-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPCH'ing Together an Understanding of Crossover Control
article has not abstract
Published in the journal: . PLoS Genet 5(7): e32767. doi:10.1371/journal.pgen.1000576
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1000576Summary
article has not abstract
Meiosis is a specialized form of cell division involving one round of chromosome replication followed by two rounds of segregation, thereby producing daughter cells with half the genomic equivalent of the progenitor. In most organisms, double-strand breaks (DSBs) are introduced into the genome following premeiotic S-phase. These breaks are repaired almost exclusively from the homologous chromosome via repair pathways that yield either a crossover or non-crossover recombination product [1],[2]. Of particular importance are the crossovers, which tether homologous chromosomes and ensure accurate segregation at the first meiotic division (MI) [3]. Chromosomes that fail to cross over have significantly higher rates of non-disjunction at MI, which produces aneuploid gametes, causing miscarriages and birth defects in humans.
It should be no surprise then that most eukaryotes possess a sophisticated mechanism to control meiotic recombination. Consider the situation in an individual mouse meiocyte. More than 200 DSBs are made; however, only a subset of these precursors are repaired as crossovers, while the rest are repaired as non-crossovers (Figure 1) [4]. Thus, central to the “crossover control” mechanism is a decision to direct a given DSB to either a crossover or non-crossover fate [5]. This process ensures that each homolog pair receives at least one crossover (often referred to as the obligate crossover), and also regulates the spatial distribution of crossovers along chromosomes such that, if a chromosome receives two or more crossovers, they tend to occur further apart than expected by chance (referred to as crossover interference) (Figure 1B) [6]. Furthermore, it has been shown that when the number of DSBs is reduced, crossovers tend to be maintained at the expense of non-crossovers (a phenomenon called crossover homeostasis) [7],[8]. It has been proposed that the obligate crossover, crossover interference, and crossover homeostasis are all manifestations of a single or closely related set of molecular processes, but this hypothesis remains to be rigorously tested [7],[9],[10].
Fig. 1. Only a subset of DSBs become crossovers. 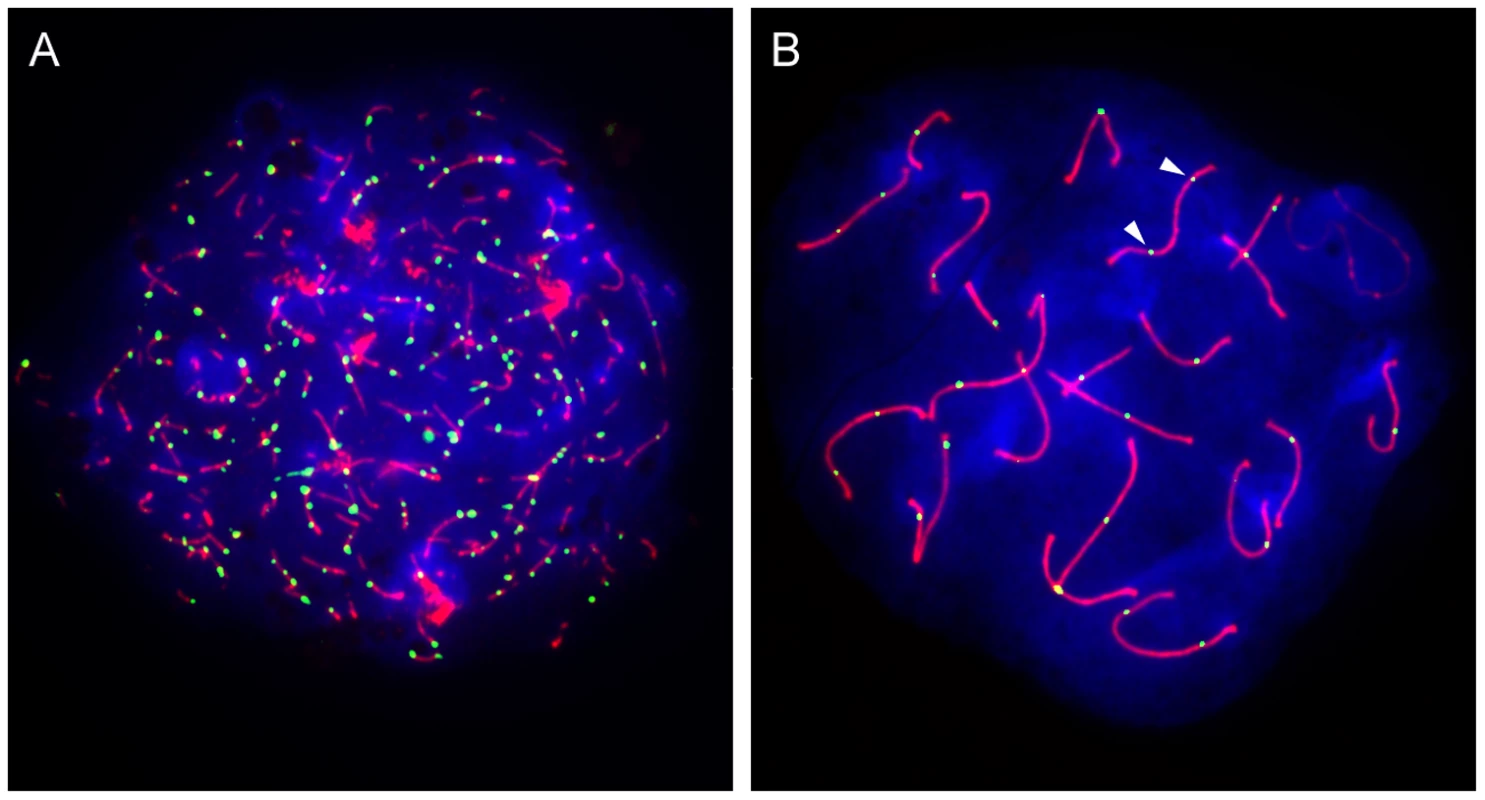
Mouse spermatocyte spreads were stained for the chromosomal axial element SYCP3 (red) and either (A) RAD51 (green) or (B) MLH1 (green). DAPI staining is shown in blue. Each DSB gives rise to a chromosome-associated RAD51 complex, whereas MLH1 complexes localize only to sites that will become crossovers. There is an approximate 9-fold excess of DSB-associated foci relative to crossover-associated foci. Arrowheads point to an example of an autosome with two widely separated MLH1 foci, characteristic of crossover interference. (Images courtesy of Ignasi Roig, Molecular Biology Program, Memorial Sloan-Kettering Cancer Center). Almost a century after the first observation of crossover control [11], we still know very little about the underlying mechanism(s). Most of the proteins that have been shown to influence crossover control in budding yeast appear to function downstream of the crossover/non-crossover decision. One such class of proteins, commonly referred to as ZMMs (Zip1/2/3/4, Msh4/5, Mer3), is specifically required for the repair of DSBs into crossovers that exhibit interference [2]. Deletion of any of the ZMM genes causes accumulation of intermediates in the crossover pathway and subsequent prophase arrest [12].
Two articles in this issue of PLoS Genetics identify a role for the Pachytene Checkpoint gene, PCH2, in crossover control in Saccharomyces cerevisiae [13],[14]. PCH2, which encodes a putative AAA+-ATPase, was initially identified in yeast as a checkpoint factor due to suppression of a zip1Δ arrest in a pch2Δ mutant. This and other observations led to the hypothesis that Pch2 helps monitor chromosome synapsis during meiotic prophase [15],[16]. However, studies in yeast, flies, and mice revealed that Pch2 is not just a checkpoint factor, but that it is also required for chromosome axis organization and DSB repair [17],[18],[19]. Interestingly, PCH2 is widely conserved in organisms that construct a synaptonemal complex and exhibit crossover interference, but is absent from organisms such as Schizosaccharomyces pombe that do not exhibit these features [15]. This observation suggested that Pch2 might also function in crossover control. Recent analysis in yeast demonstrated a small reduction in crossover numbers in pch2 mutants at the HIS4LEU2 recombination hotspot [19], but data available at the time did not allow evaluation of crossing over genome-wide and also did not address whether crossover control was normal.
In studies published in this issue of PLoS Genetics, the Alani and Börner groups [13],[14] have examined these issues in detail. When crossover frequencies were measured across several genetic intervals on chromosomes III, VII, and VIII, Zanders et al. [13] and Joshi et al. [14] observed either no difference or an increase (depending on the interval) in pch2Δ strains. Importantly, these analyses demonstrated that the crossovers formed in pch2Δ mutants show reduced interference.
Recent studies have indicated that decreased crossover interference is associated with a concomitant decrease in crossover homeostasis [8]. To investigate this relationship, both Zanders et al. and Joshi et al. measured spore viability of pch2Δ strains carrying various hypomorphic alleles of the topoisomerase-like protein, Spo11. These hypomorphic alleles decrease the number of DSBs [20]. If crossover homeostasis and crossover interference are separate manifestations of a common crossover control mechanism, then an interference-defective mutant would also be expected to show defects in homeostasis, and thus a decrease in DSBs in such a mutant should result in fewer crossovers that are randomly distributed throughout the genome. Such a scenario would in turn be expected to result in an increase in the frequency of chromosome pairs without a crossover, causing reduced spore viability because of MI non-disjunction. Indeed, although pch2 mutation has little or no effect on spore viability on its own, introducing a spo11 mutation that reduces DSB activity by ∼20% significantly reduced viability despite approximately wild-type crossover frequencies [13],[14]. This reduction in spore viability was further exacerbated in spo11 hypomorphs that reduce DSB activity up to 80%. Although this is an indirect method of measuring crossover homeostasis, these findings provide compelling evidence that Pch2 has a role in multiple aspects of crossover control during yeast meiosis.
So what role could Pch2 play in this process? Pch2 is required for differential organization of chromosome structural proteins Hop1 and Red1 relative to the synaptonemal complex central element protein Zip1 [19]. In pch2Δ mutants, Hop1/Red1 and Zip1 exhibit a more uniform axial localization pattern than is observed in wild type. Joshi et al. now demonstrate that chromosome domains that are enriched for Hop1 and Red1 tend to colocalize with future sites of crossover formation, leading to the hypothesis that Pch2 functions to stabilize alternating domains enriched for either Hop1/Red1 or Zip1. Such domains are proposed to be modules that mediate crossover designation and interference. Interestingly, when PCH2 is deleted, not only is axial organization of Hop1/Red1 and Zip1 compromised, but appearance of both crossover and non-crossover products is delayed to similar extents [19]. It is not yet clear whether these different aspects of the pch2 mutant phenotype are consequences of the same molecular defect, nor is it yet clear precisely how Pch2 protein functions in wild-type cells. Nonetheless, the current findings provide new support for the idea that higher order chromosome structure plays a key role in crossover control [9], and furthermore implicate Pch2 as an important player in coordinating recombination with large-scale chromosome structures.
Zdroje
1. AllersT
LichtenM
2001 Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106 47 57
2. BornerGV
KlecknerN
HunterN
2004 Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117 29 45
3. PageSL
HawleyRS
2003 Chromosome choreography: The meiotic ballet. Science 301 785 789
4. BuhlerC
BordeV
LichtenM
2007 Mapping meiotic single-strand DNA reveals a new landscape of DNA double-strand breaks in Saccharomyces cerevisiae. PLoS Biol 5(12) e324 doi:10.1371/journal.pbio.0050324
5. BishopDK
ZicklerD
2004 Early decision; Meiotic crossover interference prior to stable strand exchange and synapsis. Cell 117 9 15
6. JonesGH
1984 The control of chiasma distribution. Symp Soc Exp Biol 38 293 320
7. MartiniE
DiazRL
HunterN
KeeneyS
2006 Crossover homeostasis in yeast meiosis. Cell 126 285 295
8. ChenSY
TsubouchiT
RockmillB
SandlerJS
RichardsDR
2008 Global analysis of the meiotic crossover landscape. Dev Cell 15 401 415
9. KlecknerN
ZicklerD
JonesGH
DekkerJ
PadmoreR
2004 A mechanical basis for chromosome function. Proc Natl Acad Sci U S A 101 12592 12597
10. HillersKJ
2004 Crossover interference. Curr Biol 14 R1036 R1037
11. MullerHJ
1916 The mechanism of crossing over. Am Nat 50 193 221
12. LynnA
SoucekR
BornerGV
2007 ZMM proteins during meiosis: Crossover artists at work. Chromosome Res 15 591 605
13. ZandersS
AlaniE
2009 The pch2Δ mutation in baker's yeast alters meiotic crossover levels and confers a defect in crossover interference. PLoS Genet 5(7) e1000571 doi:10.1371/journal.pgen.1000571
14. JoshiN
BarotA
JamisonC
BörnerGV
2009 Pch2 links chromosome axis remodeling at future crossover sites and crossover distribution during yeast meiosis. PLoS Genet 5(7) e1000557 doi:10.1371/journal.pgen.1000557
15. WuHY
BurgessSM
2006 Two distinct surveillance mechanisms monitor meiotic chromosome metabolism in budding yeast. Curr Biol 16 2473 2479
16. BhallaN
DernburgAF
2005 A conserved checkpoint monitors meiotic chromosome synapsis in Caenorhabditis elegans. Science 310 1683 1686
17. LiX
SchimentiJC
2007 Mouse pachytene checkpoint 2 (Trip13) is required for completing meiotic recombination but not synapsis. PLoS Genet 3(8) e130 doi:10.1371/journal.pgen.0030130
18. JoyceEF
McKimKS
2009 Drosophila PCH2 is required for a pachytene checkpoint that monitors double-strand-break-independent events leading to meiotic crossover formation. Genetics 181 39 51
19. BornerGV
BarotA
KlecknerN
2008 Yeast Pch2 promotes domainal axis organization, timely recombination progression, and arrest of defective recombinosomes during meiosis. Proc Natl Acad Sci U S A 105 3327 3332
20. DiazRL
AlcidAD
BergerJM
KeeneyS
2002 Identification of residues in yeast Spo11p critical for meiotic DNA double-strand break formation. Mol Cell Biol 22 1106 1115
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2009 Číslo 7- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Scientists←Editors←Scientists: The Past, Present, and Future of
- Prediction and Interaction in Complex Disease Genetics: Experience in Type 1 Diabetes
- PCH'ing Together an Understanding of Crossover Control
- You Say You Want a Revolution: An Interview with Pat Brown
- A Missense Mutation in the Gene in Dachshunds with Osteogenesis Imperfecta
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Missense Mutation in the Gene in Dachshunds with Osteogenesis Imperfecta
- Scientists←Editors←Scientists: The Past, Present, and Future of
- Prediction and Interaction in Complex Disease Genetics: Experience in Type 1 Diabetes
- PCH'ing Together an Understanding of Crossover Control
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



