-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Multiplex Identification of Gram-Positive Bacteria and Resistance Determinants Directly from Positive Blood Culture Broths: Evaluation of an Automated Microarray-Based Nucleic Acid Test
Background:
A multicenter study was conducted to evaluate the diagnostic accuracy (sensitivity and specificity) of the Verigene Gram-Positive Blood Culture Test (BC-GP) test to identify 12 Gram-positive bacterial gene targets and three genetic resistance determinants directly from positive blood culture broths containing Gram-positive bacteria.Methods and Findings:
1,252 blood cultures containing Gram-positive bacteria were prospectively collected and tested at five clinical centers between April, 2011 and January, 2012. An additional 387 contrived blood cultures containing uncommon targets (e.g., Listeria spp., S. lugdunensis, vanB-positive Enterococci) were included to fully evaluate the performance of the BC-GP test. Sensitivity and specificity for the 12 specific genus or species targets identified by the BC-GP test ranged from 92.6%–100% and 95.4%–100%, respectively. Identification of the mecA gene in 599 cultures containing S. aureus or S. epidermidis was 98.6% sensitive and 94.3% specific compared to cefoxitin disk method. Identification of the vanA gene in 81 cultures containing Enterococcus faecium or E. faecalis was 100% sensitive and specific. Approximately 7.5% (87/1,157) of single-organism cultures contained Gram-positive bacteria not present on the BC-GP test panel. In 95 cultures containing multiple organisms the BC-GP test was in 71.6% (68/95) agreement with culture results. Retrospective analysis of 107 separate blood cultures demonstrated that identification of methicillin resistant S. aureus and vancomycin resistant Enterococcus spp. was completed an average of 41.8 to 42.4 h earlier using the BC-GP test compared to routine culture methods. The BC-GP test was unable to assign mecA to a specific organism in cultures containing more than one Staphylococcus isolate and does not identify common blood culture contaminants such as Micrococcus, Corynebacterium, and Bacillus.Conclusions:
The BC-GP test is a multiplex test capable of detecting most leading causes of Gram-positive bacterial blood stream infections as well as genetic markers of methicillin and vancomycin resistance directly from positive blood cultures.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(7): e32767. doi:10.1371/journal.pmed.1001478
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001478Summary
Background:
A multicenter study was conducted to evaluate the diagnostic accuracy (sensitivity and specificity) of the Verigene Gram-Positive Blood Culture Test (BC-GP) test to identify 12 Gram-positive bacterial gene targets and three genetic resistance determinants directly from positive blood culture broths containing Gram-positive bacteria.Methods and Findings:
1,252 blood cultures containing Gram-positive bacteria were prospectively collected and tested at five clinical centers between April, 2011 and January, 2012. An additional 387 contrived blood cultures containing uncommon targets (e.g., Listeria spp., S. lugdunensis, vanB-positive Enterococci) were included to fully evaluate the performance of the BC-GP test. Sensitivity and specificity for the 12 specific genus or species targets identified by the BC-GP test ranged from 92.6%–100% and 95.4%–100%, respectively. Identification of the mecA gene in 599 cultures containing S. aureus or S. epidermidis was 98.6% sensitive and 94.3% specific compared to cefoxitin disk method. Identification of the vanA gene in 81 cultures containing Enterococcus faecium or E. faecalis was 100% sensitive and specific. Approximately 7.5% (87/1,157) of single-organism cultures contained Gram-positive bacteria not present on the BC-GP test panel. In 95 cultures containing multiple organisms the BC-GP test was in 71.6% (68/95) agreement with culture results. Retrospective analysis of 107 separate blood cultures demonstrated that identification of methicillin resistant S. aureus and vancomycin resistant Enterococcus spp. was completed an average of 41.8 to 42.4 h earlier using the BC-GP test compared to routine culture methods. The BC-GP test was unable to assign mecA to a specific organism in cultures containing more than one Staphylococcus isolate and does not identify common blood culture contaminants such as Micrococcus, Corynebacterium, and Bacillus.Conclusions:
The BC-GP test is a multiplex test capable of detecting most leading causes of Gram-positive bacterial blood stream infections as well as genetic markers of methicillin and vancomycin resistance directly from positive blood cultures.
Please see later in the article for the Editors' SummaryIntroduction
Sepsis resulting from bacterial bloodstream infection (BSI) is a serious condition that results in up to 500,000 hospitalizations per year and accounts for 11% of intensive care unit (ICU) admissions in the United States [1],[2]. Underscoring the significance of these infections is a mortality rate of 25% to 80% in critically ill patients [1],[3],[4]. The most prevalent causes of bacterial BSI are the Gram-positive bacteria, which account for 52% to 77% of bacterial sepsis [2],[5]. Among Gram-positive organisms, coagulase negative Staphylococcus spp. (CoNS) are most commonly isolated followed by S. aureus and Enterococcus spp. [5],[6]. Streptococcus pneumoniae are less frequently isolated but are of specific concern for patients with pneumococcal pneumonia [5]. BSIs carry a high monetary cost for the ill patient and also present a resource burden to the health care facility. In comparisons of patients admitted to hospital ICUs, those acquiring a BSI spent an additional 8 d in the ICU and 24 d in the hospital at an added cost of US$36,000 to US$40,000 per patient [4],[7].
The outcome of BSI can be affected by numerous factors including patient age, number, and type of co-morbidities, and time to effective antibiotic therapy [5],[8]. Indeed, effective antibiotic therapy has been independently correlated with positive outcome following culture confirmation of BSI [8],[9]. Kumar et al. report a 7.6% mean decrease in survival for every hour effective antibiotic therapy is delayed following the onset of sepsis-related hypotension [8]. Similarly, Bauer et al. demonstrated a reduction in length of ICU stay of 6.2 d and an overall savings of US$21,000 per septic episode by accurately identifying and differentiating S. aureus, methicillin-resistant S. aureus (MRSA), and coagulase negative staphylococci directly from positive blood cultures [10]. These savings were attributed, in part, to timely administration of appropriate antimicrobial therapy based on rapid laboratory results.
The current mainstay of laboratory diagnosis for BSI is broth-based culture of patient blood samples using a continuous monitoring blood culture system. Upon culture positivity, a primary Gram stain is performed and a portion of the broth culture is inoculated to solid media. Solid media subcultures require 18 to 48 h of incubation prior to biochemical testing of isolates to reach a definitive bacterial identification. Subsequent antibiotic susceptibility testing requires an additional 12–24 h for final result. This extended delay between confirmation of BSI, final identification, and susceptibility results leaves the clinician with little actionable information during a critical phase of infection. In response, patients are routinely treated with empiric broad spectrum antimicrobials that in some instances may be ineffective [11]. For this reason, a variety of technologies have been employed to shorten the window between blood culture positivity and the availability of results useful in guiding therapy.
Molecular methods, fluorescent in situ hybridization (FISH), and more recently matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-ToF MS) have all been used to rapidly identify a variety of organisms directly from positive blood cultures [12]–[19]. These methods all significantly reduce turn-around-time (TAT) compared to routine culture, delivering results in as little as 30–60 min following blood culture positivity. Sensitivity and specificity of these methods are generally high, reaching >90% for each method. Unfortunately, current molecular methods and FISH are limited to the detection of one or few specific targets. MALDI-ToF MS has the potential to identify nearly any bacterial species present in a blood culture; however, acceptable confidence score results were obtained for only 67% to 80% of cultures containing Gram-positive bacteria when using standard protocols [12]–[14]. Further, differentiation between S. pneumoniae and S. mitis using MALDI-ToF MS remains an active area of investigation [20]–[22]. These shortcomings have been addressed primarily through optimization of spectral analysis software and modification of scoring thresholds [12],[20]–[22].
In this study we evaluate the microarray based Verigene Gram-Positive Blood Culture test (BC-GP) (Nanosphere) for the identification of 12 Gram-positive bacterial targets and three genetic markers of antibiotic resistance directly from positive blood culture broths. This test was intended for analysis of positive blood cultures confirmed to contain Gram-positive organisms upon primary Gram stain of the broth. The specific targets identified by the BC-GP test include Staphylococcus spp., S. aureus, S. epidermidis, S. lugdudensis, Streptococcus spp., S. pyogenes, S. agalactiae, S. anginosus group, S. pneumoniae, E. faecalis, E. faecium, and Listeria spp. as well as the mecA, vanA, and vanB genes. We report sensitivity and specificity of the BC-GP test compared to routine culture methods for 1,252 prospectively collected blood cultures including 95 polymicrobial cultures obtained at five medical centers using BACTEC (BD) and BacT/ALERT (bioMérieux) blood culture systems.
Materials and Methods
Collection of Blood Culture Broths
A total of 1,252 positive blood culture broths were prospectively and consecutively collected at five clinical centers located in Wisconsin, New York, Illinois, Texas, and Ohio in accordance with site-specific institutional review board (IRB)-approved study protocols. Specimens were enrolled in this study from April, 2011 to January, 2012. Compliance criteria for this study included blood cultures using BACTEC Plus Aerobic/F (BD) and BacT/ALERT FA FAN (bioMérieux) aerobic blood culture medium. Blood culture broths were eligible for study enrolment if they contained Gram-positive cocci or bacilli on primary Gram stain and could be tested on the BC-GP within 12 h of broth positivity (held at room temperature). Only one positive blood culture per patient was allowed to avoid redundancy of enrolled samples (Text S1).
Verigene Gram-Positive Blood Culture Test
The BC-GP test is a sample to result system consisting of a sample processor (SP) and microarray reader. This test was run by laboratory technologists who were trained by the manufacturer on how to perform the test. A single use extraction tray was inserted into the SP and a 350 µl aliquot of positive blood culture broth containing Gram-positive organisms was transferred to the sample well within the extraction tray. Nucleic acid was extracted from blood culture samples using magnetic bead-based extraction and reagents contained in the extraction tray. No amplification of nucleic acid is performed. Purified nucleic acid was automatically hybridized to complementary nucleic acid capture probes immobilized on a glass microarray slide within the SP. Capture probes for each BC-GP test target are present in triplicate on the array. Detection of target sequence relies on hybridization of a second, nanoparticle-conjugated, detection probe. This method allows up to 1,000-fold greater sensitivity than fluorescent probes and requires comparatively simple excitation and detection optics [23],[24]. Automated sample processing (nucleic acid extraction and array hybridization) in the SP takes 2.5 h. Reading of the array is conducted in the Verigene Reader following processing and takes 30 to 60 sec. Tests generating indeterminate results (e.g., internal control failure, extraction control failure, variation in target signal, high background signal) were repeated a single time. A total of 64 cultures generated an indeterminate result upon initial analysis resulting in an initial call rate of 94.9% (1,188/1,252). Of these, 82.8% (53/64) were resolved following a single retest for a final call rate of 99.1%.
Reference Culture Method
Reference method testing was conducted by trained laboratory technologists (Figure S1; Text S1). To avoid bias, technologists conducting reference method testing were blinded to results obtained using the BC-GP test. Broth from blood cultures that were analyzed using the BC-GP test was inoculated to Trypticase Soy Agar with 5% Sheep Blood (TSA, BBL 221261 or equivalent, BD) and incubated at 35°C for 48 h. Following 24 and 48 h incubation, two glycerol stocks (Trypticase Soy Broth with 20% glycerol, BD) were made from each unique colony type present on the plate. One stock was retained by the test lab and the other was shipped on dry ice to a third party reference lab (Dynacare Laboratories). Glycerol stocks received by the reference laboratory were thawed, inoculated to TSA, and incubated 24 h at 35°C in 5% CO2. Following incubation, an isolated colony(s) representative of each colony type present on the plate were passed a second time onto TSA and incubated 24–48 h at 35°C. Glycerol stocks were made from resulting bacterial growth and each bacterial isolate was identified according to a reference culture flow chart using biochemical tests (Figure S1; Text S1). Briefly, S. aureus was identified on the basis of a positive catalase reaction, observation of beta-hemolysis on TSA, and a positive latex agglutination test. CoNS were identified similarly; however CoNS could demonstrate either beta - or gamma -hemolysis on TSA coupled with a negative latex agglutination test. S. lugdunensis were identified with the addition of a positive test for ornithine decarboxylase and L-pyrrolidonyl arylamidase (PYR) activity. All CoNS were identified to species using Vitek 2 Gram-positive identification card (GPID). All isolates identified to species level as S. aureus or S. epidermidis were tested for resistance to methicillin using a cefoxitin disk diffusion assay according to CLSI guidelines [25]. Micrococcus spp. were identified based upon a positive catalase test, yellow colored colony, and a positive microdase disk test. Listeria spp. were identified using Gram stain (Gram-positive bacilli), the presence of “soft” beta-hemolysis on TSA, a positive catalase reaction, and Vitek GPID. Enterococcus spp. were identified by a negative catalase test and positive reactions for PYR and lucine aminipepdidase (LAP) along with the ability to grow in the presence of 6.5% NaCl in TSB broth and form black precipitate when cultured on bile esculin agar. E. faecalis and E. faecium were differentiated by the ability to utilize arabinose [E. faecium (+), E. faecalis (−)]. All isolates of E. faecium or E. faecalis were tested for resistance to vancomycin using Etest vancomycin (bioMérieux). Other species of Entercoccus were identified using Vitek 2 GPID. Streptococcus spp. were identified based upon negative catalase and PYR reactions combined with a positive LAP result. Colonies demonstrating beta hemolysis were typed using latex agglutination tests for Lancefield antigen types A (S. pyogenes) and B (S. agalactiae). If negative for type A and B antigens, S. anginosus group identification was made on the basis of positive Voges-Proskauer and arginine fermentation tests. S. pneumoniae was differentiated from other Streptococci on the basis of susceptibility to optochin (disk diffusion test) and bile solubility. Additionally, all Streptococcus spp. were identified using Vitek 2 GPID (bioMérieux).
Contrived Cultures
A challenge set of 387 contrived (n = 213) or previously characterized (n = 174) blood cultures was constructed to test BC-GP targets that are rarely encountered in clinical laboratories (e.g., Listeria spp., vanB, etc.). For contrived cultures, characterized clinical isolates were seeded into BACTEC Plus Aerobic/F blood broths supplemented with 10 ml fresh whole blood. Broths were incubated in a BACTEC continuous monitoring blood culture instrument until positivity. A 1 ml aliquot of the positive broth culture was removed, frozen, blinded, and shipped to one of the five clinical test sites for analysis using the BC-GP. Reference culture identification was conducted as described above. Since the BC-GP Listeria target is a genus only target various species were used in construction of the challenge set including L. monocytogenes (n = 21), L. innocua (n = 7), L. ivanovii (n = 2), L. seeligeri, and L. welshimeri.
Nucleic Acid Sequencing and Discrepant Analysis
All E. faecalis and E. faecium isolates testing positive for vanA or vanB by BC-GP and/or demonstrating phenotypic resistance to vancomycin were sequenced by a third-party service laboratory (ACGT, Inc.) to confirm the presence of vanA/vanB. Extracted DNA was amplified with either vanA- or vanB-specific PCR primers and run on an agarose gel to observe the presence of a specific amplification product. Specific PCR-amplified products were then sequenced with the appropriate specific primers and the raw sequence data was subjected to PHRED score analysis. Only PHRED 30 quality sequences were collected and aligned to determine the specific van allele variant.
Procedures used for the in-house PCR assay to detect mecA were those described by Paule et al., including DNA extraction, primers, real-time PCR conditions, and melt analysis to confirm amplicon identity [26]. Ribosomal 16S gene sequencing method and primer design was performed as described by Clarridge et al. and as found in CLSI MM18-A [27]. PCR and sequencing methods followed protocols for rpoB gene amplification and sequencing described by Khamis et al. [28]. 16S rRNA nucleotide sequences were analyzed and interpreted using the MEGA software [29] and BLAST algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Assessment of Time to Identification Using BC-GP and Routine Culture Methods
A retrospective comparison of time to final identification and susceptibility result was conducted using a collection of 107 consecutive blood culture broths with a positive Gram stain for Gram-positive organisms. This additional study was conducted following laboratory validation and implementation of the BC-GP test for clinical use at a single study site (Medical College of Wisconsin, Milwaukee) in accordance with a separately IRB approved protocol allowing for collection of laboratory values including time of culture positivity, standard of care identification and susceptibility results, and time of final culture report. These cultures were not part of the 1,252 cultures prospectively collected for evaluation of the performance of the BC-GP test. Cultures were selected on the basis of a positive Gram-stain (Gram-positive bacteria present). Only the initial positive culture from a patient was analyzed using the BC-GP test. Time zero was defined as the time at which the primary culture Gram stain result was reported to appropriate medical staff responsible for care of the patient. Upon completion of the BC-GP test, the medical technologist directly notified the physician responsible for patient care by telephone with a “preliminary identification.” The time to result for the BC-GP test was defined as the time that the result was reported to the physician. For culture based identification and susceptibility, the time at which final results were entered into the laboratory information management system (available to medical staff) was utilized to determine total time to identification/susceptibility. The BC-GP test was set up following primary Gram-stain confirmation of Gram-positive bacteria in broth culture. BC-GP test results were available within in 2 h of test initiation. Standard culture method involved subculture of the positive broth to TSA and chocolate agar (Remel) and incubation at 35°C for 18–24 h. Resulting colonies that tested positive for catalase activity were subjected to a Staphylococcus latex agglutination test for preliminary differentiation of S. aureus from other Staphylococcus species. Full identification and susceptibility testing of isolates was conducted using the BD Phoenix system (BD). Colonies preliminarily identified as coagulase negative Staphylococcus species were only subjected to full identification and susceptibility if they were present in multiple culture from the same patient.
Statistical Analysis
Sensitivity and specificity were calculated using standard methods. Ninety-five percent confidence intervals were calculated using binomial expansion. p-Values were calculated using matched two-tailed t-test.
Results
Prospectively Collected Monomicrobial Cultures
A total of 1,157 prospectively collected blood cultures contained a single organism as determined by reference culture method. The majority of these contained Staphylococcus spp., which accounted for 73.0% (845/1,157) of monomicrobial cultures, followed by Streptococcus spp., 11.7% (135/1,157), E. faecalis or faecium, 7.4% (86/1,157), and Listeria spp., 0.3% (3/1,154). Only 7.5% (87/1,157) of cultures contained organisms not included on the BC-GP panel. The majority of these cultures were positive for Micrococcus spp., Corynebaterium spp., Kocuria spp., and Bacillus spp.
Sensitivity of the Staphylococcus genus target was a combined 99.4% (840/845) (Table 1). Five cultures categorized as false negative were resulted as “not detected” by the BC-GP test. These cultures all contained CoNS including one methicillin susceptible S. epidermidis (MSSE), one methicillin resistant S. epidermidis (MRSE), two S. hominis, and one S. haemolyticus. Specificity of the Staphylococcus genus target was 99.7% (310/311). The false positive result was reported as “Staphylococcus spp.” by the BC-GP test but the reference method culture identified the isolate as Aerococcus viridans. The S. aureus species target was 99.7% (317/318) sensitive and 100% specific. The single false negative was identified as methicillin susceptible S. aureus (MSSA) by culture method but “Staphylococcus spp.” only by BC-GP. S. epidermidis was identified with a sensitivity of 96.5% (272/282) and specificity of 98.9% (864/874). Of ten false negative S. epidermidis results, seven were reported as “Staphylococcus spp.” only and three were reported as “not detected” by the BC-GP test. Nucleic acid sequencing used to resolve discrepant results confirmed the identification of S. epidermidis in six of seven cultures reported as “Staphylococcus spp.” (i.e., false negative). The ten false positive cultures with a BC-GP test result of “S. epidermidis” contained one S. intermedius, two each S. capitis, S. hominis, and “CoNS” and three S. haemolyticus. Nucleic acid sequencing supported the BC-GP test result (S. epidermidis) in five of ten discrepant cultures, raising the final specificity to 99.4% (864/869). Identification of S. lugdunensis was 100% sensitive and specific, however only eight prospectively collected cultures contained this organism.
Tab. 1. Detection of Staphylococcus spp. in prospectively collected monomicrobial blood cultures by Verigene BC-GP (n = 1,157). 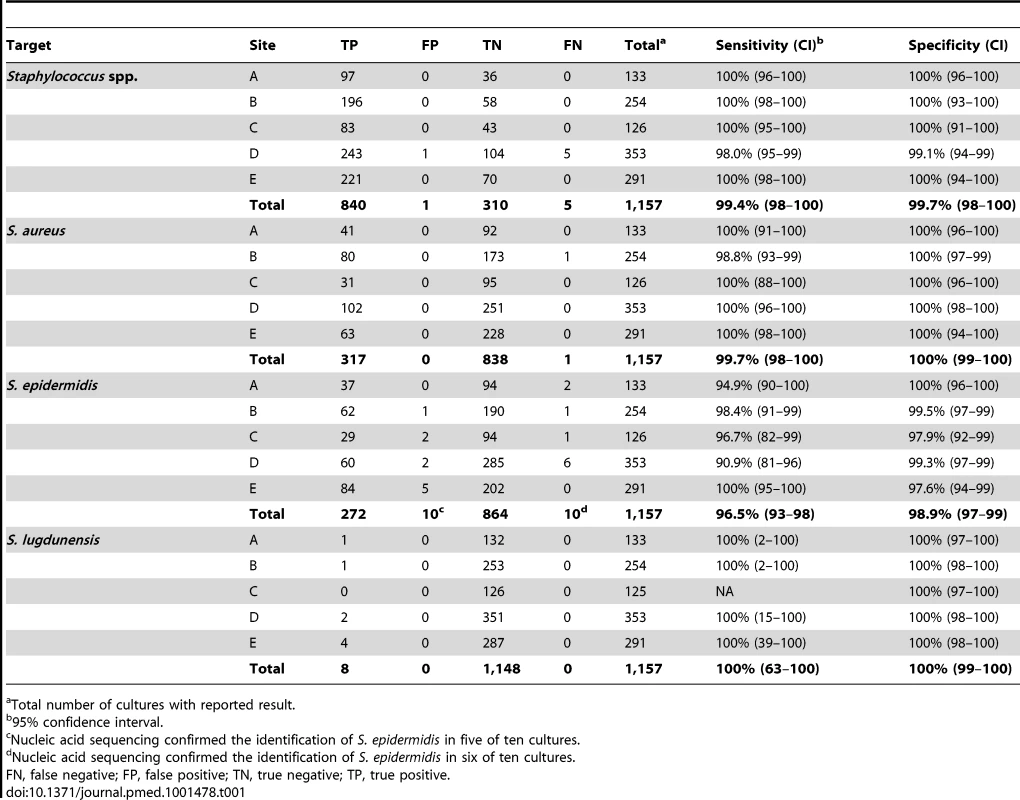
Total number of cultures with reported result. Streptococcus spp. were identified with 94.8% (128/135) sensitivity and 99.6% (1,018/1,022) specificity in prospectively collected cultures (Table 2). The seven false negative results were all reported as “not detected” by the BC-GP test and included six cultures containing alpha hemolytic streptococci (two S. mitis, S. mutans, S. sanguinis, S. salivarius, and “viridans group streptococcus”). The remaining false negative “Streptococcus spp.” result was a culture containing S. agalactiae. Four cultures incorrectly reported as positive for “Streptococcus spp.” contained Lactococcus garvieae (two), Granulicatella adjacens, and Lactococcus spp. Sensitivity for identification of S. pyogenes (9/9), S. pneumoniae (23/23), and S. anginosus group (8/8) species targets were all 100%. Five cultures incorrectly reported as “S. pneumoniae” by the BC-GP test were found to contain viridans group streptococci, including four of five that contained S. mitis as determined by the reference method.
Tab. 2. Detection of Streptococcus spp. in prospectively collected monomicrobial blood cultures by Verigene BC-GP (n = 1,157). 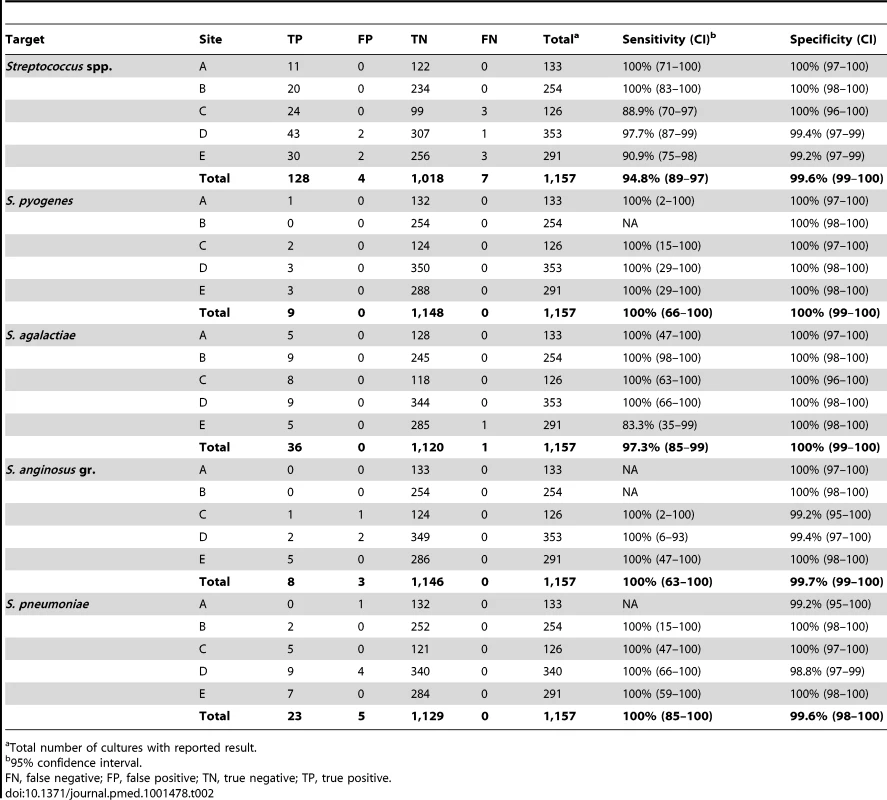
Total number of cultures with reported result. The BC-GP test does not contain a general genus target for Enterococcus spp., rather, it specifically identifies only E. faecalis and E. faecium species. Sensitivity for identification of these species was 94.8% (55/58) and 92.6% (26/28), respectively (Table 3). All five cultures categorized as false negative were reported as “not detected” by BC-GP but were positive for E. faecalis or E. faecium by the reference method. The single false positive result came from a culture reported as positive for both E. faecalis and S. pyogenes by BC-GP, however only S. pyogenes was recovered upon subculture and reference testing. Only three prospectively collected blood cultures contained Listeria spp., including two with L. monocytogenes and one with L. innocua. The BC-GP was 100% sensitive and specific for detection of these organisms.
Tab. 3. Detection of E. faecalis, E. faecium, and Listeria in prospectively collected monomicrobial blood cultures by Verigene BC-GP (n = 1,157). 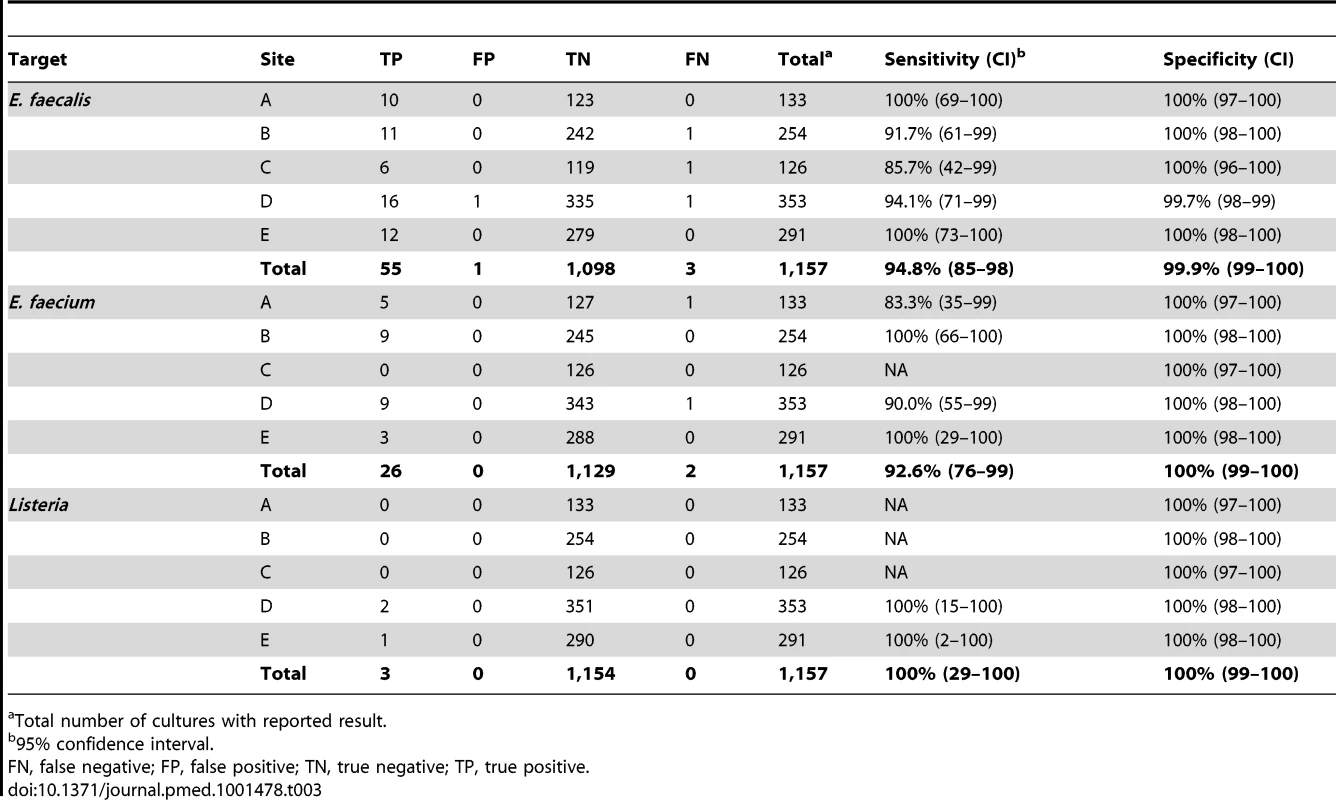
Total number of cultures with reported result. Identification of mecA, vanA, and vanB as Markers of Antibiotic Resistance
The BC-GP test identifies the mecA gene, which serves as an indicator of resistance to methicillin in Staphylococcus spp., and the vanA and vanB genes, which serve as markers of resistance to vancomycin in E. faecalis and E. faecium. Although mecA can be found in most species of Staphylococcus, the BC-GP only reports mecA positivity in S. aureus and S. epidermidis. Among the five clinical test sites, methicillin resistance in S. aureus and S. epidermidis ranged from 46.8% to 64.5% as determined by cefoxitin disk diffusion test. The BC-GP test was 98.6% (348/353) sensitive and 94.3% (232/246) specific for prediction of methicillin resistance based on mecA positivity when using cefoxitin disk test results as gold standard comparator (Table 4). The five false negative methicillin resistance results were noted for both S. epidermidis (3/5) and S. aureus (2/5). Four of these isolates were available for discrepant analysis using an alternative nucleic acid amplification test (NAAT) specific for mecA. All four isolates tested negative using the alternative NAAT, raising the post discrepant resolution sensitivity to 99.7% (348/349). False positive results were more common among S. epidermidis (13/14) compared to S. aureus (1/14). Discrepant analysis using an alternative NAAT was conducted and confirmed the presence of mecA in 8/14 of these isolates, raising the final post discrepant resolution specificity to 97.5% (232/238).
Tab. 4. Detection of resistance determinants mecA, vanA, and vanB in prospectively collected monomicrobial blood cultures by Verigene BC-GP (n = 599 S. aureus/S. epidermidis, n = 81 E. faecalis/E. faecium). 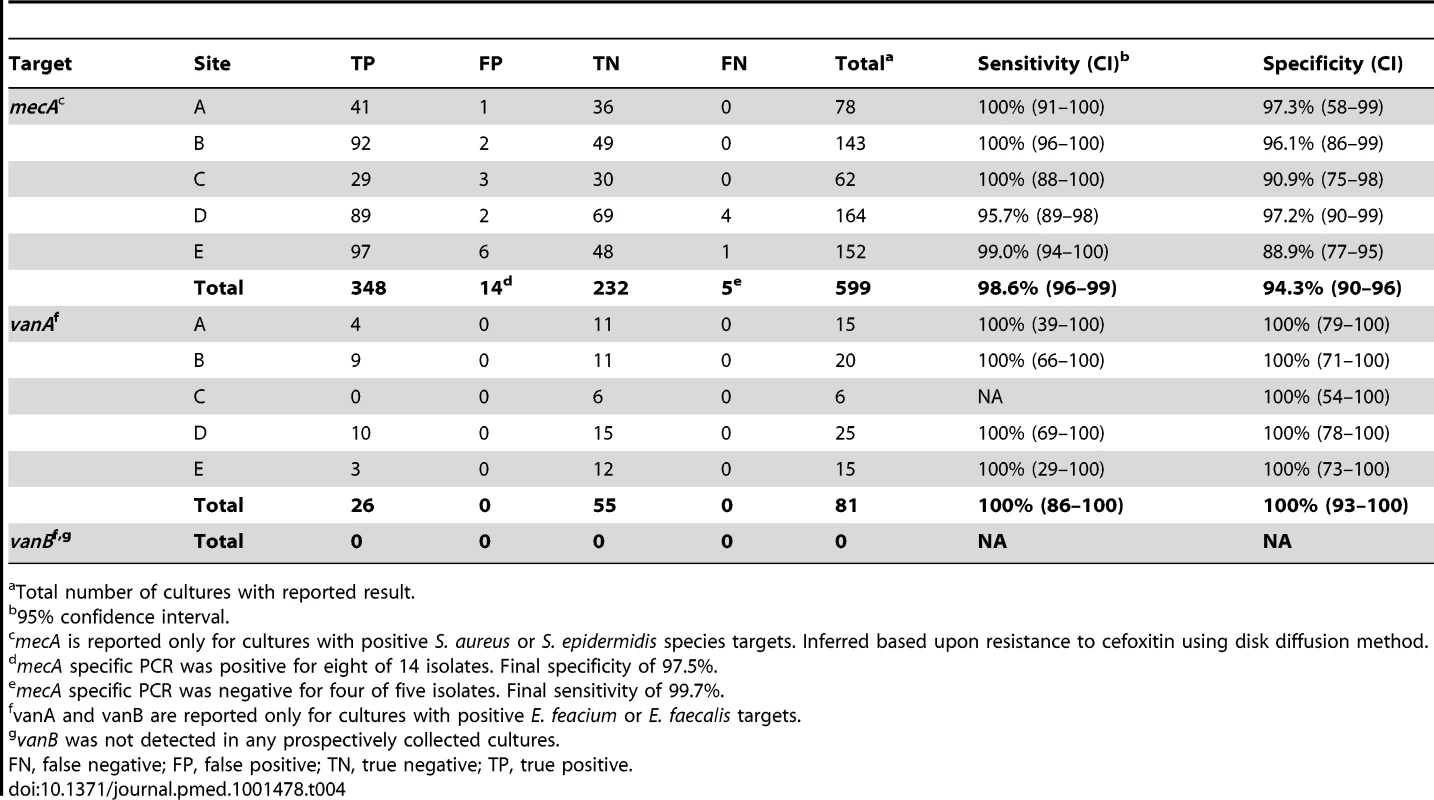
Total number of cultures with reported result. Vancomycin resistance was observed in 55 of 81 E. faecalis or E. faecium isolates recovered from positive blood culture broths as determined by Etest vancomycin reference method. The BC-GP was 100% sensitive and specific, reporting a vanA positive result for all vancomycin resistant isolates. Phenotypic resistance and genotype (vanA or vanB) was confirmed by nucleic acid sequencing, which confirmed the presence of vanA sequence variant-2 in these isolates (Table S2).
Polymicrobial Blood Cultures
Prospectively collected blood cultures included 95/1,252 (7.6%) that were found to contain more than one organism, i.e., polymicrobial, when tested by reference culture method. The majority of these (88/95) contained two different organisms while seven cultures contained three organisms (Table 5). Of the 95 polymicrobial cultures, 53 (55.8%) were composed of CoNS in addition to a second or third organism. The BC-GP was in complete agreement with the reference culture method for 68/95 (71.6%) cultures. False negative BC-GP results were far more common (n = 26) than false positive results (n = 3) among discordant cultures. The most commonly missed target was CoNS (n = 19) including 12 S. epidermidis, one S. lugdunensis, and six other cultures containing various CoNS species in addition to a second or third organism. S. aureus was missed in two polymicrobial cultures, both of which also contained Enterococcus which was correctly identified by BC-GP. The remaining five false negative results were Streptococcus spp. not detected by BC-GP that were isolated from cultures also containing CoNS or organisms not on the BC-GP panel (e.g., Rothia, Leuconostoc).
Tab. 5. Detection of Gram-positive identification and resistance determinants from polymicrobial blood cultures by Verigene BC-GP (n = 95). 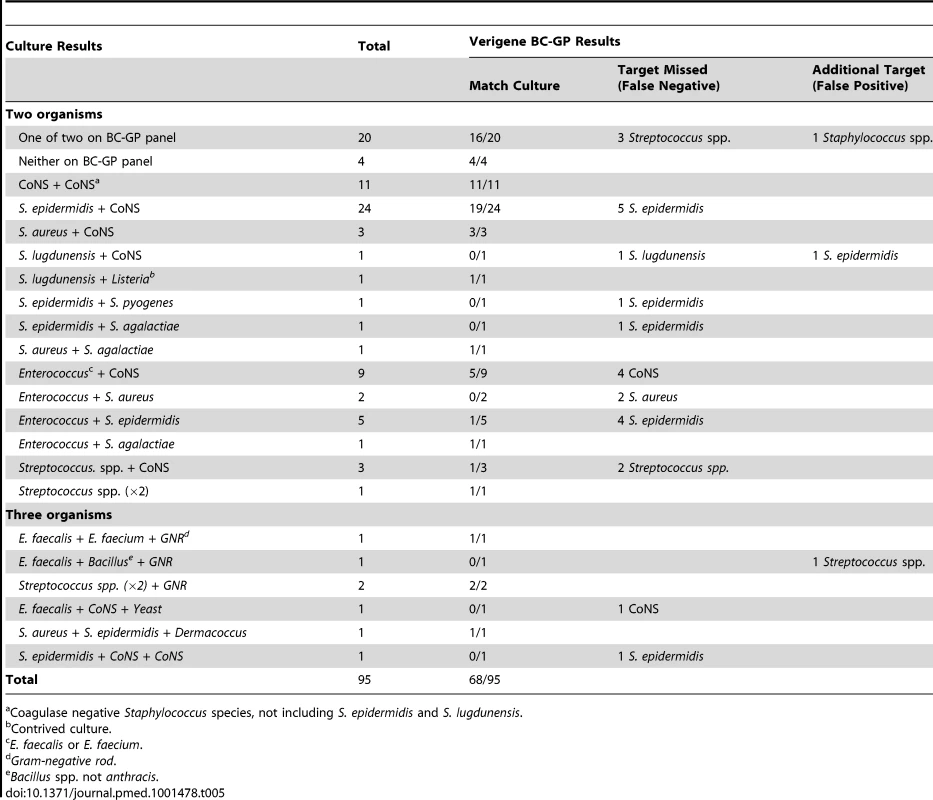
Coagulase negative Staphylococcus species, not including S. epidermidis and S. lugdunensis. Contrived Blood Cultures
Only 4/387 contrived cultures yielded a false negative identification result by BC-GP (Table 6). Two cultures contained S. pyogenes by reference culture method, one of which was reported as “not detected” and the other which was reported as “Streptococcus spp.” by BC-GP. This translates to a sensitivity of 99.3% (154/155) for Streptococcus spp. and 97.1% (66/68) for S. pyogenes among contrived cultures. The remaining false negative result was a culture containing E. faecalis that was misidentified as E. faecium by BC-GP. All other bacterial targets demonstrated 100% sensitivity. The specificity of the identification for all targets was also high in the contrived culture study. BC-GP reported positive results that were not confirmed by reference culture method in only 5/387 contrived cultures. These included a culture containing S. xylosus that was incorrectly identified as S. lugdunensis by BC-GP, an E. faecium that was incorrectly identified as E. faecalis, a culture containing only L. monocytogenes that was reported as positive for Listeria and S. anginosus (false positive for “Staphylococcus spp.” and S. anginosus group” targets), and a culture containing only E. faecalis that was reported as positive for E. faecalis and “Staphylococcus spp.”.
Tab. 6. Detection of Gram positive identification and resistance determinants from contrived blood cultures by Verigene BC-GP (n = 387). 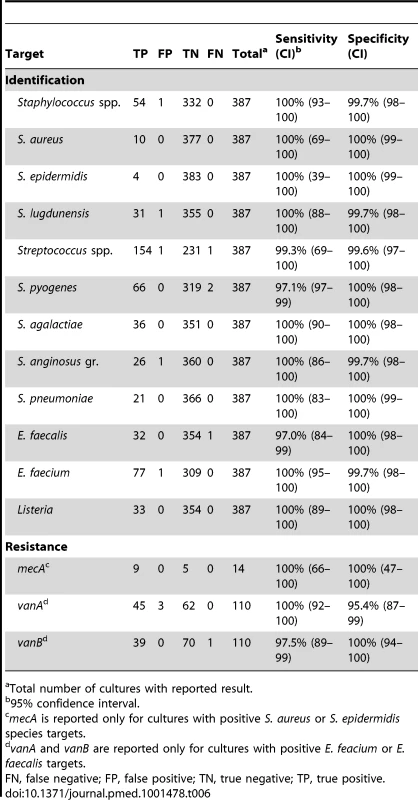
Total number of cultures with reported result. Identification of genetic resistance determinants mecA, vanA, and vanB was also assessed using contrived cultures. Eighty-five cultures contained strains of E. faecalis or E. faecium that demonstrated resistance to vancomycin by Etest. The BC-GP demonstrated an overall sensitivity of 98.8% (84/85) for the identification of vanA (100%, 45/45) or vanB (97.5%, 39/40) in these isolates. All vanA genes were confirmed as sequence variant-2 using nucleic acid sequencing. Strains that were positive for vanB were more diverse in sequence including 25 variant-6, 11 variant-14, three variant-8, and one variant-10 alleles. The single strain false negative for vanB contained a sequence variant-6 allele.
Time to Identification
The difference in time between reporting of results using the BC-GP test and final identification and antimicrobial susceptibility results was examined for 107 positive blood culture broths containing Gram-positive bacteria. Of these, 76 (71.0%) were reported as Gram-positive cocci in clusters (Staphylococcus spp., 68; Micrococcus spp., seven; Rothia spp., one). Results for cultures containing S. aureus (MRSA or MSSA) were available an average of 42.2 h (range 31 to 68 h) before final identification and susceptibility results were reported using routine culture and susceptibility testing methods (Table 7). Only eight of 43 cultures containing CoNS met criteria for full identification and susceptibility testing (>one set of blood cultures containing CoNS with different morphology), however preliminary identification of CoNS using culture methods was reported an average of 27.5 h (range 14 h to 48 h) after availability of the BC-GP test result. Among 22 positive cultures with a Gram stain indicating the presence of Gram-positive cocci in chains (Streptococcus spp., 12; Enterococcus spp., ten), BC-GP results were available an average of 53.4 h (range 31 to 127 h) before routine culture results. Importantly, this includes the identification of vancomycin-resistant Enterococcus (VRE), which was available an average of 44.4 h (range 33 to 55 h) before final results using routine culture and susceptibility testing methods.
Tab. 7. Difference in time to final identification and antimicrobial susceptibility report (n = 107). 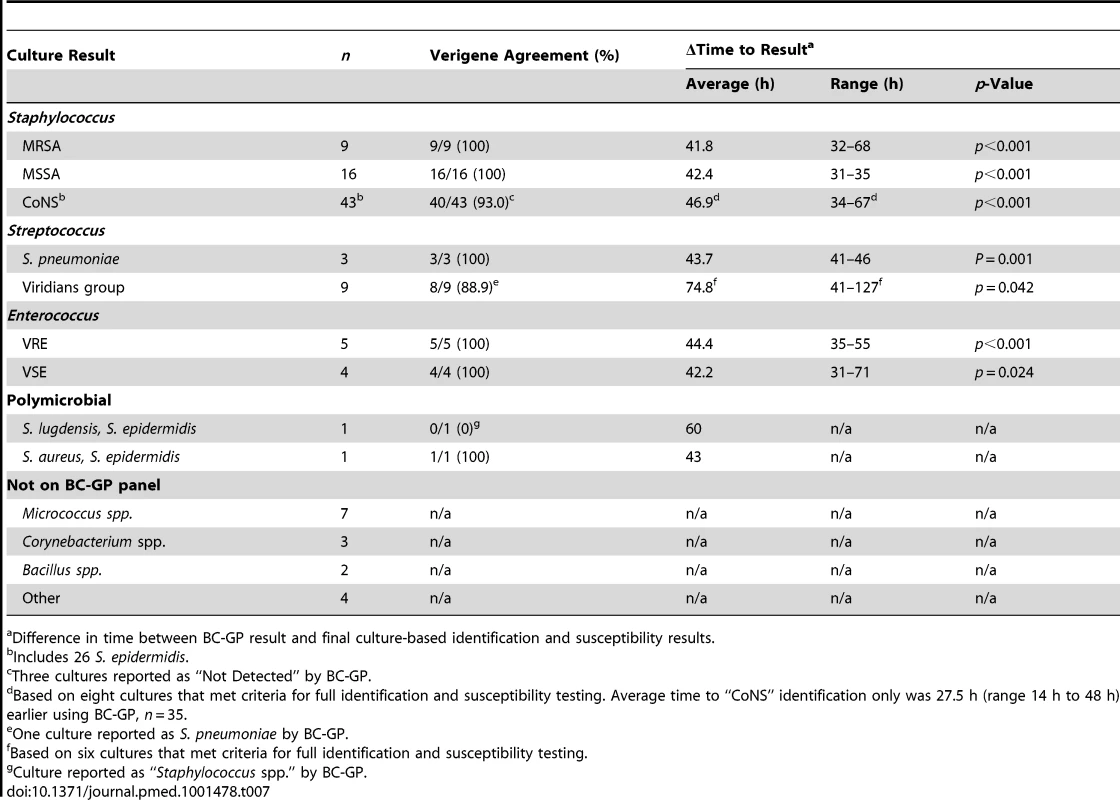
Difference in time between BC-GP result and final culture-based identification and susceptibility results. Discussion
Diagnostic methods capable of reducing the time to identification and providing antimicrobial susceptibility results for agents of BSI have great potential to positively impact patient care. Molecular and fluorescent in situ hybridization (FISH) methods have been used to dramatically reduce the TAT for identification of a variety of organisms directly from positive blood cultures [15]–[18],[30]. Specifically, FISH has been used to identify and differentiate S. aureus from CoNS with a sensitivity of 96% to 98% and specificity of 89% to 100% within 30 min of culture positivity [15],[16]. FISH technology has also been used to identify Enterococcus spp. and various yeasts directly from positive blood cultures [31],[32]. Molecular methods for the detection of various bacteria including P. aeruginosa, S. aureus, and MRSA directly from blood culture have also been reported and demonstrate high sensitivity and specificity characteristics [17]–[19]. The use of currently available FISH and molecular methods are somewhat limited by the ability to identify only single or few specific targets (e.g., Staphylococcus spp., Enterococcus spp.). In this study, Staphylococcus spp. and Enterococcus spp. comprised 79.6% of bacteria isolated from positive blood cultures. Mass spectrometry based methods such as MALDI-ToF MS have the advantage of being able to identify nearly any bacterium or yeast present in a blood culture broth within approximately 30 min [12]–[14],[33]. A current weakness of this method is a limited ability to identify the individual constituents present in polymicrobial cultures [12]. Additionally, there are no widely available methods using MALDI-ToF to reliably identify genetic determinants of antimicrobial resistance, which is critical to selection of appropriate therapy. However, novel methods are currently in development that may enable phenotypic susceptibility testing for some organisms and antimicrobials [34],[35].
A multiplex, microarray-based assay (Prove-it sepsis assay, Mobidiag) has recently been described that is capable of identifying 50 bacterial pathogens and the mecA gene directly from positive blood culture broths with >94% sensitivity and >98% specificity [36]. Using this assay, authors were able to reduce TAT by 17.5 h to 43.1 h for the identification of bacteria in positive blood cultures as compared to routine culture methods. As with our study, accurate identification of all components in polymicrobial culture was challenging, suggesting a potential weakness for all multiplexed direct-from-specimen nucleic acid tests.
The BC-GP test is a sample to result microarray-based test consisting of single use reagents marketed at approximately US$75 USD per test. A major advantage of the BC-GP test is the rapid turnaround time following blood culture positivity. The BC-GP test requires only 350 µl of blood culture broth and can be completed within 2 h with less than 5 min of hands-on time. This enables same day analysis and reporting of positive blood culture results to physicians. The initial call rate including all prospective and contrived samples was 1,563/1,639 (95.4%). The BC-GP test panel includes 12 bacterial genus and species genetic targets representing the most common causes of Gram positive BSI. Only 87 monomicrobial cultures (7.5%) contained Gram positive organisms not present on the BC-GP test panel. This illustrates the broad applicability of this test for any blood culture containing Gram positive organisms upon primary stain. The most frequently isolated organisms not present on the BC-GP test panel were Micrococcus spp., Corynebacterium spp., Kocuria spp., Bacillus spp., and Rothia spp., which are common components of normal skin flora and are often associated with contaminated blood cultures [37].
Staphylococcus spp. are the most common species isolated from blood culture [38]. A recent study found that up to 32% of Staphylococcal BSIs were treated sub optimally using empiric therapy [39]. Identification and differentiation of MRSA, MSSA, and CoNS in positive blood cultures significantly reduces health care cost, shortens time to appropriate antimicrobial therapy, and improves patient outcome [10],[40]. A major benefit of the BC-GP test is the ability to identify and differentiate several species of Staphylococcus, including S. aureus, S. epidermidis, and S. lugdunensis, as well as the mecA gene encoding resistance to methicillin. Detection of MRSA using individual probes specific for S. aureus and mecA nucleic acid targets reduces the probability of false negative results that can occur as the result of genetic SCCmec rearrangements or emergence of new SCCmec cassettes [41],[42]. The ability to rapidly identify S. lugdunensis may also aid in guiding early patient management decisions because of the increased association of this bacterium with endocarditis, joint infections, and abscess compared to other CoNS [43],[44]. Additionally, accurate identification of S. lugdunensis is important to selection of antimicrobial therapy because of different breakpoints for oxacillin resistance in S. lugdunensis (≥4 µg/ml) compared to other CoNS (≥0.5 µg/ml) [25].
Streptococcus species are another group of organisms commonly isolated from blood cultures. Many alpha haemolytic “viridans group” species are relatively innocuous contaminants. In contrast, S. pyogenes, S. agalactiae, and S. pneumoniae can cause serious infection including meningitis, necrotizing fasciitis, and pneumonia. The BC-GP test misidentified five viridans group streptococci as S. pneumoniae. This is likely due to the close genetic relationship between mitis (viridans) group streptococci and S. pneumoniae. However, the overall specificity of the BC-GP test was still 99.6% for identification of S. pneumoniae in blood culture broth. Blood cultures containing S. pneumoniae as identified by the BC-GP test may be confirmed using standard biochemical tests such as optochin disk or bile solubility test once the isolate is cultured on solid medium. Blood culture is not recommended for the routine diagnosis of community acquired pneumonia (CAP) [45]; however in patients presenting with severe CAP, 2.1% to 13% were also bacteremic [46],[47]. Culture-based identification of the infecting organism from patient blood samples resulted in modification of antimicrobial therapy in 67% to 83% of these cases, including de-escalation in 50% to 80% of cases [46],[47]. Use of a direct-from-blood culture broth identification method such as the BC-GP test could enable earlier therapy interventions in these cases. Invasive infections with S. agalactiae are becoming more prevalent in non-pregnant adults including the elderly and immunocompromised [48],[49]. In one study central nervous system (CNS) involvement was present in 15% of patients with positive blood cultures and in 54.5% of disseminated infections [48]. In another case series, 78% of patients with CNS disease also had blood cultures positive for S. agalactiae [50]. Early identification of these organisms directly from blood culture broth also enables targeted antimicrobial therapy since these species are almost universally susceptible to penicillin and cephalosporins.
Enterococcus species, specifically E. faecium and E faecalis, was the third most commonly isolated genus in this study and is the second leading cause of BSI [38]. E. faecalis is more commonly isolated in blood cultures and is typically more susceptible to antimicrobials than E. faecium [51]. Specifically, resistance to ampicillin and vancomycin are rare in E. faecalis, 1.3%, and 0.5%, respectively, while 82.4% and 9.6% of E. faecium isolates are resistant to ampicillin and vancomycin, respectively [51]. Use of vancomycin as empiric therapy for suspected BSI has led to VRE being the most frequent agents of BSI that are inadequately treated [38]. The ability to identify and differentiate E. faecalis and E. faecium, along with the vanA and vanB genes directly from positive blood culture broth has the potential to dramatically reduce the time to appropriate therapy for these organisms.
Polymicrobial blood cultures accounted for 7.6% of prospectively collected blood cultures in this study. Cultures containing multiple organisms can be a challenge for direct detection methodologies for several reasons. In the case of MALDI-ToF, cumulative protein profiles of multiple organisms must be deconvoluted before accurate comparison to single reference spectra can be made. For molecular tests that detect a limited number of targets only one of the constituents may be reported. This can be misleading in the case of cultures containing multiple organisms with similar Gram stain morphologies. The use of microarray-based detection by the BC-GP test allows the capture and detection of multiple analytes, which decreases the chance of missing individual organisms in a polymicrobial culture. Agreement between the BC-GP test and reference culture method for polymicrobial cultures was only 72%. This is substantially lower than the 98% agreement observed for bacterial targets in prospective monomicrobial cultures. The majority of discrepant results in polymicrobial cultures involved CoNS, including S. epidermidis, which were missed by the BC-GP test. The increased prevalence of false negative results in polymicrobial cultures could be due to technical limitations of the test such as signal interference between multiple capture probes on the array. False negative results could also be due to minor constituents of the polymicrobial culture being present in low quantity, near the limit of detection of the BC-GP test.
A retrospective chart review of 107 blood culture broths containing Gram-positive organisms analyzed by BC-GP test demonstrated the ability to differentiate S. aureus from common blood culture contaminants including CoNS and other organisms with similar Gram-stain morphologies (e.g., Micrococcus spp, Rothia spp.) an average of 27.8 h (range 16 to 48 h) earlier than differentiation using routine solid media culture-based methods. Further, the identification of MRSA and MSSA were made 31 to 68 h (average 42.2 h) earlier using the BC-GP test as compared to routine culture identification and antimicrobial susceptibility testing. These data are similar to results reported by Bauer et al. and Ly et al. [10],[52]. These authors demonstrated a US$19,000 to US$21,000 reduction in cost of care, 2 - to 6-d reduction in ICU stay, and a reduction in sepsis related mortality from 16.8% (usual care) to 7.9% (early identification) using other direct-from-blood culture identification methods to identify and differentiate Staphylococcus spp., including MRSA and MSSA. An additional advantage of the BC-GP test is the ability to also identify E. faecalis and E. faecium, along with the vanA and vanB genes encoding vancomycin resistance. VRE are not covered by routinely used empiric therapy for BSI. Upon primary Gram stain, these resistant organisms appear very similar to Streptococcus spp., which are susceptible to vancomycin often included as part of routine empiric therapy. In this study, use of the BC-GP test was able to reduce the time to identification of VRE by 42 to 44 h. The rapid times to identification of MRSA, MSSA, and VRE enable earlier, targeted, modification to empiric therapy, which can result in improved patient outcome [10],[52].
One weakness of the BC-GP test is the inability to assign mecA positivity to a specific organism in a mixed culture. In the case of a culture positive for “S. aureus,” “S. epidermidis,” and “mecA,” the laboratory would have to wait for solid media-based isolation of each organism and susceptibility testing to determine which organism was methicillin resistant. This would delay antimicrobial de-escalation in the case of a culture containing MSSA that was contaminated with methicillin resistant S. epidermidis. This scenario appears to be rare however, occurring in only 1/1,252 (0.07%) prospectively collected cultures in this study. A second weakness of the BC-GP test is the omission of additional targets from the test panel that are common contaminants in blood cultures. Specifically, Micrococcus spp. was present in 26/87 (29.9%) of cultures containing organisms not on the BC-GP test panel. In addition to being a common contaminant, the Gram stain morphology of Micrococcus can be similar to that of Staphylococcus. This may cause hesitation in reporting cultures containing Gram positive cocci in clusters as “negative for Staphylococcus” even if all Staphylococcus targets are reported as “not detected” by BC-GP.
There were also two potential weaknesses in the design of this study that may have influenced sensitivity and specificity characteristics for specific targets. First, species level identification of CoNS was achieved using the Vitek 2 bacterial identification system. Because of similar biochemical profiles, the accuracy of Vitek 2 for identification of CoNS species ranges from 86% to 87.5% [53],[54]. The observed sensitivity of BC-GP to identify S. epidermidis was only 96.5%, which was lower than other Staphylococcus targets (99.4% to 100% sensitive). In 7/10 cultures containing S. epidermidis according to Vitek 2 result the BC-GP reported “Staphylococcus spp.” only, apparently missing the S. epidermidis target. These false negative results could be due to failure of the BC-GP test to accurately identify the S. epidermidis-specific target, but could also be the result of misidentification by Vitek 2. The second potential weakness is the use of cefoxitin disk diffusion method as a surrogate test to indicate the presence of mecA in S. aureus and S. epidermidis. False positive results (i.e., mecA positive/cefoxitin susceptible) have been attributed specific alleles of the mecA repressor, mecI, or point mutations in the mecA promoter region that decrease resistance to cefoxitin in strains of S. aureus carrying a functional mecA [55]; however, additional mechanisms may also contribute to this phenotype [56]. Conversely, false negative results, i.e., BC-GP test negative/cefoxitin resistant, could be due to resistance mechanisms other than mecA [57],[58]. Amplification and nucleic acid sequencing of the mecA gene would be required to definitively characterize these isolates. We addressed these weaknesses using nucleic acid sequencing for definitive identification of any discrepant result (false positive or false negative) involving S. epidermidis or the mecA gene.
The strengths of this study include the large enrollment of prospectively collected blood cultures (n = 1,252) by five geographically distinct clinical centers. Four of five sites (sites A–D) used BACTEC Aerobic/F broth and one site (site E) used BacT/ALERT FA aerobic broth for blood culture prior to testing on BC-GP. The sensitivity and specificity for each BC-GP target at individual clinical sites was within the 95% confidence interval for all sites combined, indicating that the BC-GP test generates reliable results independent of external variables such as geographic region, blood culture media, test operator, and prevalence of specific agents of BSI. Each target on the BC-GP panel was tested a minimum of 33 times using unique blood cultures containing each of the targets. For rare targets such as Listeria and vanB, contrived cultures prepared using clinical isolates were utilized to fully vet the test performance. The van gene of all Enterococcus isolates demonstrating phenotypic resistance to vancomycin was sequenced to confirm the BG-GP result of vanA or vanB as well as to determine the specific genetic variant of the gene present in each isolate.
We report the performance of the FDA cleared BC-GP test for the identification of Gram-positive organisms directly from positive blood culture broths. The high sensitivity and specificity characteristics of this test, coupled with on-demand testing capability and a 2-h TAT enable reporting of both the identification and antimicrobial resistance genes of bacteria obtained from blood culture significantly faster than using routine culture methods.
Supporting Information
Zdroje
1. AngusDC, WaxRS (2001) Epidemiology of sepsis: an update. Crit Care Med 29: S109–S116.
2. MartinGS, ManninoDM, EatonS, MossM (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348 : 1546–1554.
3. LevyMM, ArtigasA, PhillipsGS, RhodesA, BealeR, et al. (2012) Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 12 : 919–924.
4. PittetD, TararaD, WenzelRP (1994) Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 271 : 1598–1601.
5. GrozdanovskiK, MilenkovicZ, DemiriI, SpasovskaK (2012) Prediction of outcome from community-acquired severe sepsis and septic shock in tertiary-care university hospital in a developing country. Crit Care Res Pract 2012 : 182324.
6. WisplinghoffH, BischoffT, TallentSM, SeifertH, WenzelRP, et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39 : 309–317.
7. StonePW, BracciaD, LarsonE (2005) Systematic review of economic analyses of health care-associated infections. Am J Infect Control 33 : 501–509.
8. KumarA, RobertsD, WoodKE, LightB, ParrilloJE, et al. (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34 : 1589–1596.
9. BryanCS, ReynoldsKL, BrennerER (1983) Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev Infect Dis 5 : 629–638.
10. BauerKA, WestJE, Balada-LlasatJM, PancholiP, StevensonKB, et al. (2010) An antimicrobial stewardship program's impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis 51 : 1074–1080.
11. DellingerRP, LevyMM, CarletJM, BionJ, ParkerMM, et al. (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 34 : 17–60.
12. BuchanBW, RiebeKM, LedeboerNA (2012) Comparison of the MALDI Biotyper system using Sepsityper specimen processing to routine microbiological methods for identification of bacteria from positive blood culture bottles. J Clin Microbiol 50 : 346–352.
13. La ScolaB, RaoultD (2009) Direct identification of bacteria in positive blood culture bottles by matrix-assisted laser desorption ionisation time-of-flight mass spectrometry. PLoS One 4: e8041 doi:10.1371/journal.pone.0008041
14. StevensonLG, DrakeSK, MurrayPR (2010) Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 48 : 444–447.
15. DeckMK, AndersonES, BucknerRJ, ColasanteG, CoullJM, et al. (2012) Multicenter evaluation of the Staphylococcus QuickFISH method for simultaneous identification of Staphylococcus aureus and coagulase-negative staphylococci directly from blood culture bottles in less than 30 minutes. J Clin Microbiol 50 : 1994–1998.
16. HensleyDM, TapiaR, EncinaY (2009) An evaluation of the advandx Staphylococcus aureus/CNS PNA FISH assay. Clin Lab Sci 22 : 30–33.
17. CattoirV, GilibertA, Le GlaunecJM, LaunayN, Bait-MerabetL, et al. (2010) Rapid detection of Pseudomonas aeruginosa from positive blood cultures by quantitative PCR. Ann Clin Microbiol Antimicrob 9 : 21.
18. JukesL, MikhailJ, Bome-MannathokoN, HadfieldSJ, HarrisLG, et al. (2010) Rapid differentiation of Staphylococcus aureus, Staphylococcus epidermidis and other coagulase-negative staphylococci and meticillin susceptibility testing directly from growth-positive blood cultures by multiplex real-time PCR. J Med Microbiol 59 : 1456–1461.
19. WolkDM, StruelensMJ, PancholiP, DavisT, Della-LattaP, et al. (2009) Rapid detection of Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) in wound specimens and blood cultures: multicenter preclinical evaluation of the Cepheid Xpert MRSA/SA skin and soft tissue and blood culture assays. J Clin Microbiol 47 : 823–826.
20. IkryannikovaLN, FilimonovaAV, MalakhovaMV, SavinovaT, FilimonovaO, et al. (2012) Discrimination between Streptococcus pneumoniae and Streptococcus mitis based on sorting of their MALDI mass spectra. Clin Microbiol Infect In press.
21. MartinyD, BussonL, WyboI, El HajRA, DedisteA, et al. (2012) Comparison of the Microflex LT and Vitek MS systems for routine identification of bacteria by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 50 : 1313–1325.
22. WernoAM, ChristnerM, AndersonTP, MurdochDR (2012) Differentiation of Streptococcus pneumoniae from nonpneumococcal streptococci of the Streptococcus mitis group by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 50 : 2863–2867.
23. ElghanianR, StorhoffJJ, MucicRC, LetsingerRL, MirkinCA (1997) Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 277 : 1078–1081.
24. StorhoffJJ, MarlaSS, BaoP, HagenowS, MehtaH, et al. (2004) Gold nanoparticle-based detection of genomic DNA targets on microarrays using a novel optical detection system. Biosens Bioelectron 19 : 875–883.
25. CLSI (2011) Performance standards for antimicrobial susceptibility testing; 21st informational supplement. M100-S21.
26. PauleSM, PasquarielloAC, ThomsonRBJr, KaulKL, PetersonLR (2005) Real-time PCR can rapidly detect methicillin-susceptible and methicillin-resistant Staphylococcus aureus directly from positive blood culture bottles. Am J Clin Pathol 124 : 404–407.
27. ClarridgeJE3rd (2004) Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 17 : 840–862 table of contents.
28. KhamisA, RaoultD, La ScolaB (2005) Comparison between rpoB and 16S rRNA gene sequencing for molecular identification of 168 clinical isolates of Corynebacterium. J Clin Microbiol 43 : 1934–1936.
29. TamuraK, PetersonD, PetersonN, StecherG, NeiM, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28 : 2731–2739.
30. PetersRP, SavelkoulPH, Simoons-SmitAM, DannerSA, Vandenbroucke-GraulsCM, et al. (2006) Faster identification of pathogens in positive blood cultures by fluorescence in situ hybridization in routine practice. J Clin Microbiol 44 : 119–123.
31. MorganMA, MarloweE, Novak-WeeklyS, MillerJM, PainterTM, et al. (2011) A 1.5 hour procedure for identification of Enterococcus Species directly from blood cultures. J Vis Exp pii: 2616.
32. ShepardJR, AddisonRM, AlexanderBD, Della-LattaP, GhernaM, et al. (2008) Multicenter evaluation of the Candida albicans/Candida glabrata peptide nucleic acid fluorescent in situ hybridization method for simultaneous dual-color identification of C. albicans and C. glabrata directly from blood culture bottles. J Clin Microbiol 46 : 50–55.
33. StevensonLG, DrakeSK, SheaYR, ZelaznyAM, MurrayPR (2010) Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of clinically important yeast species. J Clin Microbiol 48 : 3482–3486.
34. BurckhardtI, ZimmermannS (2011) Using matrix-assisted laser desorption ionization-time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J Clin Microbiol 49 : 3321–3324.
35. DuZ, YangR, GuoZ, SongY, WangJ (2002) Identification of Staphylococcus aureus and determination of its methicillin resistance by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Chem 74 : 5487–5491.
36. TissariP, ZumlaA, TarkkaE, MeroS, SavolainenL, et al. (2010) Accurate and rapid identification of bacterial species from positive blood cultures with a DNA-based microarray platform: an observational study. Lancet 375 : 224–230.
37. HallKK, LymanJA (2006) Updated review of blood culture contamination. Clin Microbiol Rev 19 : 788–802.
38. IbrahimEH, ShermanG, WardS, FraserVJ, KollefMH (2000) The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118 : 146–155.
39. RuimyR, Dos-SantosM, RaskineL, BertF, MassonR, et al. (2008) Accuracy and potential usefulness of triplex real-time PCR for improving antibiotic treatment of patients with blood cultures showing clustered gram-positive cocci on direct smears. J Clin Microbiol 46 : 2045–2051.
40. PartaM, GoebelM, ThomasJ, MatloobiM, StagerC, et al. (2010) Impact of an assay that enables rapid determination of Staphylococcus species and their drug susceptibility on the treatment of patients with positive blood culture results. Infect Control Hosp Epidemiol 31 : 1043–1048.
41. BartelsMD, BoyeK, RohdeSM, LarsenAR, TorfsH, et al. (2009) A common variant of staphylococcal cassette chromosome mec type IVa in isolates from Copenhagen, Denmark, is not detected by the BD GeneOhm methicillin-resistant Staphylococcus aureus assay. J Clin Microbiol 47 : 1524–1527.
42. SnyderJW, MunierGK, HeckmanSA, CampP, OvermanTL (2009) Failure of the BD GeneOhm StaphSR assay for direct detection of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates in positive blood cultures collected in the United States. J Clin Microbiol 47 : 3747–3748.
43. KlotchkoA, WallaceMR, LicitraC, SiegerB (2011) Staphylococcus lugdunensis: an emerging pathogen. South Med J 104 : 509–514.
44. FrankKL, Del PozoJL, PatelR (2008) From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev 21 : 111–133.
45. MandellLA, WunderinkRG, AnzuetoA, BartlettJG, CampbellGD, et al. (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44 (Suppl 2) S27–S72.
46. ShahSS, DuganMH, BellLM, GrundmeierRW, FlorinTA, et al. (2011) Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J 30 : 475–479.
47. AbeT, TokudaY, IshimatsuS, BirrerRB (2009) Usefulness of initial blood cultures in patients admitted with pneumonia from an emergency department in Japan. J Infect Chemother 15 : 180–186.
48. ChaiwarithR, JullaketW, BunchooM, NuntachitN, SirisanthanaT, et al. (2011) Streptococcus agalactiae in adults at Chiang Mai University Hospital: a retrospective study. BMC Infect Dis 11 : 149.
49. EdwardsMS, BakerCJ (2005) Group B streptococcal infections in elderly adults. Clin Infect Dis 41 : 839–847.
50. DomingoP, BarquetN, AlvarezM, CollP, NavaJ, et al. (1997) Group B streptococcal meningitis in adults: report of twelve cases and review. Clin Infect Dis 25 : 1180–1187.
51. ProtonotariouE, DimitrouliaE, PournarasS, PitirigaV, SofianouD, et al. (2010) Trends in antimicrobial resistance of clinical isolates of Enterococcus faecalis and Enterococcus faecium in Greece between 2002 and 2007. J Hosp Infect 75 : 225–227.
52. LyT, GuliaJ, PyrgosV, WagaM, ShohamS (2008) Impact upon clinical outcomes of translation of PNA FISH-generated laboratory data from the clinical microbiology bench to bedside in real time. Ther Clin Risk Manag 4 : 637–640.
53. KimM, HeoSR, ChoiSH, KwonH, ParkJS, et al. (2008) Comparison of the MicroScan, VITEK 2, and Crystal GP with 16S rRNA sequencing and MicroSeq 500 v2.0 analysis for coagulase-negative Staphylococci. BMC Microbiol 8 : 233.
54. LayerF, GhebremedhinB, ModerKA, KonigW, KonigB (2006) Comparative study using various methods for identification of Staphylococcus species in clinical specimens. J Clin Microbiol 44 : 2824–2830.
55. KobayashiN, TaniguchiK, UrasawaS (1998) Analysis of diversity of mutations in the mecI gene and mecA promoter/operator region of methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother 42 : 717–720.
56. OliveiraDC, de LencastreH (2011) Methicillin-resistance in Staphylococcus aureus is not affected by the overexpression in trans of the mecA gene repressor: a surprising observation. PLoS One 6: e23287 doi:10.1371/journal.pone.0023287
57. BargN, ChambersH, KernodleD (1991) Borderline susceptibility to antistaphylococcal penicillins is not conferred exclusively by the hyperproduction of beta-lactamase. Antimicrob Agents Chemother 35 : 1975–1979.
58. MassiddaO, MingoiaM, FaddaD, WhalenMB, MontanariMP, et al. (2006) Analysis of the beta-lactamase plasmid of borderline methicillin-susceptible Staphylococcus aureus: focus on bla complex genes and cadmium resistance determinants cadD and cadX. Plasmid 55 : 114–127.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- The Influence of Health Systems on Hypertension Awareness, Treatment, and Control: A Systematic Literature Review
- Factors Affecting the Delivery, Access, and Use of Interventions to Prevent Malaria in Pregnancy in Sub-Saharan Africa: A Systematic Review and Meta-Analysis
- Translating Translational Research into Global Health Gains
- Progress in Using Systematic Reviews of Animal Studies to Improve Translational Research
- A Comparison of Frameworks Evaluating Evidence for Global Health Interventions
- Global Burden of Sickle Cell Anaemia in Children under Five, 2010–2050: Modelling Based on Demographics, Excess Mortality, and Interventions
- The Effect of Tobacco Control Measures during a Period of Rising Cardiovascular Disease Risk in India: A Mathematical Model of Myocardial Infarction and Stroke
- Association of Lifecourse Socioeconomic Status with Chronic Inflammation and Type 2 Diabetes Risk: The Whitehall II Prospective Cohort Study
- Risk Prediction for Breast, Endometrial, and Ovarian Cancer in White Women Aged 50 y or Older: Derivation and Validation from Population-Based Cohort Studies
- Evaluation of Prediction Models for Decision-Making: Beyond Calibration and Discrimination
- The Growing Problem of Multidrug-Resistant Tuberculosis in North Korea
- Multiplex Identification of Gram-Positive Bacteria and Resistance Determinants Directly from Positive Blood Culture Broths: Evaluation of an Automated Microarray-Based Nucleic Acid Test
- Combatting Substandard and Falsified Medicines: A View from Rwanda
- Access to Drugs for Treatment of Noncommunicable Diseases
- Threats to Validity in the Design and Conduct of Preclinical Efficacy Studies: A Systematic Review of Guidelines for In Vivo Animal Experiments
- Recent Shifts in Global Governance: Implications for the Response to Non-communicable Diseases
- Reflections on the Global Burden of Disease 2010 Estimates
- Sickle Cell Anaemia in a Changing World
- Changes in Association between Previous Therapeutic Abortion and Preterm Birth in Scotland, 1980 to 2008: A Historical Cohort Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Changes in Association between Previous Therapeutic Abortion and Preterm Birth in Scotland, 1980 to 2008: A Historical Cohort Study
- Multiplex Identification of Gram-Positive Bacteria and Resistance Determinants Directly from Positive Blood Culture Broths: Evaluation of an Automated Microarray-Based Nucleic Acid Test
- Combatting Substandard and Falsified Medicines: A View from Rwanda
- Reflections on the Global Burden of Disease 2010 Estimates
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



