-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics
Background:
Traumatic brain injury (TBI) is a leading cause of death and disability. A reliable prediction of outcome on admission is of great clinical relevance. We aimed to develop prognostic models with readily available traditional and novel predictors.Methods and Findings:
Prospectively collected individual patient data were analyzed from 11 studies. We considered predictors available at admission in logistic regression models to predict mortality and unfavorable outcome according to the Glasgow Outcome Scale at 6 mo after injury. Prognostic models were developed in 8,509 patients with severe or moderate TBI, with cross-validation by omission of each of the 11 studies in turn. External validation was on 6,681 patients from the recent Medical Research Council Corticosteroid Randomisation after Significant Head Injury (MRC CRASH) trial. We found that the strongest predictors of outcome were age, motor score, pupillary reactivity, and CT characteristics, including the presence of traumatic subarachnoid hemorrhage. A prognostic model that combined age, motor score, and pupillary reactivity had an area under the receiver operating characteristic curve (AUC) between 0.66 and 0.84 at cross-validation. This performance could be improved (AUC increased by approximately 0.05) by considering CT characteristics, secondary insults (hypotension and hypoxia), and laboratory parameters (glucose and hemoglobin). External validation confirmed that the discriminative ability of the model was adequate (AUC 0.80). Outcomes were systematically worse than predicted, but less so in 1,588 patients who were from high-income countries in the CRASH trial.Conclusions:
Prognostic models using baseline characteristics provide adequate discrimination between patients with good and poor 6 mo outcomes after TBI, especially if CT and laboratory findings are considered in addition to traditional predictors. The model predictions may support clinical practice and research, including the design and analysis of randomized controlled trials.
Published in the journal: . PLoS Med 5(8): e165. doi:10.1371/journal.pmed.0050165
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.0050165Summary
Background:
Traumatic brain injury (TBI) is a leading cause of death and disability. A reliable prediction of outcome on admission is of great clinical relevance. We aimed to develop prognostic models with readily available traditional and novel predictors.Methods and Findings:
Prospectively collected individual patient data were analyzed from 11 studies. We considered predictors available at admission in logistic regression models to predict mortality and unfavorable outcome according to the Glasgow Outcome Scale at 6 mo after injury. Prognostic models were developed in 8,509 patients with severe or moderate TBI, with cross-validation by omission of each of the 11 studies in turn. External validation was on 6,681 patients from the recent Medical Research Council Corticosteroid Randomisation after Significant Head Injury (MRC CRASH) trial. We found that the strongest predictors of outcome were age, motor score, pupillary reactivity, and CT characteristics, including the presence of traumatic subarachnoid hemorrhage. A prognostic model that combined age, motor score, and pupillary reactivity had an area under the receiver operating characteristic curve (AUC) between 0.66 and 0.84 at cross-validation. This performance could be improved (AUC increased by approximately 0.05) by considering CT characteristics, secondary insults (hypotension and hypoxia), and laboratory parameters (glucose and hemoglobin). External validation confirmed that the discriminative ability of the model was adequate (AUC 0.80). Outcomes were systematically worse than predicted, but less so in 1,588 patients who were from high-income countries in the CRASH trial.Conclusions:
Prognostic models using baseline characteristics provide adequate discrimination between patients with good and poor 6 mo outcomes after TBI, especially if CT and laboratory findings are considered in addition to traditional predictors. The model predictions may support clinical practice and research, including the design and analysis of randomized controlled trials.Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability. Establishing a reliable prognosis early after injury is notoriously difficult, as is captured in the Hippocratic aphorism, “No head injury is too severe to despair of, nor too trivial to ignore.” Following the development of the Glasgow Coma Scale (GCS) [1] and the Glasgow Outcome Scale (GOS) [2], it was found that confident predictions could be made after 24 h following the injury, but were difficult to establish on admission [3]. Prognostic models with admission data are essential to support early clinical decision-making, and to facilitate reliable comparison of outcomes between different patient series and variation in results over time. Furthermore, prognostic models have an important role in randomized controlled trials (RCTs), for stratification [4] and statistical analyses that explicitly consider prognostic information, such as covariate adjustment [5,6].
Many models include data obtained after admission, and most were developed on relatively small sample sizes originating from a single center or region [7,8]. Many models lack external validation, which is essential before the broad application of a model can be advised [9,10]. Furthermore, few models are presented in a clinically practical way.
We aimed to develop prognostic models based on admission characteristics, which would allow application of the model before in-hospital therapeutic interventions. We used several large patient series for model development as available in the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) project [11], as an extension of multivariable analyses reported before [12]. External validation was possible on data from a large, recently completed RCT [13]. This RCT was used to develop a series of prediction models with a specific focus on non-Western countries [14]. In parallel with this work and as part of a collaboration between CRASH and IMPACT investigators, we developed and describe here a basic model that includes easily accessible clinical features, and additional models that included findings from computed tomography (CT) scanning, and laboratory measurements.
Methods
Patients
The IMPACT database includes patients with moderate and severe TBI (GCS ≤ 12) from eight randomized controlled trials and three observational studies conducted between 1984 and 1997 [11]. Detailed characteristics of these 11 studies and data management have been described previously [15]. The endpoint for the prognostic analyses was the 6 mo GOS, which is an ordered outcome with five categories: 1, dead; 2, vegetative state; 3, severe disability; 4, moderate disability; and 5, good recovery. In patients whose 6 mo assessment was not available we used the 3 mo GOS (n = 1,611, 19% of the patients). We selected 8,509 patients aged ≥ 14 y [12].
We externally validated prognostic models using patients enrolled in the Medical Research Council Corticosteroid Randomisation after Significant Head Injury (MRC CRASH) trial (trial registration ISRCTN74459797, ISRCTN Register, http://www.controlled-trials.com/), who were recruited between 1999 and 2004 [13]. This was a large international double-blind, randomized placebo-controlled trial of the effect of early administration of a 48-h infusion of methylprednisolone on outcome after head injury. It was found that the risks of death and disability were higher in the corticosteroid group than in the placebo group. The trial included 10,008 adults with GCS ≤ 14, who were enrolled within 8 h after injury. We selected 6,681 patients with a GCS ≤ 12 and with complete 6 mo GOS. Secondary analyses considered only placebo patients (n = 3,287) and patients from high-income countries (n = 1,588). For the validation we focused on prediction of mortality (GOS 1) versus survival (GOS 2–5) and of unfavorable (GOS 1–3) versus favorable outcome (GOS 4–5).
Predictors and Model Development
We considered patient characteristics that could be determined easily and reliably within the first few hours after injury. We initially examined a set of 26 potential predictors [12]. These included demographics (age, sex, race, education), indicators of clinical severity (cause of injury, GCS components, pupillary reactivity), secondary insults (hypoxia, hypotension, hypothermia), blood pressure (systolic, diastolic), various CT characteristics and various biochemical variables. For the present analyses, we selected predictors that were important in predicting outcome (according to the Nagelkerke R2 in multivariable analyses), and available for a substantial numbers of patients in the development cohort [12]. Three prognostic models were defined: (1) The core model included age, the motor score component from the GCS, and pupillary reactivity; (2) the extended model included the three predictors from the core model plus information on secondary insults (hypoxia, hypotension), CT characteristics (Marshall CT classification [16]), traumatic subarachnoid hemorrhage (tSAH), and epidural hematoma (EDH); and (3) the lab model included the characteristics from the extended model and additional information on glucose and hemoglobin (Hb). Definitions of predictors have been described in detail [15].
Age and motor score were available for all patients. Missing values occurred for several other predictors, especially because some predictors were not recorded in some studies. Within studies, predictor values were generally over 90% complete if the predictor was recorded [15]. Pupillary reactivity was not recorded in two trials (n = 1,045), but were nearly complete in the other studies (338 missing values among 7,474 patients).
For the extended model we excluded one trial, since hypoxia, hypotension, and the CT classification were not recorded, leaving 6,999 patients. For the development of the lab model, we were limited to four studies in which glucose and Hb had been recorded (n = 3,554). Missing values occurred for 167 glucose values (5%), and 132 Hb values (4%).
We multiply imputed ten sets of data that were identical in known information, but could differ on imputed values for missing information. We used the method of chained equations, sampling imputed values from the posterior predictive distribution of the missing data [17–20]. We used the MICE algorithm [21], which works with R software [22]. The imputation models used all the variables that we considered as potential predictors as well as the 6 mo GOS. In total, 1,383 of the required 25,527 values (5%) in the core model were imputed, 7,477 of the 55,992 required values in the extended model (13%), and 2,965 of the 35,540 required values in the lab model (8%).
Statistical Analysis
Proportional odds logistic regression analysis was performed with the 6 mo GOS as an ordinal outcome [23]. This analysis efficiently summarizes predictive relationships with an ordinal outcome such as the GOS. The proportionality assumption was checked for each selected predictor and found to be reasonable [12]. Interaction terms between predictors were examined with likelihood ratio tests, but none was of sufficient relevance to extend the models beyond the main effects for each predictor. Similarly, study-specific effects were assessed with interaction terms between study and each predictor. Final prognostic models were developed with logistic regression analysis for dichotomized versions for the GOS: mortality (versus survival) and unfavorable outcome (versus favorable outcome). All analyses were stratified by study.
For the continuous predictors age, glucose, and Hb, a linear relationship with outcome was found to be a good approximation after assessment of nonlinearity using restricted cubic splines [24]. The odds ratios (ORs) were scaled so that they corresponded to a change from the 25th percentile to the 75th percentile of the predictor distribution. This scaling allowed for a direct comparison of the prognostic value of predictors that had been recorded in different units or on different scales. Pooled ORs were estimated over the imputed datasets (fit.mult.impute function from the Harrell Design library [25]). All analyses were repeated using only complete data, which gave similar results (unpublished data).
Internal Validation
The discriminatory power of the three models was indicated by the area under the receiver operating characteristic curve (AUC). The AUC varies between 0.5 (a noninformative model) and 1.0 (a perfect model). AUC was calculated in a cross-validation procedure, where each study was omitted in turn. Results were pooled over the ten imputed datasets for eight studies with sufficient numbers for reliable validation (n > 500) [26].
External Validation
We aimed to validate all models externally using data from selected patients in the CRASH trial. However, lab values were not recorded in this trial, nor were hypoxia, hypotension, and EDH. We therefore validated the core model, and a variant of the extended model, in which only the Marshall CT classification and presence of tSAH were added to the core model (i.e., the core + CT model). Results are shown for patients with complete data (core model: n = 6,272; extended model variant, n = 5,309). Imputation of missing values was performed as for the IMPACT studies, leading to similar results (unpublished data). Performance criteria comprised discrimination (measured using the AUC) and calibration (agreement of observed outcomes with predicted risk). Calibration was assessed with the Hosmer-Lemeshow test and graphically using a calibration plot [24].
Model Presentation
The final models were presented in a score chart, with scores based on the regression coefficients in the proportional odds models [27]. Coefficients were scaled such that the same rounded score was obtained for predictors that were used across the different models (e.g., age, motor score, pupils). Logistic regression was subsequently used to calibrate the risks of mortality and unfavorable outcome according to the scores, with the model intercept referring to the Tirilazad international trial [15]. This intercept was chosen since it represented typical proportions of mortality (278/1,118, 25%) and unfavorable outcome (456/1,118, 41%). Predictions can be calculated from an Excel spreadsheet and from a Web page (Text S1 is also available at http://www.tbi-impact.org/).
Results
The characteristics of IMPACT and CRASH patients with GCS ≤ 12 were fairly comparable (Table 1). CRASH trial patients were marginally older than in IMPACT, and admission motor scores were somewhat higher. Six-month mortality was 28% in IMPACT and 32% in CRASH, and unfavorable outcomes occurred in nearly half of the patients (48% in IMPACT, 47% in CRASH). Mortality was slightly lower in the placebo group of the selected CRASH patients (mortality 988/3,287, 30%; unfavorable outcome 1,524/3,287, 46%), and in the patients from high-income countries (mortality 405/1,588, 26%; unfavorable outcome 747/1,588, 47%).
Tab. 1. 
Patient Characteristics of 11 Studies in the IMPACT Database and the CRASH Trial All predictors had statistically significant associations with 6 mo GOS in univariate and multivariable analyses (Table 2). An increase in age equal to the interquartile range (24 y) was associated with approximately a doubling of the risk of poor outcome. A poor outcome occurred especially for those with motor scores 1 (none) or 2 (extension). Pupillary reactivity, hypoxia, and hypotension also had strong prognostic effects. CT classifications showing mass lesions or signs of raised Intracranial Pressure (CT class III to VI) had similar increases in risk as the presence of tSAH (OR around 2). An EDH was a relatively favorable sign on a CT (compared to not having an EDH on CT). Higher glucose levels and lower Hb levels were associated with a poor outcomes, but effects were more moderate than, for example, for age.
Tab. 2. 
Associations between Predictors and 6-Month Outcome in the IMPACT Data (<i>n</i> = 8,509) A simple score chart for the sequential application of the models is presented in Figure 1, which can be used in combination with Figure 2 to obtain approximate predictions for individual patients. For example, a 35-y-old patient, with a motor score of 3 (abnormal flexion), and both pupils reacting, has a core model score of 1 + 4 + 0 = 5 points. According to Figure 2, this score corresponds to risks of mortality and unfavorable outcome of approximately 20% and 50%, respectively. If this patient had suffered from hypoxia but not hypotension before admission, and the CT showed a mass lesion and tSAH, the extended model score becomes 5 for the core model + 1 + 2 + 2 + 0 = 10 points. The corresponding risks are approximately 40% for mortality and 70% for unfavorable outcome. When glucose is 10 mmol/l and Hb 11 g/dl, the lab model score increases by 2 + 2 to 14 points, which corresponds to slightly higher predictions of mortality and unfavorable outcome than those estimated with the extended model (Figure 3).
Fig. 1. Score Chart for 6 Month Outcome after TBI 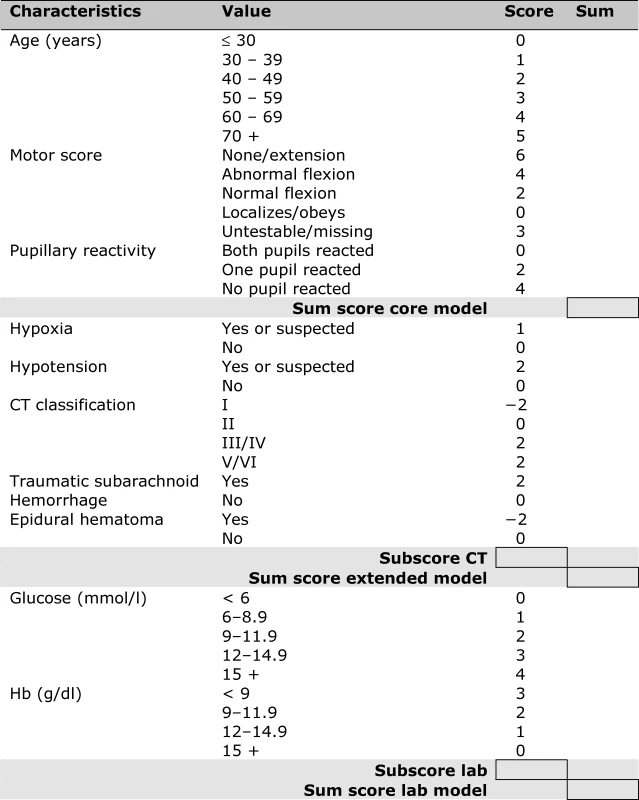
Sum scores can be calculated for the core model (age, motor score, pupillary reactivity), the extended model (core + hypoxia + hypotension + CT characteristics), and a lab model (core + hypoxia + hypotension + CT + glucose + Hb). The probability of 6 mo outcome is defined as 1 / (1 + e−LP), where LP refers to the linear predictor in a logistic regression model. Six LPs were defined as follows: Fig. 2. Predicted Probabilities of Mortality and Unfavorable Outcome at 6 Month after TBI in Relation to the Sum Scores from the Core, Extended, and Lab Models 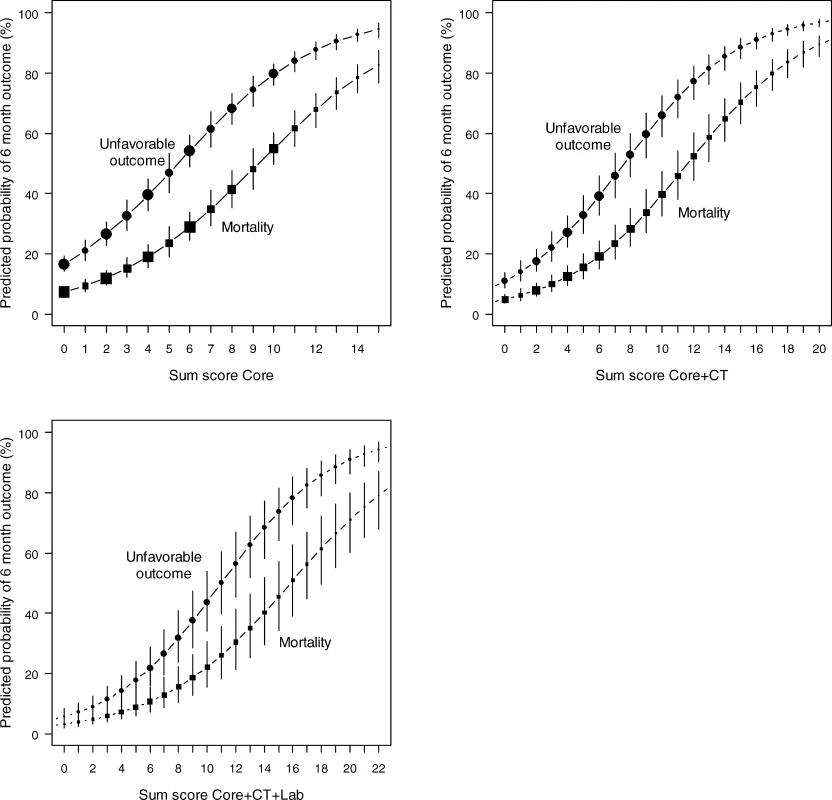
The logistic functions are plotted with 95% confidence intervals. Dot size is proportional to sample size. Sum scores can be obtained from Figure 1. Fig. 3. Screenshot of the Spreadsheet with Calculations of Probabilities for the Three Prediction Models 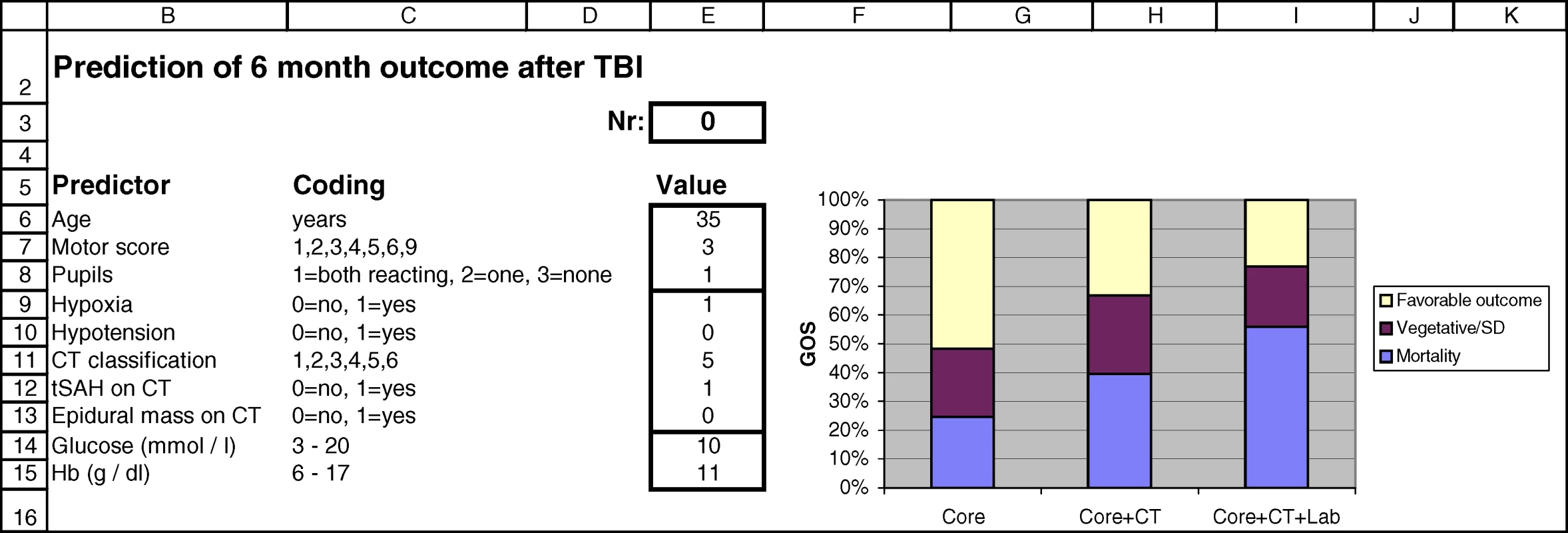
Predictions are calculated for a 35-y-old patient with motor score 3, both pupils reacting, hypoxia before admission, mass lesion and tSAH on admission CT scan, glucose 11 mmol/l, and Hb 10 g/dl. A Web-based calculator is available at http://www.tbi-impact.org/. Cross-Validation and External Validation
The discriminatory ability of the models increased with increasing complexity (Table 3). Within the IMPACT data, the best cross-validated performance was seen for the three observational studies, with AUCs over 0.80. Evaluation in the RCTs showed lower AUCs. External validation confirmed the discriminatory ability of the core model in the CRASH trial (AUC 0.776 and 0.780 for mortality and unfavorable outcome, respectively, Figures 4 and 5). When CT classification and tSAH were considered as well, the performance increased to 0.801 and 0.796 for mortality and unfavorable outcome, respectively, for 5,309 patients in CRASH. Outcomes in CRASH were systematically poorer than those predicted for both the core and core + CT models, for both mortality and unfavorable outcome (Hosmer-Lemeshow tests, p < 0.001, Figures 4 and 5). This miscalibration was slightly less but did not disappear when only the placebo patients were considered. Calibration was better for the patients from high-income countries, with near perfect calibration (Hosmer-Lemeshow tests, p > 0.1) for the extended model predicting mortality (n = 1,351, Figure 4) and the core model predicting unfavorable outcomes (n = 1,466, Figure 5).
Tab. 3. 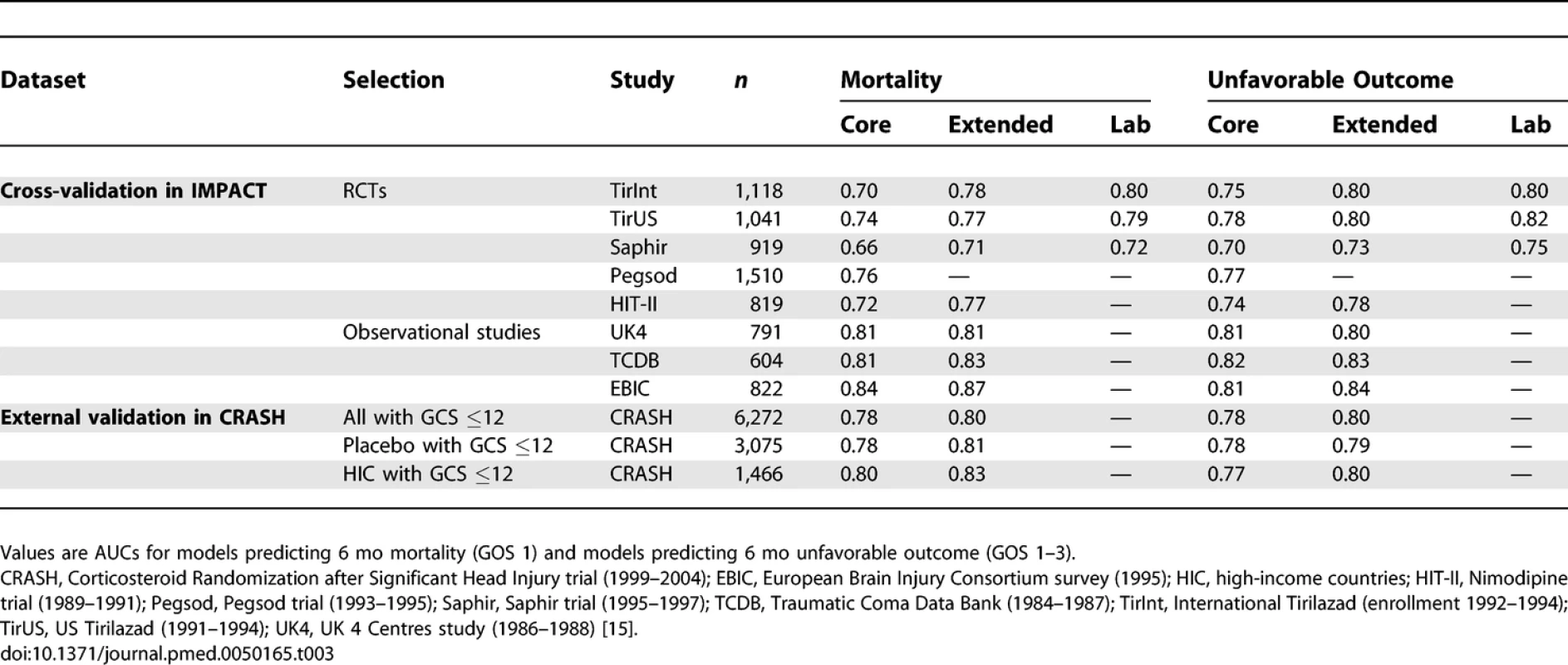
Discriminative Ability of the Models at Cross-Validation in IMPACT Patients (Studies with <i>n</i> > 500), and External Validation in Patients from the CRASH Trial Fig. 4. External Validity for the Core and Core + CT Model Characteristics for Prediction of Mortality in the CRASH Trial 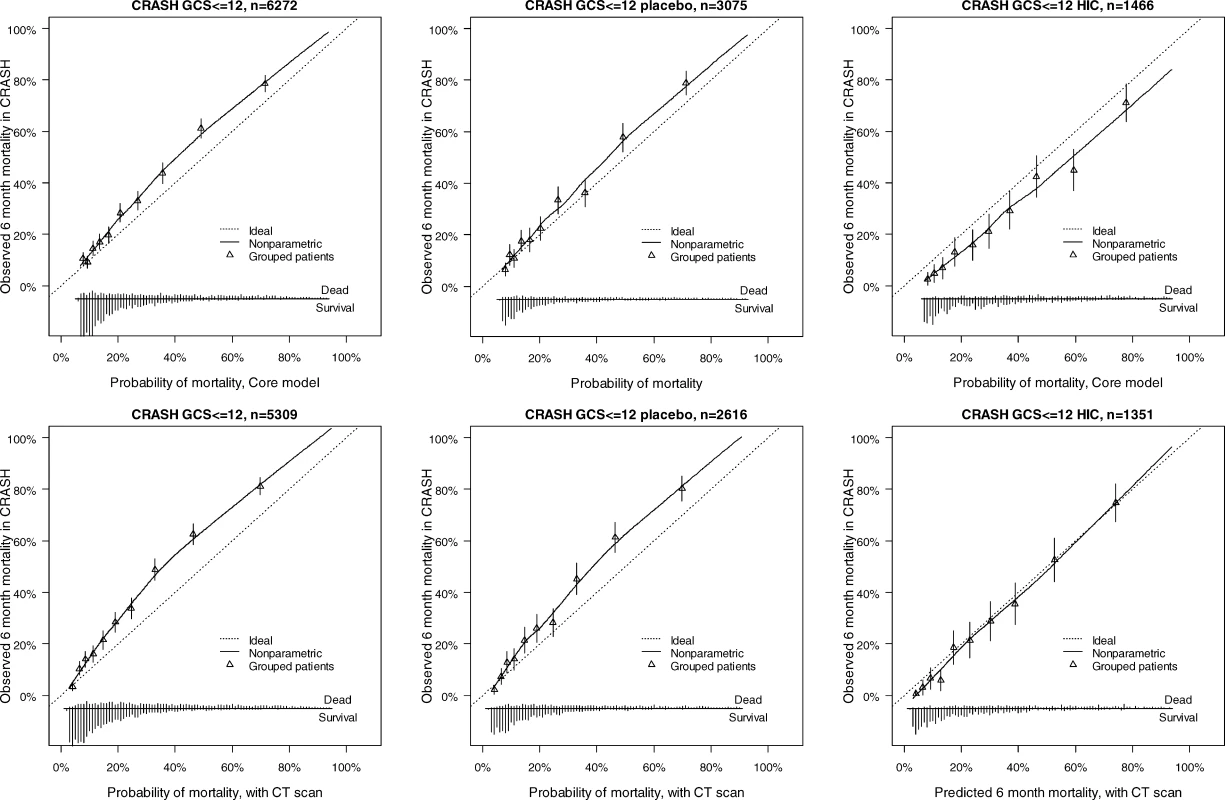
The distribution of predicted probabilities is shown at the bottom of the graphs, by 6-mo mortality. The triangles indicate the observed frequencies by decile of predicted probability. Fig. 5. External Validity for the Core and Core + CT Model Characteristics for Prediction of Unfavorable Outcomes in the CRASH Trial 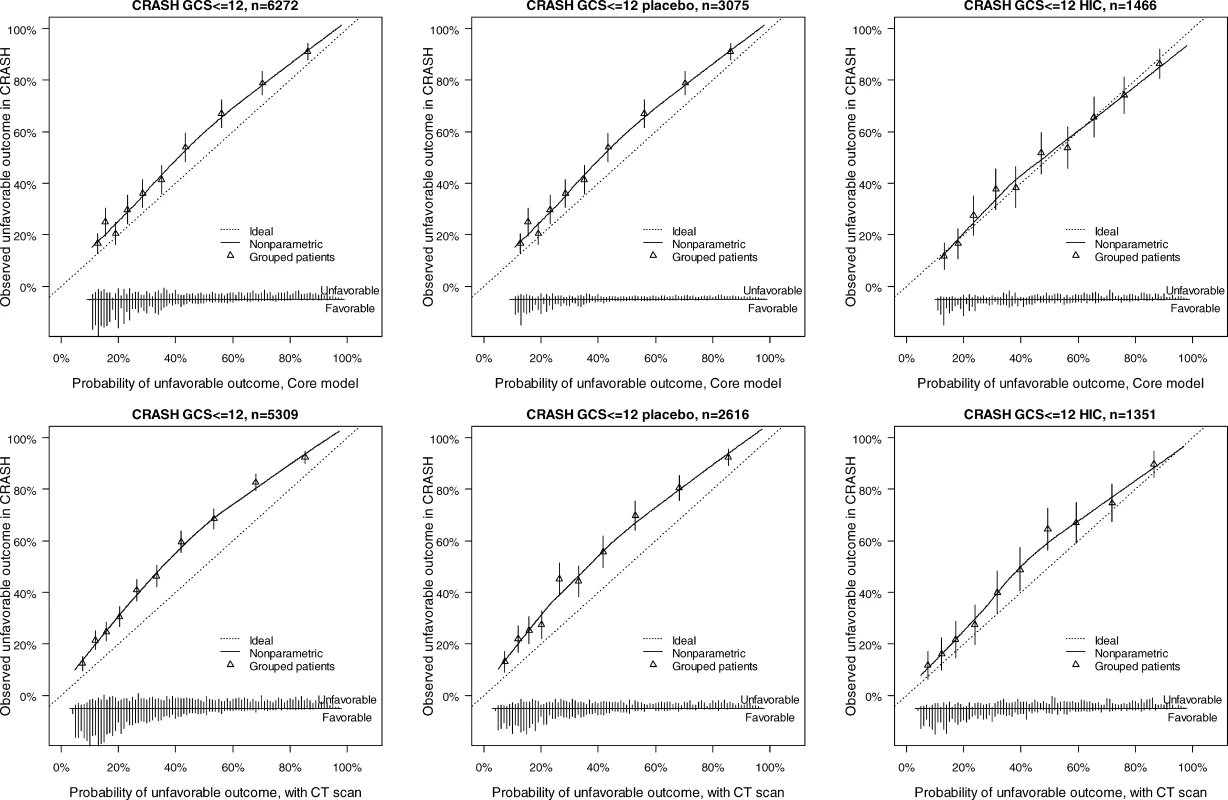
The distribution of predicted probabilities is shown at the bottom of the graphs, by 6-mo outcome. The triangles indicate the observed frequencies by decile of predicted probability. Discussion
In this paper we describe the development of a series of prognostic models of increasing complexity, based on admission characteristics, to predict the risk of 6-mo mortality and unfavorable outcomes in individual patients after moderate or severe TBI. The models discriminated adequately between patients with poor and good outcomes, especially in the relatively unselected observational studies. Patients in the randomized trials were selected according to various enrollment criteria, which led to more homogeneous samples, as reflected in a lower discriminative ability of the models. We found a small but systematic difference between predicted and observed outcome in a large, relatively recent, external validation set [13] with recently treated patients from both high - and low/middle-income countries. This miscalibration largely disappeared when we considered only patients from high-income countries.
The largest amount of prognostic information was contained in a core set of three predictors: age, motor score, and pupillary reactivity at admission. These characteristics were already considered in the first well-known model for TBI [3] and in many subsequent prognostic models [7,8]. Information from the CT scan provided additional prognostic information, although we did not exploit all the prognostic information contained in a CT scan. The Marshall CT classification combines several characteristics, and we previously proposed a more detailed scoring for prognostic purposes [28]. Further validation of this score is necessary, but the required data were not sufficiently available in most studies from IMPACT. The presence of EDH was associated with a better outcome after trauma, which may be explained by the possibility of emergent surgical evacuation of such hematomas. An EDH often disturbs brain function because of compression, although there is generally little intrinsic brain damage. If compression is relieved in time, full recovery will more likely occur. Laboratory parameters have not yet been widely used for prognosis after TBI [29]. Glucose and hemoglobin were shown to contribute to outcome prediction, although their effects are smaller than other predictors, e.g., age. Coagulation parameters may also be very relevant for outcome prediction [29], but these parameters were not sufficiently available in our studies. These biochemical parameters warrant further exploration, especially since they are amenable to intervention. For example, in critical care, intensive hyperglycemia management has been shown to reduce mortality [30]. We could not include effects of extracranial injuries, since measures such as the ISS (injury severity score) were not consistently recorded in the IMPACT studies. Major extracranial injury was included as a predictor in recently developed prognostic models from the CRASH trial [14]. It is likely that the AUC of our models would have been even better if this variable had been available [31,32]
Relationship of Our Model to Previously Published Models
Several models have been derived to estimate the probability of hospital mortality of adult intensive care unit patients with physiological characteristics collected during the first day(s), including APACHE (Acute Physiology and Chronic Health Evaluation), SAPS (Simplified Acute Physiology Score), and MPM (Mortality Prediction Model) [33–35]. Our models differ in several aspects, since we predicted long term outcome, specifically for TBI patients, and used only baseline characteristics. Recently, prognostic models for 14 d mortality and 6 mo outcome were published by the MRC CRASH trial collaborators. CRASH was a mega-trial, including mild TBI (30% of n = 10,008), with a relatively simple data collection in mostly patients from low-income countries (75% of n = 10,008) [14]. The IMPACT database involves merged individual patient data from eight clinical trials and three observational series, conducted over approximately 15 y, and focused on severe TBI. The IMPACT data are available in greater detail, especially with respect to CT scan characteristics.
We externally validated modified versions of two of our three IMPACT models in selected patients from the CRASH trial with GCS ≤ 12, similar to the external validation of two modified versions of CRASH models for unfavorable outcome at 6 mo in IMPACT [14]. Both studies confirmed the external validity of the presented models. This collaboration with reciprocal validation of CRASH and IMPACT models is important for reliable application of models outside their respective development settings.
Early prediction of outcome permits establishment of a baseline risk profile for individual patients, thus providing a reference for assessing quality of health-care delivery. Prognostic models are particularly relevant for a more efficient design and analysis of RCTs. For example, we can exclude those with a very good or a very poor prognosis [4], perform covariate adjustment of a treatment effect [6,36], and consider other analyses that lead to increases in statistical power [37].
The proposed scores may also support clinicians in their initial assessment of the severity and prognosis of a TBI patient. We note, however, that statistical models can only augment, not replace clinical judgment, although it is unlikely that any clinician has the equivalent systematic experience of the outcomes of the thousands of patients underlying our models. Predictions should be regarded with care and not directly be applied for treatment-limiting decisions [38]. The UK 4 Centres Study found that making predictions available as part of a routine clinical service altered deployment of resources [39].
The validity and applicability of the prognostic models is affected by various factors. The local level of care may vary between regions, which may result in differences in outcome. Previously we found unexplained outcome differences between the US and the international part of the Tirilazad trials [40]. In the CRASH trial, outcomes were better for patients from the high-income countries [14]. One explanation is that facilities were more extensive than in the low - and middle-income countries that participated in this trial. Predictions for TBI patients in low - and middle-income countries may best be obtained from the CRASH models that were specifically developed for these countries [14]. Our model predictions may be better than the CRASH predictions for high-income countries, because of the more detailed information in the models and larger patient numbers used in model development. Predictions may, even on average, be too poor, considering that treatment standards have improved over time, including trauma organization, diagnostic facilities such as CT scanning, and critical care management. We did not, however, find a clear trend of better outcomes in more recently treated patients when we applied identical selection criteria to the studies in the IMPACT database. Both the CRASH and IMPACT model predictions may require regular updating according to specific population characteristics, such as calendar year, treatment setting, or local trauma organization [41,42].
Limitations of These Models
Our study has several limitations. Patients in our studies were treated between 1984 and 1997. Even though evaluation in the more recent CRASH trial data confirmed the validity of the model predictions to more recent times (enrollment between 1999 and 2004), we cannot exclude that better outcomes are obtained nowadays. Also, the motor score is not always available in current clinical practice, or can be unreliable even when it is available, due to the effects of early sedation or paralysis. Furthermore, missing variables and missing values were a problem in the development of the models. Multiple imputation of the relatively few missing values allowed us to use the information from other predictors. Both theoretical and empirical support is growing for the use of such imputation methods instead of traditional complete case analyses [19,43]. However, more complete data would have been preferable. Furthermore, some misclassification may have occurred in classification of unfavorable versus favorable outcome. Mortality at 6 mo has the advantage that it suffers less from such a potential bias.
In conclusion, prognostic models are now available that provide adequate discrimination between patients with good and poor 6-mo outcome. These models may be useful for providing realistic information to relatives on expectations of outcome, for quantifying and classifying the severity of brain injury, for stratification and covariate adjustment in clinical trials, and as a reference for evaluating quality of care.
Supporting Information
Zdroje
1. TeasdaleGJennettB
1974
Assessment of coma and impaired consciousness. A practical scale.
Lancet
2
81
84
2. JennettBBondM
1975
Assessment of outcome after severe brain damage.
Lancet
1
480
484
3. JennettBTeasdaleGBraakmanRMinderhoudJKnill-JonesR
1976
Predicting outcome in individual patients after severe head injury.
Lancet
1
1031
1034
4. MachadoSGMurrayGDTeasdaleGM
1999
Evaluation of designs for clinical trials of neuroprotective agents in head injury. European Brain Injury Consortium.
J Neurotrauma
16
1131
1138
5. HernandezAVSteyerbergEWTaylorGSMarmarouAHabbemaJD
2005
Subgroup analysis and covariate adjustment in randomized clinical trials of traumatic brain injury: a systematic review.
Neurosurgery
57
1244
1253
6. HernandezAVSteyerbergEWButcherIMushkudianiNTaylorGS
2006
Adjustment for strong predictors of outcome in traumatic brain injury trials: 25% reduction in sample size requirements in the IMPACT study.
J Neurotrauma
23
1295
1303
7. PerelPEdwardsPWentzRRobertsI
2006
Systematic review of prognostic models in traumatic brain injury.
BMC Med Inform Decis Mak
6
38
8. MushkudianiNAHukkelhovenCWHernandezAVMurrayGDChoiSC
2008
A systematic review finds methodological improvements necessary for prognostic models in determining traumatic brain injury outcomes.
J Clin Epidemiol
61
331
343
9. JusticeACCovinskyKEBerlinJA
1999
Assessing the generalizability of prognostic information.
Ann Intern Med
130
515
524
10. ReillyBMEvansAT
2006
Translating clinical research into clinical practice: impact of using prediction rules to make decisions.
Ann Intern Med
144
201
209
11. MaasAIMarmarouAMurrayGDTeasdaleSGSteyerbergEW
2007
Prognosis and clinical trial design in traumatic brain injury: the IMPACT study.
J Neurotrauma
24
232
238
12. MurrayGDButcherIMcHughGSLuJMushkudianiNA
2007
Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study.
J Neurotrauma
24
329
337
13. EdwardsPArangoMBalicaLCottinghamREl-SayedH
2005
Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months.
Lancet
365
1957
1959
14. MRC CRASH Trial Collaborators,PerelPArangoMClaytonTEdwardsP
2008
Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients.
BMJ
336
425
429
15. MarmarouALuJButcherIMcHughGSMushkudianiNA
2007
IMPACT database of traumatic brain injury: design and description.
J Neurotrauma
24
239
250
16. MarshallLFMarshallSBKlauberMRVan Berkum ClarkMEisenbergH
1992
The diagnosis of head injury requires a classification based on computed axial tomography.
J Neurotrauma
9
Suppl 1
S287
292
17. McHughGSButcherISteyerbergEWLuJMushkudianiN
2007
Statistical approaches to the univariate prognostic analysis of the IMPACT database on traumatic brain injury.
J Neurotrauma
24
251
258
18. RubinDBSchenkerN
1991
Multiple imputation in health-care databases: an overview and some applications.
Stat Med
10
585
598
19. SchaferJLGrahamJW
2002
Missing data: our view of the state of the art.
Psychol Methods
7
147
177
20. EndersCK
2006
A primer on the use of modern missing-data methods in psychosomatic medicine research.
Psychosom Med
68
427
436
21. Van BuurenSOudshoornCGM
2006
mice: Multivariate Imputation by Chained Equations. R package version 1.16
Available: http://web.inter.nl.net/users/S.vanBuuren/mi/hmtl/mice.htm. Accessed 12 December 2006.
22. R Development Core Team
2006
R: A language and environment for statistical computing
Vienna (Austria)
R Foundation for Statistical Computing
Available: http://www.R-project.org/. Accessed 30 June 2008.
23. McCullaghP
1980
Regression models for ordinal data.
J R Stat Soc Ser B
42
109
142
24. HarrellFE
2001
Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis
New York
Springer
568 pp.
25. HarrellFE
2006
Design: Design Package. R package version 2.0
http://biostat.mc.vanderbilt.edu/s/Design. Accessed 30 June 2008.
26. VergouweYSteyerbergEWEijkemansMJHabbemaJD
2005
Substantial effective sample sizes were required for external validation studies of predictive logistic regression models.
J Clin Epidemiol
58
475
483
27. MoonsKGHarrellFESteyerbergEW
2002
Should scoring rules be based on odds ratios or regression coefficients.
J Clin Epidemiol
55
1054
1055
28. MaasAIHukkelhovenCWMarshallLFSteyerbergEW
2005
Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors.
Neurosurgery
57
1173
1182
29. Van BeekJGMushkudianiNASteyerbergEWButcherIMcHughGS
2007
Prognostic value of admission laboratory parameters in traumatic brain injury: results from the IMPACT study.
J Neurotrauma
24
315
328
30. van den BergheGWoutersPWeekersFVerwaestCBruyninckxF
2001
Intensive insulin therapy in the critically ill patients.
N Engl J Med
345
1359
1367
31. SignoriniDFAndrewsPJJonesPAWardlawJMMillerJD
1999
Predicting survival using simple clinical variables: a case study in traumatic brain injury.
J Neurol Neurosurg Psychiatry
66
20
25
32. SchreiberMAAokiNScottBGBeckJR
2002
Determinants of mortality in patients with severe blunt head injury.
Arch Surg
137
285
290
33. LemeshowSLe GallJR
1994
Modeling the severity of illness of ICU patients. A systems update.
JAMA
272
1049
1055
34. ChoDYWangYC
1997
Comparison of the APACHE III, APACHE II and Glasgow coma scale in acute head injury for prediction of mortality and functional outcome.
Intensive Care Med
23
77
84
35. HyamJAWelchCAHarrisonDAMenonDK
2006
Case mix, outcomes and comparison of risk prediction models for admissions to adult, general and specialist critical care units for head injury: a secondary analysis of the ICNARC Case Mix Programme Database.
Crit Care
10
Suppl 2
S2
36. ChoiSC
1998
Sample size in clinical trials with dichotomous endpoints: use of covariables.
J Biopharm Stat
8
367
375
37. MurrayGDBarerDChoiSFernandesHGregsonB
2005
Design and analysis of phase III trials with ordered outcome scales: the concept of the sliding dichotomy.
J Neurotrauma
22
511
517
38. LemeshowSKlarJTeresD
1995
Outcome prediction for individual intensive care patients: useful, misused, or abused.
Intensive Care Med
21
770
776
39. MurrayLSTeasdaleGMMurrayGDJennettBMillerJD
1993
Does prediction of outcome alter patient management.
Lancet
341
1487
1491
40. HukkelhovenCWSteyerbergEWFaraceEHabbemaJDMarshallLF
2002
Regional differences in patient characteristics, case management, and outcomes in traumatic brain injury: experience from the tirilazad trials.
J Neurosurg
97
549
557
41. SteyerbergEWBorsboomGJvan HouwelingenHCEijkemansMJHabbemaJD
2004
Validation and updating of predictive logistic regression models: a study on sample size and shrinkage.
Stat Med
23
2567
2586
42. SteyerbergEW
2005
Local applicability of clinical and model-based probability estimates.
Med Decis Making
25
678
680
43. MoonsKGDondersRAStijnenTHarrellFEJr.
2006
Using the outcome for imputation of missing predictor values was preferred.
J Clin Epidemiol
59
1092
1101
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2008 Číslo 8- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics
- Greater Response to Placebo in Children Than in Adults: A Systematic Review and Meta-Analysis in Drug-Resistant Partial Epilepsy
- Can a Topical Microbicide Prevent Rectal HIV Transmission?
- Developing a Prognostic Model for Traumatic Brain Injury—A Missed Opportunity?
- Assessing Antimalarial Efficacy in a Time of Change to Artemisinin-Based Combination Therapies: The Role of Médecins Sans Frontières
- Children Are Not Just Small Adults: The Urgent Need for High-Quality Trial Evidence in Children
- The Use of Nonhuman Primate Models in HIV Vaccine Development
- More Evidence Against a Causal Association between C-Reactive Protein and Diabetes
- Strategies to Reduce Mortality from Bacterial Sepsis in Adults in Developing Countries
- Ensuring the Involvement of Children in the Evaluation of New Tuberculosis Treatment Regimens
- An Acute Evolving Flaccid Quadriparesis in an Elderly Woman
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Ensuring the Involvement of Children in the Evaluation of New Tuberculosis Treatment Regimens
- Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics
- The Use of Nonhuman Primate Models in HIV Vaccine Development
- Strategies to Reduce Mortality from Bacterial Sepsis in Adults in Developing Countries
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



