-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
PRRG4 function reveals that Robo trafficking is evolutionarily conserved
article has not abstract
Published in the journal: . PLoS Genet 13(8): e32767. doi:10.1371/journal.pgen.1006927
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1006927Summary
article has not abstract
Achieving a correct set of neuronal connections during development is key for a healthy functioning nervous system. Autism, which is characterised by impairments in social interaction, language, and range of interests, has been hypothesised to originate from defective synaptic function and abnormal brain connectivity [1,2]. Moreover, genetic alterations such as the deficiency in proline-rich carboxyglutamic acid protein 4 (PRRG4) have been associated with autistic features present in WAGR syndrome (Wilm’s tumour, aniridia, genitourinary anomalies and “mental retardation”). Therefore, understanding the genetic mechanisms underlying the assembly of brain circuits is likely to be essential for the design of new diagnostic tools and therapeutic strategies for Autistic Spectrum Disorders (ASD). In this issue of PLOS Genetics, Justice et al. link genetic alterations and neural circuitry development, revealing a novel role for the PRRG4 as a regulator of Roundabout (Robo) receptor subcellular localization in the nervous system [3].
Both in vertebrates and invertebrates, the Slit/Robo repulsive pathway plays a major role in regulating how commissural neurons cross the midline while they navigate towards their targets on the contralateral side of the nervous system (reviewed by [4]). As the neuron extends towards and across the midline, the Robo receptor is absent from the growth cone of commissural axons, desensitizing it from the repulsive Slit signal emanating from the midline [5]. Robo extracellular membrane levels increase after the growing axon crosses the midline, guaranteeing that it will neither stall at the midline or cross back. In Drosophila, the decrease in Robo in the growth cone membrane of precrossing commissural neurons is regulated by Commissureless (Comm), which sorts Robo to the late endosome for degradation [6,7] (Fig 1). In the vertebrate hindbrain, the protein Rig1/Robo3 has been shown to regulate the responsiveness to Slit but through a different mechanism, probably dimerising with Robo1 and rendering it irresponsive to Slit [8]. So far, however, the way Robo is regulated in the vertebrate forebrain is unknown and it is tantalizing to think that a functional Comm homologue could be performing this function.
Fig. 1. Proline-rich carboxyglutamic acid protein 4 (PRRG4) is a Commissureless (Comm) homologue. 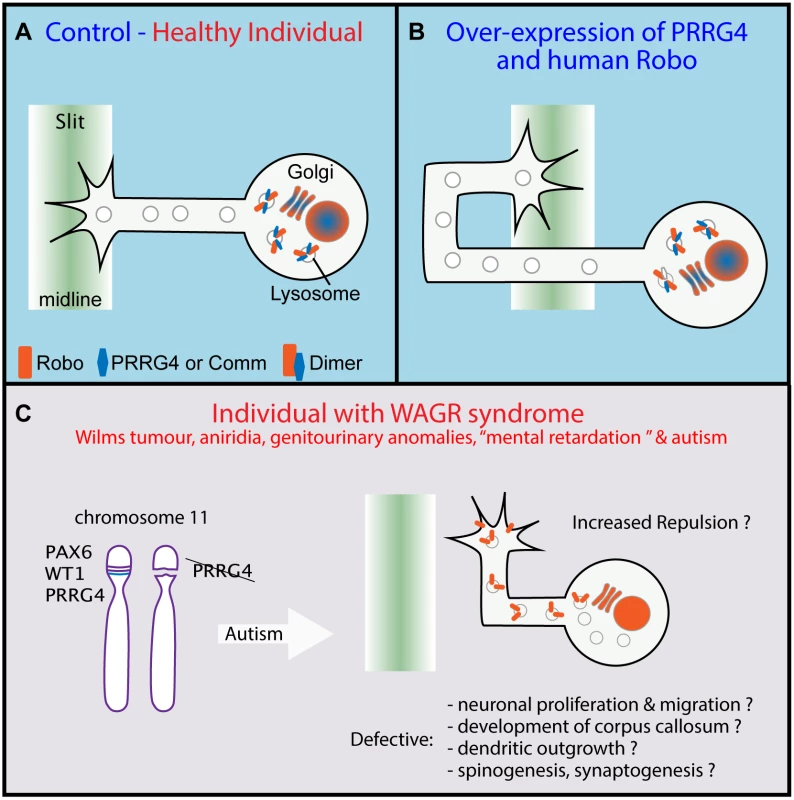
(A) In Drosophila control, before and during midline crossing, Comm dimerises with Robo and induces its degradation, preventing its trafficking to the axon growth cone extracellular membrane. A similar situation is suggested (red text) in vertebrates but relies on PRRG4-Robo dimers. (B) Overexpression of PRRG4 and human Robo induces a Drosophila Robo null phenotype with axons crossing aberrantly the midline indicating that PRRG4 acts as a Comm homologue. (C) PRRG4 deletion is associated with Autistic Spectrum Disorder (ASD) in WAGR syndrome. The results allow the hypothesis that Robo trafficking deregulation may underlie ASD through multiple mechanisms. Adapted from [6] and [3]. Justice et al. present their finding of a vertebrate functional homologue of Comm capable of regulating midline crossing in the fruit fly heterologous system. Because there are no gene sequence homologues of Comm in vertebrates, they decided to guide their search looking for the protein motif that could determine conserved function. There is little sequence requirement for Comm sorting function [6,9] and the authors embarked on an uncertain but ambitious quest focusing on an extended version of the L/PPxY motif. This strategy proved successful and they found several candidates in different species. They then used the reverse strategy and looked for a putative motif from the candidate proteins that could be found in Comm. The PRRG family of proteins presented the higher homology containing an N-terminal Gla domain that seems degenerated in Comm and the LPxY motif. To test in vivo the functional role of the candidate proteins, they overexpressed them in the nervous system of Drosophila. A functional homologue of Comm is expected to induce a Robo null phenotype that manifests itself by increased midline crossing as the consequence of decreased receptor availability [10]. PRRG4 induces midline crossing defects, the penetrance of which was magnified when overexpressed with human Robo (Fig 1). Finally, to understand the mechanism of action of PRRG4, Justice et al. overexpressed murine and Drosophila proteins in cell culture and demonstrated that PRRG4 species specifically recruits Robo to the endoplasmic reticulum/Golgi and that it induces a decrease in protein levels, probably through targeted degradation. This shared function between Comm and PRRG4 implies that the mechanism of Robo trafficking is evolutionarily conserved and that Comm/PRRG proteins may belong to an ancient family of cell surface protein effectors.
Interestingly, haploinsufficiencies of PRRG4 or Pax 6 are candidate genes underlying ASD in patients with WAGR syndrome. The finding of a likely role of PRRG4 in regulating Robo trafficking in vertebrates suggests that defects in the Slit/Robo pathway may underlie the ASD features. Considering that Slit/Robo signalling pathway is involved in several aspects of forebrain development (e.g., neuronal proliferation and migration, development of corpus callosum, dendritic outgrowth, and spinogenesis [11]), the discovery presented by Justice et al. represents a unique opportunity to further investigate the complexity of the Robo-dependent signalling pathway for the development of the vertebrate brain in health and disease.
Zdroje
1. Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9 : 341–355. doi: 10.1038/nrg2346 18414403
2. Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17 : 103–111. doi: 10.1016/j.conb.2007.01.009 17275283
3. Justice ED, Barnum SJ, Kidd T. The WAGR syndrome gene PRRG4 is a functional homologue of the commissureless axon guidance gene. PLoS Genet. 2017.
4. Dickson BJ, Gilestro GF. Regulation of Commissural Axon Pathfinding by Slit and its Robo Receptors. Annu Rev Cell Dev Biol. 2006;22 : 651–675. doi: 10.1146/annurev.cellbio.21.090704.151234 17029581
5. Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92 : 205–215. 9458045
6. Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, et al. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110 : 415–427. 12202032
7. Myat A, Henry P, McCabe V, Flintoft L, Rotin D, Tear G. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the roundabout receptor. Neuron. Elsevier; 2002;35 : 447–459. doi: 10.1016/S0896-6273(02)00795-X
8. Friocourt F, Chédotal A. The Robo3 receptor, a key player in the development, evolution, and function of commissural systems. Devel Neurobio. 2017. doi: 10.1002/dneu.22478 28033646
9. Gilestro GF. Redundant Mechanisms for Regulation of Midline Crossing in Drosophila. Schweisguth F, editor. PLoS ONE. 2008;3: e3798–11. doi: 10.1371/journal.pone.0003798 19030109
10. Kidd T, Russell C, Goodman CS, Tear G. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron. 1998;20 : 25–33. 9459439
11. Blockus H, Chédotal A. The multifaceted roles of Slits and Robos in cortical circuits: from proliferation to axon guidance and neurological diseases. Current Opinion in Neurobiology. 2014;27 : 82–88. doi: 10.1016/j.conb.2014.03.003 24698714
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2017 Číslo 8- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
- Prof. Petr Urbánek: Potřebujeme najít pacienty s nediagnostikovanou akutní intermitentní porfyrií
-
Všechny články tohoto čísla
- Deciphering the genetic control of gene expression following antigen stimulation
- Culture-induced recurrent epigenetic aberrations in human pluripotent stem cells
- Positional cloning of quantitative trait nucleotides for blood pressure and cardiac QT-interval by targeted CRISPR/Cas9 editing of a novel long non-coding RNA
- PRRG4 function reveals that Robo trafficking is evolutionarily conserved
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Deciphering the genetic control of gene expression following antigen stimulation
- Culture-induced recurrent epigenetic aberrations in human pluripotent stem cells
- Positional cloning of quantitative trait nucleotides for blood pressure and cardiac QT-interval by targeted CRISPR/Cas9 editing of a novel long non-coding RNA
- PRRG4 function reveals that Robo trafficking is evolutionarily conserved
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



