-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNeurological and MRI Screening Improves Long-term anti-TNF-α Treatment Safety in Patients with Crohn΄s Disease
Neurologický a MR skríning pacientů s Crohnovou chorobou může zvýšit bezpečnost dlouhodobé terapie anti-TNF-α
Úvod:
V minulosti bylo publikováno několik prací popisujících gliové změny na MR mozku typické pro demyelinizační onemocnění u pacientů s nespecifickými střevními záněty, kteří byli léčení monoklonální protilátkou proti tumor necrosis factor alpha (TNF-α). Některé z těchto prací dokonce poukazovaly na možný podíl anti-TNF-α terapie na rozvoj roztroušené sklerózy. Hlavním cílem naší studie bylo zhodnocení neurologického stavu pacientů s Crohnovou chorobou před zahájením terapie anti-TNF-α s cílem minimalizovat riziko progrese choroby u pacientů se zatím klinicky němou roztroušenou sklerózou. Naším druhým cílem bylo, prostřednictvím prospektivního sledování MR a neurologického nálezu, zhodnotit riziko rozvoje roztroušené sklerózy u pacientů na terapii anti-TNF-α.Metody:
Padesát pacientů, kteří byli sledováni více než 2 roky, byli zařazeni do prospektivní studie. Třicet z těchto pacientů bylo léčeno anti-TNF-α. Dvacet pacientů s Crohnovou chorobou bez této terapie bylo do studie zařazeno jako kontroly. Všichni pacienti byli neurologicky vyšetření a podstoupili MR mozku před zahájením terapie anti-TNF-α, na počátku studie a následně rok a půl po zahájení terapie/od počátku studie v případě kontrol.Závěr:
Neurologický skríning pacientů s Crohnovou chorobou a vyřazení pacientů s podezřením na roztroušenou sklerózu dle výsledků MR z terapie anti-TNF-α může zvýšit bezpečnost této terKlíčová slova:
nespecifické střevní záněty – Crohnova choroba – roztroušená skleróza – anti tumor necrosis factor-α
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors: P. Lišková 1; J. Šťovíček 2; J. Lisý 3; Š. Hlava 2; M. Šrámek 1; R. Keil 2
Authors place of work: Clinic of Neurology 1; Department of Gastroenterology 2; Charles University in Prague and nd Faculty of Medicine University Hospital Motol in Prague 2; Department of Radiology 3
Published in the journal: Cesk Slov Neurol N 2017; 80/113(1): 95-100
Category: Krátké sdělení
Supported by the project (Ministry of Health, Czech Republic) for conceptual development of research organization 00064203 (University Hospital Motol, Prague, Czech Republic).
Summary
Background:
Several reports describe MRI signs of CNS demyelination in inflammatory bowel diseases (IBD) patients treated with monoclonal antibody against tumor necrosis factor alpha (anti-TNF-α) and even indicate that anti-TNF-α can trigger multiple sclerosis (MS). The first aim of the study was to evaluate the neurological status of Crohn’s disease (CD) patients before anti-TNF-α therapy initiation in order to avoid possible complications in patients with latent demyelinating diseases. Our second aim was to evaluate, by prospectively following their neurological and MRI status, the risk of developing CNS demyelination in patients with CD treated with anti-TNF-α.Methods:
Fifty patients, followed for more than 2 years, were included in the prospective phase of the study. Thirty of these patients were treated with anti-TNF-α. Twenty patients without biological therapy were used as controls. Neurostatus and brain MRI were performed in all patients at baseline and after 1.5 years of treatment. CSF examination was performed if MRI raised suspicion of MS.Results:
54% of patients had abnormalities in neurostatus. MRI changes suggestive of demyelination were found in seven cases. In one patient, CSF-specific oligoclonal bands were found and anti-TNF-α treatment was contraindicated. No changes in neurological or MRI status were observed after 1.5 years of treatment in either group.Conclusions:
Preselection of patients at high risk of developing MS can increase the safety of anti-TNF-alpha treatment.Key words:
inflammatory bowel disease – Crohn´s disease – multiple sclerosis – anti-tumor necrosis factor-α
Chinese summary - 摘要
改变患者的微生物样本确认后细菌性脑膜炎
后脑勺 手术
摘要
开颅手术后(PCM)脑膜炎是神经外科的一个重要挑战。此本文介绍了PCM诊断为4,392的22名患者进行了开颅手术在2008年1月1日和2012年31月至12月期间在7例脑膜炎是由细菌,鲍曼不动杆菌,并在15例引起鲍曼不动杆菌未经证实的脑膜炎显着差异和本地化;头部外伤(P = 0.004 OR =5.873,95%CI1.575至17.511)的发生中枢神经系统(; P = 0.003 OR =0.872; 95%CI0.665至1.621)以外的感染。我们的报告提供医师传染性病原体模式的转变的当前视图PCM。
关键词:
脑膜炎 - 神经外科 - 鲍曼不动杆菌 - 金黄色葡萄球菌 -头部受伤
神经学与MRI筛查患者克罗恩病情可能加重长期治疗的安全性抗TNF-α
摘要
背景:在过去,曾有多篇论文描述胶质改变MR典型的脑脱髓鞘疾病患者的肠道不规范
炎,谁用单克隆抗体治疗的肿瘤坏死因子α(TNF-α)。一些这些作品甚至指出抗TNF-α的可能共享
治疗多发性硬化的发展。我们研究的主要目的是评估神经疾病克隆氏病患者开始治疗用抗前TNF-α,以尽量减少疾病进展的危险的患者的临床还沉默的多发性硬化症。我们的第二个目标是通过
MR前瞻性监控和神经系统的发现,评估发展中国家的风险多发性硬化症的患者与抗TNF-α治疗。方法:将50例随访了两年多,他们在一个前瞻性研究对象。这些三十患者用抗TNF-α治疗。 20例克罗恩病没有这个疗法作为对照。所有患者均为神经系统检查并用抗TNF-α处理之前后行MRI,在开始时研究,然后在一年和治疗开始后/在支票的情况下,一个半从基线。
结论:患者神经筛选克罗恩病的患者和退役根据MR治疗的结果与抗TNF-α多发性硬化症的怀疑可能
增加该治疗的安全性。
关键词:
炎症性肠病 - 克罗恩病 - 多发性硬化症 - 抗肿瘤
坏死因子αIntroduction
Inflammatory bowel diseases (IBD) – Crohn’s disease (CD) and ulcerative colitis (UC) – are associated with various extraintestinal manifestations (EM). These manifestations occur in 6–40% of patients and may involve virtually any organ system – most commonly joints, skin, hepatobiliary system and eyes [1]. Neurological manifestations are not regarded as very common but may be underdiagnosed. Both central and peripheral nervous system may be affected. Etiology of neurological disability in patients with IBD varies and mainly includes micronutrient deficiency, prothrombotic and immunemediated events.
Patients with IBD have higher risk of developing multiple sclerosis (MS). In Gupta’s cohort study, the incidence rate ratio for being diagnosed with MS or optic neuritis (ON) after IBD diagnosis was 2.12 in patients with CD and 2.63 in patients with UC regardless of the type of therapy [2].
The chimeric monoclonal antibody against human tumor necrosis factor alpha (anti-TNF-α, infliximab) and the fully human antibody against human tumor necrosis factor alpha (anti-TNF-α, adalimumab) are highly effective in the treatment of inflammatory diseases such as rheumatic arthritis, psoriatic arthritis, inflammatory bowel diseases and other [3].
High levels of TNF-α have been found in MS plaques and in CSF of patients with MS. This finding encouraged trials of anti-TNF-α in MS. However, clinical study with anti-TNF-α (lenercept) in relapse-remittent MS was unsuccessful, demonstrating higher frequency of relapses among treated patients [4]. Another report described two patients with progressive MS who developed new gadolinium-enhancing lesions together with lymphocytic pleocytosis and increased IgG in the CSF after each infusion of infliximab [5].
Several recent case reports describe development of central nervous system (CNS) demyelination related to initiation of therapy with an anti-TNF-α. These reports include cases of sporadic central demyelinating events as well as definite MS [6–9].
In a population-based study performed by Deepak et al. in the United States, 153 reports of CNS demyelination possibly associated with anti-TNF-α were identified among 772 reports of unwanted effects of anti-TNF-α reported to the Food and Drug Administration. However, none of these reports was identified as “definite” adverse event and evidence for causal relationship is, therefore, still missing [10,11].
Based on the studies described above, it appears that anti-TNF-α is associated with demyelination. However, it remains to be shown whether anti-TNF-α itself causes MS or merely acts as a trigger, exacerbating preexisting subclinical CNS damage during chronic anti-TNF-α treatment. Some published cases appear to support the latter [12].
These results support preselection of patients without preclinical signs of MS when choosing long-term anti-TNF-α therapy for IBD. Thus, our study had the following objectives:
- To develop neurological and magnetic resonance (MRI) screening allowing early identification of patients with clinically silent MS or those at high risk for developing MS among patients with highly active CD.
- To follow-up on the neurological and magnetic resonance imaging (MRI) status of patients with CD who were undergoing long-term therapy with anti-TNF-α.
Methods
In this prospective, single-center study, we performed rigorous baseline and follow-up examinations (both MRI and neurostatus) of patients with CD and searched for possible symptoms of MS. The study included 96 patients (Tab. 2).
Tab. 1. Patient characteristics. 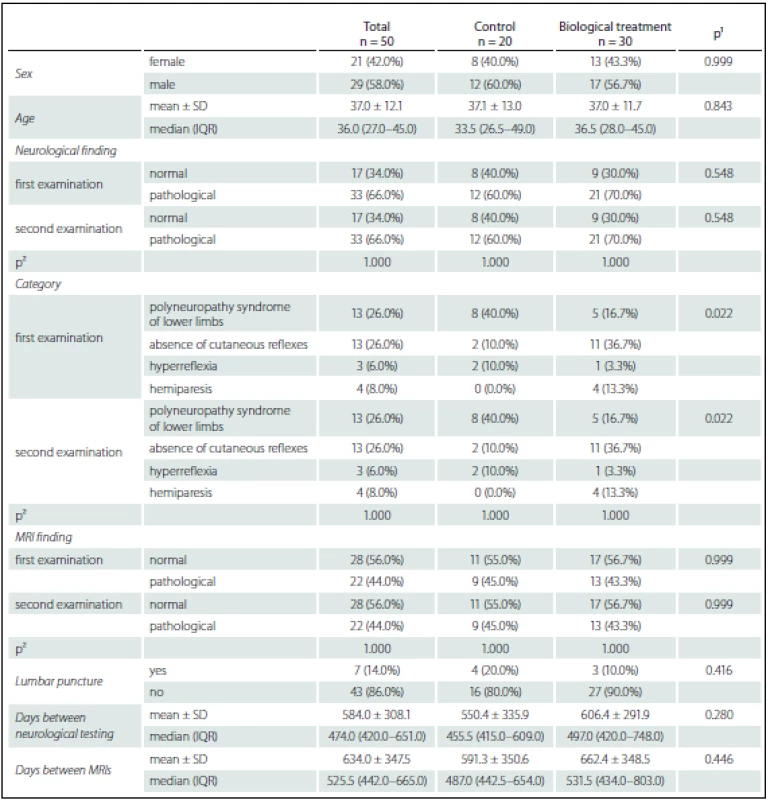
¹ Fisher’s exact test for categorical variables, Mann-Whitney U test for continuous variables. ² McNemar’s test. The inclusion criteria were: 1. history of CD for more than 1 year, 2. patients with moderate or severe CD.
The exclusion criteria were: 1. previous treatment with anti-TNF-α agents in patient’s history, 2. any serious neurological disease in patient’s history.
Treatment in these patients included azathioprine, mesalazine, corticosteroids or combination of all. Fifty patients were included in the prospective phase of the study and observed for more than 2 years. Among these patients, 30 were treated with anti-TNF-α, whereas biological treatment was not indicated in the remaining 20 and they were followed as controls. There were no differences in concurrent medications between the treated and control groups (Tab. 1).
Tab. 2. Comparison of study groups according to duration of disease and previous therapy (azathioprine, corticosteroids). 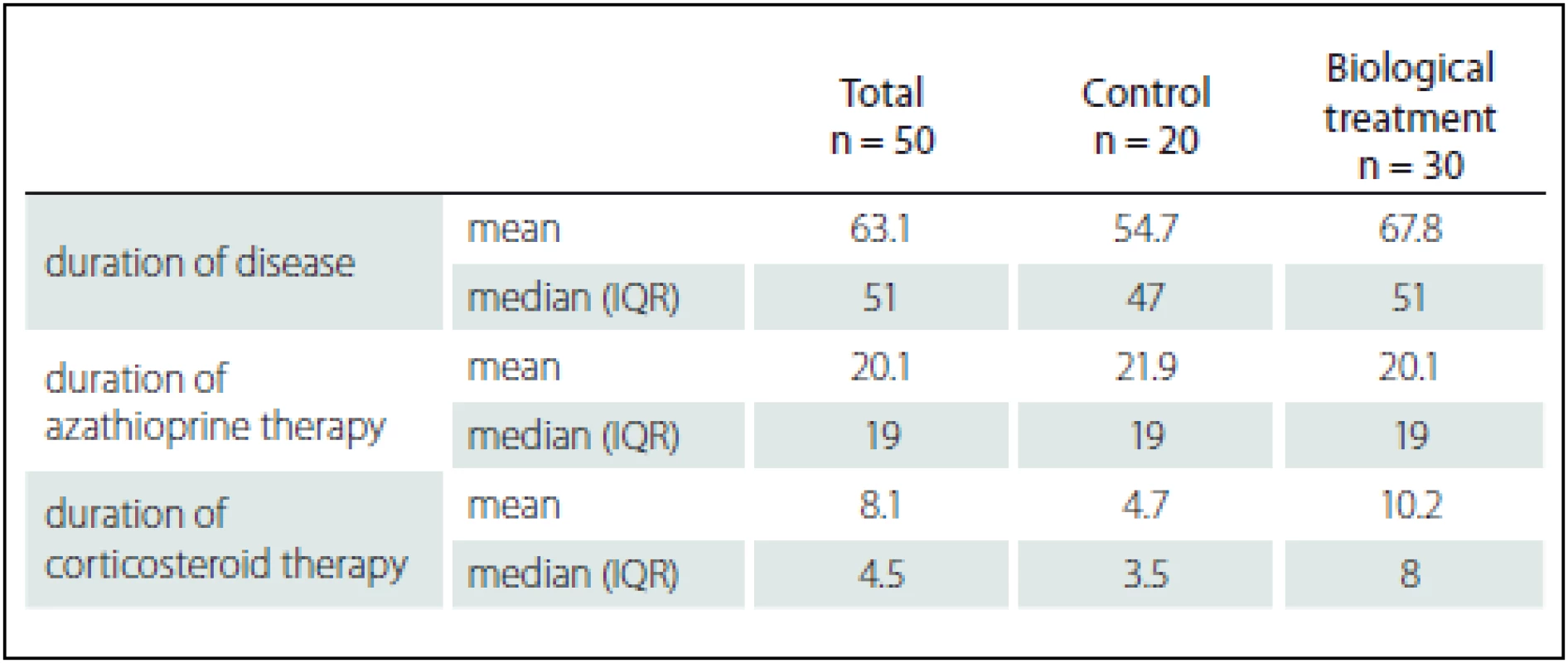
At baseline, detailed neurological examination was performed in all our patients, focusing on possible signs of a demyelinating disease. Neurological investigations were performed in a systematic way by a single neurologist with substential experience in MS diagnosis.
Subsequently, they underwent MRI of the brain using a 1.5-T system (Gyroscan Intera, Philips Medical Systems, Best, The Netherlands). The imaging protocol consisted of:
- a) axial T2-weighted, turbo spin-echo (T2-TSE) sequence (TR/ TE of 4,455/ 100 ms, slice thickness of 5mm and 1mm gap between slices, NSA 3, TSE factor 15);
- b) axial fluid-attenuated inversion-recovery(FLAIR) sequence (TR/ TE/ TI of 11,000/ 140//2,800 ms, slice thickness of 5mm and 1mm gap between slices, NSA 2, TSE factor 32);
- c) coronal T1-weighted inversion recovery turbo spin echo (IR-TSE) sequence (TR/ TE/ TI of 3,171/ 20/ 400 TI ms, 5mm thick sections at 1mm gap intervals, NSA 1, TSE factor 4);
- d) sagittal T2-weighted spin echo (SE) sequence (TR/ TE of 4,455/ 100 ms, slice thickness of 3mm and 0,3mm gap between slices, NSA 4, TSE factor), axial DWI (TR/ TE 4,063/ 93 ms, slice thickness 5mm, 1mm gap, NSA 1, epi factor 89).
Since the presence of brain lesions was the main clinical concern, gadolinium was not routinely applied. Gadolinium was applied only when appearance of brain lesions raised a suspicion of MS.
The MRI results were analyzed by independent researchers whereby both neurologists and radiologists were not aware of the findings of the other group.
We performed lumbar puncture (LP) in patients with suspicious demyelinating lesions on MRI to investigate cerebrospinal fluid (CSF).
In the prospective phase of the study, neurological examination and brain MRI (same protocol as baseline) were performed after 1.5 years of treatment.
Standard descriptive statistics were applied for analysis of results; absolute and relative frequencies for categorical variables and mean supplemented by standard deviation and median supplemented by interquartile range for continuous variables. Statistical significance of differences betweengroups of patients was tested by means of the Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. Statistical significance of time-related changes of categorical variables was tested using the McNemar’s test. Statistical analysis was computed using SPSS 22 (IBM Corporation, 2013); a = 0.05 was adopted as a level of statistical significance in all analyses.
The protocol of the study was approved by the local ethics committee.
Results
Fifty patients were prospectively examined (21 females, 29 males, mean age 36 years, range 27–45 years). Anti-TNF-α treatment was indicated in 30 of them (13 females, 17 males, mean age 36.5, range 28–45 years). The 20 patients without anti-TNF-α treatment served as controls. All patients were free of neurological problems at baseline.
Abnormalities in neurological findings were found in 33 patients (66%) – 21 patients (70%) in the treated group, 12 patients (60%) in the control group (p = 0.548, ns). Absence of abdominal cutaneous reflexes in 13 patients (26%) was the most common finding. This finding was more common among the treated group (n = 11, 36.7%) compared to controls (n = 2, 10%, p = 0.022). Polyneuropathic syndrome of the lower extremities (n = 13, 26%) was the second most frequent finding, occurring in five (16.7%) patients in the treatment group and eight controls (40%). See Table 1 for detailed results.
Abnormalities on the first MRI were detected in 29 (44%) patients. MRI abnormalities were found in 13 (43.6%) patients of the treatment group and nine patients (45%) of the control group, respectivelly (p = 0.999, ns). The majority of these pathologies were nonspecific sporadic lesions in the white matter or mild atrophy (Fig. 1). In one case, acoustic neurinoma was detected as an incidental finding. In seven cases, the character of white matter lesions raised a suspicion of demyelination (based mainly on periventricular localization and number of lesions) (Fig. 2). These patients were free of subjective symptoms but five of them had abnormalities on neurological examination. Cerebrospinal fluid (CSF) examination was performed in all seven patients, showing normal results in six cases. Isolated oligoclonal bands in the CSF were found in one patient. This patient fulfilled McDonald criteria for MS (Fig. 3). Anti-TNF-α treatment was contraindicated in this case and the patient was treated with azathioprine and corticosteroids instead. In three of the remaining six patients, anti-TNF-α treatment was indicated based on the negative results of the CSF examination.
Fig. 1. Several areas with white matter gliosis in both hemispheres of a 41-year-old woman. 
Fig. 2. Suspicious periventricular lesion in a 40-year-old man. 
Fig. 3. Scan of a 29-year-old woman with periventricular lesions typical of MS. 
After approximately 1.5 year (median 474 days, IQR 420–651 days) all patients were examined using the the same protocol (neurostatus and MRI). In the treated group, all patients received anti-TNF-α for at least one year. No significant changes in neurostatus or MRI findings were observed either in the treated or control group (Tab. 1).
Discussion
In our prospective single-center study of 50 patients with CD, we performed neurological examination and brain MRI at two time-points within approximately 1.5 years. At baseline, we found relatively high number of neurological abnormalities in the studied population. Absence of abdominal cutaneous reflexes was the most common pathology. Higher prevalence of this finding in the group treated with anti-TNF-α could be explained by more frequent history of abdominal surgery in this group. Areflexia of the lower extremities was the second most common finding, occurring in 26% of patients. This finding is probably attributable to peripheral neuropathy and is in line with many publications listing peripheral neuropathy as the most common extraintestinal neurological manifestation among patients with CD. The reported frequency of peripheral neuropathy in CD varies from 0 to 39% [13]. Nerve conduction studies are planned in all our patients presenting with clinical signs of peripheral neuropathy in order to confirm the diagnosis and possibly specify the type of neuropathy present.
The number of MRI abnormalities was also very high among our patients (44%). Most often, these were non-specific lesions not suggestive of MS. The origin of these changes is most likely vascular in the majority of cases and this would be in line with two to four times higher risk of thromboembolic complications in patients with IBD [14]. However, MRI changes suggestive of demyelination were found in a relatively high number of patients as well (14%).
Many case reports describe development of CNS demyelination after anti-TNF-α treatment, and in 2001, a recommendation to avoid anti-TNF-α treatment in patients with MS or patients with family history of MS was published [6]. Our neurological and MRI screening was aimed at identifying patients with clinically silent MS that need to be excluded from anti-TNF-α treatment. We found one such patient among 50 patients examined.
Several theories were formed about possible causes of demyelination as a result of anti-TNF-α exposure but the role of TNF-α in MS is still not fully understood. TNF-α binds with two different receptors TNFr1 and TNFr2. In animal models, demyelination is mediated by TNFr1 signals and remyelination occurred as a result of signals from TNFr2. Negative impact of anti TNF-α could be explained by blockage of remyelination TNFr2 signal pathway [15]. Furthermore, administration of anti-TNF-α can enhance function of antigen-presenting cells, decrease apoptosis of autoreactive T lymphocytes and accelerate production of proinflammatory interferon gamma (IFN - g) [16]. Polymorphisms in the gene encoding TNF receptor 1 can also play a role. People with “A” type of TNF receptor 1 have 12% higher likelihood of developing MS than individuals with “G” type receptor [17]. The “A” variant of the gene shortens the TNF receptor 1 protein. This short receptor protein is probably shunted out of the cell, where it can bind TNF-α molecules and thus inhibit intracellular signal transmission and function as an “intrinsic” anti-TNF-α treatment. With additional treatment with anti-TNF-α, the TNF-α intracellular signaling becomes very strongly inhibited and this may trigger MS. Another interesting hypothesis suggests inability of TNF-α antagonists to cross blood-brain barrier and, therefore, to prevent TNF-mediated injury in the CNS [18]. This hypothesis offers an elegant explanation for the inverse effect of anti-TNF-α on MS and IBD, as the treatment may be effective in the periphery but not in the CNS.
If we accept the possible causal relationship between anti-TNF-α treatment and CNS demyelination, it still needs to be clarified whether anti-TNF-α exposure causes MS directly, or if it only is a triggering factor that accentuates an already existing subclinical demyelinating disease. Some of the published cases are more in line with the latter [12,19]. Our observations lead us to a similar conclusion, as we did not find any differences in neurostatus or MRI findings during the observation period either in patients receiving anti-TNF-α or in patients without it. These results may also suggest that the use of anti-TNF-α is safer in preselected patients without preclinical signs of MS.
To date, there have been no general recommendations for neurological screening of IBD patients before starting an anti-TNF-α treatment. Tuberculosis screening of CD patients is standard, and incidences of these two complications are similar in the general population: 4–6 per 100,000 per year. Due to the increasing number of reports of neurological problems after anti-TNF-α treatment, an increased awareness of neurological symptoms is important [20].
The main limitation of our study is a relatively small number of patients and a shortfollow-up period. However, the majority of published cases of CNS demyelination associated with anti-TNF-α therapy occurred less than 12 month after treatment initiation [21]. Therefore, follow-up examination after approximately one and a halfyears of treatment should already exposepossible harmful effects of anti-TNF-α. Regular follow-up of our patients, nevertheless, continues.
With rigorous preselection of patients eligible for anti-TNF-α treatment, a new problem can arise, i.e. what to offer to patients in whom this therapy is contraindicated. Patients with severe CD usually fail on standard immunosuppressive treatment. Some publications suggest very good effect of natalizumab in patients with CD [20]. Taking into account the well-documented efficacy of natalizumab in MS, we regard this monoclonal antibody as suitable for patients with severe CD contraindicated for treatment with anti-TNF-α. Because of the risk of progressive multifocal leucoencephalopathy (PML), we would consider this treatment for JC virus-negative patients only.
Conclusion
In this prospective, single center study, we showed that neurological and MRI screening of patients before initiation of anti-TNF-α treatment improves patient safety by excluding those at high risk of developing clinically manifest MS. Conversely, long-term use of anti-TNF-α in preselected patients without any suspicion of demyelination (MRI and clinical) seems to be safe. However, larger multi-center prospective studies with a longer follow-up period are needed to confirm our observations.
List of used abbreviations
CD – Crohn’s Disease
CNS – Central Nervous System
CSF – Cerebrospinal Fluid
IBD – Inflammatory Bowel Disease
JCV – John Cunningham Virus
LP – Lumbar Puncture
MS – Multiple Sclerosis
ON – Optic Neuritis
TNF – Tumor Necrosis Factor
UC – Ulcerative Colitis
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Petra Lišková, MD
Clinic of Neurology
Charles University in Prague
2nd Faculty of Medicine
University Hospital Motol in Prague
V Úvalu 84
150 00 Praha
e-mail: petra.liskova@fnmotol.cz
Accepted for review: 22. 4. 2016
Accepted for print: 7. 7. 2016
Zdroje
1. Singh S, Kumar N, Loftus EV jr, et al. Neurologic complications in patients with inflammatory bowel disease: increasing relevance in the era of biologics. Inflamm Bowel Dis 2013;19(4):864–72. doi: 10.1002/ ibd.23011.
2. Gupta G, Gelfand JM, Lewis JD. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology 2005;129(3):819–26.
3. Lin J, Ziring D, Desai S, et al. TNF-alpha blockade in human diseases: an overview of efficacy and safety. Clin Immunol 2008;126(1):13–30.
4. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/ MRI Analysis Group. Neurology 1999;53(3):457–65.
5. van Oosten BW, Barkhof F, Truyen L, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 1996;47(6):1531–4.
6. Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology 2001;57(10):1885–8.
7. Theibich A, Dreyer L, Magyari M, et al. Demyelinizing neurological disease after treatment with tumor necrosis factor alpha-inhibiting agents in a rheumatological outpatient clinic: description of six cases. Clin Rheumatol 2014;33(5):719–23. doi: 10.1007/ s10067-013-2419-8.
8. Fromont A, De Seze J, Fleury MC, et al. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine 2009;45(2):55–7. doi: 10.1016/ j.cyto.2008.11.002.
9. Matsumoto T, Nakamura I, Miura A, et al. New-onset multiple sclerosis associated with adalimumab treatment in rheumatoid arthritis: a case report and literature review. Clin Rheumatol 2013;32(2):271–5. doi: 10.1007/ s10067-012-2113-2.
10. Hare NC, Hunt DP, Venugopal K, et al. Multiple sclerosis in the context of TNF blockade and inflammatory bowel disease. QJM 2012;107(1):51–5. doi: 10.1093/ qjmed/ hcr237.
11. Deepak P, Stobaugh DJ, Sherid M, et al. Neurological events with tumour necrosis factor alpha inhibitors reported to the Food and Drug Administration Adverse Event Reporting System. Aliment Pharmacol Ther 2013;38(4):388–96. doi: 10.1111/ apt.12385.
12. Seror R, Richez C, Sordet C, et al. Pattern of demyelination occurring during anti-TNF-alpha therapy: a French national survey. Rheumatology (Oxford) 2013;52(5):868–74. doi: 10.1093/ rheumatology/ kes375.
13. Moris G. Inflammatory bowel disease: an increased risk factor for neurologic complications. World J Gastroenterol 2014;20(5):1228–37. doi: 10.3748/ wjg.v20.i5.1228.
14. Zitomersky NL, Levine AE, Atkinson BJ, et al. Risk factors, morbidity, and treatment of thrombosis in children and young adults with active inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2013;57(3):343–7. doi: 10.1097/ MPG.0b013e31829ce5cd.
15. Caminero A, Comabella M, Montalban X. Tumor necrosis factor alpha (TNF-alpha), anti-TNF-alpha and demyelination revisited: an ongoing story. J Neuroimmunol 2011;234(1–2):1–6. doi: 10.1016/ j.jneuroim.2011.03.004.
16. Lin Y, Zou Q, Li H. Tipping the balance: anti-tumour necrosis factor alpha therapy may damage cerebral nerve reservation. Med Hypotheses 2009;73(6):958–60. doi: 10.1016/ j.mehy.2009.06.029.
17. Gregory AP, Dendrou CA, Attfield KE et al. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature 2012;488(7412):508–11. doi: 10.1038/ nature11307.
18. Lin CH, Kadakia S, Frieri M. New insights into an autoimmune mechanism, pharmacological treatment and relationship between multiple sclerosis and inflammatory bowel disease. Autoimmun Rev 2014;13(2):114–6. doi: 10.1016/ j.autrev.2013.09.011.
19. Enayati PJ, Papadakis KA. Association of anti-tumor necrosis factor therapy with the development of multiple sclerosis. J Clin Gastroenterol 2005;39(4):303–6.
20. Sakuraba A, Annunziata ML, Cohen RD, et al. Mucosal healing is associated with improved long-term outcome of maintenance therapy with natalizumab in Crohn‘s disease. Inflamm Bowel Dis 2013;19(12):2577–83. doi: 10.1097/ MIB.0b013e3182a8df32.
21. Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum 2001;44(12):2862–9.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2017 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Diagnostic Pitfalls of an Atypical Form of Congenital Muscular Dystrophy – Partial Merosin Deficiency – Case Reports
- Extirpation of Colloid Cyst by an Endoscopic Approach
-
Analýza dat v neurologii
LXI. Závěrečné příklady k analýzám trendů v kontingenčních tabulkách -
Zpráva o činnosti Specializační oborové rady
Neurologie v roce 2016 - Zemřel prof. MUDr. Stanislav Němeček, DrSc.
- Životné jubileum prof. MUDr. Pavla Traubnera, PhD.
- Prof. MUDr. Evžen Růžička, DrSc., FCMA, FEAN slaví 60 let
- Zpráva z výročního kongresu České neurochirurgické společnosti
- Zpráva o 9. mikrovaskulárním workshopu
- Je třeba léčit motorické tiky
- Není třeba léčit motorické tiky
-
Komentář ke kontroverzím
Je třeba léčit motorické tiky? - Stem Cell Therapy for Amyotrophic Lateral Sclerosis – an Overview of Current Clinical Experience
- Editorial
- Genetics of Atypical Parkinsonism
- Current Perception of Contraindications and Complications of Nerve Conduction Studies and Needle Electromyography
- The Evaluation of Corneal Innervation Using Corneal Confocal Microscopy
- Diabetic Retinopathy and Changes in Corneal Nerve Fibers Assessed by Confocal Microscopy
- Validation of Myasthenia Gravis Quality of Life Questionnaire – Czech Version of MG-QOL15
- Periodic Limb Movements During Sleep are More Severe in Narcolepsy with Cataplexy than in Narcolepsy without Cataplexy
- An Association Between Early Metabolic Changes in the Brain and Selected Baseline Parametres in Patients after Subarachnoid Haemorrhage Due to Ruptured Intracranial Aneurysm
- Using Transcranial Sonography to Display Intracranial Structures in the B-mode
- The Changing Microbiological Pattern in Patients with Confirmed Bacterial Meningitis after Post-craniotomy Surgery
- Essential Neurological Examination – Time for Change?
- Základní neurologické vyšetření – nastal čas pro změny?
- Základní neurologické vyšetření – nastal čas pro změny?
- Neurological and MRI Screening Improves Long-term anti-TNF-α Treatment Safety in Patients with Crohn΄s Disease
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Není třeba léčit motorické tiky
- Current Perception of Contraindications and Complications of Nerve Conduction Studies and Needle Electromyography
- Essential Neurological Examination – Time for Change?
- Extirpation of Colloid Cyst by an Endoscopic Approach
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



