-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Vedolizumab vs. ustekinumab as second-line therapy in Crohn’s disease in clinical practice
Vedolizumab vs. ustekinumab u Crohnovy choroby jako léčivo druhé linie v klinické praxi
Úvod:
Vedolizumab (VDZ) a ustekinumab (UST) nyní patří mezi dostupná biologická léčiva pro léčbu Crohnovy choroby (CD – Crohn’s disease), avšak omezené zkušenosti s těmito přípravky z klinické praxe jsou dosud limitující pro kvalifikovanou volbu optimální léčebné strategie po selhání přípravků ze skupiny anti-tumor nekotizující faktor (anti-TNF). Tato práce je zaměřena na porovnání účinnosti a bezpečnosti VDZ a UST jako biologických léčiv druhé linie u srovnatelných skupin pacientů s CD.
Metody:
Do hodnocení byli zařazeni konsekutivní pacienti s CD, kteří byli v minulosti léčeni alespoň jedním anti-TNF přípravkem. Pacienti byli sledováni v pravidelných intervalech, které odpovídaly termínům aplikace léčiv a během kterých byla hodnocena klinická aktivita onemocnění (HBI – Harvey-Bradshaw Index), zánětlivé parametry (C reaktivní protein, fekální kalprotektin) a byly zaznamenávány nežádoucí příhody. Primárním cílem bylo hodnocení podílu pacientů v klinické remisi (HBI ≤ 4) v týdnu 30– 32, klinické odpovědi ve formě poklesu HBI a podílu pacientů ukončujících léčbu.
Výsledky:
Hodnoceno bylo 45 pacientů s VDZ a 50 s UST. Obě kohorty byly srovnatelné ve všech hodnocených parametrech s výjimkou zastoupení pohlaví a podílu pacientů s penetrující formou choroby. Podíl pacientů v klinické remisi mezi nasazením léčby a týdnem 30– 32 vzrostl ve skupině s VDZ ze 44,4 na 58,1 % a ve skupině s UST z 55,1 na 63,2 %, avšak tento nárůst nedosáhl statistické významnosti. Průměrný pokles HBI u jednotlivých pacientů za sledované období ve skupině s VDZ dosáhl – 1,94 ± 5,14 (p = 0,05) a – 2,94 ± 5,91 (p = 0,01) ve skupině s UST. Podíl pacientů s UST v klinické remisi prosté kortikoterapie vzrostl ze 38,8 na 62,5 % (p = 0,04) a ve skupině s VDZ ze 33,3 na 45,2 % (p = 0,67). Terapii během sledování ukončilo 6 pacientů s VDZ a žádný s UST.
Závěr:
Z výsledků práce vyplývá srovnatelná efektivita VDZ a UST z pohledu míry dosažení klinické remise a odpovědi zánětlivých markerů, avšak v případě UST se projevil významnější steroidy šetřící účinek. Pro definici optimálního postavení nových biologik v algoritmu léčby CD bude nezbytné vyjít z prospektivních randomizovaných srovnávacích studií.
Klíčová slova:
idiopatické střevní záněty – biologická terapie – vedolizumab – ustekinumab
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Doručeno: 16. 2. 2019
Přijato: 19. 2. 2019
Authors: Kolar M. 1; Ďuricová D. 1; Bortlík M. 1,2,3; Pudilova K. 1; Hrubá V. 1; Machková N. 1; Mitrová K. 1,4; Malickova K. 1,5; Lukas M. jr. 1; Vasatko M. 1; Vanickova R. 1; Lukas M. 1,5
Authors place of work: IBD Clinical and Research Centre, ISCARE I. V. F. a. s., Prague, Czech Republic 1; Institute of Pharmacology, 1st Medical Faculty, Charles University, Prague, Czech Republic 2; Department of Internal Medicine, Military Hospital, Charles University, Prague, Czech Republic 3; Department of Paediatrics, 2nd Faculty of Medicine, Charles University in Prague and Motol University Hospital, Prague, Czech Republic 4; Institute of Medical Biochemistry and Laboratory Diagnostics, 1st Medical Faculty and General Teaching Hospital, Charles University, Prague, Czech Republic 5
Published in the journal: Gastroent Hepatol 2019; 73(1): 25-31
Category: IBD: původní práce
doi: https://doi.org/10.14735/amgh201925Summary
Background:
Vedolizumab (VDZ) and ustekinumab (UST) have become available for the treatment of Crohn’s disease (CD), however, due to limited clinical experience, the optimal treatment strategy after a failure of anti-tumor necrosis factor (anti-TNF) has yet to be elucidated. In our study, we aim to evaluate the efficiency and safety of VDZ and UST as second-line classes of biological therapy in a head-to-head manner in comparable populations of CD patients.
Methods:
Consecutive patients with CD who have previously been treated with anti-TNF therapy were included. Patients were followed at regular intervals coincident with drug applications and clinical activity (HBI – Harvey-Bradshaw Index), inflammatory markers (C-reactive protein, fecal calprotectin) and adverse events were recorded. The primary outcome was the proportion of patients in clinical remission in weeks 30– 32 (HBI ≤ 4), the clinical response in terms of HBI decrease, and the withdrawal rate.
Results:
Forty-five patients with VDZ and 50 with UST were included. Both groups were comparable in all the evaluated parameters with the exception of the male-to-female ratio and the proportion of patients with penetrating disease phenotype. The proportion of patients in clinical remission increased from 44.4% at baseline to 58.1% in weeks 30– 32 in the VDZ group and from 55.1% to 63.2% in the UST group; however, the increase was not statistically significant. The mean paired HBI difference between weeks 30– 32 and baseline reached – 1.94 ± 5.14 (p = 0.05) in the VDZ cohort and – 2.94 ± 5.91 (p = 0.01) in the UST cohort. The proportion of patients in steroid-free clinical remission increased from 38.8% to 62.5% in the UST cohort (p = 0.04), and from 33.3% to 45.2% in the VDZ (p = 0.67). Six patients on VDZ and none on UST discontinued the treatment.
Conclusion:
Our study demonstrated a comparable efficacy of VDZ and UST with respect to the rate of clinical remission and biomarker response; however, the steroid-sparing effect of UST was more prominent. There is a need for prospective randomized head-to-head trials to assess the optimal position of new biological agents in the treatment of patients with CD.
Key Words:
inflammatory bowel diseases – biological therapy – vedolizumab – ustekinumab
Introduction
Biological treatment is an established modality in the management of patients suffering from inflammatory bowel disease (IBD), however, only anti-tumor necrosis factor α (anti-TNF) antibodies were available for those patients for a long time. Despite their benefits in the management of IBD, anti-TNFs suffer from two main drawbacks: limited long-term efficacy, and a relatively high risk of adverse events [1,2].
In recent years, two new biological agents targeting different inflammatory pathways were granted marketing authorization for the treatment of Crohn’s disease (CD) – vedolizumab (VDZ) in 2014 and ustekinumab (UST) in 2016.
VDZ is a gut-selective monoclonal antibody targeting α4β7 integrin, which is preferentially expressed at a surface of a specific subgroup of T helper (Th) memory lymphocytes that take part in inducing inflammatory reactions in the gut [3]. It can be used to treat moderate to severe CD or UC after a failure or intolerance of conventional therapy or anti-TNF, and the recommended dosing regimen in CD includes 300 mg intravenous infusion at weeks 0, 2, and 6, and then every 8 weeks with the possibility of dosing at week 10 in the event of insufficient response.
UST is a fully human monoclonal antibody targeting a common subunit of interleukins (ILs) 12 and 23, thus blocking the pathway leading to the differentiation of Th cells towards Th1 and Th17, which are expected to play a central role in CD pathology [4]. UST can be used to treat moderate to severe CD with inadequate response to or intolerance or contraindication of conventional or anti-TNFα therapy. UST treatment is initiated with a single intravenous infusion of a dose based on body weight followed by 90 mg subcutaneous injection every 8 or 12 weeks.
However, the broadened spectrum of available biologics is not yet accompanied by sufficient data from clinical practice that would efficiently guide clinical decisions. Head-to-head clinical trials would provide the community with the best data on the choice of biologics in different phases of the disease. In our single-centre observational study, we retrospectively evaluated the efficiency and safety of VDZ and UST as second-line classes of biological therapy in a head-to-head manner in comparable populations of CD patients.
Methods
Patients
Consecutive patients with CD from a single tertiary centre who had been treated previously with anti-TNFα therapy (infliximab, adalimumab, or both), and in whom either VDZ or UST therapy was indicated between 2/ 2016 and 11/ 2018, were included. In this observational study, the patients were not matched, however, a comparison of all baseline characteristics in both cohorts was performed in order to reveal any potential significant differences between the groups.
Data collection
Baseline data including age, disease duration, smoking status, disease phenotype according to Montreal classification [5], previous CD-related surgery, and concomitant medications were recorded. The data were extracted from the CREdIT registry, the Czech national registry of biological and innovative therapy in IBD patients, as well as medical records, and assessed retrospectively. In our clinical practice, the patients are followed at regular intervals coincident with the drug applications. During each visit a blood sample is taken for analysis of the blood count and biochemistry, and a stool sample is collected for the measurement of fecal calprotectin (FC). The intervals between infusions as well as the dose of the drug were determined by a treating physician according to the patients’ needs. The clinical response was evaluated by the Harvey-Bradshaw index (HBI), which is routinely calculated during every visit. The need for dose optimization and the occurrence of any adverse events during the biological treatment were recorded. At the end of the follow-up the presence of concomitant medication was evaluated again.
Outcome measures
Clinical remission defined as HBI ≤ 4 and steroid-free clinical remission were assessed at week 32 for UST and week 30 for VDZ. The clinical and biological responses were evaluated as a change in HBI and a change in biomarkers (C-reactive protein (CRP) and FC) between baseline and the end of the follow-up. The withdrawal rate from both biologics due to lack of efficacy and/ or adverse events was recorded.
Statistical analysis
Standard descriptive statistical analyses were performed, including frequency distributions for categorical data and the calculation of the mean and standard deviation and/ or range for continuous variables. The differences between continuous variables at baseline were analyzed by a t-test or Mann-Whitney test, and between week 0 and weeks 30– 32, by a Wilcoxon test. The evaluation of categorical variable difference was performed by Fisher’s exact test. The statistical tests were performed using GraphPad Prism (version 8.0.1). A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
In total, 95 CD patients were analyzed, 45 patients were treated with VDZ and 50 with UST. The demographic and clinical characteristics of both cohorts at baseline are summarized in Tab. 1. Both groups were comparable in all evaluated parameters with the exception of the male-to-female ratio (75.6% females in the VDZ vs. 52.0% in the UST group; p = 0.02) and the proportion of patients with penetrating disease phenotype (6.7% in the VDZ vs. 30.0% in the UST; p = 0.004).
Tab. 1. Demographic and clinical characteristics of patients at week 0.
Tab. 1. Demografické a klinické charakteristiky pacientů v týdnu 0.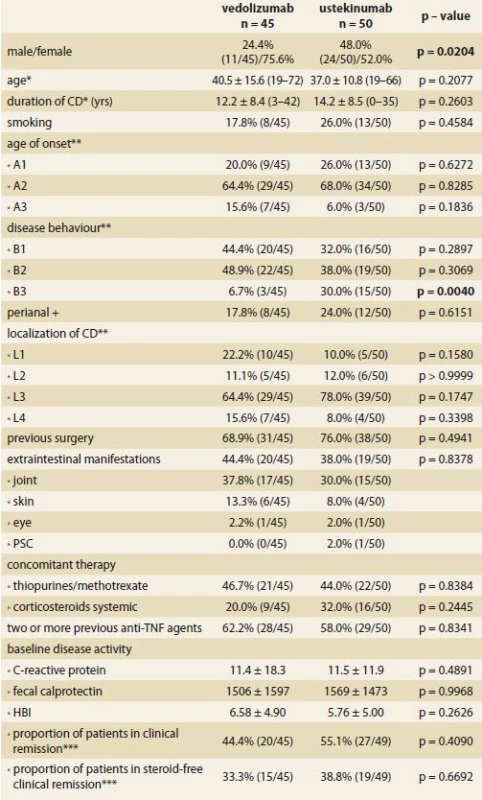
CD – Crohn’s disease, yrs – years, HBI – Harvey-Bradshaw index, * mean ± SD (range),
** Montreal classifi cation, *** HBI < 5, TNF – tumor necrosis factor, PSC – primary sclerosing cholangitisThe mean age at VDZ and UST initiation was 40.5 ± 15.6 years and 37.0 ± 10.8 years and the mean disease duration at that time reached 12.2 ± 8.4 years and 14.2 ± 8.5 years, respectively. Perianal disease was present in about 20% of the patients and the most frequent disease localization was ileocolonic. At least one previous abdominal surgery due to IBD was present in over two-thirds of the patients in both cohorts. Every patient had been treated previously by at least one anti-TNF agent, however, two or more different agents were used in about 60% of the patients in both groups. Nearly one-half of the patients had concomitant immunosuppression with either methotrexate or azathioprine. Between 20 and 30% were treated with systemic corticosteroids. The baseline inflammatory markers were similar in both groups with CRP of 11.5 mg/ L and FC of 1500 μg/ g. Clinical remission was present in 44.4 and 55.1% of the patients in the VDZ and UST groups, respectively.
Treatment efficacy
The proportion of patients in clinical remission according to HBI increased from 44.4 to 58.1% in the VDZ group and from 55.1 to 63.2% in the UST group, however, in neither of the groups did the difference reach a statistical significance (Graph 1). The mean HBI decreased significantly between baseline and week 30 in the VDZ group (6.58 ± 4.90 vs. 4.29 ± 3.67; p = 0.03), but in the case of UST the difference wasn’t significant (5.76 ± 5.00 vs. 4.68 ± 4.32; p = 0.82 (Graph 2). The mean paired HBI difference between weeks 30– 32 and baseline was, however, significantly different from zero in both biologics (– 1.94 ± 5.14; p = 0.05 in the VDZ cohort and – 2.94 ± 5.91; p = 0.01 in the UST cohort) (Graph 3).
Graph 1. Proportions of patients in clinical remission (HBI < 5) during follow-up period.
Graf 1. Podíly pacientů v klinické remisi dle HBI (< 5) v průběhu sledování.
Graph 2. Development of mean HBI from baseline to week 30–32.
Graf 2. Vývoj průměrného HBI od počátku sledování do týdne 30–32.
Graph 3. Decrease in HBI represented by mean paired HBI difference between timepoints during follow-up and baseline.
Graf 3. Pokles HBI v podobě rozdílu v párových hodnotách HBI mezi okamžiky v průběhu sledování a vstupní hodnotou.
The steroid sparing effect was well pronounced in the UST group, where the proportion of patients with systemic corticosteroids decreased from 32.0% at baseline to only 5.9% at week 32 (p = 0.01). In the VDZ group, 20.0% of the patients had systemic corticotherapy at both baseline and at week 30. The proportion of patients in steroid-free clinical remission increased from 38.8% at baseline to 62.5% at week 32 in patients with UST (p = 0.04) and from 33.3 to 45.2% in the VDZ group, however, without reaching statistical significance (Graph 4).
Graph 4. Proportions of patients in steroid-free clinical remission during follow-up period.
Graf 4. Podíly pacientů v klinické remisi prosté kortikosteroidů v průběhu sledování.
No decrease in the evaluated laboratory markers of inflammation was observed during the follow-up period in either of the cohorts. The mean CRP at baseline and weeks 30– 32 was 11.4 ± 18.3 vs. 12.1 ± 19.4 mg/ L (p = 0.48) in the VDZ cohort and 11.5 ± 11.9 vs. 9.5 ± 11.9 mg/ L (p = 0.85) in the UST group. For FC, the measured values were 1,506 ± 1,597 vs. 1,407 ± 1,799 μg/ g (p = 0.24) and 1,569 ± 1,473 vs. 929 ± 1,475 μg/ g (p = 0.15), respectively (Graph 5 and 6).
Graph 5. Development of mean C reactive protein from baseline to week 30-32.
Graf 5. Vývoj průměrné hodnoty C reaktivního proteinu od počátku sledování do týdne 30-32.
Graph 6. Development of mean fecal calprotectin from baseline to week 30-32.
Graf 6. Vývoj průměrné hodnoty fekálního kalprotektinu od počátku sledování do týdne 30–32.
The proportions of patients requiring an intensified dosing regimen was very similar at the end of the follow-up, reaching 14.3% (5/ 35) of the patients in the VDZ group and 14.7% (5/ 34) of the patients in the UST group, however, 28.6% (14/ 42) of the patients with VDZ also received an intensified induction regimen with an infusion at week 10.
In total, 6 patients had to discontinue the therapy with VDZ until the end of the follow-up – 4 due to primary non-response and 2 after adverse reactions. In the UST group none of the patients has stopped the treatment so far.
Safety
No patient with UST had discontinued treatment due to adverse events by the end of the follow-up. Two patients with vedolizumab stopped therapy as a result of adverse events, one due to an allergic infusion reaction and one refused to continue with the therapy after newly developed acne. The patient with the allergic reaction was switched directly to UST. The patient with adverse skin effects remained on thiopurines and started UST one year afterwards.
Twenty-four adverse events were registered in both biologics during the follow-up (Tab. 2). This represents 117.7 adverse events per 100 patient-years in case of UST and 103.5 per 100 patient-years in VDZ. Four cases of infusion reaction occurred in the VDZ group only. In both groups there was a single case of opportunistic mycotic infection of the esophagus and one case of molar pregnancy occurred in a patient on UST. No patients died during the follow-up.
Tab. 2. Adverse events occurring until week 32 (ustekinumab) or week 30 (vedolizumab).
Tab. 2. Nežádoucí účinky, které se vyskytly do týdne 32 (ustekinumab) nebo 30 (vedolizumab).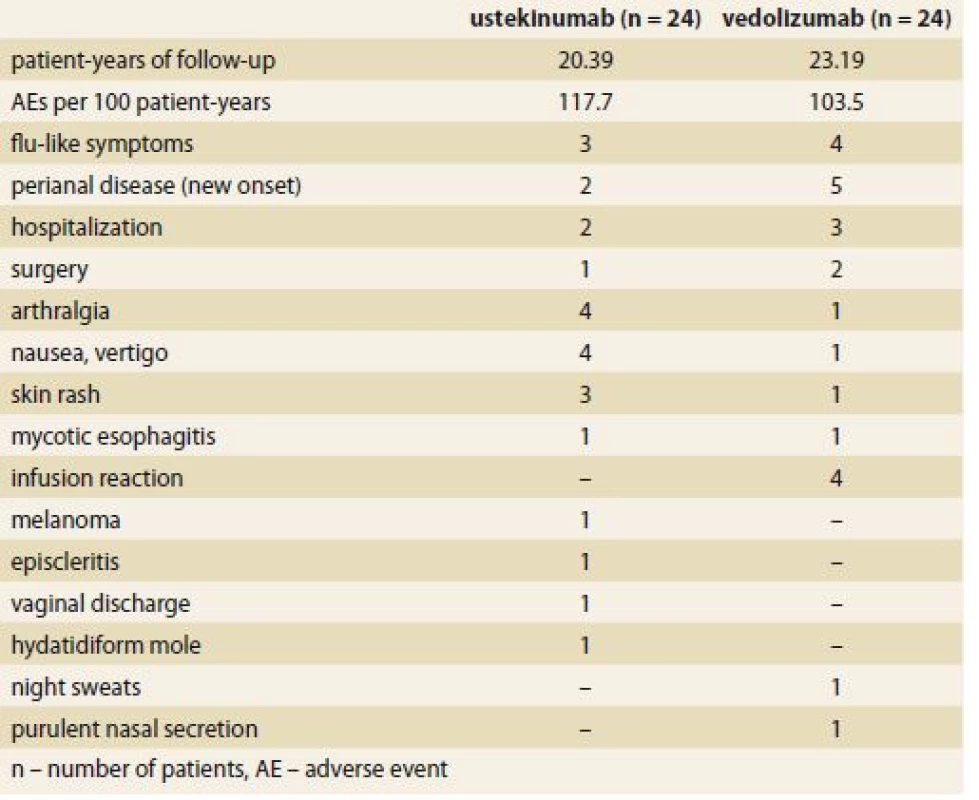
Discussion
The present study performed an indirect comparison of the efficacy and safety of new biological agents – VDZ and UST – in patients with CD in a real clinical practice. Both agents were shown to have similar mid-term efficacy with regard to clinical remission and biomarker response. However, more patients on UST were able to withdraw corticosteroids compared to VDZ. The safety profile was favorable, with no difference between the treatment groups.
Our finding of a mostly comparable efficacy between VDZ and UST is in line with the previous retrospective comparative studies reporting similar therapeutic efficacy between these agents in patients with CD [6,7]. Similarly, an indirect comparison of randomized controlled trials (RCTs) of VDZ and UST revealed no difference regarding the clinical remission of induction and maintenance therapy in patients with previous anti-TNF failure [8]. Likewise, recent network meta-analysis of RCTs comparing the relative efficacy of biologicals, including anti-TNFs, VDZ, and UST, as first - and second-line agents in patients with moderate to severe CD revealed no superiority of either agent (VDZ or UST) in individuals with prior exposure to anti-TNFs [9]. However, the relevance of these results for clinical practice is limited, since studies with different patient populations and study designs were compared. Thus, only properly designed head-to-head studies may provide the optimal estimation of the therapeutic efficacy of biological treatments.
In our study, UST was shown to have a better steroid-sparing effect than VDZ. Indeed, the proportion of patients on steroids did not change during the VDZ treatment. This is in contrast to other retrospective comparative studies, which reported similar rates of steroid-free remission between VDZ and UST [6,7]. The difference observed in our cohort probably indicates the effect of treatment selection bias despite the similar baseline characteristics of the patients. Further, mainly prospective randomized studies should address this issue.
The rate of clinical remission of more than 50% observed in both VDZ - and UST-treated patients in our study might seem too high compared to the results of clinical trials [10,11]. However, approximately one-half of our patients in each group were in clinical remission, defined by HBI, at the start of biological treatment despite high levels of biomarkers (FC, CRP), indicating the presence of active inflammation. This observation reflects the everyday clinical practice and points to a limitation of clinical indices in the estimation of therapeutic efficacy.
The evaluation of objective markers of inflammation – CRP and FC – revealed no significant decrease during the study in either treatment group. It is likely that the lack of therapeutic efficacy observed in both cohorts results from the overall treatment refractoriness of the study population, where all the patients were anti-TNF failures with more than one-half of them failing at least two anti-TNF agents.
The big advantage of both classes of new biologics over anti-TNFs seems to be their good safety profile, which was also demonstrated in our study. The gut selective mechanism of the action of VDZ provides an optimal treatment option for patients with an increased risk of infectious and non-infectious complications [12,13]. Although UST has systemic efficacy, the data from clinical trials, their extension observations, and cohort studies have reported favorable safety results thus far [14]. Furthermore, findings from the long-term registry of biological and non-biological treatment of psoriasis (PSOLAR) suggest a low risk of infectious complications in the use of UST [15].
There are several limitations to the study which have to be mentioned. Firstly, the retrospective observational design of the study precludes an adequate comparison of the treatment efficacy between the biologicals despite similar baseline patient characteristics. Furthermore, the sample size of the cohorts was small and might have led to a lack of statistical power in some comparisons. Finally, the follow-up of the cohorts was relatively short, and no endoscopic assessment of treatment efficacy was performed.
In conclusion, our study demonstrated the comparable efficacy of VDZ and UST with respect to the rate of clinical remission and biomarker response. However, UST seemed to be superior to VDZ as a steroid-sparing agent. There is a need for prospective randomized head-to-head trials to assess the optimal position of new biological agents in the treatment of patients with CD.
This research was supported by IBD-Comfort Foundation.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Submitted: 16. 2. 2019
Accepted: 19. 2. 2019
MUC. Martin Kolář
IBD Clinical and Research Centre
ISCARE I.V.F. a.s.
Jankovcova 1569/ 2c
170 04 Prague 7
Czech Republic
Zdroje
1. Roda G, Jharap B, Neeraj N et al. Loss of response to anti-tnfs: definition, epidemiology, and management. Clin Transl Gastroenterol 2016; 7: e135. doi: 10.1038/ ctg.2015.63.
2. Pagnini C, Arseneau KO, Cominelli F. Safety considerations when using anti-TNFα therapy to treat Crohn‘s disease. Expert Opin Drug Saf 2015; 14(1): 31– 44. doi: 10.1517/ 14740338. 2015.976610.
3. European Medicines Agency. Entyvio: EPAR – product information. [online]. Available from: https:/ / www.ema.europa.eu/ en/ medicines/ human/ EPAR/ entyvio.
4. European Medicines Agency. Stelara: EPAR – product information. [online]. Available from: https:/ / www.ema.europa.eu/ en/ medicines/ human/ EPAR/ stelara.
5. Satsangi J, Silverberg MS, Vermeire S et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55(6): 749– 753. doi: 10.1136/ gut.2005.082909.
6. Biemans V, van der Woude C, van der Meulen-de Jong A et al. DOP052 Vedolizumab vs. ustekinumab for Crohn’s disease: comparative effectiveness in a real-life observational cohort study. JCC 2018; 12 (Suppl 1): S066– S067. doi: 10.1093/ ecco-jcc/ jjx180.089.
7. Townsend T, Razanskaite V, Michail S et al. P586 Comparative effectiveness of vedolizumab and ustekinumab as induction therapy in anti-TNF refractory Crohn’s disease: a multi-centre retrospective cohort study. JCC 2019; 13 (Suppl 1): S407– S408. doi: 10.1093/ ecco-jcc/ jjy222.710.
8. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn‘s disease patients. J Comp Eff Res 2018; 7(2): 101– 111. doi: 10.2217/ cer-2017-0041.
9. Singh S, Fumery M, Sandborn WJ et al. Systematic review and network meta-analysis: first - and second-line biologic therapies for moderate-severe Crohn‘s disease. Aliment Pharmacol Ther 2018; 48(4): 394– 409. doi: 10.1111/ apt.14852.
10. Sandborn WJ, Feagan BG, Rutgeerts P et al. Vedolizumab as induction and maintenance therapy for Crohn‘s disease. N Engl J Med 2013; 369(8): 711– 721. doi: 10.1056/ NEJMoa1215739.
11. Feagan BG, Sandborn WJ, Gasink C et al. Ustekinumab as induction and maintenance therapy for Crohn‘s disease. N Engl J Med 2016; 375(20): 1946– 1960. doi: 10.1056/ NEJMoa160 2773.
12. Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther 2017; 46(1): 3– 15. doi: 10.1111/ apt.14075.
13. Colombel JF, Sands BE, Rutgeerts P et al. The safety of vedolizumab for ulcerative colitis and Crohn‘s disease. Gut 2017; 66(5): 839– 851. doi: 10.1136/ gutjnl-2015-311079.
14. Armuzzi A, Ardizzone S, Biancone L et al. Ustekinumab in the management of Crohn‘s disease: expert opinion. Dig Liver Dis 2018; 50(7): 653– 660. doi: 10.1016/ j.dld.2018.02.017.
15. Papp K, Gottlieb AB, Naldi L et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol 2015; 14(7): 706– 714.
Štítky
Dětská gastroenterologie Gastroenterologie a hepatologie Chirurgie všeobecná
Článek vyšel v časopiseGastroenterologie a hepatologie
Nejčtenější tento týden
2019 Číslo 1- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
-
Všechny články tohoto čísla
- Doporučení pro podávání biologické léčby pacientům s idiopatickými střevními záněty: čtvrté, aktualizované vydán
- Vedolizumab vs. ustekinumab as second-line therapy in Crohn’s disease in clinical practice
- Early vedolizumab trough levels are not associated with short-term response in patients with inflammatory bowel disease
- Inflammatory bowel disease and medical assessment service
- Treatment of a pregnant Crohn’s disease patient with ustekinumab: a case report
- Ileocaecal Crohn’s disease and familial adenomatous polyposis in one patient – a case report
- News in 2019
- Surprising etiology of terminal common bile duct stricture
- Therapeutic trial of proton pump inhibitors for management of patients with laryngopharyngeal reflux
- Biologics for inflammatory bowel disease – forth time
- Does abdominal compression improve the success rate of and tolerance to colonoscopy?
- Toxic and drug damage of the liver and kidneys
- Díl I. Editorial – zpravodajství Národního zdravotnického informačního systému
- The selection from international journals
- Kreditovaný autodidaktický test
- Správná odpověď na kvíz
- Clensia® – the first combined cleansing agent with simeticone
- Kvíz z klinické praxe
- Gastroenterologie a hepatologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Clensia® – the first combined cleansing agent with simeticone
- Toxic and drug damage of the liver and kidneys
- Surprising etiology of terminal common bile duct stricture
- Therapeutic trial of proton pump inhibitors for management of patients with laryngopharyngeal reflux
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



