-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Fishing for adaptive epistasis using mitonuclear interactions
article has not abstract
Published in the journal: . PLoS Genet 13(3): e32767. doi:10.1371/journal.pgen.1006662
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1006662Summary
article has not abstract
Mitochondria provide us with the energy to get up and do what needs to be done. They do this with 37 genes encoded in their own genome and more than 1,200 genes from the nuclear genome, whose gene products are targeted to mitochondria after being translated on cytosolic ribosomes. This complex intergenomic communication is a hallmark of eukaryotic life and has been under continuous refinement for more than 109 years, following the fusion of eubacterial and archaebacterial cells [1]. How these two genomes cooperate in the face of conflicting modes of genetic transmission, distinct evolutionary pressures of mutation and recombination, and oxidative damage brought on by the very activity of mitochondria, has puzzled and motivated biologists for decades. A central challenge is to understand the coordinated modes of communication that maintain biochemical, physiological, and evolutionary coadaptation of the nuclear and mitochondrial compartments. The traditional view is that central administration (the nucleus) maintains the activities of these hardworking laborers (mitochondria) by sending regulatory agents (nuclear gene products) to organelles spread across the cellular landscape. A growing body of literature points to diverse ways that these energy factories voice their concerns about the government of mitonuclear interactions and send retrograde signals back to the nucleus, altering its control over mitochondria [2]. Studies of these patterns and processes hope to resolve some pretty fundamental questions about life on earth: the causes of aging and disease [3–5], the metabolic bases of adaptation and Darwinian fitness [6], the genetics of speciation [7], the origin of sex and recombination [8, 9], the battle of the sexes, and genomic conflict [10].
Most questions about mitonuclear interactions focus on the oxidative phosphorylation pathway (OXPHOS), the ATP producing process that includes four multisubunit protein complexes of the electron transport chain (ETC) and a fifth complex that phosphorylates ADP to produce ATP. These OXPHOS complexes, embedded in the mitochondrial inner membrane, include all of the 13 proteins encoded in animal mitochondrial DNA (mtDNA) plus ~78 proteins encoded on nuclear chromosomes. Two other classes of mtDNA genes, transfer - and ribosomal - RNAs, are needed to express mtDNA encoded proteins, and their functions require nuclear encoded proteins for transfer RNA (tRNA) charging, ribosome function, and RNA processing. Because these sets of genes involve physical interactions between mtDNA - and nuclear-encoded gene products, they are logical targets for studies of mitonuclear communication, coordination, and coevolution. But mitochondria do much more than make ATP; for example, they maintain redox and calcium balance, mediate apoptosis, regulate amino acid and lipid metabolism via the Krebs cycle and beta-oxidation, and influence TOR signaling, among many other functions [2]. So, there are additional nuclear genes that might influence mitonuclear coadaptation by indirect interactions with mtDNA-encoded functions.
Testing hypotheses of molecular coadaptation in the age of genomics is potentially rather easy. One can sequence the genomes or transcriptomes of different organisms and ask if the interacting genes or proteins show parallel patterns of molecular evolution. Because mtDNA genes usually evolve faster than nuclear genes, those that interact with mtDNA-encoded partners should show elevated rates of divergence, implying coevolution. For functional tests, genetic replacement of a foreign mtDNA in to the nuclear background of a different species should disrupt mitonuclear coadaptation and cause fitness defects. There are some nice cases in support of these molecular [11] and genetic predictions [12], but there are also some robust examples that have not uncovered the predicted patterns from coevolution [13–15]. So, why has putatively ongoing mitonuclear coevolution not left consistent footprints of selection that should be easy to discover? Are there systems that are more likely to reveal the expected patterns?
In this issue of PLOS Genetics, Baris et al. [16] use the estuarine fish Fundulus heteroclitis to search for a signature of adaptive mitonuclear interactions, taking advantage of a natural experiment that is a geographic legacy of glacial retreat along the east coast of North America. They sampled a population of fish in Mantoloking, New Jersey, harboring two distinct mtDNA haplotypes that diverged during a period of geographic isolation imposed by the Hudson River basin that drained meltwater from the Wisconsin ice sheet. Following glacial retreat, “northern” and “southern” populations came in contact, and today, the New Jersey population has a ~50 : 50 ratio of two mtDNA haplotypes and a ~80 : 20 ratio of nuclear alleles typically found in Maine and Georgia. Baris et al. used genotyping by sequencing (GBS) to sample the nuclear genomes of fish from the New Jersey population and stratified the analysis of nuclear allelic variation by the mtDNA haplotype that each fish carried. The question they ask is whether there is evidence for population subdivision of nuclear alleles within a single population, associated with the divergent mtDNA haplotypes segregating in that population (Fig 1). Assuming neutral alleles and random mating, there should be no such mitonuclear association, especially because there is no physical linkage between nuclear chromosomes and cytoplasmic mtDNA.
Fig. 1. Nonrandom mitonuclear associations in a mixed population. 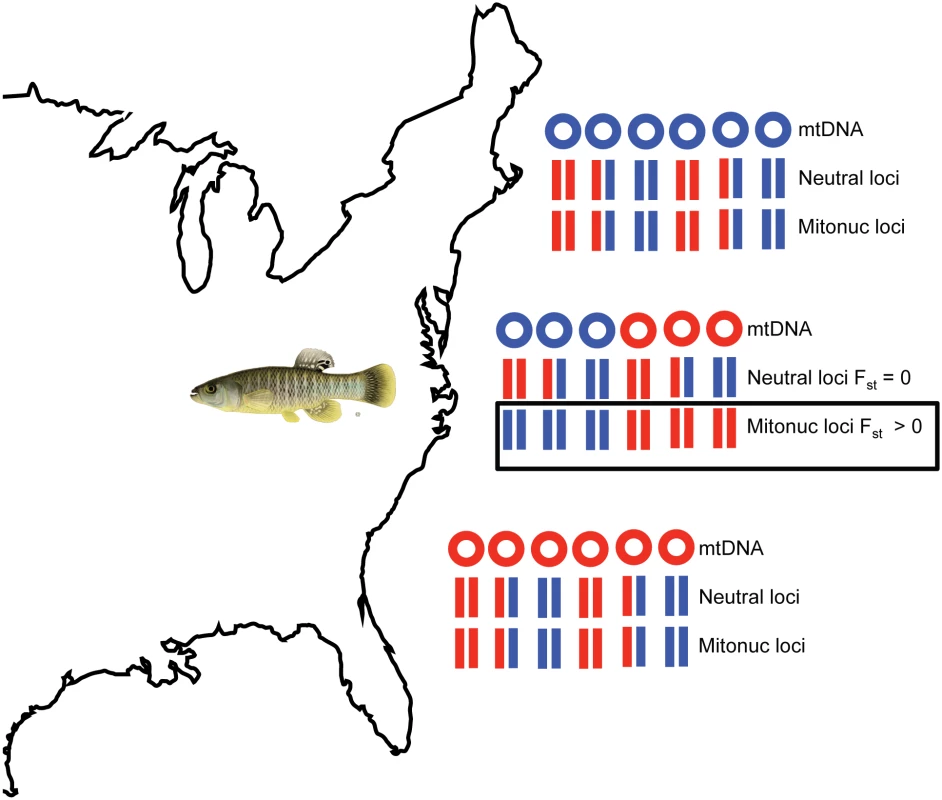
In this simplified cartoon, the northern and southern populations of F. heteroclitis each have one mitochondrial DNA (mtDNA) haplotype (blue in the north, red in the south), and alleles at neutral nuclear loci and mitonuclear loci (Mitonuc loci) associated with mitochondrial function are in Hardy-Weinberg equilibrium ([HWE], for red and blue alleles). The New Jersey population has both mtDNAs in equal frequency, and the neutral loci show the same HWE frequencies in each mtDNA background, but the mitonuclear loci show significant differences in genotype frequencies in the two mtDNA haplotype backgrounds (Fst > 0, see boxed “Mitonuc loci”). The latter suggests a selective maintenance of mitonuclear interactions for a subset of nuclear loci that have epistatic fitness interactions with mtDNA haplotypes. Map image is from http://d-maps.com/carte.php?num_car=11867&lang=en. Fish image is from http://www2.dnr.cornell.edu/cek7/nyfish/Cyprinodontidae/mummichog.html. Baris et al. found a few hundred single nucleotide polymorphisms (SNPs) spread across the nuclear chromosomes of F. heteroclitis that have significantly different allele frequencies in the two mtDNA “populations” within this single collecting locality (cf. 349 SNPs using p < 0.01 and 236 or 72 SNPs using a false discovery rate of 10% and 1%). Importantly, these SNPs show higher levels of allele frequency difference between mtDNA partitions within this population (measured as Fst “outliers”) than they do between the focal New Jersey population and geographically isolated populations in Maine and Georgia, a clear rejection of neutral expectations. Baris et al. quantified OXPHOS activity (ADP-dependent state-3 respiration) of mitochondria extracted from heart tissue from four mitonuclear genotypes that define pure parental and admixed hybrid individuals (e.g., mtDNA-Nuclear genotypes as: North-North, North-Mixed, South-Mixed, South-South) and show that these mitonuclear genotypes explain a significant portion of the variation in OXPHOS function.
It is tempting to invoke adaptive mitonuclear epistasis to explain these data, in which mtDNA haplotypes offer a sort of alternative metabolic niche for nuclear alleles within a population, akin to the classical Levene model of balancing selection via opposing selection in alternative habitats [17]. Baris et al. make a concerted effort to test alternative explanations for Fst outlier status of SNPs partitioned by mtDNA, by testing 1,000 alternative data partitions of >9,000 nuclear SNPs. None of these “nuclear-nuclear” Fst outlier tests uncovered as many outlier SNPs as the original mitonuclear outlier test, suggesting that the main result is not littered with many false positives.
One unexpected result from these analyses is that that none of the 349 Fst outlier loci map to known nuclear genes encoding subunits of the OXPHOS complexes. If this study really has identified loci important in mitonuclear coadaptation, it suggests that long-range cis- or trans-regulatory factors, or downstream pathway effects, are mediating the epistatic interactions between mtDNA haplotypes and nuclear loci other than those encoding subunits of OXPHOS complexes. Understanding the genetic basis of fitness variation in the wild is a central goal of ecological and evolutionary genetics, and the genes of central metabolism have long fascinated biologists as a logical place to search for the source of this variation [6]. Given the critical role that mitochondrial function plays in organismal performance, and the increasing knowledge of the diverse roles of mitonuclear communication in regulating homeostasis, there is a real hope that we can track down the biochemical bases of these kinds of nonneutral patterns in nature. The Baris et al. study offers a nice example of how admixed populations with divergent mtDNAs might serve as a natural genetic screen for the footprints of mitonuclear epistasis and coevolution that could point to unanticipated targets of selection on metabolic function.
Zdroje
1. Martin WF, Garg S, Zimorski V. Endosymbiotic theories for eukaryote origin. Philos Trans R Soc Lond B Biol Sci. 2015;370(1678):20140330. Epub 2015/09/02. doi: 10.1098/rstb.2014.0330 26323761
2. Quiros PM, Mottis A, Auwerx J. Mitonuclear communication in homeostasis and stress. Nature reviews Molecular cell biology. 2016;17(4):213–26. Epub 2016/03/10. doi: 10.1038/nrm.2016.23 26956194
3. Kauppila TE, Kauppila JH, Larsson NG. Mammalian Mitochondria and Aging: An Update. Cell Metab. 2017;25(1):57–71. Epub 2017/01/18. doi: 10.1016/j.cmet.2016.09.017 28094012
4. Latorre-Pellicer A, Moreno-Loshuertos R, Lechuga-Vieco AV, Sanchez-Cabo F, Torroja C, Acin-Perez R, et al. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature. 2016;535(7613):561–5. Epub 2016/07/08. doi: 10.1038/nature18618 27383793
5. Wallace DC. Genetics: Mitochondrial DNA in evolution and disease. Nature. 2016;535(7613):498–500. Epub 2016/07/08. doi: 10.1038/nature18902 27383787
6. Marden JH. Nature's inordinate fondness for metabolic enzymes: why metabolic enzyme loci are so frequently targets of selection. Mol Ecol. 2013;22(23):5743–64. Epub 2013/10/11. doi: 10.1111/mec.12534 24106889
7. Burton RS, Barreto FS. A disproportionate role for mtDNA in Dobzhansky-Muller incompatibilities? Mol Ecol. 2012;21(20):4942–57. Epub 2012/09/22. doi: 10.1111/mec.12006 22994153
8. Radzvilavicius AL, Blackstone NW. Conflict and cooperation in eukaryogenesis: implications for the timing of endosymbiosis and the evolution of sex. Journal of the Royal Society, Interface. 2015;12(111):20150584. Epub 2015/10/16. doi: 10.1098/rsif.2015.0584 26468067
9. Garg SG, Martin WF. Mitochondria, the Cell Cycle, and the Origin of Sex via a Syncytial Eukaryote Common Ancestor. Genome biology and evolution. 2016;8(6):1950–70. Epub 2016/06/28. doi: 10.1093/gbe/evw136 27345956
10. Beekman M, Dowling DK, Aanen DK. The costs of being male: are there sex-specific effects of uniparental mitochondrial inheritance? Philos Trans R Soc Lond B Biol Sci. 2014;369(1646):20130440. Epub 2014/05/28. doi: 10.1098/rstb.2013.0440 24864311
11. Barreto FS, Burton RS. Evidence for compensatory evolution of ribosomal proteins in response to rapid divergence of mitochondrial rRNA. Mol Biol Evol. 2013;30(2):310–4. Epub 2012/09/21. doi: 10.1093/molbev/mss228 22993236
12. Ellison CK, Burton RS. Interpopulation hybrid breakdown maps to the mitochondrial genome. Evolution. 2008;62(3):631–8. Epub 2007/12/18. doi: 10.1111/j.1558-5646.2007.00305.x 18081717
13. Montooth KL, Meiklejohn CD, Abt DN, Rand DM. Mitochondrial-nuclear epistasis affects fitness within species but does not contribute to fixed incompatibilities between species of Drosophila. Evolution. 2010;64(12):3364–79. Epub 2010/07/14. doi: 10.1111/j.1558-5646.2010.01077.x 20624176
14. Adrion JR, White PS, Montooth KL. The Roles of Compensatory Evolution and Constraint in Aminoacyl tRNA Synthetase Evolution. Mol Biol Evol. 2016;33(1):152–61. Epub 2015/09/30. doi: 10.1093/molbev/msv206 26416980
15. Mossman JA, Biancani LM, Zhu CT, Rand DM. Mitonuclear Epistasis for Development Time and Its Modification by Diet in Drosophila. Genetics. 2016;203(1):463–84. Epub 2016/03/12. doi: 10.1534/genetics.116.187286 26966258
16. Baris TZ, Wagner DN, Dayan DI, Du X, Blier P, Pichaud N, et al. Evolved Genetic and Phenotypic Differences due to Mitochondrial-Nuclear Interactions. PLoS Genet. 2017;13: e1006517. doi: 10.1371/journal.pgen.1006517.
17. Levene H. Genetic equilibrium when more than one ecological niche is available. Am Nat. 1953;87 : 331–3.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2017 Číslo 3- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
- Prof. Petr Urbánek: Potřebujeme najít pacienty s nediagnostikovanou akutní intermitentní porfyrií
-
Všechny články tohoto čísla
- 2016 Reviewer and Editorial Board Thank You
- The social genome: Current findings and implications for the study of human genetics
- A rare loss-of-function mutation reduces blood eosinophil counts and protects from asthma
- A variant in the gene in a dog with ichthyosis
- Excess of genomic defects in a woolly mammoth on Wrangel island
- CUZD1 is a critical mediator of the JAK/STAT5 signaling pathway that controls mammary gland development during pregnancy
- Fishing for adaptive epistasis using mitonuclear interactions
- Comparison of independent screens on differentially vulnerable motor neurons reveals alpha-synuclein as a common modifier in motor neuron diseases
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- CUZD1 is a critical mediator of the JAK/STAT5 signaling pathway that controls mammary gland development during pregnancy
- A rare loss-of-function mutation reduces blood eosinophil counts and protects from asthma
- A variant in the gene in a dog with ichthyosis
- Fishing for adaptive epistasis using mitonuclear interactions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



