-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Intermittent Preventive Treatment for Malaria in Papua New Guinean Infants Exposed to and : A Randomized Controlled Trial
Background:
Intermittent preventive treatment in infants (IPTi) has been shown in randomized trials to reduce malaria-related morbidity in African infants living in areas of high Plasmodium falciparum (Pf) transmission. It remains unclear whether IPTi is an appropriate prevention strategy in non-African settings or those co-endemic for P. vivax (Pv).Methods and Findings:
In this study, 1,121 Papua New Guinean infants were enrolled into a three-arm placebo-controlled randomized trial and assigned to sulfadoxine-pyrimethamine (SP) (25 mg/kg and 1.25 mg/kg) plus amodiaquine (AQ) (10 mg/kg, 3 d, n = 374), SP plus artesunate (AS) (4 mg/kg, 3 d, n = 374), or placebo (n = 373), given at 3, 6, 9 and 12 mo. Both participants and study teams were blinded to treatment allocation. The primary end point was protective efficacy (PE) against all episodes of clinical malaria from 3 to 15 mo of age. Analysis was by modified intention to treat. The PE (compared to placebo) against clinical malaria episodes (caused by all species) was 29% (95% CI, 10–43, p≤0.001) in children receiving SP-AQ and 12% (95% CI, −11 to 30, p = 0.12) in those receiving SP-AS. Efficacy was higher against Pf than Pv. In the SP-AQ group, Pf incidence was 35% (95% CI, 9–54, p = 0.012) and Pv incidence was 23% (95% CI, 0–41, p = 0.048) lower than in the placebo group. IPTi with SP-AS protected only against Pf episodes (PE = 31%, 95% CI, 4–51, p = 0.027), not against Pv episodes (PE = 6%, 95% CI, −24 to 26, p = 0.759). Number of observed adverse events/serious adverse events did not differ between treatment arms (p>0.55). None of the serious adverse events were thought to be treatment-related, and the vomiting rate was low in both treatment groups (1.4%–2.0%). No rebound in malaria morbidity was observed for 6 mo following the intervention.Conclusions:
IPTi using a long half-life drug combination is efficacious for the prevention of malaria and anemia in infants living in a region highly endemic for both Pf and Pv.Trial registration:
ClinicalTrials.gov NCT00285662
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 9(3): e32767. doi:10.1371/journal.pmed.1001195
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001195Summary
Background:
Intermittent preventive treatment in infants (IPTi) has been shown in randomized trials to reduce malaria-related morbidity in African infants living in areas of high Plasmodium falciparum (Pf) transmission. It remains unclear whether IPTi is an appropriate prevention strategy in non-African settings or those co-endemic for P. vivax (Pv).Methods and Findings:
In this study, 1,121 Papua New Guinean infants were enrolled into a three-arm placebo-controlled randomized trial and assigned to sulfadoxine-pyrimethamine (SP) (25 mg/kg and 1.25 mg/kg) plus amodiaquine (AQ) (10 mg/kg, 3 d, n = 374), SP plus artesunate (AS) (4 mg/kg, 3 d, n = 374), or placebo (n = 373), given at 3, 6, 9 and 12 mo. Both participants and study teams were blinded to treatment allocation. The primary end point was protective efficacy (PE) against all episodes of clinical malaria from 3 to 15 mo of age. Analysis was by modified intention to treat. The PE (compared to placebo) against clinical malaria episodes (caused by all species) was 29% (95% CI, 10–43, p≤0.001) in children receiving SP-AQ and 12% (95% CI, −11 to 30, p = 0.12) in those receiving SP-AS. Efficacy was higher against Pf than Pv. In the SP-AQ group, Pf incidence was 35% (95% CI, 9–54, p = 0.012) and Pv incidence was 23% (95% CI, 0–41, p = 0.048) lower than in the placebo group. IPTi with SP-AS protected only against Pf episodes (PE = 31%, 95% CI, 4–51, p = 0.027), not against Pv episodes (PE = 6%, 95% CI, −24 to 26, p = 0.759). Number of observed adverse events/serious adverse events did not differ between treatment arms (p>0.55). None of the serious adverse events were thought to be treatment-related, and the vomiting rate was low in both treatment groups (1.4%–2.0%). No rebound in malaria morbidity was observed for 6 mo following the intervention.Conclusions:
IPTi using a long half-life drug combination is efficacious for the prevention of malaria and anemia in infants living in a region highly endemic for both Pf and Pv.Trial registration:
ClinicalTrials.gov NCT00285662
: Please see later in the article for the Editors' SummaryIntroduction
Malaria and anemia are major causes of morbidity and mortality in children in tropical countries [1]. Several preventive strategies have proven to be effective in reducing this burden, such as insecticide-treated bed nets [2], indoor residual spraying [3], or prompt diagnosis and treatment of malaria with artemisinin combination therapy [1],[4]. Additionally, the preventative administration of a full treatment course of antimalarial drugs at fixed intervals regardless of illness episodes, named intermittent preventive treatment (IPT), has been shown to reduce malaria-related morbidity in different high risk groups [5],[6]. This strategy is increasingly widely used in pregnant women (IPTp) [7],[8], with a positive effect on antenatal parasitemia, placental malaria, maternal hemoglobin (Hb), birth weights, and newborn malaria infections [7],[9]. Several studies have shown that IPT is effective in reducing malaria incidence when given to infants at the time of routine immunizations (IPTi), or to older children in areas where malaria transmission is highly seasonal (IPTc) [10]–[13].
In the past 10 y, eight randomized controlled trials have investigated IPTi in different African countries, with various drug regimens. Sulfadoxine-pyrimethamine (SP), dispensed as a single dose three or four times during the first year of life, is the most studied drug and has been associated with a protective efficacy (PE) against clinical malaria episodes of 30% (ranging from 20% to 59%) in populations where SP resistance is not extensive. It also reduced the risk of anemia by 21% in these trials [10]. In all but one trial the preventative effect of the IPTi dose was restricted almost exclusively to the first 5 wk after an individual IPTi dose, with little effect thereafter [10]. While IPTi with SP retains efficacy in areas with moderate SP resistance [14],[15], SP has failed to prevent malaria in areas with very high resistance levels [16],[17]. Based on this evidence, the World Health Organization recommends IPTi as a suitable malaria control intervention in areas of high malaria burden and low-to-moderate SP resistance [18].
To date, all reported IPTi studies have been carried out exclusively in sub-Saharan Africa in populations where Plasmodium falciparum (Pf) is the predominant parasite and P. vivax (Pv) is uncommon because of the high prevalence of Duffy negativity [19]. However, in highly endemic areas of the Southwest Pacific [20],[21], Southeast Asia [22], the Americas [23], and the horn of Africa [24], Pv is a major cause of malaria morbidity, including severe illness and death in young children in particular. Therefore, although IPTi may have significant benefits in these regions, to date there are no data of which we are aware on IPTi efficacy in non-African settings, or on the use of IPTi for malaria due to P. vivax. Results from Africa cannot be easily extrapolated to other settings because of differences in the biology of Pf and Pv [25], in particular the ability of Pv to relapse from long-lasting liver stages, and also the ability of Pv to quickly acquire resistance to SP [26].
In order to determine the efficacy of IPTi in reducing the burden of malaria (all cause as well as Pf and Pv specific) and anemia in a Pf and Pv co-endemic setting, we undertook a three-arm randomized placebo-controlled trial of IPTi in an area of Papua New Guinea (PNG) that is highly endemic for both Pf and Pv. We tested two different drug regimens, combining a long-acting drug (SP) with either 3 d of amodiaquine (AQ) (another long half-life drug) or 3 d of artesunate (AS) (a short half-life drug), given in conjunction with routine immunization activities. These two drug regimens were chosen for two reasons: (1) they were the first and second line treatments in PNG at the time of the commencement of the study, facilitating their possible implementation into PNG standard practice as treatments for IPTi, and (2) the comparison allows differentiation between the importance of potentiallyimproved clearance of existing infections (using SP-AS) versus better prevention of new infections post-treatment (using the long-acting combination of SP-AQ).
Methods
Trial Design, Sites, and Study Participants
We undertook an individually randomized placebo-controlled trial of two different drug regimens (SP [single dose] in combination with either 3 d of AQ [SP-AQ] or 3 d of AS [SP-AS]) given as IPTi at routine vaccination time points (3, 6, and 9 mo) and at 12 mo in conjunction with routine vitamin A supplementation as part of the World Health Organization's Expanded Programme on Immunization (EPI) in PNG. Participants, field teams, and investigators were all blinded with respect to treatment allocation. The efficacy of the intervention was assessed for a 12-mo period from enrollment to a primary assessment time point at 15 mo of age, with a 6-mo extended follow-up (15–21 mo) to assess potential rebound.
The study was carried out between 6 June 2006 and 14 May 2010 on the north coast of PNG in the Mugil area of Sumkar District, Madang Province. The study site included 20 villages situated in an ∼400-km2 coastal area 30–60 km north of Madang town. The region receives over 3,000 mm of rainfall annually, with a short dry season (June to October), and is considered to have hyper-endemic malaria [27],[28]. Study villages are serviced by two major health centers (Mugil and Alexishafen) and several aid posts that are responsible for delivering EPI through monthly outreach clinics. Although ownership of bed nets was relatively high (>50%), until the start of free long-lasting insecticide-treated net distribution by the national malaria program in late 2008, the majority of the population in the study site were using untreated nets.
Originally, the trial was started as a two-site study, with a second site in Wosera (East Sepik Province). However, an interim analysis performed mid-study found that the incidence of malaria was dramatically different between the two sites, restricting the comparability of the two sites. Consequently, enrollments were ceased in Wosera in May 2008 and, in line with a revised sample size calculation, enrollments at the Madang site were increased to 334 children per arm. The data from the Wosera cohort were subsequently used only for an overall safety analysis (Table S1).
The study was carried out in accordance with Good Clinical Practice (ICH GCP E6) guidelines and externally monitored by two independent monitors and the Data Safety Monitoring Board. The study was approved by the PNG Medical Research Advisory Committee (MRAC number 05.20). The trial was registered on http://www.clinicaltrials.gov (number NCT00285662) and formed part of the IPTi Consortium (http://www.ipti-malaria.org) [29].
Randomization, Blinding, and Treatment Allocation
Children were enrolled if they fulfilled the following criteria: (1) permanent resident of the area, (2) aged 3 (±1) mo old, (3) no disability, (4) no chronic illness, (5) no known allergy to study drugs, (6) Hb>5 g/dl, and (7) no severe malnutrition (defined by the PNG national guidelines as a weight less than 60% of the Harvard reference weight-for-age value [30]). After enrollment children were randomly allocated to the placebo, SP-AQ, or SP-AS treatment group using a pre-assigned list. Randomization lists were prepared by independent statistician using SAS software and consisted of assignments in blocks of 12, each comprising a list of the same 12 letters in random order. The three arms of the trial were each assigned four randomly selected letters from among the twelve, which were used to label the packaging of the tablets. The code list was held by the statistician in a locked drawer until completion of the trial. Individual allocation codes were contained in pre-prepared opaque envelopes, which were opened only after a child was allocated to the next available ID number.
SP, AS, AQ, and their respective placebos were pre-packaged in individual blisters by the drug manufacturers (SP and AS, IPCA; AQ, Kinapharma). Study drugs and placebo were identical in appearance and color, but as drugs were administered with sweet syrup no attempt was made to match the moderately bitter taste of AQ. The quality of all drugs and corresponding placebo was independently verified. Study drugs and placebo were assigned to the respective treatment codes by an independent technician. The entire study team and principal investigators remained fully blinded for the entire study period.
Clinical Study Procedures
The three different study interventions were (1) SP-AQ, a single dose of SP (25 mg/kg sulfadoxine and 1.25 mg/kg pyrimethamine) combined with 3 d of AQ (10 mg/kg) plus 3 d of AS placebo; (2) SP-AS, a single dose of SP plus 3 d of AS (4 mg/kg) plus 3 d of AQ placebo; (3) matching placebos for all three drugs. The tablets were split (quarters or halves) according to the weight (in kilograms) of each child. The two treatment arms corresponded to the first and second line treatments of the PNG national standard guidelines for malaria at that time.
The intervention was delivered four times during the first year of life alongside the EPI at 3, 6, 9, and 12 mo of age (Table 1). The drugs were crushed and mixed with water and sweet syrup (Golden Crush Cordial, Coca-Cola Amatil) for easy administration with spoon or syringe. The first day dose was administered by study staff. The children were monitored closely, and if they vomited within 30 min of receiving treatment, the dose was repeated. The second and third doses were given by the carer at home without direct supervision. Adherence and potential adverse reactions to the study drugs were assessed by community reporters, and an adherence rate of >90% was reported (data not shown).
Tab. 1. Summary of scheduled patient contacts during study follow-up. 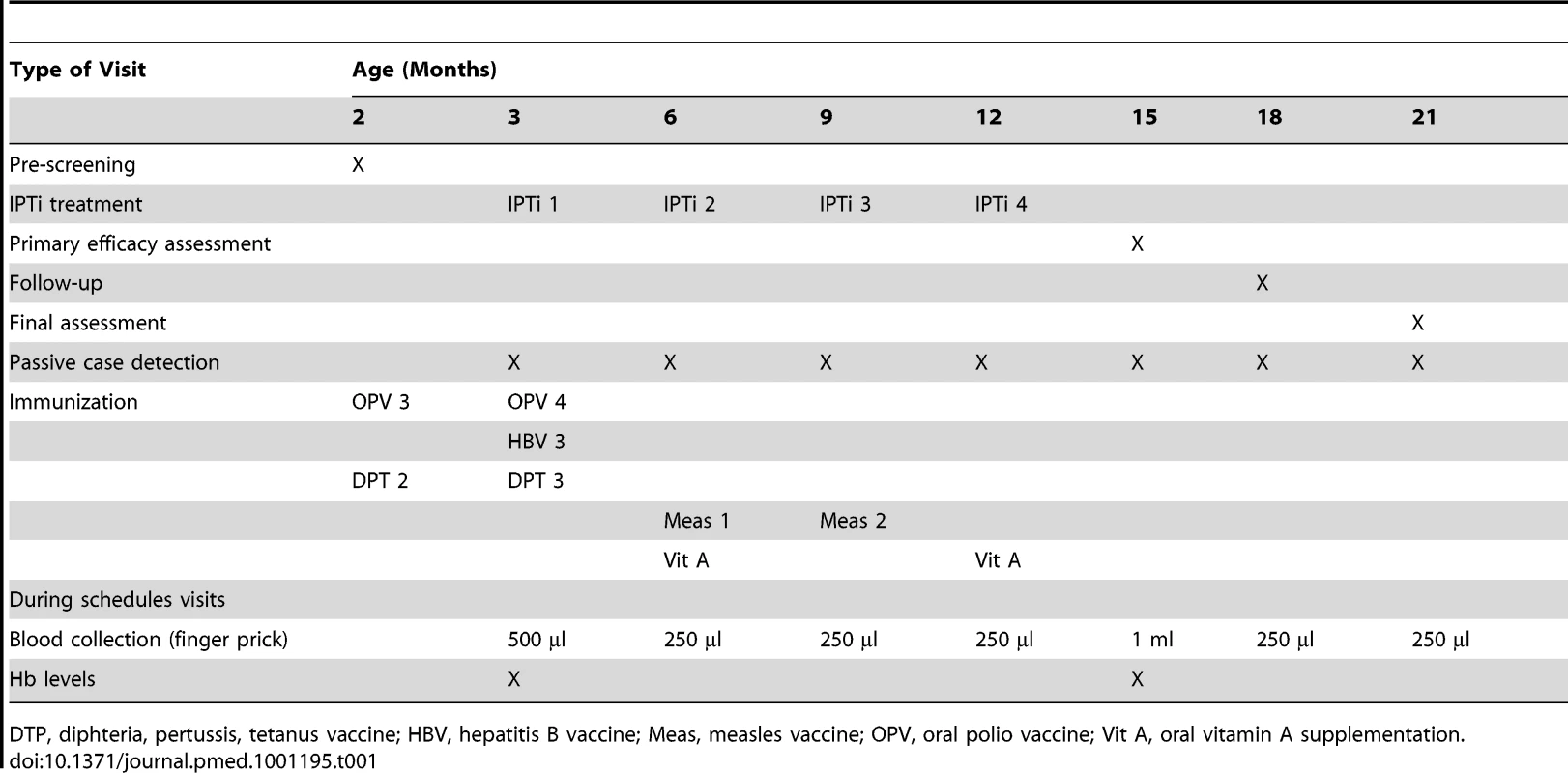
DTP, diphteria, pertussis, tetanus vaccine; HBV, hepatitis B vaccine; Meas, measles vaccine; OPV, oral polio vaccine; Vit A, oral vitamin A supplementation. The parents or guardians of potential study participants were contacted during monthly routine EPI clinics run jointly by study staff and health center staff when the child was 1–2 mo of age. The study was explained in detail to the parents through both individual and community awareness meetings. An information brochure and consent form in English and in the local language (Pidgin) was given to the parents to take home for further discussion.
A child who met the inclusion criteria was formally enrolled during the next clinic visit, when the child was 3 mo of age (±1 mo) and after at least one parent gave written consent. Upon enrollment, a concise medical history including bed net use, possible disabilities, and presence of acute illness was performed. A brief physical examination was done, including measurement of weight. Table 1 summarizes what occurred at different visits during the trial.
Throughout the study period, a passive case detection system was maintained at the Mugil health center and three outlying clinics where study participants received treatment free of charge. Each illness episode was assessed by study staff using a standard case report form. In the case of history of fever within the past 48 h or an axillary temperature >37.5°C, a rapid malaria diagnostic test (ICT Combo) was performed, two blood smears and 250 ml of whole blood were collected, and Hb level was measured using a portable HemoCue 201 machine. Only infants positive for malaria on the rapid diagnostic test were treated with artemether/lumefantrine (Coartem). The children with moderate to severe anemia (Hb<8 g/dl) received iron supplementation (ferrous sulfate, 5 mg/kg daily for 6 wk). All other illnesses were treated according to the standard treatment guidelines of PNG. Children presenting with any danger signs or symptoms were referred to the health center for admission and treatment. Study participants who attended after hours were treated by health center staff based on presumptive diagnosis. In such cases, study staff recorded the reason for admission and (when possible) performed a finger prick to check parasitemia and Hb the next day.
All illness episodes were considered adverse events (AEs) and were graded by the study clinicians according to severity from grade 1 (less severe) to grade 3 or as a serious adverse event (SAE; life-threatening or deadly, resulting in disability/incapacity, or presenting with specific clinical/laboratory features such as those that characterize important hematological disorders [as defined by the IPTi Consortium]). Hospital admissions that did not fulfill the criteria of SAE were considered grade 3. The study clinicians assessed all SAEs and causality of AEs. SAEs and AEs possibly or certainly related to the intervention were reported to the Data Safety Monitoring Board, which assessed grade and causality using IPTi Consortium guidelines.
Diagnosis of Malaria Infections
All blood slides were read by two expert microscopists. In case of discrepancies, a third read was performed. Thick blood films were examined by light microscopy for 200 thick-film fields (under 100× oil immersion lens) before being declared infection-negative. Slides were scored as light-microscopy-positive for an individual Plasmodium species, if the species was detected independently by at least two microscopists. Additionally, if the final slide readings were discrepant compared to PCR results (positivity and species), a final confirmatory read by a senior microscopist was performed. Parasite densities were recorded as the number of parasites per 200 white blood cells and converted to the number of parasites/microliter assuming 8,000 white blood cells/microliter [3]. Final parasite densities were obtained by calculating the geometric mean of positive reads.
Blood samples were collected from finger pricks in tubes coated with ethylenediaminetetraacetic acid and/or in plain tubes (15-mo visit only). Upon arrival in the laboratory, samples were separated into plasma or serum and cell pellet. Plasma and serum were stored at −80°C until further use. DNA was extracted from cell pellets using the QiaAMP 96 extraction kit (Qiagen) and the 96-well Genomic DNA Extraction Kit (Favorgen).
The presence of each of the four human malarial species was assessed in all blood samples using a semi-quantitative post-PCR ligase detection reaction/fluorescent microsphere assay (LDR-FMA) [31]. This assay combines PCR amplification of the small subunit ribosomal RNA gene (491 - to 500-bp fragments) using genus-specific primers, followed by a multiplex species-specific ligation detection reaction. The design and sensitivity of this assay have been described previously [31],[32]. The threshold of positivity of the reactions for each species was determined monthly using the mean value obtained for the negative controls (for each species), plus three times the value of its standard deviation. Samples with low-positive values (<[threshold+8× standard deviation]) were double-checked and considered as positive only if the result was confirmed.
Sample Size Calculations
Based on a predicted incidence rate of 1.2 episodes/child/year (0.7 for Pf and 0.5 for Pv), a power of 90% and alpha = 0.025, a sample size of 250 children per arm was estimated to be necessary to find at least a 28% reduction in the incidence of all malaria episodes, a 30% reduction in the incidence of Pv, and a 35%–37% reduction in the incidence of Pf episodes. Assuming a drop out of participants of 20% in conjunction with a fall in incidence of malaria of 30% to 50% due to long-lasting insecticide-treated net introduction through the national program in November 2008, a final adjusted sample size of 366 children per arm, or 1,100 children in total, was calculated.
Statistical Methods
The analysis was performed both by modified intention to treat (mITT) and according to protocol (ATP). The mITT population includes all randomized children in Mugil that received at least one dose of IPTi or placebo (i.e., 1,121 of 1,125 children). Following the mITT principle, participants were analyzed according to the preventive treatment they were assigned to at randomization. In the ATP analysis, the population includes all randomized participants who received at least three IPTi treatments (each given within 4 wk after the scheduled treatment time point) and were under passive surveillance for the entire 12-mo period.
The primary objective of the trial was to evaluate the protective effect of the two IPTi regimens. The primary outcome was measured as the incidence of symptomatic malaria due to any Plasmodium species from 3 to 15 mo of age. Secondary outcomes included (1) the specific incidence of symptomatic malaria due to Pf or Pv from 3 to 15 mo of age, (2) the incidence of symptomatic malaria (any, Pf, and Pv) from 15 to 21 mo (i.e., “rebound”), (3) the incidence of moderate-to-severe anemia (Hb<8 g/dl) and severe anemia (Hb<5 g/dl) from 3 to 15 mo and 15 to 21 mo, and (4) the prevalence of malaria parasitemia at 15 and 21 mo.
Symptomatic malaria was defined as history of fever or axillary temperature ≥37.5°C and a positive blood smear or a positive rapid diagnostic test, confirmed by positive PCR. The incidences were compared between groups using negative binomial regression to take into account possible extra-Poisson variation due to frailty at the individual level. Analyses were adjusted by sex, number of IPTi treatments received, season of enrollment (wet versus dry), average bed net use, and site (grouped by 12 recruitment zones). The time at risk was calculated starting on the date of enrollment until the date defined according to the analysis, or until the participant was withdrawn from the study. An arbitrary period of 28 d was excluded after each malaria episode. The same methodology was used for evaluating of the incidence of moderate-to-severe (Hb<8 g/dl) and severe anemia (Hb<5 g/dl). Efficacy results are presented as either incidence rate ratio (IRR) or PE (PE = 1−IRR).
The risk of malaria within 35 d following the administration of each of the treatment doses was investigated by calculating incidence rates and hazard ratios using a Cox regression model. Differences in prevalence of infection at 15 and 21 mo were evaluated using logistic regression.
The analyses were performed in Stata version 11 (StataCorp).
Results
From 6 June 2006 to 14 May 2010, 1,125 3-mo-old infants were enrolled, randomized, and followed-up in the trial in the Mugil area of Madang Province (Figure 1). Four infants were retrospectively excluded from the analysis as they were already receiving antimalarial treatments at the time of all IPTi visits and therefore did not receive any IPTi treatment doses. In all, 374 infants were allocated to the SP-AQ group, 374 to the SP-AS group, and 373 to the placebo group (Figure 1). A total of 1,079 (96%) participants completed follow-up to 15 mo, whereas 857 (76%) completed the 6-mo post-treatment follow-up (i.e., 15–21 mo).
Fig. 1. Trial profile. 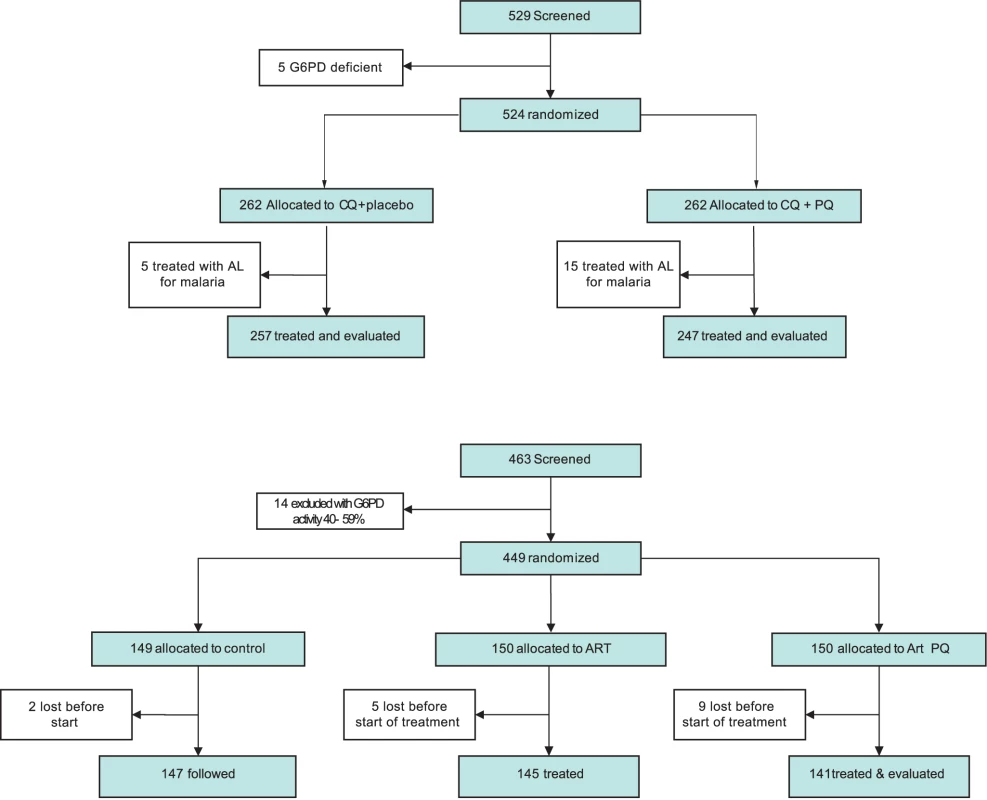
incomplete FU, incomplete follow-up (i.e., the study was terminated before the participant completed the 21-mo follow-up time period); LTF, lost to follow-up. The baseline characteristics of all study infants were similar across the three treatment arms (Table 2). No significant imbalances were observed between the three groups. In the final multivariate models, gender, village of residence, average insecticide-treated net use, and season of recruitment were included. A total of 939 children who received three or four IPTi treatments were included in the ATP population, with no significant imbalances between groups (Table S2).
Tab. 2. Baseline characteristics of study participants—intention-to-treat cohort. 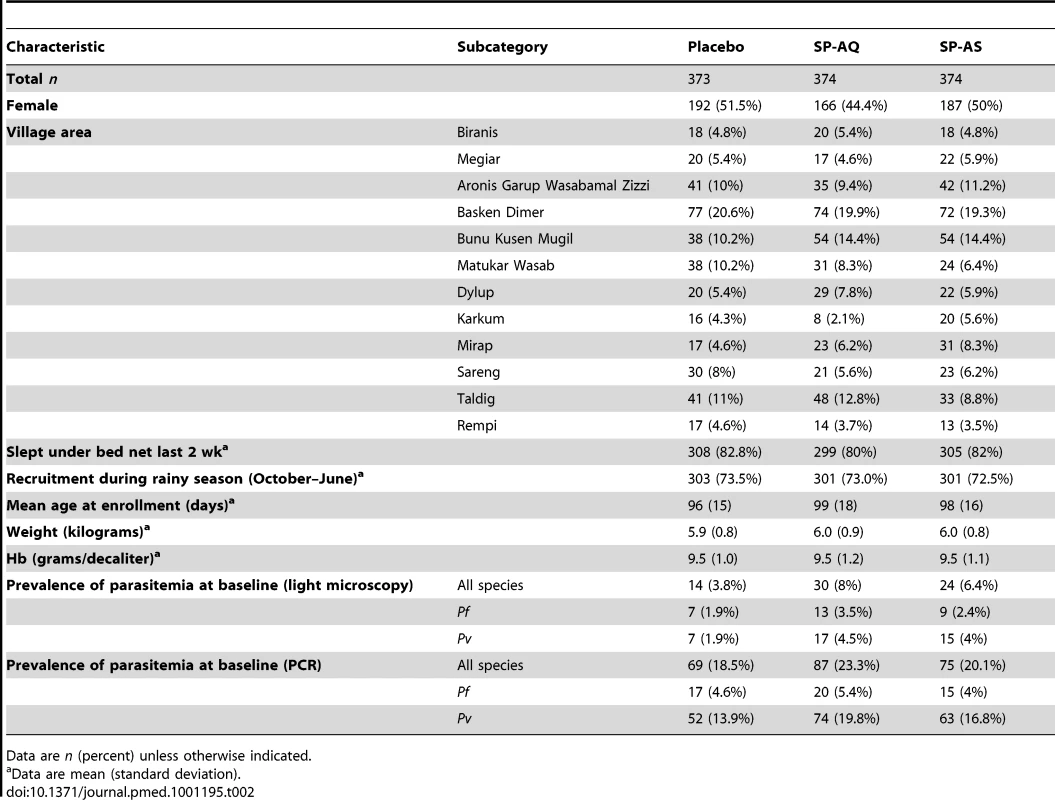
Data are n (percent) unless otherwise indicated. Protective Efficacy
The incidence of clinical malaria between the first dose at 3 mo and 15 mo in the placebo arm was 1.07 per person-year at risk (PYAR) overall, and 0.28 and 0.82 for Pf and Pv, respectively. In mITT analyses, the incidence of all cases of malaria was 29% (95% CI, 10–43, p≤0.001) lower in the SP-AQ group as compared to placebo, but not significantly reduced in the SP-AS group as compared to placebo (point estimate for change 12%, 95% CI, −11 to 30, p = 0.12). The PE was higher against Pf than Pv in both groups (Table 3). In the SP-AQ group, Pf incidence was reduced by 35% (95% CI, 9–54, p = 0.012) and Pv incidence by 23% (95% CI, 0–41, p = 0.048) in comparison to placebo. In contrast, the SP-AS group showed a significant reduction only for incidence of Pf (PE = 31%, 95% CI, 4–51, p = 0.027), not for incidence of Pv (PE = 4%, 95% CI, −26 to 24, p = 0.759).
Tab. 3. Efficacy of IPTi with SP-AQ and SP-AS against malaria and anemia, overall as well as after and between individual doses. 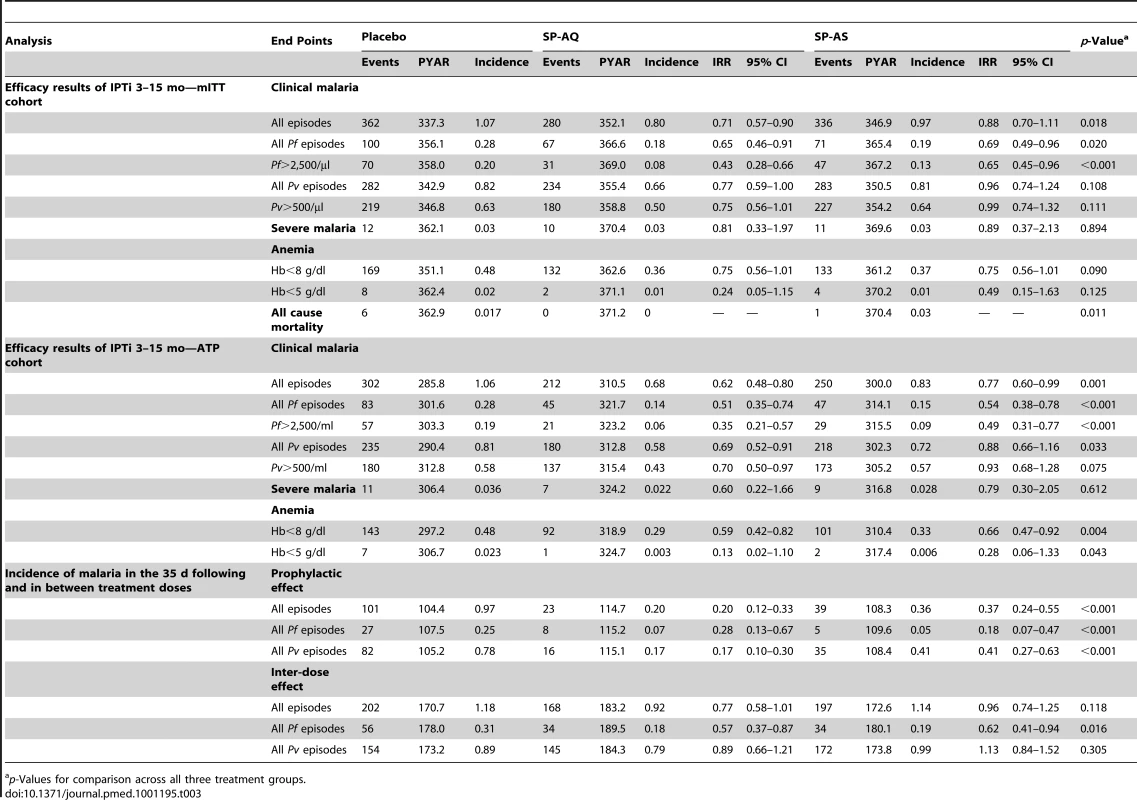
p-Values for comparison across all three treatment groups. Estimates of protection were higher when only children with three or more IPTi treatments were considered (i.e., ATP analyses; Table 3). Regular administration of SP-AQ reduced malaria episodes by 38% overall (95% CI, 20–52, p<0.001), with reductions of 49% (95% CI, 26–65, p<0.001) and 31% (95% CI, 9–48, p = 0.010) for Pf and Pv malaria episodes, respectively. Administration of SP-AS resulted in a 23% reduction in all malaria episodes (95% CI, 1–40, p = 0.041), with a statistically significant reduction for Pf (46% lower, 95% CI, 22–62, p = 0.001) but not for Pv (point estimate for change 12%, 95% CI, −16 to 34, p = 0.355).
Comparable effects were seen when more specific definitions for Pf (i.e., parasite density >2,500/µl) and Pv (i.e., parasite density >500/µl) [33] were applied (Table 3). In the ATP cohort, in the SP-AQ arm only, a substantially higher protection was observed against higher density Pf (PE = 65%, 95% CI, 43–79, p<0.001) but not against higher density Pv. The results for the mITT cohort are comparable.
IPTi with SP-AQ or SP-AS also provided protection against moderate-to-severe anemia (Hb<8 g/dl; Table 3). In the ATP population, the PE increased to 41% (95% CI, 18–58, p = 0.002) in the SP-AQ group and 34% (95% CI, 8–53, p = 0.013) in the SP-AS group. Few cases of severe anemia (<5 g/dl) were observed during the study. In the ATP analysis, there were seven cases in the placebo group, one in the SP-AQ group, and two in the SP-AS group (p = 0.04). Results for the mITT population were of similar magnitude, although in general the estimates obtained were lower and not statistically significant (p = 0.09–0.13) (see Table 3).
Adjustment for gender, village of residency, season of recruitment, and use of bed nets did not significantly alter estimates of PE (Figure 2).
Fig. 2. Summary of IPTi preventive efficacy against malaria at 15 mo of age adjusted for sex, place of residence, season of enrollment, and average insecticide-treated net use. 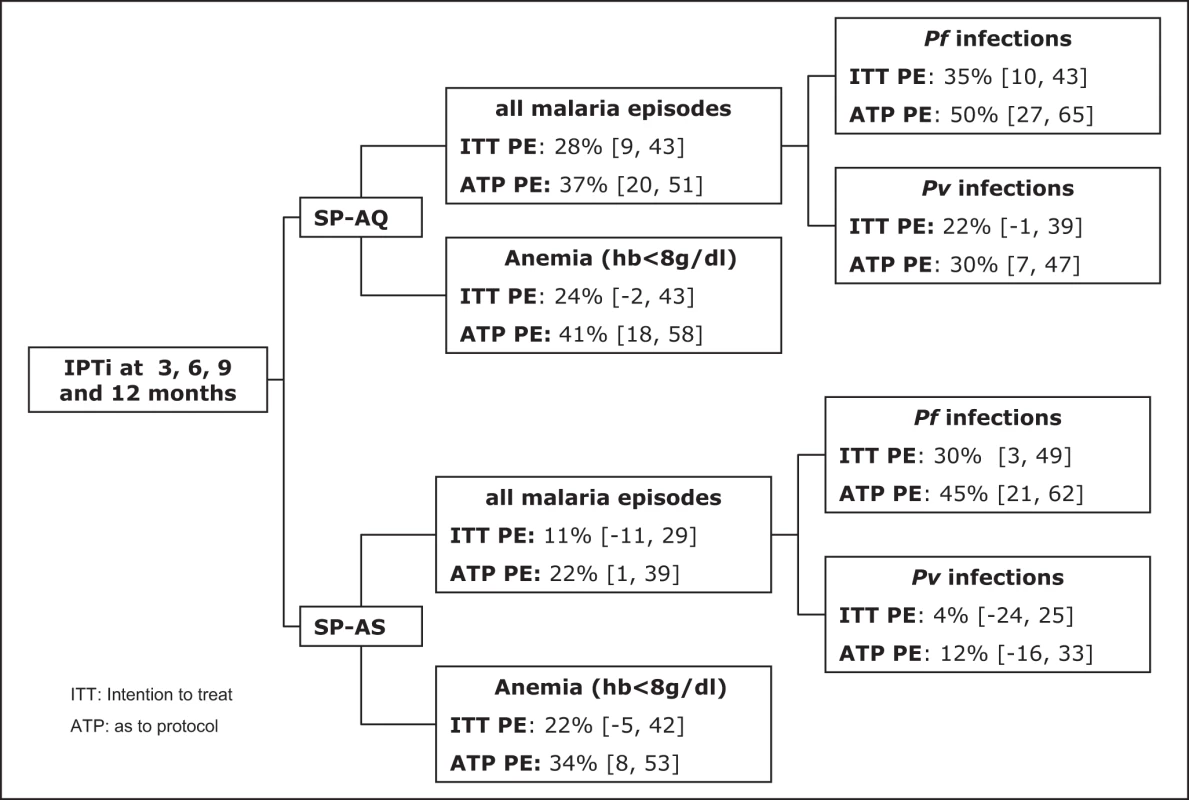
Post - and Inter-Dose Efficacy
The combined PE in the 35 d following the administration of each IPTi treatment dose showed a reduction of 80% (95% CI, 67–88, p<0.001) with SP-AQ and 63% (95% CI, 45–76, p<0.001) with SP-AS for all malaria infection (Table 3). SP-AQ had slightly lower PE against Pf (72%, 95% CI, 33–87, p<0.001) than against Pv (83%, 95% CI, 70–90, p<0.001), whereas SP-AS was more efficacious against Pf (82%, 95% CI, 53–93, p<0.001) than against Pv (59%, 95% CI, 47–73, p<0.001). Both SP-AQ (PE = 43%, 95% CI, 13–63, p = 0.010) and SP-AS (PE = 38%, 95% CI, 6–59, p = 0.026) were associated with a significant reduction in the incidence of Pf malaria between IPTi doses (i.e., the period from 35 d after a treatment dose to the next IPTi dose). However, no effect on the incidence of Pv malaria was observed for either treatment group beyond the first 35 d (Table 3).
Safety
A total of 6,165 AEs and 275 SAEs were observed during the intervention period, with no significant difference between treatment arms (Table 4). None of the SAEs were judged (by the Data Safety Monitoring Board and IPTi Safety Working Group) to be intervention-related. One child in the placebo group experienced a non-severe (grade 1) skin rash a few days following administration of the second IPTi dose. Vomiting was reported in 2% of infants receiving SP-AQ, 1.4% of infants receiving SP-AS, and 0.6% of infants receiving placebo (p = 0.001).
Tab. 4. Safety of IPTi with SP-AQ and SP-AS: adverse events and effect of incidence of malaria and anemia. 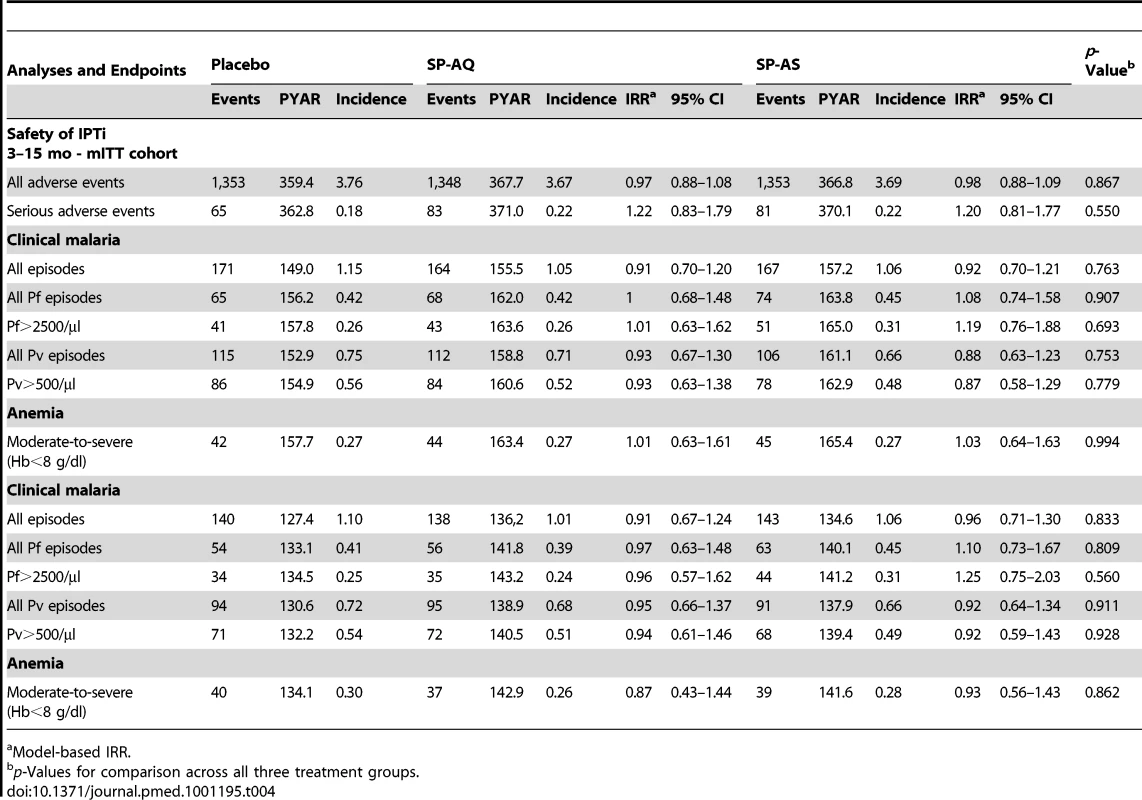
Model-based IRR. In total, ten deaths occurred among study participants. One was excluded from the analysis because he/she did not receive any IPTi treatment dose. Out of the nine study deaths, seven occurred during the intervention period (i.e., 3–15 mo of age). Of these, six occurred in the placebo group, one in the SP-AS group, and none in the SP-AQ group (p = 0.011). An additional two deaths (one each in the SP-AS and placebo groups) were observed during post-intervention follow-up. The likely diagnoses for the nine deaths were as follows: one severe Pv malaria (parasite density of 12,200/µl) with severe anemia and possible lower respiratory tract infection (severe respiratory distress and cough), three lower respiratory tract infections, two severe dehydrations, one meningitis, and two unknown diagnoses.
Post-Intervention Risk
The incidence of clinical malaria between 15 and 21 mo was 1.09 PYAR overall and 0.43 and 0.70 for Pf and Pv, respectively. No evidence of a “rebound effect” was observed in the 6 mo following the end of the intervention at the age of 15 mo. In both mITT and ATP populations, no significant difference in incidence of all malaria episodes was observed between treatment and intervention groups (Table 4; p = 0.56). Similarly, no significant differences in incidence of Pf malaria, Pv malaria, and anemia were observed.
Prevalence of Parasitemia during Follow-Up
The prevalence rates of both Pf and Pv increased from 1.5% and 8.5% at 6 mo to 3.0% and 15.8% at 15 mo and 4.4% and 20.3% at 21 mo, respectively, when detected by microscopy (Figure 3). Using molecular diagnosis, prevalence of Pf and Pv was higher, and also increased with age (Figure 3). At 15 mo, the prevalence of Pf was significantly lower in the two treatment arms (p = 0.037) by light microscopy but not by LDR-FMA. No significant differences in prevalence were observed between treatment arms at 21 mo or any other time point for either Pf or Pv. Infections with P. malariae and P. ovale were rare by microscopy (overall prevalence P. malariae: 0.1%, P. ovale: 0.0%) and LDR-FMA (P. malariae: 1.0%, P. ovale: 0.4%).
Fig. 3. Prevalence of P. falciparum and P. vivax infections during follow-up by treatment group (light microscopy) and overall (PCR). 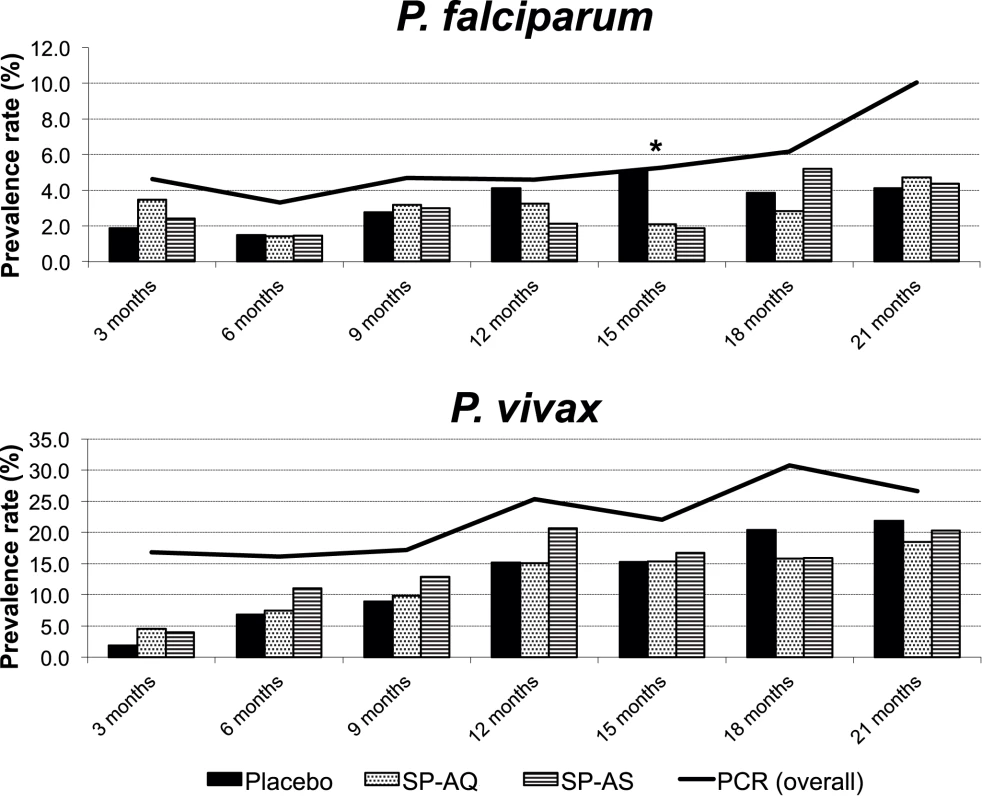
* p<0.05. Discussion
This study in PNG infants provides the first evidence of which we are aware for the efficacy of IPTi with SP-based regimens for the prevention of malaria and anemia in a non-African population, including data on the efficacy against Pv malaria. IPTi with a combination of SP plus 3 d of AQ resulted in a 29% decrease in all malaria episodes and a 25% decrease in episodes of moderate-to-severe anemia. PE was higher in children who received at least three doses of IPTi (38% for all malaria and 41% for anemia). In line with local resistance patterns and SP-AS pharmacokinetic properties, SP-AS was found to be inferior to SP-AQ. Overall, the efficacy of IPTi with SP-AQ in PNG infants is comparable to the 30% efficacy observed in other studies of African infants receiving IPTi with SP [10]. Importantly, SP-AQ has significant efficacy against both Pf and Pv malaria. Both IPTi treatments were safe and well tolerated. Therefore, these findings provide essential proof-of-principle evidence for the safety and efficacy of IPTi outside Africa, which has important implications for public health policy in non-African settings.
Prevention of Malaria
Overall, IPTi with SP-AQ was more effective in preventing Pf than Pv malaria (mITT 35% versus 23%, ATP 49% versuss 31%), and was more effective than SP-AS for Pf and Pv malaria. This was not unexpected. Two recent African clinical trials of IPTi have investigated alternative drug regimens, including at least one long half-life antimalarial such as mefloquine (125 mg single dose), a combination of SP (single dose) plus 3 d of AS, or AQ plus AS. These treatments had protective efficacies ranging from 26% to 38% [16],[34] and were comparable to those in earlier SP trials. However, a shorter acting combination, dapsone/chlorproguanil failed to significantly reduce the burden of malaria or anemia [16],[34]. Similarly, in the earlier IPTi SP trials, the preventative effect of IPTi SP was almost entirely due to a reduction of malaria in the first 5 wk after treatment [10],[35]. Together, these results suggest that IPTi works mainly through a prophylactic effect, achieved by long-acting drugs preventing new infections, rather than by eradicating existing parasitemia [35]. Consistent with the African results, in the present study both SP-containing regimens had similarly high protective effect against Pf for the first 35 d (72%–82%) as well as a moderate inter-dose effect, resulting in an overall efficacy of IPTi against Pf that was towards the higher end of those found in African studies [10],[16],[34].
SP-AQ also prevented 83% of Pv episodes in the first 35 d following treatment, indicating a similar post-treatment prophylactic effect, but the overall efficacy of IPTi against Pv malaria was lower than for Pf. SP-AS, however, showed significantly less post-treatment prophylactic effect (PE = 59%) and no effect against Pv malaria overall. The most probable reasons for this are the ability of Pv to relapse from long-lasting liver stages [36] and higher levels of SP resistance in Pv. Pv strains from New Guinea are thought to relapse very frequently [37], and Pv blood-stage infections are therefore re-established soon after drug treatment. In a concurrently conducted drug trial, 49% of PNG children 0.5–5 y treated with SP-chloroquine (3 d) for Pv malaria had recurrent Pv parasitemia, and an additional 18% had a recurrent Pv clinical episode by day 42, while 26% and 6% of children treated for Pf malaria had recurrent Pf parasitemia and clinical episodes, respectively [38].
The differential efficacy of IPTi with SP-AQ and SP-AS for the prevention of Pf and Pv malaria is consistent with the pharmacokinetic properties of the drugs used and previously observed drug resistance patterns. PCR-corrected day 28 in vivo failure rates from studies conducted concurrently or just prior to the trial were 10.3% for SP-AQ [39] and 10.0% for SP-AS [38] for Pf, and 3.6% for SP-AQ [40] and 11.8% for SP-AS [41] for Pv. As SP has never been used as monotherapy in PNG, there are no estimates of SP in vivo resistance for either Pf or Pv. However, molecular markers of SP resistance have been analyzed for both species in the study population. In Pf, the quintuple Pf dihydrofolate reductase (pfdhfr) 51–59–108/Pf dihydropteroate synthase (pfdhps) 436–547 mutant genotype is associated with a high degree of drug resistance [42], while in Pv, the 57L-58R-61M-117T pvdhfr quadruple mutation (irrespective of pvdhps mutations) has been associated with increased risk of treatment failure [43],[44]. The high frequency of the quadruple pvdhfr mutation in PNG (52% [41]) suggests that the clinical efficacy of SP for Pv may be significantly compromised. In contrast, the absence of quadruple/quintuple pfdhfr/pfdhps [45] suggests that SP retains substantial efficacy against Pf.
Although chloroquine-resistant Pv was first described in PNG [46], there is a significantly higher level of 4-aminoquinoline resistance present in Pf than in Pv. By the early 1990s, greater than 35% of Pf cases showed RII and RIII resistance [47], and mutations underlying chloroquine and AQ resistance were shown to be highly prevalent [48]. In 2003/2004, SP-AQ was 100% clinically effective, but recurrent Pv parasitemia was observed in 12% of children treated with SP-AQ [39]. The pvdhfr 57L-58R-61M-117T/Pv multidrug resistance 1 (pvmdr1) 976F quintuple mutant genotype has been associated with parasitological failure [43]. Although the pvmdr1 976F mutation was present in ∼70% of Pv isolates tested in 2006/2007, the predominantly late occurrence of recurrent parasitemia (i.e., day 28–42) following treatment with SP-chloroquine indicates that 4-aminoquinolines retained good clinical and at least partial parasitological efficacy [41].
Given the high level of AQ resistance and the very short half-life of AS [49], the efficacy of IPTi for the prevention of Pf is likely to be largely due to the effect of SP. The high level of preventative efficacy for 35 d is consistent with the terminal elimination half-life (t1/2β) of 15.6 and 9.1 d for pyrimethamine and sulfadoxine, respectively, in PNG infants [50]. With SP having a greatly reduced efficacy against Pv, the success of IPTi against Pv malaria relies largely on the efficacy of the partner drug. Whereas Pv parasites are efficiently cleared by SP-AS, the short half-life of AS provides no post-treatment prophylactic effect, and recurrent Pv infections are very common [38]. AQ on the other hand has a half-life of ∼9 d [51], and a 3-d course should therefore effectively suppress sensitive parasites effectively for 4–5 wk. The lack of any protective effect of SP-AS against Pv but not Pf adds further evidence to the observation that while SP can retain a considerable effect at low-to-moderate levels of resistance [14], it loses its prophylactic activity at high levels of resistance [16].
Prevention of Anemia, Severe Illness, and Death
Besides reducing the burden of malaria episodes, IPTi also reduced the risk of moderate-to-severe anemia (25% in the mITT and 34%–41% in the ATP analyses, respectively) and severe anemia (51%–87%). These effects are in line with previous findings on the prevention of anemia in Africa [10].
No difference was observed between the groups in number of SAEs (i.e., all cause admissions plus severe illness). Surprisingly, a significantly lower death rate was observed in the two treatment arms compared to the placebo arm. Even though this finding is very encouraging, it must be interpreted with caution. The present study was not powered to observe an effect of IPTi on mortality, and in the meta-analysis of six African studies, no effect of IPTi with SP on mortality was detected [10]. Only seven deaths occurred during the intervention period, with most children dying at home. Based on verbal autopsies and available clinical records, a majority of children might have died of severe lower respiratory tract infections and not from malaria.
Safety of SP-AQ and SP-AS
IPTi with SP-AQ or SP-AS was very safe. More than 4,000 doses of SP and 6,000 doses of AQ and AS were given, and no study-drug-related SAEs were observed, while AEs such as vomiting were rare in all groups. As reported in earlier studies, no significant increase in risk of malaria or anemia was recorded in the 6 mo after completion of the 12 mo of IPTi, indicating that IPTi does not impair the acquisition of immunity to both Pf and Pv and lead to a rebound in malaria risk. These data are very reassuring in regard to a possible use of these drug combinations in PNG and add to the safety data from African IPTi studies [10].
The use of SP as prophylaxis has been criticized because of the potential risk of Stevens-Johnson syndrome, a severe skin reaction [52], but by now >15,000 children have been treated with SP-containing IPTi regimens in well-controlled clinical trials, and not a single drug-related severe dermatological event has been observed [10],[16],[34],[53]. On the other hand, IPTi with mefloquine was highly effective, but was not well tolerated, with high rates of vomiting [16]. In the absence of novel drug regimens for IPTi, the combination of SP and AQ is the best currently available option for IPTi in PNG.
Adherence, Acceptability, and Implementation
The size of the difference in IPTi efficacy between the ATP and mITT analyses indicates that if the intervention is implemented, it will be important to achieve high coverage and high adherence in order to achieve good effectiveness.
In both the SP-AQ and SP-AS groups only the first dose was given as directly observed treatment. Parents were then counseled on the importance of giving the remaining two doses at home. With this approach a very high rate of compliance (>90%) was achieved. As a result, SP and AQ day 4 drug levels were found to be comparable (N. Senn, P. Rarau, and I. Mueller, unpublished data) to those found in pharmacological studies with directly observed drug administration [50],[54]. Acceptability of IPTi by parents and health workers of IPTi was excellent, and the administration of IPTi alongside EPI did not have negative impacts on attitudes to EPI, EPI adherence, or existing malaria prevention practices [55]. Therefore, and consistent with what was described in Africa [53], the IPTi intervention seems to be well suited for implementation in community settings in PNG, with high likelihood of both acceptability and adherence.
Conclusion
This study provides evidence of the efficacy of IPTi for the prevention of malaria and anemia in a region highly endemic for both Pf and Pv. Additionally, this study provides an essential proof of principle that IPTi is an appropriate strategy for the prevention of Pv malaria, if an effective, long half-life drug is used. Policy makers should therefore consider IPTi in areas outside Africa or in countries in the Horn of Africa, where the burden of Pv malaria is high. Which areas are suited for IPTi introduction will depend on transmission level and choice of drug, both of which will affect the cost-effectiveness of the intervention [56]. While the results from Africa indicate that IPTi is a highly cost-effective intervention even at moderate levels of transmission [57], a formal evaluation of IPTi cost-effectiveness in non-African settings will be required to assist policy makers. Given the levels of resistance to SP and AQ in many parts of the Asia-Pacific and Americas, further studies are needed to investigate other combinations of long-acting drugs with a better efficacy against Pv, in particular.
In the PNG context, the combination of SP-AQ (two long-acting drugs with well-matched half-lives and good activity against either Pf [SP] or Pv [AQ]) is an appropriate drug choice for IPTi, and its introduction into the national standard treatment guidelines should be considered. The replacement of AQ and SP by artemether-lumefantrine as the national first line treatment will reduce the selection pressure for resistance against AQ and SP. Therefore, the efficacy of the two drugs in IPTi may be retained over time. Appropriate monitoring of prophylactic efficacy of AQ and SP, as well as evaluating new regimens, such as dihydroartemisinin-piperaquine, should accompany IPTi introduction in PNG.
Supporting Information
Zdroje
1. World Health Organization 2010 Guidelines for the treatment of malaria Geneva World Health Organization
2. LengelerC 2004 Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev 2004 CD000363
3. PluessBTanserFCLengelerCSharpBL 2010 Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev 2010 CD006657
4. SinclairDZaniBDoneganSOlliaroPGarnerP 2009 Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev 2009 CD007483
5. GreenwoodB 2004 The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. Am J Trop Med Hyg 70 1 7
6. GoslingRCairnsMChicoRChandramohanD 2010 May Intermittent preventive treatment against malaria: an update. Expert Rev Anti Infect Ther 8 589 606
7. MenendezCD'AlessandroUter KuileFO 2007 Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infect Dis 7 126 135
8. CrawleyJHillJYarteyJRobaloMSerufiliraA 2007 From evidence to action? Challenges to policy change and programme delivery for malaria in pregnancy. Lancet Infect Dis 7 145 155
9. GarnerPGülmezogluA 2006 Drugs for preventing malaria-related illness in pregnant women. Cochrane Database Syst Rev 2006 CD000169
10. AponteJSchellenbergDEganABreckenridgeACarneiroI 2009 Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet 374 1533 1542
11. MeremikwuMMDoneganSEsuE 2008 Chemoprophylaxis and intermittent treatment for preventing malaria in children. Cochrane Database Syst Rev 2008 CD003756
12. Institute of Medicine of the National Academies 2008 Assessment of the role of intermittent preventive treatment for malaria in infants: letter report Washington (District of Columbia) Institute of Medicine of the National Academies
13. WilsonAL on behalf of the IPTc Taskforce 2011 A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc). PLoS ONE 6 e16976 doi:10.1371/journal.pone.0016976
14. GriffinJTCairnsMGhaniACRoperCSchellenbergD 2010 Protective efficacy of intermittent preventive treatment of malaria in infants (IPTi) using sulfadoxine-pyrimethamine and parasite resistance. PLoS ONE 5 e12618 doi:10.1371/journal.pone.0012618
15. MayorASerra-CasasESanzSAponteJJMaceteE 2008 Molecular markers of resistance to sulfadoxine-pyrimethamine during intermittent preventive treatment for malaria in Mozambican infants. J Infect Dis 197 1737 1742
16. GoslingRDGesaseSMoshaJFCarneiroIHashimR 2009 Protective efficacy and safety of three antimalarial regimens for intermittent preventive treatment for malaria in infants: a randomised, double-blind, placebo-controlled trial. Lancet 374 1521 1532
17. GesaseSGoslingRDHashimROrdRNaidooI 2009 High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at codon 581. PLoS ONE 4 e4569 doi:10.1371/journal.pone.0004569
18. World Health Organization 2010 WHO policy recommendation on intermittent preventive treatment during infancy with sulphadoxine-pyrimethamine (SP-IPTi) for Plasmodium falciparum malaria control in Africa Geneva World Health Organization
19. RosenbergR 2007 Plasmodium vivax in Africa: hidden in plain sight? Trends Parasitol 23 193 196
20. GentonBD'AcremontVRareLBaeaKReederJC 2008 Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med 5 e127 doi:10.1371/journal.pmed.0050127
21. LinEKiniboroBGrayLDobbieSRobinsonL 2010 Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS ONE 5 e9047 doi:10.1371/journal.pone.0009047
22. TjitraEAnsteyNMSugiartoPWarikarNKenangalemE 2008 Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med 5 e128 doi:10.1371/journal.pmed.0050128
23. AlexandreMAFerreiraCOSiqueiraAMMagalhaesBLMouraoMP 2010 Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis 16 1611 1614
24. LautzeJMcCartneyMKrishenPOlanaDJayasingheG 2007 Effect of a large dam on malaria risk: the Koka reservoir in Ethiopia. Trop Med Int Health 12 982 989
25. MuellerIGalinskiMRBairdJKCarltonJMKocharDK 2009 Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis 9 555 566
26. HawkinsVNSuzukiSMRungsihirunratKHapuarachchiHCMaestreA 2009 Assessment of the origins and spread of putative resistance-conferring mutations in Plasmodium vivax dihydropteroate synthase. Am J Trop Med Hyg 81 348 355
27. CattaniJA 1992 The epidemiology of malaria in Papua New Guinea. AttenboroughRAlpersM Human biology in Papua New Guinea: the small cosmos Oxford Clarendon Press 302 312
28. SchultzLWaplingJMuellerINtsukePOSennN 2010 Multilocus haplotypes reveal variable levels of diversity and population structure of Plasmodium falciparum in Papua New Guinea, a region of intense perennial transmission. Malar J 9 336
29. SchellenbergDCisseBMenendezC 2006 The IPTi Consortium: research for policy and action. Trends Parasitol 22 296 300
30. ReedRBSuartHC 1959 Patterns of growth in height and weight from birth to eighteen years of age. Pediatrics 24 904 921
31. McNamaraDTThomsonJMKasehagenLJZimmermanPA 2004 Development of a multiplex PCR-ligase detection reaction assay for diagnosis of infection by four human malaria parasite species. J Clin Microbol 42 2403 2410
32. KasehagenLJMuellerIMcNamaraDTBockarieMJKiniboroB 2006 Changing patterns of Plasmodium blood-stage infections in the Wosera region of Papua New Guinea monitored by light microscopy and high throughput PCR diagnosis. Am J Trop Med Hyg 75 588 596
33. MullerIGentonBRareLKiniboroBKastensW 2009 Three different Plasmodium species show similar patterns of clinical tolerance of malaria infection. Malar J 8 158
34. OdhiamboFOHamelMJWilliamsonJLindbladeKter KuileFO 2010 Intermittent preventive treatment in infants for the prevention of malaria in rural western Kenya: a randomized, double-blind placebo-controlled trial. PLoS ONE 5 e10016 doi:10.1371/journal.pone.0010016
35. MayJAdjeiSBuschWGaborJIssifouS 2008 Therapeutic and prophylactic effect of intermittent preventive anti-malarial treatment in infants (IPTi) from Ghana and Gabon. Malar J 7 198
36. KrotoskiWA 1989 The hypnozoite and malarial relapse. Prog Clin Parasitol 1 1 19
37. CraigeBAlvingASJonesRMerrill WhortonCPullmanTN 1947 The Chesson strain of Plasmodium vivax malaria: II. Relationship between prepatent period, latent period and relapse rate. J Infect Dis 80 228 236
38. KarunajeewaHAMuellerISennMLinELawI 2008 A trial of combination antimalarial therapies in children from Papua New Guinea. N Engl J Med 359 2545 2557
39. MarfurtJMuellerISieAMakuPGorotiM 2007 Low efficacy of amodiaquine or chloroquine plus sulfadoxine-pyrimethamine against Plasmodium falciparum and P. vivax malaria in Papua New Guinea. Am J Trop Med Hyg 77 947 954
40. KoepfliCMuellerIMarfurtJGorotiMSieA 2009 Evaluation of Plasmodium vivax genotyping markers for molecular monitoring in clinical trials. J Infect Dis 199 1074 1080
41. BarnadasCKoepfliCKarunajeewaHASibaPMDavisTME 2011 Characterization of treatment failure in efficacy trials of drugs against Plasmodium vivax by genotyping neutral and drug resistance-associated markers. Antimicrob Agents Chemother 55 4479 4481
42. PicotSOlliaroPde MonbrisonFBienvenuAPriceRN 2009 A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malaria J 8 89
43. MarfurtJde MonbrisonFBregaSBarbollatLMullerI 2008 Molecular markers of in vivo Plasmodium vivax resistance to amodiaquine plus sulfadoxine-pyrimethamine: mutations in pvdhfr and pvmdr1. J Infect Dis 198 409 417
44. HawkinsVNJoshiHRungsihirunratKNa-BangchangKSibleyCH 2007 Antifolates can have a role in the treatment of Plasmodium vivax. Trends Parasitol 23 213 222
45. WongRPKarunajeewaHMuellerISibaPZimmermanPA 2011 Molecular assessment of Plasmodium falciparum resistance to antimalarial drugs in Papua New Guinea using an extended ligase detection reaction fluorescent microsphere assay. Antimicrob Agents Chemother 55 798 805
46. RieckmannKHDavisDRHuttonDC 1989 Plasmodium vivax resistance to chloroquine? Lancet 2 1183 1184
47. MullerIBockarieMAlpersMSmithT 2003 The epidemiology of malaria in Papua New Guinea. Trends Parasitol 19 253 259
48. MehlotraRKFujiokaHRoepePDJannehOUrsosLM 2001 Evolution of a unique Plasmodium falciparum chloroquine-resistance phenotype in association with pfcrt polymorphism in Papua New Guinea and South America. Proc Natl Acad Sci U S A 98 12689 12694
49. KarunajeewaHAIlettKFDufallKKemikiABockarieM 2004 Disposition of artesunate and dihydroartemisinin after administration of artesunate suppositories in children from Papua New Guinea with uncomplicated malaria. Antimicrob Agents Chemother 48 2966 2972
50. SalmanSKoseKGriffinSBaiwogFWinmaiJ 2011 The pharmacokinetic properties of standard and double-dose sulfadoxine-pyrimethamine in infants. Antimicrob Agents Chemother 55 1693 1700
51. StepniewskaKTaylorWSirimaSBOuedraogoEBOuedraogoA 2009 Population pharmacokinetics of artesunate and amodiaquine in African children. Malar J 8 200
52. WhiteNJ 2005 Intermittent presumptive treatment for malaria. PLoS Med 2 e3 doi:10.1371/journal.pmed.0020003
53. Armstrong SchellenbergJRMShirimaKMaokolaWManziFMrishoM 2010 Community effectiveness of intermittent preventive treatment for infants (IPTi) in rural southern Tanzania. Am J Trop Med Hyg 82 772 781
54. MwesigwaJParikhSMcGeeBGermanPDrysdaleT 2010 Pharmacokinetics of artemether-lumefantrine and artesunate-amodiaquine in children in Kampala, Uganda. Antimicrob Agents Chemother 54 52 59
55. PellCStrausLPhuanukoonnonSLupiwaSMuellerI 2010 Community response to intermittent preventive treatment of malaria in infants (IPTi) in Papua New Guinea. Malar J 9 369
56. RossAMaireNSicuriESmithTContehL 2011 Determinants of the cost-effectiveness of intermittent preventive treatment for malaria in infants and children. PLoS ONE 6 e18391 doi:10.1371/journal.pone.0018391
57. ContehLSicuriEManziFHuttonGObonyoB 2010 The cost-effectiveness of intermittent preventive treatment for malaria in infants in sub-Saharan Africa. PLoS ONE 5 e10313 doi:10.1371/journal.pone.0010313
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 3- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- No Treatment versus 24 or 60 Weeks of Antiretroviral Treatment during Primary HIV Infection: The Randomized Primo-SHM Trial
- Care Seeking for Neonatal Illness in Low- and Middle-Income Countries: A Systematic Review
- Guidance for Evidence-Informed Policies about Health Systems: Rationale for and Challenges of Guidance Development
- Improving Ethical Review of Research Involving Incentives for Health Promotion
- To VBAC or Not to VBAC
- Impact of Scotland's Smoke-Free Legislation on Pregnancy Complications: Retrospective Cohort Study
- Injectable and Oral Contraceptive Use and Cancers of the Breast, Cervix, Ovary, and Endometrium in Black South African Women: Case–Control Study
- Intermittent Preventive Treatment for Malaria in Papua New Guinean Infants Exposed to and : A Randomized Controlled Trial
- CD4 Cell Count and the Risk of AIDS or Death in HIV-Infected Adults on Combination Antiretroviral Therapy with a Suppressed Viral Load: A Longitudinal Cohort Study from COHERE
- Publication Bias in Antipsychotic Trials: An Analysis of Efficacy Comparing the Published Literature to the US Food and Drug Administration Database
- Better Guidance Is Welcome, but without Blinders
- New Research on Childbirth Has the Potential to Empower Women's Decision Making, but More Is Needed
- Planned Vaginal Birth or Elective Repeat Caesarean: Patient Preference Restricted Cohort with Nested Randomised Trial
- A Comparison of -IV and -5 Panel Members' Financial Associations with Industry: A Pernicious Problem Persists
- Guidance for Evidence-Informed Policies about Health Systems: Linking Guidance Development to Policy Development
- Guidance for Evidence-Informed Policies about Health Systems: Assessing How Much Confidence to Place in the Research Evidence
- Uterine Rupture by Intended Mode of Delivery in the UK: A National Case-Control Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidance for Evidence-Informed Policies about Health Systems: Assessing How Much Confidence to Place in the Research Evidence
- Uterine Rupture by Intended Mode of Delivery in the UK: A National Case-Control Study
- Guidance for Evidence-Informed Policies about Health Systems: Linking Guidance Development to Policy Development
- Improving Ethical Review of Research Involving Incentives for Health Promotion
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



