-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Asymmetric Strand Segregation: Epigenetic Costs of Genetic Fidelity?
Asymmetric strand segregation has been proposed as a mechanism to minimize effective mutation rates in epithelial tissues. Under asymmetric strand segregation, the double-stranded molecule that contains the oldest DNA strand is preferentially targeted to the somatic stem cell after each round of DNA replication. This oldest DNA strand is expected to have fewer errors than younger strands because some of the errors that arise on daughter strands during their synthesis fail to be repaired. Empirical findings suggest the possibility of asymmetric strand segregation in a subset of mammalian cell lineages, indicating that it may indeed function to increase genetic fidelity. However, the implications of asymmetric strand segregation for the fidelity of epigenetic information remain unexplored. Here, I explore the impact of strand-segregation dynamics on epigenetic fidelity using a mathematical-modelling approach that draws on the known molecular mechanisms of DNA methylation and existing rate estimates from empirical methylation data. I find that, for a wide range of starting methylation densities, asymmetric—but not symmetric—strand segregation leads to systematic increases in methylation levels if parent strands are subject to de novo methylation events. I found that epigenetic fidelity can be compromised when enhanced genetic fidelity is achieved through asymmetric strand segregation. Strand segregation dynamics could thus explain the increased DNA methylation densities that are observed in structured cellular populations during aging and in disease.
Published in the journal: . PLoS Genet 5(6): e32767. doi:10.1371/journal.pgen.1000509
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000509Summary
Asymmetric strand segregation has been proposed as a mechanism to minimize effective mutation rates in epithelial tissues. Under asymmetric strand segregation, the double-stranded molecule that contains the oldest DNA strand is preferentially targeted to the somatic stem cell after each round of DNA replication. This oldest DNA strand is expected to have fewer errors than younger strands because some of the errors that arise on daughter strands during their synthesis fail to be repaired. Empirical findings suggest the possibility of asymmetric strand segregation in a subset of mammalian cell lineages, indicating that it may indeed function to increase genetic fidelity. However, the implications of asymmetric strand segregation for the fidelity of epigenetic information remain unexplored. Here, I explore the impact of strand-segregation dynamics on epigenetic fidelity using a mathematical-modelling approach that draws on the known molecular mechanisms of DNA methylation and existing rate estimates from empirical methylation data. I find that, for a wide range of starting methylation densities, asymmetric—but not symmetric—strand segregation leads to systematic increases in methylation levels if parent strands are subject to de novo methylation events. I found that epigenetic fidelity can be compromised when enhanced genetic fidelity is achieved through asymmetric strand segregation. Strand segregation dynamics could thus explain the increased DNA methylation densities that are observed in structured cellular populations during aging and in disease.
Introduction
Cairns proposed [1] that asymmetric strand segregation could help to minimize effective mutation rates in epithelial cells, which undergo frequent division and thus are highly susceptible to mutation. Under Cairns's model, after each round of DNA replication, the double-stranded molecule that contains the oldest DNA strand is preferentially targeted to the daughter cell that will be a somatic stem cell. The oldest DNA strands are expected to contain fewer errors than are daughter strands because some of the errors that arise on daughter strands during their synthesis fail to be repaired. Empirical findings suggest the possibility of asymmetric strand segregation in some [2]–[5] — but not all [6],[7] — mammalian cell lineages.
A few reports have discussed possible epigenetic causes and consequences of asymmetric strand segregation [8]–[15]. Klar [8] reported that epigenetic differences between DNA strands encode developmental asymmetries in fission yeast, and, more recently, suggested that breakdown of strand asymmetry could lead to disease in humans [10]. Merok et al. [11] noted that asymmetric strand segregation, which they report for cultured mammalian cells, could have consequences for the integrity of information encoded in epigenetic modifications of DNA. Cairns suggested that epigenetic changes to older strands could help to mark the stem cells that preferentially retain them [13], and Rando [14] proposed that epigenetic modifications, including DNA methylation, could provide information that would distinguish among DNA strands of different ages. Here, I use a population-epigenetic model of an epithelial crypt to investigate in detail the potential consequences of asymmetric strand segregation for the fidelity of epigenetic information.
Results
I compared the dynamics of mean and oldest-strand methylation densities under asymmetric and symmetric strand segregation (Figure 1). Three key observations held for both high (Figure 2) and low (Figure 3) initial methylation densities: (i) when de novo methylation events were permitted to occur on both parent and daughter strands, asymmetric strand segregation resulted in population-mean and oldest-strand methylation densities that increased monotonically (upper curves, Figures 2 and 3); (ii) when de novo methylation events occured on both parent and daughter strands, symmetric segregation yielded population-mean and oldest-strand methylation densities that, although dynamic, remained very near the predicted equilibrium (middle curves, Figures 2 and 3); (iii) when de novo methylation events were limited to the daughter strand, population-mean and oldest-strand methylation densities under both asymmetric and symmetric segregation remained very close to starting values (dotted lines, Figures 2 and 3). Thus, for a wide range of starting methylation densities, asymmetric — but not symmetric — strand segregation leads to systematic increases in methylation levels, if parent strands are subject to de novo methylation events.
Fig. 1. Model of two epithelial cell crypts, one showing asymmetric (A), and the other symmetric (B), strand segregation. 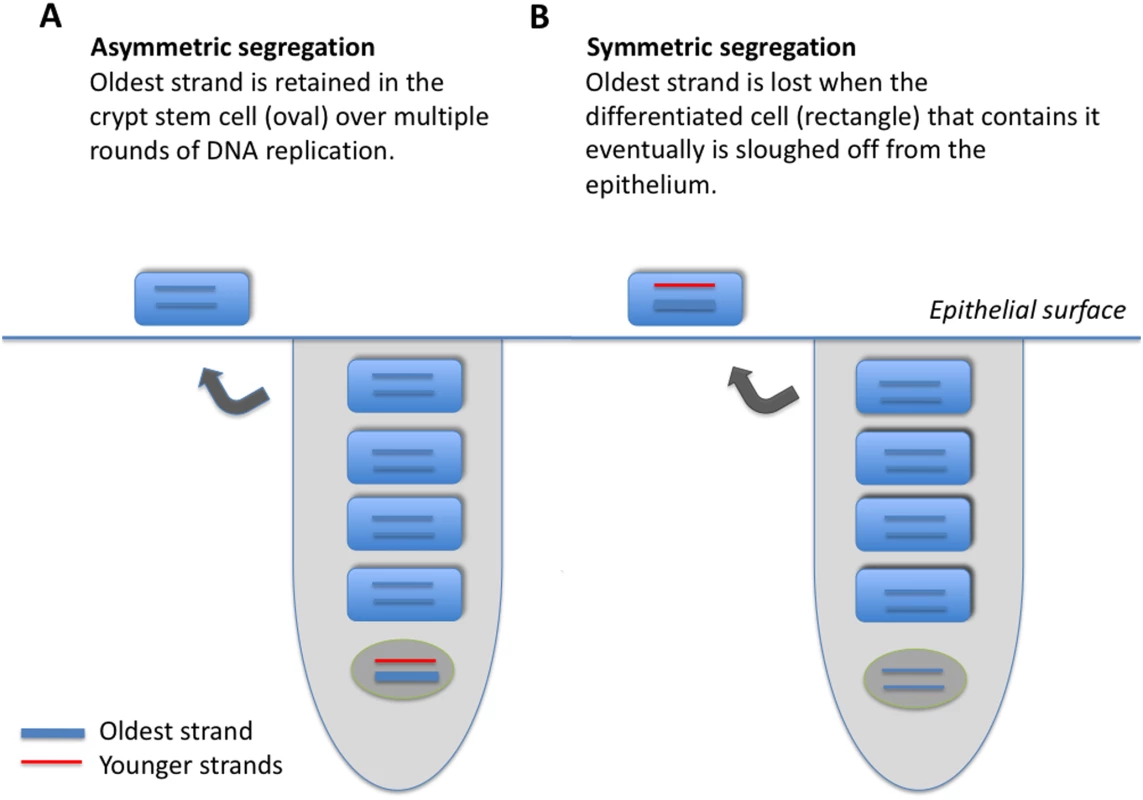
An epithelial crypt is composed of one long-lived stem cell (oval, bottom of crypt), and four daughter cells (rectangles). The oldest DNA strand is shown as a thick line; strands produced more recently are shown as thin lines. Each round of stem-cell division, produces one terminally differentiated cell, and one stem cell; a terminally differentiated cell is sloughed off at the epithelial surface. When strand segregation is asymmetric (A), the DNA molecule containing the oldest strand is retained in the somatic stem cell over multiple rounds of DNA replication and cell division. When strand segregation is symmetric (B), the oldest strand is assigned at random to the stem cell or to the differentiated cell, and is eventually lost when the terminally differentiated cell that contains it is sloughed off at the epithelial surface. Fig. 2. Trajectories of methylation densities under asymmetric or symmetric strand segregation, with high initial methylation density. 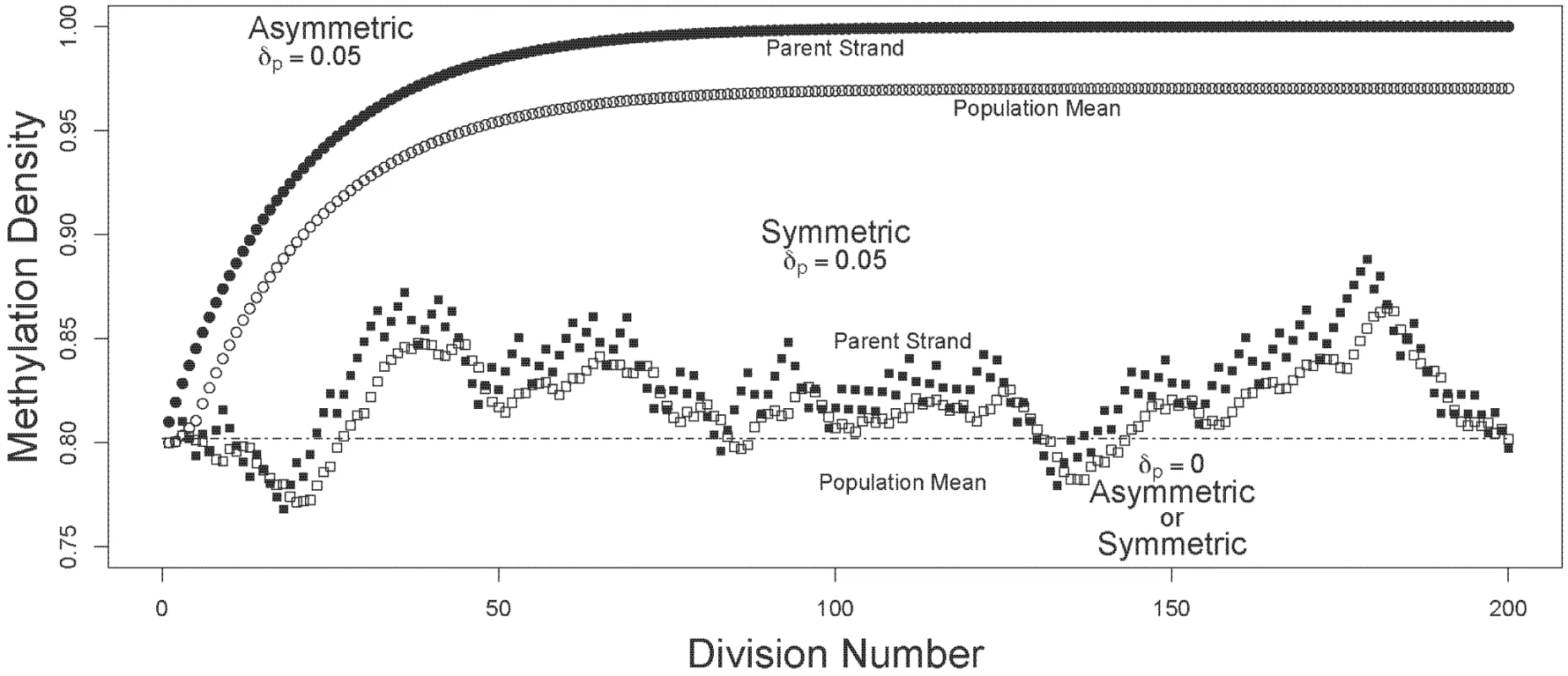
The oldest-parent strand and the population-mean methylation densities (filled and open circles, respectively), are shown for simulations run under asymmetric and symmetric modes of strand segregation (circles and squares, respectively). For the simulations shown here, I used a starting methylation density of . For the scenarios with parent strand de novo methylation, densities were calculated with , and . Under asymmetric strand segregation, these parameter values lead to monotonic increases in population-mean and oldest-parent strand methylation densities (upper curves). Under symmetric strand segregation, these parameter values lead to population-mean and oldest-parent strand DNA methylation densities that were dynamic about the starting value (middle curves). With no parent strand de novo methylation (), densities were unchanged under both symmetric and asymmetric strand segregation (dashed line). Fig. 3. Trajectories of methylation densities under asymmetric or symmetric strand segregation, with low initial methylation density. 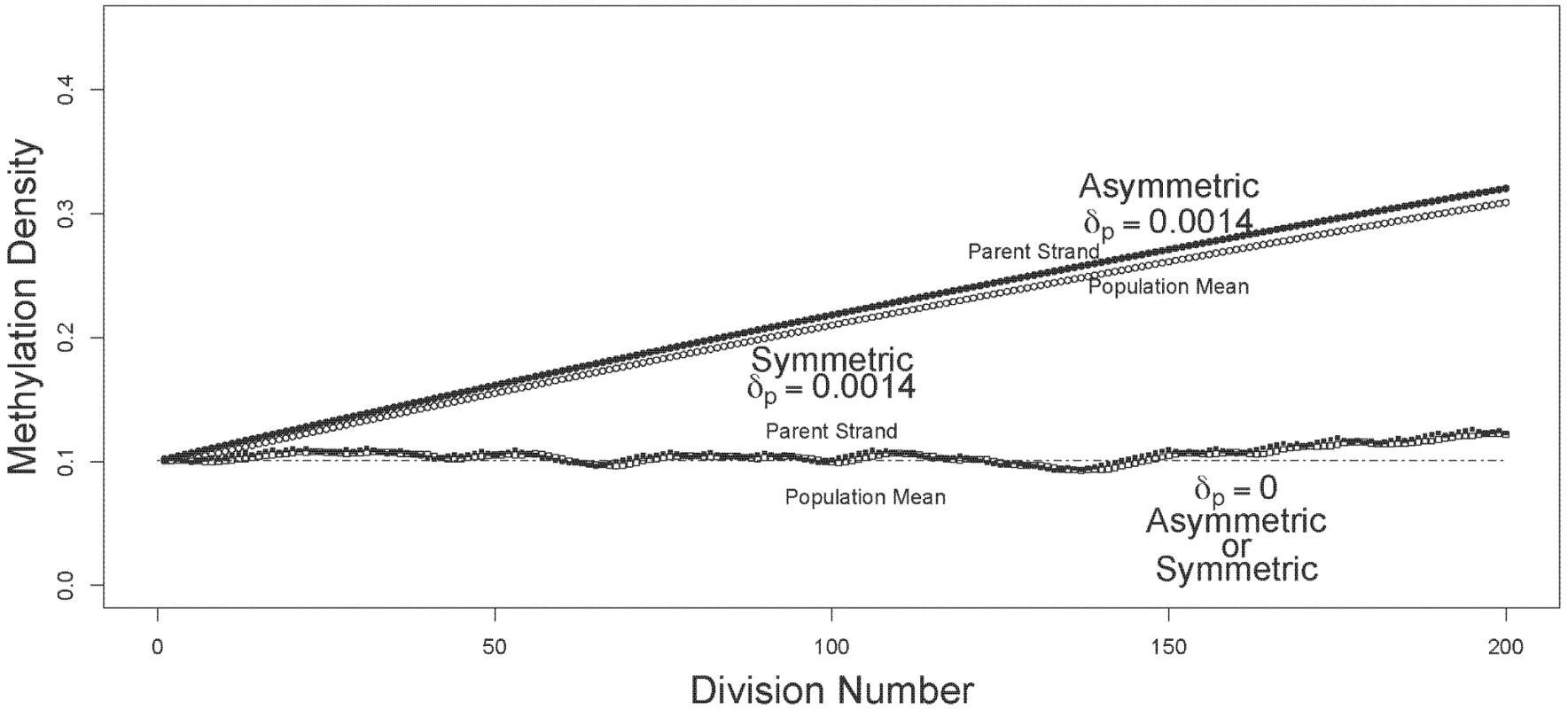
The oldest-parent strand and the population-mean methylation densities (filled and open circles, respectively), are shown for simulations run under asymmetric and symmetric modes of strand segregation (circles and squares, respectively). The simulations shown here used a starting methylation density of . For the scenarios with parent strand de novo methylation, densities were calculated with , and . Under asymmetric strand segregation, these parameter values lead to monotonic increases in population-mean and oldest-parent strand methylation densities. Under symmetric strand segregation, these parameter values lead to population-mean and oldest-parent strand DNA methylation densities that are dynamic about the starting value. With no parent-strand de novo methylation (), densities were unchanged under both symmetric and asymmetric strand segregation (dashed line). Note that the range and scale of the y-axis differ between Figure 2 and Figure 3. Discussion
The population-epigenetic model I develop here reveals that asymmetric strand segregation in somatic stem cells could lead to monotonic increases in DNA methylation densities in structured cellular populations. These increases are predicted to occur when de novo methylation occurs on parent as well as daughter strands, but not when de novo methylation events are limited to the daughter strand.
The predictions of my model are made using empirical estimates of methylation rates in differentiated cells, for which substantial amounts of data are available. Further work will be necessary directly to ascertain methylation rates in somatic stem cells. Nevertheless, the essential findings of my study are consistent across a broad range of parameter values (see, for instance, Figures 2 and 3), suggesting that these results will hold even if methylation densities and rates differ appreciably between differentiated and somatic stem cells.
The accumulation of aberrant methylation predicted by my model may have different time courses depending on the biological properties of a given lineage of somatic stem cells, and on the initial methylation density of a given locus. When somatic stem cell division always gives rise to one stem cell and one differentiated cell, as I model here, substantial increases in DNA methylation densities can occur over just a few cell divisions (Figures 2). When somatic stem cell division sometimes gives rise to one stem cell and one differentiated cell, and sometimes to two somatic stem cells, somewhat lower rates of increase could occur. The rate of increase will also depend on the initial DNA methylation density (compare, for instance, Figures 2 and 3). Lorincz et al. [16] found that progression to dense methylation is especially likely for genomic regions that have already attained intermediate methylation densities. In light of this finding, it seems plausible that even slow or transient increases in DNA methylation could raise methylation densities to a threshold sufficient to trigger more substantial increases.
What might be the functional implications of the increased DNA methylation densities predicted under asymmetric strand segregation? The accumulation of methyl groups on a long-lived DNA strand could serve as a signal to guide asymmetric strand segregation itself [17], or to distinguish stem cells from differentiated cells [13]. My findings could also help to explain the positive correlation observed between age and methylation density in endometrial [18] and intestinal [19] tissues. Both of these are rapidly-dividing tissues of the sort initially predicted by Cairns [1], and reported by some groups [4], to undergo asymmetric strand segregation. In contrast, slowly-dividing cells, such as those in the hematopoetic lineage, have constant methylation densities [20]–[23] and have been reported not to undergo asymmetric strand segregation [6]. Thus, the systematic increases in DNA methylation densities predicted here may be specific to the rapidly-dividing lineages Cairns initially discussed [1].
My results may also have implications for the etiology of cancer in humans. Several epithelial cancers are associated with reductions in epigenetic fidelity, including the accumulation of aberrant methylation and abnormal gene silencing [24],[25]. Barrett's esophagus illustrates the potential relevance of these findings. The esophageal epithelium in Barrett's esophagus contains abnormal intestinal crypt-like structures, and is characterized by abrupt increases in DNA methylation densities and consequent silencing of loci critical to cell-cycle regulation [26].
Thus, it is possible that directional change in epigenetic information may be a cost of the increased genetic fidelity achieved through asymmetric strand segregation, with implications for human disease.
Models
Modelling an Epithelial Crypt
I developed a simplified model of an epithelial crypt with which to track methylation dynamics (Figure 1). Each crypt consists of one somatic stem cell, and four differentiated cells. At each round of stem-cell division, one terminally differentiated cell is produced, and one stem cell is produced. The top-most of the terminally differentiated cells is sloughed off at the epithelial surface. Segregation of the oldest DNA strand always to the stem cell characterizes asymmetric strand segregation (Figure 1a); segregation of the oldest DNA strand at random to the stem and terminally differentiated cells characterizes symmetric segregation (Figure 1B).
Modelling DNA Methylation Events in an Epithelial Crypt
Maintenance and de novo methylation
I modelled replication and methylation dynamics for a single, methylated locus such as one of those on the hemizygous X chromosome in a human male, or on the inactive X chromosome in a human female. Prior to cell division, the locus undergoes semi-conservative replication, producing two double-stranded DNA molecules. Each molecule is composed of a parent strand from the original double-stranded molecule, and a newly-synthesized daughter strand. The model to be described in detail below compares methylation dynamics under asymmetric (Figure 1A) and symmetric (Figure 1B) strand segregation.
Methyl groups are added to CpG cytosines in DNA by two different processes: maintenance methylation and de novo methylation. Maintenance methylation is performed by maintenance methyltranferases, which exhibit a preference for hemimethylated CpG/CpG dyads, are thought to localize to the replication fork, and operate principally during DNA replication [27]. De novo methyltransferases do not seem to exhibit this preference for hemimethylated sites, and may be active throughout the mammalian cell cycle [28]–[30].
Here, the maintenance methylation rate, , is defined as the probability that a methyl group is added to a daughter-strand CpG, given that the complementary CpG on the parent strand is methylated. Maintenance events produce fully methylated CpG/CpG dyads. Maintenance methylation fails at rate , yielding hemimethylated dyads that have a methylated CpG on the parent strand, and an unmethylated, complementary CpG on the daughter strand [31]. The daughter strand de novo methylation rate, , is defined as the probability that a methyl group is added to a daughter-strand CpG that remains unmethylated after DNA replication and maintenance methylation. The parent strand de novo methylation rate, , is defined as the probability that a methyl group is added to an unmethylated CpG on the parent strand. I assume, as before [31], that methyl groups are not actively removed from DNA in differentiated cells, consistent with the lack of evidence that active demethylation occurs in epithelial cell lineages. My results would not apply in cases where there is net loss of DNA methylation from individual DNA strands, such as in early mammalian development [32].
The question of whether or not de novo methylation events can occur in vivo on the parent strand was addressed experimentally during the 1970s, yielding conflicting data [33]–[36]. More recent modelling and statistical studies of epigenetic fidelity have used a variety of approaches to accommodate this continued uncertainty. Otto and Walbot [37] assumed that de novo methylation occurs simultaneously on parent and daughter strands. In our previous model [31], we considered two scenarios: that de novo methylation occurs independently and at equal rates on the two strands, and alternately, that it is limited to the daughter strand. A new statistical study by Fu et al. (Fu et al., Manuscript in Preparation) found far greater support for the presence of parent-strand de novo methylation than for its absence, at least in lymphocytes from adult humans. Because it remains unclear whether de novo methylation on the parent strand is a universal phenomenon or whether it is, instead, limited to a subset of cell lineages or developmental stages, I here consider methylation dynamics both with and without parent-strand de novo methylation.
Mathematical Model of DNA Methylation Events and Strand Segregation in an Epithelial Crypt
(1)
I developed vector (1) to record the methylation density of each of the 10 individual strands of the five DNA molecules in the epithelial crypts (Figure 1). The initial state of the epithelial crypt is given by .
The first element of (1), “1”, is used to implement the assumption that individual methyl groups are not lost once they are incorporated. Elements two through eleven represent the methylation densities of the ten individual strands of DNA in the five double-stranded molecules of the crypt cells. These elements are best considered in pairs. Elements two and three represent the methylation states of the parent and daughter strands in the founding somatic stem cell. In particular, the second element gives the methylation density of the oldest parent strand at the start of the simulation. This strand is assumed to have started with methylation density , as calculated under our previous model [31] from the chosen parameter values for maintenance and de novo methylation events. It then acquired additional methyl groups through parent strand de novo methylation events occurring at rate , for those cases where is greater than 0. The third element gives the initial methylation density of the daughter strand in the founding somatic stem cell, as calculated from the starting methylation density of the parent strand, , using our earlier model [31]. Elements four and five represent methylation states of the parent and daughter strands in the first of the four differentiated cells, and so on up to elements ten and eleven, which give the methylation states of the parent and daughter strands in the differentiated cell closest to the epithelial surface. Because I start by modelling the establishment of the crypt, the strands represented by vector positions three through eleven have not yet been synthesized at the start of the simulation, and therefore have methylation densities of zero. Methylation densities of strands that do not yet exist are excluded from the calculation of population-mean densities.(2)
I developed matrix (2) to model the occurrence of methylation events on individual strands of DNA molecules, and to track their progression through the simplified epithelial crypt described above.
The first row of matrix is a placeholder, and is used to regenerate the “1” that is the first element of vector . The second row of the matrix is used to calculate the updated methylation density of the oldest DNA strand. The third row of the matrix is used to simulate methylation events on the daughter strand that has just been produced through replication of the oldest DNA strand. This density is determined by the maintenance methylation rate, , and the daughter-strand de novo methylation rate, . The fourth row of the matrix is used to simulate methylation events on the parent strand in the newest differentiated cell, and records methylation events that occur through parent-strand de novo methylation, at rate . The fifth row of the matrix is used to simulate methylation events on the daughter strand in the newest differentiated cell, and records methylation events that occur by daughter-strand de novo methylation, at rate . The sixth through eleventh rows of the matrix are used to simulate the progression of existing DNA molecules through the crypt. As noted above, we assume that methyl groups are never removed from a strand once they have been added, and are added to a strand either during the round in which it is synthesized, or during subsequent rounds in which it serves as a parent strand in DNA replication. Rows 6, 8 and 10 simulate the movements of strands that are parents in their respective cells; rows 7, 9 and 11 simulate the movements of strands that are daughters in their respective cells. Upon replication and cell division, cells containing the various DNA molecules advance one cell-position toward the epithelial surface.
Both asymmetric and symmetric strand segregation are modelled using matrix , with slight differences in the treatment of the resulting updated . To model a single round of DNA replication and cell division under asymmetric strand segregation, I multiplied matrix by vector . To investigate methylation trajectories over multiple rounds of DNA replication and cell division, I multiplied matrix by vector recursively up to 500 times. This large number of divisions would be unreasonable for many tissues, but is likely appropriate for the rapidly-dividing cells of the endometrium [18] and intestinal epithelium [38] over a period of several years [39].
To model a single round of DNA replication and cell division under symmetric strand segregation, I used a similar approach, multiplying matrix by vector , but included for each round a random draw of a number between 0 and 1. When the random number was less than or equal to 0.5, I retained the vector that resulted from this initial multiplication, simulating retention of the oldest DNA strand in the somatic stem cell. When the random number was greater than 0.5, I simulated the export of the oldest strand to the differentiated daughter cell by exchanging the methylation densities given in vector positions two and three with those given in vector positions four and five. I repeated this process of multiplication, random number selection, and vector rearrangement for up to 500 rounds of DNA replication, methylation events, and cell division.
Choice of Parameter Values for Maintenance and De Novo Methylation Events
Several authors have investigated the rate of maintenance methylation, , yielding estimates that range from 0.95 to 0.999 [31], [40]–[43] (Fu et al., Manuscript in Preparation). Here, I assumed .
Comparatively few studies have investigated the rate of de novo methylation. The estimates that do exist exhibit substantial variation. Pfeifer et al. [40] estimated a de novo methylation rate of 0.05, Laird et al. [41] estimated a rate of 0.17, under the assumption that de novo events are limited to the daughter strand. Results from Genereux et al. [31] suggest that part of the variation in these estimates of the de novo methylation rate may be attributable to bona fide biological variation among CpG cytosine sites, and perhaps among loci.
For de novo methylation rates, I first chose parameter values that yield an expected equilibrium methylation density of 0.8 under the model described previously [31], and assuming . To meet this condition, the sum of parent, and daughter, , de novo rates must be 0.1. To accommodate uncertainties about the strandedness of de novo methylation events, I explored two cases: de novo events occurring on the daughter strand only (, ), and de novo events occurring at equal rates on the parent and daughter strands (, ). These values were used to generate the results in Figure 2. To investigate whether or not the change in methylation density observed when starting with was limited to scenarios with high initial densities, I also conducted simulations spanning a range of initial values. For the simulation shown in Figure 3, I started with a methylation density of , and assumed the maintenance rate, , to be 0.975. To meet these conditions under our previous model [31], the sum of parent, , and daughter, , de novo rates must be 0.0028. Here, as before, I considered parameter values that included de novo methylation events on the parent strand (), and parameter values that limited de novo methylation events to the daughter strand ().
Zdroje
1. CairnsJ
1975 Mutation, selection and the natural history of cancer. Nature 255 197 200
2. LarkKG
ConsigliRA
MinochaHC
1966 Segregation of sister chromatids in mammalian cells. Science 154 1202 1205
3. KarpowiczP
MorsheadC
KamA
JervisE
RamunasJ
2005 Support for the immortal strand hypothesis: neural stem cells partition DNA asymmetrically in vitro. J Cell Biol 170 721 732
4. SmithGH
2005 Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development 132 681 687
5. ShininV
Gayraud-MorelB
GomesD
TajbakhshS
2006 Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol 8 677 687
6. KielMJ
HeS
AshkenaziR
GentrySN
TetaM
2007 Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature 449 238 242
7. SotiropoulouP
CandiA
BlanpainC
2008 The majority of multipotent epidermal stem cells do not protect their genome by asymmetrical chromosome segregation. Stem Cells 26 2964 73
8. KlarAJ
1987 Differentiated parental DNA strands confer developmental asymmetry on daughter cells in fission yeast. Nature 326 466 470
9. KlarAJ
1994 A model for specification of the left-right axis in vertebrates. Trends Genet 10 392 396
10. KlarAJS
2004 A genetic mechanism implicates chromosome 11 in schizophrenia and bipolar diseases. Genetics 167 1833 1840
11. MerokJR
LansitaJA
TunsteadJR
SherleyJL
2002 Cosegregation of chromosomes containing immortal DNA strands in cells that cycle with asymmetric stem cell kinetics. Cancer Res 62 6791 6795
12. LiuSV
2005 Linking DNA aging with cell aging and combining genetics with epigenetics. Logical Biology 5 51 55
13. CairnsJ
2006 Cancer and the immortal strand hypothesis. Genetics 174 1069 1072
14. RandoTA
2007 The immortal strand hypothesis: segregation and reconstruction. Cell 129 1239 1243
15. LewDJ
BurkeDJ
DuttaA
2008 The immortal strand hypothesis: how could it work? Cell 133 21 23
16. LorinczMC
SchubelerD
HutchinsonSR
DickersonDR
GroudineM
2002 DNA methylation density inuences the stability of an epigenetic imprint and Dnmt3a/b-independent de novo methylation. Mol Cell Biol 22 7572 7580
17. LansdorpPM
2007 Immortal strands? Give me a break. Cell 129 1244 1247
18. KimJY
TavareS
ShibataD
2005 Counting human somatic cell replications: methylation mirrors endometrial stem cell divisions. Proc Natl Acad Sci U S A 102 17739 17744
19. KimJY
BeartRW
ShibataD
2005 Stability of colon stem cell methylation after neo-adjuvant therapy in a patient with attenuated familial adenomatous polyposis. BMC Gastroenterol 5 19
20. StögerR
KajimuraTM
BrownWT
LairdCD
1997 Epigenetic variation illustrated by DNA methylation patterns of the fragile-X gene FMR1. Hum Mol Genet 6 1791 1801
21. BjornssonHT
SigurdssonMI
FallinMD
IrizarryRA
AspelundT
2008 Intra-individual change over time in DNA methylation with familial clustering. JAMA 299 2877 2883
22. SandoviciI
NaumovaAK
LeppertM
LinaresY
SapienzaC
2004 A longitudinal study of X-inactivation ratio in human females. Hum Genet 115 387 392
23. SandoviciI
LeppertM
HawkPR
SuarezA
LinaresY
2003 Familial aggregation of abnormal methylation of parental alleles at the IGF2/H19 and IGF2R differentially methylated regions. Hum Mol Genet 12 1569 1578
24. WongDJ
FosterSA
GallowayDA
ReidBJ
1999 Progressive region-specific de novo methylation of the p16 CpG island in primary human mammary epithelial cell strains during escape from M(0) growth arrest. Mol Cell Biol 19 5642 5651
25. GradyWM
2005 Epigenetic events in the colorectum and in colon cancer. Biochem Soc Trans 33 684 688
26. SmithE
De YoungNJ
PaveySJ
HaywardNK
NancarrowDJ
2008 Similarity of aberrant DNA methylation in Barrett's esophagus and esophageal adenocarcinoma. Mol Cancer 7 75
27. LeonhardtH
PageAW
WeierHU
BestorTH
1992 A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell 71 865 873
28. OkanoM
BellDW
HaberDA
LiE
1999 DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99 247 257
29. YokochiT
RobertsonKD
2002 Preferential methylation of unmethylated DNA by mammalian de novo DNA methyltransferase Dnmt3a. J Biol Chem 277 11735 11745
30. HsiehCL
1999 In vivo activity of murine de novo methyltransferases, Dnmt3a and Dnmt3b. Mol Cell Biol 19 8211 8218
31. GenereuxDP
MinerBE
BergstromCT
LairdCD
2005 A population-epigenetic model to infer site-specific methylation rates from double-stranded DNA methylation patterns. Proc Natl Acad Sci U S A 102 5802 5807
32. RazinA
WebbC
SzyfM
YisraeliJ
RosenthalA
1984 Variations in DNA methylation during mouse cell differentiation in vivo and in vitro. Proc Natl Acad Sci U S A 81 2275 2279
33. AdamsRL
1971 Methylation of newly synthesized and older deoxyribonucleic acid. Biochem J 123 38P
34. SchneidermanMH
BillenD
1973 Methylation rapidly reannealing DNA during the cell cycle of Chinese hamster cells. Biochim Biophys Acta 308 352 360
35. BirdAP
1978 Use of restriction enzymes to study eukaryotic DNA methylation: II. the symmetry of methylated sites supports semi-conservative copying of the methylation pattern. J Mol Biol 118 49 60
36. KapplerJW
1970 The kinetics of DNA methylation in cultures of a mouse adrenal cell line. J Cell Physiol 75 21 31
37. OttoSP
WalbotV
1990 DNA methylation in eukaryotes: kinetics of demethylation and de novo methylation during the life cycle. Genetics 124 429 437
38. NicolasP
KimKM
ShibataD
TavareS
2007 The stem cell population of the human colon crypt: analysis via methylation patterns. PLoS Comput Biol 3 e28
39. PottenCS
1998 Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci 353 821 830
40. PfeiferGP
SteigerwaldSD
HansenRS
GartlerSM
RiggsAD
1990 Polymerase chain reactionaided genomic sequencing of an X chromosome-linked CpG island: methylation patterns suggest clonal inheritance, CpG site autonomy, and an explanation of activity state stability. Proc Natl Acad Sci U S A 87 8252 8256
41. LairdCD
PleasantND
ClarkAD
SneedenJL
HassanKMA
2004 Hairpin-bisulfite PCR: assessing epigenetic methylation patterns on complementary strands of individual DNA molecules. Proc Natl Acad Sci U S A 101 204 209
42. HermannA
GoyalR
JeltschA
2004 The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J Biol Chem 279 48350 48359
43. VilkaitisG
SuetakeI
KlimasauskasS
TajimaS
2005 Processive methylation of hemimethylated CpG sites by mouse Dnmt1 DNA methyltransferase. J Biol Chem 280 64 72
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2009 Číslo 6- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- Cytoplasmic Streaming in : Disperse the Plug To Increase the Flow?
- The Mysteries of Chromosome Evolution in Gibbons: Methylation Is a Prime Suspect
- Meta-Analysis of 28,141 Individuals Identifies Common Variants within Five New Loci That Influence Uric Acid Concentrations
- The Impact of Divergence Time on the Nature of Population Structure: An Example from Iceland
- Is a Novel Locus for Waist Circumference: A Genome-Wide Association Study from the CHARGE Consortium
- Asymmetric Strand Segregation: Epigenetic Costs of Genetic Fidelity?
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytoplasmic Streaming in : Disperse the Plug To Increase the Flow?
- Meta-Analysis of 28,141 Individuals Identifies Common Variants within Five New Loci That Influence Uric Acid Concentrations
- Is a Novel Locus for Waist Circumference: A Genome-Wide Association Study from the CHARGE Consortium
- Asymmetric Strand Segregation: Epigenetic Costs of Genetic Fidelity?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



