-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
CD4 Cell Count and the Risk of AIDS or Death in HIV-Infected Adults on Combination Antiretroviral Therapy with a Suppressed Viral Load: A Longitudinal Cohort Study from COHERE
Background:
Most adults infected with HIV achieve viral suppression within a year of starting combination antiretroviral therapy (cART). It is important to understand the risk of AIDS events or death for patients with a suppressed viral load.Methods and Findings:
Using data from the Collaboration of Observational HIV Epidemiological Research Europe (2010 merger), we assessed the risk of a new AIDS-defining event or death in successfully treated patients. We accumulated episodes of viral suppression for each patient while on cART, each episode beginning with the second of two consecutive plasma viral load measurements <50 copies/µl and ending with either a measurement >500 copies/µl, the first of two consecutive measurements between 50–500 copies/µl, cART interruption or administrative censoring. We used stratified multivariate Cox models to estimate the association between time updated CD4 cell count and a new AIDS event or death or death alone. 75,336 patients contributed 104,265 suppression episodes and were suppressed while on cART for a median 2.7 years. The mortality rate was 4.8 per 1,000 years of viral suppression. A higher CD4 cell count was always associated with a reduced risk of a new AIDS event or death; with a hazard ratio per 100 cells/µl (95% CI) of: 0.35 (0.30–0.40) for counts <200 cells/µl, 0.81 (0.71–0.92) for counts 200 to <350 cells/µl, 0.74 (0.66–0.83) for counts 350 to <500 cells/µl, and 0.96 (0.92–0.99) for counts ≥500 cells/µl. A higher CD4 cell count became even more beneficial over time for patients with CD4 cell counts <200 cells/µl.Conclusions:
Despite the low mortality rate, the risk of a new AIDS event or death follows a CD4 cell count gradient in patients with viral suppression. A higher CD4 cell count was associated with the greatest benefit for patients with a CD4 cell count <200 cells/µl but still some slight benefit for those with a CD4 cell count ≥500 cells/µl.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 9(3): e32767. doi:10.1371/journal.pmed.1001194
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001194Summary
Background:
Most adults infected with HIV achieve viral suppression within a year of starting combination antiretroviral therapy (cART). It is important to understand the risk of AIDS events or death for patients with a suppressed viral load.Methods and Findings:
Using data from the Collaboration of Observational HIV Epidemiological Research Europe (2010 merger), we assessed the risk of a new AIDS-defining event or death in successfully treated patients. We accumulated episodes of viral suppression for each patient while on cART, each episode beginning with the second of two consecutive plasma viral load measurements <50 copies/µl and ending with either a measurement >500 copies/µl, the first of two consecutive measurements between 50–500 copies/µl, cART interruption or administrative censoring. We used stratified multivariate Cox models to estimate the association between time updated CD4 cell count and a new AIDS event or death or death alone. 75,336 patients contributed 104,265 suppression episodes and were suppressed while on cART for a median 2.7 years. The mortality rate was 4.8 per 1,000 years of viral suppression. A higher CD4 cell count was always associated with a reduced risk of a new AIDS event or death; with a hazard ratio per 100 cells/µl (95% CI) of: 0.35 (0.30–0.40) for counts <200 cells/µl, 0.81 (0.71–0.92) for counts 200 to <350 cells/µl, 0.74 (0.66–0.83) for counts 350 to <500 cells/µl, and 0.96 (0.92–0.99) for counts ≥500 cells/µl. A higher CD4 cell count became even more beneficial over time for patients with CD4 cell counts <200 cells/µl.Conclusions:
Despite the low mortality rate, the risk of a new AIDS event or death follows a CD4 cell count gradient in patients with viral suppression. A higher CD4 cell count was associated with the greatest benefit for patients with a CD4 cell count <200 cells/µl but still some slight benefit for those with a CD4 cell count ≥500 cells/µl.
: Please see later in the article for the Editors' SummaryIntroduction
More than 90% of those infected with HIV now achieve viral suppression within a year of starting a combination antiretroviral therapy (cART) [1],[2]. Patients with a suppressed viral load now represent the majority of cART recipients. Previous cohort studies have shown that the CD4 cell count when starting cART is the most important prognostic factor for clinical outcome, but these studies have focused on cART-naïve patients and have ignored treatment changes and periods of detectable viral load [3],[4].
This study considers the prognostic value of a CD4 cell count, not when starting cART, but while a patient is being successfully treated, that is, while a patient is on cART with a suppressed viral load. For many patients, viral suppression is not continuous but episodic with periods of viremia as a result of treatment interruption or treatment failure. We selected patients from the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE) database, accumulated episodes of viral suppression for each patient while on cART, and used these episodes to estimate the association between a time updated CD4 cell count and progression to a new AIDS-defining event or death, or death alone.
Methods
The COHERE Collaboration
COHERE is a collaboration of European HIV cohorts (http://www.cohere.org/). The 22 cohorts participating in this project provided data in a standardised format to one of two regional co-ordinating centres, where basic error checks were carried out and duplicate records removed for patients followed in more than one cohort. Data collected included information on patient characteristics, antiretroviral therapy, CD4 cell count, HIV RNA viral load, AIDS events, and causes of death. This analysis was based on data merged in 2010 when, for the first time, additional data were collected on co-infection with and treatment for hepatitis B or C, and on the prophylaxis and treatment of opportunistic infections. Patients included in the 2010 merger had to have this additional information and follow-up after 1 January 1997.
Patient Inclusion
Patients were eligible for our analyses if they achieved one or more episodes of viral suppression while on cART. Optimal viral suppression is defined as a viral load below the level of detection or below 20–75 copies/µl depending on the assay used; however, isolated transient detectable viral loads below 400 copies/µl are not uncommon in successfully treated patients and are not thought to represent an increased risk of virologic failure [5]. We defined the start of a suppression episode as the second of two consecutive viral load measurements below 50 copies/µl (or below the limit of detection) while on cART. We defined the end of a suppression episode as a viral load measurement below 50 copies/µl (or undetectable) then followed by either (1) a measurement greater than 500 copies/µl, (2) the first of two consecutive measurements between 50 and 500 copies/µl, (3) an interruption in cART, or (4) no further viral load measurements. Note that our definition allows for isolated viral load measurements of between 50 and 500 copies/µl within a suppression episode. We defined cART as any three antiretroviral drugs from any drug class, except that three nucleoside (or nucleotide) reverse-transcriptase inhibitors (NRTIs) was only considered cART if taken after another cART regimen.
Patients with at least one suppression episode were then included in our time to event analyses if pre-specified covariates were also available. Patients had to have a CD4 cell count measured within 6 mo prior to the start of an episode or within an episode, and CD4 cell counts were updated over time in our analyses so that each episode was represented by a set of intervals, one interval per CD4 cell count, using the counting process method of representing time to event data. Patients with more than one suppression episode contributed more than one set of intervals to our analyses, but were not at risk between episodes (see [6]). We deleted any interval where the CD4 cell count was measured before the patient was 16 y old. Other covariates were age (in the year 2000), gender, intravenous drug use as the likely mode of HIV transmission, viral load, co-infection with hepatitis B or C, cART category, and the number of prior cART regimens, with these last three covariates updated for each interval. For a first suppression episode, we used a last viral load prior to starting cART as the viral load covariate; for a subsequent episode, we used the highest viral load between the previous and current episode as the covariate.
Statistical Methods
Our primary outcome was time to a first new AIDS event or death while suppressed and on cART, with an AIDS event defined as one of the conditions listed in Appendix B of the 1993 US Centers for Disease Control (CDC) AIDS surveillance case definition [7]. We used Cox proportional hazards models to estimate the association between an AIDS event or death and CD4 cell count, with CD4 cell count represented by a linear spline with three knots at 200, 350, and 500 cells/µl [8]. These knots correspond to thresholds in treatment guidelines below; below these three thresholds, antiretroviral treatment is essential, recommended, or should be considered, respectively [5]. A hazard ratio (HR) <1.0 for any of the four components of this spline implies that a higher CD4 cell count (per 100 cells/µl) is associated with a lower risk of progression and is therefore a measure of the benefit that a patient can expect if their CD4 cell count increases above any current level within the range covered by that spline component. Our models included the baseline and time updated covariates described above. We stratified our models by cohort, so that each cohort had its own non-parametric baseline hazard function, but we assumed the effect of each covariate was the same in each cohort [9]. To assess whether the hazards associated with CD4 cell count were constant over time (i.e., proportional hazards), we fitted a model with interaction terms between log suppression time and CD4 cell count, with these interactions centred around the geometric mean suppression time [10]–[12].
We carried out six planned sensitivity analyses to check that our estimates were stable. Assays have become more sensitive over time, so we re-fitted our model with (1) a suppression episode re-defined as a viral load below 400 copies/µl—to simulate constant use over time of a less sensitive assay; and (2) with the analysis restricted to suppression episodes starting after 1 January 2001—to largely omit episodes found using less sensitive assays [13]. We varied the period of time after a last viral load measurement within which new AIDS events or death were accepted as outcomes if suppression was ongoing. We considered such events as outcomes if they occurred within 180 d of a last viral load where the patient was still suppressed at this last measurement, but in sensitivity analyses we re-fitted our model assuming (3) shorter and (4) longer periods (90 and 270 d, respectively). We dropped covariates from our model to retain episodes lost from our analyses because of missing covariates. We re-fitted our model (5) without viral load as a covariate because for many patients, we did not have a viral load measured prior to starting cART; and (6) without co-infection with hepatitis as a covariate, because then we could include additional patients in our analysis from the 2008 merger of the COHERE database [14]. Finally in a single unplanned sensitivity analysis, we assessed whether the risk of progression differed between first and subsequent episodes of viral suppression. We added an additional covariate to the analysis of the primary outcome, either taking value zero for a first suppression episode and one otherwise, or taking value zero for a first suppression episode and the number of years between successive episodes otherwise.
Our secondary outcome was time to death while both suppressed and on cART. We classified a death as “related to HIV” if death was attributed at least in part to an “AIDS defining event” or an “invasive bacterial infection.” If these two causes were not mentioned but other causes of death were given, we classified a death as “unrelated to HIV.” If no causes of death were given, we classified a death as of “unknown cause.” We then fitted a Cox model with different cause-specific hazards for CD4 cell count [15], again with CD4 cell count represented by a linear spline and with the same covariates as before.
Analyses were carried out with the PHREG procedure in SAS version 9.2; survival curves were plotted with the Survival package version 2.36-2 in R version 2.12.1. We report model estimates as HRs, each with a 95% CI.
Results
Patient Characteristics
Of the 176,585 patients in the 2010 merger of COHERE, 75,336 patients provided 104,265 suppression episodes while on cART (); 71% of these patients had just a single episode. The median length of a suppression episode was 1.7 y (interquartile range [IQR] 0.7–3.5); the median total time suppressed while on cART was 2.7 y (IQR 1.2–5.1) per patient; the estimated average gain in CD4 cell count while suppressed was 53 cells/µl per year. The main analyses of primary and secondary outcomes were based on 66,147 patients with a viral load measured prior to starting cART. Patients contributing to our main analyses tended to be slightly older, were less likely to be either female or infected through drug use, and were more likely to be recorded as of European origin than other patients in this merger of COHERE (Table 1). Few patients (1%) started their first suppression episode with a CD4 cell count below 50 cells/µl and many (34%) started with a CD4 cell count above 500 cells/µl.
Tab. 1. COHERE patients with continuous or episodic viral suppression while on cART. 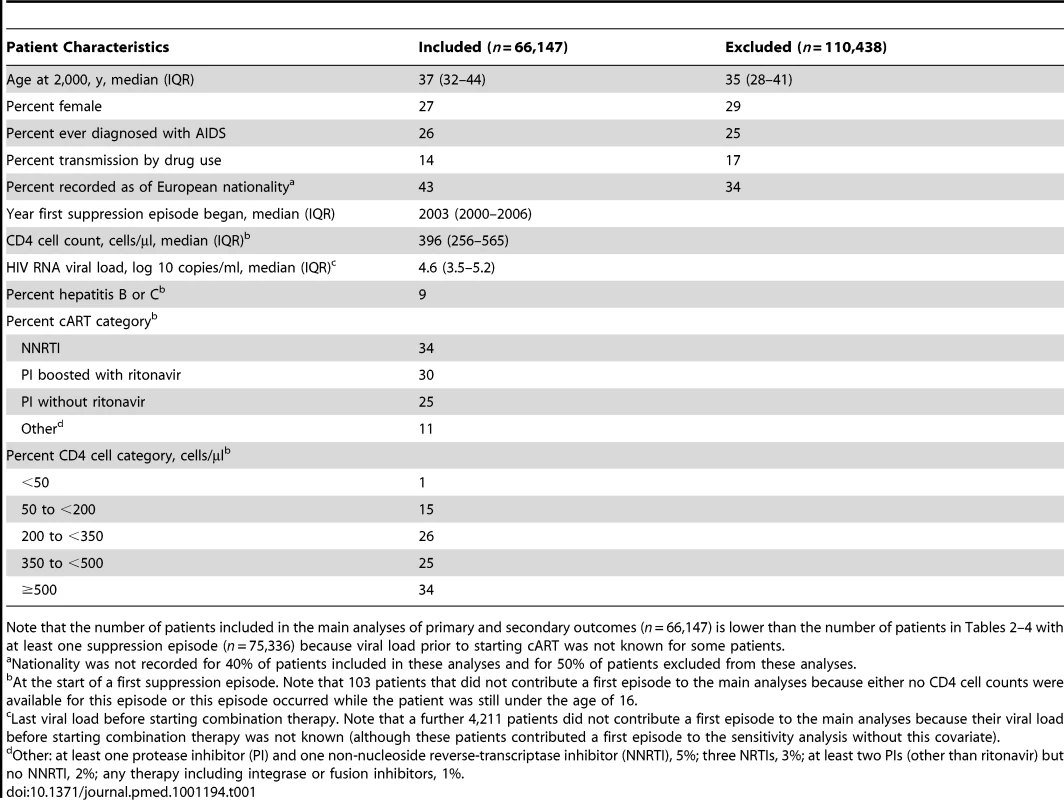
Note that the number of patients included in the main analyses of primary and secondary outcomes (n = 66,147) is lower than the number of patients in Tables 2–4 with at least one suppression episode (n = 75,336) because viral load prior to starting cART was not known for some patients. Event Rates
The rate of progression to a first new AIDS event or death was 8.9 per 1,000 y of suppression; the mortality rate was 4.8 per 1,000 y of suppression. Both rates showed a gradient that depends on CD4 cell count with the highest rates in those with <50 CD4 cells/µl at the time of the event (Table 2). Even mortality from causes thought unrelated to HIV (Table 2) and the rate of HIV related neoplasms (Table 3) increased with decreasing CD4 cell count. The rate of progression to a first new AIDS event or death decreased over time in all CD4 strata (Table 4), except where patients had a low CD4 cell count (0 to <200 CD4 cells/µl).
Tab. 2. Event rates in CD4 strata among the 75,336 patients with at least one suppression episode while on cART: event rates per 1,000 y of suppressed viral load (number of events) by outcome. 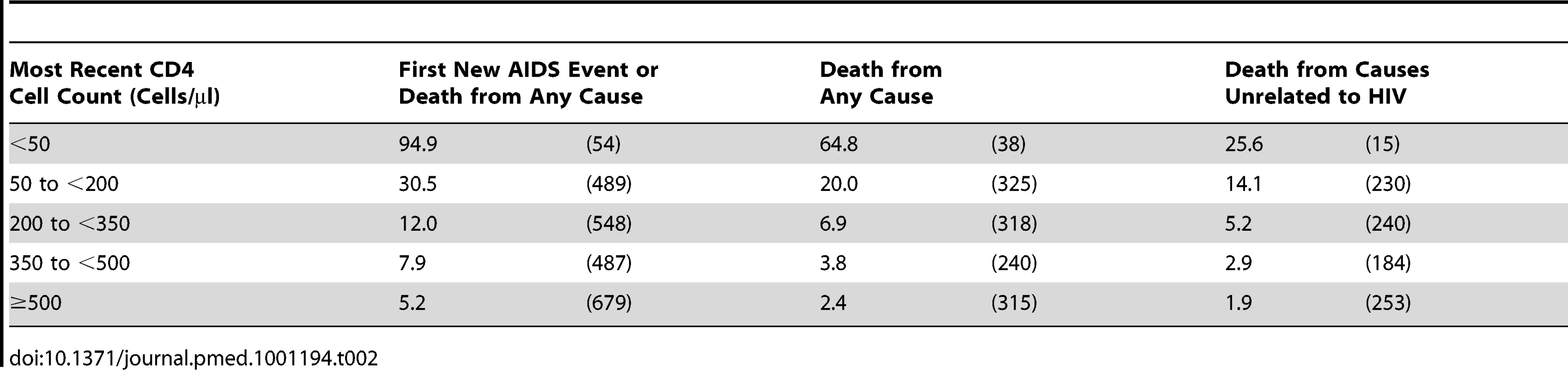
Tab. 3. Event rates in CD4 strata among the 75,336 patients with at least one suppression episode while on cART: event rates per 1,000 y of suppressed viral load (number of events) for a first new AIDS event, with each event then classified as either an opportunistic infection or a HIV related neoplasm. 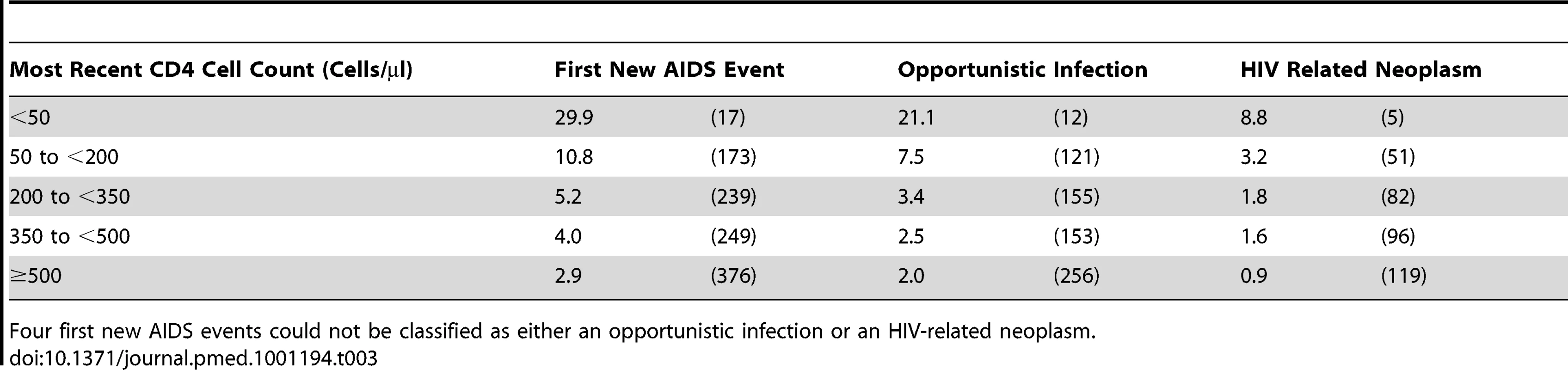
Four first new AIDS events could not be classified as either an opportunistic infection or an HIV-related neoplasm. Tab. 4. Event rates in CD4 strata among the 75,336 patients with at least one suppression episode while on cART: event rates per 1,000 y of suppressed viral load (number of events) over time for the primary outcome (a first new AIDS event or death). 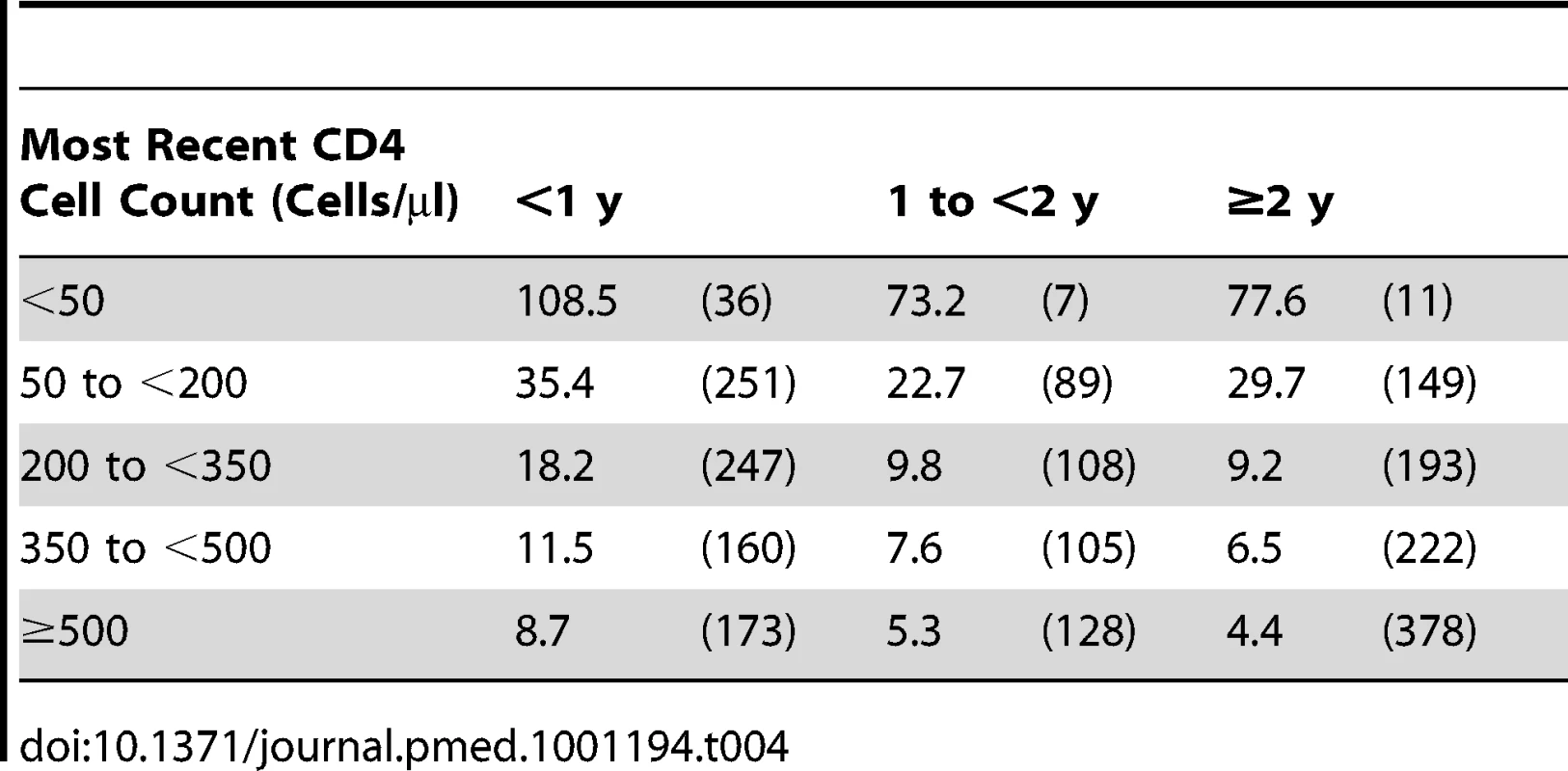
A time updated Kaplan Meier plot illustrates the relatively low probability of AIDS event-free survival—roughly 70% after 10 y of suppression—should a patient's CD4 cell count remain below 200 cells/µl while suppressed (Figure 1A) [16]. In contrast, the probability of AIDS event-free survival was roughly 95% after 10 y of suppression for patients maintaining a CD4 cell count of 500 cells/µl or more while suppressed. It is important to note that CD4 cell count was time dependent and updated when calculating these probabilities. Therefore this plot shows probabilities for hypothetical patients whose CD4 count remains within the same CD4 stratum while suppressed [16]. The roughly parallel lines in the plot of AIDS event-free survival (log log scale) against time (log scale) suggest that a proportional hazards model was appropriate for these data (Figure 1B) [11],[12].
Fig. 1. Probability plots of AIDS event-free survival over time. 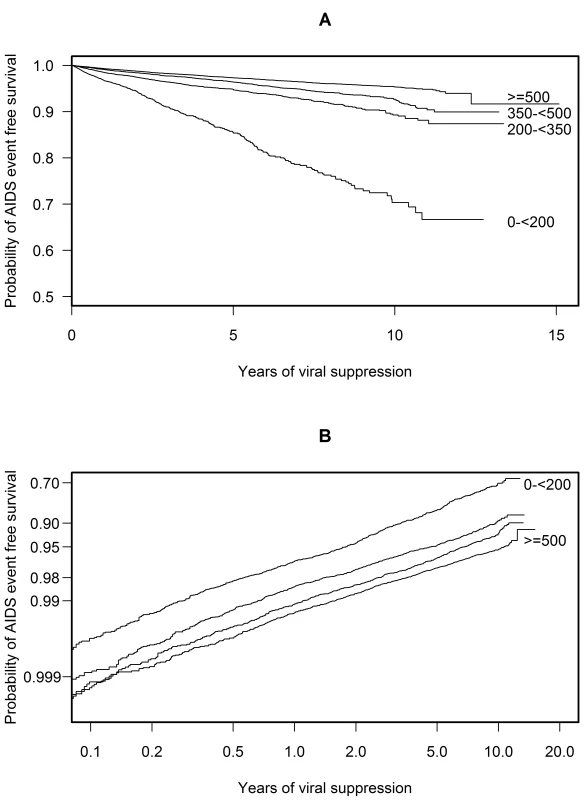
These plots apply to hypothetical patients whose CD4 cell count remains within the same CD4 stratum while on cART with a suppressed viral load. Plot (A) shows a Kaplan Meier plot of the probability of AIDS event-free survival over time. Plot (B) shows a plot of log(−log [probability of AIDS event-free survival]) against log(time). The roughly parallel lines of plot (B) suggest that a proportional hazards model is appropriate for these data. Both plots use a method appropriate for a time-dependent CD4 cell count (see [16]). Time to AIDS or Death
A Cox proportional hazards model for time to a first new AIDS event or death also showed a gradient that depends on CD4 cell count (Table 5). A higher CD4 cell count was associated with a much greater decrease in the risk of progression when a patient had a CD4 cell count below 200 cells/µl (HR 0.35, 0.30–0.40, per 100 cells/µl) than when a patient had a CD4 cell count above 500 cells/µl. However, even at a CD4 cell count above 500 cells/µl, a higher CD4 cell count was associated with a slightly reduced risk of progression (HR 0.96, 0.92–0.99, per 100 cells/µl). A higher CD4 cell count had intermediate benefit for CD4 cell counts in the range from 200 to 350 and from 350 to 500 cells/µl.
Tab. 5. HR estimates and their 95% CIs from multivariate Cox proportional hazard models for both the primary and secondary outcome in 66,147 patients on cART with a suppressed viral load. 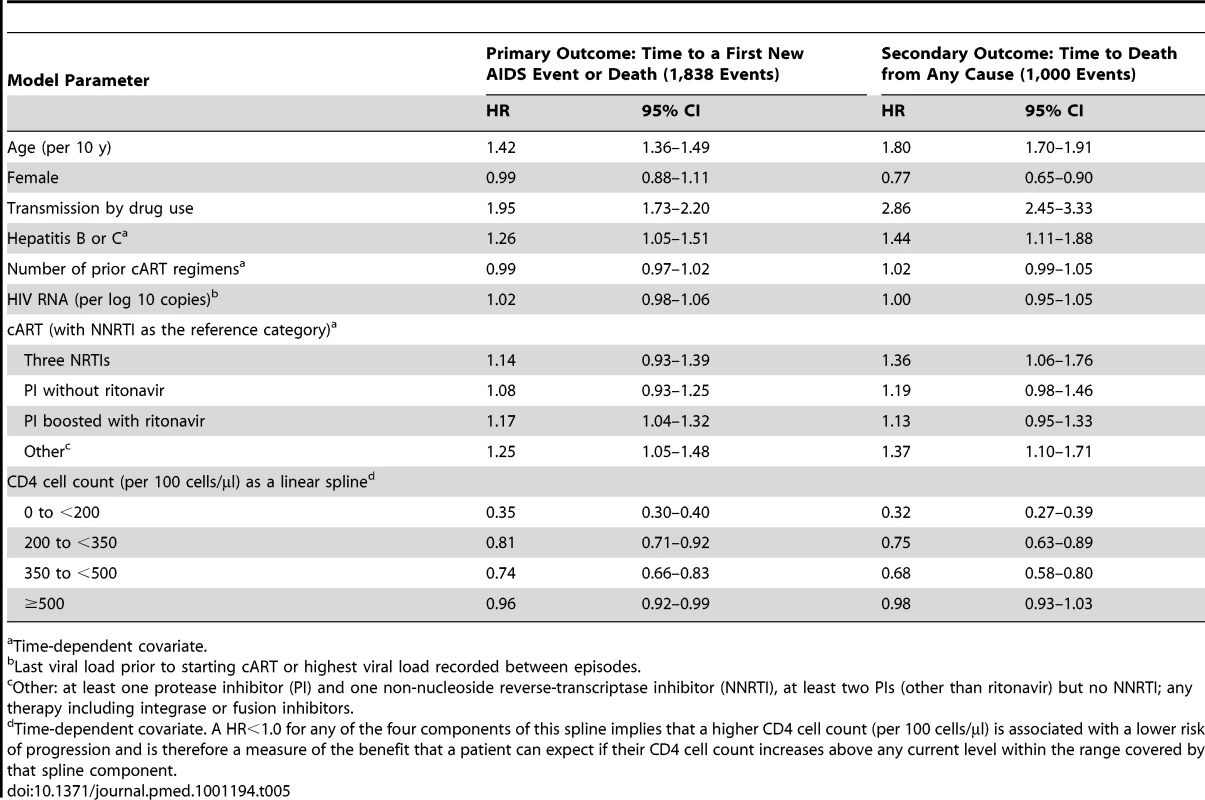
Time-dependent covariate. The same model suggests that progression was more likely for older patients (HR 1.42, 1.36–1.49, per 10 y), for those infected by drug use (HR 1.95, 1.73–2.2), and for those with hepatitis B or C (HR 1.26, 1.05–1.51). Progression was also more likely for patients on cART regimens typically used after virologic failure (boosted protease inhibitor or other cART) compared to non-NRTI-based cART (the reference category).
Plots of weighted Schoenfeld residuals (not shown) suggest a proportional hazards assumption was reasonable for these data [11]. However we also fitted a reduced model, with CD4 cell count represented by a linear spine with a just single knot at 200 cells/µl and with interaction terms between each of the two components of this spline and log suppression time. For patients with a CD4 cell count below 200 cells/µl (HR 0.21, 0.19–0.24, per 100 cells/µl), the interaction (HR 0.51, 0.48–0.54) implied that with a higher CD4 cell count, the risk of progression was not constant but decreased over time. For patients with a CD4 cell count above 200 cells/µl (HR 0.92, 0.90–0.94, per 100 cells/µl), the interaction (HR 1.02, 1.00–1.05) implied that the risk of progression was constant over time. The increasing benefit over time of a higher CD4 cell count for patients with low CD4 cell counts is consistent with the increased event rate after 2 y in Table 4 for patients with low CD4 cell counts and the slight increase in slope after 2 y in Figure 1B for patients with low CD4 cell counts.
HRs for the spline representing CD4 cell count were similar in all six planned sensitivity analyses (Text S1). In the unplanned sensitivity analysis, there was no evidence that the risk of progression differed between first and subsequent episodes of viral suppression (Text S1). The estimated average loss in CD4 cell count between the end of one suppression episode and the beginning of the next was 23 cells/µl per year.
Time to Death
A Cox proportional hazards model for time to death from any cause showed a similar gradient with respect to CD4 cell count (Table 5). There was, however, no real benefit in a higher CD4 cell count for patients with a CD4 cell count above 500 cells/µl (HR 0.98, 0.93–1.03, per 100 cells/µl). And, unlike the primary outcome, women had a lower risk of death (HR 0.77, 0.65–0.90) and cART with three NRTIs was associated with a higher risk of death (HR 1.36, 1.06–1.76).
In a competing risks analysis, we fitted a reduced model with CD4 cell count represented by a linear spine with a just single knot at 200 cells/µl. For patients with a CD4 cell count below 200 cells/µl, a higher CD4 cell count had the most benefit for deaths attributed at least in part to HIV and for deaths of unknown cause (HR 0.20, 0.14–0.30, and 0.22, 0.15–0.32, per 100 cells/µl, respectively), but still had appreciable benefit for deaths thought unrelated to HIV (HR 0.32, 0.26–0.38, per 100 cells/µl). For patients with a CD4 cell count above 200 cells/µl, a higher CD4 cell count had the most benefit for deaths attributed at least in part to HIV (HR 0.58, 0.49–0.70, per 100 cells/µl), but still had some benefit for deaths of unknown cause and for deaths thought unrelated to HIV (HR 0.86, 0.79–0.94, and 0.88, 0.85–0.91, per 100 cells/µl, respectively).
Discussion
This study shows that a higher CD4 cell count is associated with a reduced risk of clinical progression in patients on cART with a suppressed viral load. For patients with a low CD4 cell count, a higher CD4 cell count becomes even more beneficial over time. The benefits associated with a higher CD4 cell count are similar for patients with a CD4 cell count either between 200 and 350 cells/µl or between 350 and 500 cells/µl. Even patients with a CD4 cell count above 500 cells/µl will benefit to a slight extent from a higher CD4 cell count, although there is little if any association between this and the risk of death. Absolute risk reductions in this highest CD4 cell category, however, will be small at best and of little clinical relevance for most patients.
The benefits seen here appear to apply irrespective of whether viral suppression is continuous or episodic. Additional results from the unplanned sensitivity analysis suggest that, having adjusted for other covariates (including a time updated CD4 cell count), patients with episodic suppression were no more likely to progress than patients with continuous suppression. This does not imply that a period of viremia is without negative consequences. Rather these results are consistent with immunological and epidemiological evidence that the negative consequences of viremia are damage to the immune system and a subsequent decline in CD4 cell count [17]–[20]. For those patients with more than one episode of viral suppression, the estimated loss in CD4 cell count between the end of one suppression episode and the beginning of the next was 23 cells/µl per year.
Our estimates of the benefit associated with a higher CD4 cell count have relatively narrow CIs, are robust across sensitivity analyses, and show logical differences between different outcomes and different causes of death. Although many patients were excluded from this merger of COHERE or from the main analysis because of missing covariate information, sensitivity analyses without these covariates and with these patients included suggest that these exclusions have not had a material effect on estimates. We used time updated CD4 cell count to model the risk of progression because in clinical practice decisions are based on the most recent data [10],[21]. We would underestimate the benefit of a higher CD4 cell count were we to base an analysis on the CD4 cell count at the beginning of a suppression episode because of the decay over time in the predictive value of a first observation [10],[19],[22]. Nevertheless we may still underestimate the benefit associated with a higher CD4 cell count to some extent, possibly because of infrequent updating in some patients but more likely because of the considerable measurement error in CD4 cell counts [21],[23],[24]. We did not adjust for primary prophylaxis as this is on a causal pathway between a low CD4 cell count and outcome (see [25]). The use of prophylactic drugs will result in an underestimate of the benefit associated with a higher CD4 cell count for patients with a low CD4 cell count relative to the benefit one would expect in the absence of any prophylaxis.
Previous studies have shown an increased risk of AIDS or death with lower time updated CD4 cell count in untreated patients and in treatment experienced patients [19],[26], and with lower CD4 cell count at the start of treatment or after 6 mo of treatment in treatment-naive patients [3],[4],[22]. In all these studies, CD4 cell count was the strongest prognostic factor for disease progression; viral load was at best only weakly predictive of progression in models with time updated CD4 cell counts [19],[21]. Here we show an increased risk of AIDS or death with lower time updated CD4 cell count in successfully treated patients. The mortality rate in this study was 4.8 per 1,000 y of suppression; lower than the rate of 12 or 14 per 1,000 y in treatment-naive patients starting cART [3],[27]. The event rates in Tables 2–4 show that CD4 cell count gradients are seen in unadjusted rates; otherwise these rates are of limited value to clinicians because of differences between cohorts in rates of AIDS and death, with differences probably due to different methods of diagnosing disease and ascertaining death [28]. However the association between CD4 cell count and AIDS or death appears much more stable across cohorts [28], consistent with our analytic approach where each cohort had a separate baseline hazard but covariate effects were assumed to be the same in each cohort.
The results of this study provide further indirect evidence for starting cART when a patient's CD4 cell count is between 350 and 500 cells/µl [29],[30]. In this study the benefits associated with a higher CD4 cell count were similar over a range of CD4 cell counts from 200 to 500 cells/µl. Above a count of 500 cells/µl, a higher CD4 cell count was associated with a slightly reduced risk of an AIDS event but had little association with the risk of death; hence even earlier treatment with a CD4 cell count above 500 cells/µl might be appropriate for patients with characteristics associated with slower immune recovery—older patients, those with a drug addiction, or co-infected with viral hepatitis [31]–[33]; such patients had a greater risk of progression in our study. A higher CD4 cell count was also associated with a reduced risk of death from causes thought unrelated to HIV. This finding suggests that the distinction between causes of death related and unrelated to HIV is rather arbitrary in successfully treated patients, and that there is a need for more sophisticated recording and review of causes of death to avoid underestimating the burden of HIV infection [28],[34],[35].
In several studies, a CD4 cell count of around 200 cells/µl has been seen as an important threshold [3],[21],[22]. The strength of time updated CD4 cell count as a prognostic factor for survival has led to a suggestion that “there is a threshold beyond which immune reconstitution may be compromised” [22]. Others argue that patients starting treatment with low counts do not seem to remain disadvantaged if the CD4 cell count at the start of treatment is not predictive of survival once adjusted for a value at 6 mo [4]. We see our results—with a higher CD4 cell count becoming even more important over time for patients with low CD4 cell counts—as more consistent with the idea of lasting damage below some threshold from which recovery is difficult [18],[36]. Many patients starting therapy with a CD4 cell count below 200 cells/µl never achieve a normal CD4 cell count even after 10 y of otherwise effective antiretroviral therapy [37], although this failure to recover could be due to factors other than a low CD4 cell count per se. Various treatment intensification strategies have failed to show any benefit in patients with low CD4 cell counts [38],[39]. Despite improvements, the majority of patients in resource-limited settings still start therapy with a CD4 cell count below 200 cells/µl [40], so that along with improved access to treatment, earlier diagnosis and earlier treatment are also needed to reduce mortality in this setting [41].
This study shows that even though new AIDS events and death are uncommon in patients on cART with a suppressed viral load, these patients still benefit from a higher CD4 cell count. There is support in this study for starting cART when a patient's CD4 cell count is between 350 and 500 cells/µl and for continued vigilance when treating patients with sustained viral suppression but a low CD4 cell count.
Supporting Information
Zdroje
1. MayMTSterneJACostagliolaDSabinCAPhillipsAN 2006 HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet 368 451 458
2. VoTTLedergerberBKeiserOHirschelBFurrerH 2008 Durability and outcome of initial antiretroviral treatments received during 2000–2005 by patients in the Swiss HIV Cohort Study. J Infect Dis 197 1685 1694
3. EggerMMayMCheneGPhillipsANLedergerberB 2002 Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 360 119 129
4. CheneGSterneJAMayMCostagliolaDLedergerberB 2003 Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet 362 679 686
5. Panel on Antiretroviral Guidelines for Adults and Adolescents 2011 Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents Department of Health and Human Services Available: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 17 October 2011
6. TherneauTM 1996 Extending the Cox model. Mayo Clinic, Rochester, Minnesota, Department of Health Science Research. Available: http://mayoresearch.mayo.edu/mayo/research/biostat/techreports.cfm. Accessed 22 August 2011
7. CastroKGWardJWSlutskerLBuehlerJWJaffeHW 1992 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults Department of Health and Human Services Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm. Accessed 12 November 2011
8. GreenlandS 1995 Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology 6 356 365
9. GliddenDVVittinghoffE 2004 Modelling clustered survival data from multicentre clinical trials. Stat Med 23 369 388
10. AltmanDGDe StavolaBL 1994 Practical problems in fitting a proportional hazards model to data with updated measurements of the covariates. Stat Med 13 301 341
11. HessKR 1995 Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 14 1707 1723
12. BelleraCAMacGroganGDebledMde LaraCTBrousteV 2010 Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol 10 20
13. SabinCASmithCJd'ArminioMABattegayMGabianoC 2008 Response to combination antiretroviral therapy: variation by age. AIDS 22 1463 1473
14. LodwickRCostagliolaDReissPTortiCTeiraR 2010 Triple-class virologic failure in HIV-infected patients undergoing antiretroviral therapy for up to 10 years. Arch Intern Med 170 410 419
15. PutterHFioccoMGeskusRB 2007 Tutorial in biostatistics: competing risks and multi-state models. Stat Med 26 2389 2430
16. SnapinnSMJiangQIglewiczB 2005 Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. American Statistician 59 301 307
17. DouekDCRoedererMKoupRA 2009 Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med 60 471 484
18. EstesJDHaaseATSchackerTW 2008 The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol 20 181 186
19. LedergerberBLundgrenJDWalkerASSabinCJusticeA 2004 Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet 364 51 62
20. KaufmannGRElziLWeberRFurrerHGiulieriS 2011 Interruptions of cART limits CD4 T-cell recovery and increases the risk for opportunistic complications and death. AIDS 25 441 451
21. PhillipsANLundgrenJD 2006 The CD4 lymphocyte count and risk of clinical progression. Curr Opin HIV AIDS 1 43 49
22. HoggRSYipBChanKJWoodECraibKJ 2001 Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 286 2568 2577
23. BycottPTaylorJ 1998 A comparison of smoothing techniques for CD4 data measured with error in a time-dependent Cox proportional hazards model. Stat Med 17 2061 2077
24. AndersenPKLiestolK 2003 Attenuation caused by infrequently updated covariates in survival analysis. Biostatistics 4 633 649
25. HernanMAHernandez-DiazSWerlerMMMitchellAA 2002 Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 155 176 184
26. PhillipsAPezzottiP 2004 Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug-naive individuals and those treated in the monotherapy era. AIDS 18 51 58
27. MocroftALedergerberBZilmerKKirkOHirschelB 2007 Short-term clinical disease progression in HIV-1-positive patients taking combination antiretroviral therapy: the EuroSIDA risk-score. AIDS 21 1867 1875
28. Antiretroviral Therapy Cohort Collaboration 2010 Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 50 1387 1396
29. SterneJAMayMCostagliolaDde WolfFPhillipsAN 2009 Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 373 1352 1363
30. KitahataMMGangeSJAbrahamAGMerrimanBSaagMS 2009 Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 360 1815 1826
31. WolbersMBattegayMHirschelBFurrerHCavassiniM 2007 CD4+ T-cell count increase in HIV-1-infected patients with suppressed viral load within 1 year after start of antiretroviral therapy. Antivir Ther 12 889 897
32. GreubGLedergerberBBattegayMGrobPPerrinL 2000 Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet 356 1800 1805
33. PotterMOdueyungboAYangHSaeedSKleinMB 2010 Impact of hepatitis C viral replication on CD4+ T-lymphocyte progression in HIV-HCV coinfection before and after antiretroviral therapy. AIDS 24 1857 1865
34. JusticeAC 2010 Commentary: Treated HIV infection is a chronic disease: the case against cause of death analyses. Int J Epidemiol 39 146 148
35. SmithCSabinCALundgrenJDThiebautRWeberR 2010 Factors associated with specific causes of death amongst HIV-positive individuals in the D∶A∶D Study. AIDS 24 1537 1548
36. LoutfyMRGenebatMMooreDRaboudJChanK 2010 A CD4+ cell count <200 cells per cubic millimeter at 2 years after initiation of combination antiretroviral therapy is associated with increased mortality in HIV-infected individuals with viral suppression. J Acquir Immune Defic Syndr 55 451 459
37. KelleyCFKitchenCMHuntPWRodriguezBHechtFM 2009 Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 48 787 794
38. AbramsDLevyYLossoMHBabikerACollinsG 2009 Interleukin-2 therapy in patients with HIV infection. N Engl J Med 361 1548 1559
39. HatanoHHayesTLDahlVSinclairELeeTH 2011 A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis 203 960 968
40. KeiserOAnastosKSchechterMBalestreEMyerL 2008 Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health 13 870 879
41. BartlettJAShaoJF 2009 Successes, challenges, and limitations of current antiretroviral therapy in low-income and middle-income countries. Lancet Infect Dis 9 637 649
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 3- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- No Treatment versus 24 or 60 Weeks of Antiretroviral Treatment during Primary HIV Infection: The Randomized Primo-SHM Trial
- Care Seeking for Neonatal Illness in Low- and Middle-Income Countries: A Systematic Review
- Guidance for Evidence-Informed Policies about Health Systems: Rationale for and Challenges of Guidance Development
- Improving Ethical Review of Research Involving Incentives for Health Promotion
- To VBAC or Not to VBAC
- Impact of Scotland's Smoke-Free Legislation on Pregnancy Complications: Retrospective Cohort Study
- Injectable and Oral Contraceptive Use and Cancers of the Breast, Cervix, Ovary, and Endometrium in Black South African Women: Case–Control Study
- Intermittent Preventive Treatment for Malaria in Papua New Guinean Infants Exposed to and : A Randomized Controlled Trial
- CD4 Cell Count and the Risk of AIDS or Death in HIV-Infected Adults on Combination Antiretroviral Therapy with a Suppressed Viral Load: A Longitudinal Cohort Study from COHERE
- Publication Bias in Antipsychotic Trials: An Analysis of Efficacy Comparing the Published Literature to the US Food and Drug Administration Database
- Better Guidance Is Welcome, but without Blinders
- New Research on Childbirth Has the Potential to Empower Women's Decision Making, but More Is Needed
- Planned Vaginal Birth or Elective Repeat Caesarean: Patient Preference Restricted Cohort with Nested Randomised Trial
- A Comparison of -IV and -5 Panel Members' Financial Associations with Industry: A Pernicious Problem Persists
- Guidance for Evidence-Informed Policies about Health Systems: Linking Guidance Development to Policy Development
- Guidance for Evidence-Informed Policies about Health Systems: Assessing How Much Confidence to Place in the Research Evidence
- Uterine Rupture by Intended Mode of Delivery in the UK: A National Case-Control Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidance for Evidence-Informed Policies about Health Systems: Assessing How Much Confidence to Place in the Research Evidence
- Uterine Rupture by Intended Mode of Delivery in the UK: A National Case-Control Study
- Guidance for Evidence-Informed Policies about Health Systems: Linking Guidance Development to Policy Development
- Improving Ethical Review of Research Involving Incentives for Health Promotion
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



