-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Effect of Statins on Venous Thromboembolic Events: A Meta-analysis of Published and Unpublished Evidence from Randomised Controlled Trials
Background:
It has been suggested that statins substantially reduce the risk of venous thromboembolic events. We sought to test this hypothesis by performing a meta-analysis of both published and unpublished results from randomised trials of statins.Methods and Findings:
We searched MEDLINE, EMBASE, and Cochrane CENTRAL up to March 2012 for randomised controlled trials comparing statin with no statin, or comparing high dose versus standard dose statin, with 100 or more randomised participants and at least 6 months' follow-up. Investigators were contacted for unpublished information about venous thromboembolic events during follow-up. Twenty-two trials of statin versus control (105,759 participants) and seven trials of an intensive versus a standard dose statin regimen (40,594 participants) were included. In trials of statin versus control, allocation to statin therapy did not significantly reduce the risk of venous thromboembolic events (465 [0.9%] statin versus 521 [1.0%] control, odds ratio [OR] = 0.89, 95% CI 0.78–1.01, p = 0.08) with no evidence of heterogeneity between effects on deep vein thrombosis (266 versus 311, OR 0.85, 95% CI 0.72–1.01) and effects on pulmonary embolism (205 versus 222, OR 0.92, 95% CI 0.76–1.12). Exclusion of the trial result that provided the motivation for our meta-analysis (JUPITER) had little impact on the findings for venous thromboembolic events (431 [0.9%] versus 461 [1.0%], OR = 0.93 [95% CI 0.82–1.07], p = 0.32 among the other 21 trials). There was no evidence that higher dose statin therapy reduced the risk of venous thromboembolic events compared with standard dose statin therapy (198 [1.0%] versus 202 [1.0%], OR = 0.98, 95% CI 0.80–1.20, p = 0.87). Risk of bias overall was small but a certain degree of effect underestimation due to random error cannot be ruled out.
Please see later in the article for the Editors' Summary.
Conclusions:
The findings from this meta-analysis do not support the previous suggestion of a large protective effect of statins (or higher dose statins) on venous thromboembolic events. However, a more moderate reduction in risk up to about one-fifth cannot be ruled out.
Published in the journal: . PLoS Med 9(9): e32767. doi:10.1371/journal.pmed.1001310
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001310Summary
Background:
It has been suggested that statins substantially reduce the risk of venous thromboembolic events. We sought to test this hypothesis by performing a meta-analysis of both published and unpublished results from randomised trials of statins.Methods and Findings:
We searched MEDLINE, EMBASE, and Cochrane CENTRAL up to March 2012 for randomised controlled trials comparing statin with no statin, or comparing high dose versus standard dose statin, with 100 or more randomised participants and at least 6 months' follow-up. Investigators were contacted for unpublished information about venous thromboembolic events during follow-up. Twenty-two trials of statin versus control (105,759 participants) and seven trials of an intensive versus a standard dose statin regimen (40,594 participants) were included. In trials of statin versus control, allocation to statin therapy did not significantly reduce the risk of venous thromboembolic events (465 [0.9%] statin versus 521 [1.0%] control, odds ratio [OR] = 0.89, 95% CI 0.78–1.01, p = 0.08) with no evidence of heterogeneity between effects on deep vein thrombosis (266 versus 311, OR 0.85, 95% CI 0.72–1.01) and effects on pulmonary embolism (205 versus 222, OR 0.92, 95% CI 0.76–1.12). Exclusion of the trial result that provided the motivation for our meta-analysis (JUPITER) had little impact on the findings for venous thromboembolic events (431 [0.9%] versus 461 [1.0%], OR = 0.93 [95% CI 0.82–1.07], p = 0.32 among the other 21 trials). There was no evidence that higher dose statin therapy reduced the risk of venous thromboembolic events compared with standard dose statin therapy (198 [1.0%] versus 202 [1.0%], OR = 0.98, 95% CI 0.80–1.20, p = 0.87). Risk of bias overall was small but a certain degree of effect underestimation due to random error cannot be ruled out.
Please see later in the article for the Editors' Summary.
Conclusions:
The findings from this meta-analysis do not support the previous suggestion of a large protective effect of statins (or higher dose statins) on venous thromboembolic events. However, a more moderate reduction in risk up to about one-fifth cannot be ruled out.Introduction
Venous thromboembolic disease (i.e., pulmonary embolism and deep vein thrombosis) is a common cause of premature death and morbidity [1]–[3], yet our knowledge about how to safely prevent it is limited. During recent years, statins have emerged as one of the most effective treatments to reduce the burden of arterial cardiovascular disease worldwide [4]. Because of their remarkably good safety profile and declining costs, there has been some interest in their potential use for prevention of other conditions, such as venous thromboembolic events [5]–[7]. Venous and arterial thrombosis often co-occur [8],[9] and seem to share some common risk factors [10]. These epidemiological findings together with experimental evidence revealing novel mechanisms for the beneficial effect of statins unrelated to their low density lipoprotein (LDL) cholesterol lowering effect [11]–[13] have raised hopes that statins may also protect against venous thromboembolic events.
Until recently, clinical evidence for the effect of statins on venous thromboembolism was largely confined to non-randomised studies (with somewhat contradictory conclusions) [14],[15]. In 2009, however, secondary analyses of the JUPITER trial, in which 17,802 apparently healthy men and women were randomly allocated to receive either rosuvastatin 20 mg daily or matching placebo, provided direct randomised evidence that statin therapy might reduce the risk of venous thromboembolic events [5]. In this trial, allocation to rosuvastatin was associated with a reduction in the risk of venous thromboembolic events of 43% (95% CI 14%–63%) during a median 1.9 y follow-up [5], but this was based on relatively few patients with a venous thromboembolic event (34 versus 60), and so may partly (or even wholly) reflect the play of chance [16]. This uncertainty was reflected by calls for confirmatory evidence from other studies [17],[18]. An opportunity to obtain such evidence is provided by the routinely collected adverse event reports in existing statin trials. We therefore performed a meta-analysis of all larger scale trials of a statin versus control, and of a more intensive versus a less intensive statin regimen [19], which collected, but did not necessarily publish, information on the incidence of venous thromboembolic events during follow-up.
Methods
Ethics Statement
Ethics approval was not required for this work.
Search Strategy for Identification of Relevant Studies
PRISMA checklist is provided as Text S1. Study methods have been summarised in the study protocol (Text S2) and have been published previously [20]. In brief, we searched MEDLINE (January 1966 to March 2012), EMBASE (January 1985 to 2012 week 10), and the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 2, March 2012) for articles with a subject term “hydroxymethylglutaryl-coenzyme A reductase inhibitor” or any of the following terms: “hydroxymethylglutaryl-co A reductase inhibitor,” “statin,” “fluvastatin,” “pravastatin,” “lovastatin,” “simvastatin,” “atorvastatin,” or “rosuvastatin.” The search was limited to randomised controlled trials and human studies with no language restrictions.
Review Methods and Selection Criteria
Four reviewers, working in pairs, independently screened all titles and abstracts for randomised controlled trials with either a parallel or factorial design, with at least one comparison of a statin versus a control regimen or a more versus less intensive statin regimen, and with a total of 100 or more randomised participants followed for at least 6 mo. Reviewing process was piloted for the first 100 abstracts by all four reviewers (KR, NB, Paul McGale, and William Majoni) to assess comparability and difficulties. Then, each abstract was independently reviewed by two researchers and disagreements resolved by retrieval of the full text article and discussion with a third person. There were no restrictions placed on participant characteristics or study outcomes. We also hand-searched the reference lists of these studies to ensure that other relevant articles, such as meta-analyses of statin trials or other types of articles related to statins and venous thromboembolic events, were not missed. After removing duplicate reports, full text articles of all remaining reports were examined.
Data Abstraction
For each trial, the following information was recorded: study or investigator's name; mean follow-up duration; year of publication of the primary findings; randomised treatments; mean LDL cholesterol at 1 y; summary information about the studied population (number of participants, mean age, number of men, and prevalence of myocardial infarction or heart failure at randomisation); and the primary outcome of the study. The number of patients with at least one reported episode of deep vein thrombosis or pulmonary embolism was recorded. In trials where information on such outcomes had not previously been published, we asked the investigators to abstract the relevant numbers from their routine records of adverse events. Non-responders were sent at least one reminder after about 3 wk and were also contacted by telephone.
Assessment of Risk of Bias
To identify potential sources of bias in the reported events we followed the Cochrane Collaboration's risk of bias framework [21] and considered for each trial the following risk domains: (i) selection bias (random sequence generation and allocation concealment); (ii) performance bias (blinding of participants and study investigators for the outcomes of interest); (iii) detection bias (blinding of outcome assessors); (iv) attrition bias (incomplete outcome data); (v) reporting bias (selective outcome reporting). Risk of bias for each domain was categorised as low, unclear, or high. This information was used to make judgements about the overall risk of bias for each trial. We followed the Cochrane Collaboration's recommendation to make judgements on the basis of whether the ranking of the level of bias across domains could have led to any material bias on the outcomes of interests and, where applicable, what the direction of the bias would likely be [21].
Statistical Analysis
Our primary hypothesis was to test whether statin treatment reduced the risk of venous thromboembolic events. The primary analyses were, therefore, restricted to trials of statin versus control (i.e., placebo or usual care). However, since the anti-inflammatory effect of statins (which could be a potential mechanism for any venous anti-thrombotic effects) have been suggested to be more pronounced in high-dose statin therapy [22] and since there is some non-randomised evidence to suggest a greater reduction in risk of venous thromboembolic events with higher doses of statins [19],[23], we also performed secondary analyses on the basis of the trials that had compared a more intensive versus a standard statin regimen. Odds ratios (ORs) for each trial and summary estimates of ORs across trials were estimated using Peto's one-step method (see Text S3) [24]. Estimates of heterogeneity between trials were presented together with I2 statistics and their confidence intervals.
We performed three subgroup analyses: (i) to estimate effects separately for pulmonary embolism and deep vein thrombosis; (ii) to estimate effects separately in trials that specifically excluded patients with a known history of vascular disease compared with other trials; and (iii) to estimate effects separately according to the type of statin tested. The summary ORs for subgroups were compared using a standard chi-squared test. In a sensitivity analysis, we performed a meta-analysis of the individual trial results weighted by the absolute LDL cholesterol difference in each trial at 1 y (Text S3) [4].
Statistical analyses were done using R version 2.11.1 [25]. All statistical tests were two-sided and all analyses were done on an intention-to-treat basis.
Results
Out of 4,736 abstracts reviewed, 231 papers describing 112 trials were retrieved for further examination, of which 92 had both a follow-up duration of 6 mo or longer and had included 100 patients or more (Figure 1). Of these 92 trials, 47 trials comprising 54,643 participants and about 189,800 person-years' follow-up were excluded either because no venous thromboembolic events were recorded (i.e., zero events in both groups after interrogation of trial database) or because such information was not accessible to the trial investigators at the time. A further 16 trials comprising 7,846 participants and 18,200 person-years follow-up were excluded because there was no response to the data request. Our final database therefore included 29 trials comprising 146,353 participants and about 613,800 person-years follow-up. Of these, published information about venous thromboembolic events was available (at the time of our database search) from just two trials [5],[26], but unpublished information was provided by authors for a further 27 trials. 22 trials compared the effect of statin with control (105,759 randomised participants and 422,000 person-years follow-up) [26]–[46], and seven trials compared a more intensive with a standard dose statin (40,594 randomised participants and 191,000 person-years follow-up) [47]–[53], with no overlap between the two trial groups [39]. The characteristics of the 29 included trials are shown in Table 1. Risk of bias for individual trials is summarised in Table 2. Risk of bias for venous thromboembolic events was deemed low for all included trials.
Fig. 1. Flow-diagram of search retrieval process. 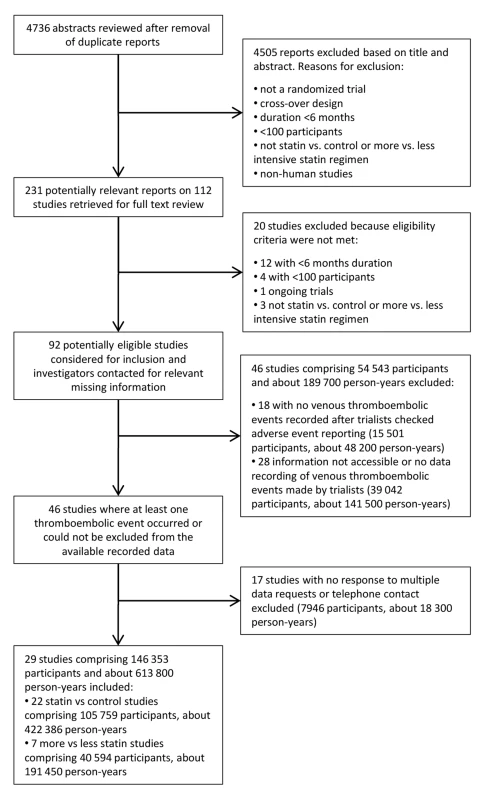
Tab. 1. Summary of trials characteristics. 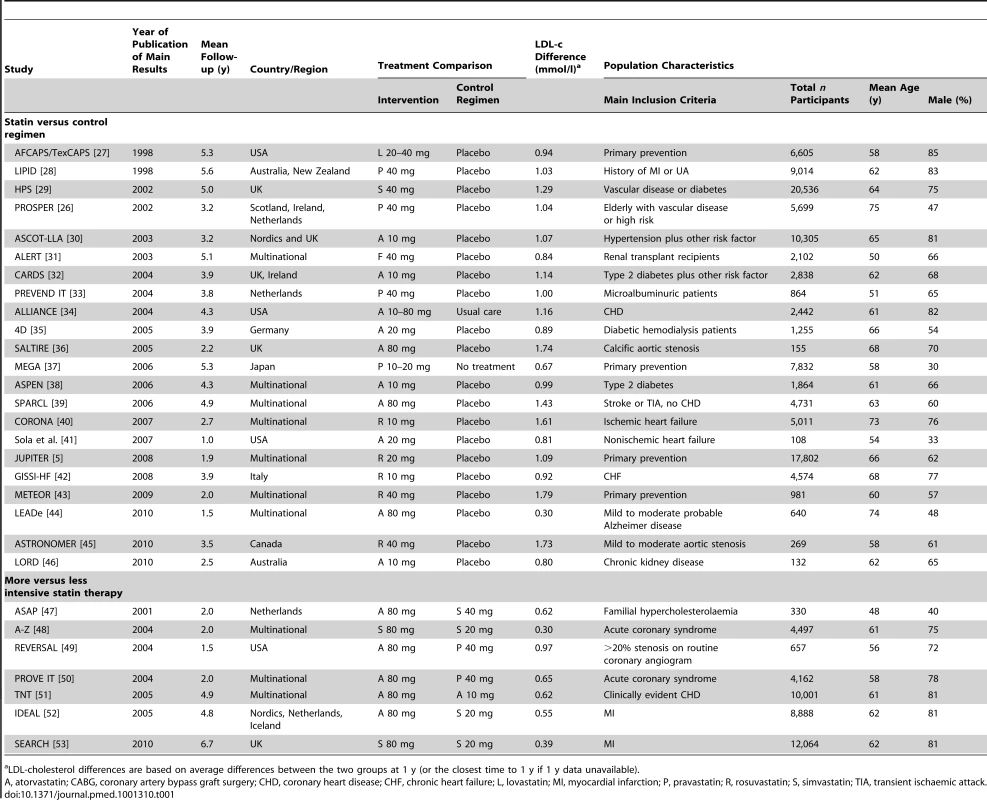
LDL-cholesterol differences are based on average differences between the two groups at 1 y (or the closest time to 1 y if 1 y data unavailable). Tab. 2. Outcome determination and risk of bias for venous thromboembolic events. 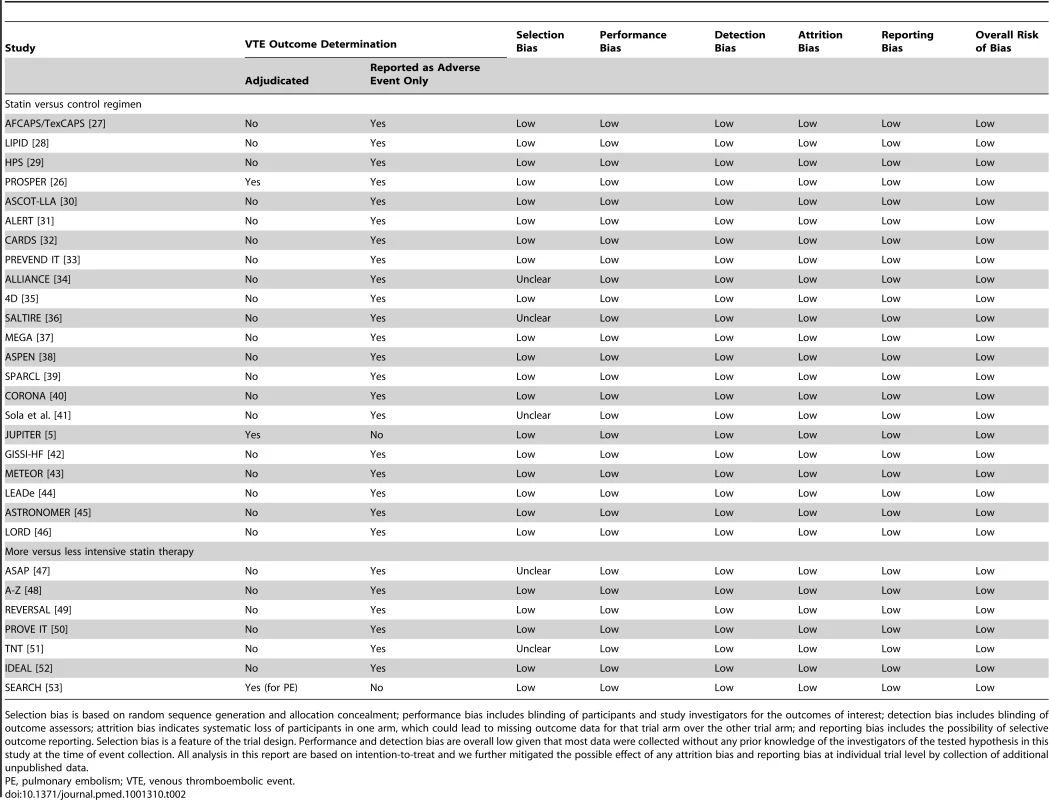
Selection bias is based on random sequence generation and allocation concealment; performance bias includes blinding of participants and study investigators for the outcomes of interest; detection bias includes blinding of outcome assessors; attrition bias indicates systematic loss of participants in one arm, which could lead to missing outcome data for that trial arm over the other trial arm; and reporting bias includes the possibility of selective outcome reporting. Selection bias is a feature of the trial design. Performance and detection bias are overall low given that most data were collected without any prior knowledge of the investigators of the tested hypothesis in this study at the time of event collection. All analysis in this report are based on intention-to-treat and we further mitigated the possible effect of any attrition bias and reporting bias at individual trial level by collection of additional unpublished data. The primary analyses were restricted to the 22 trials that compared a statin with a control regimen. In these trials, an episode of venous thromboembolic event occurred in 986 patients. Overall, there was no clear evidence that statin therapy reduced the risk of venous thromboembolic events (465 [0.9%] statin versus 521 [1.0%] control, OR = 0.89 [95% CI 0.78–1.01]; p = 0.08) (Figure 2). There was no evidence of heterogeneity in estimated effect size between the trials (heterogeneity χ221 = 23; p = 0.34) but a moderate degree of statistical inconsistency between the trials could not be ruled out (I2 = 0%, 95% CI 0%–43%). Since it was the result from the JUPITER trial that motivated us to perform this meta-analysis, it could be argued that that result should be considered only as “hypothesis generating,” and that including it in the main analyses may have led to a summary point estimate, CI, and p-value that are appreciably biased [54],[55]. Excluding this trial, however, had little effect on the overall result (431 [0.9%] versus 461 [1.0%], OR 0.93; 95% CI 0.82–1.07; p = 0.32) (Figure 2). Virtually identical results were seen when the individual trial results were weighted by the 1-y LDL cholesterol difference (in all 22 trials: OR 0.90 per mmol/l LDL cholesterol reduction, 95% CI 0.81–1.00; p = 0.05).
Fig. 2. Effect of statin therapy on venous thromboembolism. 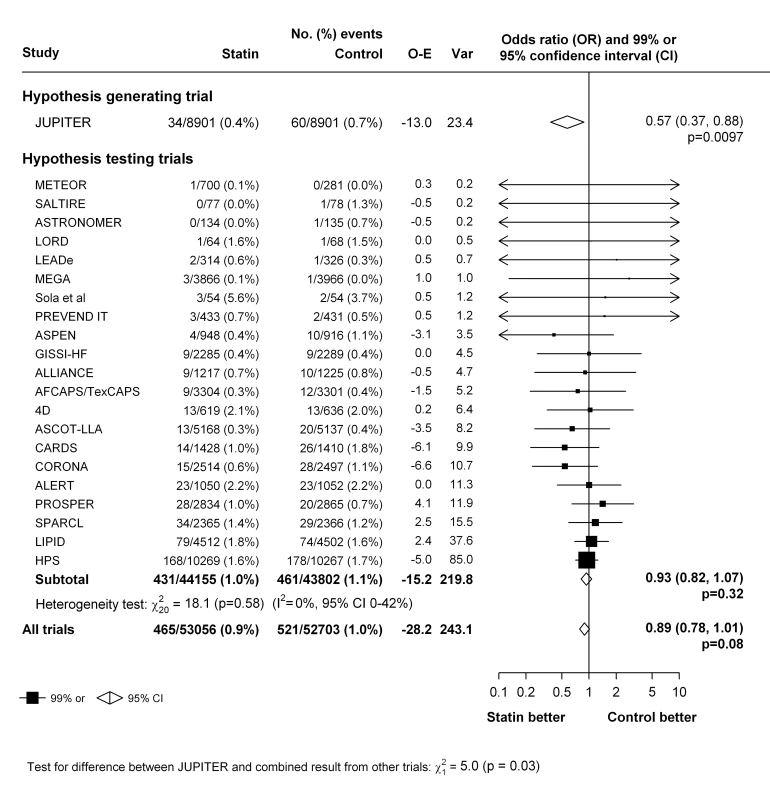
In the seven trials that compared a more intensive versus a standard statin regimen, there was no evidence that higher dose statin therapy reduced the risk of venous thromboembolic events compared with standard dose statin therapy (198 [1.0%] versus 202 [1.0%], respectively, OR 0.98; 95% CI 0.80–1.20; p = 0.87) and there was no evidence that the effect varied within these trials (heterogeneity χ62 = 4.5; p = 0.61) (Figure 3). However a moderate to large degree of statistical inconsistency between the trials could not be ruled out (I2 = 0%, 95% uncertainty level 0%–61%). The effect estimates weighted for 1-y LDL cholesterol differences were similar (weighted OR 0.99, 95% CI 0.66–1.51, per 1 mmol/l LDL cholesterol reduction).
Fig. 3. Effect of more intensive versus standard statin therapy on venous thromboembolism. 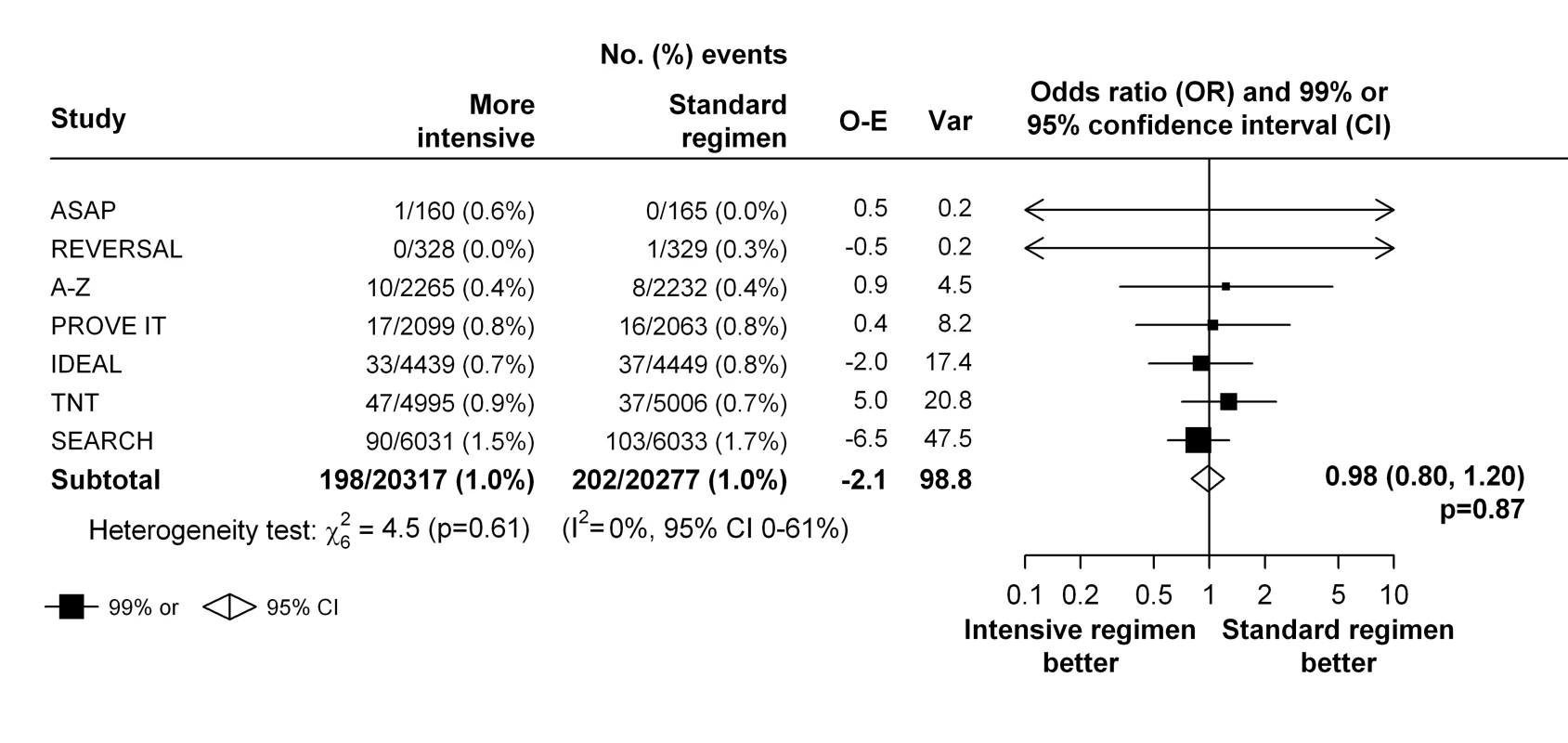
To assess a possible differential effect of statins (or higher dose statins) on pulmonary embolism and deep vein thrombosis, we estimated the effects on each outcome separately. There was no good evidence that the effect of statin therapy differed by the type of outcome (χ21 = 0.4, p = 0.54 for heterogeneity for the trials of statin versus control, and χ21 = 3.4, p = 0.06 for heterogeneity for the trials of more intensive versus standard dose statin) (Figure 4). Nor was there evidence that either statin therapy or higher dose statin therapy significantly reduced the risk of either type of outcome in isolation (deep vein thrombosis: 266 versus 311, OR 0.85 [99% CI 0.69–1.06] for trials of statin versus control and 88 versus 106, OR 0.83 [99% CI 0.57–1.21] for trials of more versus less statin; pulmonary embolism: 205 versus 222, OR 0.92 [99% CI 0.72–1.19] for trials of statin versus control and 127 versus 107, OR 1.19 [99% CI 0.84–1.68] for trials of more versus less statin) (Figure 4).
Fig. 4. Effect of statin therapy on separate components of venous thromboembolism. 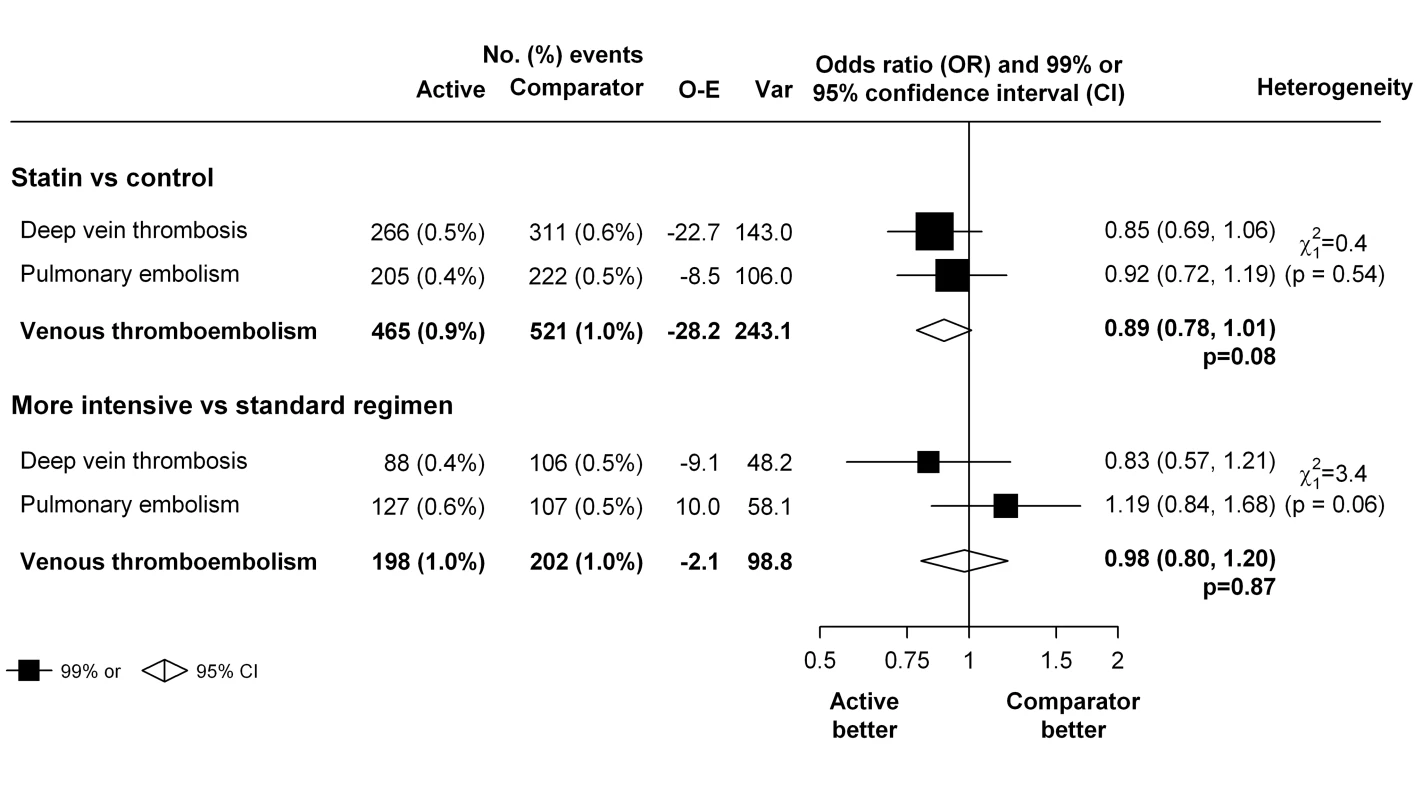
In trials that exclusively studied patients with no previous history of cardiovascular disease [5],[27],[32],[37],[43]–[45], statin therapy was associated with a significant 38% reduction in the risk of venous thromboembolism (OR 0.62, 99% CI 0.41–0.94), which appeared to differ from the non-significant 4% reduction (OR 0.96, 99% CI 0.80–1.15) seen in other trials (p-value for heterogeneity between two groups of trials = 0.01) (Figure 5). However, there were no significant differences between the two groups of trials when the hypothesis-generating JUPITER trial was excluded from these analyses (p-value for heterogeneity between two groups of trials after exclusion of JUPITER = 0.20).
Fig. 5. Effect of statin therapy on venous thromboembolism in primary cardiovascular prevention trials compared with other trials. 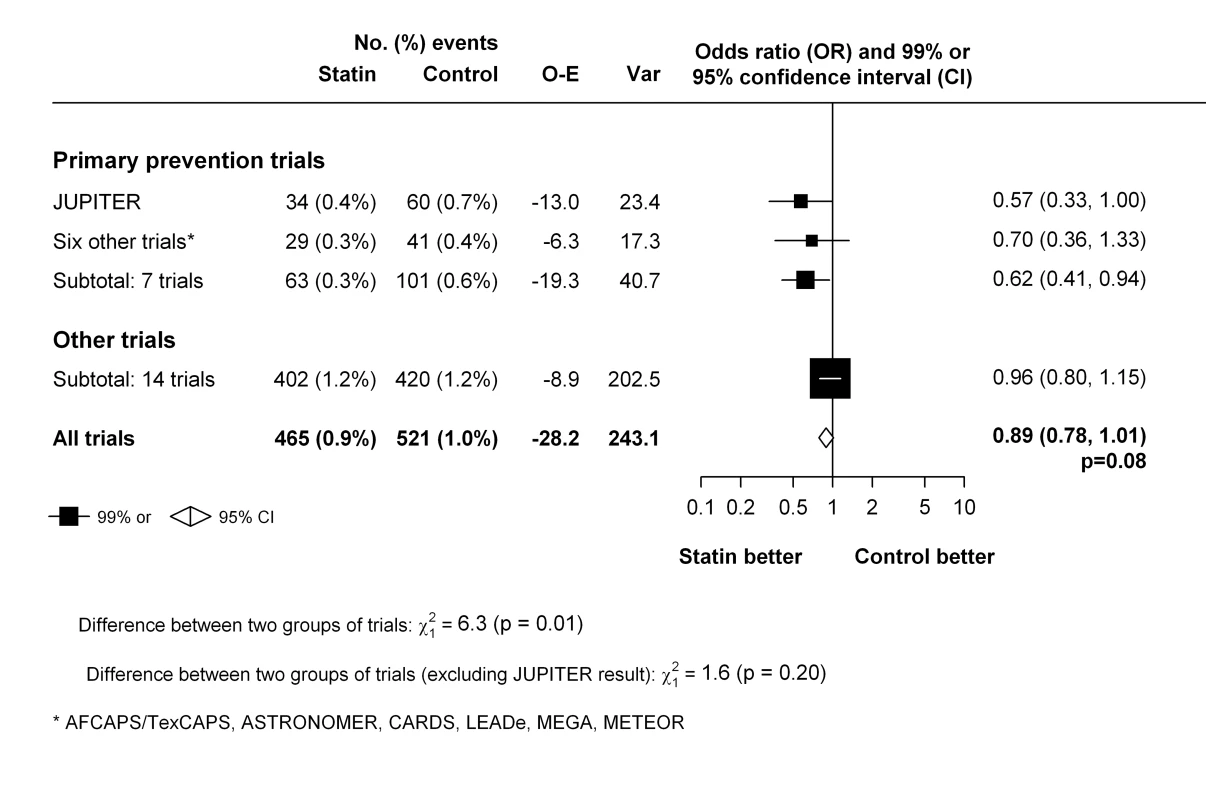
There was no good evidence that the effect of statin therapy on venous thromboembolism varied depending on type of statin studied (χ25 = 10.7, p = 0.06) (Figure S1), particularly when the results from the JUPITER trial were excluded (χ25 = 5.8, p = 0.32) (Figure S2).
Discussion
In this study, we gathered information from over 100,000 participants in 22 randomised trials of statin therapy versus control and 40,000 participants in seven randomised trials of intensive versus standard dose statin therapy, which together involve about 14 times as many venous thromboembolic events as previously reported in the JUPITER study [5]. Overall, the results from this meta-analysis do not support the suggestion that statins [5] (or higher doses of statins [19],[23]) reduce the risk of venous thromboembolic events substantially, although a more moderate reduction in risk up to about one-fifth cannot be ruled out.
Our meta-analysis has several strengths compared to previous reports. Compared with previous publications that are either based on non-randomised comparisons or have been based on one single randomised trial with limited events, our findings are based on substantially more first events from randomised trials and, importantly, include previously unpublished as well as published data. Inclusion of unpublished data helps avoid the well-documented problems caused by the preferential publication of positive findings (i.e., publication bias) [16],[56]. However, there is a perception among some that such unpublished data may be of inferior quality compared with data already in the public domain, despite the lack of evidence supporting such a view [21],[57]. In our study, reports from all trials had previously been subject to external peer review and the risk of bias in these trials for the outcomes of interest was judged to be low in our assessments (Table 2). Most of the trials had a double-blind design (i.e., trial participants and investigators were unaware of the treatment allocation) and, perhaps more importantly, venous thromboembolic events had been collected routinely as part of the safety and efficacy monitoring done in each trial (at which point there were no specific hypotheses related to venous thromboembolic events) [26]. The use of routine unadjudicated events from these properly designed and conducted randomised trials is also unlikely to have resulted in any substantial biases because any under - or over-reporting of events (or, for that matter, lack of independent confirmation) would be expected to affect both treatment arms equally [20],[58],[59]. While systematic over-reporting of events unaffected by statin therapy would tend to bias treatment effects towards the null, the magnitude of the bias would again likely be small because OR estimates are surprisingly robust to such errors. For example, even if as much as one-quarter of the reported venous thromboembolic events in our study were “false” events that were unaffected by statin treatment (and hence equally distributed by treatment group), the estimate of the OR among the trials of statin versus control would have been expected to change from 0.89 (shown in Figure 2) to 0.86 as result of removal of the false events. Confirmation of diagnosis of symptomatic venous thromboembolism in routine clinical settings already depends on objective measures such as imaging and biochemistry. It therefore seems implausible that over-reporting of unrelated outcomes in these trials of the magnitude needed to result in substantial bias in OR estimates would have occurred. This is further supported by a recent study in which treatment effects based on adjudicated versus unadjudicated vascular events in ten trials involving over 9,000 events were compared, and found to be virtually identical (OR for reported versus adjudicated outcomes 1.00, 95% CI 0.97–1.02) [59]. Indeed, if anything, one might expect the reported outcomes in our study to be more consistent with “real-world” settings in effectiveness studies and, therefore, more relevant to policy and practice.
Another concern about inclusion of unpublished data in meta-analysis is incompleteness in trials gathered, which may itself become a source of bias [57]. In our study, we sought to obtain data from all larger trials, including repeated attempts at contacting study authors. However, we might still have missed relevant event information from at least 16 further trials where there was no response from investigators (in another 47 trials, either no events occurred or events were not available to the investigators themselves so would therefore not constitute a source of bias). However, these 16 trials would have contributed only about an additional 18,000 person-years of exposure (compared to about 600,000 person-years of exposure in the 29 included trials). In addition, if an important reduction in venous thromboembolism had been observed in any single trial for which data were not made available, it seems likely that the result may already have been published (as most of these trials were completed several years ago), and hence identified by our literature search. Thus, the relatively small amount of missing information is unlikely to have resulted in any material change to our conclusions. This is illustrated by a recent similar study that assessed the effect of statin on atrial fibrillation [20]. Additional data on this outcome from a larger statin trial became available only after the meta-analysis that combined both published and unpublished data were published and this new information was entirely consistent with the pooled evidence [60].
Nonetheless, it should be recognised that the total number of first events in our meta-analysis (about 1,000 first reports of venous thromboembolism) is still relatively modest, as reflected by the confidence intervals that are consistent with anything from no effect to a real reduction in risk of about one-fifth. This makes the results of any subgroup analyses particularly unreliable, and so they need to be interpreted with due caution [54]. Consequently, this study cannot reliably investigate the potential differential effect of statins (or higher dose statins) in certain subgroups of patients based on important baseline characteristics such as prior history of venous thromboembolic events and use of anti-platelet or anti-coagulant therapy, or by the underlying cause of such events (e.g., provoked by cancer or other events versus unprovoked events).
Similarly, the suggested heterogeneity in effects on venous thromboembolism by prior vascular disease (Figure 5) is far from definitive. While it may be considered biologically plausible for there to be a proportionally smaller effect among people with pre-existing vascular disease or other chronic conditions (if the risk of venous thromboembolic events were less amenable to statin therapy as the proportion of events that are provoked by causes such cancer or immobility increases), the difference seen between trials that included healthier populations and those that did not was not statistically significant when the hypothesis-generating JUPITER trial was excluded (Figure 5). The same observation was made with regards to type of statins used (Figures S1 and S2). Thus, to demonstrate such differences (if one were to exist) would require further evidence from randomised trials.
Might the results be biased in favour of statin therapy owing to the interdependency of venous and arterial thrombosis? Statins reduce the risk of arterial vascular events (including hospitalisations) substantially [4], and more intensive regimens produce further definite reductions in risk [61]. One might therefore expect the number of venous thromboembolic events (some of which are related to arterial cardiovascular events [8],[9]) to have been greater among patients allocated placebo/less intensive statin regimens. While this may be plausible, it seems likely that the incidence of venous thromboembolic events following a cardiovascular event would be low (for example only six out of the 94 events in JUPITER occurred following a cardiovascular event) and so unlikely to have introduced any substantial bias in favour of statins.
In conclusion, this study provides a more detailed assessment of the potential effects of statins (or higher dose statins) on venous thromboembolic events than has previously been possible. We were unable to confirm the large proportional reduction in risk suggested by some previous studies. However, a more modest but perhaps clinically worthwhile reduction in venous thromboembolic events in some or all types of patient cannot be ruled out.
Supporting Information
Zdroje
1. SilversteinMD, HeitJA, MohrDN, PettersonTM, O'FallonWM, et al. (1998) Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 158 : 585–593.
2. HeitJA (2005) Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost 3 : 1611–1617.
3. NaessIA, ChristiansenSC, RomundstadP, CannegieterSC, RosendaalFR, et al. (2007) Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 5 : 692–699.
4. Cholesterol Treatment Trialists' Collaboration (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366 : 1267–1278.
5. GlynnRJ, DanielsonE, FonsecaFAH, GenestJ, GottoAM, et al. (2009) A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 360 : 1851–1861.
6. GradyD, WengerNK, HerringtonD, KhanS, FurbergC, et al. (2000) Postmenopausal hormone therapy increases risk for venous thromboembolic disease: The Heart and Estrogen/progestin Replacement Study. Ann Intern Med 132 : 689–696.
7. RayJG, MamdaniM, TsuyukiRT, AndersonDR, YeoEL, et al. (2001) Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med 161 : 1405–1410.
8. PrandoniP, BiloraF, MarchioriA, BernardiE, PetrobelliF, et al. (2003) An association between atherosclerosis and venous thrombosis. N Engl J Med 348 : 1435–1441.
9. SrensenHT, Horvath-PuhoE, PedersenL, BaronJA, PrandoniP (2007) Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet 370 : 1773–1779.
10. AgenoW, BecattiniC, BrightonT, SelbyR, KamphuisenPW (2008) Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 117 : 93–102.
11. UndasA, Brummel-ZiedinsKE, MannKG (2005) Statins and blood coagulation. Arterioscler Thromb Vasc Biol 25 : 287–294.
12. Sen-BanerjeeS, MirS, LinZ, HamikA, AtkinsGB, et al. (2005) Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 112 : 720–726.
13. TehraniS, MobarrezF, AntovicA, SantessonP, LinsP-E, et al. (2010) Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thromb Res 126: e225–e231.
14. RamcharanAS, Van StralenKJ, SnoepJD, Mantel-TeeuwisseAK, RosendaalFR, et al. (2009) HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost 7 : 514–520.
15. SerensenHT, Horvath-PuhoE, SogaardKK, ChristensenS, JohnsenSP, et al. (2009) Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost 7 : 521–528.
16. IoannidisJPA (2005) Contradicted and initially stronger effects in highly cited clinical research. JAMA 294 : 218–228.
17. EvansNS, GreenD (2009) ASH evidence-based guidelines: statins in the prevention of venous thromboembolism. ASH Education Program Book 2009 : 273–274.
18. PerezA, BartholomewJR (2010) Interpreting The JUPITER Trial: statins can prevent VTE, but more study is needed. Cleve Clin J Med 77 : 191–194.
19. KhemasuwanD, ChaeYK, GuptaS, CarpioA, YunJH, et al. (2011) Dose-related effect of statins in venous thrombosis risk reduction. Am J Med 124 : 852–859.
20. RahimiK, EmbersonJ, McGaleP, MajoniW, MerhiM, et al. (2011) Effect of statins on atrial fibrillation: collaborative meta-analysis of published and unpublished evidence from randomised controlled trials. BMJ 342: d1250.
21. (2011) Cochrane handbook for systematic reviews of interventions. Higgins JPT, Green S, editors. Version 5.1.0 (updated March 2011) London: The Cochrane Collaboration.
22. NissenSE (2005) Effect of intensive lipid lowering on progression of coronary atherosclerosis: evidence for an early benefit from the reversal of atherosclerosis with aggressive lipid lowering (REVERSAL) trial. Am J Cardiol 96 : 61f–68f.
23. DoggenCJ, LemaitreRN, SmithNL, HeckbertSR, PsatyBM (2004) HMG CoA reductase inhibitors and the risk of venous thrombosis among postmenopausal women. J Thromb Haemost 2 : 700–701.
24. YusufS, PetoR, LewisJ, CollinsR, SleightP (1985) Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 27 : 335–371.
25. R Development Core Team (2005) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
26. FreemanD, RobertsonM, BrownEA, RumleyA, TobiasE, et al. (2011) Incident venous thromboembolic events in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). BMC Geriatrics 11 : 8.
27. DownsJR, ClearfieldM, WeisS, WhitneyE, ShapiroDR, et al. (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279 : 1615–1622.
28. (1998) Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 339 : 1349–1357.
29. Heart Protection Study Collaborative Group (2002) MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360 : 7–22.
30. SeverPS, DahlofB, PoulterNR, WedelH, BeeversG, et al. (2003) Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trials-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361 : 1149–1158.
31. FellstromB, HoldaasH, JardineAG, HolmeI, NybergG, et al. (2004) Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) trial. Kidney Int 66 : 1549–1555.
32. ColhounHM, BetteridgeDJ, DurringtonPN, HitmanGA, NeilHA, et al. (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364 : 685–696.
33. AsselbergsFW, DiercksGFH, HillegeHL, Van BovenAJ, JanssenWMT, et al. (2004) Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation 110 : 2809–2816.
34. KorenMJ, HunninghakeDB (2004) Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: The ALLIANCE study. J Am Coll Cardiol 44 : 1772–1779.
35. WannerC, KraneV, MarzW, OlschewskiM, MannJF, et al. (2005) Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353 : 238–248.
36. CowellSJ, NewbyDE, PrescottRJ, BloomfieldP, ReidJ, et al. (2005) A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 352 : 2389–2397.
37. NakamuraH, ArakawaK, ItakuraH, KitabatakeA, GotoY, et al. (2006) Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 368 : 1155–1163.
38. KnoppRH, D'EmdenM, SmildeJG, PocockSJ (2006) Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care 29 : 1478–1485.
39. AmarencoP, BogousslavskyJ, CallahanA3rd, GoldsteinLB, HennericiM, et al. (2006) High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 355 : 549–559.
40. KjekshusJ, ApetreiE, BarriosV, BohmM, ClelandJG, et al. (2007) Rosuvastatin in older patients with systolic heart failure. N Engl J Med 357 : 2248–2261.
41. SolaS, MirMQS, LerakisS, TandonN, KhanBV (2006) Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol 47 : 332–337.
42. (2008) GISSI-HF investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372 : 1231–1239.
43. CrouseJR3rd, RaichlenJS, RileyWA, EvansGW, PalmerMK, et al. (2007) Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA 297 : 1344–1353.
44. FeldmanHH, DoodyRS, KivipeltoM, SparksDL, WatersDD, et al. (2010) Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease. Neurology 74 : 956–964.
45. ChanKL, TeoK, DumesnilJG, NiA, TamJ, et al. (2010) Effect of Lipid Lowering With Rosuvastatin on Progression of Aortic Stenosis: Results of the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) Trial. Circulation 121 : 306–314.
46. FassettRG, RobertsonIK, BallMJ, GeraghtyDP, CoombesJS (2010) Effect of atorvastatin on kidney function in chronic kidney disease: a randomised double-blind placebo-controlled trial. Atherosclerosis 213 : 218–224.
47. SmildeTJ, van WissenS, WollersheimH, TripMD, KasteleinJJ, et al. (2001) Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 357 : 577–581.
48. De LemosJA, BlazingMA, WiviottSD, LewisEF, FoxKAA, et al. (2004) Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase Z of the A to Z trial. JAMA 292 : 1307–1316.
49. NissenSE, TuzcuEM, SchoenhagenP, BrownBG, GanzP, et al. (2004) Effect of Intensive Compared with Moderate Lipid-Lowering Therapy on Progression of Coronary Atherosclerosis: A Randomized Controlled Trial. JAMA 291 : 1071–1080.
50. CannonCP, BraunwaldE, McCabeCH, RaderDJ, RouleauJL, et al. (2004) Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 350 : 1495–1504.
51. LaRosaJC, GrundySM, WatersDD, ShearC, BarterP, et al. (2005) Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 352 : 1425–1435.
52. PedersenTR, FaergemanO, KasteleinJJP, OlssonAG, TikkanenMJ, et al. (2005) High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction. JAMA 294 : 2437–2445.
53. (2010) SEARCH Collaborative Group. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12 064 survivors of myocardial infarction: a double-blind randomised trial. Lancet 376 : 1658–1669.
54. CollinsR, MacMahonS (2001) Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet 357 : 373–380.
55. CollinsR, ArmitageJ (2002) High-risk elderly patients PROSPER from cholesterol-lowering therapy. Lancet 360 : 1618–1619.
56. Baigent C, Peto R, Gray R, Collins R (2010) Large-scale randomized evidence: trials and meta-analyses of trials. Warrell DA, Cox TM, Firth JD, editors. Oxford textbook of medicine. 5th edition. Oxford: Oxford University Press.
57. CookDJ, GuyattGH, RyanG, CliftonJ, BuckinghamL, et al. (1993) Should unpublished data be included in meta-analyses? current convictions and controversies. JAMA 269 : 2749–2753.
58. GrangerCB, VogelV, CummingsSR, HeldP, FiedorekF, et al. (2008) Do we need to adjudicate major clinical events? Clin Trials 5 : 56–60.
59. PogueJ, WalterSD, YusufS (2009) Evaluating the benefit of event adjudication of cardiovascular outcomes in large simple RCTs. Clin Trials 6 : 239–251.
60. SchwartzGG, ChaitmanBR, GoldbergerJJ, MessigM (2010) High-dose atorvastatin and risk of atrial fibrillation in patients with prior stroke or transient ischemic attack: Analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Am Heart J 161 : 993–999.
61. Cholesterol Treatment Trialists' Collaboration (2010) Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376 : 1670–1681.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Misrepresentation of Randomized Controlled Trials in Press Releases and News Coverage: A Cohort Study
- Cryptococcal Meningitis Treatment Strategies in Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effect of Statins on Venous Thromboembolic Events: A Meta-analysis of Published and Unpublished Evidence from Randomised Controlled Trials
- The Challenges and Possibilities of Reducing Deaths from Cryptococcal Meningitis in Sub-Saharan Africa
- The World Health Report 2012 That Wasn't
- External Financial Aid to Blood Transfusion Services in Sub-Saharan Africa: A Need for Reflection
- Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- And Middle-Income Countries
- A Systematic Review and Meta-Analysis of Utility-Based Quality of Life in Chronic Kidney Disease Treatments
- Statins and Venous Thrombosis: A Story Too Good to Be True?
- Who Sets the Global Health Research Agenda? The Challenge of Multi-Bi Financing
- Reduced Risk of Malaria in Papua New Guinean Children with Southeast Asian Ovalocytosis in Two Cohorts and a Case-Control Study
- Gender Differences in Survival among Adult Patients Starting Antiretroviral Therapy in South Africa: A Multicentre Cohort Study
- Lipid-Based Nutrient Supplements: How Can They Combat Child Malnutrition?
- The Effect of Adding Ready-to-Use Supplementary Food to a General Food Distribution on Child Nutritional Status and Morbidity: A Cluster-Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lipid-Based Nutrient Supplements: How Can They Combat Child Malnutrition?
- Cryptococcal Meningitis Treatment Strategies in Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effect of Statins on Venous Thromboembolic Events: A Meta-analysis of Published and Unpublished Evidence from Randomised Controlled Trials
- The Effect of Adding Ready-to-Use Supplementary Food to a General Food Distribution on Child Nutritional Status and Morbidity: A Cluster-Randomized Controlled Trial
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



