-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Reduced Risk of Malaria in Papua New Guinean Children with Southeast Asian Ovalocytosis in Two Cohorts and a Case-Control Study
Background:
The erythrocyte polymorphism, Southeast Asian ovalocytosis (SAO) (which results from a 27-base pair deletion in the erythrocyte band 3 gene, SLC4A1Δ27) protects against cerebral malaria caused by Plasmodium falciparum; however, it is unknown whether this polymorphism also protects against P. vivax infection and disease.Methods and Findings:
The association between SAO and P. vivax infection was examined through genotyping of 1,975 children enrolled in three independent epidemiological studies conducted in the Madang area of Papua New Guinea. SAO was associated with a statistically significant 46% reduction in the incidence of clinical P. vivax episodes (adjusted incidence rate ratio [IRR] = 0.54, 95% CI 0.40–0.72, p<0.0001) in a cohort of infants aged 3–21 months and a significant 52% reduction in P. vivax (blood-stage) reinfection diagnosed by PCR (95% CI 22–71, p = 0.003) and 55% by light microscopy (95% CI 13–77, p = 0.014), respectively, in a cohort of children aged 5–14 years. SAO was also associated with a reduction in risk of P. vivax parasitaemia in children 3–21 months (1,111/µl versus 636/µl, p = 0.011) and prevalence of P. vivax infections in children 15–21 months (odds ratio [OR] = 0.39, 95% CI 0.23–0.67, p = 0.001). In a case-control study of children aged 0.5–10 years, no child with SAO was found among 27 cases with severe P. vivax or mixed P. falciparum/P. vivax malaria (OR = 0, 95% CI 0–1.56, p = 0.11). SAO was associated with protection against severe P. falciparum malaria (OR = 0.38, 95% CI 0.15–0.87, p = 0.014) but no effect was seen on either the risk of acquiring blood-stage infections or uncomplicated episodes with P. falciparum. Although Duffy antigen receptor expression and function were not affected on SAO erythrocytes compared to non-SAO children, high level (>90% binding inhibition) P. vivax Duffy binding protein–specific binding inhibitory antibodies were observed significantly more often in sera from SAO than non-SAO children (SAO, 22.2%; non-SAO, 6.7%; p = 0.008).Conclusions:
In three independent studies, we observed strong associations between SAO and protection against P. vivax malaria by a mechanism that is independent of the Duffy antigen. P. vivax malaria may have contributed to shaping the unique host genetic adaptations to malaria in Asian and Oceanic populations.
Please see later in the article for the Editors' Summary.
Published in the journal: . PLoS Med 9(9): e32767. doi:10.1371/journal.pmed.1001305
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001305Summary
Background:
The erythrocyte polymorphism, Southeast Asian ovalocytosis (SAO) (which results from a 27-base pair deletion in the erythrocyte band 3 gene, SLC4A1Δ27) protects against cerebral malaria caused by Plasmodium falciparum; however, it is unknown whether this polymorphism also protects against P. vivax infection and disease.Methods and Findings:
The association between SAO and P. vivax infection was examined through genotyping of 1,975 children enrolled in three independent epidemiological studies conducted in the Madang area of Papua New Guinea. SAO was associated with a statistically significant 46% reduction in the incidence of clinical P. vivax episodes (adjusted incidence rate ratio [IRR] = 0.54, 95% CI 0.40–0.72, p<0.0001) in a cohort of infants aged 3–21 months and a significant 52% reduction in P. vivax (blood-stage) reinfection diagnosed by PCR (95% CI 22–71, p = 0.003) and 55% by light microscopy (95% CI 13–77, p = 0.014), respectively, in a cohort of children aged 5–14 years. SAO was also associated with a reduction in risk of P. vivax parasitaemia in children 3–21 months (1,111/µl versus 636/µl, p = 0.011) and prevalence of P. vivax infections in children 15–21 months (odds ratio [OR] = 0.39, 95% CI 0.23–0.67, p = 0.001). In a case-control study of children aged 0.5–10 years, no child with SAO was found among 27 cases with severe P. vivax or mixed P. falciparum/P. vivax malaria (OR = 0, 95% CI 0–1.56, p = 0.11). SAO was associated with protection against severe P. falciparum malaria (OR = 0.38, 95% CI 0.15–0.87, p = 0.014) but no effect was seen on either the risk of acquiring blood-stage infections or uncomplicated episodes with P. falciparum. Although Duffy antigen receptor expression and function were not affected on SAO erythrocytes compared to non-SAO children, high level (>90% binding inhibition) P. vivax Duffy binding protein–specific binding inhibitory antibodies were observed significantly more often in sera from SAO than non-SAO children (SAO, 22.2%; non-SAO, 6.7%; p = 0.008).Conclusions:
In three independent studies, we observed strong associations between SAO and protection against P. vivax malaria by a mechanism that is independent of the Duffy antigen. P. vivax malaria may have contributed to shaping the unique host genetic adaptations to malaria in Asian and Oceanic populations.
Please see later in the article for the Editors' Summary.Introduction
The populations of the southwest Pacific are highly diverse and exhibit a range of unique red blood cell (RBC) polymorphisms. Within Papua New Guinea (PNG), a variety of RBC variants are found that have geographical patterns paralleling malaria endemicity [1], suggesting selective pressure by this disease [2]. In particular, Southeast Asian ovalocytosis (SAO) (caused by band 3 deletion SLC4A1Δ27) has a distribution that is closely correlated with malaria prevalence [3]. Even though the SLC4A1Δ27 deletion is lethal in the homozygous state [4], prevalence of heterozygosity reaches 35% in some PNG coastal populations [3]. Therefore, the SLC4A1Δ27 deletion is thought to be associated with improved survival against malaria in these populations. Indeed, SAO has been associated with complete protection against cerebral but not other forms of severe P. falciparum malaria in previous epidemiological studies in PNG [5]. SAO was, however, found to have little or no association with reduced prevalence, parasitaemia, or uncomplicated falciparum malaria [6]–[8].
Although non-falciparum parasites are often considered to cause only mild disease, recent data from the island of New Guinea [9]–[11], Brazil [12], and India [13] show that P. vivax infections are associated with severe disease and mortality. In addition, mortality rates of 5% to 15% were regularly observed in patients challenged with P. vivax for therapy of tertiary syphilis [14]–[18]. Thus, P. vivax may be responsible for, or contribute to, natural selection of erythrocyte polymorphisms. This selective pressure is suggested by the emergence of the unique, non-African Duffy-negative allele (FY*AES) in PNG and the observation that PNG children expressing heterozygotes (FY*A/FY*AES) are protected from P. vivax blood-stage infection [19]. The proposed Austronesian origin of SLC4A1Δ27 [20], and its geographical restriction to Southeast Asia and the southwest Pacific, regions that are co-endemic for all four human malaria parasite species, led us to hypothesize that P. vivax malaria could have contributed to selection of this genetic polymorphism. An early cross-sectional [21] and a case-control study [6] indicated that Melanesians with hereditary ovalocytosis experienced lower parasitaemia and frequency of infection with P. vivax and/or P. malariae. While three field studies have investigated associations between SLC4A1Δ27 and prevalence of P. vivax blood-stage infection, no significant relationship with susceptibility to infection was observed [7],[22],[23]. However, these were small cross-sectional studies with insufficient statistical power to unmask an association.
In vitro studies have shown that SAO red cells are relatively resistant to invasion by some P. falciparum isolates [24],[25], with the degree of resistance influenced by the “receptor preferences” of the isolate [26] and deformability of the red cell membrane [25]. Ovalocytes exhibit reduced susceptibility to invasion by P. knowlesi in vitro, with the suggestion that this is mediated by diminished adherence [27]. These observations indicate that SAO may protect against infection with P. vivax malaria in vivo.
In order to assess the relationship between SAO (i.e., SLC4A1Δ27) and risk of infection and/or disease with P. vivax, we genotyped 1,975 children participating in three separate studies conducted in the Madang area of PNG: (i) a cohort of infants participating in a clinical trial of intermittent preventative treatment (IPTi) [28], (ii) a pediatric severe malaria case-control study [29], and (iii) a cohort of children aged 5–14 y that took part in a prospective longitudinal cohort study in which all individuals were initially treated to clear blood-stage infection and subsequently evaluated for a delay in time-to-reinfection by all Plasmodium species [30].
Methods
Description of Field Studies
Infant cohort
Between July 2006 and June 2009, a total of 1,121 infants 3 mo of age were enrolled in a randomized, placebo-control trial of intermittent preventive treatment for the prevention of malaria and anemia [28]. After screening, consent, and enrolment children were randomized to receive a full treatment course of either amodiaquine+sulphadoxine/pyramethamine (SP), artesunate+SP, or placebo at 3, 6, 9, and 12 mo. A total of 1,079 children completed follow-up until 15 mo of age, with an additional 857 followed to 21 mo. At each treatment/follow-up time point, bednet usage was assessed, recent antimalarial treatment documented, and a 250-µl finger-prick blood sample was collected from all children for later detection of infection (by light microscopy [LM] and post-PCR ligase detection reaction-fluorescent microsphere assay [LDR-FMA] [31]). A full clinical examination was only conducted on children spontaneously reporting signs of clinical illness. A passive case detection system was maintained at the local health centre, three associated aid posts, and a system of monthly study clinics for the entire study period.
All children presenting with self-reported signs of a febrile illness were promptly assessed for presence of malaria infection by rapid diagnostic test (RDT) (ICT Combo test), and only RDT positive children treated with arthemeter-lumefantrine (Coartem, Novartis). A finger-prick sample was collected from all febrile cases for confirmation of malarial infections by LM and PCR. For clinical management and analysis purposes, malarial illness was defined as axillary temperature >37.5°C, or history of fever in preceding 48 h, plus an infection of any density by LM and/or a positive RDT with speciation subsequently confirmed by PCR. A more detailed description of the study and its primary outcomes is given elsewhere [28].
Pediatric severe malaria case-control study
Between October 2006 and December 2009, a total of 318 children of Madang or Sepik parentage aged 0.5–10 y admitted to the pediatric ward of Modilon Hospital, Madang Town with a diagnosis of severe malaria and 330 age-, location-, and ethnicity - matched healthy controls were enrolled in a case-control study designed to investigate the associations of host genetic polymorphism with protection against severe malaria. Cases were defined as severe malaria if they fulfilled the World Health Organization (WHO) definition of severe malarial illness [32]. Inclusion criteria included any of: (i) impaired consciousness/coma (Blantyre Coma Score [BCS]<5 [23]); (ii) prostration (inability to sit/stand unaided); (iii) multiple seizures; (iv) hyperlactatemia (blood lactate >5 mmol/l); (v) severe anemia (hemoglobin <50 g/l); (vi) dark urine; (vii) hypoglycemia (blood glucose <2.2 mmol/l); (viii) jaundice; (ix) respiratory distress; (x) persistent vomiting; (xi) abnormal bleeding; or (xii) signs of shock [29]. Where clinically indicated, CSF (n = 124) and blood (n = 281 cultures were taken on admission. All bacterial cultures from severe malaria cases were sterile [29]. Healthy community controls were recruited from the village of origin of the matched cases. At enrolment, a blood sample for determination of malaria (by LM and nested PCR [33]) and for host genotyping was collected from each case and control. A detailed description of all study procedures and in-depth clinical description of all cases is given elsewhere [29]. As specified in the study protocol, the primary case definition of a severe malaria case for analysis of host genetic associations included the following additional criteria: parasitaemia (>1,000 P. falciparum/µl or >500 P. vivax/µl) and parents from the PNG North Coast (Madang, Morobe, and Sepik) (http://www.malariagen.net/node/242). Consequently, severe cases with mixed P. falciparum/P. vivax infections by LM or PCR were only included as mixed cases if both species exceeded their respective thresholds. The parasitaemia cut-offs were based on local attributable fraction-based definitions of malaria episodes [34] and were included to increase the specificity of case definition.
Treatment time-to-reinfection study
The study population of 206 children for this treatment time-to-reinfection study has been described previously [30]. Briefly, 206 elementary and primary school students from Madang Province (Mugil and Megiar) participated in this study conducted from June to December, 2004. At enrolment, a peripheral venous blood sample was collected for determination of Plasmodium species infection status by LM and LDR-FMA. All children irrespective of infection status were treated with a 7-d course of artesunate monotherapy (4 mg/kg on day 1 and 2 mg/kg on days 2–7) that successfully cleared all but one P. vivax infection [30]. As no primaquine was given, subsequent P. vivax reinfections of the blood stream can either be from newly acquired infection via sporozoites or relapsing infections from hypnozoites.
Following treatment, children were followed up by active surveillance every 2 wk and passive surveillance at the local health centre for a total of 25 wk. Children were monitored for acquisition of new infections until they completed follow-up, withdrew from the study, or did not provide two consecutive bi-weekly blood samples. At the time of the study, bednet usage in the area was limited, most nets were untreated, and their use was not associated with differences in risk of reinfection [30].
At each bi-weekly follow-up visit, a 250-µl finger-prick blood sample was collected from each child for detection of malaria by LM and LDR-FMA. If a child presented with clinical malaria symptoms at a follow-up visit or during the intervening period, a peripheral venous blood sample was taken and treatment given in accordance with 2000 PNG guidelines (amodiaquine [3 d] plus sulfadoxine/pyrimethamine on day 1).
These studies were reviewed and approved by institutional review boards of PNG Medical Research Advisory Council, the IMR Institutional Review Board, the Walter & Eliza Hall Institute, and the Veterans Affairs Medical Center (Cleveland, Ohio, US).
Detection of Plasmodium Species Infection
Standard procedures were used for reading the blood smears and estimating parasite densities [28]–[30]. All blood smears were read independently by two experienced microscopists with parasites counted against 200 white cells. Discrepant results were adjudicated by a third microscopist. Blood smears were scored as LM positive for an individual Plasmodium species if the species was detected independently by at least two microscopists and/or subsequent PCR-based analysis confirmed the presence of the species. Densities were converted to the number of parasites per µl of blood assuming 8,000 white blood cells/µl [35].
DNA was extracted using the QIAamp96 DNA Blood kit (Qiagen) from all blood samples. Infection by each of the four human malaria species co-endemic in PNG (P. falciparum, P. vivax, P. malariae, and P. ovale) was assessed in all blood samples collected in the two cohorts using a semi-quantitative post-PCR LDR-FMA [31] and by nested PCR (nPCR) [36] in samples from the case-control study. Both assays are based on the amplification of the small subunit (SSU) ribosomal RNA gene.
PCR-Based Genotyping
Deletion and SNP genotyping of SLC4A1Δ27 (Chromosome 17) associated with SAO, glycophorin C exon 3 deletion (GYPCΔex3; Chromosome 2) associated with Gerbich-negativity [37],[38], the common Melanesian 3.7 kb (α−3.7) and 4.2 kb (α−4.2) α-globin (Chromosome 16) deletions associated with α+-thalassaemia [39], and Duffy blood group polymorphisms were all performed using methods that have been described previously [40].
Flow Cytometry-Based Analyses of Duffy Antigen on RBCs
Twelve of 21 SAO children participating in the treatment time-to-reinfection study agreed to provide an additional finger-prick blood sample for flow cytometry-based analyses of Duffy antigen on their red cells. For each SAO child, a control sample was collected from a non-SAO (wild-type) child living in the same hamlet. All samples collected were separated into plasma and cell pellets. One sample from an SAO child was subsequently discarded because of excessive cell lysis. Pelleted blood was suspended in 1 ml of 10% DMSO+90% fetal calf serum solution and frozen to −80°C. Prior to antibody staining and protein binding, cells were rapidly thawed for 1 min in 37°C water bath and washed (2×) by first resuspending the red cell pellet in 1.0 ml of RPMI-1640 followed by centrifugation at 600g for 10 min. In order to assess Duffy receptor RBC surface expression for individual blood donors, 1×106 RBC from each sample were incubated with 50 µl (1∶50) of mouse anti-human Fy6 antibody for 15 min at 37°C. Cells were then washed with RPMI-1640, stained with PE-conjugated goat anti-mouse antibody (Molecular Probes), and analyzed using a Becton Dickinson LSR II (Franklin Lakes).
To assess possibility that there are differences in the ability of P. vivax parasites to bind to SAO versus non-SAO RBC, we evaluated the level of P. vivax Duffy binding protein (PvDBP) binding using the same set of blood samples as described above [41]. Briefly, 1×106 washed RBC from each sample were re-suspended in 200 µl of RPMI-1640 and exposed to 0.5 µg of recombinant PvDBP region II (PvDBPII, Sal I variant) for 15 min at 37°C. Excess protein was removed by centrifugation of erythrocytes at 3,000g for 2 min, resuspended in RPMI-1640 followed by centrifugation at 3,000g for 2 min (2×), and then resuspended in 100 µl of RPMI-1640 to which rabbit anti-PvDBPII antibody (1∶5,000 final dilution) was added and incubated for 30 min at 37°C, washed (2×) as above and resuspended in 100 µl of RPMI-1640 to which 1∶4 to 1∶16 dilution of PE-conjugated goat anti-rabbit antibody (Sigma-Aldrich Co., depending on the lot of conjugated antibody). All data were evaluated as normalized mean fluorescence index (nMFI = % of positives cells×MFI) [42].
Measurement of Antibody Titers to Recombinant PvDBPII Variants and Blocking Antibodies
Measurement of binding inhibitory antibodies (BIAbs) and total antibodies to PvDBPII PvMSP1, PvRBP1, PvRBP2, and PvRBP3 was performed as previously described [43],[44].
Statistical Analyses
The association of malaria incidence rates with SAO genotype in children 3–21 mo was assessed using negative binomial regression. Analyses were adjusted for gender, treatment effect, season (wet versus dry), and village of enrolment (grouped by 12 recruitment zones). The time-at-risk was calculated starting on the date of enrolment until the child either reached the final study time point at 21 mo of age, or was withdrawn from the study [45]. Associations between SAO and the prevalence and density of P. vivax and P. falciparum infections were investigated using generalized estimating equation models (XTLOGIT and XTREG in STATA 10.0) that allowed accurate assessment of both variations in the outcome and the correlations between repeated measurements in individual children (modeled using an exchangeable correlation structure). A semi-robust Huber/White/sandwich estimator of variance was used to assure valid standard errors. Best fitting models were determined by backward elimination using Wald's Chi-square tests for individual variables.
In the study of children 5–14 y, log-rank tests were used to assess differences in Kaplan-Meier curves of time-to-first reinfection as detected by LDR-FMA or LM. Cox regression was employed to test for differences in time-to-first reinfection after adjusting for all other factors that were found to be associated with difference in time-to-first reinfection [30]. In these analyses children were considered at risk of acquiring a Plasmodium spp. infection until they either reached the end point, missed two consecutive bi-weekly follow-ups, were re-treated with antimalarials, or withdrew from the study.
Difference in frequency of SAO genotype among severe malaria cases and controls were tested using χ2 tests. Association of SAO genotype with other common RBC polymorphisms and potential confounders were assessed using χ2 and Student t-tests (for continuous, normally distributed variables). The associations between different genetic traits and prevalence of BIAbs against PvDBPII were assessed using Chi-square and Fisher exact tests. Due to non-normality, differences in both expression of the Duffy receptor on RBC and in binding of recombinant PvDBPII on SAO and non-SAO RBCs were compared using non-parametric Mann-Whitney U-tests.
All 95% confidence intervals are model based. Further details on statistical approaches employed are given elsewhere [30],[45]. All statistical analyses were performed using STATA 8 & 10 (Stata Corporation) statistical analysis software.
Results
Association of SAO with Incidence of Clinical Malaria
A total of 1,121 children (48.3% female) were enrolled in the IPTi trial, with equal numbers in each treatment arm. Of these, 857 were followed up to 21 mo. Over the entire 18-mo follow-up period, children on average experienced 0.74 P. vivax and 0.28 P. falciparum episodes/child/year. IPTi with amodiaquine-SP reduced the incidence of P. vivax by 23% (95% CI 0–41, p = 0.048) for the first year of follow-up (3–15 mo) and incidence of P. falciparum by 35% (95% CI 9–54, p = 0.012), whereas IPTi with artesunate-SP only protected against P. falciparum (31%, 95% CI 4–51, p = 0.027) but not against P. vivax episodes (6%, 95% CI −24 to 26, p = 0.759) [28]. No significant differences were observed between all three arms for the 15–21-mo extended follow-up period (p>0.75).
SAO genotypes were available for all 1,121 children with 130 heterozygous for SLC4A1Δ27 (11.6%), with no significant difference in frequency of SAO among treatment arms (p = 0.30). SLC4A1Δ27 heterozygocity was associated with neither α+thalassaemia nor Gerbich-negative deletion (GPYCΔex3, p>0.95) (Table S1). There were no differences between SAO and wild-type children in bednet usage or village of residence, gender, or season of recruitment (p>0.36).
Over the total follow-up period (3–21 mo), SAO was associated with a significant 32% reduction in incidence of any form of malaria (95% CI 11–49, p = 0.0068) (Table 1). The protection was exclusively directed against episodes of P. vivax malaria, with the SAO genotype associated with 43% reduction (95% CI 22–59, p = 0.0006) in incidence of P. vivax episodes of any density and a 55% reduction (95% CI 34–59, p<0.0001) in incidence of P. vivax episodes with >500 parasites/µl. There were no significant associations with all P. falciparum episodes (incidence rate ratio [IRR] = 1.03, 95% CI 0.73–1.45, p = 0.89) (Table 1) or episodes with P. falciparum >2,500 parasites/µl (IRR = 1.03, 95% CI 0.69–1.55, p = 0.86).
Tab. 1. Associations between SAO and incidence of malaria during follow-up in infants 3–21 mo. 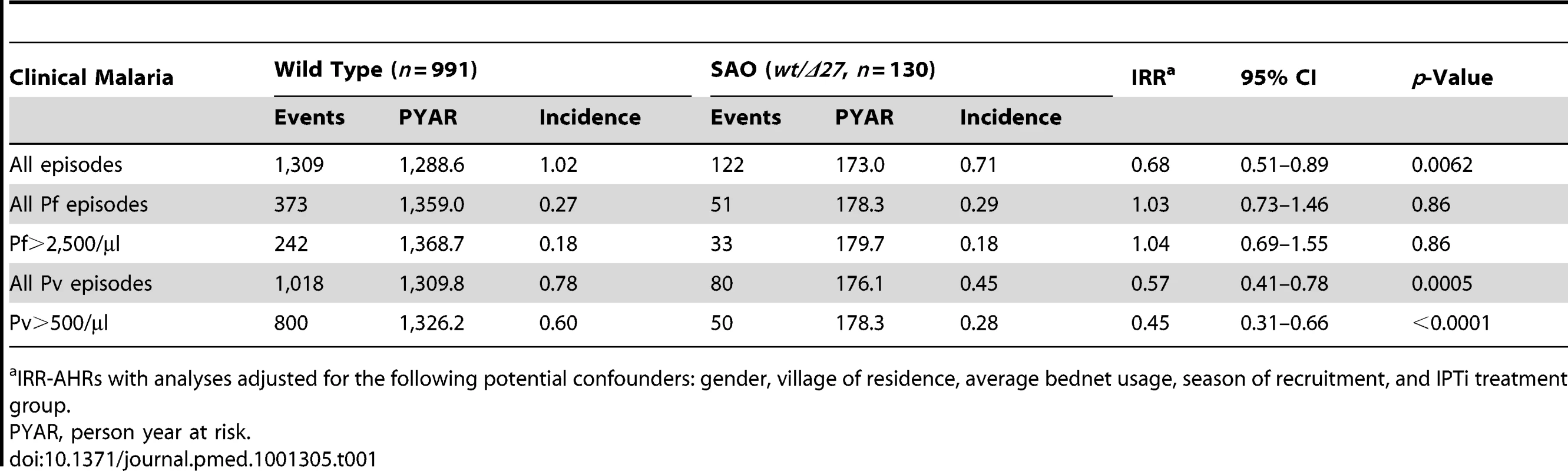
IRR-AHRs with analyses adjusted for the following potential confounders: gender, village of residence, average bednet usage, season of recruitment, and IPTi treatment group. SAO and Prevalence and Density of Malaria Infection
In children 3–21 mo, the prevalence and density of P. vivax and P. falciparum were evaluated in 6,269 blood samples collected at scheduled 3-monthly study contacts. Of these, 1,435 (22.9%) and 352 (5.6%) were positive for P. vivax and P. falciparum, respectively, by LDR-FMA and 843 (12.9%) and 197 (3.0%) by LM. Infections with P. malariae and P. ovale were rare even by LDR-FMA (1.3% and 0.5%, respectively). There was no significant difference in the prevalence P. vivax infections in SAO and wild-type children ≤12 mo (Table 2). However, in SLC4A1Δ27 heterozygous children aged 15–21 mo, significantly fewer P. vivax infections were detected by both LDR-FMA (adjusted odds ratio [aOR] 0.71, p = 0.041) and LM (aOR 0.39, p = 0.001). In children of all ages, P. vivax parasite densities (by LM) were significant lower in SLC4A1Δ27 heterozygous children (1,111/µl versus 636/µl, p = 0.011) (Figure 1). By contrast, the prevalence of P. falciparum was higher in SLC4A1Δ27 heterozygous children (reaching statistical significance only for LDR-FMA positive infections) (Table 1), but parasite densities were comparable (1,642/µl versus 2,104/µl, p = 0.59).
Fig. 1. Parasite density (by LM) in species in SAO (wt/Δ27, red squares) and non-SAO children (wt/wt, blue rhombi). 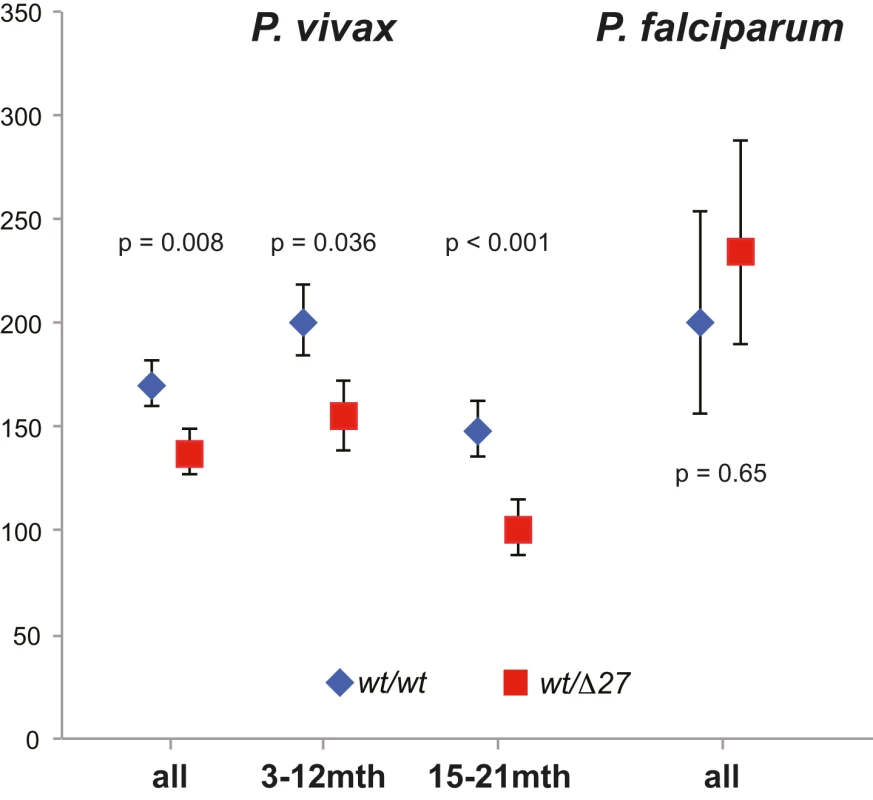
Significance levels adjusted for IPTi treatment, insecticide-treated net use, and village of residence. Tab. 2. Associations between SAO and prevalence of P. vivax and P. falciparum infection in infants 3–21 mo. 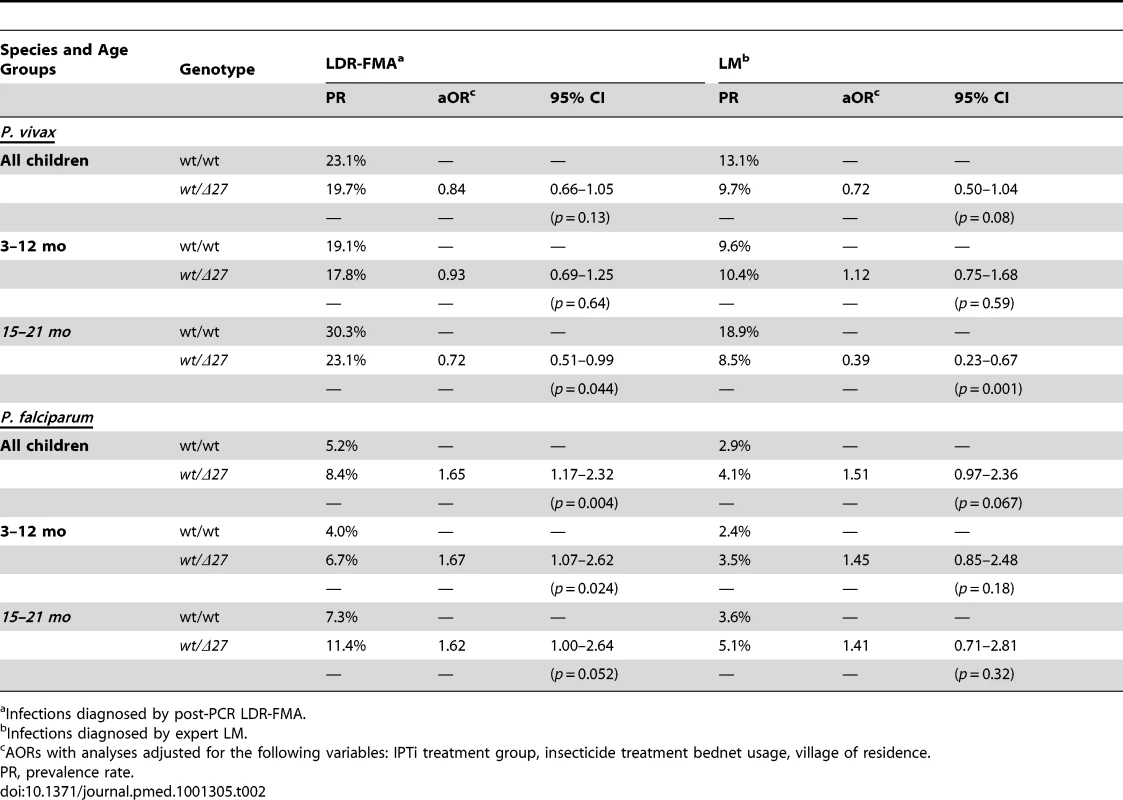
Infections diagnosed by post-PCR LDR-FMA. Of the 206 children 5–14 y of age enrolled in the treatment-reinfection cohort 106 (51.5%) were female; 116 (56.9%) were over 8 y of age. 27 children (13.1%) were heterozygous for SLC4A1Δ27. SLC4A1Δ27 heterozygous children, were similar in the basic characteristics to wild-type children except that significantly more of them were attending Mugil Elementary School (96.3% versus 70.4%, p = 0.004) (Table S2). There were no significant associations between SAO and α+-thalassaemia or GPYCΔex3 genotypes [46]. Genotype frequencies did not differ with age and sex (Fisher exact test, p>0.4 [8]). All children were wild-type (FY*A/FY*A) for the Duffy antigen.
Following initial blood-stage malaria therapy, children rapidly acquired LM-detectable blood-stage infections, with 156/192 (81.2%), 102/206 (49.5%), and 17/206 (8.3%) acquiring one or more P. falciparum, P. vivax, and P. malariae infections, respectively, over the 26 wk of observation. Similarly, using LDR-FMA diagnosis, the proportions of reinfected children rose to 91.6%, 82%, and 29.3%, respectively [30].
While SLC4A1Δ27 heterozygous and wild-type children had similar times to reinfection with P. falciparum [8], a significantly lower proportion of heterozygous children acquired P. vivax and P. malariae reinfections during the follow-up time period (Figure 2). Among SLC4A1Δ27 heterozygotes, 70.3% (19/27) acquired at least one LDR-FMA-positive P. vivax infection compared to 83.8% (149/179) of non-SAO children (log rank test, χ2 = 9.68, df = 1, p = 0.002) over the period of observation. Similarly, P. vivax infections were observed in only 37% (10/27) of SLC4A1Δ27 heterozygotes by microscopy, compared to 54.7% (98/179) of non-SAO children (log rank test, χ2 = 6.5, df = 1, p = 0.011). After correction for all other factors found to be associated with differences in risk of reinfection [30], SLC4A1Δ27 heterozygosity was associated with a significant 52% reduction in time to first LDR-FMA (adjusted hazard ratio (aHR): 0.48, p = 0.003) (Table 3) and 55% reduction in time to first LM-detectable P. vivax infections (aHR: 0.45, p = 0.014), as determined by Cox regression analysis. Similarly, SAO was associated with a 71% reduction in time to first LDR-FMA-detectable (aHR: 0.29, p = 0.03) P. malariae infections. SAO was however not associated with protection against P. falciparum (Table 3) [8].
Fig. 2. Time-to-first blood-stage infections with different Plasmodium species in SAO (dashed) and non-SAO children (solid). 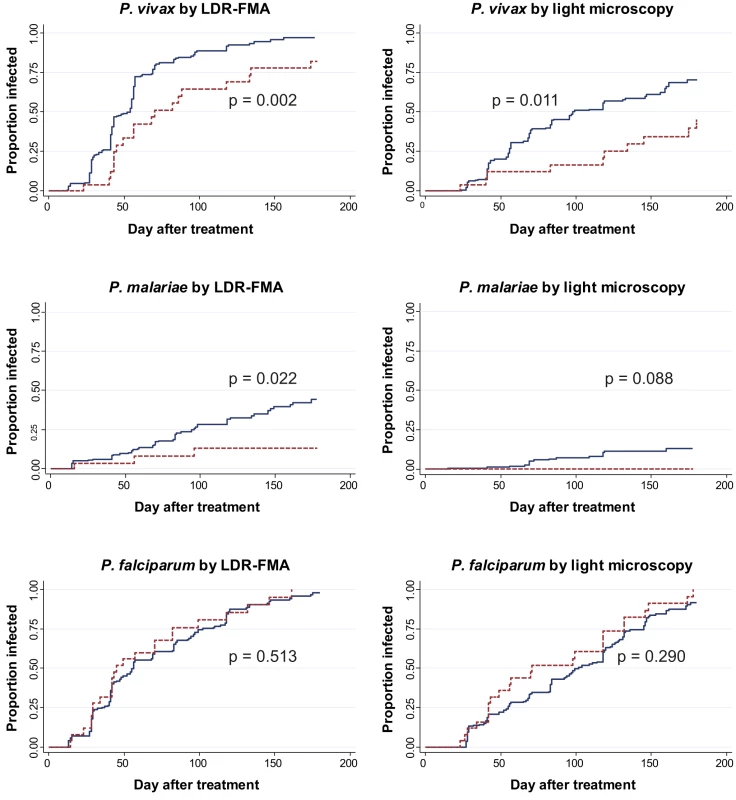
Kaplan-Meier Curves with log-rank test for difference. Tab. 3. Associations between SAO and to first Plasmodium spp. infection during follow-up in children 5–14 y. 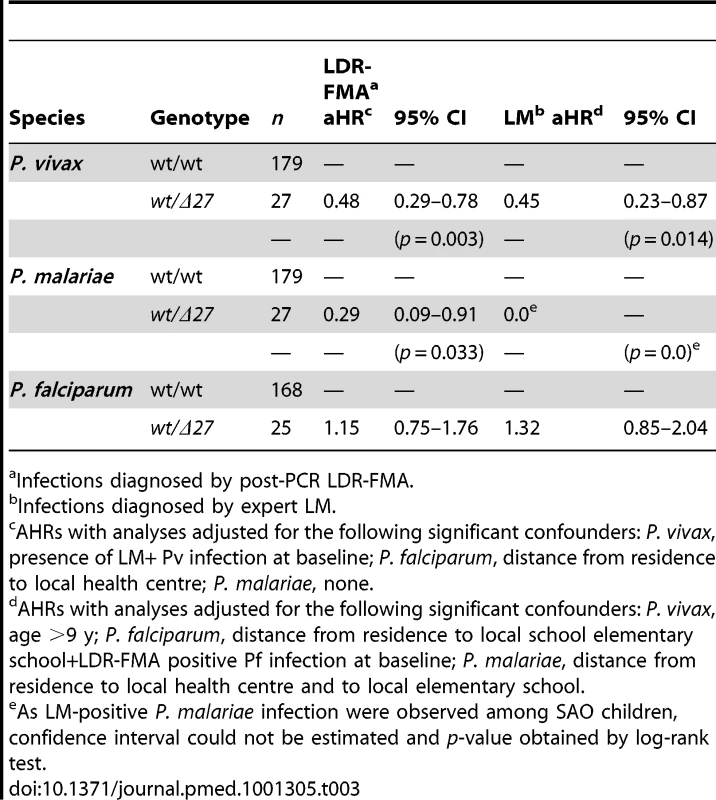
Infections diagnosed by post-PCR LDR-FMA. Duffy Expression and PvDBP Binding on SAO Red Cells
A possible mechanism whereby SLC4A1Δ27 may confer protection against P. vivax may be through reduced expression and altered functionality of the Duffy antigen receptor on RBCs. Accordingly, levels of Duffy expression and ability to bind recombinant PvDBPII were compared on red cells from a subset SAO (n = 11) and non-SAO (n = 12) study children. Erythrocytes with SLC4A1Δ27 showed no significant decrease in expression of the Duffy receptor on the surface (Figure 3, left panel, mean ± standard error of the mean [SEM] normalized mean fluorescence index [nMFI] = 36,881, inter-quartile range [IQR] 8,455–47,750, versus 18,360, IQR 9,555–27,040, p = 0.42 for SAO and non-SAO cells, respectively). The ability of PvDBPII to bind to the different SAO and non-SAO cells was similar (nMFI = 13,755, IQR 9,757–23,603, versus 12,069, IQR 6,292–20,740, p = 0.36) (Figure 2, right panel).
Fig. 3. Duffy receptor expression on erythrocytes as measured by mAb Fy6 on SAO and non-SAO cells (left panel) and binding of PvDBPII to its' receptor on SAO and non-SAO cells (right panel). 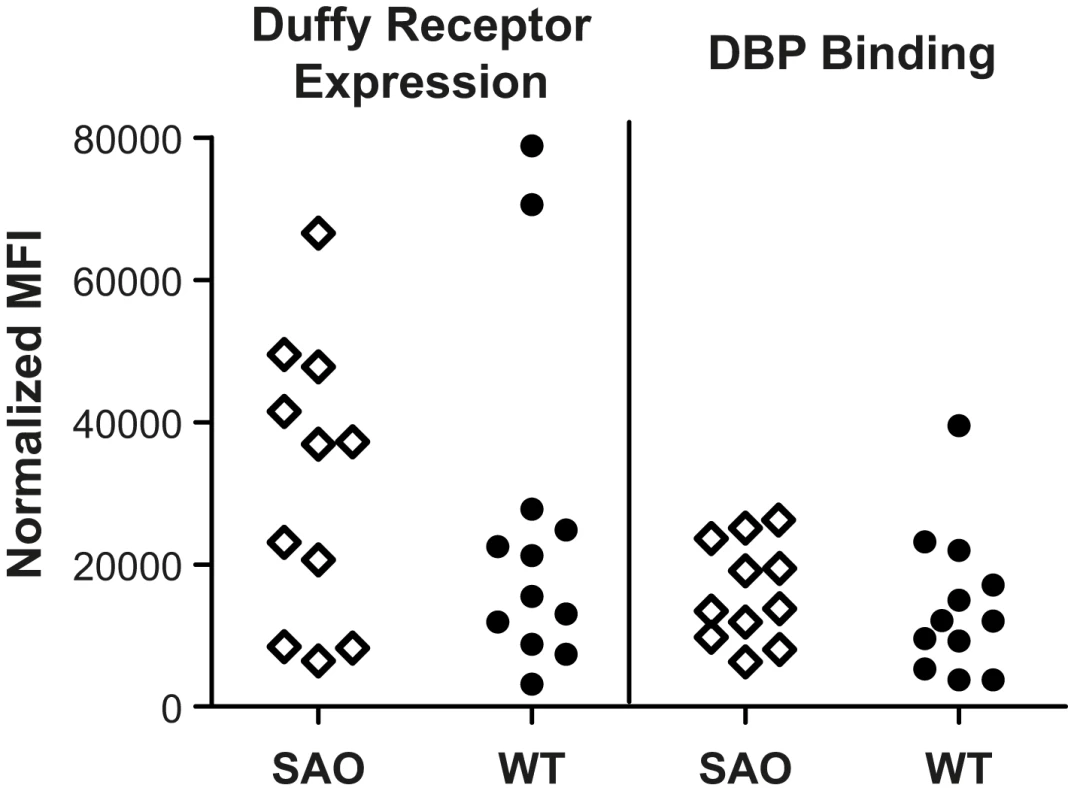
Each spot represents analysis of 5×105 erythrocytes from a single donor. SAO and Generation of P. vivax-Specific Antibody Responses
Since we had previously shown that high binding inhibitory antibodies to PvDBPII (>90% inhibitory activity) correlated with protection against P. vivax in this population [43], we examined whether SAO affects a generation of protective anti-DBPII antibodies. Among SLC4A1Δ27 heterozygous children 6/27 (22.2%) had high levels of PvDBPII BIAbs (>90% strain-transcending binding inhibition [43]) compared to 12/179 (6.7%, p = 0.008) in non-SAO children. Adjusting for the presence of PvDBP-specific BIAbs did not significantly alter the strength of protection of SAO against P. vivax infections detected by either LDR-FMA (aHR = 0.49, 95% CI 0.30–0.81, p = 0.008) or LM (aHR = 0.49, 95% CI 0.25–0.95, p = 0.036).
In contrast to the above findings, total IgG antibodies as measured by ELISA to the main variants of PvDBPII (AH, O, and P) as well as to other P. vivax merozoite surface proteins (PvRBP1, PvRBP2, and PvMSP119) were not significantly different in SAO compared with non-SAO children (unpublished data).
Prevalence of SAO among Severe Malaria Cases
Of 318 cases fulfilling the standard WHO definition of severe malaria, 273 were infected with P. falciparum, 34 with P. vivax, ten with P. falciparum plus P. vivax, and one with P. malariae. Of these 264 (83.0%) also fulfilled the pre-defined, more stringent criteria (parasitaemia cut-offs plus of local Madang or Sepik parentage) for the host genetic study: 236 were infected with P. falciparum (>1,000/µl), 25 with P. vivax (>500/µl), two with P. falciparum plus P. vivax, and one with P. falciparum plus P. malariae. SLC4A1Δ27 genotyping was successfully preformed in cases and healthy community controls.
28 of 330 (8.5%) health community controls were heterozygous for SLC4A1Δ27 compared with eight of 236 (3.4%) in children with P. falciparum single infections (OR = 0.38, 95% CI 0.15–0.87, p = 0.014) and 0/27 (0%) for children with P. vivax or mixed P. vivax/P. falciparum infection (OR = 0, 95% CI 0–1.56, p = 0.11, exact test).
When all cases fulfilling WHO criteria for severe malaria are considered, two cases of P. vivax infection (case 1 : 429 parasites/µl with prostration and mildly impaired consciousness (Blantyre coma score = 4); case 2 : 98 parasites/µl (mixed with P. falciparum by PCR), severe anemia (Hb = 1.7 g/dl), and hyperlactemia (lactate = 6.3 mmol/l) were observed in children with SAO genotype.
Among the eight SAO children with P. falciparum mono-infection, three presented with deep coma (Blantyre coma scores of 2, 1, and 2 respectively) in presence of P. falciparum densities of 4,266, 219, and 309/µl. All three children had clear CSF and negative CSF and blood cultures. These three cases are consistent with the accepted clinical definitions for cerebral malaria caused by P. falciparum [23],[29]. A detailed clinical description of each case is given in Text S1.
Discussion
This study shows that a deletion in an RBC integral membrane protein SLC4A1Δ27 causing SAO was associated with a 43% reduction in risk of clinical P. vivax episodes in a large cohort of infants followed from age 3 to 21 mo and a 52%–55% reduction in P. vivax reinfection diagnosed by PCR-LDR-FMA and LM, respectively, in a cohort of children aged 5–14 y. This is the first in vivo evidence that SAO may afford partial protection against P. vivax malaria in two independent, longitudinal cohort studies. In addition, SAO was associated with a lower P. vivax parasitaemia in children aged 3–21 mo and a reduced prevalence of P. vivax infections in children 15–21 mo. Last but not least, no child SAO genotype was found among 27 cases with severe P. vivax or mixed P. falciparum/P. vivax malaria. However, SAO was not associated with protection against P. falciparum infection and uncomplicated disease. While it was associated with a decreased risk of severe P. falciparum malaria, at least one severe malaria case with SAO genotype was admitted with deep coma (Blantyre Coma score = 2), refuting the earlier assertion that SAO provides complete protection against cerebral P. falciparum malaria [5],[47].
SAO is a dominant phenotype with respect to RBC morphology. All individuals who are SLC4A1Δ27 heterozygous have erythrocytes that are ovalocytic and more rigid than normal [48]. Although it has been suggested that the mutated band 3 protein retains its normal secondary structure [49], the membrane domain is modified [49] and the variant protein does not conduct anion transport normally [50]. While SAO erythrocytes exhibit approximately half of the anion transport activity of normal erythrocytes [50], this does not contribute to anemia or significantly impair erythrocyte function [51]. Despite being lethal in its homozygous state [4], the prevalence of heterozygosity reaches 35% in some coastal populations in PNG [3], indicating a strong selective advantage of the heterozygous genotype. Until now protection against P. falciparum cerebral malaria has been considered the most likely cause of selection for SAO [5],[47]. The strong reduction in the incidence of P. vivax disease and infections observed in our study opens up the possibility that protection against P. vivax malaria could at least have contributed to the heterozygote advantage of SAO in PNG.
It is now well established that both P. falciparum and P. vivax are associated with severe disease and death in Melanesian populations [9],[10],[29],[52]. In our studies in PNG, although P. vivax infections were less likely to result in severe symptoms, children admitted with severe P. vivax had the same phenotype as those with severe P. falciparum infections, while those with mixed P. falciparum/P. vivax presented with the most severe illness and the greatest mortality [29]. Absence of any cases of severe vivax malaria with a parasitaemia >500/µl indicates that SAO could result in a P. vivax-specific mortality benefit. However, the limited number of both SAO children and severe P. vivax infections in our case-control study restricts our ability to assess this association. Larger, appropriately powered studies are therefore required to confirm this finding.
The mechanism by which SAO may protect against P. vivax infection and disease is unknown. The changes to the RBC membrane and anion transport across the membrane caused by SAO could impact development of malarial parasites in several ways. SAO may alter the ability of the parasite to develop within the erythrocyte. The altered membrane characteristics of SAO erythrocytes may impair the parasite's ability to remodel the RBC surface [53] and affect deformability of the infected erythrocytes resulting in impaired transit through capillaries, while the changes induced by the decrease in anion transport and gas exchange [54] might inhibit growth of parasites inside the SAO RBC. Alternatively, SAO may alter the ability of P. vivax to attach to and/or invade reticulocytes Although SAO reduced parasite densities even in infants aged ≤12 mo, this resulted in a reduced prevalence of infection only in the second year of life. This indicates that SAO cells may not be inherently resistant to P. vivax infection, but that children acquire this protective effect only in concert with increasing acquisition of anti-blood-stage immunity, either by increasing the acquisition and/or by enhancing the protective effect of immune response. A similar interaction between host genetics and age-specific immune status has recently be shown for the sickle cell trait (HbAS), where in cohort of Ugandan children the youngest children were best protected against high density parasitaemia, while only older children were protected against establishment of parasitaemia [55].
In contrast to normal RBCs where band 3 is mainly found as dimers, protein expressed from the SLC4A1Δ27 allele may induce conformational changes in the normal band 3 protein resulting in the predominance of band 3 hetero-tetramers, higher order hetero-oligomers, and aggregates in SAO RBCs [56]. How this influences the distribution of other RBC membrane proteins is not known. The predominance of higher order band 3 aggregates in SAO RBCs could, however, have a significant impact on the interaction between parasite ligands and RBC proteins in general, and specifically on the invasion of P. vivax via the Duffy antigen that is thought to be part of a 4.1R-based macromolecular complex that contains band 3 as a dimer [42].
In our studies that further explored the relationship between SAO and the Duffy antigen, fluorescence activated cell sorting (FACS)-based analyses revealed that SAO and non-SAO red cells expressed similar amounts of surface-level Duffy antigen and that PvDBPII bound to SAO and non-SAO cells equally well. Interestingly, we found that while antibody levels to PvDBPII measured by ELISA did not differ, SAO children were 3.3 times more likely than non-SAO children to have high levels of PvDBPII-specific BIAbs. This preferential production of functional BIAbs could occur because critical binding regions of PvDBP might be exposed for a longer period of time during less efficient invasion of SAO cells, making the protein more accessible to the host immune response that generate broadly binding inhibitory antibodies. Adjusting for blocking antibodies did not, however, change the magnitude of the protection provided by SAO against reinfection with P. vivax. Thus, while SAO increases the likelihood that high activity Duffy blocking antibodies are acquired, the protection attributed to SAO is distinct from that provided by Duffy blocking antibodies. Further study of SAO-based protection against P. vivax illness, in particular in small children who suffer the highest morbidity burdens of P. vivax morbidity [9], is required to determine how this mutation imparts its selective advantage.
Irrespective of the mechanism by which this mutation evolved, our observations highlight the potential contribution of P. vivax malaria in shaping the unique RBC polymorphisms found in Asian and Pacific populations. Future studies on host genetic adaptation in the Asia Pacific region should thus not exclusively focus on P. falciparum but need to include other human malaria species.
Supporting Information
Zdroje
1. MullerI, BockarieM, AlpersM, SmithT (2003) The epidemiology of malaria in Papua New Guinea. Trends Parasitol 19 : 253–259.
2. HaldaneJBS (1949) The rate of mutation of human genes. Hereditas 35 : 267–273.
3. MgoneCS, KokiG, PaniuMM, KonoJ, BhatiaKK, et al. (1996) Occurrence of the erythrocyte band 3 (AE1) gene deletion in relation to malaria endemicity in Papua New Guinea. Trans R Soc Trop Med Hyg 90 : 228–231.
4. LiuSC, JarolimP, RubinHL, PalekJ, AmatoD, et al. (1994) The homozygous state for the band 3 protein mutation in Southeast Asian Ovalocytosis may be lethal. Blood 84 : 3590–3591.
5. GentonB, Al YamanF, MgoneCS, AlexanderN, PaniuMM, et al. (1995) Ovalocytosis and cerebral malaria. Nature 378 : 564–565.
6. CattaniJA, GibsonFD, AlpersMP, CraneGG (1987) Hereditary ovalocytosis and reduced susceptibility to malaria in Papua New Guinea. Trans R Soc Trop Med Hyg 81 : 705–709.
7. PatelSS, KingCL, MgoneCS, KazuraJW, ZimmermanPA (2004) Glycophorin C (Gerbich antigen blood group) and band 3 polymorphisms in two malaria holoendemic regions of Papua New Guinea. Am J Hematol 75 : 1–5.
8. LinE, MichonP, RichardsJS, TavulL, DabodE, et al. (2012) Minimal association of common red blood cell polymorphisms with P. falciparum infection and uncomplicated malaria in Papua New Guinean children. Am J Trop Med Hyg In press.
9. GentonB, D'AcremontV, RareL, BaeaK, ReederJC, et al. (2008) Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med 5: e127 doi:10.1371/journal.pmed.0050127.
10. TjitraE, AnsteyNM, SugiartoP, WarikarN, KenangalemE, et al. (2008) Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med 5: e128 doi:10.1371/journal.pmed.0050128..
11. PriceRN, DouglasNM, AnsteyNM (2009) New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis 22 : 430–435.
12. AlexandreMA, FerreiraCO, SiqueiraAM, MagalhaesBL, MouraoMP, et al. (2010) Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis 16 : 1611–1614.
13. KocharDK, SaxenaV, SinghN, KocharSK, KumarSV, et al. (2005) Plasmodium vivax malaria. Emerg Infect Dis 11 : 132–134.
14. JamesSP, NicolWD, ShutePG (1936) Clinical and parasitological observations on induced malaria. Proc Roy Soc Med 29 : 879–894.
15. KraussW (1932) Analysis of reports of 8,354 cases of IMPF-Malaria. Southern Med J 25 : 537–541.
16. O'LearyPA, WelshAL (1933) Treatment of neurosyphilis with malaria: observations on nine hundred and eighty-four cases in the last nine years. JAMA 101 : 495–501.
17. FongTCC (1937) A study of the mortality rate and complications following therapeutic malaria. Southern Med J 30 : 1084–1088.
18. PaulianD (1935) Statistique sur dix ans de malariathérapie. Rev Neurol 63.
19. KasehagenLJ, MuellerI, KiniboroB, BockarieMJ, ReederJC, et al. (2007) Reduced Plasmodium vivax erythrocyte infection in PNG Duffy-negative heterozygotes. PLoS ONE 2: e336 doi:10.1371/journal.pone.0000336..
20. TsukaharaT, HombhanjeFW, LumJK, HwaihwanjeI, MastaA, et al. (2006) Austronesian origin of the 27-bp deletion of the erythrocyte band 3 gene in East Sepik, Papua New Guinea inferred from mtDNA analysis. J Hum Genet 51 : 244–248.
21. SerjeantsonS, BrysonK, AmatoD, BabonaD (1977) Malaria and hereditary ovalocytosis. Hum Genet 37 : 161–167.
22. KimuraM, SoemantriA, IshidaT (2002) Malaria species and Southeast Asian ovalocytosis defined by a 27-bp deletion in the erythrocyte band 3 gene. Southeast Asian J Trop Med Public Health 33 : 4–6.
23. ShimizuH, TamamM, SoemantriA, IshidaT (2005) Glucose-6-phosphate dehydrogenase deficiency and Southeast Asian ovalocytosis in asymptomatic Plasmodium carriers in Sumba island, Indonesia. J Hum Genet 50 : 420–424.
24. KidsonC, LamontG, SaulA, NurseGT (1981) Ovalocytic erythrocytes from Melanesians are resistant to invasion by malaria parasites in culture. Proc Natl Acad Sci USA 78 : 5829–5832.
25. MohandasN, Lie-InjoLE, FriedmanM, MakJW (1984) Rigid membranes of Malayan ovalocytes: a likely genetic barrier against malaria. Blood 63 : 1385–1392.
26. CortesA, BenetA, CookeBM, BarnwellJW, ReederJC (2004) Ability of Plasmodium falciparum to invade Southeast Asian ovalocytes varies between parasite lines. Blood 104 : 2961–2966.
27. HadleyT, SaulA, LamontG, HudsonDE, MillerLH, et al. (1983) Resistance of Melanesian elliptocytes (ovalocytes) to invasion by Plasmodium knowlesi and Plasmodium falciparum malaria parasites in vitro. J Clin Invest 71 : 780–782.
28. SennN, RarauP, StanisicD, RobinsonL, BarnadasC, et al. (2012) Efficacy of intermittent preventive treatment for malaria in Papua New Guinean infants exposed to Plasmodium falciparum and P. vivax. PLoS Medicine 9: e1001195 doi:10.1371/journal.pmed.1001195.
29. ManningL, LamanM, LawI, BonaC, AipitS, et al. (2012) Features and prognosis of severe malaria caused by Plasmodium falciparum, Plasmodium vivax and mixed Plasmodium species in Papua New Guinean children. PLoS One 6: e29203 doi:10.1371/journal.pone.0029203..
30. MichonP, Cole-TobianJL, DabodE, SchoepflinS, IguJ, et al. (2007) The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg 76 : 997–1008.
31. McNamaraDT, KasehagenLJ, GrimbergBT, Cole-TobianJ, CollinsWE, et al. (2006) Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay. Am J Trop Med Hyg 74 : 413–421.
32. WHO (2000) Severe falciparum malaria. Trans R Soc Trop Med Hyg 94 Suppl 1: S1–S90.
33. Rosanas-UrgellA, MuellerD, BetuelaI, BarnadasC, IgaJ, et al. (2010) Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malaria J 9 : 361.
34. MullerI, GentonB, RareL, KiniboroB, KastensW, et al. (2009) Three different Plasmodium species show similar patterns of clinical tolerance of malaria infection. Malar J 8 : 158.
35. GentonB, Al YamanF, BeckHP, HiiJ, MellorS, et al. (1995) The epidemiology of malaria in the Wosera area, East Sepik Province, Papua New Guinea, in preparation for vaccine trials. I. Malariometric indices and immunity. Ann Trop Med Parasitol 89 : 359–376.
36. SnounouG, PinheiroL, GoncalvesA, FonsecaL, DiasF, et al. (1993) Identification of the four human malaria parasite species in field samples by polimerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol 58 : 283–292.
37. JarolimP, PalekJ, AmatoD, HassanK, SapakP, et al. (1991) Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc Natl Acad Sci USA 88 : 11022–11026.
38. TavulL, MuellerI, RareL, LinE, ZimmermanPA, et al. (2008) Glycophorin C Δexon3 is not associated with protection against severe anaemia in Papua New Guinea. PNG Med J 51 : 149–154.
39. ImrieH, FowkesFJ, MichonP, TavulL, HumeJC, et al. (2006) Haptoglobin levels are associated with haptoglobin genotype and alpha+ -Thalassemia in a malaria-endemic area. Am J Trop Med Hyg 74 : 965–971.
40. MénardD, BarnadasC, BouchierC, Henry-HalldinC, GrayL, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A In press.
41. GrimbergBT, UdomsangpetchR, XainliJ, McHenryA, PanichakulT, et al. (2007) Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PLoS Med 4: e337 doi: 10.1371/journal.pmed.0040337..
42. DarrahPA, PatelDT, De LucaPM, LindsayRWB, DaveyDF, et al. (2007) Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13 : 843–850.
43. KingCL, MichonP, ShakriAR, MarcottyA, StanisicD, et al. (2008) Naturally acquired Duffy-binding protein-specific binding inhibitory antibodies confer protection from blood-stage Plasmodium vivax infection. Proc Natl Acad Sci U S A 105 : 8363–8368.
44. Cole-TobianJL, MichonP, BiasorM, RichardsJS, BeesonJG, et al. (2009) Strain-specific duffy binding protein antibodies correlate with protection against infection with homologous compared to heterologous plasmodium vivax strains in Papua New Guinean children. Infect Immun 77 : 4009–4017.
45. SennN, Luang-SuarkiaD, ManongD, SibaPM, McBrideWJ (2011) Contribution of dengue fever to the burden of acute febrile illnesses in Papua New Guinea: an age-specific prospective study. Am J Trop Med Hyg 85 : 132–137.
46. LinE, MichonP, RichardsJS, TavulL, DabodE, et al. (2010) Minimal association of common red blood cell polymorphisms with P. falciparum infection and uncomplicated malaria in Papua New Guinean children. Am J Trop Med Hyg 83 : 828–833.
47. AllenSJ, O'DonnellA, AlexanderND, MgoneCS, PetoTE, et al. (1999) Prevention of cerebral malaria in children in Papua New Guinea by southeast Asian ovalocytosis band 3. Am J Trop Med Hyg 60 : 1056–1060.
48. MohandasN, Lie-InjoLE, FriedmanM, MakJW (1984) Rigid membranes of Malayan ovalocytes: a likely genetic barrier against malaria. Blood 63 : 1385–1392.
49. MoriyamaR, IdeguchiH, LombardoCR, Van DortHM, LowPS (1992) Structural and functional characterization of band 3 from Southeast Asian ovalocytes. J Biol Chem 267 : 25792–25797.
50. SchofieldAE, ReardonDM, TannerMJ (1992) Defective anion transport activity of the abnormal band 3 in hereditary ovalocytic red blood cells. Nature 355 : 836–838.
51. O'DonnellA, AllenSJ, MgoneCS, MartinsonJJ, CleggJB, et al. (1998) Red cell morphology and malaria anaemia in children with Southeast-Asian ovalocytosis band 3 in Papua New Guinea. Br J Haematol 101 : 407–412.
52. BarcusMJ, BasriH, PicarimaH, ManyakoriC, Sekartuti, et al. (2007) Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern Indonesian Papua. Am J Trop Med Hyg 77 : 984–991.
53. AikawaM (1988) Morphological changes in erythrocytes induced by malarial parasites. Biol Cell 64 : 173–181.
54. BruceLJ (2008) Red cell membrane transport abnormalities. Curr Opin Hematol 15 : 184–190.
55. GongL, Maiteki-SebuguziC, RosenthalPJ, HubbardAE, DrakeleyCJ, et al. (2012) Evidence for both innate and acquired mechanisms of protection from Plasmodium falciparum in children with sickle cell trait. Blood 119 : 3808–3814.
56. SarabiaVE, CaseyJR, ReithmeierRAF (1993) Molecular characterization of the band-3 protein from Southeast-Asian ovalocytes. J Biol Chem 268 : 10676–10680.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Misrepresentation of Randomized Controlled Trials in Press Releases and News Coverage: A Cohort Study
- Cryptococcal Meningitis Treatment Strategies in Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effect of Statins on Venous Thromboembolic Events: A Meta-analysis of Published and Unpublished Evidence from Randomised Controlled Trials
- The Challenges and Possibilities of Reducing Deaths from Cryptococcal Meningitis in Sub-Saharan Africa
- The World Health Report 2012 That Wasn't
- External Financial Aid to Blood Transfusion Services in Sub-Saharan Africa: A Need for Reflection
- Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- And Middle-Income Countries
- A Systematic Review and Meta-Analysis of Utility-Based Quality of Life in Chronic Kidney Disease Treatments
- Statins and Venous Thrombosis: A Story Too Good to Be True?
- Who Sets the Global Health Research Agenda? The Challenge of Multi-Bi Financing
- Reduced Risk of Malaria in Papua New Guinean Children with Southeast Asian Ovalocytosis in Two Cohorts and a Case-Control Study
- Gender Differences in Survival among Adult Patients Starting Antiretroviral Therapy in South Africa: A Multicentre Cohort Study
- Lipid-Based Nutrient Supplements: How Can They Combat Child Malnutrition?
- The Effect of Adding Ready-to-Use Supplementary Food to a General Food Distribution on Child Nutritional Status and Morbidity: A Cluster-Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lipid-Based Nutrient Supplements: How Can They Combat Child Malnutrition?
- Cryptococcal Meningitis Treatment Strategies in Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effect of Statins on Venous Thromboembolic Events: A Meta-analysis of Published and Unpublished Evidence from Randomised Controlled Trials
- The Effect of Adding Ready-to-Use Supplementary Food to a General Food Distribution on Child Nutritional Status and Morbidity: A Cluster-Randomized Controlled Trial
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



