causally implicated in a disease, then genetic variants associated with that biomarker (but not other disease risk factors) should be associated with the disease in the direction predicted by observational analyses [12–15].
Fig. 1. Design of the study. 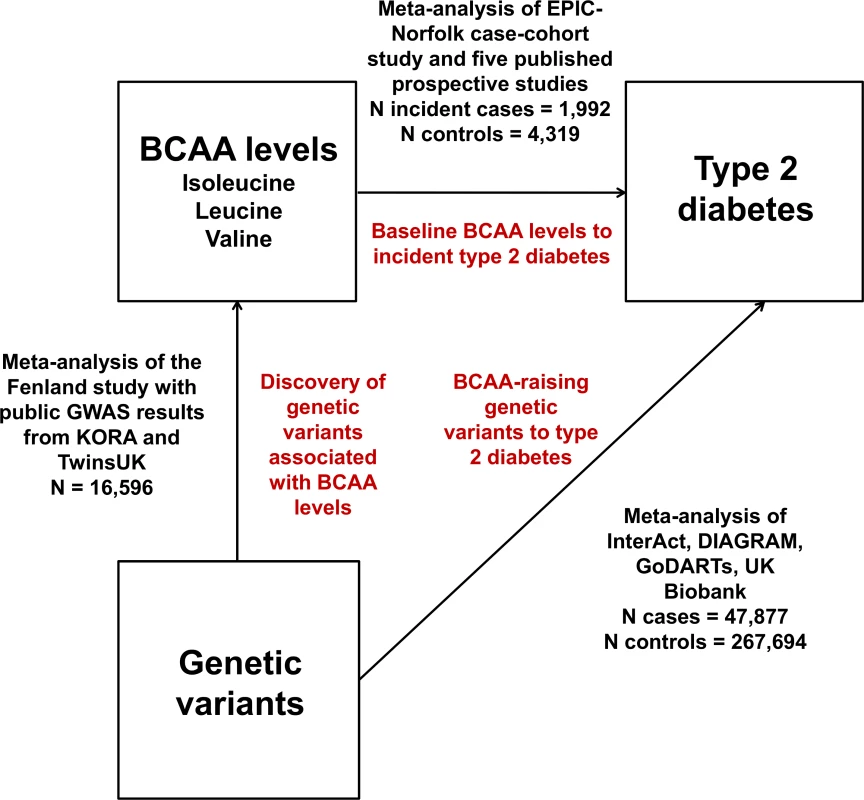
DIAGRAM, DIAbetes Genetics Replication And Meta-analysis. Therefore, we conducted meta-analyses of (a) GWASs of plasma levels of isoleucine, leucine, and valine; (b) studies of the associations of BCAA-raising genetic variants with type 2 diabetes; and (c) prospective studies of the associations of baseline isoleucine, leucine, and valine levels with incident type 2 diabetes.
The investigations described in this study were approved by local ethical committees, and participants provided their informed consent.
Participating Studies
We performed GWASs of the plasma levels of isoleucine, leucine, and valine in the Fenland study (n = 9,237; S1 Text; S1 Table). Fenland GWAS results were then meta-analysed with publicly available results from a meta-analysis of the KORA and TwinsUK studies [24]. The total sample size of the genome-wide meta-analysis was 16,596 individuals.
We estimated the association with type 2 diabetes of the lead single nucleotide polymorphism (SNP) at each BCAA-associated genomic locus. We meta-analysed SNP association results from the DIAbetes Genetics Replication And Meta-analysis [25] and the EPIC-InterAct [26], GoDARTs [21], and UK Biobank studies [27] (S1 Text; S2 Table). The total sample size of this analysis was 47,877 type 2 diabetes cases and 267,694 controls.
We conducted a systematic review of the literature of prospective studies of the association of BCAA levels and incident type 2 diabetes (S1 Text). The results of five prospective population-based studies [3–6] were meta-analysed with the unpublished results of the EPIC-Norfolk case-cohort study using fixed effect models (S3 Table) [28]. The I-squared statistic was used to quantify heterogeneity. In the EPIC-Norfolk study, the association of metabolite levels with incident type 2 diabetes was estimated using multivariable Cox proportional hazards regression with Prentice weighting and robust standard errors. The total sample size of the observational analysis was 1,992 incident cases of type 2 diabetes and 4,319 non-cases.
Metabolite Measurements
In the Fenland study, BCAA levels were measured using liquid chromatography coupled with tandem mass spectrometry (S1 Fig; AbsoluteIDQ p180 Kit, Biocrates Life Sciences [29]). In the KORA and TwinsUK studies, BCAA levels were measured as part of untargeted metabolomic measurements using gas chromatography mass spectrometry or ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS) [24]. In the EPIC-Norfolk study, BCAA levels were measured by UPLC-MS/MS (S1 Fig; DiscoveryHD4 platform, Metabolon [30]). This platform also measured an additional 15 metabolites related to BCAA metabolism. In the Southall And Brent REvisited Study (SABRE), the levels of BCAAs were measured by nuclear magnetic resonance at 0 and 120 min during the course of an oral glucose tolerance test (OGTT) [31,32].
Genome-Wide Association Analyses
Genome-wide association analyses were conducted using SNPTEST v2 [33], and study results were meta-analysed using METAL [34], which was chosen as the analysis software because studies included in the meta-analysis were focused on individuals of European ancestry. All genetic association results are reported per allele and assuming an additive effect. In the meta-analysis of GWASs of BCAA levels, we meta-analysed Z-scores rather than betas and standard errors in order to minimise the influence of the different platforms used to measure amino acid levels in participating studies. The conventional threshold for genome-wide statistical significance (p < 5 × 10−8) was used to identify associated loci. The SNP with the lowest p-value within a 1 million base-pair window was chosen as the lead SNP at a given genomic locus.
Variance Explained and Genetic Correlation Analyses
For each amino acid, we estimated the variance in amino acid level explained by the identified lead SNPs using linear regression models in the Fenland study. We used the BOLT-REML algorithm of the BOLT-LMM v2.2 software [35] to estimate chip-based heritability. This was estimated directly from variants captured across the genome on the genome-wide genotyping chip. We also used estimates of family-based genetic heritability from twin studies from Shin and colleagues [24]. We calculated the proportion of heritability explained by the lead SNPs by dividing the explained variance estimates by the familial and chip-based heritability estimates. Using genome-wide association results and LD score regression [36], we estimated genetic correlations of BCAA levels with type 2 diabetes, continuous glycaemic traits (fasting glucose, fasting insulin, glucose at 2 h in a 75-g OGTT, HbA1c, HOMA-B, and HOMA-IR), and anthropometric traits (body mass index [BMI] and waist-to-hip ratio) on the LD Hub platform (http://ldsc.broadinstitute.org/#; accessed 6 October 2016) [37]. Isoleucine estimates were not generated by the software due to low levels of genetic heritability estimated by the LD Hub platform.
Mendelian Randomisation Analysis
We estimated the association between a genetically predicted difference of 1 standard deviation (SD) in isoleucine, leucine, or valine plasma level and type 2 diabetes risk. For each of the BCAAs, we constructed weighted genetic risk scores including the lead SNP (independent genetic variants analysis) or genome-wide significant SNPs in imperfect linkage disequilibrium (r2 < 0.8 for all pairwise SNP comparisons; correlated genetic variants analysis) at each locus identified by the genome-wide meta-analysis for that metabolite. Fenland study weights were used to scale the effect of each genetic score to 1 SD. We used Fenland study weights as this was the largest study and we had access to individual-level data, allowing the standardisation of genetic effect estimates. Lead SNPs (nearest gene) included in the genetic scores used in the independent variants analysis were as follows: rs7678928 (PPM1K), rs75950518 (DDX19A), rs58101275 (TRMT61A), and rs1420601 (CBLN1) for isoleucine; rs1440581 (PPM1K) for leucine; and rs1440581 (PPM1K) for valine. Analyses of correlated genetic variants modelled the estimates of 12 SNPs at four loci (PPM1K, DDX19A, TRMT61A, and CBLN1) for isoleucine, seven SNPs at the PPM1K locus for leucine, and eight SNPs at the PPM1K locus for valine (details in S1 Text).
We combined estimates of “SNP to metabolite” and “SNP to type 2 diabetes” associations to calculate estimates of each “genetically predicted metabolite level to type 2 diabetes” association [13,14]. Estimates of multiple SNPs contributing to a given genetic score were pooled using an inverse-variance-weighted method [13,14]. For the analysis of correlated genetic variants, estimates were pooled with a weighted generalised linear regression method that accounts for the correlation between genetic variants [13]. The correlation values were obtained using the SNAP software [38]. Results were scaled to represent the odds ratio [OR] per 1-SD genetically predicted difference in amino acid level. Given that Mendelian randomisation assumes no pleiotropic effect beyond that on the risk factor of interest (i.e., BCAA levels), we excluded the lead SNP (rs1260326) at the known pleiotropic [39–43] GCKR locus from the isoleucine genetic score (Section 1 of S2 Text).
In order to assess the specificity of the genetic scores, we performed large-scale association testing of BCAA-raising alleles with the levels of blood metabolites: 175 metabolites in the Fenland study, 453 metabolites in KORA and TwinsUK, and 18 metabolites of the BCAA pathway in the EPIC-Norfolk study (S1 Text). We also investigated the association of BCAA-raising alleles with cardiometabolic traits in large-scale GWAS meta-analyses of up to 328,036 individuals [39–43].
Type 2 Diabetes Disease Mechanisms and BCAA Metabolism
We investigated other potential links between BCAA levels and type 2 diabetes. We tested the association with BCAA levels of a genetic predisposition to higher BMI, greater insulin resistance, and impaired insulin secretion using previously validated unweighted genetic scores for these traits [44–46] (S4 Table). Using linear regression models, we estimated the association of fasting insulin with BCAA levels during the course of an OGTT in the SABRE study.
Expression of PPM1K in Muscle Biopsies
We investigated changes in levels of PPM1K gene expression in skeletal muscle during the course of an OGTT. Fifty age-matched men with either normal glucose tolerance (n = 25) or type 2 diabetes (n = 25) were recruited at the Karolinska Institutet, Sweden. Following an overnight fast, a skeletal muscle biopsy was taken from the vastus lateralis muscle under local anaesthesia with a Weil-Blakesley conchotome tong instrument (Agntho’s). Participants ingested a standardised solution containing 75 g of glucose, and 2 h later a second biopsy was taken from the vastus lateralis of the contralateral leg. Biopsies were frozen immediately and stored in liquid nitrogen until processed. Total RNA was extracted from biopsies using the mirVana miRNA Isolation Kit (Thermo Fisher). Equal amounts of total RNA were used to synthesize cDNA using random primers and the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher). Quantitative PCR was performed with a ViiA 7 Real-Time PCR System with Fast SYBR Green Master Mix (Thermo Fisher). The ViiA 7 Software (version 1.1) was used to determine threshold cycle (Ct) values, and relative gene expression was calculated with the comparative Ct method relative to a reference gene, with dCt being the difference in expression between the gene of interest and the reference gene. The most suitable reference genes were determined to be GUSB, RPLP0, and TBP (out of four measured genes) using the NormFinder algorithm (Multid Analyses) [47]. Statistical analysis was performed on dCt values since these were normally distributed.
Statistical Analysis
Both Mendelian randomisation and observational association estimates are reported per 1-SD increase in metabolite level. Standardisation was necessary in light of the different platforms used to measure BCAA levels. Analyses were conducted using STATA v13.1 (StataCorp) and R (https://cran.r-project.org/). Further details of the methods used in this study are reported in S1 Text.
Results
Genome-Wide Association Studies of Isoleucine, Leucine, and Valine
Genome-wide meta-analyses of 10.5 million genetic variants in 16,596 individuals revealed five independent genomic loci associated with BCAA levels at the genome-wide level of statistical significance (Table 1; Figs 2, S2 and S3). The strongest signal was 21 kb upstream of the PPM1K gene on Chromosome 4q22.1, a locus previously reported for BCAA levels [24,48] (Fig 2).
Fig. 2. Manhattan plot of the association of genetic variants with the levels of branched-chain amino acids. 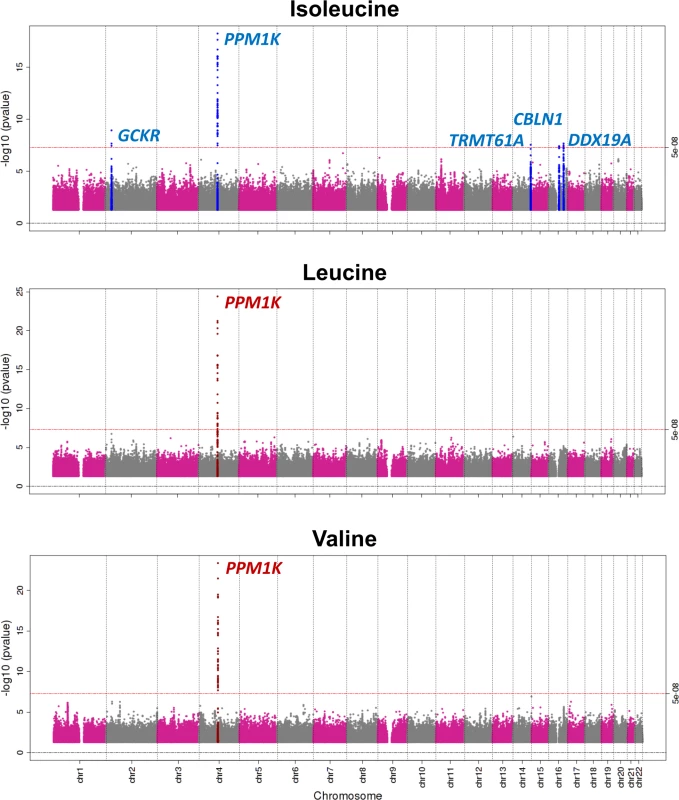
Tab. 1. Genetic variants associated with the levels of branched-chain amino acids in the genome-wide meta-analysis. 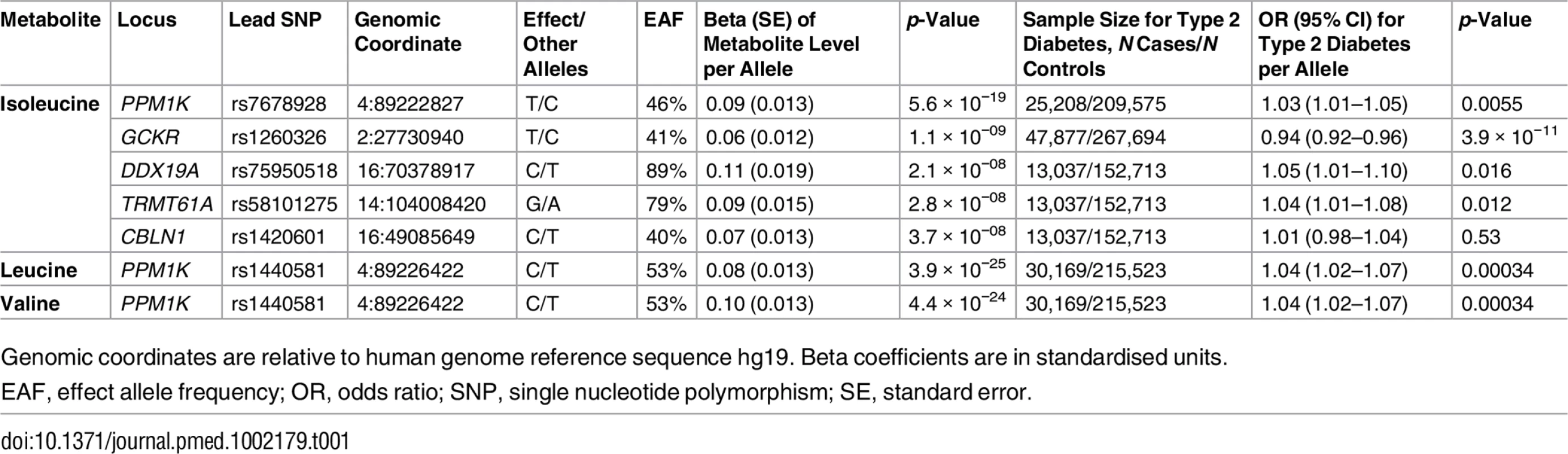
Genomic coordinates are relative to human genome reference sequence hg19. Beta coefficients are in standardised units. PPM1K encodes the mitochondrial phosphatase that activates the branched-chain alpha-ketoacid dehydrogenase (BCKD) complex [49–51]. This catalytic complex is responsible for the rate-limiting step of BCAA catabolism, i.e., the irreversible oxidative decarboxylation of branched-chain alpha-ketoacids [49–51]. In addition to PPM1K, we identified four novel loci associated with isoleucine (Table 1; Fig 2). Lead SNPs at these loci were also associated with the levels of the other two BCAAs in a consistent direction, albeit not at the genome-wide level of significance (S5 Table).
We estimated that identified lead genetic variants explain 7.5%, 6.3%, and 5.3% of the chip-based heritability of isoleucine, leucine, and valine levels estimated in the Fenland study using BOLT-LMM [35] and 2.2%, 0.8%, and 1.2% of the family-based heritability of isoleucine, leucine, and valine levels estimated in twin studies [24], respectively.
Association of BCAA-Raising Alleles with Type 2 Diabetes
A genetic predisposition to a higher level of isoleucine, leucine, or valine was strongly associated with higher odds for type 2 diabetes (for isoleucine, OR 1.44, 95% CI 1.26–1.65, p = 9.5 × 10−8; for leucine, OR 1.85, 95% CI 1.41–2.42, p = 7.3 × 10−6; for valine, OR 1.54, 95% CI 1.28–1.84, p = 4.2 × 10−6; Fig 3A). Relative risk estimates from genetic analyses based on the identified genetic variants were similar to those from the prospective studies of the association between baseline amino acid levels and incident type 2 diabetes (Figs 3A, S4 and S5; Section 2 of S2 Text). For isoleucine, the results of the genetic analyses were almost identical when considering genetic variants at the PPM1K locus only or genetic variants at all four loci (S6 Table). At the PPM1K locus, both the lead SNPs in the BCAA association analyses, rs1440581 (r2 = 0.88) and rs7678928 (r2 = 0.70), were in linkage disequilibrium with the lead type 2 diabetes SNP at the locus, rs1975393 (Fig 3B). There was a dose-response relationship between the association with amino acid level and the relative increase in diabetes risk for the four lead SNPs that were used for the construction of the isoleucine genetic score (Fig 3C). Similarly, the association between isoleucine level and incident type 2 diabetes in prospective studies showed a graded dose-response relationship (Fig 3D; S7 Table).
Fig. 3. Genetically predicted or measured levels of the branched-chain amino acids and risk of type 2 diabetes. 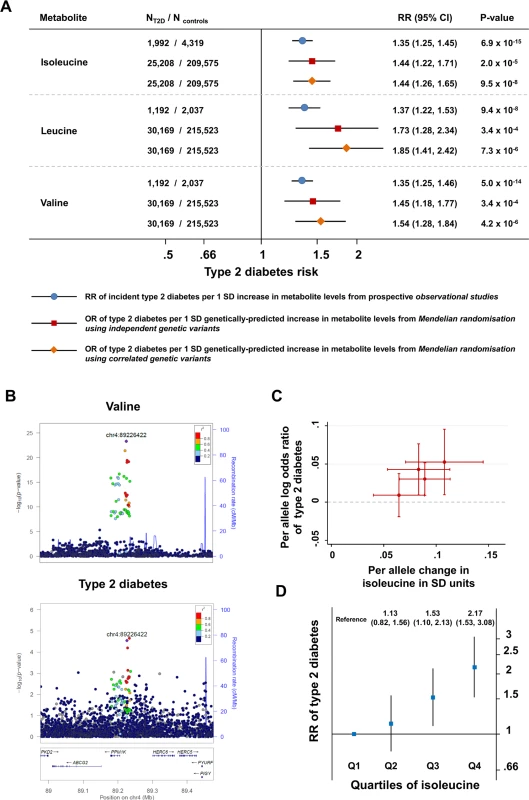
(A) Comparison of (i) the association of a difference of 1 SD in the levels of BCAAs at baseline with incident type 2 diabetes in prospective observational studies (bars with blue circles) and (ii) the association of a genetically predicted difference of 1 SD in BCAA levels with type 2 diabetes in genetic Mendelian randomisation studies (bars with red squares for analyses of independent genetic variants and bars with orange diamonds for analyses of correlated genetic variants). (B) Regional association plots for the association of variants at the PPM1K locus with valine (top; representative of the three BCAAs) and type 2 diabetes (bottom). Associations were characterised by a peak of signal upstream of the PPM1K gene, with the lead rs1440581 polymorphism and other polymorphisms in high linkage disequilibrium, as well as a peripheral signal overlaying the gene, with variants in lower linkage disequilibrium with the lead polymorphism. (C) Scatter plot of the association of the isoleucine-raising alleles included in the isoleucine genetic score with amino acid levels and with type 2 diabetes risk. (D) The association of the quartiles of isoleucine level at baseline with incident type 2 diabetes in the EPIC-Norfolk case-cohort study, the Framingham Offspring Study, and the Malmö Diet and Cancer Study (1,025 cases of incident type 2 diabetes and 1,182 controls). Error bars represent the 95% confidence intervals around the central estimates. BCAA, branched-chain amino acid; OR, odds ratio; RR, relative risk; SD, standard deviation; T2D, type 2 diabetes. BCAA-Raising Alleles, Continuous Metabolic Traits, and Plasma Metabolites
The isoleucine, leucine, and valine genetic scores were specifically associated with the three BCAAs and not with any of the remaining 172 metabolites measured in the Fenland study (Fig 4). Consistent with these results, the rs1440581 SNP at PPM1K was associated with only eight out of 453 metabolites studied by Shin et al. [24], all of which belonged to the BCAA pathway (S8 Table). These results illustrate the high specificity to BCAA metabolism of these genetic scores.
Fig. 4. Association of branched-chain amino acid genetic scores with metabolites in the Fenland study. 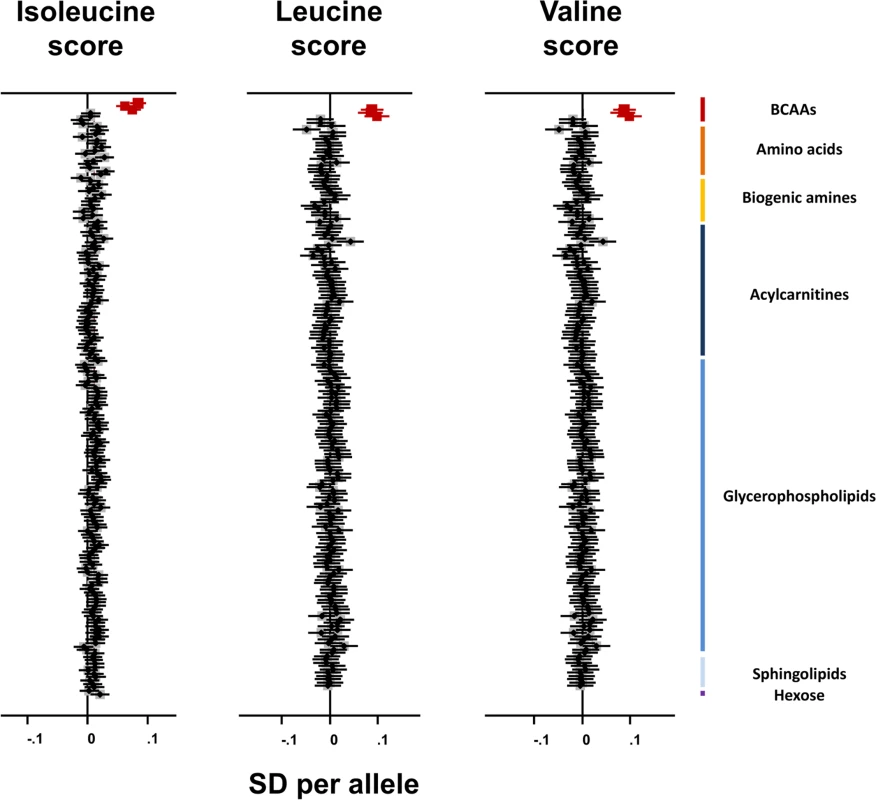
Associations of the BCAA scores with the BCAAs are highlighted in dark red. Error bars represent the 95% confidence interval around the central estimate.BCAA, branched-chain amino acid; SD, standard deviation. The BCAA-raising genetic scores and their constituent SNPs were not associated with continuous metabolic traits in large-scale meta-analyses. There was an association of the isoleucine-raising allele of rs1420601 near CBLN1 with higher BMI (S9 Table), but this did not affect the main analysis results (OR for type 2 diabetes after exclusion of rs1420601 = 1.50, 95% CI 1.25–1.80, p = 0.000013).
We assessed genome-wide genetic correlations of BCAA levels with type 2 diabetes and continuous metabolic traits and found statistically significant (p < 0.05) positive correlations of (a) leucine level with type 2 diabetes (rgenetic = 0.34, p = 0.0004), HbA1c (rgenetic = 0.34, p = 0.0038), BMI (rgenetic = 0.25, p = 1.5 × 10−5), and waist-to-hip ratio (rgenetic = 0.29, p = 2.6 × 10−6) and of (b) valine level with type 2 diabetes (rgenetic = 0.54, p = 8.8 × 10−5), fasting insulin (rgenetic = 0.42, p = 0.013), BMI (rgenetic = 0.43, p = 1.8 × 10−5), waist-to-hip ratio (rgenetic = 0.47, p = 6.4 × 10−6), HOMA-B (rgenetic = 0.40, p = 0.016), and HOMA-IR (rgenetic = 0.45, p = 0.012).
Type 2 Diabetes Disease Mechanisms and BCAA Metabolism
In the Fenland study, both higher BMI and higher fasting insulin level, a measure of fasting-state insulin resistance, were associated with higher levels of BCAAs (S10 Table). In addition, genetic predispositions to higher BMI and insulin resistance, but not to impaired insulin secretion, were associated with higher levels of BCAAs (S11 and S12 Tables; S6 Fig). The association of genetic predisposition to greater adiposity with high BCAA levels appeared to be mediated by insulin resistance (S11 Table). In the SABRE study, a glucose challenge resulted in a reduction of circulating BCAA levels. Individuals with higher fasting insulin showed a diminished reduction of all three BCAA levels in response to a glucose challenge (S7 Fig; S13 and S14 Tables). In muscle biopsies collected during an oral glucose challenge, the expression of PPM1K increased at 2 h in normoglycaemic individuals, but not in age-matched patients with type 2 diabetes (S8 Fig). These results are in line with what has been reported in previous investigations [52,53] and suggest that greater adiposity and impaired insulin sensitivity result in exposure to higher levels of BCAAs both in the fasting state and after glucose intake, which could be mediated at least in part by impaired BCKD activation.
BCAA Pathway Analysis
Because BCAA-raising alleles were strongly associated with metabolites specific to the BCAA pathway, we hypothesized that studying their pattern of association might provide additional insights into the mechanisms affected by these genetic variants. Using untargeted metabolomics in up to 8,693 individuals, we investigated the association of BCAA-raising alleles with the levels of 18 metabolites involved in BCAA metabolism (Tables 2 and S15; Fig 5). BCAA-raising genetic variants were strongly associated with higher levels of the branched-chain alpha-ketoacids and other metabolites upstream of the irreversible branched-chain alpha-ketoacid dehydrogenation (Fig 5). Metabolites downstream of this important biochemical reaction were largely unaffected by BCAA-raising alleles (Tables 2 and S15; Fig 5).
Fig. 5. Schematic representation of the branched-chain amino acid pathway and associations with type 2 diabetes. 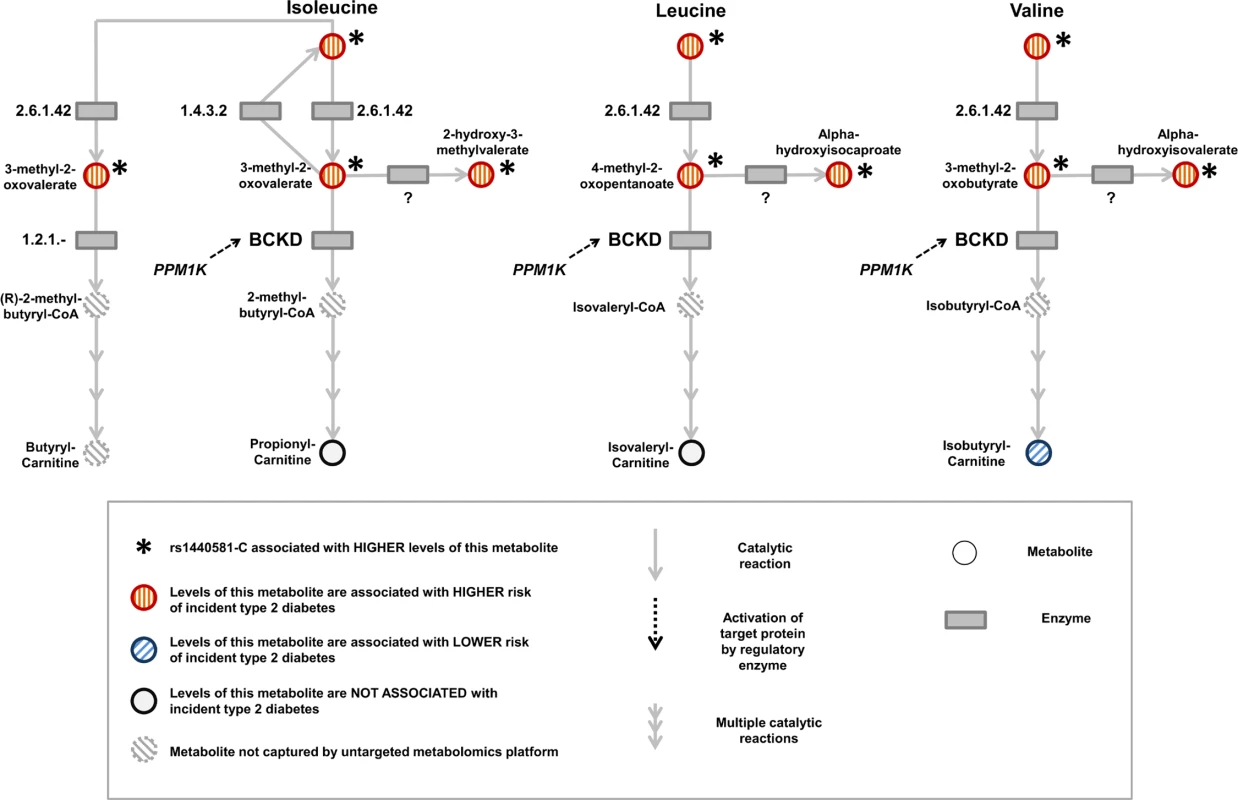
BCKD, branched-chain alpha-ketoacid dehydrogenase. Tab. 2. BCAA pathway associations. 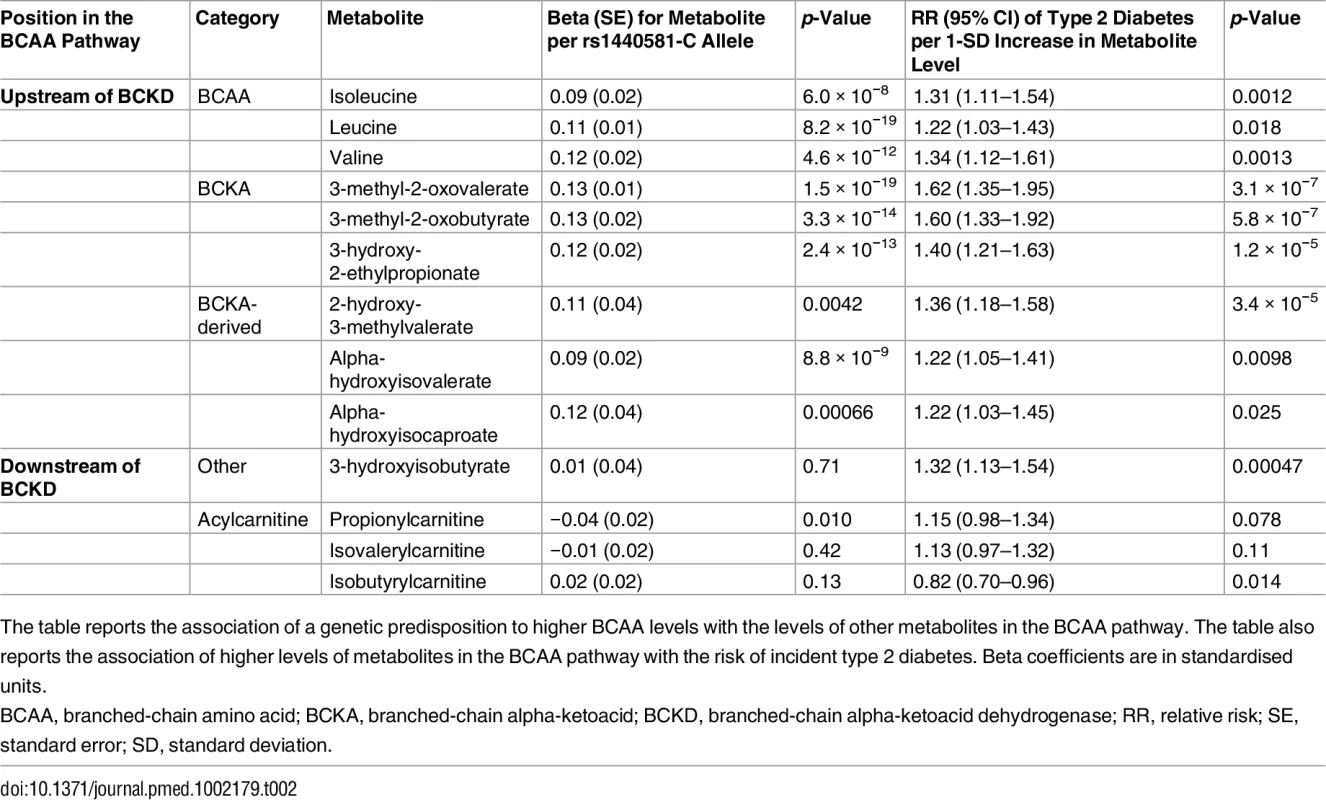
The table reports the association of a genetic predisposition to higher BCAA levels with the levels of other metabolites in the BCAA pathway. The table also reports the association of higher levels of metabolites in the BCAA pathway with the risk of incident type 2 diabetes. Beta coefficients are in standardised units. Higher levels of branched-chain alpha-ketoacids and other metabolites upstream of BCKD action were also strongly associated with a higher risk of incident type 2 diabetes in the EPIC-Norfolk case-cohort study, whereas the associations of downstream metabolites were inconsistent (Tables 2 and S15; Fig 5).
Discussion
In this large-scale human genetic study, BCAA-raising polymorphisms identified with a genome-wide approach were associated with a higher risk of type 2 diabetes. Our findings suggest that mechanisms leading to impaired BCAA metabolism are implicated in the pathophysiology of type 2 diabetes. Given the strong and specific association with the BCAA pathway observed after testing more than 500 metabolic phenotypes, it is unlikely that the identified genetic variants affect type 2 diabetes risk via mechanisms outside of this metabolic pathway. The relative increase in diabetes risk associated with these genetic variants appeared to be proportional to the size of the association with amino acid levels, which would further support a possible causal link. The diabetes risk increase estimated in genetic analyses was also consistent with the direction and magnitude of association between baseline amino acid levels and incident diabetes in observational prospective studies.
A genetic predisposition to insulin resistance was associated with higher plasma BCAA levels, suggesting that previously reported associations between genetic susceptibility to higher BMI and BCAA levels [11] may be mediated by insulin resistance mechanisms. Consistent with recent studies in mice [54], the expression of PPM1K in muscle biopsies during an oral glucose challenge failed to increase in people with type 2 diabetes, suggesting that the link between insulin resistance and higher BCAA levels may be partly mediated by impaired BCKD activation. Therefore, part of the contribution of insulin resistance mechanisms to type 2 diabetes may be exerted via impaired BCAA metabolism.
In metabolome-wide investigations of BCAA-raising alleles, we found evidence of an accumulation of BCAAs and BCAA-derived metabolites upstream of the oxidative dehydrogenation of branched-chain alpha-ketoacids. This reaction is the irreversible and rate-limiting step in BCAA metabolism, catalysed by the mitochondrial BCKD complex [55]. The pattern of association observed in this study mirrors that observed in maple syrup urine disease (MSUD), an inborn error of metabolism caused by rare loss-of-function mutations in genes encoding components of the BCKD complex [55] or its regulatory phosphatase [56]. In our GWAS of BCAA levels, the strongest association signal was located 21 kb upstream of the PPM1K gene, which encodes the mitochondrial phosphatase that activates BCKD [49–51]. Loss-of-function mutations of PPM1K in humans [56] or the knock-out of its ortholog Ppm1k in mice models [35] results in impaired BCKD activity and high levels of BCAAs and branched-chain alpha-ketoacids, a pattern that resembles that observed for common PPM1K genetic variants in our study. Therefore, it is plausible that the genetic variants identified in this study act by impairing the catabolism of BCAAs, hence leading to higher circulating levels of these amino acids. We found that levels of all metabolites accumulated upstream of BCKD action were associated with incident type 2 diabetes. This further supports the hypothesis that reduced BCKD activity could be one of the mechanistic links between BCAA metabolism and type 2 diabetes.
Our findings have public health and clinical implications as they indicate that modulation of BCAA metabolism may impact diabetes risk. The activity of BCKD is a major determinant of the rate of BCAA catabolism and is amenable to modulation by pharmacological intervention [57–62]. Improving insulin sensitivity may also ameliorate BCAA metabolism, resulting in reduced diabetes risk. This approach is supported by the well-described reduction of amino acid levels following the administration or secretion of insulin [63,64], by our finding of higher BCAA levels in individuals with a genetic predisposition to insulin resistance, and by the observation of reduced BCAA levels following insulin-sensitising interventions [65–67].
Future studies will clarify the molecular mechanisms linking impaired BCAA metabolism and increased risk of type 2 diabetes. The lack of a strong association between BCAA-raising alleles and continuous metabolic traits in publicly available GWAS meta-analyses possibly reflects a lack of statistical power or the unavailability of data about specific glycaemic traits altered by these alleles, similar to several other type 2 diabetes risk variants [25]. We found positive genetic correlations of BCAA levels with type 2 diabetes and several glycaemic and anthropometric traits, which points to shared genetic determinants between BCAA levels and greater adiposity, hyperinsulinaemia, and hyperglycaemia.
A growing body of evidence from human, cellular, and animal models is beginning to shed light on the possible mechanisms linking BCAA metabolism and diabetes risk [2,7,8,68]. Emerging mechanistic explanations include a synergistic interference of BCAAs and lipids with the response of peripheral tissues to insulin [8]. In animal feeding studies, BCAA supplementation requires the background of a high-fat diet to promote insulin resistance [8]. The rs1440581-C variant, i.e., the lead BCAA-raising allele in our genome-wide association analysis, was found to be associated with less weight loss and insulin sensitisation following a calorie-restricted, high-fat diet in a dietary intervention study in humans [69]. Recent research has shown that higher BCAA levels are associated with a gut microbiome pattern characterised by enriched BCAA biosynthetic potential, including Prevotella copri and Bacteroides vulgatus species, pointing to a possible role of the gut flora in the relationship between BCAA levels and insulin resistance [70]. BCAAs and their by-products have also been linked to beta-cell dysfunction [7–9]. The knock-down of PPM1K in clonal INS-1 cell lines has been shown to impair glucose-stimulated insulin secretion [9]. It has also been suggested that a chronic exposure to high levels of BCAAs may result in a constant hyperinsulinaemic response, eventually leading to beta-cell exhaustion [8]. The increased oxidative stress associated with the accumulation of BCAA-derived alpha-ketoacids is consistent with the link between superoxide generation and beta-cell dysfunction [71,72].
Another aspect to be elucidated is whether patients with MSUD have altered glycaemic metabolism. Alterations of glucose metabolism were not reported as a clinical feature in a review of the phenotype of the disease [55]. However, Mogos et al. reported hypoglycaemia in a case of MSUD, which could perhaps be consistent with the hypothesis of BCAA-mediated hyperinsulinaemia [73]. MSUD can be fatal early in life, is often associated with severe cognitive impairment, and is managed with a tightly controlled therapeutic diet [55]. Therefore, MSUD may not be an optimal model to study the risk of a late-life disease with important dietary determinants such as type 2 diabetes.
Limitations of the genetic approach used in this study affect its interpretation. Mendelian randomisation assumes that the genetic variants used as instruments are associated with the disease exclusively via the risk factor of interest. For this reason, we excluded the pleiotropic GCKR locus from analysis. We also assessed the association of genetic variants with more than 500 phenotypes, without finding evidence of pleiotropy. While this reduces the possibility that pleiotropy has influenced our findings, we cannot entirely exclude the possibility of pleiotropic associations. Similar to other complex traits, the genetic variants identified in this study explain only a fraction of the heritability of BCAA levels. Therefore, it is possible that only some of the mechanisms that increase BCAA levels or affect their metabolism are implicated in type 2 diabetes.
While our genetic approach investigated the relationships between circulating BCAA levels and diabetes risk, BCAA metabolism is a complex biological phenomenon closely linked to other metabolic pathways. It is plausible that changes in this metabolic pathway may influence diabetes risk only in a certain metabolic context. Some suggestions come from the emerging biology of the glucokinase regulatory protein (GCKR), the genetic variants of which had to be excluded from our study for methodological reasons. GCKR is known to switch liver metabolism from production of glucose towards that of triglycerides, in particular triglycerides with lower carbon content and double bonds [74,75]. These lipid species are associated with higher diabetes incidence in observational studies [76]. Therefore, the activation of specific pathways (such as those that increase circulating BCAAs or short-chain, highly saturated triglycerides) may typically increase type 2 diabetes risk, except when such activation is caused by GCKR. These findings suggest that (a) the mechanisms by which the concentrations of these biomarkers are raised, not their higher concentrations per se, are implicated in higher metabolic risk and (b) higher BCAA levels might be tolerated as long as other protective pathways are activated. Larger-scale genetic studies and randomised controlled trials will help to further qualify the causal nature of the relationship between BCAA metabolism and type 2 diabetes risk.
Conclusions
In this study, BCAA-raising polymorphisms identified with a genome-wide approach were associated with a higher risk of type 2 diabetes, consistent with a causal role of BCAA metabolism in the aetiology of this common complex disease.
Supporting Information
Zdroje
1. Felig P, Marliss E, Cahill GF Jr (1969) Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 281 : 811–816. doi: 10.1056/NEJM196910092811503 5809519
2. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, et al. (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9 : 311–326. doi: 10.1016/j.cmet.2009.02.002 19356713
3. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, et al. (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17 : 448–453. doi: 10.1038/nm.2307 21423183
4. Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, et al. (2013) Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 62 : 639–648. doi: 10.2337/db12-0495 23043162
5. Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, et al. (2012) Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol 8 : 615. doi: 10.1038/msb.2012.43 23010998
6. Palmer ND, Stevens RD, Antinozzi PA, Anderson A, Bergman RN, et al. (2015) Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab 100: E463–E468. doi: 10.1210/jc.2014-2357 25423564
7. Lynch CJ, Adams SH (2014) Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10 : 723–736. doi: 10.1038/nrendo.2014.171 25287287
8. Newgard CB (2012) Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15 : 606–614. doi: 10.1016/j.cmet.2012.01.024 22560213
9. Taneera J, Lang S, Sharma A, Fadista J, Zhou Y, et al. (2012) A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab 16 : 122–134. doi: 10.1016/j.cmet.2012.06.006 22768844
10. Shin AC, Fasshauer M, Filatova N, Grundell LA, Zielinski E, et al. (2014) Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab 20 : 898–909. doi: 10.1016/j.cmet.2014.09.003 25307860
11. Wurtz P, Wang Q, Kangas AJ, Richmond RC, Skarp J, et al. (2014) Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med 11: e1001765. doi: 10.1371/journal.pmed.1001765 25490400
12. Smith GD, Ebrahim S (2003) ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32 : 1–22. 12689998
13. Burgess S, Dudbridge F, Thompson SG (2016) Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 35 : 1880–1906. doi: 10.1002/sim.6835 26661904
14. Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37 : 658–665. doi: 10.1002/gepi.21758 24114802
15. Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, et al. (2012) Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 380 : 572–580. doi: 10.1016/S0140-6736(12)60312-2 22607825
16. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH (2006) Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 354 : 1264–1272. doi: 10.1056/NEJMoa054013 16554528
17. Myocardial Infarction Genetics Consortium Investigators, Stitziel NO, Won HH, Morrison AC, Peloso GM, et al. (2014) Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med 371 : 2072–2082. doi: 10.1056/NEJMoa1405386 25390462
18. Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD (2015) Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 x 2 factorial Mendelian randomization study. J Am Coll Cardiol 65 : 1552–1561. doi: 10.1016/j.jacc.2015.02.020 25770315
19. Lotta LA, Sharp SJ, Burgess S, Perry JR, Stewart ID, et al. (2016) Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: a meta-analysis. JAMA 316 : 1383–1391. doi: 10.1001/jama.2016.14568 27701660
20. Scott RA, Freitag DF, Li L, Chu AY, Surendran P, et al. (2016) A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease. Sci Transl Med 8 : 341ra376.
21. Menni C, Fauman E, Erte I, Perry JR, Kastenmuller G, et al. (2013) Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 62 : 4270–4276. doi: 10.2337/db13-0570 23884885
22. World Health Organization (2016 Jun) Obesity and overweight. Fact sheet. Available: http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 10 Oct 2016.
23. World Health Organization (2016 Jun) Diabetes. Fact sheet. Available: http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed 10 Oct 2016.
24. Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, et al. (2014) An atlas of genetic influences on human blood metabolites. Nat Genet 46 : 543–550. doi: 10.1038/ng.2982 24816252
25. Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, et al. (2012) Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 44 : 981–990. doi: 10.1038/ng.2383 22885922
26. InterAct Consortium, Langenberg C, Sharp S, Forouhi NG, Franks PW, et al. (2011) Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia 54 : 2272–2282. doi: 10.1007/s00125-011-2182-9 21717116
27. Collins R (2012) What makes UK Biobank special? Lancet 379 : 1173–1174. doi: 10.1016/S0140-6736(12)60404-8 22463865
28. Day N, Oakes S, Luben R, Khaw KT, Bingham S, et al. (1999) EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer 80 (Suppl 1): 95–103.
29. Biocrates Life Sciences (2016) Absolute/DQ p180 Kit. Available: http://www.biocrates.com/images/stories/pdf/Folders/produktfolder_180-6_einzelseiten_1.pdf. Accessed 10 Oct 2016.
30. Evans AM, Bridgewater BR, Liu Q, Mitchell MW, Robinson RJ, et al. (2014) High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics (Los Angel) 4 : 132.
31. Tillin T, Hughes AD, Wang Q, Wurtz P, Ala-Korpela M, et al. (2015) Diabetes risk and amino acid profiles: cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 58 : 968–979. doi: 10.1007/s00125-015-3517-8 25693751
32. Soininen P, Kangas AJ, Wurtz P, Suna T, Ala-Korpela M (2015) Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet 8 : 192–206. doi: 10.1161/CIRCGENETICS.114.000216 25691689
33. Marchini J, Howie B (2010) Genotype imputation for genome-wide association studies. Nat Rev Genet 11 : 499–511. doi: 10.1038/nrg2796 20517342
34. Willer CJ, Li Y, Abecasis GR (2010) METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26 : 2190–2191. doi: 10.1093/bioinformatics/btq340 20616382
35. Loh PR, Bhatia G, Gusev A, Finucane HK, Bulik-Sullivan BK, et al. (2015) Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat Genet 47 : 1385–1392. doi: 10.1038/ng.3431 26523775
36. Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, et al. (2015) An atlas of genetic correlations across human diseases and traits. Nat Genet 47 : 1236–1241. doi: 10.1038/ng.3406 26414676
37. Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, et al. (2016) LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. Epub ahead of print. doi: 10.1093/bioinformatics/btw613 27663502
38. Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O’Donnell CJ, et al. (2008) SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 24 : 2938–2939. doi: 10.1093/bioinformatics/btn564 18974171
39. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, et al. (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518 : 197–206. doi: 10.1038/nature14177 25673413
40. Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, et al. (2015) New genetic loci link adipose and insulin biology to body fat distribution. Nature 518 : 187–196. doi: 10.1038/nature14132 25673412
41. Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, et al. (2012) Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet 44 : 991–1005. doi: 10.1038/ng.2385 22885924
42. Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, et al. (2012) A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 44 : 659–669. doi: 10.1038/ng.2274 22581228
43. Global Lipids Genetics Consortium, Willer CJ, Schmidt EM, Sengupta S, Peloso GM, et al. (2013) Discovery and refinement of loci associated with lipid levels. Nat Genet 45 : 1274–1283. doi: 10.1038/ng.2797 24097068
44. Scott RA, Fall T, Pasko D, Barker A, Sharp SJ, et al. (2014) Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independent of obesity. Diabetes 63 : 4378–4387. doi: 10.2337/db14-0319 24947364
45. Ostergaard SD, Mukherjee S, Sharp SJ, Proitsi P, Lotta LA, et al. (2015) Associations between potentially modifiable risk factors and Alzheimer disease: a Mendelian randomization study. PLoS Med 12: e1001841. doi: 10.1371/journal.pmed.1001841 26079503
46. Nead KT, Sharp SJ, Thompson DJ, Painter JN, Savage DB, et al. (2015) Evidence of a causal association between insulinemia and endometrial cancer: a Mendelian randomization analysis. J Natl Cancer Inst 107: djv178. doi: 10.1093/jnci/djv178 26134033
47. Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64 : 5245–5250. doi: 10.1158/0008-5472.CAN-04-0496 15289330
48. Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, et al. (2012) Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet 44 : 269–276. doi: 10.1038/ng.1073 22286219
49. Lu G, Sun H, She P, Youn JY, Warburton S, et al. (2009) Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest 119 : 1678–1687. doi: 10.1172/JCI38151 19411760
50. Zhao Y, Hawes J, Popov KM, Jaskiewicz J, Shimomura Y, et al. (1994) Site-directed mutagenesis of phosphorylation sites of the branched chain alpha-ketoacid dehydrogenase complex. J Biol Chem 269 : 18583–18587. 8034607
51. Wynn RM, Kato M, Machius M, Chuang JL, Li J, et al. (2004) Molecular mechanism for regulation of the human mitochondrial branched-chain alpha-ketoacid dehydrogenase complex by phosphorylation. Structure 12 : 2185–2196. doi: 10.1016/j.str.2004.09.013 15576032
52. Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, et al. (2008) Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol 4 : 214. doi: 10.1038/msb.2008.50 18682704
53. Ho JE, Larson MG, Vasan RS, Ghorbani A, Cheng S, et al. (2013) Metabolite profiles during oral glucose challenge. Diabetes 62 : 2689–2698. doi: 10.2337/db12-0754 23382451
54. Lian K, Du C, Liu Y, Zhu D, Yan W, et al. (2015) Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes 64 : 49–59. doi: 10.2337/db14-0312 25071024
55. /Strauss KA, Puffenberger EG, Morton DH (2013 May 9) Maple syrup urine disease. GeneReviews [Internet]. Available: https://www.ncbi.nlm.nih.gov/books/NBK1319/. Accessed 27 Oct 2016.
56. Oyarzabal A, Martinez-Pardo M, Merinero B, Navarrete R, Desviat LR, et al. (2013) A novel regulatory defect in the branched-chain alpha-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease. Hum Mutat 34 : 355–362. doi: 10.1002/humu.22242 23086801
57. Tso SC, Qi X, Gui WJ, Chuang JL, Morlock LK, et al. (2013) Structure-based design and mechanisms of allosteric inhibitors for mitochondrial branched-chain alpha-ketoacid dehydrogenase kinase. Proc Natl Acad Sci U S A 110 : 9728–9733. doi: 10.1073/pnas.1303220110 23716694
58. Tso SC, Gui WJ, Wu CY, Chuang JL, Qi X, et al. (2014) Benzothiophene carboxylate derivatives as novel allosteric inhibitors of branched-chain alpha-ketoacid dehydrogenase kinase. J Biol Chem 289 : 20583–20593. doi: 10.1074/jbc.M114.569251 24895126
59. Brunetti-Pierri N, Lanpher B, Erez A, Ananieva EA, Islam M, et al. (2011) Phenylbutyrate therapy for maple syrup urine disease. Hum Mol Genet 20 : 631–640. doi: 10.1093/hmg/ddq507 21098507
60. Burrage LC, Jain M, Gandolfo L, Lee BH, Members of the Urea Cycle Disorders Consortium, et al. (2014) Sodium phenylbutyrate decreases plasma branched-chain amino acids in patients with urea cycle disorders. Mol Genet Metab 113 : 131–135. doi: 10.1016/j.ymgme.2014.06.005 25042691
61. Xiao C, Giacca A, Lewis GF (2011) Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes 60 : 918–924. doi: 10.2337/db10-1433 21270237
62. Danner DJ, Davidson ED, Elsas LJ 2nd (1975) Thiamine increases the specific activity of human liver branched chain alpha-ketoacid dehydrogenase. Nature 254 : 529–530. 1121328
63. Lukens FD (1964) Insulin and protein metabolism. Diabetes 13 : 451–461. 14208234
64. Luck JM, Morrison G, Wilbur LF (1928) The effect of insulin on the amino acid content of blood. J Biol Chem 77 : 151–156.
65. Lips MA, Van Klinken JB, van Harmelen V, Dharuri HK, ‘t Hoen PA, et al. (2014) Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Diabetes Care 37 : 3150–3156. doi: 10.2337/dc14-0195 25315204
66. Kakazu E, Kondo Y, Ninomiya M, Kimura O, Nagasaki F, et al. (2013) The influence of pioglitazone on the plasma amino acid profile in patients with nonalcoholic steatohepatitis (NASH). Hepatol Int 7 : 577–585. doi: 10.1007/s12072-012-9395-y 26201790
67. Glynn EL, Piner LW, Huffman KM, Slentz CA, Elliot-Penry L, et al. (2015) Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia 58 : 2324–2335. doi: 10.1007/s00125-015-3705-6 26254576
68. Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, et al. (2013) Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341 : 1241214. doi: 10.1126/science.1241214 24009397
69. Xu M, Qi Q, Liang J, Bray GA, Hu FB, et al. (2013) Genetic determinant for amino acid metabolites and changes in body weight and insulin resistance in response to weight-loss diets: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 127 : 1283–1289. doi: 10.1161/CIRCULATIONAHA.112.000586 23446828
70. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, et al. (2016) Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535 : 376–381. doi: 10.1038/nature18646 27409811
71. Tang C, Han P, Oprescu AI, Lee SC, Gyulkhandanyan AV, et al. (2007) Evidence for a role of superoxide generation in glucose-induced beta-cell dysfunction in vivo. Diabetes 56 : 2722–2731. doi: 10.2337/db07-0279 17682092
72. Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV, Wheeler MB (2010) Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes 59 : 448–459. doi: 10.2337/db09-0129 19903739
73. Mogos T, Cheta CP, Mincu IT (1994) Clinical consequences of disorders in the intermediate metabolism of branched chain amino acids (valine, leucine and isoleucine). Rom J Intern Med 32 : 57–61. 8081313
74. Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PR, et al. (2009) The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet 18 : 4081–4088. doi: 10.1093/hmg/ddp357 19643913
75. Rhee EP, Ho JE, Chen MH, Shen D, Cheng S, et al. (2013) A genome-wide association study of the human metabolome in a community-based cohort. Cell Metab 18 : 130–143. doi: 10.1016/j.cmet.2013.06.013 23823483
76. Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, et al. (2011) Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 121 : 1402–1411. doi: 10.1172/JCI44442 21403394
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 11- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Effectiveness of Seasonal Malaria Chemoprevention in Children under Ten Years of Age in Senegal: A Stepped-Wedge Cluster-Randomised Trial
- Lifestyle Advice Combined with Personalized Estimates of Genetic or Phenotypic Risk of Type 2 Diabetes, and Objectively Measured Physical Activity: A Randomized Controlled Trial
- Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
- Projected Impact of Mexico’s Sugar-Sweetened Beverage Tax Policy on Diabetes and Cardiovascular Disease: A Modeling Study
- Promoting Partner Testing and Couples Testing through Secondary Distribution of HIV Self-Tests: A Randomized Clinical Trial
- Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
- Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050?
- Educational Outreach with an Integrated Clinical Tool for Nurse-Led Non-communicable Chronic Disease Management in Primary Care in South Africa: A Pragmatic Cluster Randomised Controlled Trial
- Measures of Malaria Burden after Long-Lasting Insecticidal Net Distribution and Indoor Residual Spraying at Three Sites in Uganda: A Prospective Observational Study
- Leukocyte Telomere Length in Relation to 17 Biomarkers of Cardiovascular Disease Risk: A Cross-Sectional Study of US Adults
- Under-prescribing of Prevention Drugs and Primary Prevention of Stroke and Transient Ischaemic Attack in UK General Practice: A Retrospective Analysis
- The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study
- Three Steps to Improve Management of Noncommunicable Diseases in Humanitarian Crises
- A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project
- Improving the Pipeline for Developing and Testing Pharmacological Treatments in Pregnancy
- Seasonal Malaria Chemoprevention: An Evolving Research Paradigm
- Exposure Patterns Driving Ebola Transmission in West Africa: A Retrospective Observational Study
- Minimally Invasive Autopsy: A New Paradigm for Understanding Global Health?
- Towards Equity in Health: Researchers Take Stock
- Willingness to Know the Cause of Death and Hypothetical Acceptability of the Minimally Invasive Autopsy in Six Diverse African and Asian Settings: A Mixed Methods Socio-Behavioural Study
- Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels
- Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis
- Validity of a Minimally Invasive Autopsy for Cause of Death Determination in Adults in Mozambique: An Observational Study
- The Dengue Vaccine Dilemma: Balancing the Individual and Population Risks and Benefits
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
- Three Steps to Improve Management of Noncommunicable Diseases in Humanitarian Crises
- Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
- A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



