-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Psychosocial Interventions for Perinatal Common Mental Disorders Delivered by Providers Who Are Not Mental Health Specialists in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
Background:
Perinatal common mental disorders (PCMDs) are a major cause of disability among women. Psychosocial interventions are one approach to reduce the burden of PCMDs. Working with care providers who are not mental health specialists, in the community or in antenatal health care facilities, can expand access to these interventions in low-resource settings. We assessed effects of such interventions compared to usual perinatal care, as well as effects of interventions based on intervention type, delivery method, and timing.Methods and Findings:
We conducted a systematic review, meta-analysis, and meta-regression. We searched databases including Embase and the Global Health Library (up to 7 July 2013) for randomized and non-randomized trials of psychosocial interventions delivered by non-specialist mental health care providers in community settings and antenatal health care facilities in low - and middle-income countries. We pooled outcomes from ten trials for 18,738 participants. Interventions led to an overall reduction in PCMDs compared to usual care when using continuous data for PCMD symptomatology (effect size [ES] −0.34; 95% CI −0.53, −0.16) and binary categorizations for presence or absence of PCMDs (odds ratio 0.59; 95% CI 0.26, 0.92). We found a significantly larger ES for psychological interventions (three studies; ES −0.46; 95% CI −0.58, −0.33) than for health promotion interventions (seven studies; ES −0.15; 95% CI −0.27, −0.02). Both individual (five studies; ES −0.18; 95% CI −0.34, −0.01) and group (three studies; ES −0.48; 95% CI −0.85, −0.11) interventions were effective compared to usual care, though delivery method was not associated with ES (meta-regression β coefficient −0.11; 95% CI −0.36, 0.14). Combined group and individual interventions (based on two studies) had no benefit compared to usual care, nor did interventions restricted to pregnancy (three studies). Intervention timing was not associated with ES (β 0.16; 95% CI −0.16, 0.49). The small number of trials and heterogeneity of interventions limit our findings.Conclusions:
Psychosocial interventions delivered by non-specialists are beneficial for PCMDs, especially psychological interventions. Research is needed on interventions in low-income countries, treatment versus preventive approaches, and cost-effectiveness.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(10): e32767. doi:10.1371/journal.pmed.1001541
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001541Summary
Background:
Perinatal common mental disorders (PCMDs) are a major cause of disability among women. Psychosocial interventions are one approach to reduce the burden of PCMDs. Working with care providers who are not mental health specialists, in the community or in antenatal health care facilities, can expand access to these interventions in low-resource settings. We assessed effects of such interventions compared to usual perinatal care, as well as effects of interventions based on intervention type, delivery method, and timing.Methods and Findings:
We conducted a systematic review, meta-analysis, and meta-regression. We searched databases including Embase and the Global Health Library (up to 7 July 2013) for randomized and non-randomized trials of psychosocial interventions delivered by non-specialist mental health care providers in community settings and antenatal health care facilities in low - and middle-income countries. We pooled outcomes from ten trials for 18,738 participants. Interventions led to an overall reduction in PCMDs compared to usual care when using continuous data for PCMD symptomatology (effect size [ES] −0.34; 95% CI −0.53, −0.16) and binary categorizations for presence or absence of PCMDs (odds ratio 0.59; 95% CI 0.26, 0.92). We found a significantly larger ES for psychological interventions (three studies; ES −0.46; 95% CI −0.58, −0.33) than for health promotion interventions (seven studies; ES −0.15; 95% CI −0.27, −0.02). Both individual (five studies; ES −0.18; 95% CI −0.34, −0.01) and group (three studies; ES −0.48; 95% CI −0.85, −0.11) interventions were effective compared to usual care, though delivery method was not associated with ES (meta-regression β coefficient −0.11; 95% CI −0.36, 0.14). Combined group and individual interventions (based on two studies) had no benefit compared to usual care, nor did interventions restricted to pregnancy (three studies). Intervention timing was not associated with ES (β 0.16; 95% CI −0.16, 0.49). The small number of trials and heterogeneity of interventions limit our findings.Conclusions:
Psychosocial interventions delivered by non-specialists are beneficial for PCMDs, especially psychological interventions. Research is needed on interventions in low-income countries, treatment versus preventive approaches, and cost-effectiveness.
Please see later in the article for the Editors' SummaryIntroduction
Common mental disorders, defined as depressive, anxiety, and somatic disorders, are a major cause of disability among women during the perinatal period, and may have consequences for children's growth and development [1]–[4]. In low - and lower middle-income countries an estimated 16% (95% CI 15.0%, 16.8%) of women suffer from these disorders in pregnancy, and around 20% (95% CI 19.2%, 20.6%) in the postnatal period [5]. To date, most reviews of interventions for perinatal common mental disorders (PCMDs) have focused on interventions for depression, and on evidence from high-income countries [6]–[10]. Their results may not be generalizable to low-resource settings, where specialists and financial resources for mental health care are scarce [11]–[13]. In these settings, the World Health Organization Mental Health Gap Action Programme recommends a cost-effective package of interventions to treat depression that includes antidepressant, psychoeducation, and problem-solving therapies [14]. A recent meta-analysis showed that interventions for PCMDs in low - and middle-income countries are effective (effect Size [ES] −0.38; 95% CI −0.56, −0.21), with benefits for children's health and cognitive development, and for the quality of mother–infant interactions [15]. The findings from this review, though useful, are limited by the diversity of interventions included and high statistical heterogeneity (I2 = 79.9%). Effects of different intervention types and statistical heterogeneity were not fully investigated.
We have conducted a systematic review and meta-analysis of interventions for PCMDs in low - and middle-income countries that address the limitations of previous reviews. We include interventions for all PCMDs since depression and anxiety often coexist, and subcategories of common mental disorder may lack conceptual validity in some cultures [16]–[18]. We focus on psychosocial interventions (i.e., non-pharmacological interventions to influence thoughts, behaviors, skills, and associated feelings), given concerns about the safety of pharmacotherapy during the perinatal period and because access to psychotropic drugs and trained personnel to prescribe them can be limited in low-resource settings [19]–[22]. We also focus on interventions delivered by providers without specialized mental health training (“non-mental health specialists”) in community and primary care settings because of the lack of mental health professionals in low - and middle-income countries, and to address calls for integration of mental health interventions into existing community and maternal and child health programs [23],[24]. We investigate the effects of these interventions based on the type of intervention, timing, and delivery mode, in order to make practical policy recommendations.
Methods
We conducted the systematic review in accordance with the 2009 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Text S1) [25]. The protocol was finalized prior to conducting the systematic review and meta-analysis (Protocol S1). The review was not registered with PROSPERO or any other database.
Criteria for Trials Considered for the Review
Study types and origins
We considered published and unpublished, randomized and non-randomized controlled trials. Publication dates were not restricted, but only trials written in English, French, or Spanish were included. We restricted the review to trials conducted in low - and middle-income countries according to World Bank country classifications at the time of the search [26]. We included studies from mainland China because it is a middle-income country. However, we excluded studies from Taiwan and Hong Kong Special Administrative Region because economic conditions and health infrastructure in these regions of China are comparable with those of high-income settings.
Participants
We included trials that enrolled pregnant or postnatal women (≤12 mo after delivery), or women who were not pregnant at recruitment but became pregnant during the trial.
Interventions
We considered preventive and treatment interventions involving a psychological or social component, delivered prior to pregnancy, during pregnancy, and/or postnatally. We included interventions delivered by non-mental health specialists, including lay persons (i.e., those without any health training), health workers and health volunteers (with some health training), and nurses and doctors with no specialized mental health training. We excluded interventions delivered by psychiatrists, psychologists (undergraduate or postgraduate level), and psychosocial workers, as these practitioners are not commonly available in low - and middle-income settings. We considered interventions in community settings (e.g., villages) and, knowing that the antenatal period is the time when most women are likely to come into contact with health services, the most commonly accessible health provider of antenatal care for their location (e.g., health posts, primary care centers, and hospitals).
Types of outcome measures
We included antenatal and postnatal PCMDs since a large proportion of PCMDs identified in the postnatal period are also present during pregnancy [27]–[29]. There is no consistent definition of the perinatal period in the psychiatric literature, so we adopted a working definition of pregnancy plus the first 12 mo after birth, in line with a number of trials [30]–[32]. We included trials measuring depressive, anxiety, panic, and somatic disorders, as well as perinatal psychological distress as a proxy measure of PCMDs [1]. We considered trials where outcomes were defined and measured using structured clinical interviews, such as the Clinical Interview Schedule–Revised [33], or validated screening questionnaires, for example, the Edinburgh Postnatal Depression Scale [34], the Kessler 10-Item Scale [35], and the 12-item General Health Questionnaire [36]. Outcomes included in the review were binary categorizations indicating the presence or absence of a PCMD (“caseness”), and reduction of symptoms of PCMDs as a continuous outcome.
Search Methods for Identifying Trials
Between 5 and 7 July 2013 we searched the following online bibliographic databases for trials that met the inclusion criteria detailed above: Medline, Embase, Cumulative Index to Nursing and Allied Health Literature, the Cochrane Library, PsycINFO, Web of Science, Scopus, Popline, Maternity and Infant Care, and the Global Health Library. We searched for unpublished completed or ongoing trials in the World Health Organization International Clinical Trials Registry. Customized search strategies were developed for each database. We used controlled vocabulary (e.g., MeSH terms) and search filters to identify randomized controlled trials, and trials from low - and middle-income countries where these were available. Our search of Embase (via OVID), including the exact search terms, is included in Text S2. The search mainly identified journal articles, but also reports, conference proceedings, and theses. We contacted experts in the field to identify further relevant trials, specifically unpublished or ongoing trials.
Data Collection and Analysis
Trial selection and data extraction
We removed duplicates and articles not written in English, French, or Spanish, and reviewed the abstracts of the remaining articles. Trials of interventions were retained, and observational studies excluded. We searched reviews of appropriate interventions for relevant citations. We contacted authors to request full articles where they were not available online, as well as further details of interventions as required. One reviewer (K. C.) independently screened full articles that appeared to meet the search criteria to assess the trial setting and design. We resolved any uncertainty about the inclusion of specific trials through discussions between reviewers, and documented reasons for exclusion.
Using a spreadsheet, two reviewers (K. C. and A. P.) independently recorded the following data for included trials: date of extraction, source reference and type, authors, publication year, article title, source of funding, trial design and methods, study setting and population, details about interventions and control conditions, participant inclusion and exclusion criteria and characteristics, sample size, definitions of PCMDs, screening tools, timing of assessments, variables adjusted for in the analyses, and results.
Assessment of methodological quality and small study effects
We did not exclude papers from the systematic review on the basis of methodological quality but assessed risk of bias for each study in terms of sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting, using the Cochrane Risk of Bias Tool [37]. We defined trials at high risk of bias as those found to be at high risk or unclear risk of bias across five or more bias domains. Trials at low risk of bias were defined as those using adequate sequence generation and allocation concealment methods [37]. In reality these definitions were arbitrary, and studies may lie anywhere along the continuum from “free of bias” to “undoubtedly biased” [38]. We assessed potential small study effects using a funnel plot and the Egger test [38]. However, we attempted to limit small study effects by searching the World Health Organization International Clinical Trials Registry, and by asking expert informants about unpublished and ongoing trials.
Data synthesis and statistical analysis
We identified more than six studies that were not at high risk of bias and were comparable in terms of intervention content and study population [39]. We therefore conducted a meta-analysis to assess effects of psychosocial interventions versus usual care. We used the main outcomes reported in each publication, adjusted for clustering, baseline differences, and other covariates where appropriate. We conducted separate meta-analyses for binary and continuous outcomes. Odds ratios (ORs) were pooled for trials reporting binary outcomes. Where studies reported binary outcomes from both clinical interviews and screening questionnaires, we selected the former as the superior measure of PCMDs. One study reported a categorical outcome for the presence of PCMD (none/mild, moderate, or severe) [40]. Data were therefore reanalyzed to calculate a binary outcome (none/mild versus moderate/severe) using the same methods reported in the publication. For continuous outcomes, standardized mean differences were calculated because different screening questionnaires were used to report the outcome [38]. We estimated statistical heterogeneity using the I2 statistic and calculated confidence intervals around these estimates [41],[42]. We used a random effects model to account for unexplained heterogeneity and because we assumed that the effects being estimated in the different trials were not identical [38]. We planned to exclude trials at high risk of bias from the meta-analysis (although all trials were included in the systematic review), and to conduct one sensitivity analysis including only trials at low risk of bias [38] and another including only results from the last follow-up assessment in each trial. We conducted a further post hoc sensitivity analysis excluding a study that was not peer-reviewed.
We planned to conduct the following subgroup analyses: psychological interventions versus usual care, health promotion interventions versus usual care, group-based interventions versus usual care, individual-based interventions versus usual care, and combined (group - and individual-based) interventions versus usual care. We carried out two further post hoc subgroup analyses: antenatal interventions versus usual care, and antenatal and postnatal interventions versus usual care.
We wanted to compare treatment and preventive approaches versus usual care, but this was not possible because only one of the retrieved studies was a trial of a treatment intervention. In order to maximize power for subgroup analyses, we pooled results from all trials by converting ORs to ESs—comparable with the standardized mean difference—where studies did not report a continuous outcome [43]. In order to examine differences in ES between intervention subgroups we conducted a series of univariable random effects meta-regression analyses [44].
All data analyses were conducted with Stata (version 12.1) using metan and metareg commands.
Results
The database search identified 6,177 abstracts, which we screened according to the process outlined in Figure 1. We also identified five trials through personal communication with researchers. Two abstracts were unavailable online through University of London or British Library accounts. We were unable to obtain these abstracts through colleagues working in Asian institutions and could not locate the authors' e-mail addresses to contact them directly [45],[46]. We screened 37 full-text articles, and trials excluded at this stage are shown in Table S1, with reasons for exclusion. Six ongoing trials, including one conducted in a low-income country, were not included in the review but are described in Table S2. In total, 11 trials were included in the review and are described in Table 1. Results from one trial are reported in two separate publications [47],[48].
Fig. 1. Flow diagram of search results. 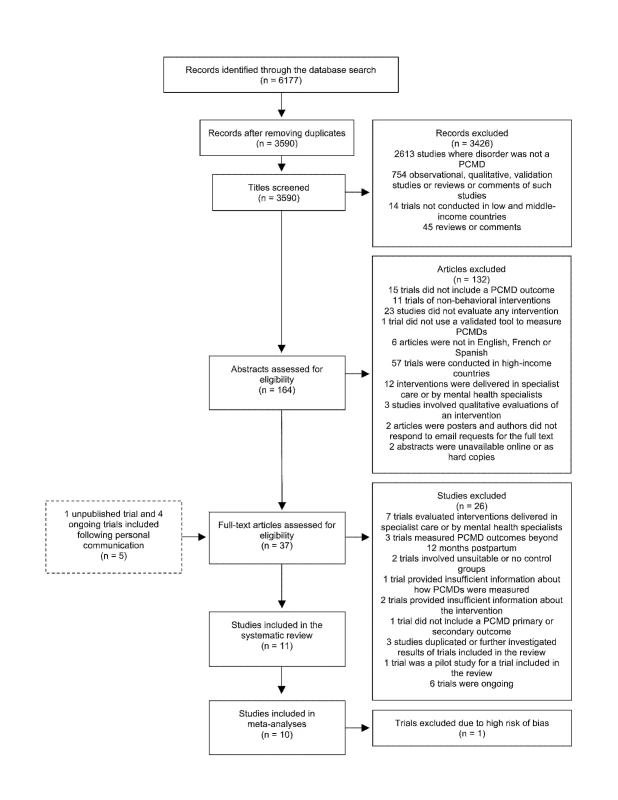
Out of 6,177 abstracts retrieved through a search of electronic databases, 11 articles were included in the systematic review, including one unpublished trial identified following personal communication with the author. Tab. 1. Components of psychosocial interventions for PCMDs delivered by non-mental health specialists in middle-income countries. 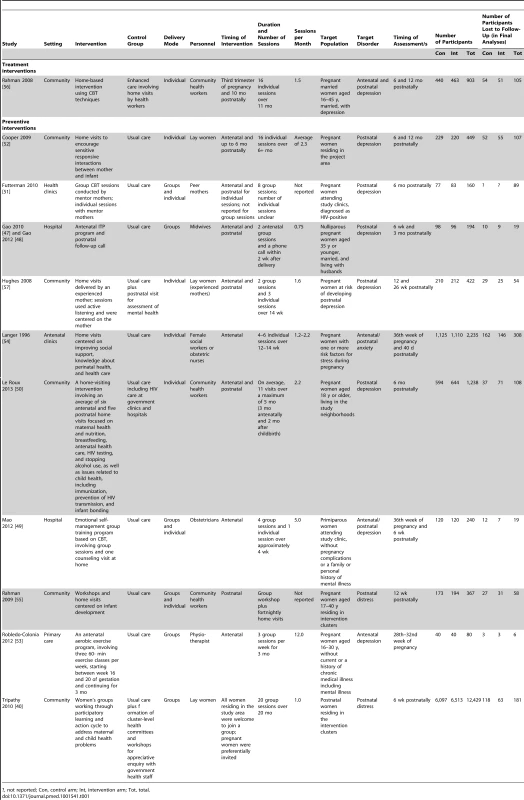
?, not reported; Con, control arm; Int, intervention arm; Tot, total. Included Trials
None of the trials included were conducted in a low-income country. Seven of the included trials were conducted in upper middle-income countries: China [47],[49], South Africa [50]–[52], Columbia [53], Mexico, Argentina, Cuba and Brazil [54]. Four trials were set in lower middle-income countries: Pakistan [55],[56] and India [40],[57].
Trial Characteristics
Outcomes
Depression was an outcome in eight trials [47],[49]–[53],[56],[57], and anxiety in only one trial [54]. Three trials measured general common mental disorders [40],[47],[55]. All trials employed validated self-report measures to assess PCMD symptomatology: five used the Edinburgh Postnatal Depression Scale [47],[49],[50],[52],[57], others used self-report measures such as the Center for Epidemiological Studies Depression Scale [51],[53], the Kessler 10-Item Scale [40], the Self Reporting Questionnaire [55], the Hamilton Depression Rating Scale [56], the nine-item Patient Health Questionnaire [49], and the 12-item General Health Questionnaire [47]. Only four trials used a clinical interview in addition to self-report measures [49],[52],[56],[57].
Target populations
Table 1 details components of interventions included in the review. We defined treatment interventions as those that targeted women diagnosed with a PCMD, and preventive interventions as those sampling from the general population, women at risk of developing a PCMD, and women with symptoms but not meeting the full criteria for a PCMD [58]. We identified only one treatment intervention [56].
In ten trials, participants were recruited during pregnancy [47],[49]–[57]. In Tripathy et al., all mothers who had delivered in the study area were interviewed approximately 6 wk after childbirth [40]. The intervention (participatory women's groups) was delivered at a community level: 18% of participants attended groups in the first year, rising to 55% in the third year. Overall for the included studies, participants' initial exposure to the intervention may therefore have occurred prior to or during pregnancy, in the postnatal period, or not at all.
Interventions
Six trials involved community-based interventions, three of which were conducted in resource-limited, rural settings [40],[50],[52],[55]–[57]. Five trials involved interventions based in health facilities, including primary care facilities [53], antenatal clinics [51],[54], and hospitals [47],[49]. Four out of five facility-based interventions were conducted in urban populations.
The timing of interventions varied: three trials tested interventions limited to pregnancy [49],[53],[54], and one to the postnatal period [55]. In six trials, interventions began antenatally and continued into the postnatal period [40],[47],[50],[52],[56],[57]. One trial did not report the timing of the intervention [51].
The duration of interventions ranged from 4 wk [49] to 20 mo [40]. Where appropriate, we compared their intensity by calculating the number of scheduled contact events (group or individual) per month (Table 1). Of six trials, the least intensive intervention involved two group sessions plus a follow-up telephone call over a period of 9.5 mo [47]. The most intensive intervention involved three group exercise sessions per week for 3 mo [53].
Intervention Content
The treatment intervention and three of the ten preventive interventions involved psychological components [47],[49],[51],[56]. Psychological interventions were defined as interventions incorporating a structured and explicitly psychological approach, such as cognitive behavior therapy (CBT) or interpersonal therapy (IPT).
Seven trials tested health promotion interventions [40],[50],[52]–[55],[57]. Health promotion approaches were defined by the absence of a structured and explicitly psychological approach, and incorporation of one of the following components: communication techniques to positively influence individuals and communities; education to improve knowledge and skills conducive to health; sharing of common experiences or problems and social support; creation of better environments to promote healthier living; community development and mobilization to address health problems; or advocacy and health policy development [59].
Two health promotion interventions involved educational workshops and/or home visits, specifically focusing on mother–infant interactions and attachment [52],[55]. One intervention was a participatory learning and action cycle to improve maternal and newborn health, through women's groups [40]. Groups were also used to deliver an antenatal exercise program incorporating motivating techniques, including support by a physiotherapist, exercise with other women, and music [53]. Two interventions used home visits to communicate information to participants about topics including perinatal health care, nutrition, and mother–infant interaction [50],[57]. One of these interventions promoted infant gender equality and had a strong emphasis on listening to participants [57]. Home visits were used in another intervention to disseminate information about pregnancy and delivery to participants and their chosen “support persons” [54].
Details of care received by control groups are included in Table 1.
Intervention Delivery Mode and Personnel
Psychological interventions were delivered by health workers [56], lay persons (“mentor mothers” [51]), and doctors or midwives [47],[49]. Three out of four psychological interventions were predominantly delivered in a group context [47],[49],[51]; one psychological intervention was delivered during individual home visits [56].
Health promotion interventions were delivered to groups and individuals by community health workers, social workers, physiotherapists, obstetric nurses, and lay women.
Methodological Quality of Trials and Risk of Bias
All but one of the studies had been peer-reviewed [57]. Most used self-report measures validated in the study population, and ten used a measure validated in the country in which they were conducted [40],[47],[49]–[55],[57]. One trial in Pakistan used the Hamilton Depression Rating Scale, which had not been formally validated in this context but which was translated, culturally adapted, and administered by experienced mental health professionals [56]. Statistical analysis in three trials included in the intervention did not take account of clustering [47],[49],[53].
For each trial we assessed risk of bias, as summarized in Table 2. Sequence generation for randomization was adequate in nine trials [40],[47],[49],[50],[52],[54]–[57], unclear in one trial [53], and absent in one non-randomized trial in which participants were allocated by clinic [51]. Method of allocation concealment was adequate in seven trials [40],[50],[52],[54]–[57], unclear in three trials [47],[49],[53], and absent in one [51].
Tab. 2. Assessment of risk of bias for trials included in the review. 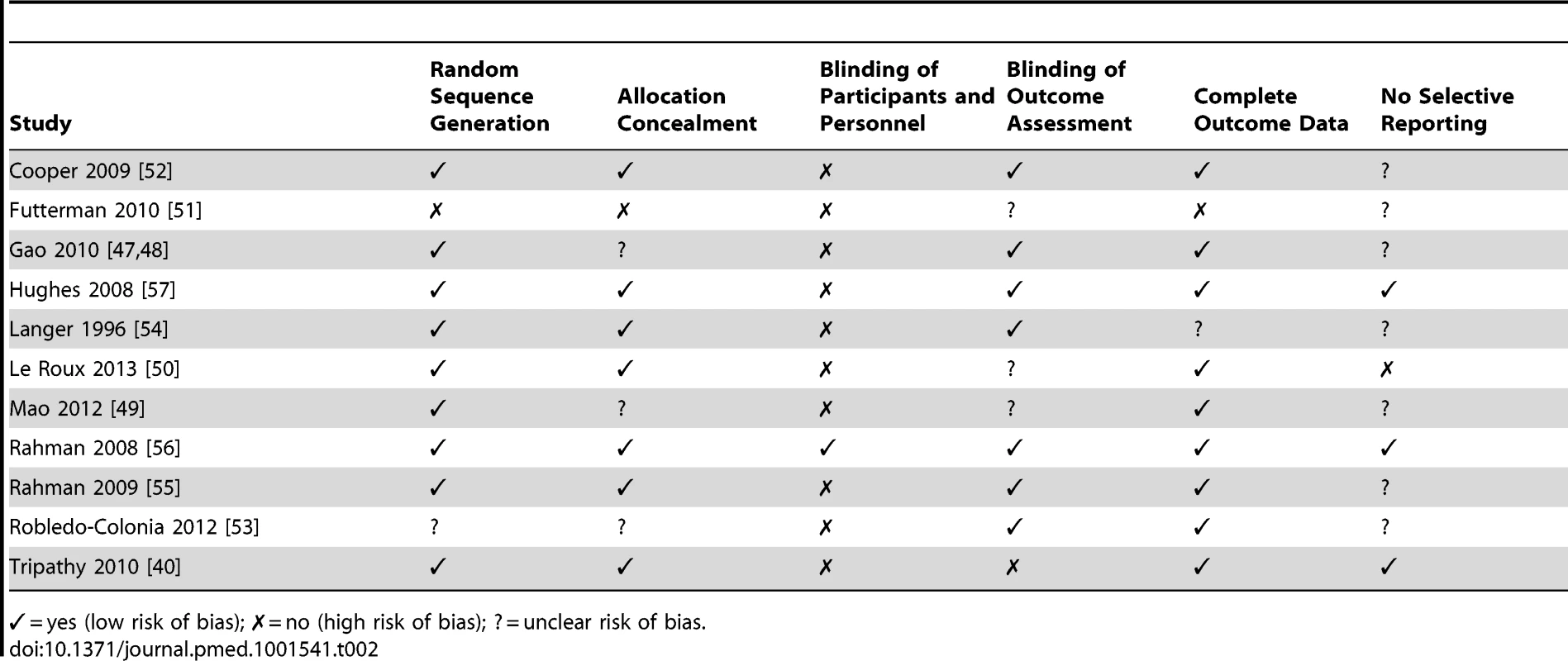
✓ = yes (low risk of bias); ✗ = no (high risk of bias); ? = unclear risk of bias. The nature of interventions inhibited blinding of participants and personnel in most trials; however, blinding of outcome assessors occurred in seven trials [47],[52]–[57]. Outcome assessments were not blinded in one trial [40], and three provided insufficient details [49]–[51].
With regards to completeness of follow-up data, the information provided was adequate in nine trials [40],[47],[49],[50],[52],[53],[55]–[57] and inadequate in one trial [51]; in another trial, reasons for loss to follow-up were not discussed [54]. Two trials reported high attrition rates: 24% at 12 mo [52] and 55.6% at 6 mo [51]. We were unable to assess selective reporting (defined as the occurrence of one of the following: not all of a study's prespecified outcomes reported, primary outcomes not prespecified, outcomes incompletely reported, or key outcomes expected to be reported not reported) in the majority of trials for which the study protocol was not available.
Intervention Effects: Meta-Analysis
Out of the 11 trials that met the inclusion criteria, ten had useable outcomes for 18,738 participants [40],[47],[49],[50],[52]–[57]. One trial found to be at high risk of bias (which had not used adequate sequence generation and allocation concealment methods) was excluded from the meta-analysis to reduce the impact of bias on the results [38],[51].
Comparison 1: All Interventions versus Usual Care
Figures 2 and 3 show the pooled effects of any intervention (ten in total) versus usual care, for dichotomous (Figure 2) and continuous outcomes (Figure 3), after intervention. There was evidence that interventions delivered by non-mental health specialists compared to usual perinatal care were associated with a reduction in PCMD symptoms (ES −0.34; 95% CI −0.53, −0.16) and caseness (OR 0.59; 95% CI 0.26, 0.92) immediately after the intervention. Heterogeneity was high (I2 = 84.1% and 79.3%, respectively) and statistically significant.
Fig. 2. Effects of psychosocial interventions on continuous PCMD outcomes. 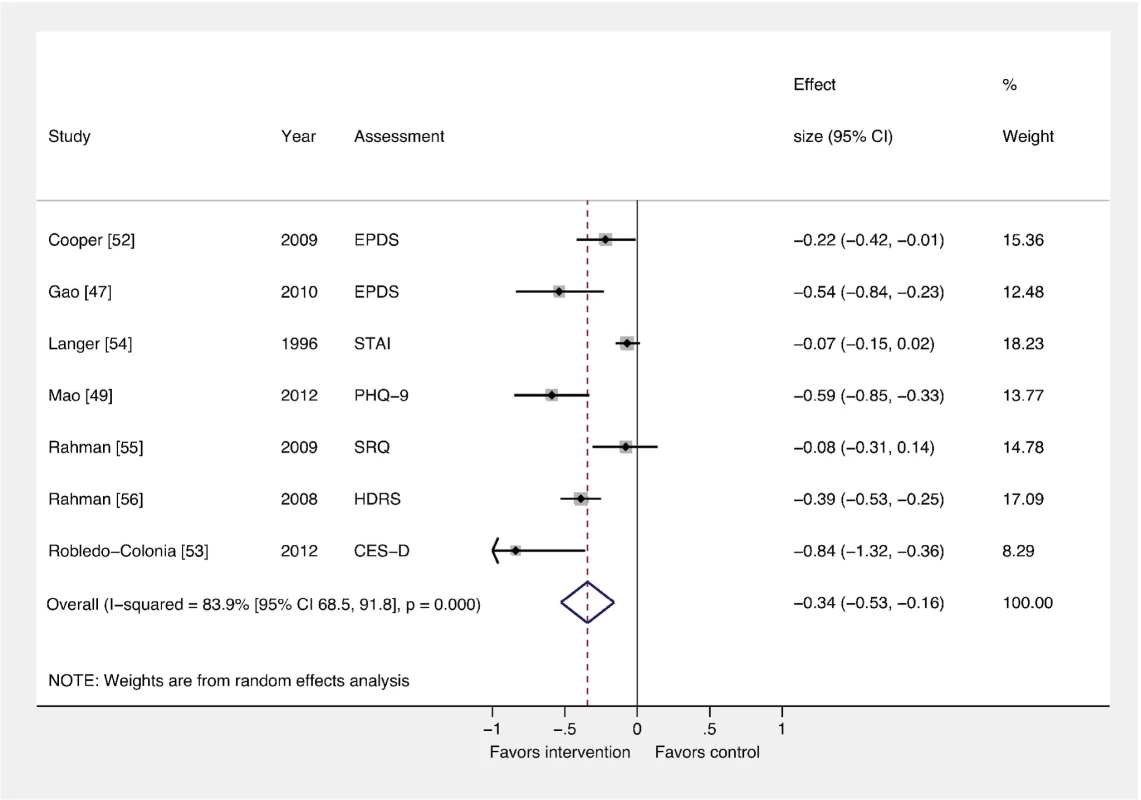
The pooled effect of interventions delivered by non-mental health specialists compared to usual perinatal care was a reduction in PCMD symptomatology compared to usual care, using effect estimates from assessments immediately following delivery of the intervention (ES −0.34; 95% CI −0.53, −0.16). CES-D, Center for Epidemiological Studies Depression Scale; EPDS, Edinburgh Postnatal Depression Scale; HDRS, Hamilton Depression Rating Scale; PHQ-9, nine-item Patient Health Questionnaire; SRQ, Self Reporting Questionnaire; STAI, State-Trait Anxiety Inventory. Fig. 3. Effects of psychosocial interventions on binary PCMD outcomes. 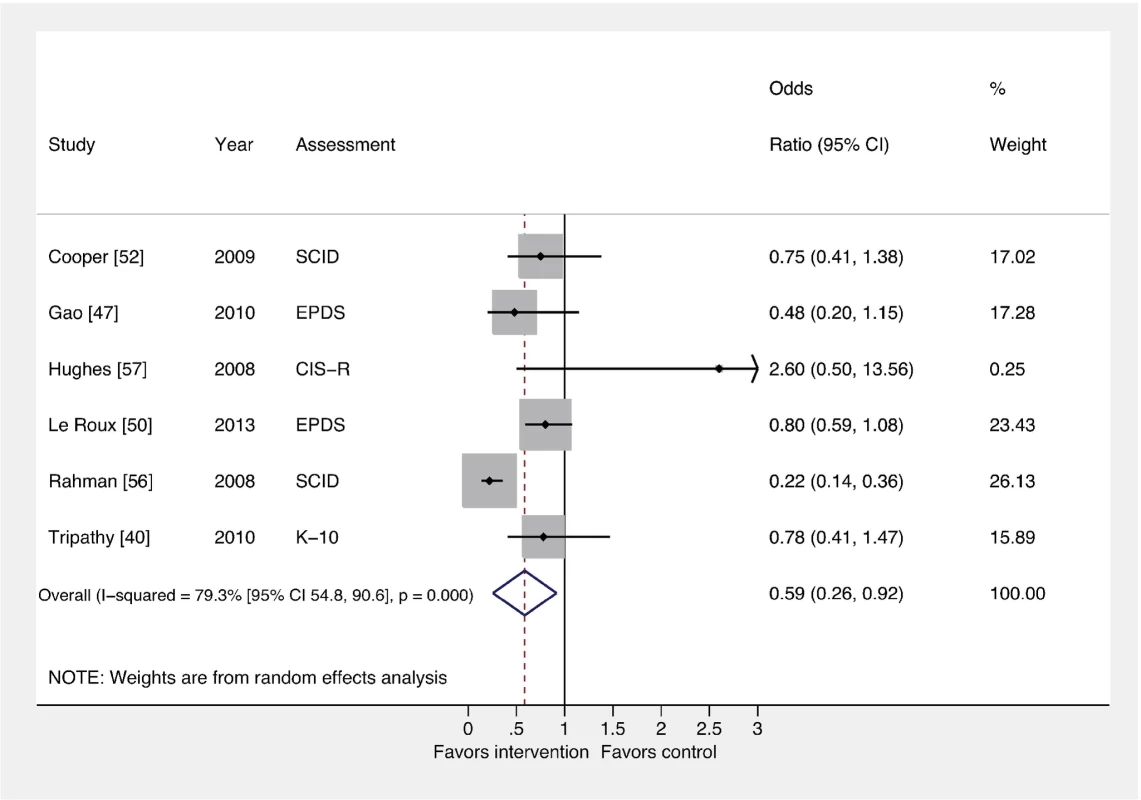
Using binary PCMD categorizations from assessments immediately following delivery of the intervention, the pooled effect for all interventions was significant (OR 0.59; 95% CI 0.26, 0.92) compared to usual care. CIS-R, Clinical Interview Schedule–Revised; EPDS, Edinburgh Postnatal Depression Scale; K-10, Kessler 10-Item Scale; SCID, Structured Clinical Interview for DSM Disorders. We conducted sensitivity analyses excluding the study that was not peer-reviewed [57], and using binary and continuous outcomes associated with the final assessment, as opposed to the assessment immediately after the intervention. These analyses resulted in similar ESs. We also performed a sensitivity analysis using studies with low risk of bias and found that the ES was reduced for PCMD symptoms and caseness (ES −0.19; 95% CI −0.36, −0.02; OR 0.61; 95% CI 0.22, 1.01). Statistical heterogeneity was not significantly reduced in any of these sensitivity analyses.
In order to pool results from all ten trials of psychosocial interventions, we converted ORs to ESs where trials did not report a continuous outcome. The pooled ES of converted and unconverted outcomes was significant (ES −0.27; 95% CI −0.42, −0.13). A funnel plot of these outcomes was broadly symmetric (Figure S1), though the power to detect small study effects with this method was low given the small number of trials included in the meta-analysis. The Egger test provided no evidence of small study bias on PCMD symptoms (p = 0.205).
Comparison 2: Interventions by Type versus Usual Care
We conducted a subgroup analysis to assess whether ESs differed by intervention type (Figure 4). This analysis was important because heterogeneity was high in the main comparison (Comparison 1), and subgroup analyses can provide explanations for heterogeneity. Health promotion interventions for PCMDs were evaluated in seven trials with a total of 17,401 participants, and these interventions were beneficial compared to usual care (ES −0.15; 95% CI −0.27, −0.02). Psychological interventions, evaluated in three trials with a total of 1,337 participants, had a larger effect (ES −0.46; 95% CI −0.58, −0.33). In a meta-regression analysis, the ESs for psychological and health promotion interventions were significantly different (β coefficient −0.33; 95% CI −0.09, −0.58) (Table S3).
Fig. 4. Effects of psychological and health promotion interventions on continuous PCMD outcomes. 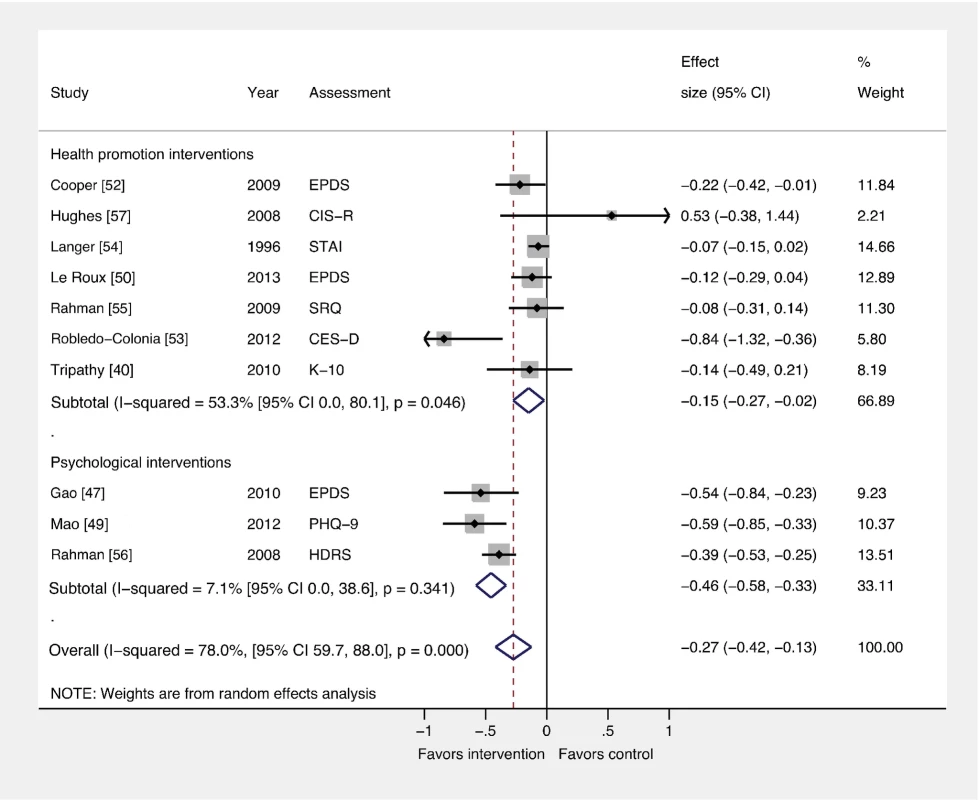
The pooled effect of three health promotion interventions delivered by non-mental health specialists was significant compared to usual care (ES −0.15; 95% CI −0.27, −0.02). Three psychological interventions were associated with a larger overall ES (−0.46; 95% CI −0.58, −0.33). CES-D, Center for Epidemiological Studies Depression Scale; CIS-R, Clinical Interview Schedule–Revised; EPDS, Edinburgh Postnatal Depression Scale; HDRS, Hamilton Depression Rating Scale; K-10, Kessler 10-Item Scale; PHQ-9, nine-item Patient Health Questionnaire; SCID, Structured Clinical Interview for DSM Disorders; SRQ, Self Reporting Questionnaire; STAI, State-Trait Anxiety Inventory. Comparison 3: Interventions by Delivery Method versus Usual Care
We also conducted subgroup analyses to examine whether ESs differed by intervention delivery method (Figure 5). Five trials (n = 5,247) and three trials (n = 12,884) evaluated individual and group-based interventions, respectively. Although individual (ES −0.18; 95% CI −0.34, −0.01) and group (ES −0.48; 95% CI −0.85, −0.11) interventions for PCMDs were effective compared to usual care, delivery method was not associated with ES (β −0.11; 95% CI −0.36, 0.14). Interventions with combined group and individual components had no benefits compared to usual care (ES −0.33; 95% CI −0.83, 0.17).
Fig. 5. Effects of group and individually based psychosocial interventions on continuous PCMD outcomes. 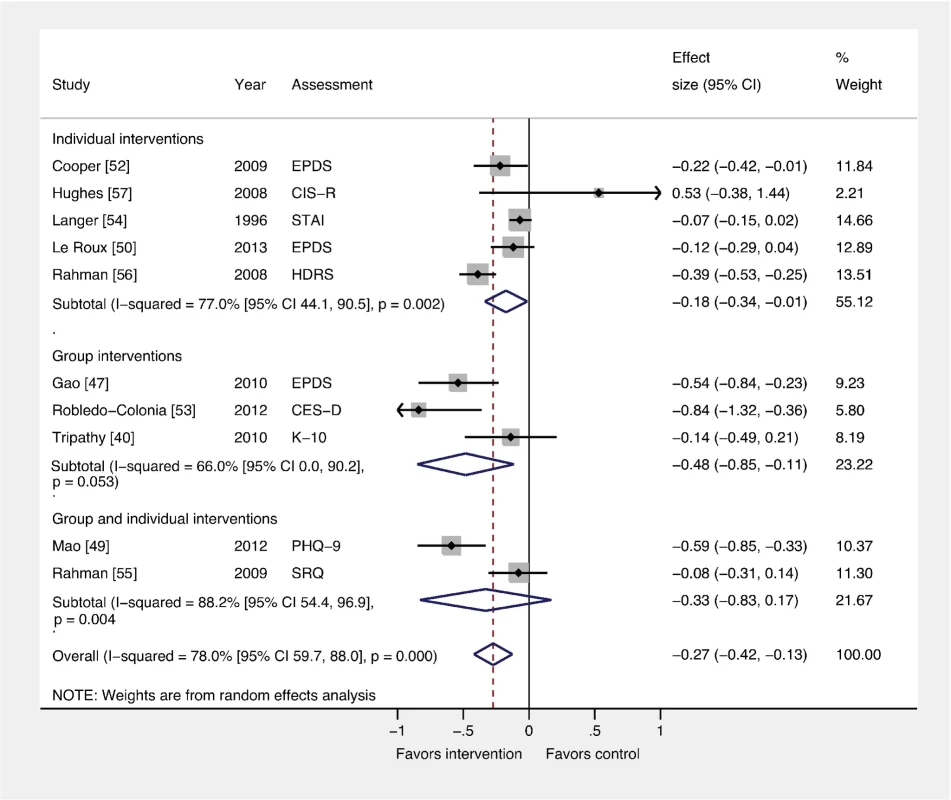
Individual (ES −0.18; 95% CI −0.34, −0.01) and group-based (ES −0.48; 95% CI −0.85, −0.11) psychosocial interventions were associated with significant ESs for PCMDs compared to usual care. Interventions combining group and individual components had no significant effect compared to usual care. CES-D, Center for Epidemiological Studies Depression Scale; CIS-R, Clinical Interview Schedule–Revised; EPDS, Edinburgh Postnatal Depression Scale; HDRS, Hamilton Depression Rating Scale; K-10, Kessler 10-Item Scale; PHQ-9, nine-item Patient Health Questionnaire; SRQ, Self Reporting Questionnaire; STAI, State-Trait Anxiety Inventory. Comparison 4: Interventions by Timing versus Usual Care
We found that interventions delivered during pregnancy and postnatally had a significant overall effect compared to usual care (n = 15,816; ES −0.26; 95% CI −0.42, −0.10), whereas those delivered only during pregnancy did not (n = 2,555; ES −0.46 95% −0.94, 0.01) (Figure 6). Only one trial evaluated an intervention restricted to the postnatal period [55]. Intervention timing was not associated with ES in a meta-regression analysis (β 0.16; 95% CI −0.16, 0.49).
Fig. 6. Effects of antenatal and postnatal psychosocial interventions on continuous PCMD outcomes. 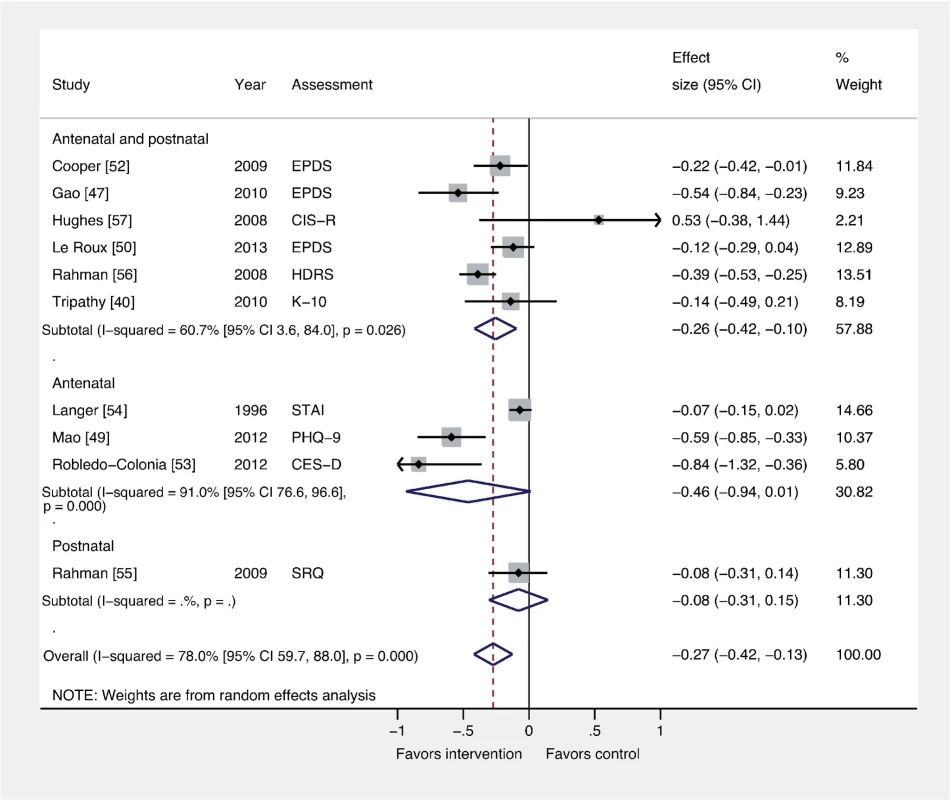
Antenatal interventions were not effective for PCMDs compared to usual care (ES −0.46; 95% CI −0.94, 0.01), whereas interventions delivered both antenatally and postnatally were (ES −0.26; 95% CI −0.42, −0.10). Only one trial assessed an intervention delivered in the postnatal period only. CES-D, Center for Epidemiological Studies Depression Scale; CIS-R, Clinical Interview Schedule–Revised; EPDS, Edinburgh Postnatal Depression Scale; HDRS, Hamilton Depression Rating Scale; K-10, Kessler 10-Item Scale; PHQ-9, nine-item Patient Health Questionnaire; SRQ, Self Reporting Questionnaire; STAI, State-Trait Anxiety Inventory. Discussion
Our results show there is promise for psychosocial interventions delivered by non-mental health specialists for PCMDs in middle-income countries, and corroborate findings from a previous meta-analysis [15]. We identified a group of trials distinct from this previous meta-analysis through exclusion of trials that did not meet our inclusion criteria [32],[60]–[63], exclusion of a pilot study [64] of a trial that we included [52], and inclusion of recent [50],[53] and additional [51],[54] trials. In both meta-analyses, the lack of trials from low-income countries is striking, and research to determine the feasibility and effectiveness of delivering such interventions in these countries is urgently needed.
Study Limitations
Our findings are exploratory and should be interpreted with caution for several reasons. First, only ten trials were included, some of which were associated with an unclear risk of bias. The small number of trials made it difficult to assess small study effects, which, if present, may have led to overestimation of the true effect of interventions. Statistical analysis in three trials included in the meta-analysis did not take account of clustering [47],[49],[53]. The exclusion of these trials in the sensitivity analysis that included only trials at low risk of bias reduced the overall ES for PCMD symptoms and caseness, suggesting that trials that did not take account of clustering may have received more weight in the meta-analysis than is appropriate. Second, interventions differed in terms of participants, timing, setting, personnel, duration, and delivery mode, and meta-analyses showed high levels of statistical heterogeneity. However, the overall impact of psychosocial interventions on PCMDs was clear, and heterogeneity was reduced in subgroup analyses of psychological and health promotion interventions. Third, we excluded trials reported in all languages other than English, French, or Spanish. Fourth, the comparison group in most trials was usual perinatal care, which, in many settings, is likely to amount to no care. Beneficial effects of interventions in these trials are therefore not surprising, and future trials should consider more active comparison groups to control for nonspecific effects of contact with health workers and for ethical reasons. Finally, trials included in the meta-analysis were all from middle-income countries, and most were from Asia. The generalizability of the study findings for low-income and non-Asian countries is therefore limited.
Addressing PCMDs through Psychological Intervention
Our results suggest that psychological interventions for PCMDs are effective. Because we identified only three trials of psychological interventions, it is not possible to recommend one form of psychological therapy over another. However, meta-analyses combining trials from high-income countries and low - and middle-income countries have shown that CBT-based interventions are effective in reducing levels of PCMDs [7],[9]. Moreover, a meta-analysis of psychological interventions for general adult depression and anxiety disorders in low - and middle-income countries found that CBT-based interventions had significantly larger ESs than interventions incorporating other therapies [65]. IPT-based interventions have also shown promise in resource-constrained settings: two trials in rural Uganda showed strong benefits of group IPT interventions delivered by non-mental health specialists for treating general depression in adults and adolescents [66],[67].
Despite these apparent benefits of psychological interventions for common mental disorders, the interventions must be adapted for individual contexts [68]. For example, where strong gender inequalities exist, it may be unrealistic to expect a psychological intervention to empower women in a way that they are individually able to negotiate for change in their lives. Also, there may be stigma associated with participation in an intervention explicitly for mental illness. The Thinking Healthy CBT intervention for depressed Pakistani mothers addressed these contextual factors by using infant health to mobilize family members to improve conditions for the infant's mother, and by integrating the intervention into an existing community health program [56].
We included one psychological intervention to treat participants with established PCMDs and two to prevent PCMDs. Psychological interventions may be more human-resource intensive than other interventions, since they require qualified trainers and supervisors, as well as multiple sessions to build rapport between the participant and “therapist.” Delivering preventive psychological interventions to all pregnant women or new mothers is unlikely to be cost-effective, particularly in remote rural contexts without access to mental health care. Further data on the sustainability and affordability of these programs is therefore required.
Health Promotion Interventions: Addressing Determinants of PCMDs
Although psychological interventions were associated with a significantly larger ES, we found that health promotion interventions also reduced symptoms of PCMDs compared to usual care. Health promotion interventions were diverse but had two common components: sharing information and developing skills to enhance perinatal health—though not specifically perinatal mental health—and giving women an opportunity to share concerns and feelings and receive social support in the context of a group or individually. Tripathy et al. hypothesized that their participatory intervention with women's groups also developed problem-solving skills and empowered women in their communities; this may account for the positive result reported in this trial in its final year [40]. Evidence from qualitative studies suggests that women with common mental disorders do not consider themselves to be ill but attribute their symptoms to social difficulties [69]–[71]. More social and less individual-focused interventions involving health promotion approaches may therefore be more acceptable.
Although health promotion interventions did not directly address mental illness, they did address determinants of PCMDs, such as poor maternal health, infant mortality, and lack of social support. Numerous general community-based interventions in low - and middle-income countries have successfully addressed risk factors for PCMDs, for example, domestic violence [72], poor access to maternal health care [73], and neonatal mortality [74],[75]. More explicit recognition of women's mental health as both a mediator and consequence of these outcomes may increase the effectiveness of such interventions (both in terms of improving women's mental health as well as other targets including reduction in domestic violence), and future trials should consider incorporating a mental health outcome.
We were unable to carry out a subgroup analysis of treatment versus preventive interventions because only one treatment intervention was identified [56]. However, all seven health promotion interventions adopted a preventive approach. In the context of low - and middle-income countries, preventive interventions have several advantages over treatment interventions. First, the chance of detecting PCMDs in low - and middle-income countries is low if access to health care is low in the perinatal period. An intervention involving assessment of mental health status prior to participation may therefore be unrealistic. Preventive interventions are not necessarily dependent on detection of mental illness. Second, some of the effects of PCMDs on infants are thought to begin within the first few months after birth [76]. Delayed diagnosis and treatment could therefore lead to early disruption of the mother–infant relationship, as well as an extended period of distress for the mother. A preventive intervention might avoid these harmful effects. Third, training and supervising personnel to deliver psychological or pharmacological treatment interventions may be more laborious and costly than training personnel to deliver preventive interventions addressing social determinants of PCMDs. Finally, preventive interventions that reduce population levels of domestic violence, poverty, and reproductive ill health that perpetuate mental illness are likely to have a long-term impact on the prevalence of PCMDs.
Delivery of Psychosocial Interventions
Although delivery method was not associated with ES, we found evidence that interventions delivered through groups reduced symptoms of PCMDs compared to usual care, and that group-based interventions were associated with a larger ES than individual interventions. The “one-to-many” approach employed by group interventions is attractive in resource-constrained settings and where it is more culturally appropriate for women to come together to discuss their problems rather than having one-to-one discussions with a health professional. Previous meta-analyses that included subgroup analyses of group interventions for PCMDs in high-income countries reported inconsistent results: one study reported no overall reduction in postnatal depressive symptoms; another study showed a significant effect of group interventions compared to individual interventions [7],[8]. Such contradictory results have led some authors to question the efficacy of psychological group interventions for mothers with young children [77]. In contrast, three meta-analyses of trials from high-income countries all reported that individual interventions reduced levels of PCMD compared to usual care [7]–[9]. The fact that interventions incorporating both group and individual components did not have an impact on PCMDs warrants further investigation. However, only two trials were included in this subgroup, and the finding should therefore be interpreted with caution.
Onset, Duration, and Intensity of Intervention
We found that interventions delivered during pregnancy and postnatally were associated with a reduction in symptoms of PCMDs compared to usual care. The fact that interventions restricted to pregnancy had no significant effect on PCMDs compared to usual care suggests that intervention in the postnatal period is important. In support of this, a meta-analysis of trials from high-income countries showed that psychosocial interventions delivered postnatally prevented postnatal depression compared to usual care, whereas those beginning antenatally and continuing postnatally had no effect [8]. Postnatal psychosocial interventions may be more beneficial because women rely on social support and emotional resilience in the postnatal period to care for a newborn, recover from childbirth, and resume their daily routines. Interventions addressing anxieties around childbirth and perinatal health may be more appropriate for pregnant women.
In the current review the duration and intensity of interventions was variable but did not appear to be correlated with ES. There is little evidence in the literature for an optimum, or even minimum, number or frequency of sessions, although findings from a meta-analysis of trials in high-income countries indicated that interventions involving a single contact event do not prevent postnatal depression, whereas interventions with multiple contact events are efficacious [8].
Personnel
A recent review of possible packages of care for depression in low - and middle-income countries included routine screening for detection of depression, psychoeducation, and problem-solving [78]. This meta-analysis and other key trials of interventions for general common mental disorders provide some evidence that community health workers or lay workers can deliver these non-pharmacological interventions [67],[79]–[81]. Advantages of working with these cadres are that interventions can be delivered at the community level and in areas without access to mental health care. However, community health workers are already indispensable in the provision of perinatal care, family planning, health education, HIV/AIDs care, and immunization programs. Their existing workload may limit their availability for mental health interventions. Referral of severe mental illness must also be considered, and nesting interventions in existing health care services where specialist care and pharmacotherapy can be provided is one potential strategy. Trials of interventions integrated into primary care settings in India and Chile have reported promising results [32],[81],[82]. Further consideration is needed to adapt existing mental health care packages for PCMDs. For example, routine screening for PCMDs has been demonstrated to be not cost-effective in high-income countries, and could overwhelm weak health systems in low - and middle-income countries [83].
Conclusions
Evidence supports the implementation of psychosocial interventions for PCMDs delivered by non-mental health specialists in middle-income countries. We found stronger evidence for the efficacy of psychological interventions, compared to health promotion interventions. More research is needed to evaluate the impact of such interventions in low-income countries, as well as research to compare treatment and preventive approaches, and antenatal versus postnatal interventions.
Supporting Information
Zdroje
1. Goldberg D, Huxley P (1992) Common mental disorders: a biosocial model. London: Tavistock/Routledge.
2. MedhinG, HanlonC, DeweyM, AlemA, TesfayeF, et al. (2010) The effect of maternal common mental disorders on infant undernutrition in Butajira, Ethiopia: the P-MaMiE study. BMC Psychiatry 10 : 32.
3. SurkanPJ, KennedyCE, HurleyKM, BlackMM (2011) Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bull World Health Organ 89 : 608–615.
4. ParsonsCE, YoungKS, RochatTJ, KringelbachML, SteinA (2012) Postnatal depression and its effects on child development: a review of evidence from low - and middle-income countries. Br Med Bull 101 : 57–79.
5. FisherJ, MelloCD, PatelV, RahmanA, TranT, et al. (2012) Prevalence and determinants of common perinatal mental disorders in women in low - and lower-middle - income countries: a systematic review. Bull World Health Organ 90 : 139–149.
6. BoathE, HenshawC (2001) The treatment of postnatal depression: a comprehensive literature review. J Reprod Infant Psychol 19 : 215–248.
7. CuijpersP, BrännmarkJG, van StratenA (2008) Psychological treatment of postpartum depression: a meta-analysis. J Clin Psychol 64 : 103–118.
8. DennisC-L, CreedyD (2004) Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev 2004: CD001134.
9. DennisC-L, HodnettED (2007) Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst Rev 2007: CD006116.
10. MorrellCJ (2006) Review of interventions to prevent or treat postnatal depression. Clin Eff Nurs 9S2: e135–e161.
11. DemyttenaereK, BruffaertsR, Posada-VillaJ (2004) Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA 291 : 2581–2590.
12. PatelV (2000) The need for treatment evidence for common mental disorders in developing countries. Psychol Med 30 : 743–746.
13. SaxenaS, ThornicroftG, KnappM, WhitefordH (2007) Resources for mental health: scarcity, inequity, and inefficiency. Lancet 370 : 878–889.
14. World Health Organization (2010) mhGAP implementation guide for mental, neurological and substance use disorders in non-specialized health settings. Geneva: World Health Organization.
15. RahmanA, FisherJ, BowerP, LuchtersS, TranT, et al. (2013) Interventions for common perinatal mental disorders in women in low - and middle-income countries: a systematic review and meta-analysis. Bull World Health Organ 91 : 593I–601I.
16. PatelV (1996) Recognition of common mental disorders in primary care in African countries: should “mental” be dropped? Lancet 347 : 742–744.
17. SartoriusN, UstünTB, LecrubierY, WittchenHU (1996) Depression comorbid with anxiety: results from the WHO study on psychological disorders in primary health care. Br J Psychiatry Suppl 30 : 38–43.
18. WetzlerS, KatzMM (1989) Problems with the differentiation of anxiety and depression. J of Psychiatr Res 23 : 1–12.
19. AlwanS, ReefhuisJ, RasmussenSA, OlneyRS, FriedmanJM (2007) Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med 356 : 2684–2692.
20. BonariL, KorenG, EinarsonTR, JasperJD, TaddioA, et al. (2005) Use of antidepressants by pregnant women: evaluation of perception of risk, efficacy of evidence based counseling and determinants of decision making. Arch Womens Ment Health 8 : 214–220.
21. McBainR, NortonDJ, MorrisJ, YasamyT, BetancourtT (2012) The role of health systems factors in facilitating access to psychotropic medicines: a cross-sectional analysis of the WHO-AIMS in 63 low - and middle-income countries. PLoS Med 9: e1001166. e1001166 doi:10.1371/journal.pmed.1001166
22. TohS, MitchellAA, LouikC, WerlerMM, ChambersCD, et al. (2009) Selective serotonin reuptake inhibitor use and risk of gestational hypertension. Am J Psychiatry 166 : 320–328.
23. HanlonC (2012) Maternal depression in low - and middle - income countries. Int Health 5 : 4–5.
24. RahmanA (2013) Grand challenges: integrating maternal mental health into maternal and child health programmes. PLoS Med 10: e1001442 doi:10.1371/journal.pmed.1001442
25. MoherD, LiberatiA, TetzlaffJ, AltmanDG (2009) The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097 doi:10.1371/journal.pmed.1000097
26. World Bank (2013) Country and lending groups. Washington (District of Columbia): World Bank.
27. CooperPJ, TomlinsonM, SwartzL, WoolgarM, MurrayL, et al. (1999) Postpartum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry 175 : 554–548.
28. EvansJ, HeronJ, FrancombH (2001) Cohort study of depressed mood during pregnancy and after childbirth. BMJ 231 : 257–260.
29. RahmanA, IqbalZ, HarringtonR (2003) Life events, social support and depression in childbirth: perspectives from a rural community in the developing world. Psychol Med 33 : 1161–1167.
30. MillerL (2002) Postpartum depression. JAMA 287 : 762–765.
31. O'Hara MW, Zekoski EM (1988) Postpartum depression: a comprehensive review. In: Kumar R, Brockington IF, editors. Motherhood and mental illness. London: John Wright.
32. RojasG, FritschR, SolisJ, JadresicE, CastilloC, et al. (2007) Treatment of postnatal depression in low-income mothers in primary-care clinics in Santiago, Chile: a randomised controlled trial. Lancet 370 : 1629–1637.
33. LewisG, PelosiAJ, ArayaR, DunnG (1992) Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med 22 : 465–486.
34. CoxJL, HoldenJM, SagovskyR (1987) Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 150 : 782–786.
35. KesslerRC, AndrewsG, ColpeLJ, HripiE, MroczekDK, et al. (2002) Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 32 : 959–976.
36. Goldberg DP, Williams P (1988) A user's guide to the General Health Questionnaire. Windsor (United Kingdom): NFER-Nelson.
37. HigginsJPT, AltmanDG, GøtzschePC, JüniP, MoherD, et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343: d5828.
38. Higgins JPT, Green S (2011 Mar) Cochrane handbook for systematic reviews of interventions, version 5.1.0.
39. FuR, GartlehnerG, GrantM, ShamilyanT, SedrakyanA, et al. (2011) Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 64 : 1187–1197.
40. TripathyP, NairN, BarnettS, MahapatraR, BorghiJ, et al. (2010) Effect of a participatory intervention with women's groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster-randomised controlled trial. Lancet 375 : 1182–1192.
41. HigginsJPT, ThompsonSG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21 : 1539–1558.
42. ThorlundK, ImbergerG, JohnstonBC, WalshM, AwadT, et al. (2012) Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS ONE 7: e39471 doi:10.1371/journal.pone.0039471
43. ChinnS (2000) A simple method for converting an odds ratio to effect size for use in meta-analysis. Statistics in Medicine 19 : 3127–3131.
44. Harbord RM, Higgins JPT (2009) Meta-regression in Stata. In: Sterne JAC, editor. Meta-analysis in Stata: an updated collection from the Stata Journal. College Station (Texas): Stata Press. pp. 70–96.
45. SharmaS, SangarK, BajwaG (1998) A psycho-educational programme for the primigravidae. Nurs J India 89 : 53–55.
46. WuL, HeZ, WangL, HanD, ZhaoH, et al. (2007) Effectiveness of informational support for maternal anxiety and postpartum depression in Chinese mothers of premature infants: a quasi-experimental study. Asian J Nurs 10 : 251–256.
47. GaoL, ChanSW, LiX, ChenS, HaoY (2010) Evaluation of an interpersonal psychotherapy-oriented childbirth education programme for Chinese first-time childbearing women: a randomised controlled trial. Int J Nurs Stud 47 : 1208–1216.
48. GaoL, ChanSW, SunK (2012) Effects of an interpersonal-psychotherapy-oriented childbirth education programme for Chinese first-time childbearing women at 3-month follow up: randomised controlled trial. Int J Nurs Stud 49 : 274–281.
49. MaoH-J, LiH-J, ChiuH, ChanW-C, ChenS-L (2012) Effectiveness of antenatal emotional self-management training program in prevention of postnatal depression in Chinese women. Perspect Psychiatr Care 48 : 218–224.
50. Le RouxIM, TomlinsonM, HarwoodJM, O'ConnorMJ, WorthmanCM, et al. (2013) Outcomes of home visits for pregnant mothers and their infants: a cluster randomized controlled trial. AIDS 27 : 1461–1471.
51. FuttermanD, SheaJ, BesserM, StaffordS, DesmondKA, et al. (2010) Mamekhaya: a pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care 22 : 1093–1100.
52. CooperPJ, TomlinsonM, SwartzL, LandmanM, MoltenoC, et al. (2009) Improving quality of mother-infant relationship and infant attachment in socioeconomically deprived community in South Africa: randomised controlled trial. BMJ 338 : 997.
53. Robledo-ColoniaAF, Sandoval-RestrepoN, Mosquera-ValderramaYF, Escobar-HurtadoC, Ramírez-VélezR (2012) Aerobic exercise training during pregnancy reduces depressive symptoms in nulliparous women: a randomised trial. J Physiother 58 : 9–15.
54. LangerA, FarnotU, GarciaC, BarrosF, VictoraCG, et al. (1996) The Latin American trial of psychosocial support during pregnancy: effects on mothers' wellbeing and satisfaction. Soc Sci Med 42 : 1589–1597.
55. RahmanA, IqbalZ, RobertsC, HusainN (2009) Cluster randomized trial of a parent-based intervention to support early development of children in a low-income country. Child Care Health Dev 35 : 56–62.
56. RahmanA, MalikA, SikanderS, RobertsC, CreedF (2008) Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet 372 : 902–909.
57. Hughes MW (2008) A randomised controlled trial of a perinatal psycho-social interventoin for postnatal depression in Goa, India [PhD dissertation]. London: Kings College London.
58. Mrazek PJ, Haggerty RJ (1994) Reducing risks for mental disorders—frontiers for prevention intervention research. Washington (District of Columbia): National Academy Press.
59. NutbeamD (1998) Health promotion glossary. Health Promot Int 13 : 349–364.
60. Baker-HenninghamH, PowellC, WalkerS, Grantham-McGregorS (2005) The effect of early stimulation on maternal depression: a cluster randomised controlled trial. Arch Dis Child 90 : 1230–1234.
61. HoSM, HehSS, JevittCM, HuangLH, FuYY, et al. (2009) Effectiveness of a discharge education program in reducing the severity of postpartum depression: a randomized controlled evaluation study. Patient Educ Couns 77 : 68–71.
62. Lara MaA, NavarroC, NavarreteL (2010) Outcome results of a psycho-educational intervention in pregnancy to prevent PPD: a randomized control trial. J Affect Disord 122 : 109–117.
63. MorrisJ, JonesL, BerrinoA, JordansMJD, OkemaL, et al. (2012) Does combining infant stimulation with emergency feeding improve psychosocial outcomes for displaced mothers and babies? A controlled evaluation from northern Uganda. Am J Orthopsychiatry 82 : 349–357.
64. CooperPJ, LandmanM, TomlinsonM, MoltenoC, SwartzL, et al. (2002) Impact of a mother-infant intervention in an indigent peri-urban South African context: pilot study. Br J Psychiatry 180 : 76–81.
65. van't HofE, CuijpersP, WaheedW, SteinDJ (2011) Psychological treatments for depression and anxiety disorders in low - and middle - income countries: a meta-analysis. Afr J Psychiatry (Johannesbg) 14 : 200–207.
66. BoltonP, BassJ, BetancourtT, SpeelmanL, OnyangoG, et al. (2007) Interventions for depression symptoms among adolescent survivors of war and displacement in northern Uganda. JAMA 298 : 519–527.
67. BoltonP, BassJ, NeugebauerR, VerdeliH, CloughertyKF, et al. (2003) Group interpersonal psychotherapy for depression in rural Uganda: a randomized controlled trial. JAMA 289 : 3117–3124.
68. PatelV, ChowdharyN, RahmanA, VerdeliH (2011) Improving access to psychological treatments: lessons from developing countries. Behav Res Ther 49 : 523–528.
69. HanlonC, WhitleyR, WondimagegnD, AlemA, PrinceM (2009) Postnatal mental distress in relation to the sociocultural practices of childbirth: an exploratory qualitative study from Ethiopia. Soc Sci Med 69 : 1211–1219.
70. RodriguesM, PatelV, JaswalS, de SouzaN (2003) Listening to mothers: qualitative studies on motherhood and depression from Goa, India. Soc Sci Med 57 : 1797–1806.
71. SelimN (2010) Cultural dimensions of depression in Bangladesh: a qualitative study in two villages of Matlab. J Health Popul Nutr 28 : 95–106.
72. JewkesR, NdunaM, LevinJ, JamaN, DunkleK, et al. (2008) Impact of stepping stones on incidence of HIV and HSV-2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. BMJ 337: a506.
73. BarberSL, GertlerPJ (2009) Empowering women to obtain high quality care: evidence from an evaluation of Mexico's conditional cash transfer programme. Health Policy Plan 24 : 18–25.
74. BangAT, BangRA, BaituleSB, ReddyMH, DeshmukhMD (1999) Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet 354 : 1955–1961.
75. ManandharDS, OsrinD, ShresthaBP, MeskoN, MorrisonJ, et al. (2004) Effect of a participatory intervention with women's groups on birth outcomes in Nepal: cluster-randomised controlled trial. Lancet 364 : 970–979.
76. MurrayL, CooperPJ (1997) The impact of postnatal depression on child development. Psychol Med 27 : 253–260.
77. SmallR, LumleyJ (2007) Reduction of maternal depression: much remains to be done. Lancet 370 : 1593–1595.
78. PatelV, SimonG, ChowdharyN, KaayaSF, ArayaR (2009) Packages of care for depression in low - and middle-income countries. PLoS Med 6: e1000159 doi:10.1371/journal.pmed.1000159
79. AliNS, AliBS, AzamIS, KhuwajaAK (2010) Effectiveness of counseling for anxiety and depression in mothers of children ages 0–30 months by community workers in Karachi, Pakistan: a quasi experimental study. BMC Psychiatry 10 : 57.
80. ChibandaD, MesuP, KajawuL, CowanF, ArayaR, et al. (2011) Problem-solving therapy for depression and common mental disorders in Zimbabwe: piloting a task-shifting primary mental health care intervention in a population with a high prevalence of people living with HIV. BMC Public Health 11 : 828.
81. PatelV, WeissHA, ChowdharyN, NaikS, PednekarS, et al. (2010) Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet 376 : 2086–2095.
82. ArayaR, RojasG, FritschR, GaeteJ, RojasM, et al. (2003) Treating depression in primary care in low-income women in Santiago, Chile: a randomised controlled trial. Lancet 361 : 995–1000.
83. PauldenM, PalmerS, HewittC, GilbodyS (2009) Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ 339: b5203.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 10- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Modelling the Strategic Use of Antiretroviral Therapy for the Treatment and Prevention of HIV
- Psychosocial Interventions for Perinatal Common Mental Disorders Delivered by Providers Who Are Not Mental Health Specialists in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
- Predicting Patterns of Long-Term CD4 Reconstitution in HIV-Infected Children Starting Antiretroviral Therapy in Sub-Saharan Africa: A Cohort-Based Modelling Study
- Use of Expert Panels to Define the Reference Standard in Diagnostic Research: A Systematic Review of Published Methods and Reporting
- Elimination of HIV in South Africa through Expanded Access to Antiretroviral Therapy: A Model Comparison Study
- A Transcriptional Signature for Active TB: Have We Found the Needle in the Haystack?
- Poor Health in Rich Countries: A Role for Open Access Journals
- The 2003 Iraq War and Avoidable Death Toll
- Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data
- The Final Push for Polio Eradication: Addressing the Challenge of Violence in Afghanistan, Pakistan, and Nigeria
- Saving Lives in Health: Global Estimates and Country Measurement
- Complexity in Mathematical Models of Public Health Policies: A Guide for Consumers of Models
- Mortality in Iraq Associated with the 2003–2011 War and Occupation: Findings from a National Cluster Sample Survey by the University Collaborative Iraq Mortality Study
- Why We Must Provide Better Support for Pakistan's Female Frontline Health Workers
- Effect on Postpartum Hemorrhage of Prophylactic Oxytocin (10 IU) by Injection by Community Health Officers in Ghana: A Community-Based, Cluster-Randomized Trial
- The Prevention of Postpartum Hemorrhage in the Community
- Pregnancy Weight Gain and Childhood Body Weight: A Within-Family Comparison
- Detection of Tuberculosis in HIV-Infected and -Uninfected African Adults Using Whole Blood RNA Expression Signatures: A Case-Control Study
- A New Approach to Psychiatric Drug Approval in Europe
- Utility of the Xpert MTB/RIF Assay for Diagnosis of Tuberculous Meningitis
- Methodological and Policy Limitations of Quantifying the Saving of Lives: A Case Study of the Global Fund's Approach
- Diagnostic Accuracy of Quantitative PCR (Xpert MTB/RIF) for Tuberculous Meningitis in a High Burden Setting: A Prospective Study
- Assessing Optimal Target Populations for Influenza Vaccination Programmes: An Evidence Synthesis and Modelling Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effect on Postpartum Hemorrhage of Prophylactic Oxytocin (10 IU) by Injection by Community Health Officers in Ghana: A Community-Based, Cluster-Randomized Trial
- Utility of the Xpert MTB/RIF Assay for Diagnosis of Tuberculous Meningitis
- Modelling the Strategic Use of Antiretroviral Therapy for the Treatment and Prevention of HIV
- Diagnostic Accuracy of Quantitative PCR (Xpert MTB/RIF) for Tuberculous Meningitis in a High Burden Setting: A Prospective Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



