-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetic Disruption of the Copulatory Plug in Mice Leads to Severely Reduced Fertility
Seminal fluid proteins affect fertility at multiple stages in reproduction. In many species, a male's ejaculate coagulates to form a copulatory plug. Although taxonomically widespread, the molecular details of plug formation remain poorly understood, limiting our ability to manipulate the structure and understand its role in reproduction. Here I show that male mice knockouts for transglutaminase IV (Tgm4) fail to form a copulatory plug, demonstrating that this gene is necessary for plug formation and lending a powerful new genetic tool to begin characterizing plug function. Tgm4 knockout males show normal sperm count, sperm motility, and reproductive morphology. However, very little of their ejaculate migrates into the female's reproductive tract, suggesting the plug prevents ejaculate leakage. Poor ejaculate migration leads to a reduction in the proportion of oocytes fertilized. However, Tgm4 knockout males fertilized between 3–11 oocytes, which should be adequate for a normal litter. Nevertheless, females mated to Tgm4 knockout males for approximately 14 days were significantly less likely to give birth to a litter compared to females mated to wild-type males. Therefore, it appears that the plug also affects post-fertilization events such as implantation and/or gestation. This study shows that a gene influencing the viscosity of seminal fluid has a major influence on male fertility.
Published in the journal: . PLoS Genet 9(1): e32767. doi:10.1371/journal.pgen.1003185
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003185Summary
Seminal fluid proteins affect fertility at multiple stages in reproduction. In many species, a male's ejaculate coagulates to form a copulatory plug. Although taxonomically widespread, the molecular details of plug formation remain poorly understood, limiting our ability to manipulate the structure and understand its role in reproduction. Here I show that male mice knockouts for transglutaminase IV (Tgm4) fail to form a copulatory plug, demonstrating that this gene is necessary for plug formation and lending a powerful new genetic tool to begin characterizing plug function. Tgm4 knockout males show normal sperm count, sperm motility, and reproductive morphology. However, very little of their ejaculate migrates into the female's reproductive tract, suggesting the plug prevents ejaculate leakage. Poor ejaculate migration leads to a reduction in the proportion of oocytes fertilized. However, Tgm4 knockout males fertilized between 3–11 oocytes, which should be adequate for a normal litter. Nevertheless, females mated to Tgm4 knockout males for approximately 14 days were significantly less likely to give birth to a litter compared to females mated to wild-type males. Therefore, it appears that the plug also affects post-fertilization events such as implantation and/or gestation. This study shows that a gene influencing the viscosity of seminal fluid has a major influence on male fertility.
Introduction
The non-sperm component of an ejaculate can have large effects on male reproductive fitness. In internally fertilizing species, seminal proteins can modify female receptivity [1]–[3], egg laying behavior [4]–[6], implantation and gestation [7], and the female's immune response to sperm and embryo [7]–[11]. Seminal proteins can also interact with the ejaculates of competitor males to influence the outcomes of fertilization [12]–[14]. In many internally fertilizing taxa, ejaculated proteins coagulate to form a hardened copulatory plug in the vaginal-cervical region of the female [15]–[22]. In spite of its wide taxonomic distribution, the molecular details that underlie its formation remain poorly understood, which limits investigations into its function. After reviewing previous biochemical insights, I present a new genetic model that offers unprecedented power to being dissecting the function of the plug.
Since the first published observation of a copulatory plug in a rodent nearly 165 years ago [19], several groups have attempted to characterize its molecular basis. Camus and Gley [23] showed that fluids extracted from the seminal vesicles coagulated in vitro upon contact with extract from the anterior lobe of the prostate (also referred to as the coagulating gland) [24], [25]. Building from the Camus & Gley experiment, Williams-Ashman and colleagues showed that the rate of coagulation depended on the concentrations of seminal vesicle and/or prostate protein extracts in vitro [26]. Because these early experiments were based on crude extracts, the general term “vesiculase” was coined to describe the unknown prostate-derived protein(s) responsible for inducing the coagulation of seminal vesicle proteins. More detailed biochemical investigations suggested the unknown vesiculase(s) was a transglutaminase [27], [28], a protein that crosslinks glutamines and lysines via γ-glutamyl-ε-lysine dipeptide bonds and causes the bound proteins to become insoluble and coagulate. A prostate-specific transglutaminase, transglutaminase IV (Tgm4), was later characterized from humans [29]–[31], and its protein is found in human ejaculates [32]. The ortholog in mouse is also ejaculated [33], and functionally analogous transglutaminases have been found in mosquito ejaculates [16].
In spite of these early advances, it remains unknown whether Tgm4 is necessary for the formation of the copulatory plug. It has been suggested that some seminal vesicle proteins self-coagulate in the absence of Tgm4 [34], that proteins other than Tgm4 induce the coagulation [35], and that female-derived proteins may be necessary for coagulation [36]. Furthermore, there is evidence that more than one transglutaminase exists in the male reproductive tract of rodents [33], [37], [38], though this could also be due to post-translational modifications [39]. Interestingly, human ejaculates do not coagulate strongly even though they have large amounts of Tgm4 [32], calling into question its role in seminal fluid coagulation.
More fully characterizing the biochemical basis of seminal fluid coagulation is critical for understanding the function of the copulatory plug. Early attempts to study copulatory plug function necessarily relied on surgical removal of male accessory glands [40]–[47]. Although copulatory plugs were abnormal and male fertility compromised in some cases, inferences were limited by the invasiveness of the procedures, the confounding effects associated with the potential alteration of hundreds of ejaculated proteins, and the failure to fully prevent a copulatory plug-like structure from forming. Other experiments showed that manual removal of the plug soon after copulation did not prevent pregnancy or parturition [48], [49]. However, the copulatory plug may have affected fertility prior to experimental removal. To address these early experimental limitations requires a method to fully prevent the formation of a copulatory plug with minimal invasiveness.
Here, I use Tgm4 knockout mice to better understand the molecular basis and functional importance of the copulatory plug, and report two main findings. First, Tgm4 knockout males failed to produce a copulatory plug after mating, demonstrating for the first time that this gene is necessary for the coagulation of seminal fluid in mice. Tgm4 knockout males therefore provide a powerful model to investigate the function of the copulatory plug. Second, in spite of normal sperm count, sperm motility, and reproductive morphology, Tgm4 knockout males sired significantly fewer litters than their wild type brothers. Analyses presented below suggest Tgm4 knockout males suffer fertility defects at two important stages: 1) less of their ejaculate migrates into the female's reproductive tract, and 2) females mated to Tgm4 knockout males produce significantly fewer litters even though a “normal” absolute number of oocytes were fertilized, suggesting additional defects in implantation and/or gestation. This study demonstrates that a gene influencing the viscosity of semen has major affects on male reproductive success.
Results
Heterozygous “knockout first” mice were acquired from the Knockout Mouse Project (see [50],[51], and Materials and Methods). Heterozygotes were crossed in the laboratory to generate homozygous and heterozygous knockout males, as well as homozygous wild type males that were used as controls in all experiments. All females used throughout the manuscript were homozygous wild type. All mice were essentially genetically identical except for the ∼7 kb “knockout first” cassette that spans exons 2–3 of Tgm4.
Tgm4 knockout males (homozygous for the “knockout first” allele) did not form a copulatory plug (Table 1), demonstrating for the first time that this gene is necessary for seminal fluid coagulation. From 13 successful 3-hour pairings to Tgm4 knockout males (“success” being defined as the presence of sperm somewhere in the female's reproductive tract after three hours of pairing), complete dissection of each female's reproductive tract failed to yield a copulatory plug or plug-like structure (Table 1). In contrast, 14 of 16 successful 3-hour pairings to wild type males resulted in a copulatory plug, which normally occupies most of the vaginal canal and extends into the cervix, appearing “glued” to the epithelium. Herein, “wild type” includes males that were either heterozygous or homozygous for the wild type allele, as they were phenotypically indistinguishable from each other. I obtained similar results from 20-hour long male-female pairings: 0 of 8 females successfully paired with Tgm4 knockout males, and 11 of 15 paired to wild type males, yielded a plug. Because they cannot form a plug, Tgm4 knockout males represent a powerful genetic tool to investigate its role in reproduction.
Tab. 1. Results from 3 h and 20 h pairings of experimental males to homozygous wild type 6N females. 
Females were paired with homozygous knockout (KO) or wild type (+, possessing at least one functional allele) males for 3 hours or 20 hours (see text). In the absence of a plug, the ejaculates of Tgm4 knockout males did not traverse the female reproductive tract properly. After mating to wild type males, female uterine horns appeared swollen, full of sperm and seminal fluid (Figure 1A). In contrast, after mating to Tgm4 knockout males, female uterine horns did not swell and sperm were difficult to locate upon dissection (Figure 1B). The difference in uterine horn width was statistically significant between females mated to wild type (N = 6) vs. Tgm4 knockout males (N = 15) (wild type: 2.64 mm, SD = 0.30; Tgm4 knockout: 2.14 mm, SD = 0.43; t = 2.98, df = 19, P = 0.01).
Fig. 1. The appearance of female uterine horns after mating. 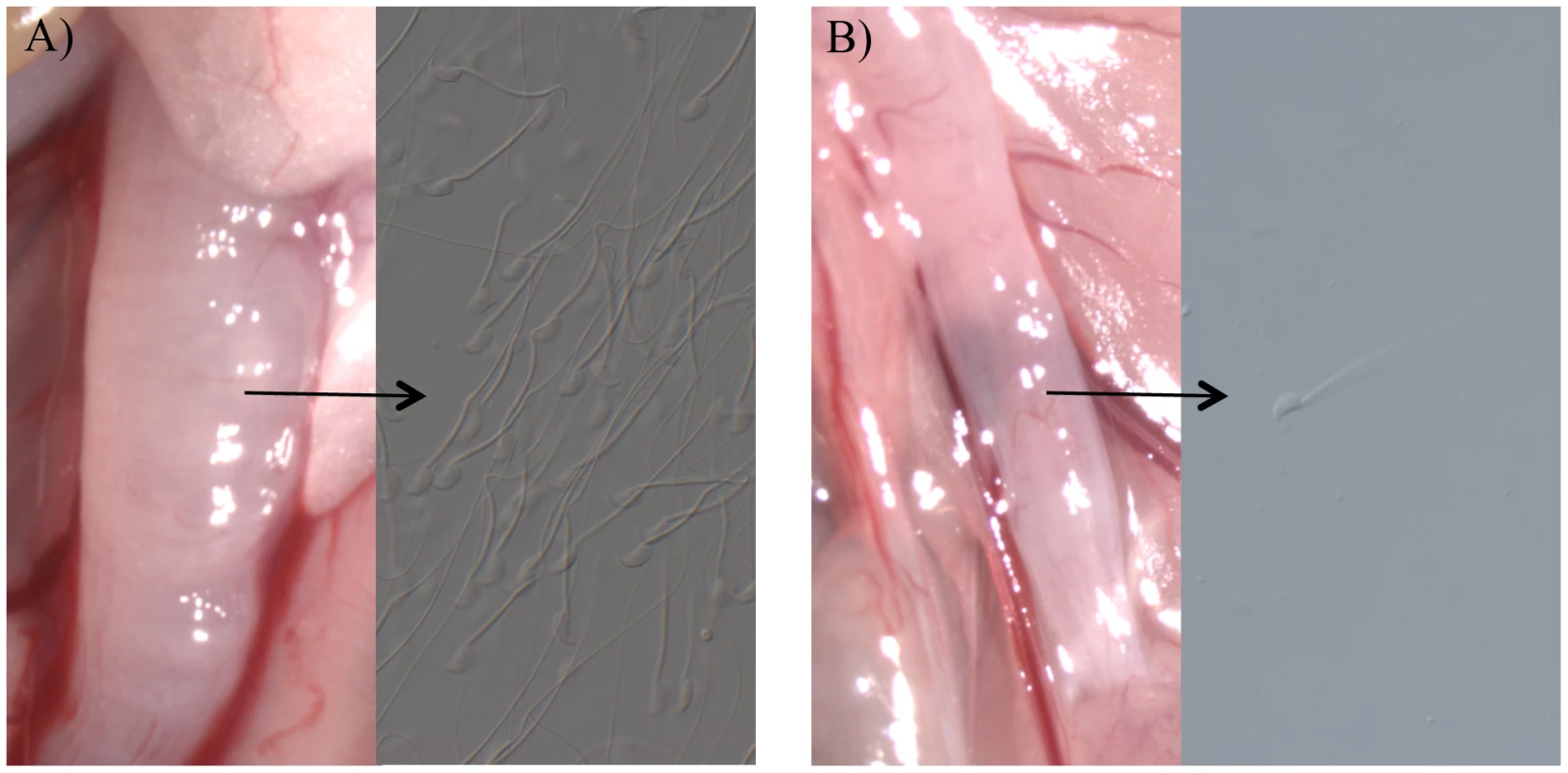
The appearance of female uterine horns after mating to A) a wild type male, where uterine horns swelled with the male's ejaculate, or B) a Tgm4 knockout male, where uterine horns qualitatively resembled unmated females, with no swelling. Very few sperm were observed in females mated to Tgm4 knockout males. The defect in ejaculate migration cannot be explained by defects in reproductive morphology of Tgm4 knockout males. Sperm count was not statistically different between wild type (N = 19) vs. Tgm4 knockout (N = 10) males (mean = 133,900 sperm/µl, SD = 55,000 vs. mean = 106,000 sperm/µl, SD = 43,000, respectively: t = 1.39, df = 27, P = 0.18), nor was sperm motility (mean = 0.96 sperm/sec, SD = 0.28 vs. mean = 0.87 sperm/sec, SD = 0.27, respectively: t = 0.78, df = 27, P = 0.44). From these same males, testis and seminal vesicle weight were analyzed in a full factorial ANCOVA to account for the potential covariation with body weight. There was not a significant difference in testis weight between Tgm4 knockout vs. wild type males (F1,25 = 0.02, P = 0.88), nor was there a genotype×body weight interaction effect on testis weight (F1, 25 = 0.95, P = 0.34). Similarly, there was no difference in seminal vesicle weight between genotypes (F1, 25<0.01, P = 0.97), nor was there a genotype×body weight interaction effect on seminal vesicle weight (F1, 25 = 0.03, P = 0.86). Furthermore, Tgm4 knockout males successfully copulated at a rate similar to wild type; from the 3-hour pairings, 16/26 to wild type, and 13/28 pairings to Tgm4 knockout males succeeded (Table 1, χ2 = 0.7, P = 0.4). Therefore, both genotypes display normal copulatory behavior.
As might be expected from the reduced number of sperm that make it into the female's uterus, Tgm4 knockout males fertilized significantly fewer oocytes in 20-hour assays. Among successful 20-hour pairings, Tgm4 knockout males fertilized 45 of 122 oocytes dissected from the female's oviducts (36.9%), compared to wild type males, which fertilized 153 of 231 oocytes (66.2%) (Table 1). Fertilized oocytes from all successful pairings appeared healthy, with almost no signs of fragmentation. Oocytes originating from the same female are not independent observations, so I compared the proportion of fertilized oocytes on a per-female basis. Seven females successfully mated to Tgm4 knockout males yielded a mean 39.4% fertilized oocytes (range 21.1%–72.7%), significantly lower than 12 females successfully mated to wild type (mean = 67.9%, range 12.5%–93.3%) (t = 2.78, df = 17, P = 0.01). The number of females analyzed (7 mated to Tgm4 knockout and 12 mated to wild type) does not add up to the numbers in Table 1 (8 mated to Tgm4 knockout and 15 mated to wild type) because scorable oocytes were not always recovered from oviduct dissections. It should be noted that even though Tgm4 knockout males fertilized a lower proportion of oocytes compared to wild type, they always fertilized at least 3 oocytes (mean = 6.4, range 3–11), suggesting they should be able to impregnate females without difficulty.
In contrast to this prediction, after being paired with females for 10–14 days, Tgm4 knockout males sired significantly fewer litters than wild type (Table 2). Of 30 pairings with Tgm4 knockout males, only 17 produced litters, significantly fewer than wild type males, which produced litters in 135 of 165 pairings (χ2 = 7.93, P = 0.005) (Table 2). Among all litters born to wild type fathers, 42/106 (39.6%) yielded 6 or fewer pups (the mean number of oocytes fertilized by Tgm4 knockout males, see previous paragraph), and 14/106 (13.2%) resulted in 3 or fewer pups (the minimum number of oocytes fertilized by Tgm4 knockout males, see previous paragraph). In other words, Tgm4 knockout males sired significantly fewer litters in spite of the fact that they appeared to fertilize enough oocytes for a healthy litter.
Tab. 2. Results of pairing experimental males to homozygous wild type 6N females for 10–14 days. 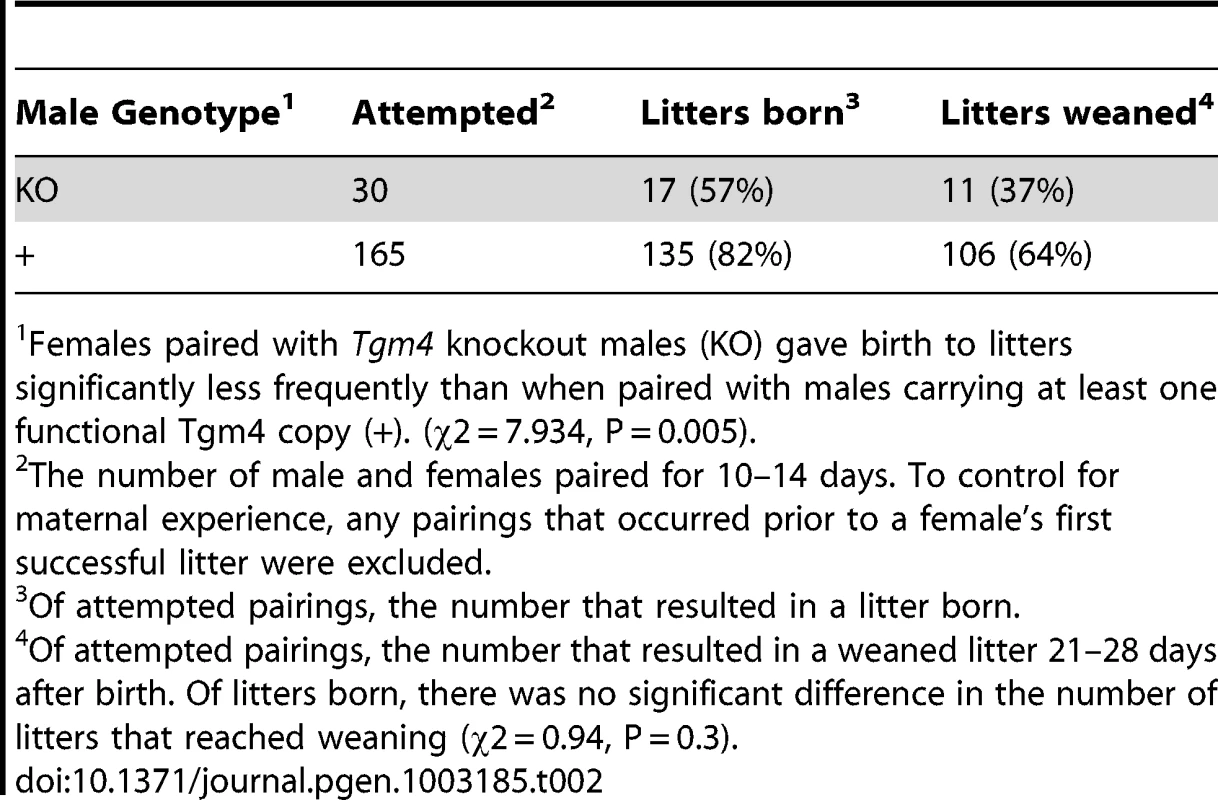
Females paired with Tgm4 knockout males (KO) gave birth to litters significantly less frequently than when paired with males carrying at least one functional Tgm4 copy (+). (χ2 = 7.934, P = 0.005). Although Tgm4 knockout males sired significantly fewer litters (Table 2), there were no signs of maternal neglect, as judged by the likelihood a litter reached weaning age, the litter size, and the size of offspring at weaning. Specifically, 11 of 17 (65%) litters sired by Tgm4 knockout males reached weaning age (21–28 days old), compared to 106 of 135 (79%) litters born to a wild type male (Table 2; χ2 = 0.94, P = 0.3). Sometimes litters do not reach weaning age because of maternal neglect. Furthermore, the number of offspring weaned per litter was not significantly different among the two male genotypes (mean = 6.0 vs. 6.4 pups weaned per litter, SD = 3.5 vs. 2.4, range 1–12 for both, from N = 11 vs. 106 weaned litters sired by Tgm4 knockout or wild type males, respectively: Welch's t = 0.59, df = 10.97, P = 0.57), nor was weanling weight (mean weight = 11.61 g vs. 12.14 g, SD = 3.0 vs. 3.8 from N = 66 pups weighed from 11 litters vs. 77 pups weighed from 13 litters sired by Tgm4 knockout or wild type males, respectively: Welch's t = 0.93, df = 140.0, P = 0.35). The lack of statistical significance may be due to small sample sizes, but suggests that once litters are born, the pups have an equal chance of reaching healthy weaning age regardless of sire genotype.
Discussion
Tgm4 knockout males failed to produce a copulatory plug, demonstrating for the first time that this gene is necessary for plug formation. In spite of normal sperm count, motility and reproductive morphology, Tgm4 knockout males suffered reduced fertility, most importantly in the significant reduction of litters born compared to wild type. Taking all the data into consideration, a model of the copulatory plug acting at two important stages of reproduction seems to explain the fertility defects of Tgm4 knockout males. First, the plug may facilitate passage of the ejaculate through the cervix and into the uterine horns and oviducts (Fig. 1, Table 1), perhaps by sealing off the vagina and preventing backflow of the ejaculate [16], [41], [42], [52]–[54]. Second, the plug may enhance the embryos' ability to implant in the female's uterus, and/or reduce the chances of abortion after implantation (Table 2). For example, the plug may contribute to the physical stimulation necessary to shift the female's physiology towards “pseudopregnancy” [53], [55]–[57], a state where the uterus becomes primed for implantation in mice. This second aspect of the model is supported by the reduced number of litters born to Tgm4 knockout males in spite of the fact that they fertilized between 3–11 oocytes in 20-hour assays. There does not appear to be any fertility defects that arise from differential maternal investment post-parturition.
Four observations suggest that the fertility defects observed in the current study arose from the absence of the copulatory plug rather than from additional pleiotropic functions of Tgm4. First, Tgm4 expression has so far only been detected in the prostate [58]–[60], and never in any other tissues of a male or a female [61], [62], thus it should only affect ejaculate composition. Second, the only annotated domains in the Tgm4 protein are related to the formation of γ-glutamyl-ε-lysine bridges in its target proteins (www.ensembl.org), suggesting that it has a limited biological role. Third, although transglutaminases may alter the sperm surface in vitro [63], [64], Tgm4 has never been detected on the sperm surface [65], [66], suggesting it does not directly affect the gamete. Fourth, Tgm4 has accumulated multiple loss-of-function mutations in some species that do not form a plug [67], which is not predicted if Tgm4 functions outside the context of plug formation.
Although the present study demonstrates the importance of the copulatory plug in non-competitive matings, it does not reject the hypothesis that the copulatory plug evolved in response to sperm competition [20], which occurs when a female mates with more than one male during a single fertile period [68]. Copulatory plugs are larger and show stronger coagulation intensity in species with high levels of inferred sperm competition [21], [22], [69], and have been lost in some species that experience low levels of sperm competition [67], [70], [71]. Some copulatory plug proteins evolve rapidly in species with high levels of inferred sperm competition, which is predicted if the plug inhibits female remating [67], [70]–[73]. In mice, the copulatory plug forms immediately upon ejaculation and remains intact for approximately 24 hours [20 and unpublished data], which is longer than the 4–12 hours that a female is able to be fertilized during her estrus cycle. Males contribute protease inhibitors in their ejaculates, which may function to preserve their copulatory plugs from female degradation [33]. Interestingly, males missing one of these protease inhibitors make a plug that degrades more quickly than wild type, which is associated with fertility defects [74]. Although the above patterns suggest the plug inhibits female remating, over 20% of wild caught pregnant females carry a litter sired by more than one male [75], [76], suggesting the plug is an imperfect barrier, and females or competitor males sometimes remove the plug [77]–[81].
Interestingly, copulatory plugs do not always bias fertilizations towards the first male to mate in one-female-two-male mating experiments [82]–[85], and some evolutionary patterns do not fit the sperm competition hypothesis. For example, the socially and genetically monogamous rodent Peromyscus polionotus forms a plug [86], [87]. By showing that the copulatory plug is correlated with normal fertility in one-male-one-female matings, the current study offers an explanation for the evolutionary maintenance of the copulatory plug in the absence of intense sperm competition. For example, the copulatory plug may prevent loss of semen [52], promote transport of semen through the female's reproductive tract [16], [41], [42], [53], [54], contribute to the threshold stimulation females require for proper implantation and pregnancy [55], and/or serve as a reservoir for the slow release of sperm in the female reproductive tract [88]. In reality, the copulatory plug may have multiple functions and the genetic model presented here enables unprecedented power to begin dissecting these hypotheses.
Many human seminal fluid proteins have orthologs in mouse ejaculates, including Tgm4 [33]. Even though human ejaculates do not form copulatory plugs, human seminal fluid enters a phase of coagulation and liquefaction [89], and defects in these transitions have been associated with subfertility [90]. There are 250 known nucleotide polymorphisms in human Tgm4 mRNA, including 120 missense mutations (www.ensembl.org version 69), and Tgm4 was not detected in all ejaculates of five humans [91]. Future studies may reveal genetic and proteomic variation in Tgm4 associated with differences in human male fertility.
Materials and Methods
Ethics statement
All mouse husbandry techniques, experimental methods, and personnel involved were approved by the University of Southern California's Institute for Animal Care and Use Committee, protocols #11394 and #11777.
Tgm4 “knockout first” allele
The Tgm4 knockout mouse model was constructed by the multi-institutional Knockout Mouse Project [50], [51]. A ∼7 kb “knockout first” cassette was inserted into the C57BL/6N (6N) genetic background (project #CSD30105). Alternative crossing to Cre and/or FLP mice allows for further genetic modification of the knockout allele, but was unnecessary in the present study.
Mouse husbandry
All experimental males used in this study had 6N parents that were heterozygous for the knockout (KO) and wild type (+) allele. When possible, all three genotypes were taken from the same litter to control for simple maternal effects.
Sires and dams were paired for one to two weeks, then separated so the dam gave birth in isolation. Between 21–28 days after birth, males were weaned in groups until genotyping, at which point they were separated into their own cages to avoid dominance interactions between brothers. Sexually mature males show reduced fertility when grouped together, presumably as a result of dominance interactions [92]. Females were weaned in groups of up to three individuals. All three possible male 6N genotypes - but only homozygous wild type 6N females - were used in various experiments described below.
Shortly after weaning, ear snips were taken for PCR-based genotyping. DNA isolated from ear snips was genotyped with four PCR reactions. Two PCR reactions specifically amplified the wild type allele: Reaction 1 primers (5′-AGGTGAAAAACCAAGAAATACCATC-3′ and 5′-CTATTCCAAAACCACCAGACAGTAC-3′) amplified a 704 bp fragment and Reaction 2 primers (GTGGACAGATATTCACTCTGAAGGT and GGAAACACCAATAGAAAAGTGAGTC) amplified a 1,170 bp fragment. Two PCR reactions specifically amplified the knockout first allele: Reaction 3 primers (GCTTTACATGTGTTTAGTCGAGGTT and GTTAAAGTTGTTCTGCTTCATCAGC) amplified a 1,244 bp fragment and Reaction 4 primers (GATTAAATATGATGAAAACGGCAAC and ATTATTTTTGACACCAGACCAACTG) amplified a 1,349 bp fragment. DNA was amplified using 35 cycles of denaturation (94 C, 20 seconds), annealing (58 C, 20 seconds) and extension (70 C and 40 seconds for Reaction 1, 70 C and 80 seconds for the other three reactions). All PCR reactions used Fermentas 2× PCR premix. Presence/absence of bands was scored on agarose gels. Only genotypes consistent across all four reactions were included in experiments.
Male reproductive phenotypes, in vivo
All experimental males were individually paired with homozygous wild type 6N females. Males were between 60 and 90 days old. For the 3-hour and 20-hour assays (see below), ∼28 day-old females were induced to ovulate using standard techniques [93], [94]. Briefly, females were administered 5U Pregnant Mare's Serum Gonadotropin (PMSG) followed 48 hours later by 5U Human Chorionic Gonadotropin (hCG). For the 10–14 day assays (see below), females between 2 and 10 months old were used and ovulation was not artificially induced.
3–hour assays
Twelve hours after administration of hCG, males and females were paired, left together for three hours, and then females were removed and sacrificed. Female reproductive tracts, extending from the vagina through the cervix and uterine horns, were dissected and examined under a dissecting microscope to assess i) the presence/absence of sperm, ii) the presence/absence of a copulatory plug, and iii) the number of oocytes with two pronuclei, a sign of recent fertilization [following 94]. Two different researchers who were blind to genotype measured uterine horns digitally, from a subset of females. Since their measurements were significantly correlated (Pearson's product-moment correlation coefficient = 0.84, P = 10−11), mean values were used for subsequent statistical analyses.
20–hour assays
Males and females were paired immediately after administration of hCG and females were removed 20 hours later and the same phenotypes were gathered as for the 3-hour assays.
10–14 day assays
Males and females were paired for 10 to 14 days, without hormonal induction of estrus, and then separated. Females were monitored for pregnancy and parturition, and litters were monitored to determine if they reached weaning age. All weanlings were sexed and counted. For a subset of randomly chosen litters, weanlings were weighed. To control for maternal experience, no pairings were included if they occurred prior to a female's first successful litter.
Male reproductive phenotypes, in vitro
Between 2–6 months of age, a subset of experimental males were sacrificed, standard measurements taken, and testes and seminal vesicles dissected and weighed.
Sperm count and sperm motility
To determine sperm numbers and sperm motility, one caudal epididymis was placed in 100 µl pre-warmed Dulbecco's and minced with 27G needles. The minced epididymis was placed at 37 degrees C for one hour to allow sperm to swim free of cellular debris. The medium was swirled once, cellular debris allowed to settle, and 5 µl of this mixture placed on a Makler Chamber for quantification of sperm motility [95], [96]. Sperm motility was quantified as the average number of sperm that entered a 0.1 µl cell in a Makler Chamber per second. A total of 10 cells were observed for 10 seconds each. An additional 5 µl of heat-shocked sperm suspensions was used to quantify sperm count on a Makler Chamber.
Statistical analyses
Unless otherwise stated, Student's t-tests were used to compare phenotypes among groups. In all t-tests, assumptions of normality and equal variances were confirmed using Shapiro-Wilk tests and F-tests, respectively. In a few comparisons indicated above, the two groups being compared had significantly different variances; in these cases Welch's t-test [97] was used. Importantly, no conclusions changed if Student's t-tests, Welch's t-test, or non-parametric Mann-Whitney U tests were used in any comparisons.
Testis and seminal vesicle weight were each analyzed in a full factorial ANCOVA using male genotype (knockout vs. wild type) and body weight as factors. An ANCOVA was employed to account for the potential covariation of testis or seminal vesicle weight with body weight. To test for differences in the number of litters born to Tgm4 knockout vs. wild type males, a 2×2 contingency table was tested against a χ2 distribution. All statistical analyses were performed in R (www.r-project.org) or customized Python scripts (www.python.org).
Zdroje
1. AigakiT, FleischmannI, ChenPS, KubliE (1991) Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster. Neuron 7 : 557–563.
2. ChenPS, Stumm-ZollingerE, AigakiT, BalmerJ, BienzM, et al. (1988) A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell 54 : 291–298.
3. WolfnerMF (1997) Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem Mol Biol 27 : 179–192.
4. ChapmanT, HerndonLA, HeifetzY, PartridgeL, WolfnerMF (2001) The Acp26Aa seminal fluid protein is a modulator of early egg hatchability in Drosophila melanogaster. Proc R Soc Lond B Biol Sci 268 : 1647–1654.
5. HeifetzY, TramU, WolfnerMF (2001) Male contributions to egg production: the role of accessory gland products and sperm in Drosophila melanogaster. Proc R Soc Lond B Biol Sci 268 : 175–180.
6. HerndonLA, WolfnerMF (1995) A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc Natl Acad Sci U S A 92 : 10114–10118.
7. RobertsonSA (2007) Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs. J Anim Sci 85: E36–44.
8. PoianiA (2006) Complexity of seminal fluid: a review. Behav Ecol Sociobiol 60 : 289–310.
9. Rodríguez-MartínezH, KvistU, ErnerudhJ, SanzL, CalveteJJ (2011) Seminal plasma proteins: what role do they play? Am J Reprod Immunol 66 : 11–22.
10. AvilaFW, SirotLK, LaFlammeBA, RubinsteinCD, WolfnerMF (2011) Insect seminal fluid proteins: identification and function. Annu Rev Entomol 56 : 21–40.
11. Mann T, Lutwak-Mann C (1981) Male reproductive function and semen. New York, New York: Springer-Verlag. 495 p.
12. WigbyS, SirotLK, LinklaterJR, BuehnerN, CalboliFCF, et al. (2009) Seminal fluid protein allocation and male reproductive success. Curr Biol 19 : 751–757.
13. HarshmanLG, ProutT (1994) Sperm displacement without sperm transfer in Drosophila melanogaster. Evolution 48 : 758–766.
14. SirotLK, WolfnerMF, WigbyS (2011) Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster. Proc Natl Acad Sci U S A 108 : 9922.
15. BarkerDM (1994) Copulatory plugs and paternity assurance in the nematode Caenorhabditis elegans. Anim Behav 48 : 147–156.
16. RogersDW, BaldiniF, BattagliaF, PanicoM, DellA, et al. (2009) Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol 7: e1000272 doi:10.1371/journal.pbio.1000272.
17. DevineMC (1975) Copulatory plugs in snakes: enforced chastity. Science 187 : 844–845.
18. Price D, Williams-Ashman HG (1961) The accessory reproductive glands of mammals; Young WC, editor. Baltimore: Williams and Wilkins Inc. 366–448 p.
19. Leuckart R (1847) Zur morphologie und anatomie der geschlechtsorgane. Göttingen: Vandenhoeck und Ruprecht. 130 p.
20. VossR (1979) Male accessory glands and the evolution of copulatory plugs in rodents. Occas Pap Mus Zool Univ Mich 689 : 1–27.
21. HartungTG, DewsburyDA (1978) A comparative analysis of copulatory plugs in muroid rodents and their relationship to copulatory behavior. J Mammal 59 : 717–723.
22. DixsonAF, AndersonMJ (2002) Sexual selection, seminal coagulation and copulatory plug formation in primates. Folia Primatol 73 : 63–69.
23. CamusL, GleyE (1896) Action coagulante du liquide prostatique sur le contenu des vésicules séminales. Compt Rend Hebd Seances Acad Sci 123 : 194–195.
24. WalkerG (1910) A special function discovered in a glandular structure hitherto supposed to form a part of the prostate gland in rats and guinea-pigs. Johns Hopkins Hosp Reports 21 : 182–185.
25. WalkerG (1910) The nature of the secretion of the vesiculae seminales and of an adjacent glandular structure in the rat and guinea-pig, with special reference to the occurrence of histone in the former. Johns Hopkins Hosp Reports 21 : 185–192.
26. GottererG, GinsbergD, SchulmanT, BanksJ, Williams-AshmanHG (1955) Enzymatic coagulation of semen. Nature 176 : 1209–1211.
27. NotidesAC, Williams-AshmanHG (1967) The basic protein responsible for the clotting of guinea pig semen. Proc Natl Acad Sci U S A 58 : 1991–1995.
28. Williams-AshmanHG, NotidesAC, PabalanSS, LorandL (1972) Transamidase reactions involved in the enzymic coagulation of semen: isolation of γ-glutamyl-ε-lysine dipeptide from clotted secretion protein of guinea pig seminal vesicle. Proc Natl Acad Sci U S A 69 : 2322–2325.
29. GentileV, GrantF, PortaR (1995) Localization of the human prostate transglutaminase (type IV) gene (TGM4) to chromosome 3p21. 33-p22 by fluorescence in situ hybridization. Genomics 27 : 219–220.
30. GrantFJ, TaylorDA, SheppardPO, MathewesSL, LintW, et al. (1994) Molecular cloning and characterization of a novel transglutaminase cDNA from a human prostate cDNA library. Biochem Biophys Res Commun 203 : 1117–1123.
31. DubbinkHJ, VerkaikNS, FaberPW, TrapmanJ, SchroderFH, et al. (1996) Tissue specific and androgen-regulated expression of human prostate-specific transglutaminase. Biochem J 315(Pt 3): 901–908.
32. PilchB, MannM (2006) Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol 7: R40.
33. DeanMD, FindlayGD, HoopmannMR, WuCC, MacCossMJ, et al. (2011) Identification of ejaculated proteins in the house mouse (Mus domesticus) via isotopic labeling. BMC Genomics 12 : 306.
34. TsengH-C, TangJ-B, Sudhakar GandhiP, LuoC-W, OuC-M, et al. (2011) Mutual adaptation between mouse transglutaminase 4 and its native substrates in the formation of copulatory plug. Amino Acids 42 : 951–960.
35. HartRG, GreensteinJS (1968) A newly discovered role for Cowper's gland secretion in rodent semen coagulation. J Reprod Fertil 17 : 87–94.
36. HartmanCG (1924) Observations on the motility of the opossum genital tract and the vaginal plug. Anat Rec 27 : 293–303.
37. Williams-AshmanHG, BeilRE, WilsonJ, HawkinsM, GrayhackJ, et al. (1980) Transglutaminases in mammalian reproductive tissues and fluids: relation to polyamine metabolism and semen coagulation. Adv Enzyme Regul 18 : 239–246, IN213–IN214, 247–258.
38. Williams-AshmanHG (1984) Transglutaminases and the clotting of mammalian seminal fluids. Mol Cell Biochem 58 : 51–61.
39. PortaR, EspositoC, MetaforaS, MalorniA, PucciP, et al. (1991) Mass spectrometric identification of the amino donor and acceptor sites in a transglutaminase protein substrate secreted from rat seminal vesicles. Biochemistry 30 : 3114–3120.
40. WalkerG (1911) The effect on breeding of the removal of the prostate gland or of the vesiculae seminales, or of both; together with observations on the condition of the testes after such operations on white rats. Johns Hopkins Hosp Reports 16 : 223–255.
41. BlandauRJ (1945) Is the copulation plug necessary for the en masse transport of spermatozoa into the uterine cornua of the albino rat? Anat Rec 91 : 266–267.
42. CarballadaR, EspondaP (1992) Role of fluid from seminal vesicles and coagulating glands in sperm transport into the uterus and fertility in rats. J Reprod Fertil 95 : 639–648.
43. CukierskiMA, SinaJL, PrahaladaS, RobertsonRT (1991) Effects of seminal vesicle and coagulating gland ablation on fertility in rats. Reprod Toxicol 5 : 347–352.
44. OW-S, ChenHQ, ChowPH (1988) Effects of male accessory sex gland secretions on early embryonic development in the golden hamster. J Reprod Fertil 84 : 341–344.
45. QueenK, DhabuwalaCB, PierrepointCG (1981) The effect of the removal of the various accessory sex glands on the fertility of male rats. J Reprod Fertil 62 : 423–426.
46. PeitzB, Olds-ClarkeP (1986) Effects of seminal vesicle removal on fertility and uterine sperm motility in the house mouse. Biol Reprod 35 : 608–617.
47. PangSF, ChowPH, WongTM (1979) The role of the seminal vesicles, coagulating glands and prostate glands on the fertility and fecundity of mice. J Reprod Fertil 56 : 129–132.
48. MartanJ, ShepherdBA (1976) The role of the copulatory plug in reproduction of the guinea pig. J Exp Zool 196 : 79–83.
49. FirmanRC, SimmonsLW (2009) Experimental evolution of sperm quality via postcopulatory sexual selection in house mice. Evolution 64 : 1245–1256.
50. AustinCP, BatteyJF, BradleyA, BucanM, CapecchiM, et al. (2004) The knockout mouse project. Nat Genet 36 : 921–924.
51. TestaG, SchaftJ, van der HoevenF, GlaserS, AnastassiadisK, et al. (2004) A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles. Genesis 38 : 151–158.
52. BlandauRJ (1945) On the factors involved in sperm transport through the cervix uteri of the albino rat. Am J Anat 77 : 253–272.
53. TonerJP, AttasAI, AdlerNT (1987) Transcervical sperm transport in the rat: the roles of pre-ejaculatory behavior and copulatory plug fit. Physiol Behav 39 : 371–375.
54. MatthewsMKJr, AdlerNT (1978) Systematic interrelationship of mating, vaginal plug position, and sperm transport in the rat. Physiol Behav 20 : 303–309.
55. BallJ (1934) Demonstration of a quantitative relation between stimulus and response in pseudopregnancy in the rat. Am J Physiol 107 : 698–703.
56. WestJD, FrelsWI, PapaioannouVE, KarrJP, ChapmanVM (1977) Development of interspecific hybrids of Mus. J Embryol Exp Morphol 41 : 233–243.
57. LeckiePA, WatsonJG, ChaykinS (1973) An improved method for the artificial insemination of the mouse (Mus musculus). Biol Reprod 9 : 420–425.
58. EngleET (1926) A morphological and experimental study of the proximal lobes of the prostate of the guinea-pig, cavia cobaya. Anat Rec 34 : 75–90.
59. DeanMD, ClarkNL, FindlayGD, KarnRC, YiX, et al. (2009) Proteomics and comparative genomic investigations reveal heterogeneity in evolutionary rate of male reproductive proteins in mice (Mus domesticus). Mol Biol Evol 26 : 1733–1743.
60. DubbinkHJ, de WaalL, van HaperenR, VerkaikNS, TrapmanJ, et al. (1998) The human prostate-specific transglutaminase gene (TGM4): genomic organization, tissue-specific expression, and promoter characterization. Genomics 51 : 434–444.
61. SuAI, CookeMP, ChingKA, HakakY, WalkerJR, et al. (2002) Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A 99 : 4465–4470.
62. SuAI, WiltshireT, BatalovS, LappH, ChingKA, et al. (2004) A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A 101 : 6062–6067.
63. PaonessaG, MetaforaS, TajanaG, AbresciaP, SantisAD, et al. (1984) Transglutaminase-mediated modifications of the rat sperm surface in vitro. Science 226 : 852–855.
64. MukherjeeDC, AgrawalAK, ManjunathR, MukherjeeAB (1983) Suppression of epididymal sperm antigenicity in the rabbit by uteroglobin and transglutaminase in vitro. Science 219 : 989–991.
65. DorusS, WasbroughER, BusbyJ, WilkinEC, KarrTL (2010) Sperm proteomics reveals intensified selection on mouse sperm membrane and acrosome genes. Mol Biol Evol 27 : 1235–1246.
66. BakerMA, ReevesG, HetheringtonL, MüllerJ, BaurI, et al. (2007) Identification of gene products present in Triton X-100 soluble and insoluble fractions of human spermatozoa lysates using LC-MS/MS analysis. Proteomics Clin Appl 1 : 524–532.
67. CarnahanSJ, Jensen-SeamanMI (2008) Hominoid seminal protein evolution and ancestral mating behavior. Am J Primatol 70 : 939–948.
68. ParkerGA (1970) Sperm competition and its evolutionary consequences in the insects. Biol Rev Camb Philos Soc 45 : 525–567.
69. RammSA, ParkerGA, StockleyP (2005) Sperm competition and the evolution of male reproductive anatomy in rodents. Proc Biol Sci 272 : 949–955.
70. KinganSB, TatarM, RandDM (2003) Reduced polymorphism in the chimpanzee semen coagulating protein, semenogelin I. J Mol Evol 57 : 159–169.
71. Jensen-SeamanMI, LiWH (2003) Evolution of the hominoid semenogelin genes, the major proteins of ejaculated semen. J Mol Evol 57 : 261–270.
72. RammSA, McDonaldL, HurstJL, BeynonRJ, StockleyP (2009) Comparative proteomics reveals evidence for evolutionary diversification of rodent seminal fluid and its functional significance in sperm competition. Mol Biol Evol 26 : 189–198.
73. DorusS, EvansPD, WyckoffGJ, ChoiSS, LahnBT (2004) Rate of molecular evolution of the seminal protein gene SEMG2 correlates with levels of female promiscuity. Nat Genet 36 : 1326–1329.
74. MurerV, SpetzJF, HengstU, AltroggeLM, de AgostiniA, et al. (2001) Male fertility defects in mice lacking the serine protease inhibitor protease nexin-1. Proc Natl Acad Sci U S A 98 : 3029–3033.
75. FirmanRC, SimmonsLW (2008) The frequency of multiple paternity predicts variation in testes size among island populations of house mice. J Evol Biol 21 : 1524–1533.
76. DeanMD, ArdlieKG, NachmanMW (2006) The frequency of multiple paternity suggests that sperm competition is common in house mice (Mus domesticus). Mol Ecol 15 : 4141–4151.
77. KoprowskiJL (1992) Removal of copulatory plugs by female tree squirrels. J Mammal 73 : 572–576.
78. PargaJA (2003) Copulatory plug displacement evidences sperm competition in Lemur catta. Int J Primatol 24 : 889–899.
79. PargaJA, MagaM, OverdorffDJ (2006) High-resolution X-ray computed tomography scanning of primate copulatory plugs. Am J Phys Anthropol 129 : 567–576.
80. O'HanlonJK, SachsBD (1986) Fertility of mating in rats (Rattus norvegicus): contributions of androgen-dependent morphology and actions of the penis. J Comp Psychol 100 : 178–187.
81. WallachSJR, HartBL (1983) The role of the striated penile muscles of the male rat in seminal plug dislodgement and deposition. Physiol Behav 31 : 815–821.
82. DewsburyDA (1988) A test of the role of copulatory plugs in sperm competition in deer mice (Peromyscus maniculatus). J Mammal 69 : 854–857.
83. LanierDL, EstepDQ, DewsburyDA (1979) Role of prolonged copulatory behavior in facilitating reproductive success in a competitive mating situation in laboratory rats. J Comp Physiol Psychol 93 : 781–792.
84. OglesbyJM, LanierDL, DewsburyDA (1981) The role of prolonged copulatory behavior in facilitating reproductive success in male Syrian golden hamsters (Mesocricetus auratus) in a competitive mating situation. Behav Ecol Sociobiol 8 : 47–54.
85. HuckUW, ToniasBA, LiskRD (1989) The effectiveness of competitive male inseminations in golden hamsters, Mesocricetus auratus, depends on an interaction of mating order, time delay between males, and the time of mating relative to ovulation. Anim Behav 37 : 674–680.
86. FoltzDW (1981) Genetic evidence for long-term monogamy in a small rodent, Peromyscus polionotus. Am Nat 117 : 665–675.
87. BaumgardnerDJ, HartungTG, SawreyDK, WebsterDG, DewsburyDA (1982) Muroid copulatory plugs and female reproductive tracts: a comparative investigation. J Mammal 63 : 110–117.
88. Asdell SA (1946) Patterns of mammalian reproduction. Ithaca, New York: Comstock Publishing Company. 437 p.
89. HugginsC, NealW (1942) Coagulation and liquefaction of semen. J Exp Med 76 : 527.
90. MikhailichenkoVV, EsipovAS (2005) Peculiarities of semen coagulation and liquefaction in males from infertile couples. Fertil Steril 84 : 256–259.
91. MilardiD, GrandeG, VincenzoniF, MessanaI, PontecorviA, et al. (2012) Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil Steril 97 : 67–73.
92. Snyder RL (1967) Fertility and reproductive performance of grouped male mice. In: Benirschke K, editor. Comparative aspects of reproductive failure. New York: Springer-Verlag. pp. 458–472.
93. Nagy A, Gertsenstein M, Vintersten K, Behringer R (2003) Manipulating the mouse embryo. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. 764 p.
94. DeanMD, NachmanMW (2009) Faster fertilization rate in conspecific versus heterospecific matings in house mice. Evolution 63 : 20–28.
95. GoodJM, DeanMD, NachmanMW (2008) A complex genetic basis to X-linked hybrid male sterility between two species of house mice. Genetics 179 : 2213–2228.
96. GoodJM, HandelMA, NachmanMW (2008) Asymmetry and polymorphism of hybrid male sterility during the early stages of speciation in house mice. Evolution 62 : 50–65.
97. WelchBL (1947) The generalization of “Student's” problem when several different population variances are involved. Biometrika 34 : 28–35.
Štítky
Genetika Reprodukční medicína
Článek Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across PathogensČlánek TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association StudiesČlánek Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization inČlánek Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA ExpressionČlánek The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of GenesČlánek The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 1- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- A Model of High Sugar Diet-Induced Cardiomyopathy
- Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across Pathogens
- Emerging Function of Fat Mass and Obesity-Associated Protein (Fto)
- Positional Cloning Reveals Strain-Dependent Expression of to Alter Susceptibility to Bleomycin-Induced Pulmonary Fibrosis in Mice
- Genetics of Ribosomal Proteins: “Curiouser and Curiouser”
- Transposable Elements Re-Wire and Fine-Tune the Transcriptome
- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- MAML1 Enhances the Transcriptional Activity of Runx2 and Plays a Role in Bone Development
- Predicting Mendelian Disease-Causing Non-Synonymous Single Nucleotide Variants in Exome Sequencing Studies
- A Systematic Mapping Approach of 16q12.2/ and BMI in More Than 20,000 African Americans Narrows in on the Underlying Functional Variation: Results from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- Transcription of the Major microRNA–Like Small RNAs Relies on RNA Polymerase III
- Histone H3K56 Acetylation, Rad52, and Non-DNA Repair Factors Control Double-Strand Break Repair Choice with the Sister Chromatid
- Genome-Wide Association Study Identifies a Novel Susceptibility Locus at 12q23.1 for Lung Squamous Cell Carcinoma in Han Chinese
- Genetic Disruption of the Copulatory Plug in Mice Leads to Severely Reduced Fertility
- The [] Prion Exists as a Dynamic Cloud of Variants
- Adult Onset Global Loss of the Gene Alters Body Composition and Metabolism in the Mouse
- Fis Protein Insulates the Gene from Uncontrolled Transcription
- The Meiotic Nuclear Lamina Regulates Chromosome Dynamics and Promotes Efficient Homologous Recombination in the Mouse
- Genome-Wide Haplotype Analysis of Expression Quantitative Trait Loci in Monocytes
- TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association Studies
- Structural Basis of a Histone H3 Lysine 4 Demethylase Required for Stem Elongation in Rice
- The Ecm11-Gmc2 Complex Promotes Synaptonemal Complex Formation through Assembly of Transverse Filaments in Budding Yeast
- MCM8 Is Required for a Pathway of Meiotic Double-Strand Break Repair Independent of DMC1 in
- Comparative Genomic Analysis of the Endosymbionts of Herbivorous Insects Reveals Eco-Environmental Adaptations: Biotechnology Applications
- Integration of Nodal and BMP Signals in the Heart Requires FoxH1 to Create Left–Right Differences in Cell Migration Rates That Direct Cardiac Asymmetry
- Pharmacodynamics, Population Dynamics, and the Evolution of Persistence in
- A Hybrid Likelihood Model for Sequence-Based Disease Association Studies
- Aberration in DNA Methylation in B-Cell Lymphomas Has a Complex Origin and Increases with Disease Severity
- Multiple Opposing Constraints Govern Chromosome Interactions during Meiosis
- Transcriptional Dynamics Elicited by a Short Pulse of Notch Activation Involves Feed-Forward Regulation by Genes
- Dynamic Large-Scale Chromosomal Rearrangements Fuel Rapid Adaptation in Yeast Populations
- Heterologous Gln/Asn-Rich Proteins Impede the Propagation of Yeast Prions by Altering Chaperone Availability
- Gene Copy-Number Polymorphism Caused by Retrotransposition in Humans
- An Incompatibility between a Mitochondrial tRNA and Its Nuclear-Encoded tRNA Synthetase Compromises Development and Fitness in
- Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization in
- Single-Stranded Annealing Induced by Re-Initiation of Replication Origins Provides a Novel and Efficient Mechanism for Generating Copy Number Expansion via Non-Allelic Homologous Recombination
- Tbx2 Controls Lung Growth by Direct Repression of the Cell Cycle Inhibitor Genes and
- Suv4-20h Histone Methyltransferases Promote Neuroectodermal Differentiation by Silencing the Pluripotency-Associated Oct-25 Gene
- A Conserved Helicase Processivity Factor Is Needed for Conjugation and Replication of an Integrative and Conjugative Element
- Telomerase-Null Survivor Screening Identifies Novel Telomere Recombination Regulators
- Genome-Wide Analysis Reveals Selection for Important Traits in Domestic Horse Breeds
- Coordinated Degradation of Replisome Components Ensures Genome Stability upon Replication Stress in the Absence of the Replication Fork Protection Complex
- Nkx6.1 Controls a Gene Regulatory Network Required for Establishing and Maintaining Pancreatic Beta Cell Identity
- HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in
- Delineating a Conserved Genetic Cassette Promoting Outgrowth of Body Appendages
- The Telomere Capping Complex CST Has an Unusual Stoichiometry, Makes Multipartite Interaction with G-Tails, and Unfolds Higher-Order G-Tail Structures
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Loci Associated with -Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers
- Switchgrass Genomic Diversity, Ploidy, and Evolution: Novel Insights from a Network-Based SNP Discovery Protocol
- Centromere-Like Regions in the Budding Yeast Genome
- Sequencing of Loci from the Elephant Shark Reveals a Family of Genes in Vertebrate Genomes, Forged by Ancient Duplications and Divergences
- Mendelian and Non-Mendelian Regulation of Gene Expression in Maize
- Mutational Spectrum Drives the Rise of Mutator Bacteria
- Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA Expression
- The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of Genes
- Sex-Specific Signaling in the Blood–Brain Barrier Is Required for Male Courtship in
- A Newly Uncovered Group of Distantly Related Lysine Methyltransferases Preferentially Interact with Molecular Chaperones to Regulate Their Activity
- Is Required for Leptin-Mediated Depolarization of POMC Neurons in the Hypothalamic Arcuate Nucleus in Mice
- Unlocking the Bottleneck in Forward Genetics Using Whole-Genome Sequencing and Identity by Descent to Isolate Causative Mutations
- The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
- MTERF3 Regulates Mitochondrial Ribosome Biogenesis in Invertebrates and Mammals
- Downregulation and Altered Splicing by in a Mouse Model of Facioscapulohumeral Muscular Dystrophy (FSHD)
- NBR1-Mediated Selective Autophagy Targets Insoluble Ubiquitinated Protein Aggregates in Plant Stress Responses
- Retroactive Maintains Cuticle Integrity by Promoting the Trafficking of Knickkopf into the Procuticle of
- Phenome-Wide Association Study (PheWAS) for Detection of Pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network
- Genetic and Functional Modularity of Activities in the Specification of Limb-Innervating Motor Neurons
- A Population Genetic Model for the Maintenance of R2 Retrotransposons in rRNA Gene Loci
- A Quartet of PIF bHLH Factors Provides a Transcriptionally Centered Signaling Hub That Regulates Seedling Morphogenesis through Differential Expression-Patterning of Shared Target Genes in
- A Genome-Wide Integrative Genomic Study Localizes Genetic Factors Influencing Antibodies against Epstein-Barr Virus Nuclear Antigen 1 (EBNA-1)
- Mutation of the Diamond-Blackfan Anemia Gene in Mouse Results in Morphological and Neuroanatomical Phenotypes
- Life, the Universe, and Everything: An Interview with David Haussler
- Alternative Oxidase Expression in the Mouse Enables Bypassing Cytochrome Oxidase Blockade and Limits Mitochondrial ROS Overproduction
- An Evolutionarily Conserved Synthetic Lethal Interaction Network Identifies FEN1 as a Broad-Spectrum Target for Anticancer Therapeutic Development
- The Flowering Repressor Underlies a Novel QTL Interacting with the Genetic Background
- Telomerase Is Required for Zebrafish Lifespan
- and Diversified Expression of the Gene Family Bolster the Floral Stem Cell Network
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Increased Maternal Genome Dosage Bypasses the Requirement of the FIS Polycomb Repressive Complex 2 in Arabidopsis Seed Development
- WNK1/HSN2 Mutation in Human Peripheral Neuropathy Deregulates Expression and Posterior Lateral Line Development in Zebrafish ()
- Synergistic Interaction of Rnf8 and p53 in the Protection against Genomic Instability and Tumorigenesis
- Dot1-Dependent Histone H3K79 Methylation Promotes Activation of the Mek1 Meiotic Checkpoint Effector Kinase by Regulating the Hop1 Adaptor
- A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Reproductive Tract
- Starvation, Together with the SOS Response, Mediates High Biofilm-Specific Tolerance to the Fluoroquinolone Ofloxacin
- Directed Evolution of a Model Primordial Enzyme Provides Insights into the Development of the Genetic Code
- Genome-Wide Screens for Tinman Binding Sites Identify Cardiac Enhancers with Diverse Functional Architectures
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



