-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Decompressive craniectomy as treatment for a rat model of „malignant“ middle cerebral artery infarction
Dekompresní kraniektomie jako léčba pro krysí model „maligního“ infarktu střední mozkové tepny
Zkoumali jsme účinky dekompresní kraniektomie u krysího modelu „maligního“ infarktu střední mozkové tepny (ACM). Celkem 55 samců krys Sprague-Dawley o váze 290 až 340 g bylo rozděleno do těchto skupin: 1) simulovaně operovaná skupina (skupina 1, n = 14, jako kontrolní); 2) skupina s okluzí ACM (OACM) (skupina 2, n = 26, zůstala neléčená po permanentní OACM); 3) skupina s dekompresní kraniektomií (skupina 3, n = 15, vhodná dekomprese do 1 hodiny po OACM). Po 48 hodinách bylo zjištěno, že dekompresní kraniektomie může znamenat jednoznačný přínos z hlediska přežití, lepších neurologických výsledků, jakož i menšího rozsahu infarktu po OACM u krys. Výrazně snižuje odumírání neuronů v kůře, ne však v hlubokých strukturách, jako je hippocampus a striatum.
Klíčová slova:
dekompresní kraniektomie – mozkový infarkt – střední mozková tepna (ACM) – krysa
Authors: H. Weiwei 1; Y. Yu 2; L. Weiguo 1
Authors place of work: Department of Neurosurgery, nd Affiliated Hospital, School of Medicine, Zhejiang University Hangzhou, P. R. China 1; Department of Neurosurgery, Fudan University Affiliated Huashan Hospital, Shanghai, P. R. China 2
Published in the journal: Cesk Slov Neurol N 2007; 70/103(4): 388-391
Category: Původní práce
Summary
We investigated the effects of decompressive craniectomy in a rat model of „malignant“ middle cerebral artery (MCA) infarction. A total of 55 male Sprague-Dawley rats weighing 290–340 g were allocated to the following groups: (1) Sham operated group (group 1, n = 14, as controls); (2) MCA occlusion (MCAO) group (group 2, n = 26, remained untreated after right permanently MCAO); (3) Decompressive craniectomy group (group 3, n = 15, received right decompression at 1 hour after MCAO). 48 hours later we found that decompressive craniectomy can offer a clear survival benefit, a better neurological score as well as a reduced infarct volume after MCAO in rats. It significantly decreases neuronal death in cortex but not in deep structures such as hippocampus and striatum.
Key words:
decompressive craniectomy – cerebral infarction – middle cerebral artery (MCA) – ratIntroduction
There is still a subset of patients who deteriorate rapidly after hospital admission for cerebral infarction, with a mortality approaching 80% when treated conservatively [1,2]. This occurs in 10 to 15% of supratentorial infarction cases and is involved in the entire middle cerebral artery (MCA) territory. Patients with this „malignant“ MCA infarction suffer from coma or death because of large space-occupying brain edema and brain herniation. They may have concomitant anterior cerebral artery (ACA) or posterior cerebral artery (PCA) territory involvement. Thereby, this „malignant“ MCA infarction is also called large space-occupying infarction [1,2]. This patient subpopulation is a particularly difficult challenge for clinicians in charge. In the management of such cases, decompressive craniectomy has been recommended and indeed may be an appropriate, lifesaving procedure [1/3]. Our previous study also suggests patients' mortality may be better after surgery [3]. However so far, few experimental data have been published about the effects of decompressive craniectomy on neuronal death after acute stroke. The aim of this study, in which we used an endovascular model for the occlusion of MCA (MCAO) in rats, is to show the effects of decompressive craniectomy on mortality, infarct size, neurological score and the density of neurons. These findings may offer rich experimental data for the clinical management of malignant MCA infarction.
Materials and methods
Animal preparation
The study was approved by the local animal protection committee. Fifty-five male Sprague-Dawley rats weighing 290–340 g were allocated to 3 groups (group 1 to group 3). All animals were anesthetized with chloral hydrate (10%) by intra-peritoneal injection. MCAO was induced in 41 animals by an endovascular occlusion technique first described by Koizumi et al [4]. Briefly, the right common carotid artery and the right external carotid artery were exposed through a midline neck incision. A 4-0 monofilament nylon suture, whose tip had been coated with silicone, was then inserted through an arteriotomy of the common carotid artery and gently advanced into the internal carotid artery to a point approximately 18 mm distal to the carotid bifurcation. The common carotid artery was loosely ligated just distal to the arteriotomy, and after that the neck wound was closed. Sham-operated rats were used as control (group 1, n = 14,). Rats in group 2 remained untreated after permanent MCAO (n = 26). Animals received decompressive craniectomy at 1 hour after MCAO were enrolled in group 3 (n = 15).
Decompressive cranioctomy
In group 3, right decompressive craniectomy was performed as described by Forsting et al [5]. A bone flap (10 × 5 mm) was created in the parietal and temporal bone, and additional bone was removed down to the floor of the middle cerebral fossa. The dura covering the frontal, parietal, and temporal lobes was then opened by a large incision. At the end of the operation the temporalis muscle and skin flap were adapted and sutured in place.
Mortality, neurological score and infarction volume
At 48 hours after MCAO, we calculated three groups' mortality and examined all surviving animals neurologically using an established scoring system first introduced by Menzies et al6. The detailed are as the following: 0, no apparent deficits; 1, contralateral forelimb flexion; 2, decreased grip of contralateral forelimb while pulled by tail; 3, spontaneous movement in all directions „or“ or „and“ contralateral circling only if pulled by tail; 4, spontaneous contralateral circling; 5, death.
Part of animals were then killed, the brains were rapidly removed, and 2-mm brain slices were incubated for 30 minutes in a 4% solution of 2,3,5-triphenyltetrazolium chloride (TTC) at 37°C and fixed by immersion into 10% buffered formalin solution before photographed. TTC stains normal brain tissue (intact cellular membranes) red, while ischemic tissue turns pink and necrotic tissue turns grayish. After the digitization of the photographs, we quantified the size of infarction. To avoid the overestimation of the infarction volume, we used the corrected infarction volume (CIV) described by Lin et al [7] as the indicator. The ischemic lesion volume was expressed as absolute volume (mm3).
Neuronal density
At 48 hours later other sacrificed animals were perfused through the ascending aorta with saline followed by a solution of paraformaldehyde 4% in 0.1M phosphate buffer (PB). Twelve-micrometer thick brain coronal sections were cut by a freezing microtome and stored in PB at 4 °C. Sections were prepared with toluidine blue staining for light microscopic studies. They were examined by a pathologist who was blinded to the groups. Neuronal densities in cortex, hippocampus and striatum were respectively counted.
Data analysis
All values are expressed as mean ± SD. Data analysis was performed using SPSS version 10.0 for windows (SPSS Inc., Chicago, IL, USA). Rates were compared by the Fisher exact test. Kruskal-Wallis test was used to compare means between groups. Statistical significance was assigned to a P value of less than .05.
Results
Animal mortality at 48h after MCA occlusion was 46.2% (group 2), however, it was significant lower in the rats received decompression (group 3) (p<0.05). Meanwhile, rats in group 3 had better (ie. lower) neurological score (3.1 ±1.0 vs 4.0 ± 1.1, p < 0.05) and less infarct volume (172.28 ± 74.40 mm3 vs 253.22 ± 60.19 mm3, p < 0.05) (Figure 1). Decompressive craniectomy also significantly decreases neuronal death in cortex but not in deep structure such as hippocampus and striatum (Table 1).
Figure 1. Infarction volume in the three groups. 
Tab. 1. Comparison of mortality, neurological score, corrected infarction volume (CIV) and density of neurons 48 hours later between normal control group (group 1), MCAO group (group 2) and surgical decompression group (group 3). 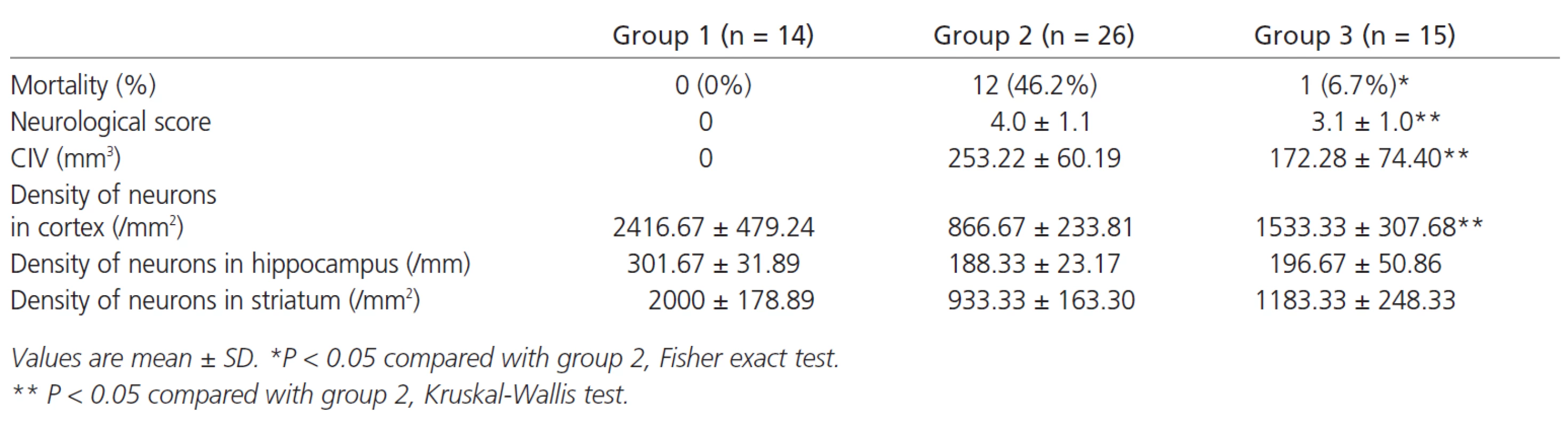
Discussion
Decompressive craniectomy with durotomy is usually performed as a last resort in patients with malignant brain edema caused by infarction or trauma [1/3]. Although decompressive craniectomy for supratentorial infarction has been done in a few patients sporadically over 4 decades, it is only in the last 2 decades that the treatment has been studied systematically. Several authors worldwide have shown encouraging results both in survival rate and functional outcome [1/3]. Despite these encouraging results, decompressive craniectomy has not gained widespread acceptance, especially in developing countries like China, where the incidence of stroke is much higher. Furthermore, few experimental findings have been published thus far about the effects of decompressive craniectomy on neuronal death after acute stroke.
In this study, we used a rat model of endovascular occlusion to simulate MCA occlusion nearly perfectly in humans. In its correct position the intraluminal suture reaches the proximal segment of the ACA. At this point the suture has blocked the origin of the MCA, occluding all sources of collateral blood flow from the ACA and PCA, resulting in large hemispheric ischemia lesions [4]. We found the mortality of rats with permanent MCA occlusion for 48 hours (46.2%) was really high which is very similar to the situation of clinical setting. So we choices this time point to examine decompression's value while other authors performed biopsy not early than 5 days [5/8]. We found decompressive surgery could also show its merit after 2 days. Craniectomy was performed at 1 hour because early surgery has shown satisfying neurological outcome [8] The mortality rate in our experimental study was 6.7% for all animals treated by decompressive craniectomy versus 46.2% in the MCAO group. Meanwhile our study validates the previous findings which suggest that neurological score and infarct volume may be better after surgery. The mechanism of better neurological outcome after decompression is that craniectomy can reduce ICP and the vicious circle of extensive edema, and can improve cerebral perfusion [5,9]. However, are there any changes at cellular level after decompressive craniectomy? We then analyzed neuronal densities of three sites through twelve-micrometer thick brain coronal sections. We found that decompressive surgery made an increase of survival cortical neurons from 866.67 ± ± 233.81/mm2 to 1533.33 ± 307.68/mm2 (p < 0.05),while lost its protection in the field of deep cerebral structures such as hippocampus and striatum. The possible mechanism is that craniectomy can only significantly improve cortical perfusion [9], which may offer more survival chances for cortical neurons as well as whole bodies. On the other hand, the phenomenon that the insulted deep brain structures can not benefit from decompressive craniectomy may explain why functional outcome and level of independence of patients are poor after surgery although survival rates are improved in our clinical setting [3].
Accepted for review: 16.10. 2006
Accepted for publication: 27. 2. 2007
Yao Yu, M.D Ph.D
Department of Neurosurgery,
Fudan University Affiliated
Huashan Hospital,
12# Wu Lu Mu Qi Zhong Road,
Shanghai, 200040, P.R.China
Email: yu_yao03@126.com
Zdroje
1. Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke 1998; 29 : 1888–1893.
2. Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. „Malignant“ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 2003; 53 : 309–315.
3. Yu Yao, Weiguo Liu, Xiaofeng Yang, Weiwei Hu, Gu Li. Is decompressive craniectomy for malignant middle cerebral artery infarction any benefits for elderly patients? Surg Neurol 2005; 64 : 165–169.
4. Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema, I: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke 1986; 8 : 1–8.
5. Forsting M, Reith W, Schaebitz WR, Heiland S, von Kummer R, Hacke W et al. Decompressive craniectomy for cerebral infarction: an experimental study in rats. Stroke 1995; 26 : 259–264.
6. Menzies SA, Hoff JT, Betz LA. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery 1992; 31 : 100–107.
7. Lin TN, He YY, Wu G, Khan M, Hsu CY. Effects on brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 1993; 24 : 117–121.
8. Engelhorn T, von kummer R, Reith W, Forsting M, Doerfler A. What is effective in malignant middle cerebral artery infarction: reperfusion, craniectomy, or both? An experiment study in rats. Stroke 2002; 33 : 617–622.
9. Engelhorn T, Doerfler A, Kastrup A, Beaulieu C, de Crespigny A, Forsting M et al. Decompressive craniectomy, reperfusion, or a combination for early treatment of acute „Malignant“ cerebral hemispheric stroke in rats? Potential mechanisms studied by MRI. Stroke 1999; 30 : 1456–1463.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2007 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Cervical dystonia
- Repetitive transcranial magnetic stimulation and chronic subjective tinnitus
- Levels of D-dimers in patients with acute ischaemic stroke
- Komentář k pilotní studii autorů D. Školoudíka et al. Změny kognitivních funkcí u pacientů s akutní cévní mozkovou příhodou testovaných pomocí Mini-Mental State Examination (MMSE) a Clock Drawing Test (CDT)
- Changes in congintive functions in patients with acute cerebrovascular event who tested by Mini-Mental State Examination and the Clock Drawing Test
- Decompressive craniectomy as treatment for a rat model of „malignant“ middle cerebral artery infarction
- Correlation of the IgG index and oligoclonal bands in the CSF of patients with multiple sclerosis
- Muscular biopsy in myotonic dystrophy in the era of molecular genetics
- Surgical treatment of hormonally active hypophysial adenomas
- Analysis of 1775 Patients Treated by Percutaneous Radiofrequency Rhizotomy for Trigeminal Neuralgia
- Regulation of mRNA expression of the SMN2 gene by histone deacetylase inhibitors and their influence on the phenotype of type I and II spinal muscular atrophy
- Komentář ke článku Balcer LJ, Galetta SL, Calabresi PA et al. Natalizumab reduces visual loss in patiens with relapsing multiple sclerosis. Neurology 2007; 68: 1299–1304.
- Poliomyelitis-like syndrome caused by tick-meningoencephalitis
- Satelitní anatomický worhshop Transtemporal approaches
- Thrombosis of the sigmoid sinus – current views on diagnosing and treatment
- The treatment of sleep apnea in young children using bilevel positive airway pressure
- Swallowing difficulties in diffuse idiopathic skeletal hyperostosis
- Hennerici MG, Daffertshofer M, Caplan LR, Szabo K (Eds). Case Studies in Stroke. Common and Uncommon Presentations. Cambridge: Cambridge University Press 2007. 272 p. ISBN 0-521-67367-4.
- Lze bez pochybností interpretovat výsledky lumbálního infuzního testu?
- Zpráva z 8. sjezdu Evropské společnosti báze lební
-
Analýza dat v neurologii. IV.
Variabilita měření není vždy „chyba“ - Webové okénko
- XVIII. neuromuskulární sympozium
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cervical dystonia
- Levels of D-dimers in patients with acute ischaemic stroke
- Thrombosis of the sigmoid sinus – current views on diagnosing and treatment
- Repetitive transcranial magnetic stimulation and chronic subjective tinnitus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



