-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Greater Response to Placebo in Children Than in Adults: A Systematic Review and Meta-Analysis in Drug-Resistant Partial Epilepsy
Background:
Despite guidelines establishing the need to perform comprehensive paediatric drug development programs, pivotal trials in children with epilepsy have been completed mostly in Phase IV as a postapproval replication of adult data. However, it has been shown that the treatment response in children can differ from that in adults. It has not been investigated whether differences in drug effect between adults and children might occur in the treatment of drug-resistant partial epilepsy, although such differences may have a substantial impact on the design and results of paediatric randomised controlled trials (RCTs).Methods and Findings:
Three electronic databases were searched for RCTs investigating any antiepileptic drug (AED) in the add-on treatment of drug-resistant partial epilepsy in both children and adults. The treatment effect was compared between the two age groups using the ratio of the relative risk (RR) of the 50% responder rate between active AEDs treatment and placebo groups, as well as meta-regression. Differences in the response to placebo and to active treatment were searched using logistic regression. A comparable approach was used for analysing secondary endpoints, including seizure-free rate, total and adverse events-related withdrawal rates, and withdrawal rate for seizure aggravation. Five AEDs were evaluated in both adults and children with drug-resistant partial epilepsy in 32 RCTs. The treatment effect was significantly lower in children than in adults (RR ratio: 0.67 [95% confidence interval (CI) 0.51–0.89]; p = 0.02 by meta-regression). This difference was related to an age-dependent variation in the response to placebo, with a higher rate in children than in adults (19% versus 9.9%, p < 0.001), whereas no significant difference was observed in the response to active treatment (37.2% versus 30.4%, p = 0.364). The relative risk of the total withdrawal rate was also significantly lower in children than in adults (RR ratio: 0.65 [95% CI 0.43–0.98], p = 0.004 by metaregression), due to higher withdrawal rate for seizure aggravation in children (5.6%) than in adults (0.7%) receiving placebo (p < 0.001). Finally, there was no significant difference in the seizure-free rate between adult and paediatric studies.Conclusions:
Children with drug-resistant partial epilepsy receiving placebo in double-blind RCTs demonstrated significantly greater 50% responder rate than adults, probably reflecting increased placebo and regression to the mean effects. Paediatric clinical trial designs should account for these age-dependent variations of the response to placebo to reduce the risk of an underestimated sample size that could result in falsely negative trials.
Published in the journal: . PLoS Med 5(8): e166. doi:10.1371/journal.pmed.0050166
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.0050166Summary
Background:
Despite guidelines establishing the need to perform comprehensive paediatric drug development programs, pivotal trials in children with epilepsy have been completed mostly in Phase IV as a postapproval replication of adult data. However, it has been shown that the treatment response in children can differ from that in adults. It has not been investigated whether differences in drug effect between adults and children might occur in the treatment of drug-resistant partial epilepsy, although such differences may have a substantial impact on the design and results of paediatric randomised controlled trials (RCTs).Methods and Findings:
Three electronic databases were searched for RCTs investigating any antiepileptic drug (AED) in the add-on treatment of drug-resistant partial epilepsy in both children and adults. The treatment effect was compared between the two age groups using the ratio of the relative risk (RR) of the 50% responder rate between active AEDs treatment and placebo groups, as well as meta-regression. Differences in the response to placebo and to active treatment were searched using logistic regression. A comparable approach was used for analysing secondary endpoints, including seizure-free rate, total and adverse events-related withdrawal rates, and withdrawal rate for seizure aggravation. Five AEDs were evaluated in both adults and children with drug-resistant partial epilepsy in 32 RCTs. The treatment effect was significantly lower in children than in adults (RR ratio: 0.67 [95% confidence interval (CI) 0.51–0.89]; p = 0.02 by meta-regression). This difference was related to an age-dependent variation in the response to placebo, with a higher rate in children than in adults (19% versus 9.9%, p < 0.001), whereas no significant difference was observed in the response to active treatment (37.2% versus 30.4%, p = 0.364). The relative risk of the total withdrawal rate was also significantly lower in children than in adults (RR ratio: 0.65 [95% CI 0.43–0.98], p = 0.004 by metaregression), due to higher withdrawal rate for seizure aggravation in children (5.6%) than in adults (0.7%) receiving placebo (p < 0.001). Finally, there was no significant difference in the seizure-free rate between adult and paediatric studies.Conclusions:
Children with drug-resistant partial epilepsy receiving placebo in double-blind RCTs demonstrated significantly greater 50% responder rate than adults, probably reflecting increased placebo and regression to the mean effects. Paediatric clinical trial designs should account for these age-dependent variations of the response to placebo to reduce the risk of an underestimated sample size that could result in falsely negative trials.Introduction
Epilepsy is a common disorder in children that often requires antiepileptic drug (AED) treatment for many years [1]. However, most AEDs have inadequate paediatric use information [2]. Indeed, AEDs are evaluated primarily in adult patients [3], and only a few randomised controlled trials (RCTs) have been performed in paediatric population [1]. Furthermore, despite reviews and guidelines establishing the importance of comprehensive drug development programs in children [2,4], most pivotal paediatric trials have been completed in Phase IV as a postapproval replication of adult data. Formulations, target doses, and expected effect size—which determines trial design and sample size—have been largely extrapolated from data collected in adult studies. Overall, the typical practice has been to extend the use of AEDs approved for adult epilepsy to children [5].
It has been shown that unpredictable differences may exist in drug metabolism and treatment response between children and adults [4,6]. For instance, the clearance of modern AEDs was found to be 20% to 170% higher in children than in adults [7]. Trial design could be affected as well—reluctance to recruit children with epilepsy into AEDs trials [8] might result in the selection of more severe epilepsy in paediatric than in adult RCTs. Furthermore, it has been suggested that the size of the placebo effect may be greater than usually expected in children with developmental disabilities [9] or migraine [10–12].
Whether age differences might exist in the treatment of drug-resistant partial epilepsy has never been investigated, despite the possibility that they may have an important impact on the design of paediatric trials. Specifically, a greater placebo effect in children than in adults would narrow the expected effect size of AED treatment in the former population, and it could lead to falsely negative RCT outcomes if the sample size is directly extrapolated from data collected in adult studies. To test this issue we performed a meta-analysis that compared AED efficacy in paediatric and adult RCTs.
Materials and Methods
Literature Search
See Text S1 for the review protocol and Text S2 for the QUOROM checklist.
Three electronic databases (MEDLINE, EMBASE, and the Cochrane Library) were searched from 1960 to 30 June 2007, for RCTs investigating any AED in the add-on treatment of drug-resistant partial epilepsy. A detailed search strategy is provided in Text S3. We searched for any additional studies (i) in the clinical trials registries ISRCTN (http://isrctn.org/), Current Controlled Trials (http://www.controlled-trials.com/mrct/), ClinicalTrials.gov (http://www.ClinicalTrials.gov/), and the Centre for Reviews and Dissemination (University of York, UK) Database of Abstracts of Reviews of Effectiveness (DARE; http://www.crd.york.ac.uk/); (ii) in the abstracts of the International Epilepsy Congress and the European Epilepsy Congress, the annual meeting of the American Epileptic Society (http://www.aesnet.org/), and the annual meeting of the American Academy of Neurology (http://www.aan.com/); (iii) in the references of all identified publications, including previous relevant meta-analyses and narrative reviews; and (iv) by contacting the pharmaceutical companies involved in the development of modern licensed AEDs (including gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, vigabatrin, and zonisamide) to exclude the possibility of relevant unpublished RCTs. No language restrictions were applied.
Study Selection
The following criteria were used to select relevant RCTs: double-blind placebo-controlled design; patients with drug-resistant partial epilepsy with or without secondary generalization; adequate method of concealment of randomization (e.g., allocation of sequentially numbered sealed packages of medication, sealed opaque envelopes, telephone randomization) minimum baseline period 4 wk; minimum treatment phase 8 wk; efficacy as a primary endpoint; available data regarding the 50% responder rate; and number of withdrawals due to loss to follow up unlikely to affect the robustness of the results. Only drugs that have been evaluated in both adult and paediatric populations were included in further analyses.
We focused on drug-resistant partial epilepsy for several reasons. AEDs are first evaluated in patients with this condition before being tested in those with newly diagnosed epilepsy and refractory generalised epilepsies [3]. Furthermore, in RCTs in patients with newly diagnosed epilepsy, for ethical reasons AEDs are usually not compared to placebo but to another active treatment, whereas the nine RCTs performed in patients with refractory generalized epilepsy included a mixed population of children and adults without providing detailed results by age groups [13–21].
Drug-resistant partial epilepsy is defined by the International League Against Epilepsy (ILAE; http://www.ilae.org/) as an epileptic disorder characterised by: (i) seizures whose initial signs and symptoms indicate, or are consistent with, initial involvement of only part of one hemisphere [22] and (ii) persistent seizures during a minimum of 2 y despite treatment with trials of at least two different AEDs used at the therapeutic level [23–26]. However, the inclusion criteria used in RCTs are more stringent and usually require that the patient had failed three or more AEDs, while the actual average number of failed AEDs reported in those trials is often eight or more [23].
Data Abstraction
The following information were extracted and entered independently into databases by two investigators (SR and PR): (i) study design—number of treatment arms, masking description, randomisation description, baseline description, titration period, and treatment period duration; (ii) patients' characteristics—age, type of seizures (partial or generalized), number of patients per arm, percentage of male sex, number of concomitant AEDs, and median seizure frequency during the baseline period (28 d); (iii) intervention—type and mean dose; (iv) outcomes — primary and secondary outcomes of the study, number of 50% responder patients per arm in an intention to treat (ITT) analysis on the whole treatment period, number of withdrawals per arm, and number of patients excluded from the published analysis with reasons.
For crossover studies, the first treatment period was treated as a parallel trial (i.e., only data from the first treatment period were used), and we discarded the second treatment period. If data provided by the authors in tables differed slightly from published figures, the tabular data were used. If some information about study design or seizure outcomes were not reported, the authors were contacted to obtain the required information.
Trials were finally classified as paediatric or adult trials according to the age of the patients. Paediatric trials were defined by a maximum age of patients < 18 y [27]. Adult trials included a majority of patients ≥ 18 y, but some also included a few adolescents (age > 12 y [27]).
The results of the two resulting databases were compared and disagreements resolved by consensus. The quality of the reports was assessed by the Jadad score [28].
Endpoints
The primary endpoint was the 50% responder rate (50% or greater reduction in seizure frequency during the treatment period as compared to baseline). All doses evaluated in trials were included. A secondary analysis was restricted to comparable doses between paediatric and adult studies. Dosages were considered “comparable” when the difference between paediatric and adult doses (expressed in mg/kg) was < 35%. In adult trials, where the target dose was never adjusted to body weight, we calculated the mean dose (mg/kg) by dividing the target dose (mg) by the average body weight of the population. When this latter information was not provided, we used the average body weight observed in the other trials included in our study (i.e., 70 kg).
Secondary endpoints included the seizure-free rate (no seizure during the entire treatment period); the total withdrawal rate, which included all dropouts occurring during the treatment period; the adverse events withdrawal rate, which included only dropouts related to side effects; and the withdrawal rate for seizure aggravation.
The 50% responder and withdrawal rates for active AEDs treatment and placebo arms were extracted in an ITT analysis on the whole treatment period.
The seizure-free rate for active AEDs treatment and placebo arms was extracted in a modified ITT analysis. Only patients who remained seizure free during the entire treatment period were considered to achieve seizure-free outcome. Indeed, the alternative use of standard ITT analysis, based on the principle of last observation carried forward, has been considered inappropriate for analysing seizure-free outcome [29].
Statistical Methods
To evaluate efficacy of AEDs, we performed a meta-analysis that compared the 50% responder rate in active AEDs treatment group with the one observed in placebo group, and used the logarithm of relative risk method. This analysis was done separately in paediatric and in adult populations. Treatment effect and heterogeneity tests were performed, and a p-value of < 0.05 from a treatment effect test and of < 0.10 from a heterogeneity test (Cochrane Q-test) were considered statistically significant. We also used the I2 statistic and its 95% CI, which is independent of the number of studies and quantifies heterogeneity on a scale of 0% to 100%. Very large heterogeneity between studies is usually denoted by I2 values of 75% or more [30]. In the absence of a clear explanation for heterogeneity, a random-effect model for the relative risk (RR) was planned.
To compare efficacy of AEDs in children and in adults, we calculated for each drug the ratio of the RR of the 50% responder rate observed in paediatric and in adult trials. To further assess the robustness of this ratio, we used a meta-regression approach with the log odds ratio as a dependent variable and a restricted maximum likelihood method for the estimation. Subanalyses were planned a priori to determine whether doses of AEDs or methodological quality was responsible for significant heterogeneity. The same statistical methods were used to analyse the seizure-free rate, the total withdrawal rate, the adverse events withdrawal rate, and the withdrawal rate for seizure aggravation.
Meta-analyses were performed using the software EasyMA [31,32] and their results are presented graphically, including RRs and corresponding 95% CIs, as well as RR ratio for the comparison between adult and children.
The baseline risk of the primary and secondary endpoints was compared between adult and paediatric trials using logistic regression, with a level of significance at p < 0.05.
Results
The initial search for RCTs investigating any AED in the add-on treatment of drug-resistant partial epilepsy yielded 2,122 articles. A review of the abstracts and exclusion of irrelevant and duplicate articles yielded 109 articles (Figure 1). Of the 109 articles examined, we excluded 25 open-label trials or trials with inappropriate blinding methods, seven studies with inappropriate randomization, two studies with a response conditional design, two studies with a period of treatment phase shorter than 8 wk, one monotherapy study, ten studies with inappropriate baseline, five review articles, five studies for which we were unable to determine the 50% responder rate, and two duplicate studies, leaving 50 potentially eligible studies (Figure 1). Among these 50 studies, we excluded 18 that investigated AEDs that have not been evaluated in paediatric populations (Figure 1). Two trials were only published in abstract [33,34]. However, the main data regarding the study design and the 50% responder rate were directly available in these abstracts, and missing information could be retrieved in the corresponding systematic review performed by the Cochrane Collaboration Group [35,36]. No other unpublished RCTs fulfilling our criteria was notified by pharmaceutical companies.
Fig. 1. Flow Diagrams of Trials 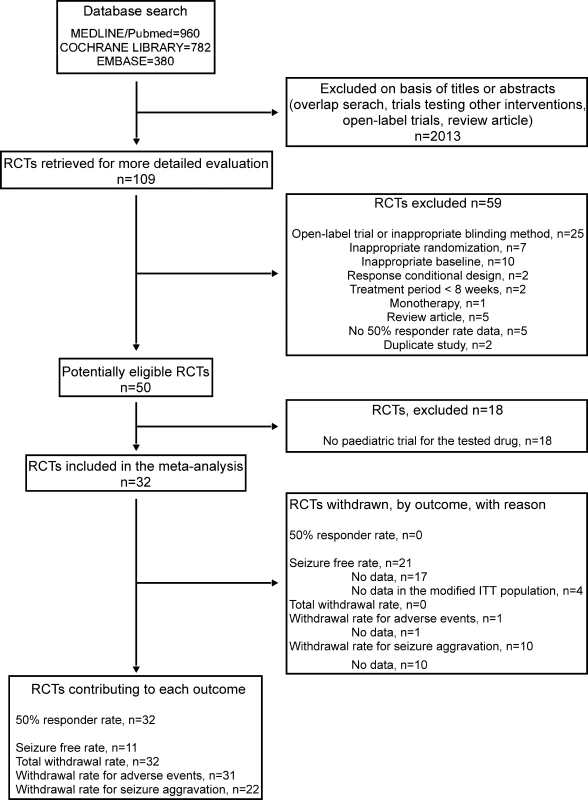
Five drugs—gabapentin (GBP), levetiracetam (LEV), lamotrigine (LTG), oxcarbazepine (OXC), and topiramate (TPM)—were evaluated in 32 RCTs fulfilling our inclusion criteria, with at least one trial in children and another one in adults for each AED. Five RCTs included children; the other 27 included adults. The main characteristics and results of these trials are given in Tables 1 and 2, as well as in Figure 2, while their detailed characteristics are presented in Text S4. The mean age of patients was 10 y in paediatric trials and 33 y in adult trials. In children, it ranged from 2 to 17 y. However, information about baseline characteristics and seizure outcome in different age subgroups (i.e., < 6, 6–12, and > 12 y) was never provided. Study designs were similar between paediatric and adult trials. All paediatric trials were performed with parallel groups. In adults, there were nine crossover trials and 18 trials with parallel groups. The mean duration of the baseline was nonsignificantly shorter in children than in adults (7.6 and 9.5 wk, respectively; p = 0.067). The mean duration of the treatment period was 15 wk in both paediatric and adult trials. With the exception of LTG trials, in which the paediatric dose was similar to the adult one, the mean dose studied was different in children and adults trials. It was 57% higher in the paediatric GBP trial than in adults, 100% higher in the paediatric LEV trial than in adults, and 60% higher in the paediatric OXC study than in adults. By contrast, for TPM, the mean dose studied was 50% lower in paediatric trials than in adults. We did not observe differences in the baseline patient characteristics, suggesting substantial clinical heterogeneity between adult and paediatric trials, although some data were missing in some trials (Table 1). The sex ratio, seizure types, baseline seizure frequency, and number of background AEDs were similar in both populations. An average of 85% of adult patients and 82% of children suffered from complex partial seizures, whereas 41% of adults and 47% of children suffered from secondarily generalised partial seizures (p = 0.44). The median seizure frequency per month was 11.8 in children and 10.1 in adults (p = 0.69). When these figures were compared for each drug, separately, comparable median seizure frequency were found in children and adults for GBP, LTG, LEV, and OXC. By contrast, in TPM trials, children had a median seizure frequency twice that of adults (21 and 10.5 per month, respectively). The aetiology of the disease was rarely specified, and could not be compared between age groups. However, it should be stressed that 40% of the children included in the LTG paediatric trial were diagnosed as suffering from idiopathic epilepsy. This form of epilepsy was not reported in other trials, and is often considered an exclusion criterion in RCTs performed in adult patients with drug-resistant partial epilepsy.
Tab. 1. 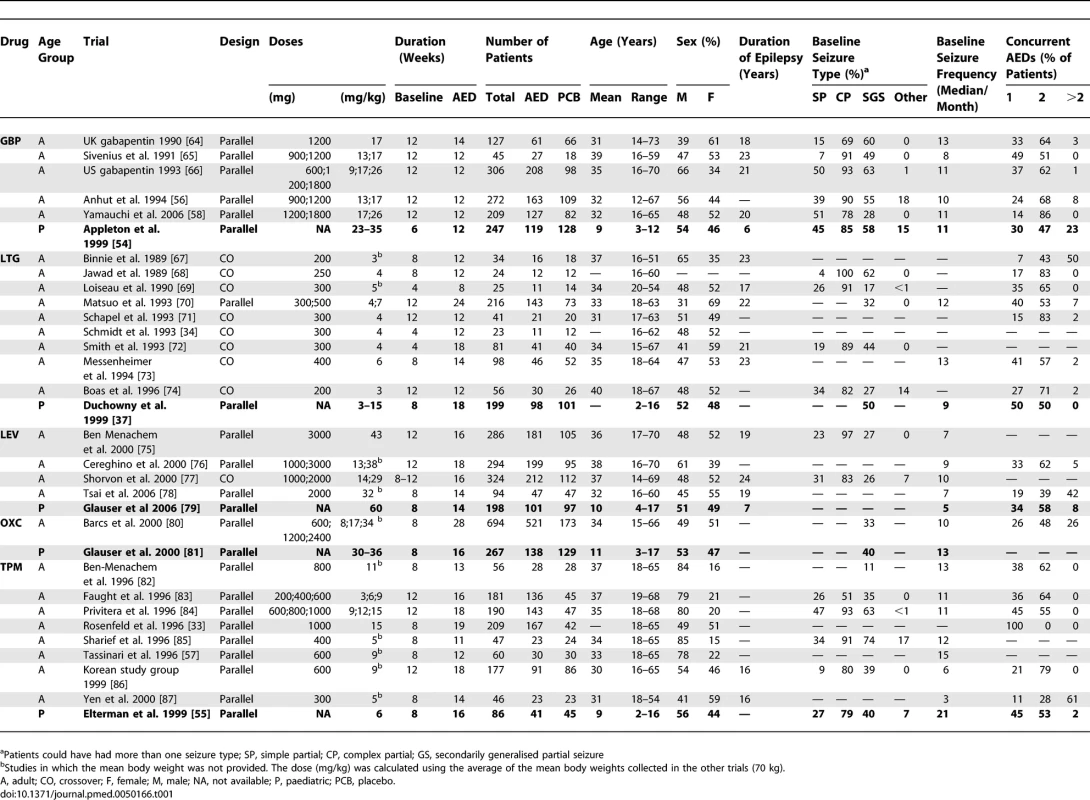
Characteristics of Studies Included in the Meta-Analysis Tab. 2. 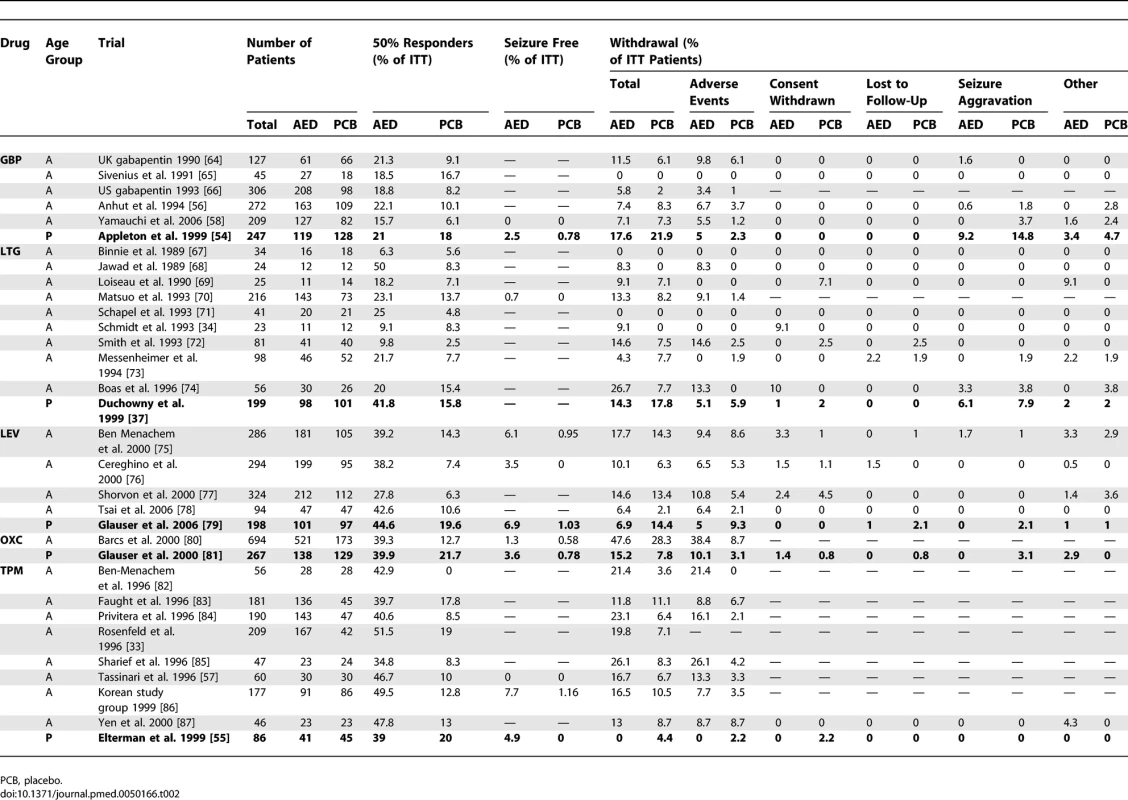
Percentages of 50% Responders, Seizure-Free Patients, and Withdrawals Fig. 2. Proportion of 50% Responders in Each Trial 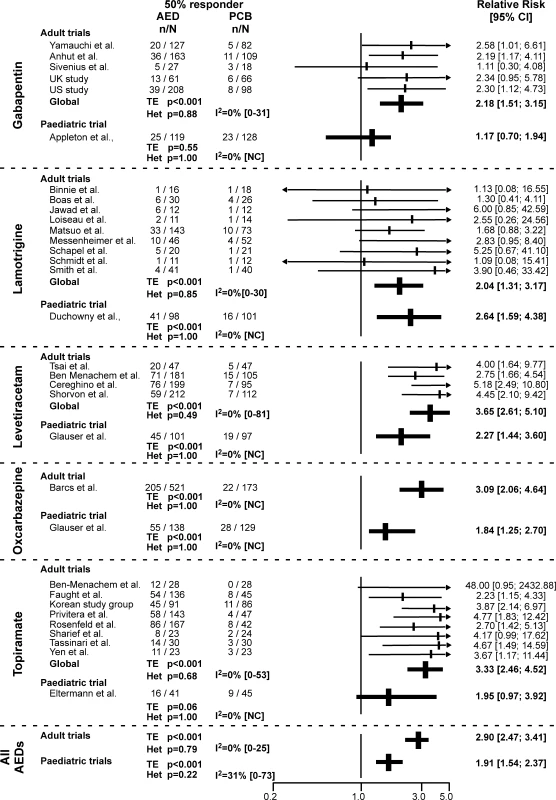
I2, point estimates of Higgins I2 with confidence interval; p Het, value of the heterogeneity test; p TE, value of the treatment effect test. Primary Endpoint: Comparison of the 50% Responder Rate
Indirect comparison of the RR of the 50% responder rate in adults and children showed lower values in children (1.91 [95% CI 1.54–2.37]) than in adults (2.90 [2.47–3.41]), with confidence intervals that did not overlap (Figures 2 and 3A). When we tested this issue further in a meta-analysis that calculated the RR ratio of the 50% responder rate between adults and children for each of the five drugs, we confirmed a significant difference (0.67 [95% CI 0.51–0.89], p = 0.007) pointing to lower values in children (Figure 3A). No significant heterogeneity was observed between drugs in this analysis (Figures 2 and 3A). These findings were further confirmed by the meta-regression, demonstrating a significant relation between the age of patients and the treatment effect (p = 0.02).
Fig. 3. Analysis of the 50% Responder Rate Including All Dosages 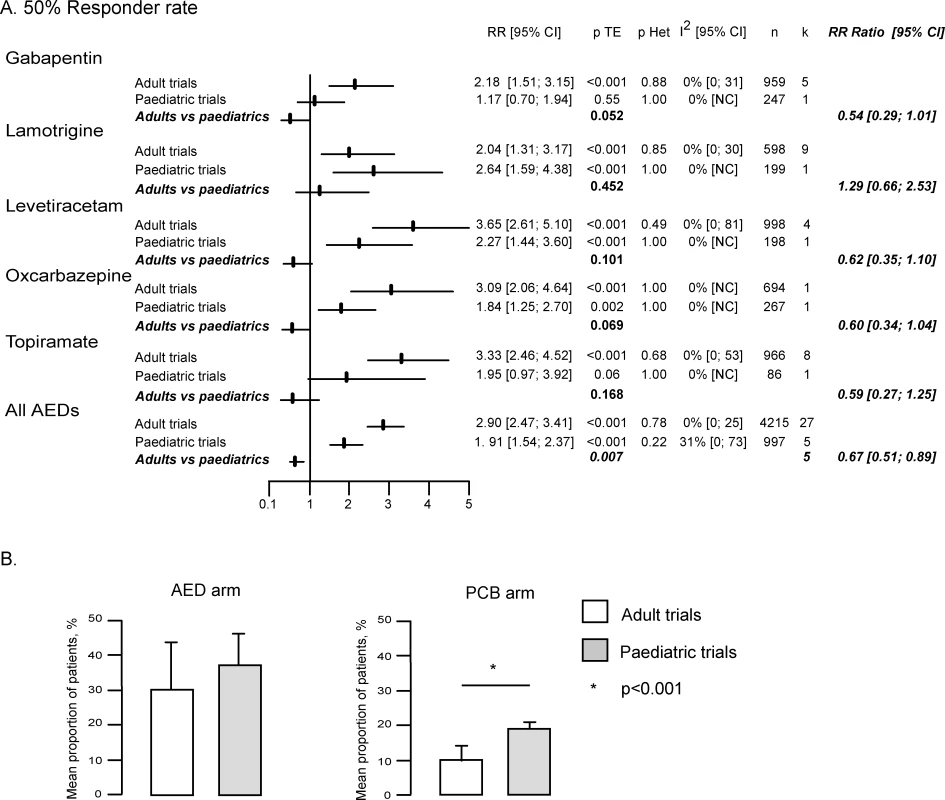
(A) RR of 50% responder rate in adult and paediatric trials and comparison of RR of 50% responder rate in children and adults. The above findings were found to primarily reflect differences in the response of the two populations to placebo. Indeed, logistic regression showed that the mean 50% responder rate in patients receiving placebo was significantly higher in children (19% ± 2.3%) than in adults (9.9% ± 4.6%) (p < 0.001), whereas no significant difference was observed in the response to AEDs between these two populations (37.2% ± 9.4% in children versus 30.4% ± 13.9% in adults, p = 0.364) (Figure 3B). The 1.9 times greater 50% responder rate observed in children receiving placebo as compared to adult was a consistent finding across all AEDs, including LTG (1.7 for OXC and GBP, 1.8 for TPM, 1.9 for LEV, and 2.0 for LTG).
All above results remained valid when the same analyses were restricted to comparable doses between paediatric and adult trials (Figure S1). The RR ratio of the 50% responder rate between adults and children was 0.63 (95% CI 0.47–0.84) (p = 0.002). These findings were confirmed by the meta-regression, demonstrating a significant relation between the age of patients and the treatment effect (p = 0.02). Similarly, the 50% responder rate in patients receiving placebo remained significantly higher in children (19% ± 2.3%) than in adults (10.1% ± 4.1%) (p < 0.001, Figure S1B).
Secondary Endpoints
Seizure-free rate (Figure 4).
The seizure-free rate was provided in only 15 of the 32 trials, including 11 of the 27 adults trials and four of the five paediatric trials (Table 2). Furthermore, the published data did not allow to calculate the number of seizure-free patients in the modified ITT population (see methods) in seven trials. However, these data could be retrieved for three of these seven trials from a recently published meta-analysis that focused on seizure-free outcome [29]. Overall, the seizure-free rate in the modified ITT population was available for four AEDs (GBP, LEV, OXC, and TPM) in seven adult and four children trials (Table 2).
The RR of seizure-free rate was 4.19 (95% CI 1.45–12.14) in adult trials and 5.00 (1.52–16.42) in paediatric trials. There was no significant difference between adult and paediatric studies (RR ratio 1.42 [95% CI 0.23–8.77], p = 0.704; p = 0.68 by meta-regression). In addition, the seizure-free rate in treatment and placebo arms were similar in both populations, with 2.8% of adults and 4.5% of children receiving AEDs (p = 0.256), and 0.4% of adults and 0.6% of children receiving placebo (p = 0.607) achieving seizure freedom during the entire treatment period.
Fig. 4. Analysis of the Seizure-Free Rate 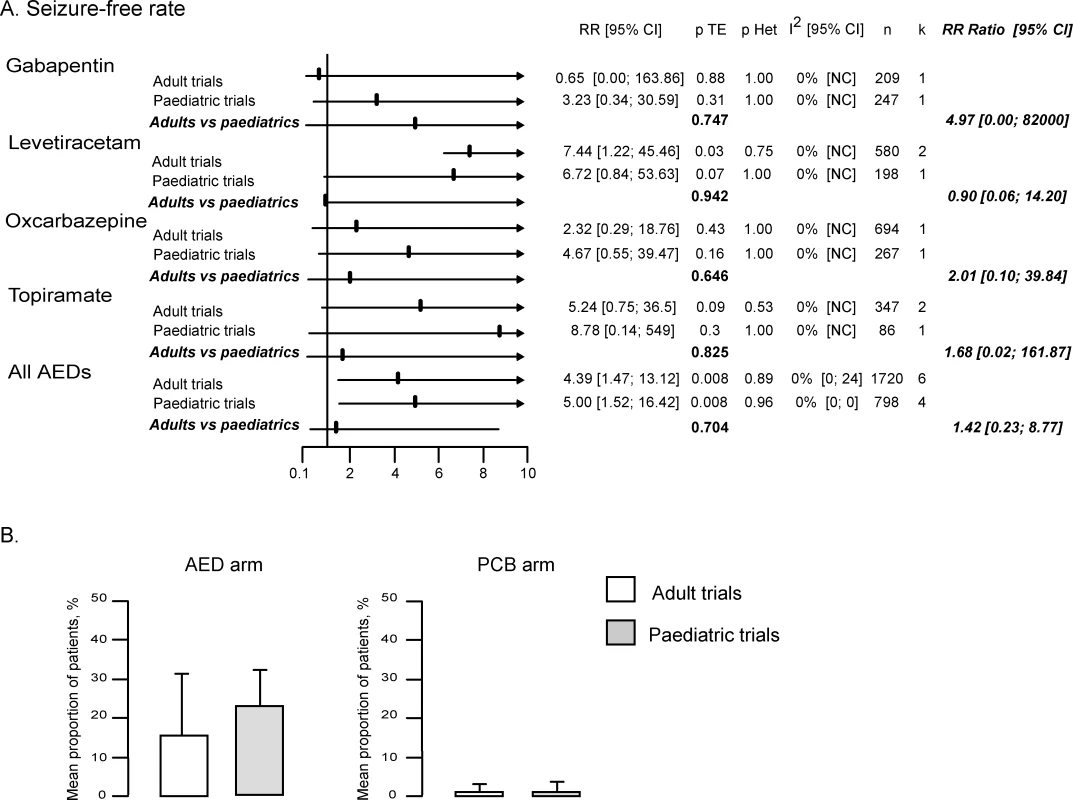
(A) RR of seizure-free rate in adult and paediatric trials and comparison of RR of seizure-free rate in children and adults. Withdrawal rates (Figure 5).
In adults, both the total and adverse events withdrawal rates were significantly more frequent in treatment than in placebo arms. Indeed, the RR of the withdrawal rates for any reason and for adverse events were respectively 1.57 (95% CI 1.33–1.86) and 2.49 (1.90–3.28) (Figures 5A and 5B). By contrast, in children, neither the total nor the adverse events withdrawal rates differed between treatment and placebo arms (RR 0.89 [95% CI 0.64–1.22] for total withdrawal rate and of 1.24 [0.70–2.20] for adverse events withdrawal rate). When comparing the RRs of the total withdrawal rate between the two populations, there was no overlap between the confidence intervals observed in children (0.64–1.22) and adults (1.33–1.86). Accordingly, meta-analysis of the RR ratio demonstrated significant differences between the two populations for the total withdrawal rate, with a ratio of 0.65 (95% CI 0.43–0.98, p = 0.037) pointing to lower RR values in paediatric trials (Figure 5A), a finding confirmed by the meta-regression (p = 0.004). When analysing the above differences, we found a nonsignificant higher total withdrawal rate in children receiving placebo (13.3%) than in adults (7%) (p = 0.08, Figure 5D), whereas no difference was observed between the two populations in the active treatment arm (13.4% in adults and 11.8% in children, p = 0.775).
Fig. 5. Analysis of the Withdrawal Rates 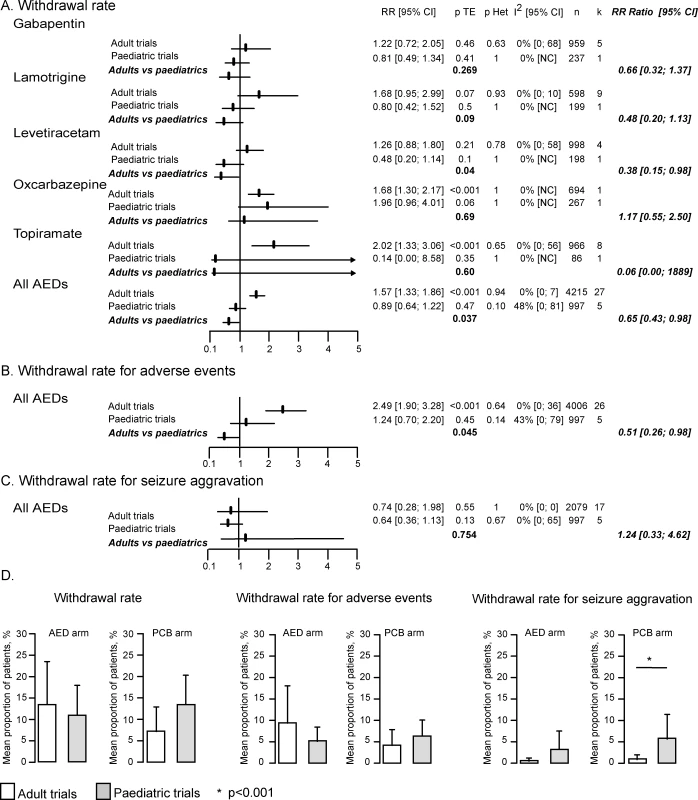
(A) RR of withdrawal for any reason in adult and paediatric trials and comparison of RR of the total withdrawal rate in children and adults. The meta-analysis of the RR ratio for the adverse events withdrawal rate also demonstrated significantly lower values in children than in adult RCTs, with a ratio of 0.51 (95% CI 0.26–0.98, p = 0.045). However, this finding was not confirmed by the meta-regression (p = 0.08).
There was no difference in the RR of withdrawal rate for seizure aggravation between adults (0.74 [95% CI 0.28–1.98], p = 0.55) and children (1.24 [0.70–2.20], p = 0.13), as further demonstrated by the RR ratio (1.24 [0.33–4.62], p = 0.754, Figure 5C) and the meta-regression (p = 0.605). However, logistic regression showed that children receiving placebo dropped out for an aggravation of seizures (5.6%) more frequently than adults (0.7%) (p < 0.001, Figure 5D).
There was no difference between paediatric and adult trials in loss to follow-up and consent withdrawal (Table 2).
Discussion
In this meta-analysis, we found that the RR of the 50% responder rate for active AEDs over placebo, one of the main measurements of AED efficacy in drug-resistant partial epilepsy, was significantly lower in paediatric trials than in adult ones. This difference was primarily related to a 2-fold higher placebo responder rate in children than in adults. Such a difference, if ignored, could well result in an underestimation of the placebo response and type II errors in paediatric RCTs.
Study Limitations
Only five RCTs fulfilling our selection criteria were performed in children, representing one trial per AED. Thus, each paediatric trial might have had an important weight in the meta-analysis. However, the very consistent findings observed among four of the five selected AEDs strongly suggest that our main result did not depend on any single paediatric trial, but rather on the consistency of the majority of RCTs in children.
Our study focused on refractory partial epilepsy, for which AEDs are usually tested during Phases II and III before further evaluation is considered in newly diagnosed epilepsy and refractory generalised epileptic syndromes. Diagnosis of partial seizures was always based on the association of ictal signs and symptoms suggesting an underlying focal neuronal discharge and consistent neurophysiological findings on a recent electroencephalogram. According to these inclusion criteria, it is very unlikely that the patients included in the selected RCTs could have been misdiagnosed.
However, differences in the type of partial epilepsy might account for the opposite trend observed for LTG as compared to the four other AEDs, with higher RR of 50% responder rate in children than in adults. Idiopathic partial epilepsies, which are typically benign conditions, are excluded in Phase III RCTs performed in adult patients with drug-resistant partial epilepsy, and are also unlikely to meet the inclusion criteria of RCTs performed in children with refractory partial seizures. Accordingly, there was no indication that patients with idiopathic epilepsy were included in the RCTs selected for this meta-analysis, with the exception of the LTG paediatric trial in which 40% of patients were classified as having idiopathic epilepsy [37]. This rate could partly account for the 2-fold higher responder rate observed in children receiving LTG compared to adults (42% versus 20%), a difference not observed for the four other AEDs (GBP, 21% versus 19%; OXC, 40% versus 39%; TPM, 39% versus 44%; LEV, 45% versus 37%).
More generally, we cannot exclude the possibility that clinical heterogeneity between adults and children as well as between trials in the same age group might have interfered with our results. It has recently been suggested that the statistical tests available for evaluating heterogeneity between studies (i.e., Cochran Q and Higgins I2) may suffer some limitations [38,39]. Although the I2 did not demonstrate significant heterogeneity in our meta-analysis, its upper 95% CI ranged between 50% and 75% in some analyses, and more rarely exceeded the 75% threshold. Comparable confidence intervals of I2 have been reported in 83% of the Cochrane meta-analyses [39], but no recommendation on the management of this limitation has yet been published.
The reluctance to recruit children with epilepsy into AEDs trials [8] might have resulted in the selection of more severe epilepsy in paediatric than in adult RCTs. In fact, the baseline median monthly seizure frequency was comparable in trials with children and adults for the entire study as well as for four of the five tested AEDs (GBP, LTG, LEV, and OXC). Furthermore, the important issue of poor seizure count reliability [40,41], which might differ between children and adults, is also unlikely to explain a difference in the 50% responder rate selectively to placebo.
Drug dosages differed between adult and paediatric trials, partly reflecting the fact that the clearance of modern AEDs is 20%–170% higher in children than in adults [7]. However, our main findings remain unchanged when we reprocessed all our analyses among a selection of RCTs and study arms that resulted in comparable doses in the two age groups.
We used an ITT assessment that may be overly conservative. However, it must be stressed that the same method was used in all the Cochrane systematic reviews of AEDs [35,36,42–48], as well as in previously published meta-analyses [49–51]. The major reason for preferring ITT rather than per-protocol analysis is that the criteria used for defining the latter greatly vary between trials and AEDs.
Finally, whether the age-dependent variation of the response to placebo observed in this study might apply to generalized epilepsies remains to be determined.
Higher Response to Placebo in Children Than in Adults
The overall difference in the RR of being a 50% responder between adult and paediatric trials was primarily related to an age-dependent variation in the response to placebo. Indeed, placebo 50% responder rate was 1.9 times higher in children than in adults, in a very consistent way across all AEDs, including LTG. It has been suggested that the response to placebo may depend on the age of patients [9–12]. It should be remembered, however, that the RCTs considered in our meta-analysis did not include a patient group receiving no intervention. Thus, we did not measure the placebo effect per se but all nonpharmacological effects that include the placebo effect, but also the Hawthorne effect, the regression to the mean, and the clinical course of epilepsy.
The reasons underlying the higher response to placebo in paediatric populations remains largely unknown and mostly speculative. In placebo-controlled studies of antimigraine agents, adolescents were more likely to believe that the treatment received was an effective pain relieving medication than were adults [12]. A placebo effect by proxy may also be suggested in younger populations, since parents play an important role in reporting the outcome of their affected child [9]. The higher 50% responder rate observed in our study in children compared to adults may also partly reflect a regression to the mean effect. This regression to the mean effect might be greater in paediatric than in adults RCTs due to several factors, including the marked reluctance of parents to enrol their affected child in placebo-controlled trials [5], the trend observed in this meta-analysis toward shorter baseline duration in paediatric than in adult RCTs, and the possibility of greater short-term changes in seizure frequency in children than in adults.
In contrast to the difference in the RR of the 50% responder rate observed between children and adults, the RR of the seizure-free rate was found to be comparable in the two populations. Seizure-free rates in placebo groups were quite low in both children (0.6%) and adults (0.4%), suggesting that this outcome is insensitive to nonpharmacological effects. However, the seizure-free rate was also low in patients receiving AEDs (4.5% in children and 2.8% in adults), accounting for the fact that this outcome failed to show a significant difference between active treatment and placebo arms in the 11 series in which it was available. Thus, the seizure-free rate, though representing a more clinically relevant and less placebo-sensitive outcome than the 50% responder rate, appears inappropriate for Phase III RCTs in patients with drug-resistant partial epilepsy.
We observed a significantly lower RR of the withdrawal rate in children than in adults, primarily reflecting a greater dropout rate from placebo arms in the former population (13.3%) than in the latter (7%). This greater dropout rate in placebo-treated children might have theoretically contributed to the increased 50% responder rate observed in the same population. Indeed, according to the method of last observation carried forward used in all the selected RCTs, patients who withdraw early from the study because of adverse events might be more likely to achieve a 50% reduction in seizure frequency during the shorter time spent in the trial. However, this possibility does not seem to apply to our findings, since the greater withdrawal rate observed in children receiving placebo was primarily due to more frequent aggravation of seizures in these patients (5.6%) than in adults allocated to placebo (0.7%).
Impacts on Future AEDs Trials in Paediatric Populations
The impact of our findings on future AED development programs in children needs to be emphasized. Recent reviews and guidelines have established that comprehensive drug development is needed in paediatric populations, including double-blind RCTs, but also have stressed the difficulties in achieving this goal [2,4]. Both the United States and the European Union have developed paediatric investigation plans, including financial incentives for pharmaceutical companies to perform specific drug trials in children [4,52,53]. These regulations aim at facilitating the development and use of medicinal products in children and at ensuring that medicinal products used to treat the paediatric population are subject to ethical, high-quality research [52]. These regulations also establish that the above objectives should be achieved without delaying the authorisation of medicinal products for other age populations [27,52] and without subjecting the paediatric population to unnecessary clinical trials [52]. In that context, one can hardly afford the risk of an underestimated sample size and related type II error that would result in a falsely negative trial. In fact, two of the five selected RCTs performed in children with drug-resistant partial epilepsy failed to demonstrate a significant difference in the 50% responder rate between the active drug (GBP and TPM) and placebo [54,55], whereas adults trials with the same AEDs and comparable or slightly lower sample sizes all demonstrated significant differences in 50% responder rate between active treatment and placebo [56–58]. It would also be unethical to grossly overestimate the number of children to be included in a double-blind, placebo-controlled study, in order to ensure sufficient statistical power. We thus need to provide as much precise data as we can to optimally design paediatric RCTs in drug-resistant partial epilepsy. According to the results of this meta-analysis, we recommend calculating statistical power for paediatric RCTs on the premise of a 50% responder rate comparable to that observed in adults for the active treatment group, and of 20% (or twice that observed in the adult RCTs for the same AED) for placebo.
The value of add-on placebo-controlled RCTs in children with drug-resistant partial epilepsy may also be in question. Other trial designs such as the comparison of low and high doses of the same drug [59] or response-conditional design [60–63] have been proposed, but both have possible statistical [8] or ethical issues [62]. Thus, it is likely that classic add-on placebo-controlled RCTs will continue to be used for the development of AEDs in the near future, and will benefit from more precise knowledge regarding the response to placebo in the paediatric population.
Supporting Information
Zdroje
1. GuerriniR
2006
Epilepsy in children.
Lancet
367
499
524
2. GarofaloE
2006
Obtaining pediatric indications for new anti-epileptic drugs: how and when.
Epilepsy Res
68
38
42
3. SchmidtB
2007
Clinical development of antiepileptic drugs in adults.
Neurotherapeutics
4
62
69
4. CaldwellPHMurphySBButowPNCraigJC
2004
Clinical trials in children.
Lancet
364
803
811
5. PellockJM
1998
Pediatric trials: practical issues. Special populations and trial design.
Adv Neurol
76
167
171
6. SteinbrookR
2002
Testing medications in children.
N Engl J Med
347
1462
1470
7. PeruccaE
2006
Clinical pharmacokinetics of new-generation antiepileptic drugs at the extremes of age.
Clin Pharmacokinet
45
351
363
8. ShinnarSPellockJM
2005
The trials and tribulations of pediatric drug trials.
Neurology
65
1348
1349
9. SandlerA
2005
Placebo effects in developmental disabilities: implications for research and practice.
Ment Retard Dev Disabil Res Rev
11
164
170
10. FernandesRFerreiraJJSampaioC
2008
The placebo response in studies of acute migraine.
J Pediatr
152
527
33
533.e.l
11. LewisDWWinnerPWasiewskiW
2005
The placebo responder rate in children and adolescents.
Headache
45
232
239
12. RothnerADWasiewskiWWinnerPLewisDStankowskiJ
2006
Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents.
Headache
46
101
109
13. [No authors listed]
1993
Efficacy of felbamate in childhood epileptic encephalopathy (Lennox-Gastaut syndrome). The Felbamate Study Group in Lennox-Gastaut Syndrome.
N Engl J Med
328
29
33
14. BeranRGBerkovicSFDunaganFMVajdaFJDantaG
1998
Double-blind, placebo-controlled, crossover study of lamotrigine in treatment-resistant generalised epilepsy.
Epilepsia
39
1329
1333
15. BerkovicSFKnowltonRCLeroyRFSchiemannJFalterU
2007
Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy.
Neurology
69
1751
1760
16. BitonVMontourisGDRitterFRivielloJJReifeR
1999
A randomized, placebo-controlled study of topiramate in primary generalized tonic-clonic seizures. Topiramate YTC Study Group.
Neurology
52
1330
1337
17. BitonVSackellaresJCVuongAHammerAEBarrettPS
2005
Double-blind, placebo-controlled study of lamotrigine in primary generalized tonic-clonic seizures.
Neurology
65
1737
1743
18. ChadwickDLeidermanDBSauermannWAlexanderJGarofaloE
1996
Gabapentin in generalized seizures.
Epilepsy Res
25
191
197
19. GlauserTKlugerGSachdeoRKraussGPerdomoC
2008
Rufinamide for generalized seizures associated with Lennox-Gastaut syndrome.
Neurology
17
1950
1958
20. MotteJTrevathanEArvidssonJFBarreraMNMullensEL
1997
Lamotrigine for generalized seizures associated with the Lennox-Gastaut syndrome. Lamictal Lennox-Gastaut Study Group.
N Engl J Med
337
1807
1812
21. SachdeoRCGlauserTARitterFReifeRLimP
1999
A double-blind, randomized trial of topiramate in Lennox-Gastaut syndrome. Topiramate YL Study Group.
Neurology
52
1882
1887
22. [No authors listed]
1989
Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy.
Epilepsia
30
389
399
23. FrenchJAKannerAMBautistaJAbou-KhalilBBrowneT
2004
Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society.
Neurology
62
1261
1273
24. GumnitRJWalczakTS
2001
Guidelines for essential services, personnel, and facilities in specialized epilepsy centers in the United States.
Epilepsia
42
804
814
25. PerrucaE
1998
Pharmacoresistance in epilepsy: how should it be defined.
CNS Drugs
10
171
179
26. RegestaGTanganelliP
1999
Clinical aspects and biological bases of drug-resistant epilepsies.
Epilepsy Res
34
109
122
27. European Medicines Agency
2001
International Conference on Harmonisation.
ICH topic E11 clinical investigation of medicinal products in the paediatric population
Available: http://www.emea.eu/pdfs/human/ich/271199EN.pdf. Accessed 07 July 2008.
28. JadadARMooreRACarrollDJenkinsonCReynoldsDJ
1996
Assessing the quality of reports of randomized clinical trials: is blinding necessary.
Control Clin Trials
17
1
12
29. GazzolaDMBalcerLJFrenchJA
2007
Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs.
Epilepsia
48
1303
1307
30. HigginsJPThompsonSG
2002
Quantifying heterogeneity in a meta-analysis.
Stat Med
21
1539
1558
31. CucheratM
2000
EasyMA Sofware Version 2000
Available: http://www.spc.univ-lyon1.fr/easyma.net/. Accessed 07 July 08.
32. CucheratMBoisselJPLeizoroviczAHaughMC
1997
EasyMA: a program for the meta-analysis of clinical trials.
Comput Methods Programs Biomed
53
187
190
33. RosenfeldWAbou-KhalilBReifeRHegadusRPledgerG
1996
Placebo-controlled trial of topiramate as adjunctive therapy to carbamazepine or phenitoine for partial onset seizures.
Epilepsia
37
153
34. SchmidtDRiedRSRappP
1993
Add-on treatment with Lamotrigine for intractable partial epilepsy: a placebo-controlled, cross-over trial.
Epilepsia
34
66
35. JetteNJMarsonAGHuttonJL
2002
Topiramate add-on for drug-resistant partial epilepsy.
Cochrane Database Syst Rev CD001417
Available: http://www.cochrane.org/reviews/en/ab001417.html. Accessed 07 July 08.
36. RamaratnamSMarsonAGBakerGA
2001
Lamotrigine add-on for drug-resistant partial epilepsy.
Cochrane Database Syst Rev CD001909
Available: http://www.cochrane.org/reviews/en/ab001909.html. Accessed 07 July 08.
37. DuchownyMPellockJMGrafWDBillardCGilmanJ
1999
A placebo-controlled trial of lamotrigine add-on therapy for partial seizures in children. Lamictal Pediatric Partial Seizure Study Group.
Neurology
53
1724
1731
38. Huedo-MedinaTBSanchez-MecaFMarin-MartinezFBotellaJ
2006
Assessing heterogenity in meta-analysi: Q statistic or I2 index.
Psychol Methods
11
193
206
39. IoannidisJPatsopoulosNEvangelouE
2007
Uncertainty in heterogeneity estimates in meta-analyses.
BMJ
335
914
916
40. HoppeCPoepelAElgerCE
2007
Epilepsy: accuracy of patient seizure counts.
Arch Neurol
64
1595
1599
41. KerlingFMuellerSPauliEStefanH
2006
When do patients forget their seizures? An electroclinical study.
Epilepsy Behav
9
281
285
42. CastilloSSchmidtDBWhiteS
2000
Oxcarbazepine add-on for drug-resistant partial epilepsy.
Cochrane Database Syst Rev CD002028
Available: http://www.cochrane.org/reviews/en/ab002028.html. Accessed 07 July 08.
43. ChadwickDWMarsonAG
2005
Zonisamide add-on for drug-resistant partial epilepsy.
Cochrane Database Syst Rev CD001416
Available: http://www.cochrane.org/reviews/en/ab001416.html. Accessed 07 July 08.
44. ChaisewikulRPriviteraMDHuttonJLMarsonAG
2001
Levetiracetam add-on for drug-resistant localization related (partial) epilepsy.
Cochrane Database Syst Rev CD001901
http://www.cochrane.org/reviews/en/ab001901.html. Accessed 07 July 08.
45. LeachJPMarsonAGHuttonJL
2002
Remacemide for drug-resistant localization related epilepsy.
Cochrane Database Syst Rev CD001900
Available: http://www.cochrane.org/reviews/en/ab001900.html. Accessed 07 July 08.
46. LozsadiDHemmingKMarsonA
2008
Pregabalin add-on for drug-resistant partial epilepsy.
Cochrane Database Syst Rev CD005612
Available: http://www.cochrane.org/reviews/en/ab005612.html. Accessed 07 July 08.
47. MarsonAGKadirZAHuttonJLChadwickDW
2000
Gabapentin add-on for drug-resistant partial epilepsy.
Cochrane Database Syst Rev CD001415
Available: http://www.cochrane.org/reviews/en/ab001415.html. Accessed 07 July 08.
48. PereiraJMarsonAGHuttonJL
2002
Tiagabine add-on for drug-resistant partial epilepsy.
Cochrane Database Syst Rev CD001908
Available: http://www.cochrane.org/reviews/en/ab001908.html. Accessed 07 July 08.
49. MarsonAGHuttonJLLeachJPCastilloSSchmidtD
2001
Levetiracetam, oxcarbazepine, remacemide and zonisamide for drug resistant localization-related epilepsy: a systematic review.
Epilepsy Res
46
259
270
50. MarsonAGKadirZAHuttonJLChadwickDW
1997
The new antiepileptic drugs: a systematic review of their efficacy and tolerability.
Epilepsia
38
859
880
51. OtoulCArrigoCvan RijckevorselKFrenchJA
2005
Meta-analysis and indirect comparisons of levetiracetam with other second-generation antiepileptic drugs in partial epilepsy.
Clin Neuropharmacol
28
72
78
52. [No authors listed]
2006
Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medical products for paediatric use
Official Journal of the European Union
Available: http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/vol-1/reg_2006_1901/reg_2006_1901_en.pdf. Accessed 07 July 08.
53. National Institutes of Health
1998
NIH policy and guidelines on the inclusion of children as participants in research involving human subjects
Available: http://grants.nih.gov/grants/guide/notice-files/not98–024.html. Accessed 07 July 08.
54. AppletonRFichtnerKLaMoreauxLAlexanderJHalsallG
1999
Gabapentin as add-on therapy in children with refractory partial seizures: a 12-week, multicentre, double-blind, placebo-controlled study. Gabapentin Paediatric Study Group.
Epilepsia
40
1147
1154
55. EltermanRDGlauserTAWyllieEReifeRWuSC
1999
A double-blind, randomized trial of topiramate as adjunctive therapy for partial-onset seizures in children. Topiramate YP Study Group.
Neurology
52
1338
1344
56. AnhutHAshmanPFeuersteinTJSauermannWSaundersM
1994
Gabapentin (Neurontin) as add-on therapy in patients with partial seizures: a double-blind, placebo-controlled study. The International Gabapentin Study Group.
Epilepsia
35
795
801
57. TassinariCAMichelucciRChauvelPChodkiewiczJShorvonS
1996
Double-blind, placebo-controlled trial of topiramate (600 mg daily) for the treatment of refractory partial epilepsy.
Epilepsia
37
763
768
58. YamauchiTKanekoSYagiKSaseS
2006
Treatment of partial seizures with gabapentin: double-blind, placebo-controlled, parallel-group study.
Psychiatry Clin Neurosci
60
507
515
59. Pina-GarzaJEEspinozaRNordliDBennettDASpiritoS
2005
Oxcarbazepine adjunctive therapy in infants and young children with partial seizures.
Neurology
65
1370
1375
60. ChironCTonnelierSReyEBrunetMLTranA
2006
Stiripentol in childhood partial epilepsy: randomized placebo-controlled trial with enrichment and withdrawal design.
J Child Neurol
21
496
502
61. ChironCDulacOGramL
1996
Vigabatrin withdrawal randomized study in children.
Epilepsy Res
25
209
215
62. ChironCDulacOPonsG
2008
Antiepileptic drug development in children: considerations for a revisited strategy.
Drugs
68
17
25
63. Pina-GarzaJELevisohnPGucuyenerKMikatiMAWarnockCR
2007
Adjunctive lamotrigine for partial seizures in patients aged 1 to 24 months.
Neurology
64. [No authors listed]
1990
Gabapentin in partial epilepsy. UK Gabapentin Study Group.
Lancet
335
1114
1117
65. SiveniusJKalviainenRYlinenARiekkinenP
1991
Double-blind study of Gabapentin in the treatment of partial seizures.
Epilepsia
32
539
542
66. [No authors listed]
1993
Gabapentin as add-on therapy in refractory partial epilepsy: a double-blind, placebo-controlled, parallel-group study. The US Gabapentin Study Group No. 5.
Neurology
43
2292
2298
67. BinnieCDDebetsRMEngelsmanMMeijerJWMeinardiH
1989
Double-blind crossover trial of lamotrigine (Lamictal) as add-on therapy in intractable epilepsy.
Epilepsy Res
4
222
229
68. JawadSRichensAGoodwinGYuenWC
1989
Controlled trial of lamotrigine (Lamictal) for refractory partial seizures.
Epilepsia
30
356
363
69. LoiseauPYuenAWDucheBMenagerTArne-BesMC
1990
A randomised double-blind placebo-controlled crossover add-on trial of lamotrigine in patients with treatment-resistant partial seizures.
Epilepsy Res
7
136
145
70. MatsuoFBergenDFaughtEMessenheimerJADrenAT
1993
Placebo-controlled study of the efficacy and safety of lamotrigine in patients with partial seizures. U.S. Lamotrigine Protocol 0.5 Clinical Trial Group.
Neurology
43
2284
2291
71. SchapelGJBeranRGVajdaFJBerkovicSFMashfordML
1993
Double-blind, placebo controlled, crossover study of lamotrigine in treatment resistant partial seizures.
J Neurol Neurosurg Psychiatry
56
448
453
72. SmithDBakerGDaviesGDeweyMChadwickDW
1993
Outcomes of add-on treatment with lamotrigine in partial epilepsy.
Epilepsia
34
312
322
73. MessenheimerJRamsayREWillmoreLJLeroyRFZielinskiJJ
1994
Lamotrigine therapy for partial seizures: a multicenter, placebo-controlled, double-blind, cross-over trial.
Epilepsia
35
113
121
74. BoasJDamMFriisMLKristensenOPedersenB
1996
Controlled trial of lamotrigine (Lamictal) for treatment-resistant partial seizures.
Acta Neurol Scand
94
247
252
75. Ben-MenachemEFalterU
2000
Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory partial seizures: a multicenter, double-blind, responder-selected study evaluating monotherapy. European Levetiracetam Study Group.
Epilepsia
41
1276
1283
76. CereghinoJJBitonVAbou-KhalilBDreifussFGauerLJ
2000
Levetiracetam for partial seizures: results of a double-blind, randomized clinical trial.
Neurology
55
236
242
77. ShorvonSDLowenthalAJanzDBielenELoiseauP
2000
Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group.
Epilepsia
41
1179
1186
78. TsaiJJYenDJHsihMSChenSSHiersemenzelR
2006
Efficacy and safety of levetiracetam (up to 2000 mg/day) in Taiwanese patients with refractory partial seizures: a multicenter, randomized, double-blind, placebo-controlled study.
Epilepsia
47
72
81
79. GlauserTAAyalaREltermanRDMitchellWGVan OrmanCB
2006
Double-blind placebo-controlled trial of adjunctive levetiracetam in pediatric partial seizures.
Neurology
66
1654
1660
80. BarcsGWalkerEBElgerCEScaramelliAStefanH
2000
Oxcarbazepine placebo-controlled, dose-ranging trial in refractory partial epilepsy.
Epilepsia
41
1597
1607
81. GlauserTANigroMSachdeoRPasterisLAWeinsteinS
2000
Adjunctive therapy with oxcarbazepine in children with partial seizures. The Oxcarbazepine Pediatric Study Group.
Neurology
54
2237
2244
82. Ben-MenachemEHenriksenODamMMikkelsenMSchmidtD
1996
Double-blind, placebo-controlled trial of topiramate as add-on therapy in patients with refractory partial seizures.
Epilepsia
37
539
543
83. FaughtEWilderBJRamsayREReifeRAKramerLD
1996
Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 200-, 400-, and 600-mg daily dosages. Topiramate YD Study Group.
Neurology
46
1684
1690
84. PriviteraMFinchamRPenryJReifeRKramerL
1996
Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 600-, 800-, and 1,000-mg daily dosages. Topiramate YE Study Group.
Neurology
46
1678
1683
85. ShariefMViteriCBen-MenachemEWeberMReifeR
1996
Double-blind, placebo-controlled study of topiramate in patients with refractory partial epilepsy.
Epilepsy Res
25
217
224
86. Korean
1999
Topiramate in medically intractable partial epilepsies: double-blind placebo-controlled randomized parallel group trial. Korean Topiramate Study Group.
Epilepsia
40
1767
1774
87. YenDJYuHYGuoYCChenCYiuCH
2000
A double-blind, placebo-controlled study of topiramate in adult patients with refractory partial epilepsy.
Epilepsia
41
1162
1166
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2008 Číslo 8- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics
- Greater Response to Placebo in Children Than in Adults: A Systematic Review and Meta-Analysis in Drug-Resistant Partial Epilepsy
- Can a Topical Microbicide Prevent Rectal HIV Transmission?
- Developing a Prognostic Model for Traumatic Brain Injury—A Missed Opportunity?
- Assessing Antimalarial Efficacy in a Time of Change to Artemisinin-Based Combination Therapies: The Role of Médecins Sans Frontières
- Children Are Not Just Small Adults: The Urgent Need for High-Quality Trial Evidence in Children
- The Use of Nonhuman Primate Models in HIV Vaccine Development
- More Evidence Against a Causal Association between C-Reactive Protein and Diabetes
- Strategies to Reduce Mortality from Bacterial Sepsis in Adults in Developing Countries
- Ensuring the Involvement of Children in the Evaluation of New Tuberculosis Treatment Regimens
- An Acute Evolving Flaccid Quadriparesis in an Elderly Woman
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Ensuring the Involvement of Children in the Evaluation of New Tuberculosis Treatment Regimens
- Predicting Outcome after Traumatic Brain Injury: Development and International Validation of Prognostic Scores Based on Admission Characteristics
- The Use of Nonhuman Primate Models in HIV Vaccine Development
- Strategies to Reduce Mortality from Bacterial Sepsis in Adults in Developing Countries
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



